Optimized Approaches for the Induction of Putative Canine Induced Pluripotent Stem Cells from Old Fibroblasts Using Synthetic RNAs
Simple Summary
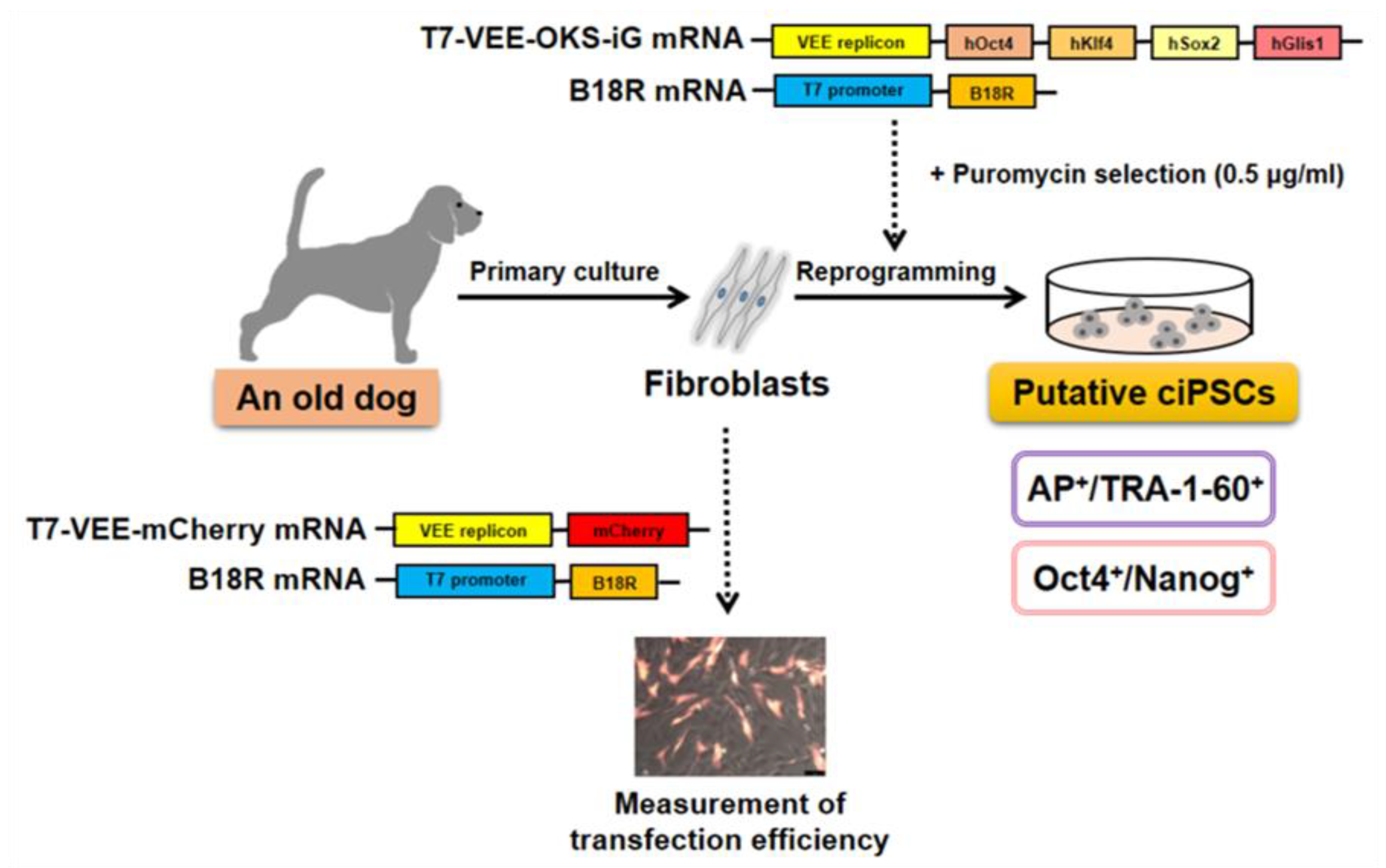
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Chemicals
2.3. Cell Culture
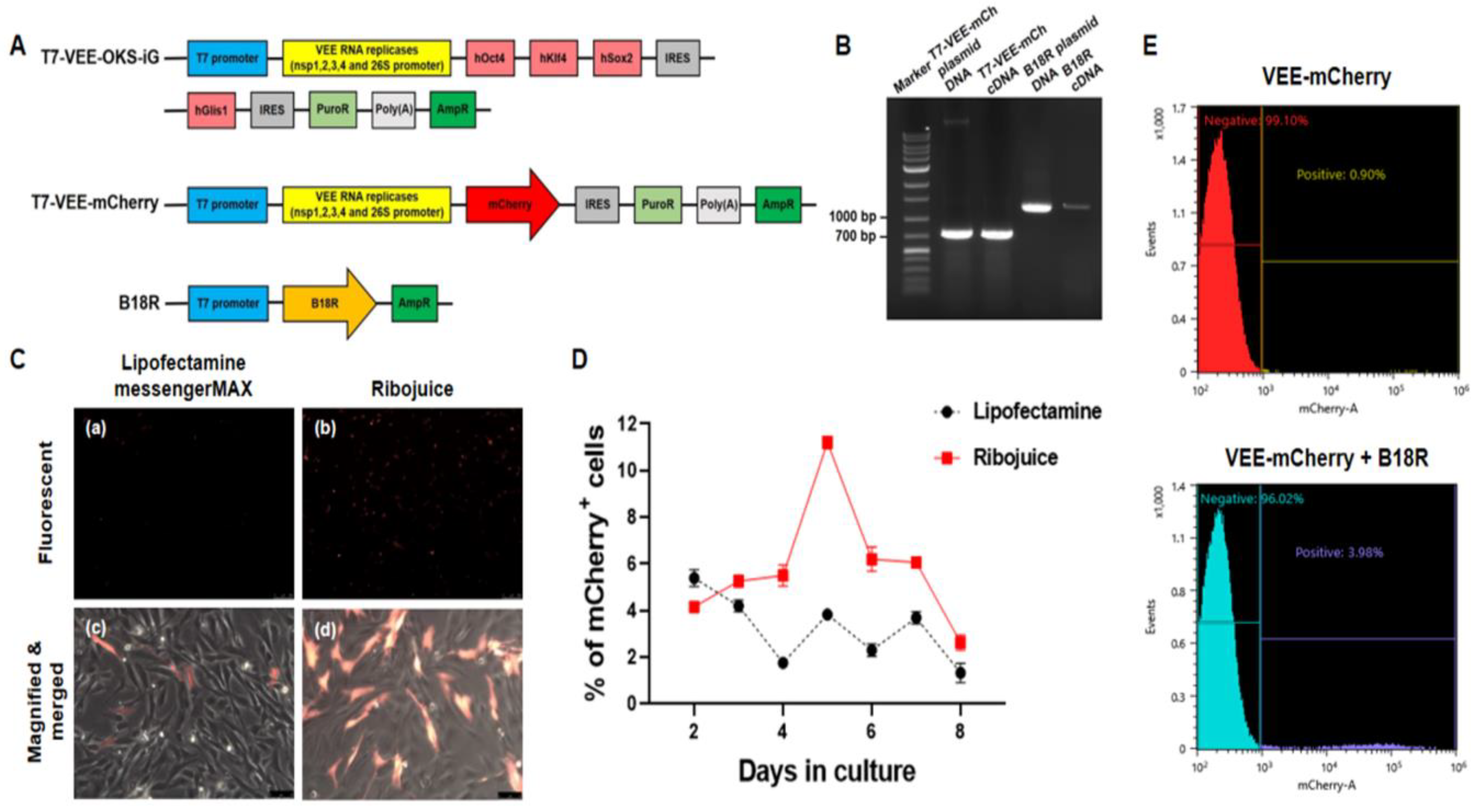
2.4. Plasmid Construction and RNA Synthesis
2.5. Determination of Optimal Puromycin Concentration
2.6. Measurement of Transfection Efficiencies by Flow Cytometry
2.7. Preparation of Feeder Cells
2.8. Preparation of Mouse Embryonic Fibroblast Conditioned Medium (MEF-CM)
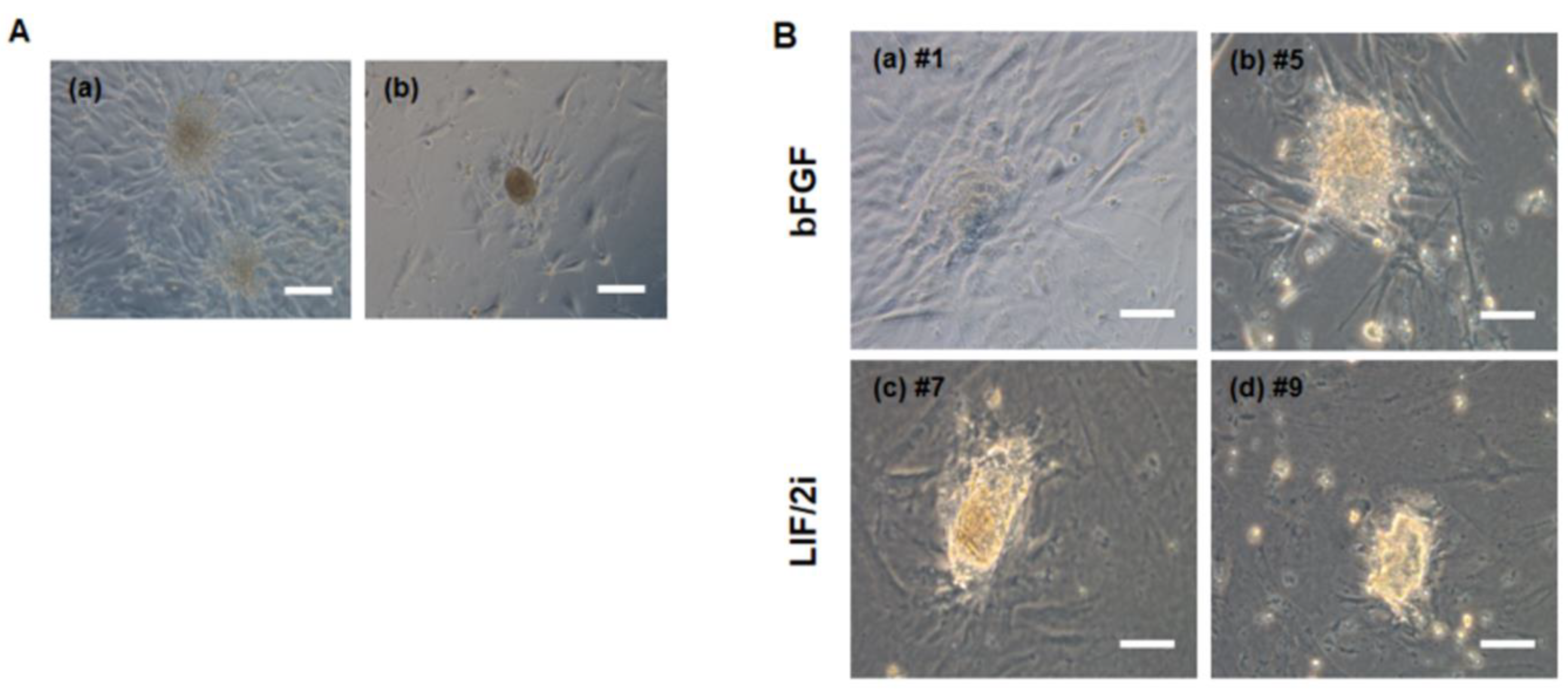
2.9. Generation and Culture of the Putative ciPSCs
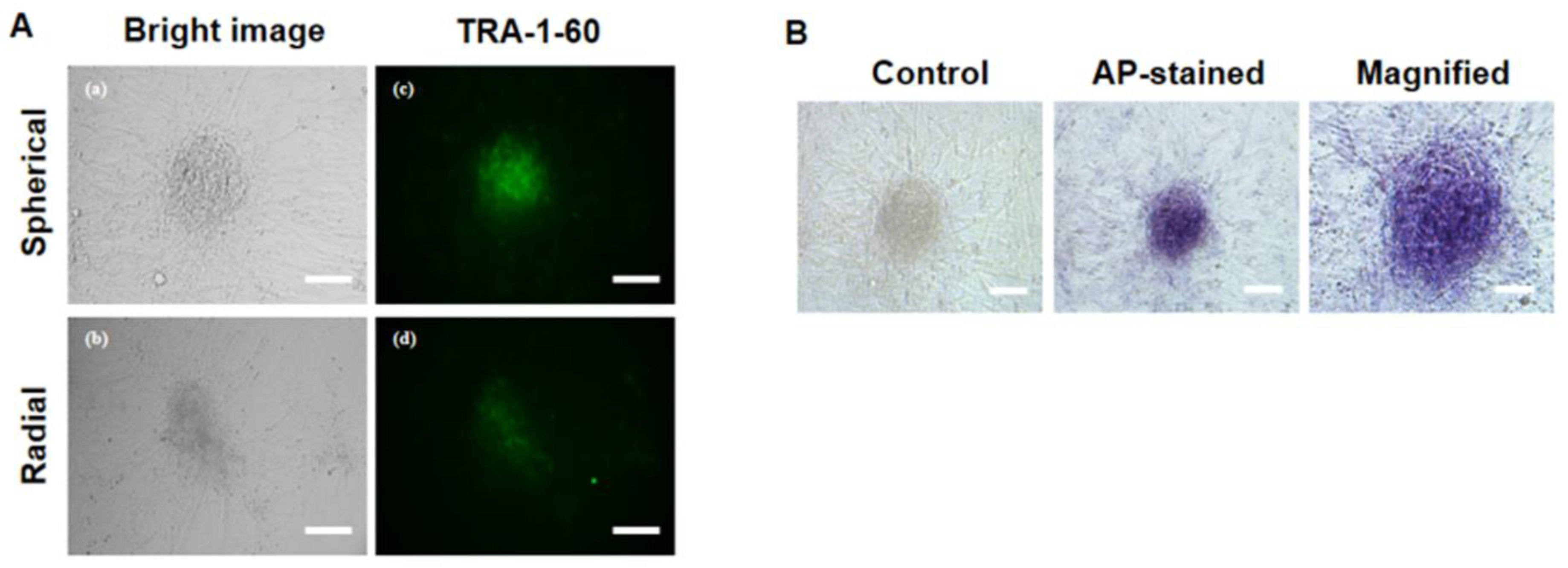
2.10. Primary iPSC Colony Staining with Alkaline Phosphatase (AP) and TRA-1-60
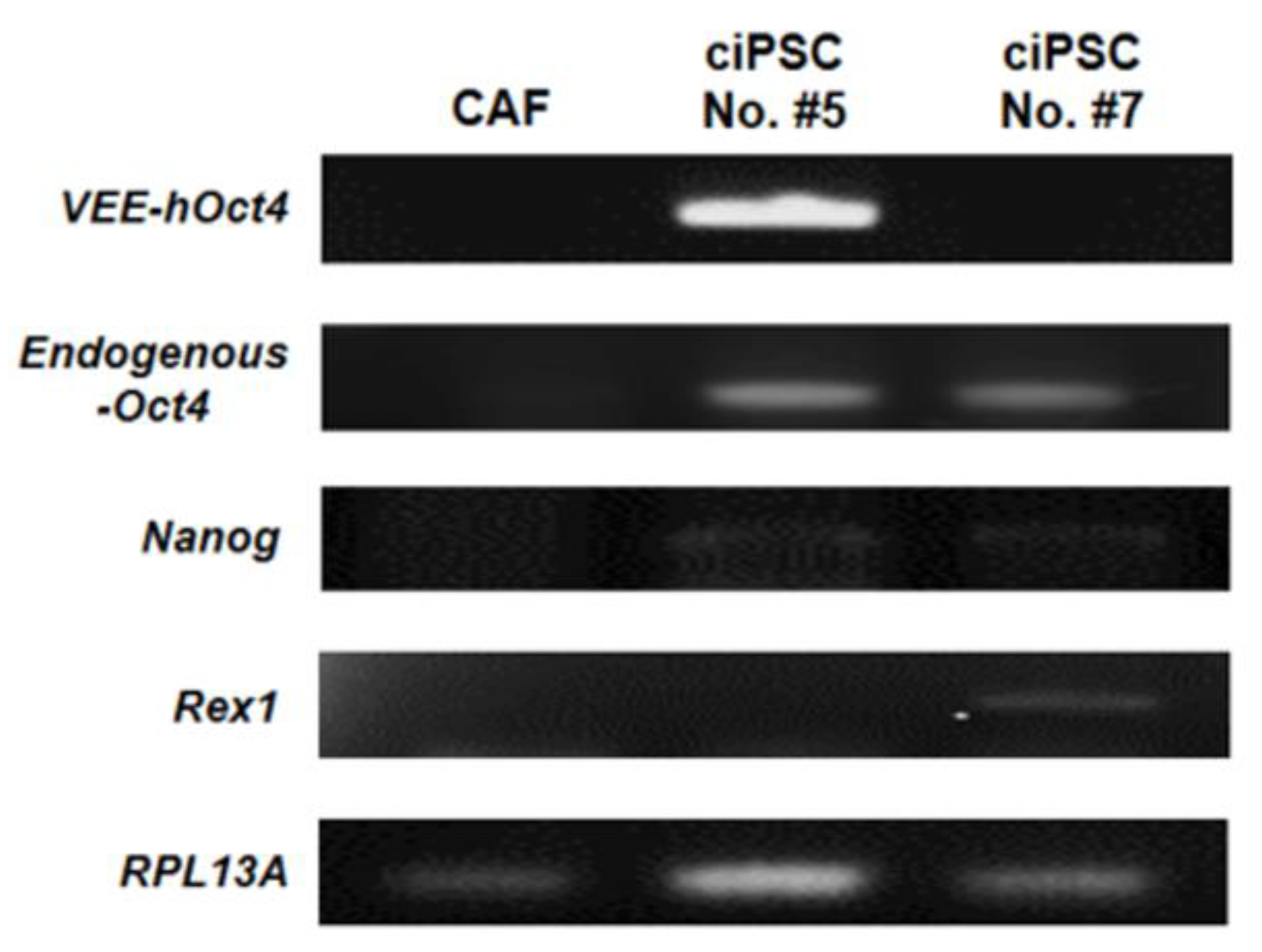
2.11. Gene Expression Analysis of Putative ciPSCs by Reverse Transcription PCR (RT-PCR)
2.12. Statistical Analysis
3. Results
3.1. Determination of Optimal Puromycin Concentration
3.2. Synthesis of RNA by In Vitro Transcription (IVT) and Measurement of Transfection Efficiencies
3.3. Generation of Putative ciPSCs Using Fibroblasts from an Aged Dog
3.4. Characterization of Pluripotency Markers in Primary ciPSC Colonies
3.5. Gene Expression Analysis of Putative ciPSCs by RT-PCR
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-H.; Zhao, R.; West, J.A.; Yabuuchi, A.; Huo, H.; Ince, T.A.; Lerou, P.H.; Lensch, M.W.; Daley, G.Q. Reprogramming of human somatic cells to pluripotency with defined factors. Nature 2008, 451, 141. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Zucconi, E.; Vieira, N.M.; Bueno, D.F.; Secco, M.; Jazedje, T.; Ambrosio, C.E.; Passos-Bueno, M.R.; Miglino, M.A.; Zatz, M. Mesenchymal stem cells derived from canine umbilical cord vein—a novel source for cell therapy studies. Stem Cells Dev. 2010, 19, 395–402. [Google Scholar] [CrossRef]
- Shearin, A.L.; Ostrander, E.A. Leading the way: Canine models of genomics and disease. Dis. Models Mech. 2010, 3, 27–34. [Google Scholar] [CrossRef]
- Tsai, K.L.; Clark, L.A.; Murphy, K.E. Understanding hereditary diseases using the dog and human as companion model systems. Mamm. Genome 2007, 18, 444–451. [Google Scholar] [CrossRef]
- Nishimura, T.; Hatoya, S.; Kanegi, R.; Wijesekera, D.P.H.; Sanno, K.; Tanaka, E.; Sugiura, K.; Tamada, H.; Kawate, N.; Imai, H. Feeder-independent canine induced pluripotent stem cells maintained under serum-free conditions. Mol. Reprod. Dev. 2017, 84, 329–339. [Google Scholar] [CrossRef]
- Shimada, H.; Nakada, A.; Hashimoto, Y.; Shigeno, K.; Shionoya, Y.; Nakamura, T. Generation of canine induced pluripotent stem cells by retroviral transduction and chemical inhibitors. Mol. Reprod. Dev. 2010, 77, 2. [Google Scholar] [CrossRef]
