Simple Summary
The present work focuses on stem-cell assessment as a therapeutic approach on cardiovascular diseases, both in terms of safety and efficacy. In particular, this is a systematic review of the relevant literature about the use of stem-cell treatment against acute or chronic ischemic cardiomyopathies in large animal models and a meta-analysis on collected data with regard to the left ventricular ejection fraction (LVEF) as functional parameter. This approach is compliant with the “3Rs” (replacement, reduction and refinement) principle about the use of animal experimentation in preclinical trials to predict evidences and perform the future translational researches.
Abstract
Stem-cell therapy provides a promising strategy for patients with ischemic heart disease. In recent years, numerous studies related to this therapeutic approach were performed; however, the results were often heterogeneous and contradictory. For this reason, we conducted a systematic review and meta-analysis of trials, reporting the use of stem-cell treatment against acute or chronic ischemic cardiomyopathies in large animal models with regard to Left Ventricular Ejection Fraction (LVEF). The defined research strategy was applied to the PubMed database to identify relevant studies published from January 2011 to July 2021. A random-effect meta-analysis was performed on LVEF mean data at follow-up between control and stem-cell-treated animals. In order to improve the definition of the effect measure and to analyze the factors that could influence the outcomes, a subgroup comparison was conducted. Sixty-six studies (n = 1183 animals) satisfied our inclusion criteria. Ischemia/reperfusion infarction was performed in 37 studies, and chronic occlusion in 29 studies; moreover, 58 studies were on a pig animal model. The meta-analysis showed that cell therapy increased LVEF by 7.41% (95% Confidence Interval 6.23–8.59%; p < 0.001) at follow-up, with significative heterogeneity and high inconsistency (I2 = 82%, p < 0.001). By subgroup comparison, the follow-up after 31–60 days (p = 0.025), the late cell injection (>7 days, p = 0.005) and the route of cellular delivery by surgical treatment (p < 0.001) were significant predictors of LVEF improvement. This meta-analysis showed that stem-cell therapy may improve heart function in large animal models and that the swine specie is confirmed as a relevant animal model in the cardiovascular field. Due to the significative heterogeneity and high inconsistency, future translational studies should be designed to take into account the evidenced predictors to allow for the reduction of the number of animals used.
1. Introduction
Myocardial infarction is a leading cause of mortality and morbidity worldwide [1]. This pathology leads to death by necrosis of myocardial cells, due to prolonged ischemia, usually following coronary atherosclerosis [2]. In particular, coronary occlusion causes a loss of myocardial perfusion with consequent morphological, biochemical and functional alterations of the affected area, thus establishing ischemia, which, based on its extent and duration, can cause cell necrosis. Cell death leads to dramatic consequences because it triggers an acute inflammatory reaction. Subsequently, the damaged area is replaced by intensely vascularized granulation tissue, which then evolves into a process of fibrosis and, consequently, scar formation. Hyperplastic scar tissue is not functional, but the surviving patient’s heart must still find a way to function while maintaining adequate cardiac output. To do this, it undergoes a series of structural and dynamic changes which are referred to as “ventricular remodeling”. In fact, both the necrotic area and the non-infarcted segment of the ventricle progressively change in size, thickness and shape. All of this can then lead to heart failure [3]. Effective treatment strategies for myocardial infarction are designed to limit adverse ventricular remodeling to attenuate myocardial scar expansion and promote improvement of cardiac function and myocardial regeneration [4,5]. Among many therapies proposed, stem cells represent a promising option to repair the injured heart. Several cell types, including embryonic stem cells, skeletal myoblasts, mesenchymal stem cells (MSCs), cardiac stem cells (CSCs) and induced pluripotent stem cells (iPSCs), have been employed to re-functionalize the injured heart [5]. It has been shown that CSCs can differentiate into endothelial cells (ECs), vascular smooth-muscle cells (VSMCs) and cardiomyocytes (CMs) [6,7]. MSCs can differentiate into cardiomyocytes and induce angiogenesis [8,9]. Nevertheless, in vivo studies show that the percentage of inoculated stem cells that are stably implanted in the infarcted region and the related rate of cardiomyogenesis and angiogenesis are very slow to support myocardial regeneration [10]. However, studies from the past 20 years have clearly shown that it has been demonstrated that transplanted stem cells are able to release soluble factors that act in a paracrine way, contributing to the repair and regeneration of the infarcted myocardium [11]. These factors include a variety of growth factors, cytokines and extracellular matrix proteins [12]. Moreover, paracrine effects also include the recruitment activation and proliferation of resident endothelial progenitor cells (EPCs), cardiac progenitor cells (CPCs) and/or resident CSCs [12,13]. Furthermore, paracrine factors influence the contractile abilities of CM [6], promote cytoprotection (inhibition of apoptosis and necrosis) and formation of new blood vessels [7,12], prevent degradation of extracellular matrix (ECM), inhibit fibrosis and release of granulation factors [7]. Currently the most promising results have been obtained through the paracrine action rather than the direct action of cell differentiation [10,14,15,16].
In the last 20 years, in addition to the numerous experiments performed with in vitro models, numerous studies have been performed with large animal models with ischemic cardiomyopathies. These preclinical studies evaluated the risk of this new cell therapy, considering safety, feasibility and efficacy. In addition, they tried to answer the unsolved problems in clinical cell therapy (cell-type selection, number of cells, method of administration, time of administration and follow-up after cell transplantation); however, the results obtained were often heterogeneous and contradictory [17]. Studies based on large animal models often suffer from extremely limited sample sizes, due to ethical reasons, costs and management difficulties. Systematic reviews and meta-analysis substantially increase the statistical power, and the different experimental settings in the studies make it possible to obtain an estimate with a much higher external validity of the model. The present systematic review is an update of the previous work published by van der Spoel and colleagues [17] and aims to summarize trials reported in the literature about the use of stem-cell treatment against acute or chronic ischemic cardiomyopathies in large animal models and perform a meta-analysis on collected data from 2011 to 2021, with regard to Left Ventricular Ejection Fraction (LVEF) as a functional parameter.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
The international principles of preferred reporting items for systematic reviews and meta-analyses (PRISMA) 2020 guidelines were followed throughout the study [18]. Research was conducted in PubMed [19] to identify all the relevant publication from the period January 2011 to July 2021 by using the following search terms: “(pig OR porcine OR swine OR canine OR dog OR sheep OR ovine) AND (stem cells OR progenitor cells OR bone marrow) AND (myocardial infarction OR heart failure OR coronary artery disease OR cardiac repair OR myocardial regeneration)”. Only articles published in English were included. The collected studies were carefully examined, and duplicates were removed.
2.2. Eligibility Criteria
The primary literature used to conduct the systematic review was compliant with the following inclusion and exclusion criteria. Studies that used large animal models with acute myocardial infarction (MI) or chronic ischemic cardiomyopathies, randomized controlled trials (RCTs) or no RCT studies were included to investigate the effect of stem-cell therapy on cardiac function as determined by left ventricular ejection fraction (LVEF). In addition, a placebo or sham-operated control group was included in the study. Studies using reporter genes (for stem-cell-imaging purposes only) were also included. In vitro studies, studies using genetically engineered or transfected stem cells with altered cellular behavior and studies using only conditioned media were excluded. Reviews, editorials, comments, letters and reports were excluded.
2.3. Data Extraction
Two reviewers (D.L.M. and C.W.) independently selected the studies by reading titles, abstracts and full manuscripts and applying the criteria mentioned above, and the resulting list of studies was approved by a third reviewer (M.F.). Then the following information was extracted from the full text of the selected studies: basal characteristics of the studies and left ventricular ejection fraction (LVEF) outcomes. If necessary, LVEF data were recalculated as follows: (EDV − ESV)/EDV × 100% (EDV, end-diastolic volume, ESV, end-systolic volume). Accordingly, the standards deviations (SD) were determined or recalculated from the standard errors of mean (SEM).
