Effect of Scrapie Prion Infection in Ovine Bone Marrow-Derived Mesenchymal Stem Cells and Ovine Mesenchymal Stem Cell-Derived Neurons
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Collection
2.2. Ovine Mesenchymal Stem Cell Isolation and Culture
2.3. Neurogenic Differentiation
2.4. Scrapie Inocula and Infection of oBM-MSC and Neuron-Like Cultures
2.5. Proliferation Potential and Cell Viability
2.6. PrPSc Detection
3. Results
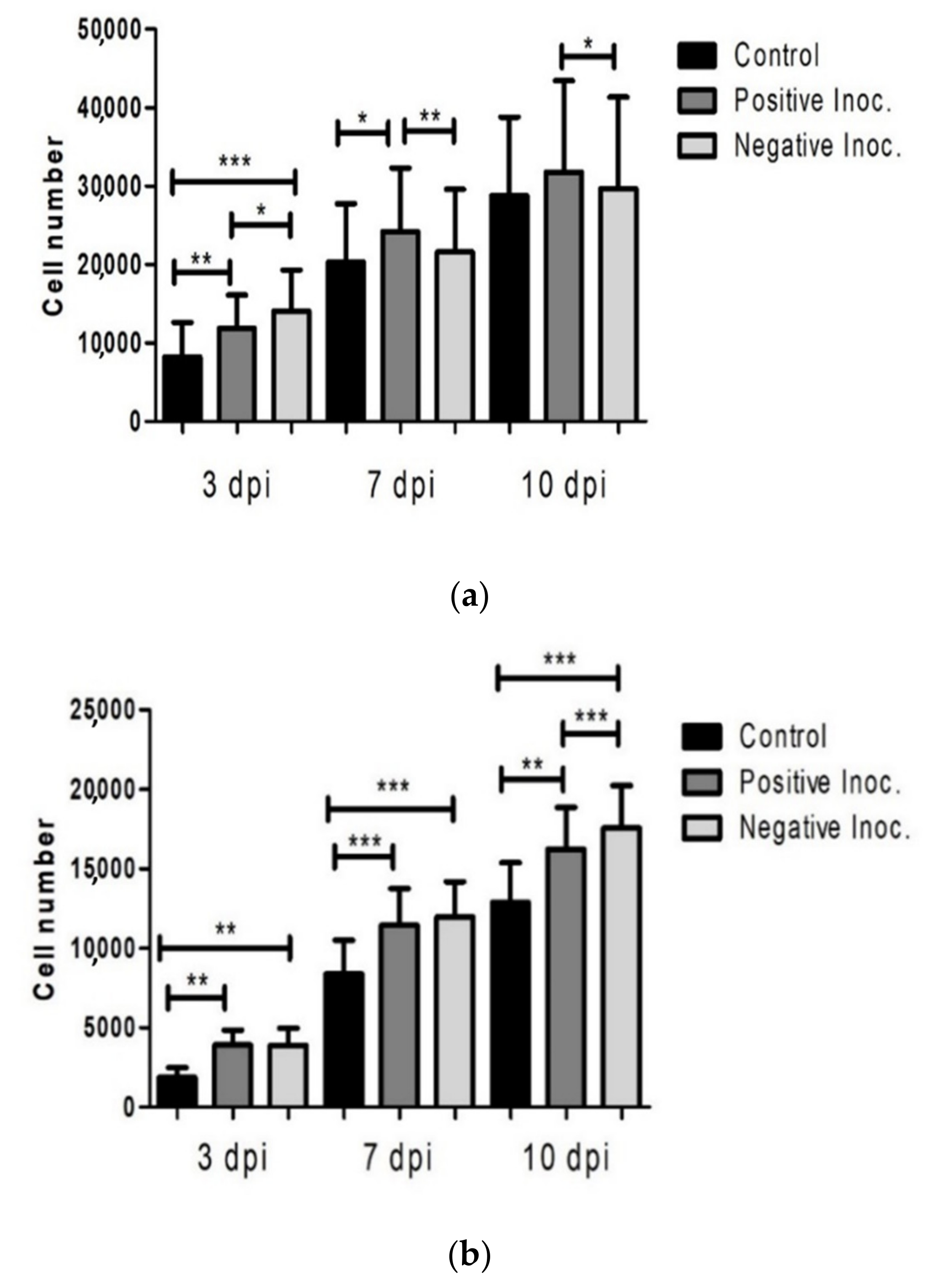
3.1. Proliferation Potential of Infected oBM-MSC
3.2. Cell Viability of Infected Cultures
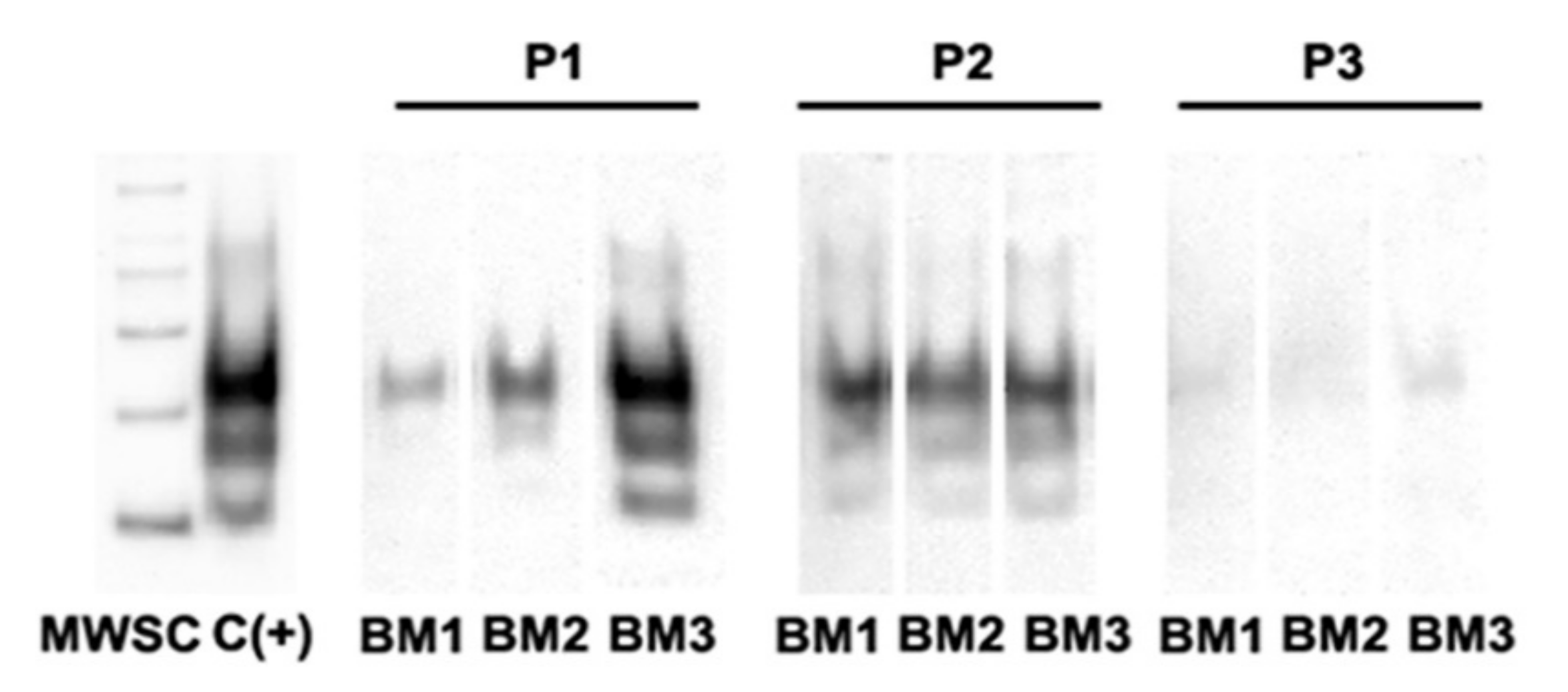
3.3. PrPSc Detection in Infected oBM-MSCs, Analyzed by Western Blotting
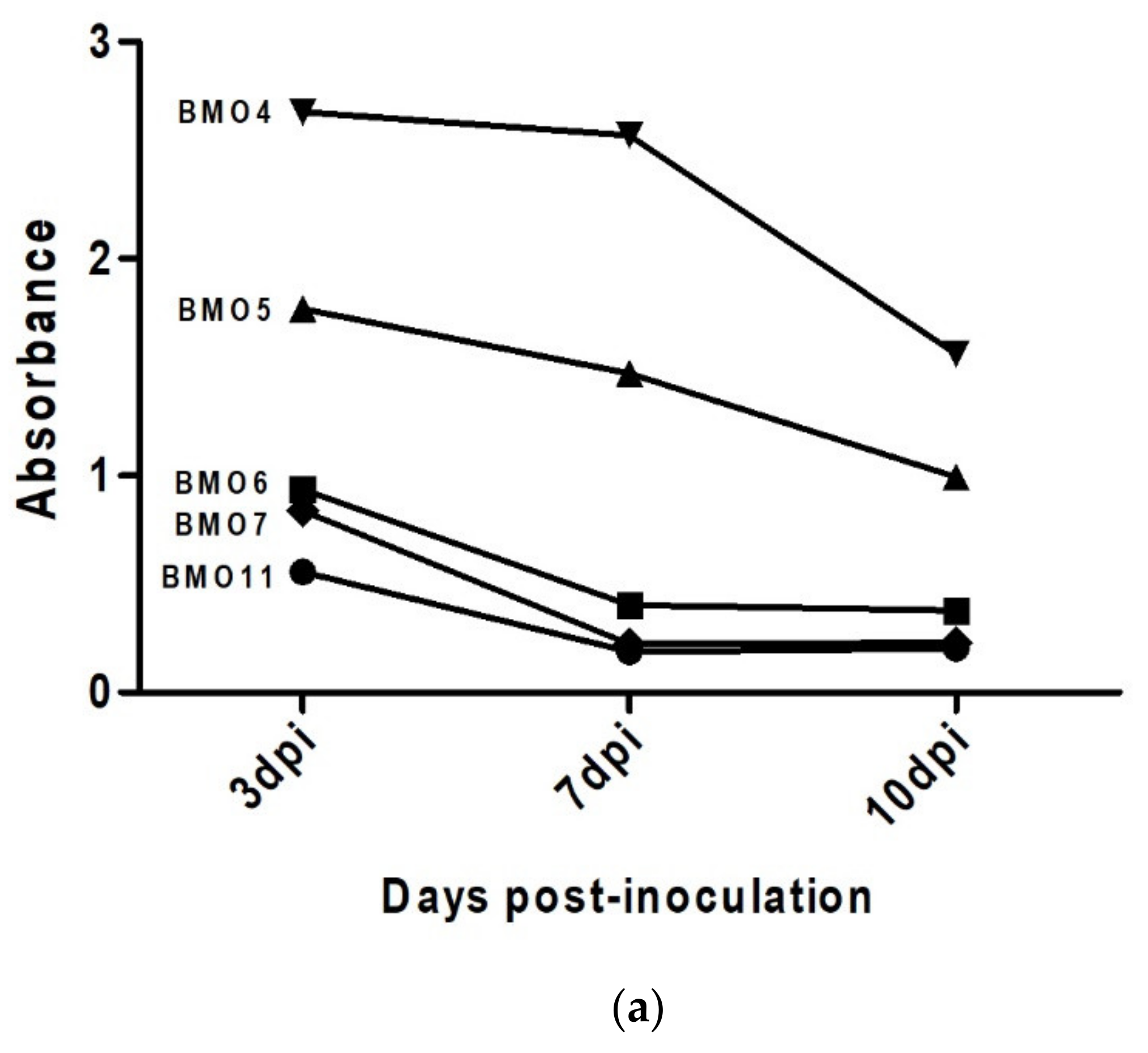
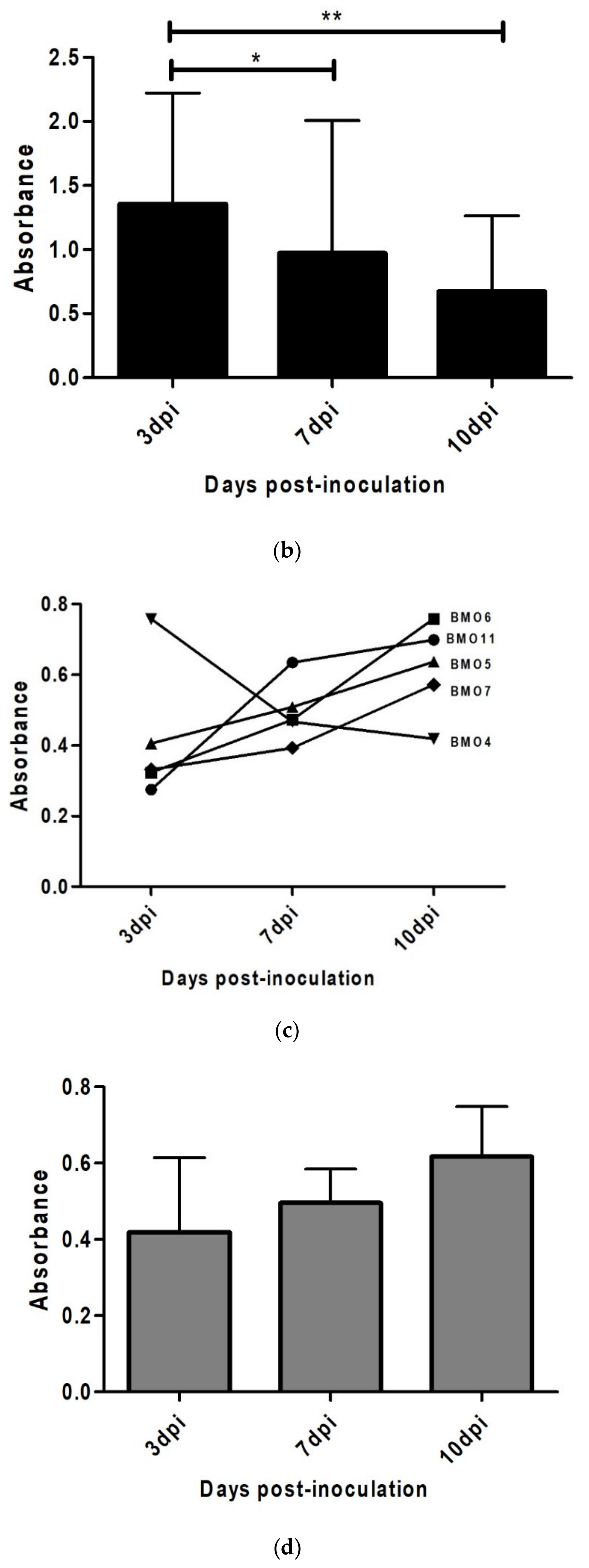
3.4. Prion Detection by ELISA in oBM-MSC and Neuron-Like Cultures Infected with Scrapie
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prusiner, S.B. The prion diseases. Brain Pathol. 1998, 8, 499–513. [Google Scholar] [CrossRef]
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef]
- Bell, J.E.; Ironside, J.W. Neuropathology of spongiform encephalopathies in humans. Br. Med. Bull. 1993, 49, 738–777. [Google Scholar] [CrossRef] [PubMed]
- Pattison, I.H.; Jones, K.M. The astrocytic reaction in experimental scrapie in the rat. Res. Vet. Sci. 1967, 8, 160–165. [Google Scholar] [CrossRef]
- Zabel, M.D.; Reid, C. A brief history of prions. Pathog. Dis. 2015, 73. [Google Scholar] [CrossRef] [PubMed]
- McMahon, H.E. Cell culture methods for screening of prion therapeutics. In Methods in Molecular Biology; Humana Press Inc.: Totowa, NJ, USA, 2017; Volume 1658, pp. 295–304. [Google Scholar]