- Hainsworth, A.H.; Allan, S.M.; Boltze, J.; Cunningham, C.; Farris, C.; Head, E.; Ihara, M.; Isaacs, J.D.; Kalaria, R.N.; Oberstein, S.A.L. Translational models for vascular cognitive impairment: A review including larger species. BMC Med. 2017, 15, 16. [Google Scholar] [CrossRef]
- Whitworth, D.J.; Ovchinnikov, D.A.; Wolvetang, E.J. Generation and characterization of LIF-dependent canine induced pluripotent stem cells from adult dermal fibroblasts. Stem Cells Dev. 2012, 21, 2288–2297. [Google Scholar] [CrossRef]
- Tecirlioglu, R.T.; Trounson, A.O. Embryonic stem cells in companion animals (horses, dogs and cats): Present status and future prospects. Reprod. Fertil. Dev. 2007, 19, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Hytönen, M.K.; Lohi, H. Canine models of human rare disorders. Rare Dis. 2016, 4, e1006037. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.H.; Ollier, W.E.; Nuttall, T.; McEwan, N.A.; Carter, S.D. Despite identifying some shared gene associations with human atopic dermatitis the use of multiple dog breeds from various locations limits detection of gene associations in canine atopic dermatitis. Vet. Immunol. Immunopathol. 2010, 138, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Paoloni, M.; Khanna, C. Translation of new cancer treatments from pet dogs to humans. Nat. Rev. Cancer 2008, 8, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A.; Pugh, R.N. Dogs, zoonoses and immunosuppression. J. R. Soc. Promot. Health 2002, 122, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Betts, D.H.; Tobias, I.C. Canine Pluripotent Stem Cells: Are They Ready for Clinical Applications? Front. Vet. Sci. 2015, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Debowski, K.; Warthemann, R.; Lentes, J.; Salinas-Riester, G.; Dressel, R.; Langenstroth, D.; Gromoll, J.; Sasaki, E.; Behr, R. Non-viral generation of marmoset monkey iPS cells by a six-factor-in-one-vector approach. PLoS ONE 2015, 10, e0118424. [Google Scholar] [CrossRef]
- Schlaeger, T.M.; Daheron, L.; Brickler, T.R.; Entwisle, S.; Chan, K.; Cianci, A.; DeVine, A.; Ettenger, A.; Fitzgerald, K.; Godfrey, M. A comparison of non-integrating reprogramming methods. Nat. Biotechnol. 2015, 33, 58–63. [Google Scholar] [CrossRef]
- Hoffman, B.; Liebermann, D.A. The proto-oncogene c-myc and apoptosis. Oncogene 1998, 17. [Google Scholar] [CrossRef]
- Hoffman, B.; Amanullah, A.; Shafarenko, M.; Liebermann, D.A. The proto-oncogene c-myc in hematopoietic development and leukemogenesis. Oncogene 2002, 21, 3414–3421. [Google Scholar] [CrossRef]