2.4. Statistical Analysis
Primary analysis consisted of calculating the LVEF mean difference (reported in %) at follow-up between the control and stem-cell-treated groups when exposed to acute myocardial infarction or chronic ischemic cardiomyopathies. Continuous variables were reported as weighted mean differences with the 95% confidence intervals (CIs) between the treated and control groups. In the presence of multiple experimental groups alongside the control group within a study, the control group was used as control for each experimental group. A random-effect model (DerSimonian–Laird) was applied for the meta-analysis. Heterogeneity was assessed by using the I2 statistics. Values for 25%, 50% and 75% for I2 represented low, moderate and high heterogeneity, respectively [20]. In addition, the following subgroup analyses were performed: type of study (RCT or cohort); MI model (ischemia/reperfusion (I/R) or chronic occlusion); location of infarct-related artery (left anterior descending artery (LAD) or left circumflex artery (LCX)); autologous cell therapy (yes or no); cell type (adipose-derived stem cells (ASCs), bone-marrow mononuclear cells (BMMNC), bone-marrow-derived mononuclear cells (BMDMNCs), bone-marrow stem cells (BMSCs), cardiosphere-derived cells (CDCs), cardiac-derived progenitor cells (CPCs), cardiac stem cells (CSCs), multipotent adult progenitor cells (MAPCs), mesenchymal precursor cells (MPCs), induced pluripotent stem cells (iPSCs), mesenchymal stem cells (MSCs) or other types of stem cells (SC)); number of cells injected (<107, 107–108, ≥108); timing of cell therapy after heart attack (<1 day, 1–7 days, >7 days); follow-up after cell therapy (≤30 days, 31–60 days, >60 days); type of animal (pig, dog or sheep); and route of delivery (intramyocardial (IM), intracoronary (IC), trans-endocardial (TE), surgical or other routes)). Welch’s t-test or ANOVA test was applied to compare subgroups. A funnel plot for LVEF was drawn to explore publication bias. All analyses were performed by using JASP software (JASP Team, 2022; version 0.14.1; Amsterdam, The Netherlands).
3. Results
3.1. Study Selection
The database search yielded 435 publications. After removing articles not in English and reviews, 401 publications were identified and assessed for eligibility. Based on the defined criteria, 289 studies were excluded and 112 studies were reviewed in detail. Only 66 studies met our inclusion criteria. The study search and selection processes are described in detail in Figure 1.
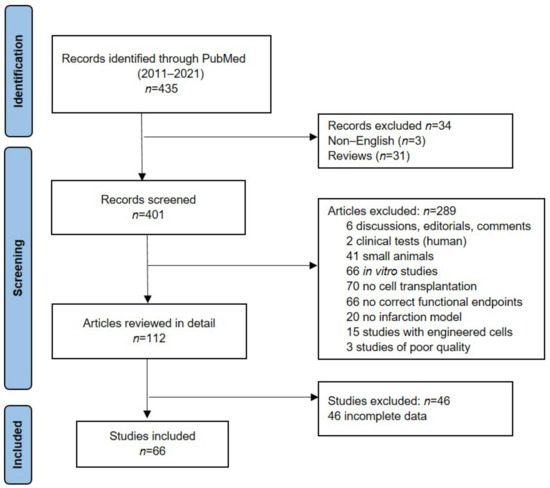
Figure 1.
PRISMA workflow of the study selection process, records screened and studies included.
3.2. Included Studies Characteristics
In total, 1183 animals met the inclusion criteria, and the data derived from them were analyzed. Table 1 provides the characteristics of the included studies. Most studies used the porcine model (58 studies). In 37 studies, ischemia/reperfusion was used as an MI model. MI was mainly induced in the LAD (60 studies), but the site of ligation/constriction of the vessel (proximal, mid or distal) varied. Ten different types of cells were studied (25 studies used MSCs), but the number of stem cells administered varied (from 106 to 109); 26 studies used autologous cells. The main routes of delivery were IC infusion, IM, TE injection and surgical. Cell therapy was performed at different times after MI: <1 day (21 studies), 1–7 days (12 studies) and >7 days (33 studies). Follow-ups after cell therapy varied from 1 day to 180 days. The median and interquartile range of follow-up time was 51 days (28–60 days).

Table 1.
Characteristics of the included studies.
3.3. Meta-Analysis
Meta-analysis showed a LVEF difference of 7.41% at follow-up after stem-cell therapy vs. control (95% CI, 6.23–8.59%; p < 0.001), with significative heterogeneity (p < 0.001) and high inconsistency (I2: 82%) (Figure 2). At follow-up, the mean LVEF after stem-cell treatment and control was 48% and 40.7%, respectively.
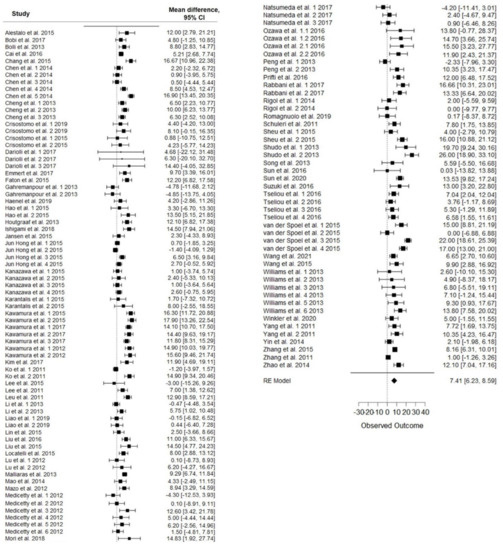
Figure 2.
Forest plot showing the effect of stem-cell therapy on LVEF improvement compared with controls. Note: 95% CI, 95% confidence interval.
3.4. Subgroup Analysis
LVEF mean difference values were compared for subgroup analysis, and a Welch’s t-test or ANOVA test was applied. The analysis showed that follow-up after cell therapy (p = 0.025), time between infarction and cell injection (p = 0.005) and the route of delivery (p < 0.001) are independent significant predictors of LVEF improvement. Figure 3d,f,i shows a trend toward greater improvements after cell therapy in the following aspects: follow-up at 31–60 days, since, after that period, the effect of cell therapy appeared to decline over time; and the late cell injection after MI (>7 days) and the surgical treatment. In addition, less benefit was observed in the ischemia/reperfusion MI model compared to the chronic MI models (p = 0.063), and there was an improvement with autologous cell treatment (p = 0.079) (Figure 3b,c). No significant differences in LVEF were observed in the following cases: animal model (p = 0.355), type of infarction (p = 0.257), type of study (p = 0.345), cell number (p = 0.39) and cell type (p = 0.361) (Figure 3a,e,g,h,j). An additional subgroup analysis was performed to analyze the three predictors at the significant levels (Follow up 31–60 days, timing of cell therapy after MI > 7 days and surgical as route of delivery) to understand whether the effect on LVEF improvement was related to a specific cell type. No significative differences were detected (Figure 4).
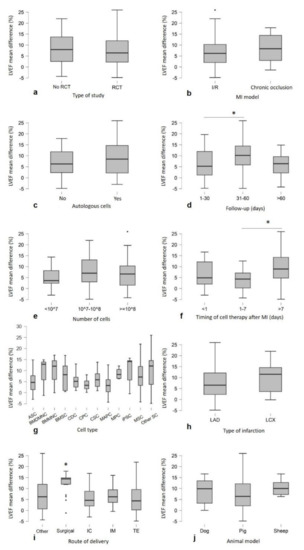
Figure 3.