- Bedecs, K. Cell culture models to unravel prion protein function and aberrancies in prion diseases. Methods Mol. Biol. 2008, 459, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Solassol, J.; Crozet, C.; Lehmann, S. Prion propagation in cultured cells. Br. Med. Bull. 2003, 66, 87–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Woodbury, D.; Reynolds, K.; Black, I.B. Adult bone marrow stromal stem cells express germline, ectodermal, endodermal, and mesodermal genes prior to neurogenesis. J. Neurosci. Res. 2002, 69, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.R.; Duan, W.M.; Reyes, M.; Keene, C.D.; Verfaillie, C.M.; Low, W.C. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp. Neurol. 2002, 174, 11–20. [Google Scholar] [CrossRef]
- Takakura, Y.; Yamaguchi, N.; Nakagaki, T.; Satoh, K.; Kira, J.; Nishida, N. Bone marrow stroma cells are susceptible to prion infection. Biochem. Biophys. Res. Commun. 2008, 377, 957–961. [Google Scholar] [CrossRef]
- Shi, F.; Yang, Y.; Wang, T.; Kouadir, M.; Zhao, D.; Hu, S. Cellular Prion Protein Promotes Neuronal Differentiation of Adipose-Derived Stem Cells by Upregulating miRNA-124. J. Mol. Neurosci. 2016, 59, 48–55. [Google Scholar] [CrossRef]
- Martellucci, S.; Santacroce, C.; Manganelli, V.; Santilli, F.; Piccoli, L.; Cassetta, M.; Misasi, R.; Sorice, M.; Mattei, V. Isolation, propagation, and prion protein expression during neuronal differentiation of human dental pulp stem cells. J. Vis. Exp. 2019, 2019, e59282. [Google Scholar] [CrossRef]