- Vafa, O.; Wade, M.; Kern, S.; Beeche, M.; Pandita, T.K.; Hampton, G.M.; Wahl, G.M. c-Myc can induce DNA damage, increase reactive oxygen species, and mitigate p53 function: A mechanism for oncogene-induced genetic instability. Mol. Cell 2002, 9, 1031–1044. [Google Scholar] [CrossRef]
- Maekawa, M.; Yamaguchi, K.; Nakamura, T.; Shibukawa, R.; Kodanaka, I.; Ichisaka, T.; Kawamura, Y.; Mochizuki, H.; Goshima, N.; Yamanaka, S. Direct reprogramming of somatic cells is promoted by maternal transcription factor Glis1. Nature 2011, 474, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008, 26, 101. [Google Scholar] [CrossRef]
- Chow, L.; Johnson, V.; Regan, D.; Wheat, W.; Webb, S.; Koch, P.; Dow, S. Safety and immune regulatory properties of canine induced pluripotent stem cell-derived mesenchymal stem cells. Stem Cell Res. 2017, 25, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Nishimura, T.; Yodoe, K.; Kanegi, R.; Tsujimoto, Y.; Alam, M.E.; Kuramochi, M.; Kuwamura, M.; Ohtaka, M.; Nishimura, K. Generation of footprint-free canine induced pluripotent stem cells using auto-erasable Sendai virus vector. Stem Cells Dev. 2018, 27, 1577–1586. [Google Scholar] [CrossRef]
- Yoshioka, N.; Gros, E.; Li, H.-R.; Kumar, S.; Deacon, D.C.; Maron, C.; Muotri, A.R.; Chi, N.C.; Fu, X.-D.; Benjamin, D.Y. Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell 2013, 13, 246–254. [Google Scholar] [CrossRef]
- Yoshioka, N.; Dowdy, S.F. Enhanced generation of iPSCs from older adult human cells by a synthetic five-factor self-replicative RNA. PLoS ONE 2017, 12, e0182018. [Google Scholar] [CrossRef]
- Kinney, R.M.; Johnson, B.J.; Welch, J.B.; Tsuchiya, K.R.; Trent, D.W. The full-length nucleotide sequences of the virulent Trinidad donkey strain of Venezuelan equine encephalitis virus and its attenuated vaccine derivative, strain TC-83. Virology 1989, 170, 19–30. [Google Scholar] [CrossRef]
- Greenway, T.E.; Eldridge, J.H.; Ludwig, G.; Staas, J.K.; Smith, J.F.; Gilley, R.M.; Michalek, S.M. Enhancement of protective immune responses to Venezuelan equine encephalitis (VEE) virus with microencapsulated vaccine. Vaccine 1995, 13, 1411–1420. [Google Scholar] [CrossRef]
- Paessler, S.; Ni, H.; Petrakova, O.; Fayzulin, R.Z.; Yun, N.; Anishchenko, M.; Weaver, S.C.; Frolov, I. Replication and clearance of Venezuelan equine encephalitis virus from the brains of animals vaccinated with chimeric SIN/VEE viruses. J. Virol. 2006, 80, 2784–2796. [Google Scholar] [CrossRef]