Subgroup analysis showing the LVEF trends toward more improvements after cell therapy compared with control: (d) follow-up at 31–60 days (p = 0.025), (f) the late cell injection (>7 days, p = 0.005), (i) surgical administration (p < 0.001), (b) chronic occlusion model (p = 0.063) and (c) autologous cells (p = 0.079). No significant differences were observed in (j) animal model (p = 0.355), (h) type of infarction (p = 0.257), (a) type of study (p = 0.345), (e) number of cells (p = 0.39) and (g) cell type (n ≥ 3 studies) (p = 0.361). Graphs are represented as Boxplots; the two segments that delimit the rectangle represent the 25th and 75th percentiles; the central segment is the median; the bars represent the minimum and maximum values, respectively; and the external points are the outliers. Note: (* p <0.05) represents statistical significance resulting from one-way ANOVA, followed by post hoc Tukey comparison test.
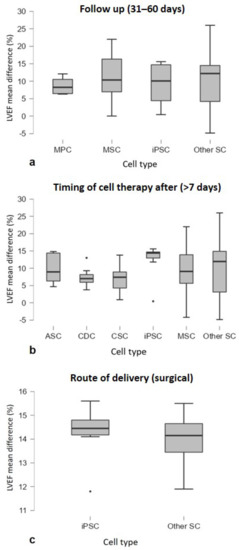
Figure 4.
Subgroup analysis showing LVEF trends of cell type (n > 3 studies) by considering studies of the three significant predictors, in particular (a) follow-up (30–60 days) (p = 0.904), (b) timing of cell therapy after MI (>7 days) (p = 0.690) and (c) route of delivery (surgical) (p = 0.729). Graphs are represented as Boxplots; the two segments that delimit the rectangle represent the 25th and 75th percentiles; the central segment is the median; the bars represent the minimum and maximum values, respectively; and the external points the outliers.
The funnel plot for LVEF mean difference shows that there is no publication bias (Figure 5), as values are evenly distributed around the effect estimate, as evidenced by the regression test for funnel plot asymmetry (Egger’s test) (p = 0.657).
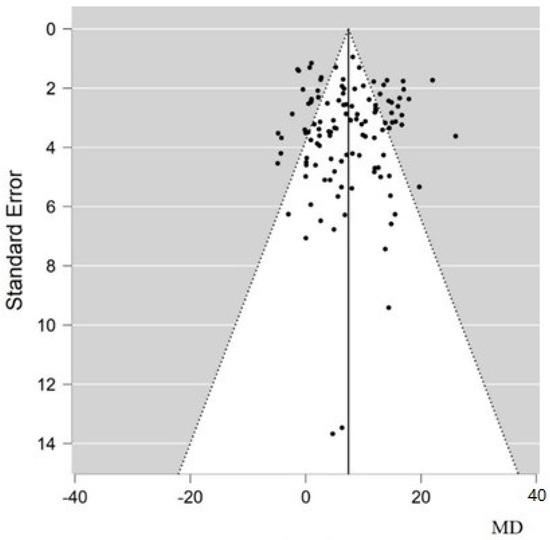
Figure 5.
Funnel plot for LVEF improvement showing the absence of publication biases. The vertical solid line represents the estimated overall mean difference; black dots are the standard error of each study. MD, mean difference.
4. Discussion
In the present systematic review and meta-analysis, we assessed the effect of stem-cell therapy against ischemic cardiomyopathies in large animal models; this is an update of a previous work published by van der Spoel et al. [17] that reviewed the same topic in studies performed from 1980 to 2010. The analysis includes data from 66 published pre-clinical studies (2011–2021) that used large animal models treated with stem cells in order to study the effects of cell therapy of ischemic cardiomyopathies by reporting outcomes derived from left ventricular ejection fraction (LVEF) as functional parameter. The meta-analysis showed a significant improvement of LVEF by 7.41% (95% CI 6.23–8.59%) after stem-cell therapy against control group confirming the positive effect reported in the previous meta-analysis [17], in which LVEF effect size was 7.51% (95% CI 6.15–8.87%). Given the number of the studies included, a random-effect model was applied by resulting a significative heterogeneity with high inconsistency (I2: 82%). For this reason, a comparison between subgroups was investigated in order to analyze clinically relevant parameters. The sub-analysis revealed that time of follow-up, time between infarction and cell injection, and the route of cellular delivery are independent significant predictors of LVEF improvement. In detail, in large animals, the effect of cell therapy achieved better results at 31–60 days, after which it fades over time; this phenomenon is in accordance with the previous analysis [17]. This finding could suggest the use of new applications and therapeutic strategies to increase cell survival over time, such as the use of slow-release molecules by cell pre-conditioning [86,87], the application of biomaterials [88,89] or the genetic stem-cell modifications [90]. Late cell injection assumed better benefit if applied 7 days after MI; our findings are comparable with the previous meta-analyses both in large animals [17] and human [91]. Optimal stem-cell therapy depends not only on engraftment and survival of the transplanted cells but also on successful delivery. By comparing different types of cellular delivery, our results demonstrated that surgical treatment is the route that significantly improves the heart functionality. In general, stem cells can be delivered by intravenous or intracoronary routes after coronary revascularization in the setting of acute MI to avoid the risk of invasive procedure; however, both IV and IC routes seems to be not applicable for patients with chronic myocardial ischemia not amenable to coronary revascularization, so direct intramyocardial injection via either surgical epicardial or transcatheter endocardial approaches may be necessary, as they allow for the direct visualization of the site of injection [92]. In addition, our findings showed that less benefit in LVEF improvement was observed in ischemia/reperfusion MI model compared to the chronic occlusion models, but without reaching significance. This is compliant with the findings obtained by van der Spoel et al. [17] both in large and small animal models [93]. Autologous cell therapy resulted in better results on LVEF improvement, but not significative; a similar effect was shown in a meta-analysis performed in large animal models about autologous and allogeneic cell therapy for ischemic heart disease by using BM-MNCs, MSCs and cardiac stem-cell types [38]. Furthermore, the use of autologous BM- or MSC-derived cells is confounded by the functional impairment of those stem cells associated with aging and because of the restricted immediate availability; the use of allogenic cell products with limited immunogenicity, such as MSC derived from different tissues, or standardized non-cellular products, such as conditional medium, may overcome these problems in terms of efficacy and safety [38,92,94].
No significative differences in LVEF were observed in animal species, infarct type, type of study, number of cells and cell type. Regarding the cell type, the result obtained contrasts with that of van der Spoel et al. [17]. We could not exclude that this result is due to the high difference in the studies’ number between the group analyzed. The same consideration regarding the lack of significative results deserves to be made for the number of cells (<107 n = 6, 107–108 n = 41, and ≥ 108 n = 19) and the animal species (porcine n = 58 others n = 8).
Although no difference was observed between species, we would sustain the widely accepted porcine animal model as the one to be recommended to evaluate the effect of the cell therapy, confirming the swine as a relevant animal model in the cardiovascular field and in translational research in a broader sense [95]. This is because most of the published studies (89% of those included in our SR) are based on this model, and the data are, therefore, available for comparison as a fundamental tool in future experimental designs, in particular, in relation to the Reduction aspects. Furthermore, we wanted to investigate what could contribute to the statistical significance of the improving predictors of LVEF. In particular, we analyzed whether the effect of LVEF improvement was attributed to a specific cell type. No significative difference was observed in cell type in large animal studies with a significative improvement in follow-up (31–60 days), timing of cell therapy after MI (>7 days) and surgical treatment (Figure 4).