- Martellucci, S.; Santacroce, C.; Santilli, F.; Piccoli, L.; Monache, S.D.; Angelucci, A.; Misasi, R.; Sorice, M.; Mattei, V. Cellular and molecular mechanisms mediated by recPrP C involved in the neuronal differentiation process of mesenchymal stem cells. Int. J. Mol. Sci. 2019, 20, 345. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Hirai, Y.; Nakayama, M.; Hayashi, R.; Yamasaki, T.; Hasebe, R.; Song, C.H.; Horiuchi, M. Therapeutic effect of autologous compact bone-derived mesenchymal stem cell transplantation on prion disease. J. Gen. Virol. 2017, 98, 2615–2627. [Google Scholar] [CrossRef] [PubMed]
- Akimov, S.; Vasilyeva, I.; Yakovleva, O.; McKenzie, C.; Cervenakova, L. Murine bone marrow stromal cell culture with features of mesenchymal stem cells susceptible to mouse-adapted human TSE agent, Fukuoka-1. Folia Neuropathol. 2009, 47, 205–214. [Google Scholar] [PubMed]
- Mediano, D.R.; Sanz-Rubio, D.; Bolea, R.; Marín, B.; Vázquez, F.J.; Remacha, A.R.; López-Pérez, Ó.; Fernández-Borges, N.; Castilla, J.; Zaragoza, P.; et al. Characterization of mesenchymal stem cells in sheep naturally infected with scrapie. J. Gen. Virol. 2015, 96, 3715–3726. [Google Scholar] [CrossRef] [PubMed]
- Groveman, B.R.; Foliaki, S.T.; Orru, C.D.; Zanusso, G.; Carroll, J.A.; Race, B.; Haigh, C.L. Sporadic Creutzfeldt-Jakob disease prion infection of human cerebral organoids. Acta Neuropathol. Commun. 2019, 7, 90. [Google Scholar] [CrossRef]
- Krejciova, Z.; Alibhai, J.; Zhao, C.; Krencik, R.; Rzechorzek, N.M.; Ullian, E.M.; Manson, J.; Ironside, J.W.; Head, M.W.; Chandran, S. Human stem cell-derived astrocytes replicate human prions in a PRNP genotype-dependent manner. J. Exp. Med. 2017, 214, 3481–3495. [Google Scholar] [CrossRef]