- Gonçalves, N.; Bressan, F.; Roballo, K.; Meirelles, F.; Xavier, P.; Fukumasu, H.; Williams, C.; Breen, M.; Koh, S.; Sper, R. Generation of LIF-independent induced pluripotent stem cells from canine fetal fibroblasts. Theriogenology 2017, 92, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Cibelli, J.B. Conserved Role of bFGF and a Divergent Role of LIF for Pluripotency Maintenance and Survival in Canine Pluripotent Stem Cells. Stem Cells Dev. 2016, 25, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Kalkan, T.; Olova, N.; Roode, M.; Mulas, C.; Lee, H.J.; Nett, I.; Marks, H.; Walker, R.; Stunnenberg, H.G.; Lilley, K.S. Tracking the embryonic stem cell transition from ground state pluripotency. Development 2017, 144, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Heo, J.S.; Kwon, S.; Kim, J.; Hwang, J.; Kang, P.J.; Kim, A.; Kim, H.O.; Whang, K.Y.; Yoon, B.S. Two-step generation of induced pluripotent stem cells from mouse fibroblasts using Id3 and Oct4. J. Mol. Cell Biol. 2011, 4, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.M.; Ratanasirintrawoot, S.; Park, I.-H.; Manos, P.D.; Loh, Y.-H.; Huo, H.; Miller, J.D.; Hartung, O.; Rho, J.; Ince, T.A. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat. Biotechnol. 2009, 27, 1033–1037. [Google Scholar] [CrossRef]
- Lee, A.S.; Xu, D.; Plews, J.R.; Nguyen, P.K.; Nag, D.; Lyons, J.K.; Han, L.; Hu, S.; Lan, F.; Liu, J. Preclinical derivation and imaging of autologously transplanted canine induced pluripotent stem cells. J. Biol. Chem. 2011, 286, 32697–32704. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Suhr, S.T.; Chang, E.A.; Wang, K.; Ross, P.J.; Nelson, L.L.; Venta, P.J.; Knott, J.G.; Cibelli, J.B. Generation of leukemia inhibitory factor and basic fibroblast growth factor-dependent induced pluripotent stem cells from canine adult somatic cells. Stem Cells Dev. 2011, 20, 1669–1678. [Google Scholar] [CrossRef]
- You, S.; Moon, J.-H.; Kim, T.-K.; Kim, S.-C.; Kim, J.-W.; Yoon, D.-H.; Kwak, S.; Hong, K.-C.; Choi, Y.-J.; Kim, H. Cellular characteristics of primary and immortal canine embryonic fibroblast cells. Exp. Mol. Med. 2004, 36, 325. [Google Scholar] [CrossRef]
- Steinle, H.; Weber, M.; Behring, A.; Mau-Holzmann, U.; Schlensak, C.; Wendel, H.P.; Avci-Adali, M. Generation of iPSCs by nonintegrative RNA-based reprogramming techniques: Benefits of self-replicating RNA versus synthetic mRNA. Stem Cells Int. 2019. [Google Scholar] [CrossRef]
- Alcamí, A.; Symons, J.A.; Smith, G.L. The vaccinia virus soluble alpha/beta interferon (IFN) receptor binds to the cell surface and protects cells from the antiviral effects of IFN. J. Virol. 2000, 74, 11230–11239. [Google Scholar] [CrossRef]