Limitations
The limitations of meta-analysis are well-known [96]. Meta-analyses and systematic reviews are statistical and scientific techniques that can highlight areas where evidence is lacking, but they cannot overcome these deficiencies [97]. Publication bias and search bias are potential problems in all meta-analyses [97]; this arises from the fact that unpublished studies may contradict the results due to the tendency not to publish negative studies, thus leading to the over-representation of “positive” ones [98]. In this meta-analysis, the funnel plot (Figure 4) for the LVEF mean difference showed that there is no publication bias. Thus, based on the results we obtained, we can affirm that, in the future, stem-cell-transplant studies in large animal models with ischemic cardiomyopathies should therefore focus on late (>7 days) surgical treatments and 31–60-day follow-up. The analysis of the subgroups shows that the greater heterogeneity of the included studies could be mainly due to the different amounts of data in the different comparison groups, such as in the case of cell number, cell type and animal species.
5. Conclusions
In conclusion, in the present systematic review and meta-analysis, we evaluated the effect of stem-cell transplantation in large animal models with ischemic cardiomyopathies, showing that stem-cell therapy could improve LVEF. The SR is therefore confirmed as a reliable method for obtaining a complete contextual framework from which to start for further experimentation, and future translational studies should be designed by considering the evidenced predictors to allow for the reduction of the number of animals used in preclinical trials. Large animal models, especially the swine, are a translational step necessary to predict outcomes of clinical trials in the cardiovascular field.
Author Contributions
Conceptualization, D.L.M., C.B. and M.F.; methodology, D.L.M. and M.F.; software, D.L.M. and C.W.; validation, M.F.; writing—original draft preparation D.L.M. and C.B.; writing—review and editing, A.Z., R.S. and S.B.; supervision, C.B. and M.F.; funding acquisition, M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was funded by RFO 2020–University of Bologna to M.F.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cardiovascular Diseases. Available online: https://www.who.int/westernpacific/health-topics/cardiovascular-diseases (accessed on 19 January 2022).
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D. Writing Group on behalf of the Joint For the Universal Definition of Myocardial Infarction Third Universal Definition of Myocardial Infarction. Glob. Heart 2012, 7, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; He, X.; Wang, B.; Pan, J.; Shi, C.; Li, J.; Wang, L.; Zhao, Y.; Dai, J.; Wang, D. Injectable Collagen Scaffold Promotes Swine Myocardial Infarction Recovery by Long-Term Local Retention of Transplanted Human Umbilical Cord Mesenchymal Stem Cells. Sci. China Life Sci. 2021, 64, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Perea-Gil, I.; Prat-Vidal, C.; Bayes-Genis, A. In Vivo Experience with Natural Scaffolds for Myocardial Infarction: The Times They Are a-Changin’. Stem Cell Res. Ther. 2015, 6, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, P.; Katare, R. Challenges in Identifying the Best Source of Stem Cells for Cardiac Regeneration Therapy. Stem Cell Res. Ther. 2015, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leri, A.; Kajstura, J.; Anversa, P. Cardiac Stem Cells and Mechanisms of Myocardial Regeneration. Physiol. Rev. 2005, 85, 1373–1416. [Google Scholar] [CrossRef] [Green Version]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Tomita, S.; Li, R.-K.; Weisel, R.D.; Mickle, D.A.G.; Kim, E.-J.; Sakai, T.; Jia, Z.-Q. Autologous Transplantation of Bone Marrow Cells Improves Damaged Heart Function. Circulation 1999, 100, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Pittenger, M.F.; Martin, B.J. Mesenchymal Stem Cells and Their Potential as Cardiac Therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef]
- Yang, D.; Wang, W.; Li, L.; Peng, Y.; Chen, P.; Huang, H.; Guo, Y.; Xia, X.; Wang, Y.; Wang, H.; et al. The Relative Contribution of Paracine Effect versus Direct Differentiation on Adipose-Derived Stem Cell Transplantation Mediated Cardiac Repair. PLoS ONE 2013, 8, e59020. [Google Scholar] [CrossRef]
- Sid-Otmane, C.; Perrault, L.P.; Ly, H.Q. Mesenchymal stem cell mediates cardiac repair through autocrine, paracrine and endocrine axes. J. Transl. Med. 2020, 18, 336. [Google Scholar] [CrossRef]
- Burchfield, J.S.; Dimmeler, S. Role of Paracrine Factors in Stem and Progenitor Cell Mediated Cardiac Repair and Tissue Fibrosis. Fibrogenesis Tissue Repair 2008, 1, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segers, V.F.M.; Tokunou, T.; Higgins, L.J.; MacGillivray, C.; Gannon, J.; Lee, R.T. Local Delivery of Protease-Resistant Stromal Cell Derived Factor-1 for Stem Cell Recruitment After Myocardial Infarction. Circulation 2007, 116, 1683–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chimenti, I.; Smith, R.R.; Li, T.-S.; Gerstenblith, G.; Messina, E.; Giacomello, A.; Marbán, E. Relative Roles of Direct Regeneration Versus Paracrine Effects of Human Cardiosphere-Derived Cells Transplanted Into Infarcted Mice. Circ. Res. 2010, 106, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Rajabi-Zeleti, S.; Annabi, N.; Aghdami, N.; Baharvand, H. Stem Cells and Injectable Hydrogels: Synergistic Therapeutics in Myocardial Repair. Biotechnol. Adv. 2016, 34, 362–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, K.R.; Frisén, J.; Fritsche-Danielson, R.; Melton, D.A.; Murry, C.E.; Weissman, I.L. Regenerating the Field of Cardiovascular Cell Therapy. Nat. Biotechnol. 2019, 37, 232–237. [Google Scholar] [CrossRef]
- Van der Spoel, T.I.G.; Jansen of Lorkeers, S.J.; Agostoni, P.; van Belle, E.; Gyöngyösi, M.; Sluijter, J.P.G.; Cramer, M.J.; Doevendans, P.A.; Chamuleau, S.A.J. Human Relevance of Pre-Clinical Studies in Stem Cell Therapy: Systematic Review and Meta-Analysis of Large Animal Models of Ischaemic Heart Disease. Cardiovasc. Res. 2011, 91, 649–658. [Google Scholar] [CrossRef]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 Explanation and Elaboration: Updated Guidance and Exemplars for Reporting Systematic Reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 24 January 2022).