- Lyahyai, J.; Mediano, D.R.; Ranera, B.; Sanz, A.; Remacha, A.R.; Bolea, R.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Isolation and characterization of ovine mesenchymal stem cells derived from peripheral blood. BMC Vet. Res. 2012, 8, 169. [Google Scholar] [CrossRef]
- Hortells, P.; Monzón, M.; Monleón, E.; Acín, C.; Vargas, A.; Bolea, R.; Luján, L.; Badiola, J.J. Pathological findings in retina and visual pathways associated to natural Scrapie in sheep. Brain Res. 2006, 1108, 188–194. [Google Scholar] [CrossRef]
- O’Rourke, K.I.; Baszler, T.V.; Besser, T.E.; Miller, J.M.; Cutlip, R.C.; Wells, G.A.H.; Ryder, S.J.; Parish, S.M.; Hamir, A.N.; Cockett, N.E.; et al. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J. Clin. Microbiol. 2000, 38, 3254–3259. [Google Scholar] [CrossRef] [PubMed]
- Ranera, B.; Ordovás, L.; Lyahyai, J.; Bernal, M.L.; Fernandes, F.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Osta, R.; Cons, C.; et al. Comparative study of equine bone marrow and adipose tissue-derived mesenchymal stromal cells. Equine Vet. J. 2012, 44, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bolea, R.; Monleón, E.; Schiller, I.; Raeber, A.J.; Acín, C.; Monzón, M.; Martín-Burriel, I.; Struckmeyer, T.; Oesch, B.; Badiola, J.J. Comparison of immunohistochemistry and two rapid tests for detection of abnormal prion protein in different brain regions of sheep with typical scrapie. J. Vet. Diagn. Investig. 2005, 17, 467–469. [Google Scholar] [CrossRef]