- Warren, L.; Manos, P.D.; Ahfeldt, T.; Loh, Y.-H.; Li, H.; Lau, F.; Ebina, W.; Mandal, P.K.; Smith, Z.D.; Meissner, A. Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 2010, 7, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Mirae Kim, S.-U.H.; Hyun, S.-H. An integration-free method for the generation of putative induced pluripotent stem cells derived from aged dogs. In Proceedings of the ISSCR 2018 Annual Meeting, Melbourne, Australia, 20 June 2018; p. 282. [Google Scholar]
- Kalkan, T.; Smith, A. Mapping the route from naive pluripotency to lineage specification. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130540. [Google Scholar] [CrossRef] [PubMed]
- Sosa, E.; Kim, R.; Rojas, E.J.; Hosohama, L.; Hennebold, J.D.; Orwig, K.E.; Clark, A.T. An integration-free, virus-free rhesus macaque induced pluripotent stem cell line (riPSC89) from embryonic fibroblasts. Stem Cell Res. 2016, 17, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Iwamoto, M.; Suzuki, S.-i.; Fuchimoto, D.; Honma, D.; Nagai, T.; Hashimoto, M.; Yazaki, S.; Sato, M.; Onishi, A. A novel method for the production of transgenic cloned pigs: Electroporation-mediated gene transfer to non-cultured cells and subsequent selection with puromycin. Biol. Reprod. 2005, 72, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.G.; Oh, H.J.; Park, J.E.; Kim, M.J.; Kim, G.A.; Koo, O.J.; Jang, G.; Lee, B.C. Production of transgenic canine embryos using interspecies somatic cell nuclear transfer. Zygote 2012, 20, 67–72. [Google Scholar] [CrossRef]
- Techangamsuwan, S.; Kreutzer, R.; Kreutzer, M.; Imbschweiler, I.; Rohn, K.; Wewetzer, K.; Baumgärtner, W. Transfection of adult canine Schwann cells and olfactory ensheathing cells at early and late passage with human TERT differentially affects growth factor responsiveness and in vitro growth. J. Neurosci. Methods 2009, 176, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sayed, N.; Hunter, A.; Au, K.F.; Wong, W.H.; Mocarski, E.S.; Pera, R.R.; Yakubov, E.; Cooke, J.P. Activation of innate immunity is required for efficient nuclear reprogramming. Cell 2012, 151, 547–558. [Google Scholar] [CrossRef]
- Weinberger, L.; Ayyash, M.; Novershtern, N.; Hanna, J.H. Dynamic stem cell states: Naive to primed pluripotency in rodents and humans. Nat. Rev. Mol. Cell Biol. 2016, 17, 155–169. [Google Scholar] [CrossRef]
- Silva, J.; Nichols, J.; Theunissen, T.W.; Guo, G.; van Oosten, A.L.; Barrandon, O.; Wray, J.; Yamanaka, S.; Chambers, I.; Smith, A. Nanog is the gateway to the pluripotent ground state. Cell 2009, 138, 722–737. [Google Scholar] [CrossRef]
- Wen, Y.; Wani, P.; Zhou, L.; Baer, T.; Phadnis, S.M.; Reijo Pera, R.A.; Chen, B. Reprogramming of fibroblasts from older women with pelvic floor disorders alters cellular behavior associated with donor age. Stem Cells Transl. Med. 2013, 2, 118–128. [Google Scholar] [CrossRef]