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Alestalo, K.; Korpi, R.; Mäkelä, J.; Lehtonen, S.; Mäkelä, T.; Yannopoulos, F.; Ylitalo, K.; Haapea, M.; Juvonen, T.; Anttila, V.; et al. High Number of Transplanted Stem Cells Improves Myocardial Recovery after AMI in a Porcine Model. Scand. Cardiovasc. J. 2015, 49, 82–94. [Google Scholar] [CrossRef]
- Bobi, J.; Solanes, N.; Fernández-Jiménez, R.; Galán-Arriola, C.; Dantas, A.P.; Fernández-Friera, L.; Gálvez-Montón, C.; Rigol-Monzó, E.; Agüero, J.; Ramírez, J.; et al. Intracoronary Administration of Allogeneic Adipose Tissue-Derived Mesenchymal Stem Cells Improves Myocardial Perfusion But Not Left Ventricle Function, in a Translational Model of Acute Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e005771. [Google Scholar] [CrossRef] [Green Version]
- Bolli, R.; Tang, X.-L.; Sanganalmath, S.K.; Rimoldi, O.; Mosna, F.; Abdel-Latif, A.; Jneid, H.; Rota, M.; Leri, A.; Kajstura, J. Intracoronary Delivery of Autologous Cardiac Stem Cells Improves Cardiac Function in a Porcine Model of Chronic Ischemic Cardiomyopathy. Circulation 2013, 128, 122–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, M.; Shen, R.; Song, L.; Lu, M.; Wang, J.; Zhao, S.; Tang, Y.; Meng, X.; Li, Z.; He, Z.-X. Erratum: Bone Marrow Mesenchymal Stem Cells (BM-MSCs) Improve Heart Function in Swine Myocardial Infarction Model through Paracrine Effects. Sci. Rep. 2016, 6, 31528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, X.; Liu, J.; Liao, X.; Liu, G. Ultrasound-Mediated Microbubble Destruction Enhances the Therapeutic Effect of Intracoronary Transplantation of Bone Marrow Stem Cells on Myocardial Infarction. Int. J. Clin. Exp. Pathol. 2015, 8, 2221–2234. [Google Scholar] [PubMed]
- Chen, Y.; Teng, X.; Chen, W.; Yang, J.; Yang, Z.; Yu, Y.; Shen, Z. Timing of Transplantation of Autologous Bone Marrow Derived Mesenchymal Stem Cells for Treating Myocardial Infarction. Sci. China Life Sci. 2014, 57, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Yi, G.; Conditt, G.B.; Sheehy, A.; Kolodgie, F.D.; Tellez, A.; Polyakov, I.; Gu, A.; Aboodi, M.S.; Wallace-Bradley, D.; et al. Catheter-Based Endomyocardial Delivery of Mesenchymal Precursor Cells Using 3D Echo Guidance Improves Cardiac Function in a Chronic Myocardial Injury Ovine Model. Cell Transplant. 2013, 22, 2299–2309. [Google Scholar] [CrossRef] [Green Version]
- Crisostomo, V.; Baez, C.; Abad, J.L.; Sanchez, B.; Alvarez, V.; Rosado, R.; Gómez-Mauricio, G.; Gheysens, O.; Blanco-Blazquez, V.; Blazquez, R.; et al. Dose-Dependent Improvement of Cardiac Function in a Swine Model of Acute Myocardial Infarction after Intracoronary Administration of Allogeneic Heart-Derived Cells. Stem Cell Res. Ther. 2019, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Crisostomo, V.; Baez-Diaz, C.; Maestre, J.; Garcia-Lindo, M.; Sun, F.; Casado, J.G.; Blazquez, R.; Abad, J.L.; Palacios, I.; Rodriguez-Borlado, L.; et al. Delayed Administration of Allogeneic Cardiac Stem Cell Therapy for Acute Myocardial Infarction Could Ameliorate Adverse Remodeling: Experimental Study in Swine. J. Transl. Med. 2015, 13, 156. [Google Scholar] [CrossRef] [Green Version]
- Dariolli, R.; Naghetini, M.V.; Marques, E.F.; Takimura, C.K.; Jensen, L.S.; Kiers, B.; Tsutsui, J.M.; Mathias, W.; Lemos Neto, P.A.; Krieger, J.E. Allogeneic PASC Transplantation in Humanized Pigs Attenuates Cardiac Remodeling Post-Myocardial Infarction. PLoS ONE 2017, 12, e0176412. [Google Scholar] [CrossRef]
- Emmert, M.Y.; Wolint, P.; Jakab, A.; Sheehy, S.P.; Pasqualini, F.S.; Nguyen, T.D.L.; Hilbe, M.; Seifert, B.; Weber, B.; Brokopp, C.E.; et al. Safety and Efficacy of Cardiopoietic Stem Cells in the Treatment of Post-Infarction Left-Ventricular Dysfunction—From Cardioprotection to Functional Repair in a Translational Pig Infarction Model. Biomaterials 2017, 122, 48–62. [Google Scholar] [CrossRef] [Green Version]
- Fanton, Y.; Robic, B.; Rummens, J.-L.; Daniëls, A.; Windmolders, S.; Willems, L.; Jamaer, L.; Dubois, J.; Bijnens, E.; Heuts, N.; et al. Cardiac Atrial Appendage Stem Cells Engraft and Differentiate into Cardiomyocytes in Vivo: A New Tool for Cardiac Repair after MI. Int. J. Cardiol. 2015, 201, 10–19. [Google Scholar] [CrossRef]
- Gahremanpour, A.; Vela, D.; Zheng, Y.; Silva, G.V.; Fodor, W.; Cardoso, C.O.; Baimbridge, F.; Fernandes, M.R.; Buja, L.M.; Perin, E.C. Xenotransplantation of Human Unrestricted Somatic Stem Cells in a Pig Model of Acute Myocardial Infarction. Xenotransplantation 2013, 20, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Haenel, A.; Ghosn, M.; Karimi, T.; Vykoukal, J.; Shah, D.; Valderrabano, M.; Schulz, D.G.; Raizner, A.; Schmitz, C.; Alt, E.U. Unmodified Autologous Stem Cells at Point of Care for Chronic Myocardial Infarction. World J. Stem Cells 2019, 11, 831–858. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Hao, J.; Fang, W.; Han, C.; Zhang, K.; Wang, X. Dual Isotope Simultaneous Imaging to Evaluate the Effects of Intracoronary Bone Marrow-Derived Mesenchymal Stem Cells on Perfusion and Metabolism in Canines with Acute Myocardial Infarction. Biomed. Rep. 2015, 3, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houtgraaf, J.H.; de Jong, R.; Kazemi, K.; de Groot, D.; van der Spoel, T.I.G.; Arslan, F.; Hoefer, I.; Pasterkamp, G.; Itescu, S.; Zijlstra, F.; et al. Intracoronary Infusion of Allogeneic Mesenchymal Precursor Cells Directly after Experimental Acute Myocardial Infarction Reduces Infarct Size, Abrogates Adverse Remodeling, and Improves Cardiac Function. Circ. Res. 2013, 113, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Ishigami, M.; Masumoto, H.; Ikuno, T.; Aoki, T.; Kawatou, M.; Minakata, K.; Ikeda, T.; Sakata, R.; Yamashita, J.K.; Minatoya, K. Human IPS Cell-Derived Cardiac Tissue Sheets for Functional Restoration of Infarcted Porcine Hearts. PLoS ONE 2018, 13, e0201650. [Google Scholar] [CrossRef] [Green Version]
- Jansen of Lorkeers, S.J.; Gho, J.M.I.H.; Koudstaal, S.; van Hout, G.P.J.; Zwetsloot, P.P.M.; van Oorschot, J.W.M.; van Eeuwijk, E.C.M.; Leiner, T.; Hoefer, I.E.; Goumans, M.-J.; et al. Xenotransplantation of Human Cardiomyocyte Progenitor Cells Does Not Improve Cardiac Function in a Porcine Model of Chronic Ischemic Heart Failure. Results from a Randomized, Blinded, Placebo Controlled Trial. PLoS ONE 2015, 10, e0143953. [Google Scholar] [CrossRef] [PubMed]