- Filali, H.; Vidal, E.; Bolea, R.; Márquez, M.; Marco, P.; Vargas, A.; Pumarola, M.; Martin-Burriel, I.; Badiola, J.J. Gene and protein patterns of potential prion-related markers in the central nervous system of clinical and preclinical infected sheep. Vet. Res. 2013, 44, 14. [Google Scholar] [CrossRef] [PubMed]
- Zvaifler, N.J.; Marinova-Mutafchieva, L.; Adams, G.; Edwards, C.J.; Moss, J.; Burger, J.A.; Maini, R.N. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000, 2, 477–488. [Google Scholar] [CrossRef]
- Sanchez-Ramos, J.; Song, S.; Cardozo-Pelaez, F.; Hazzi, C.; Stedeford, T.; Willing, A.; Freeman, T.B.; Saporta, S.; Janssen, W.; Patel, N.; et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp. Neurol. 2000, 164, 247–256. [Google Scholar] [CrossRef]
- Akimov, S.; Yakovleva, O.; Vasilyeva, I.; McKenzie, C.; Cervenakova, L. Persistent Propagation of Variant Creutzfeldt-Jakob Disease Agent in Murine Spleen Stromal Cell Culture with Features of Mesenchymal Stem Cells. J. Virol. 2008, 82, 10959–10962. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cervenakova, L.; Akimov, S.; Vasilyeva, I.; Yakovleva, O.; McKenzie, C.; Cervenak, J.; Piccardo, P.; Asher, D.M. Fukuoka-1 strain of transmissible spongiform encephalopathy agent infects murine bone marrow-derived cells with features of mesenchymal stem cells. Transfusion 2011, 51, 1755–1768. [Google Scholar] [CrossRef]
- Song, C.-H.; Honmou, O.; Ohsawa, N.; Nakamura, K.; Hamada, H.; Furuoka, H.; Hasebe, R.; Horiuchi, M. Effect of Transplantation of Bone Marrow-Derived Mesenchymal Stem Cells on Mice Infected with Prions. J. Virol. 2009, 83, 5918–5927. [Google Scholar] [CrossRef] [PubMed]