- Neely, M.D.; Tidball, A.M.; Aboud, A.A.; Ess, K.C.; Bowman, A.B. Induced Pluripotent Stem Cells (iPSCs): An emerging model system for the study of human neurotoxicology. In Cell Culture Techniques; Springer: New York, NY, USA, 2011; pp. 27–61. [Google Scholar]
- Rosselló, R.A.; Chen, C.-C.; Dai, R.; Howard, J.T.; Hochgeschwender, U.; Jarvis, E.D. Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. eLife 2013, 2, e00036. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Thomas, R.; Tsai, S.; Bischoff, S.; Lim, J.-H.; Breen, M.; Olby, N.J.; Piedrahita, J.A. Growth requirements and chromosomal instability of induced pluripotent stem cells generated from adult canine fibroblasts. Stem Cells Dev. 2012, 22, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Hatoya, S.; Kanegi, R.; Sugiura, K.; Wijewardana, V.; Kuwamura, M.; Tanaka, M.; Yamate, J.; Izawa, T.; Takahashi, M. Generation of functional platelets from canine induced pluripotent stem cells. Stem Cells Dev. 2013, 22, 2026–2035. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Polo, J.M. Phases of reprogramming. Stem Cell Res. 2014, 12, 754–761. [Google Scholar] [CrossRef]
- Hassani, S.-N.; Totonchi, M.; Gourabi, H.; Schöler, H.R.; Baharvand, H. Signaling roadmap modulating naive and primed pluripotency. Stem Cells Dev. 2014, 23, 193–208. [Google Scholar] [CrossRef]
- Angel, M.; Yanik, M.F. Innate immune suppression enables frequent transfection with RNA encoding reprogramming proteins. PLoS ONE 2010, 5, e11756. [Google Scholar] [CrossRef]
- Warren, L.; Ni, Y.; Wang, J.; Guo, X. Feeder-free derivation of human induced pluripotent stem cells with messenger RNA. Sci. Rep. 2012, 2, 657. [Google Scholar] [CrossRef]
- Poleganov, M.A.; Eminli, S.; Beissert, T.; Herz, S.; Moon, J.-I.; Goldmann, J.; Beyer, A.; Heck, R.; Burkhart, I.; Barea Roldan, D. Efficient reprogramming of human fibroblasts and blood-derived endothelial progenitor cells using nonmodified RNA for reprogramming and immune evasion. Hum. Gene Ther. 2015, 26, 751–766. [Google Scholar] [CrossRef]
- Pushko, P.; Bray, M.; Ludwig, G.V.; Parker, M.; Schmaljohn, A.; Sanchez, A.; Jahrling, P.B.; Smith, J.F. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 2000, 19, 142–153. [Google Scholar] [CrossRef]
- Lapasset, L.; Milhavet, O.; Prieur, A.; Besnard, E.; Babled, A.; Aït-Hamou, N.; Leschik, J.; Pellestor, F.; Ramirez, J.-M.; De Vos, J. Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev. 2011, 25, 2248–2253. [Google Scholar] [CrossRef]