- Jun Hong, S.; Rogers, P.I.; Kihlken, J.; Warfel, J.; Bull, C.; Deuter-Reinhard, M.; Feng, D.; Xie, J.; Kyle, A.; Merfeld-Clauss, S.; et al. Intravenous Xenogeneic Transplantation of Human Adipose-Derived Stem Cells Improves Left Ventricular Function and Microvascular Integrity in Swine Myocardial Infarction Model. Catheter. Cardiovasc. Interv. Off. J. Soc. Card. Angiogr. Interv. 2015, 86, E38–E48. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, H.; Tseliou, E.; Malliaras, K.; Yee, K.; Dawkins, J.F.; De Couto, G.; Smith, R.R.; Kreke, M.; Seinfeld, J.; Middleton, R.C.; et al. Cellular Postconditioning: Allogeneic Cardiosphere-Derived Cells Reduce Infarct Size and Attenuate Microvascular Obstruction When Administered after Reperfusion in Pigs with Acute Myocardial Infarction. Circ. Heart Fail. 2015, 8, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karantalis, V.; Suncion-Loescher, V.Y.; Bagno, L.; Golpanian, S.; Wolf, A.; Sanina, C.; Premer, C.; Kanelidis, A.J.; McCall, F.; Wang, B.; et al. Synergistic Effects of Combined Cell Therapy for Chronic Ischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2015, 66, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Toda, K.; Daimon, T.; Shimizu, T.; Okano, T.; Sawa, Y. Xenotransplantation of Bone Marrow-Derived Human Mesenchymal Stem Cell Sheets Attenuates Left Ventricular Remodeling in a Porcine Ischemic Cardiomyopathy Model. Tissue Eng. Part A 2015, 21, 2272–2280. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, M.; Miyagawa, S.; Fukushima, S.; Saito, A.; Miki, K.; Funakoshi, S.; Yoshida, Y.; Yamanaka, S.; Shimizu, T.; Okano, T.; et al. Enhanced Therapeutic Effects of Human IPS Cell Derived-Cardiomyocyte by Combined Cell-Sheets with Omental Flap Technique in Porcine Ischemic Cardiomyopathy Model. Sci. Rep. 2017, 7, 8824. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Miyagawa, S.; Miki, K.; Saito, A.; Fukushima, S.; Higuchi, T.; Kawamura, T.; Kuratani, T.; Daimon, T.; Shimizu, T.; et al. Feasibility, Safety, and Therapeutic Efficacy of Human Induced Pluripotent Stem Cell-Derived Cardiomyocyte Sheets in a Porcine Ischemic Cardiomyopathy Model. Circulation 2012, 126, S29–S37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.C.; Kim, Y.S.; Kang, W.S.; Lee, K.H.; Cho, M.; Hong, M.H.; Lim, K.S.; Jeong, M.H.; Ahn, Y. Intramyocardial Injection of Stem Cells in Pig Myocardial Infarction Model: The First Trial in Korea. J. Korean Med. Sci. 2017, 32, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-F.; Yip, H.-K.; Lee, C.-C.; Sheu, J.-J.; Sun, C.-K.; Ng, S.-H.; Huang, C.-C.; Lin, Y.-C.; Chang, L.-T.; Chen, M.-C. Immediate Intramyocardial Bone Marrow-Derived Mononuclear Cells Implantation in Minipig Myocardium after Permanent Coronary Artery Ligation: Magnetic Resonance Imaging with Histopathologic and Immunochemical Correlation. Investig. Radiol. 2011, 46, 495–503. [Google Scholar] [CrossRef]
- Lee, H.W.; Lee, H.C.; Park, J.H.; Kim, B.W.; Ahn, J.; Kim, J.H.; Park, J.S.; Oh, J.-H.; Choi, J.H.; Cha, K.S.; et al. Effects of Intracoronary Administration of Autologous Adipose Tissue-Derived Stem Cells on Acute Myocardial Infarction in a Porcine Model. Yonsei Med. J. 2015, 56, 1522–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-T.; White, A.J.; Matsushita, S.; Malliaras, K.; Steenbergen, C.; Zhang, Y.; Li, T.-S.; Terrovitis, J.; Yee, K.; Simsir, S.; et al. Intramyocardial Injection of Autologous Cardiospheres or Cardiosphere-Derived Cells Preserves Function and Minimizes Adverse Ventricular Remodeling in Pigs with Heart Failure Post-Myocardial Infarction. J. Am. Coll. Cardiol. 2011, 57, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leu, S.; Sun, C.-K.; Sheu, J.-J.; Chang, L.-T.; Yuen, C.-M.; Yen, C.-H.; Chiang, C.-H.; Ko, S.-F.; Pei, S.-N.; Chua, S.; et al. Autologous Bone Marrow Cell Implantation Attenuates Left Ventricular Remodeling and Improves Heart Function in Porcine Myocardial Infarction: An Echocardiographic, Six-Month Angiographic, and Molecular-Cellular Study. Int. J. Cardiol. 2011, 150, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, F.; Song, G.; Gu, W.; Chen, M.; Yang, B.; Li, D.; Wang, D.; Cao, K. Intramyocardial Injection of Pig Pluripotent Stem Cells Improves Left Ventricular Function and Perfusion: A Study in a Porcine Model of Acute Myocardial Infarction. PLoS ONE 2013, 8, e66688. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Ting, S.; Zhen, Z.; Luo, F.; Zhu, Z.; Jiang, Y.; Sun, S.; Lai, W.-H.; Lian, Q.; et al. Potent Immunomodulation and Angiogenic Effects of Mesenchymal Stem Cells versus Cardiomyocytes Derived from Pluripotent Stem Cells for Treatment of Heart Failure. Stem Cell Res. Ther. 2019, 10, 78. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.-D.; Chang, M.-Y.; Cheng, B.; Liu, Y.-W.; Lin, L.-C.; Chen, J.-H.; Hsieh, P.C.H. Injection of Peptide Nanogels Preserves Postinfarct Diastolic Function and Prolongs Efficacy of Cell Therapy in Pigs. Tissue Eng. Part A 2015, 21, 1662–1671. [Google Scholar] [CrossRef]
- Liu, C.-B.; Huang, H.; Sun, P.; Ma, S.-Z.; Liu, A.-H.; Xue, J.; Fu, J.-H.; Liang, Y.-Q.; Liu, B.; Wu, D.-Y.; et al. Human Umbilical Cord-Derived Mesenchymal Stromal Cells Improve Left Ventricular Function, Perfusion, and Remodeling in a Porcine Model of Chronic Myocardial Ischemia. Stem Cells Transl. Med. 2016, 5, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Peng, K.-Y.; Chiu, Y.-W.; Ho, Y.-L.; Wang, Y.-H.; Shun, C.-T.; Huang, S.-Y.; Lin, Y.-S.; de Vries, A.A.F.; Pijnappels, D.A.; et al. Human Placenta-Derived Multipotent Cells (HPDMCs) Modulate Cardiac Injury: From Bench to Small and Large Animal Myocardial Ischemia Studies. Cell Transplant. 2015, 24, 2463–2478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locatelli, P.; Olea, F.D.; Hnatiuk, A.; De Lorenzi, A.; Cerdá, M.; Giménez, C.S.; Sepúlveda, D.; Laguens, R.; Crottogini, A. Mesenchymal Stromal Cells Overexpressing Vascular Endothelial Growth Factor in Ovine Myocardial Infarction. Gene Ther. 2015, 22, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Zhao, S.; Liu, Q.; Jiang, S.; Song, P.; Qian, H.; Zhang, Y.; Ling, J.; Yan, C.; Cheng, H.; et al. Transplantation with Autologous Mesenchymal Stem Cells after Acute Myocardial Infarction Evaluated by Magnetic Resonance Imaging: An Experimental Study. J. Thorac. Imaging 2012, 27, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Smith, R.R.; Kanazawa, H.; Yee, K.; Seinfeld, J.; Tseliou, E.; Dawkins, J.F.; Kreke, M.; Cheng, K.; Luthringer, D.; et al. Validation of Contrast-Enhanced Magnetic Resonance Imaging to Monitor Regenerative Efficacy after Cell Therapy in a Porcine Model of Convalescent Myocardial Infarction. Circulation 2013, 128, 2764–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.; Lin, C.; Gao, J.; Liang, X.; Gao, W.; Shen, L.; Kang, L.; Xu, B. Mesenchymal Stem Cells Overexpressing Integrin-Linked Kinase Attenuate Left Ventricular Remodeling and Improve Cardiac Function after Myocardial Infarction. Mol. Cell. Biochem. 2014, 397, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Mazo, M.; Hernández, S.; Gavira, J.J.; Abizanda, G.; Araña, M.; López-Martínez, T.; Moreno, C.; Merino, J.; Martino-Rodríguez, A.; Uixeira, A.; et al. Treatment of Reperfused Ischemia with Adipose-Derived Stem Cells in a Preclinical Swine Model of Myocardial Infarction. Cell Transplant. 2012, 21, 2723–2733. [Google Scholar] [CrossRef] [Green Version]