- Chopp, M.; Li, Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002, 1, 92–100. [Google Scholar] [CrossRef]
- Song, C.-H.; Honmou, O.; Furuoka, H.; Horiuchi, M. Identification of Chemoattractive Factors Involved in the Migration of Bone Marrow-Derived Mesenchymal Stem Cells to Brain Lesions Caused by Prions. J. Virol. 2011, 85, 11069–11078. [Google Scholar] [CrossRef] [PubMed]
- Bonab, M.M.; Alimoghaddam, K.; Talebian, F.; Ghaffari, S.H.; Ghavamzadeh, A.; Nikbin, B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006, 7, 14. [Google Scholar] [CrossRef]
- Calloni, R.; Viegas, G.S.; Türck, P.; Bonatto, D.; Henriques, J.A.P. Mesenchymal stromal cells from unconventional model organisms. Cytotherapy 2014, 16, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaghami, S.; Phuan, P.W.; Perkins, B.; Ullman, J.; May, B.C.H.; Cohen, F.E.; Prusiner, S.B. Cell division modulates prion accumulation in cultured cells. Proc. Natl. Acad. Sci. USA 2007, 104, 17971–17976. [Google Scholar] [CrossRef] [PubMed]
- Kanu, N.; Imokawa, Y.; Drechsel, D.N.; Williamson, R.A.; Birkett, C.R.; Bostock, C.J.; Brockes, J.P. Transfer of scrapie prion infectivity by cell contact in culture. Curr. Biol. 2002, 12, 523–530. [Google Scholar] [CrossRef]
- Mediano, D.R. Caracterización de Células Madre Mesenquimales Ovinas Infectadas Por Scrapie; University of Zaragoza: Zaragoza, Spain, 2016. (In Spanish) [Google Scholar]

| Sheep | Genotype | Sex | Age (Years) | Scrapie Status | Breed | Assay |
|---|---|---|---|---|---|---|
| BMO1 | ARQ/ARQ | Female | 7 | Exposed, not detected | Rasa Aragonesa | WB, PA |