- Heng, B.C.; Heinimann, K.; Miny, P.; Iezzi, G.; Glatz, K.; Scherberich, A.; Zulewski, H.; Fussenegger, M. mRNA transfection-based, feeder-free, induced pluripotent stem cells derived from adipose tissue of a 50-year-old patient. Metab. Eng. 2013, 18, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Tabar, M.S.; Hesaraki, M.; Esfandiari, F.; Samani, F.S.; Vakilian, H.; Baharvand, H. Evaluating electroporation and lipofectamine approaches for transient and stable transgene expressions in human fibroblasts and embryonic stem cells. Cell J. (Yakhteh) 2015, 17, 438. [Google Scholar]
- Buganim, Y.; Faddah, D.A.; Jaenisch, R. Mechanisms and models of somatic cell reprogramming. Nat. Rev. Genet. 2013, 14, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Hackett, J.A.; Dietmann, S.; Murakami, K.; Down, T.A.; Leitch, H.G.; Surani, M.A. Synergistic mechanisms of DNA demethylation during transition to ground-state pluripotency. Stem Cell Rep. 2013, 1, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Buganim, Y.; Faddah, D.A.; Cheng, A.W.; Itskovich, E.; Markoulaki, S.; Ganz, K.; Klemm, S.L.; van Oudenaarden, A.; Jaenisch, R. Single-cell expression analyses during cellular reprogramming reveal an early stochastic and a late hierarchic phase. Cell 2012, 150, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Polo, J.M.; Anderssen, E.; Walsh, R.M.; Schwarz, B.A.; Nefzger, C.M.; Lim, S.M.; Borkent, M.; Apostolou, E.; Alaei, S.; Cloutier, J. A molecular roadmap of reprogramming somatic cells into iPS cells. Cell 2012, 151, 1617–1632. [Google Scholar] [CrossRef] [PubMed]
- Folmes, C.D.; Nelson, T.J.; Martinez-Fernandez, A.; Arrell, D.K.; Lindor, J.Z.; Dzeja, P.P.; Ikeda, Y.; Perez-Terzic, C.; Terzic, A. Somatic oxidative bioenergetics transitions into pluripotency-dependent glycolysis to facilitate nuclear reprogramming. Cell Metab. 2011, 14, 264–271. [Google Scholar] [CrossRef]
- Panopoulos, A.D.; Yanes, O.; Ruiz, S.; Kida, Y.S.; Diep, D.; Tautenhahn, R.; Herrerías, A.; Batchelder, E.M.; Plongthongkum, N.; Lutz, M. The metabolome of induced pluripotent stem cells reveals metabolic changes occurring in somatic cell reprogramming. Cell Res. 2012, 22, 168–177. [Google Scholar] [CrossRef]
- Van Den Hurk, M.; Kenis, G.; Bardy, C.; Van Den Hove, D.L.; Gage, F.H.; Steinbusch, H.W.; Rutten, B.P. Transcriptional and epigenetic mechanisms of cellular reprogramming to induced pluripotency. Epigenomics 2016, 8, 1131–1149. [Google Scholar] [CrossRef]
- Wood, S.H.; Clements, D.N.; McEwan, N.A.; Nuttall, T.; Carter, S.D. Reference genes for canine skin when using quantitative real-time PCR. Vet. Immunol. Immunopathol. 2008, 126, 392–395. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Peñalosa-Ruiz, G.; Mulder, K.W.; Veenstra, G.J.C. The corepressor NCOR1 and OCT4 facilitate early reprogramming by suppressing fibroblast gene expression. PeerJ 2020, 8, e8952. [Google Scholar] [CrossRef] [PubMed]