- Medicetty, S.; Wiktor, D.; Lehman, N.; Raber, A.; Popovic, Z.B.; Deans, R.; Ting, A.E.; Penn, M.S. Percutaneous Adventitial Delivery of Allogeneic Bone Marrow-Derived Stem Cells via Infarct-Related Artery Improves Long-Term Ventricular Function in Acute Myocardial Infarction. Cell Transplant. 2012, 21, 1109–1120. [Google Scholar] [CrossRef]
- Mori, D.; Miyagawa, S.; Yajima, S.; Saito, S.; Fukushima, S.; Ueno, T.; Toda, K.; Kawai, K.; Kurata, H.; Nishida, H.; et al. Cell Spray Transplantation of Adipose-Derived Mesenchymal Stem Cell Recovers Ischemic Cardiomyopathy in a Porcine Model. Transplantation 2018, 102, 2012–2024. [Google Scholar] [CrossRef]
- Natsumeda, M.; Florea, V.; Rieger, A.C.; Tompkins, B.A.; Banerjee, M.N.; Golpanian, S.; Fritsch, J.; Landin, A.M.; Kashikar, N.D.; Karantalis, V.; et al. A Combination of Allogeneic Stem Cells Promotes Cardiac Regeneration. J. Am. Coll. Cardiol. 2017, 70, 2504–2515. [Google Scholar] [CrossRef]
- Ozawa, H.; Miyagawa, S.; Fukushima, S.; Itoh, E.; Harada, A.; Saito, A.; Ueno, T.; Toda, K.; Kuratani, T.; Sawa, Y. Sirtuin1 Regulates the Stem Cell Therapeutic Effects on Regenerative Capability for Treating Severe Heart Failure in a Juvenile Animal Model. Ann. Thorac. Surg. 2016, 102, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, C.; Yang, K.; Xiang, P.; Zhang, C.; Zou, L.; Wu, X.; Gao, Y.; Kang, Z.; He, K.; Liu, J.; et al. Effect of Transplantation with Autologous Bone Marrow Stem Cells on Acute Myocardial Infarction. Int. J. Cardiol. 2013, 162, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Prifti, E.; Di Lascio, G.; Harmelin, G.; Bani, D.; Briganti, V.; Veshti, A.; Bonacchi, M. Cellular Cardiomyoplasty into Infracted Swine’s Hearts by Retrograde Infusion through the Venous Coronary Sinus: An Experimental Study. Cardiovasc. Revasc. Med. Mol. Interv. 2016, 17, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, S.; Soleimani, M.; Sahebjam, M.; Imani, M.; Nassiri, S.M.; Atashi, A.; Daliri Joupari, M.; Ghiaseddin, A.; Latifpour, M.; Ahmadi Tafti, S.H. Effects of Endothelial and Mesenchymal Stem Cells on Improving Myocardial Function in a Sheep Animal Model. J. Tehran Heart Cent. 2017, 12, 65–71. [Google Scholar] [PubMed]
- Rigol, M.; Solanes, N.; Roura, S.; Roqué, M.; Novensà, L.; Dantas, A.P.; Martorell, J.; Sitges, M.; Ramírez, J.; Bayés-Genís, A.; et al. Allogeneic Adipose Stem Cell Therapy in Acute Myocardial Infarction. Eur. J. Clin. Investig. 2014, 44, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Romagnuolo, R.; Masoudpour, H.; Porta-Sánchez, A.; Qiang, B.; Barry, J.; Laskary, A.; Qi, X.; Massé, S.; Magtibay, K.; Kawajiri, H.; et al. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Rep. 2019, 12, 967–981. [Google Scholar] [CrossRef] [Green Version]
- Schuleri, K.H.; Centola, M.; Choi, S.H.; Evers, K.S.; Dawoud, F.; George, R.T.; Lima, J.A.C.; Lardo, A.C. CT for Evaluation of Myocardial Cell Therapy in Heart Failure: A Comparison with CMR Imaging. JACC Cardiovasc. Imaging 2011, 4, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Sheu, J.-J.; Lee, F.-Y.; Yuen, C.-M.; Chen, Y.-L.; Huang, T.-H.; Chua, S.; Chen, Y.-L.; Chen, C.-H.; Chai, H.-T.; Sung, P.-H.; et al. Combined Therapy with Shock Wave and Autologous Bone Marrow-Derived Mesenchymal Stem Cells Alleviates Left Ventricular Dysfunction and Remodeling through Inhibiting Inflammatory Stimuli, Oxidative Stress & Enhancing Angiogenesis in a Swine Myocardial Infarction Model. Int. J. Cardiol. 2015, 193, 69–83. [Google Scholar] [CrossRef]
- Shudo, Y.; Miyagawa, S.; Nakatani, S.; Fukushima, S.; Sakaguchi, T.; Saito, A.; Asanuma, T.; Kawaguchi, N.; Matsuura, N.; Shimizu, T.; et al. Myocardial Layer-Specific Effect of Myoblast Cell-Sheet Implantation Evaluated by Tissue Strain Imaging. Circ. J. Off. J. Jpn. Circ. Soc. 2013, 77, 1063–1072. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Yang, Y.-J.; Dong, Q.-T.; Qian, H.-Y.; Gao, R.-L.; Qiao, S.-B.; Shen, R.; He, Z.-X.; Lu, M.-J.; Zhao, S.-H.; et al. Atorvastatin Enhance Efficacy of Mesenchymal Stem Cells Treatment for Swine Myocardial Infarction via Activation of Nitric Oxide Synthase. PLoS ONE 2013, 8, e65702. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.-W.; Zhen, L.; Wang, Q.; Sun, Y.; Yang, J.; Li, Y.-J.; Li, R.-J.; Ma, N.; Li, Z.-A.; Wang, L.-Y.; et al. Assessment of Retrograde Coronary Venous Infusion of Mesenchymal Stem Cells Combined with Basic Fibroblast Growth Factor in Canine Myocardial Infarction Using Strain Values Derived from Speckle-Tracking Echocardiography. Ultrasound Med. Biol. 2016, 42, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jiang, Y.; Zhen, Z.; Lai, W.-H.; Liao, S.; Tse, H.-F. Establishing a Swine Model of Post-Myocardial Infarction Heart Failure for Stem Cell Treatment. J. Vis. Exp. JoVE 2020, 159, e60392. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, G.; Young, R.F.; Leiker, M.M.; Suzuki, T. Heart-Derived Stem Cells in Miniature Swine with Coronary Microembolization: Novel Ischemic Cardiomyopathy Model to Assess the Efficacy of Cell-Based Therapy. Stem Cells Int. 2016, 2016, 6940195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseliou, E.; Kanazawa, H.; Dawkins, J.; Gallet, R.; Kreke, M.; Smith, R.; Middleton, R.; Valle, J.; Marbán, L.; Kar, S.; et al. Widespread Myocardial Delivery of Heart-Derived Stem Cells by Nonocclusive Triple-Vessel Intracoronary Infusion in Porcine Ischemic Cardiomyopathy: Superior Attenuation of Adverse Remodeling Documented by Magnetic Resonance Imaging and Histology. PLoS ONE 2016, 11, e0144523. [Google Scholar] [CrossRef]
- Van der Spoel, T.I.G.; Gathier, W.A.; Koudstaal, S.; van Slochteren, F.; Of Lorkeers, S.J.; Sluijter, J.P.G.; Hoefer, I.E.; Steendijk, P.; Cramer, M.J.M.; Doevendans, P.A.; et al. Autologous Mesenchymal Stem Cells Show More Benefit on Systolic Function Compared to Bone Marrow Mononuclear Cells in a Porcine Model of Chronic Myocardial Infarction. J Cardiovasc. Transl. Res. 2015, 8, 393–403. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Zhen, L.; Miao, H.; Sun, Q.; Yang, Y.; Que, B.; Lopes Lao, E.P.; Wu, X.; Ren, H.; Shi, S.; et al. Concomitant Retrograde Coronary Venous Infusion of Basic Fibroblast Growth Factor Enhances Engraftment and Differentiation of Bone Marrow Mesenchymal Stem Cells for Cardiac Repair after Myocardial Infarction. Theranostics 2015, 5, 995–1006. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.R.; Hatzistergos, K.E.; Addicott, B.; McCall, F.; Carvalho, D.; Suncion, V.; Morales, A.R.; Da Silva, J.; Sussman, M.A.; Heldman, A.W.; et al. Enhanced Effect of Combining Human Cardiac Stem Cells and Bone Marrow Mesenchymal Stem Cells to Reduce Infarct Size and to Restore Cardiac Function after Myocardial Infarction. Circulation 2013, 127, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Winkler, J.; Lukovic, D.; Mester-Tonczar, J.; Zlabinger, K.; Gugerell, A.; Pavo, N.; Jakab, A.; Szankai, Z.; Traxler, D.; Müller, C.; et al. Quantitative Hybrid Cardiac [18F]FDG-PET-MRI Images for Assessment of Cardiac Repair by Preconditioned Cardiosphere-Derived Cells. Mol. Ther. Methods Clin. Dev. 2020, 18, 354–366. [Google Scholar] [CrossRef]
- Yang, K.; Xiang, P.; Zhang, C.; Zou, L.; Wu, X.; Gao, Y.; Kang, Z.; He, K.; Liu, J.; Peng, C. Magnetic Resonance Evaluation of Transplanted Mesenchymal Stem Cells after Myocardial Infarction in Swine. Can. J. Cardiol. 2011, 27, 818–825. [Google Scholar] [CrossRef]
- Yin, Q.; Pei, Z.; Wang, H.; Zhao, Y. Cyclosporine A-Nanoparticles Enhance the Therapeutic Benefit of Adipose Tissue-Derived Stem Cell Transplantation in a Swine Myocardial Infarction Model. Int. J. Nanomed. 2014, 9, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.-W.; Gu, T.-X.; Guan, X.-Y.; Sun, X.-J.; Jiang, D.-Q.; Tang, R.; Qi, X.; Li, X.-Y. Delayed Enrichment for C-Kit and Inducing Cardiac Differentiation Attenuated Protective Effects of BMSCs’ Transplantation in Pig Model of Acute Myocardial Ischemia. Cardiovasc. Ther. 2015, 33, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-W.; Liu, X.-C.; Li-Ling, J.; Luan, Y.; Ying, Y.-N.; Wu, X.-S.; Zhao, C.-H.; Liu, T.-J.; Lü, F. Mechanisms of the Protective Effects of BMSCs Promoted by TMDR with Heparinized BFGF-Incorporated Stent in Pig Model of Acute Myocardial Ischemia. J. Cell. Mol. Med. 2011, 15, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-J.; Liu, X.-C.; Kong, F.; Qi, T.-G.; Cheng, G.-H.; Wang, J.; Sun, C.; Luan, Y. Bone Marrow Mesenchymal Stem Cells Improve Myocardial Function in a Swine Model of Acute Myocardial Infarction. Mol. Med. Rep. 2014, 10, 1448–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noiseux, N.; Borie, M.; Desnoyers, A.; Menaouar, A.; Stevens, L.M.; Mansour, S.; Danalache, B.A.; Roy, D.-C.; Jankowski, M.; Gutkowska, J. Preconditioning of Stem Cells by Oxytocin to Improve Their Therapeutic Potential. Endocrinology 2012, 153, 5361–5372. [Google Scholar] [CrossRef]
- Tan, S.C.; Gomes, R.S.; Yeoh, K.K.; Perbellini, F.; Malandraki-Miller, S.; Ambrose, L.; Heather, L.C.; Faggian, G.; Schofield, C.J.; Davies, K.E.; et al. Preconditioning of Cardiosphere-Derived Cells With Hypoxia or Prolyl-4-Hydroxylase Inhibitors Increases Stemness and Decreases Reliance on Oxidative Metabolism. Cell Transpl. 2016, 25, 35–53. [Google Scholar] [CrossRef]
- Araña, M.; Gavira, J.J.; Peña, E.; González, A.; Abizanda, G.; Cilla, M.; Pérez, M.M.; Albiasu, E.; Aguado, N.; Casado, M.; et al. Epicardial Delivery of Collagen Patches with Adipose-Derived Stem Cells in Rat and Minipig Models of Chronic Myocardial Infarction. Biomaterials 2014, 35, 143–151. [Google Scholar] [CrossRef]
- Rashedi, I.; Talele, N.; Wang, X.-H.; Hinz, B.; Radisic, M.; Keating, A. Collagen Scaffold Enhances the Regenerative Properties of Mesenchymal Stromal Cells. PLoS ONE 2017, 12, e0187348. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Lemcke, H.; David, R. Stem Cell Therapy in Heart Diseases—Cell Types, Mechanisms and Improvement Strategies. Cell. Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef]
- Xu, J.; Liu, D.; Zhong, Y.; Huang, R. Effects of Timing on Intracoronary Autologous Bone Marrow-Derived Cell Transplantation in Acute Myocardial Infarction: A Meta-Analysis of Randomized Controlled Trials. Stem Cell Res. Ther. 2017, 8, 231. [Google Scholar] [CrossRef] [Green Version]
- Siu, C.-W.; Liao, S.-Y.; Liu, Y.; Lian, Q.; Tse, H.-F. Stem Cells for Myocardial Repair. Thromb. Haemost. 2010, 104, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Zwetsloot, P.P.; Végh, A.M.D.; Jansen of Lorkeers, S.J.; van Hout, G.P.J.; Currie, G.L.; Sena, E.S.; Gremmels, H.; Buikema, J.W.; Goumans, M.-J.; Macleod, M.R.; et al. Cardiac Stem Cell Treatment in Myocardial Infarction. Circ. Res. 2016, 118, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
- Korf-Klingebiel, M.; Kempf, T.; Sauer, T.; Brinkmann, E.; Fischer, P.; Meyer, G.P.; Ganser, A.; Drexler, H.; Wollert, K.C. Bone Marrow Cells Are a Rich Source of Growth Factors and Cytokines: Implications for Cell Therapy Trials after Myocardial Infarction. Eur. Heart J. 2008, 29, 2851–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spannbauer, A.; Mester-Tonczar, J.; Traxler, D.; Kastner, N.; Zlabinger, K.; Hašimbegović, E.; Riesenhuber, M.; Pavo, N.; Goliasch, G.; Gyöngyösi, M. Large Animal Models of Cell-Free Cardiac Regeneration. Biomolecules 2020, 10, 1392. [Google Scholar] [CrossRef] [PubMed]
- Petitti, D.B. Meta-Analysis, Decision Analysis, and Cost-Effectiveness Analysis: Methods for Quantitative Synthesis in Medicine; Oxford University Press: New York, NY, USA, 2000; ISBN 978-0-19-513364-6. [Google Scholar]
- Gurevitch, J.; Koricheva, J.; Nakagawa, S.; Stewart, G. Meta-Analysis and the Science of Research Synthesis. Nature 2018, 555, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Spector, T.D.; Thompson, S.G. The Potential and Limitations of Meta-Analysis. J. Epidemiol. Community Health 1991, 45, 89–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).