- Radzisheuskaya, A.; Chia, G.L.B.; Dos Santos, R.L.; Theunissen, T.W.; Castro, L.F.C.; Nichols, J.; Silva, J.C. A defined Oct4 level governs cell state transitions of pluripotency entry and differentiation into all embryonic lineages. Nat. Cell Biol. 2013, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Golipour, A.; David, L.; Liu, Y.; Jayakumaran, G.; Hirsch, C.L.; Trcka, D.; Wrana, J.L. A late transition in somatic cell reprogramming requires regulators distinct from the pluripotency network. Cell Stem Cell 2012, 11, 769–782. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.; Smith, A. Naive and primed pluripotent states. Cell Stem Cell 2009, 4, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.A.; Ross, P.J. Pluripotent stem cells and livestock genetic engineering. Transgenic Res. 2016, 25, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Rony, I.; Baten, A.; Bloomfield, J.A.; Islam, M.; Billah, M.; Islam, K. Inducing pluripotency in vitro: Recent advances and highlights in induced pluripotent stem cells generation and pluripotency reprogramming. Cell Prolif. 2015, 48, 140–156. [Google Scholar] [CrossRef]
- Brouwer, M.; Zhou, H.; Kasri, N.N. Choices for induction of pluripotency: Recent developments in human induced pluripotent stem cell reprogramming strategies. Stem Cell Rev. Rep. 2016, 12, 54–72. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Hwang, S.-U.; Yoon, J.D.; Jeong, Y.W.; Kim, E.; Hyun, S.-H. Optimized Approaches for the Induction of Putative Canine Induced Pluripotent Stem Cells from Old Fibroblasts Using Synthetic RNAs. Animals 2020, 10, 1848. https://doi.org/10.3390/ani10101848
Kim M, Hwang S-U, Yoon JD, Jeong YW, Kim E, Hyun S-H. Optimized Approaches for the Induction of Putative Canine Induced Pluripotent Stem Cells from Old Fibroblasts Using Synthetic RNAs. Animals. 2020; 10(10):1848. https://doi.org/10.3390/ani10101848
Chicago/Turabian StyleKim, Mirae, Seon-Ung Hwang, Junchul David Yoon, Yeon Woo Jeong, Eunhye Kim, and Sang-Hwan Hyun. 2020. "Optimized Approaches for the Induction of Putative Canine Induced Pluripotent Stem Cells from Old Fibroblasts Using Synthetic RNAs" Animals 10, no. 10: 1848. https://doi.org/10.3390/ani10101848
APA StyleKim, M., Hwang, S.-U., Yoon, J. D., Jeong, Y. W., Kim, E., & Hyun, S.-H. (2020). Optimized Approaches for the Induction of Putative Canine Induced Pluripotent Stem Cells from Old Fibroblasts Using Synthetic RNAs. Animals, 10(10), 1848. https://doi.org/10.3390/ani10101848