| BMO2 | ARQ/ARQ | Female | 4 | Exposed, not detected | Rasa Aragonesa | WB, PA |
| BMO3 | ARQ/ARQ | Female | 4 | Exposed, not detected | Rasa Aragonesa | WB, PA |
| BMO4 | ARQ/ARQ | Male | 1 | Exposed, not detected | Rasa Aragonesa | MTT, ELISA |
| BMO5 | ARQ/ARQ | Male | 2 | Preclinical | Crossbreed | MTT, ELISA |
| BMO6 | ARQ/ARQ | Female | 6 | Exposed, not detected | Ojinegra | MTT, ELISA |
| BMO7 | ARQ/ARQ | Male | 3 | Exposed, not detected | Crossbreed | MTT, ELISA |
| BMO8 | ARQ/ARQ | Female | 7 | Exposed, not detected | Rasa Aragonesa | MTT |
| BMO9 | ARR/ARQ | Female | 5 | Exposed, not detected | Rasa Aragonesa | MTT |
| BMO10 | ARQ/VRQ | Female | 4 | Exposed, not detected | Crossbreed | MTT |
| BMO11 | ARQ/VRQ | Male | 2 | Preclinical | Crossbreed | MTT, ELISA |
| Passage | Inocula | ||
|---|---|---|---|
| Healthy | Scrapie | ||
| 1 | CD DT (days) | 3.150 ± 0.286 * | 2.949 ± 0.219 * |
| 1.714 ± 0.355 ** | 1.825 ± 0.343 ** | ||
| 2 | CD DT (days) | 3.22 ± 0.651 | 2.870 ± 0.531 |
| 2.054 ± 0.653 | 2.291 ± 0.681 | ||
| 3 | CD DT (days) | 1.93 ± 0.390 | 1.807 ± 0.027 |
| 2.116 ± 0.428 | 2.214 ± 0.033 | ||
| Av | CD DT (days) | 2.871 ± 0.711 * | 2.634 ± 0.597 * |
| 1.942 ± 0.469 | 2.097 ± 0.469 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Mendívil, L.; Mediano, D.R.; Hernaiz, A.; Sanz-Rubio, D.; Vázquez, F.J.; Marín, B.; López-Pérez, Ó.; Otero, A.; Badiola, J.J.; Zaragoza, P.; et al. Effect of Scrapie Prion Infection in Ovine Bone Marrow-Derived Mesenchymal Stem Cells and Ovine Mesenchymal Stem Cell-Derived Neurons. Animals 2021, 11, 1137. https://doi.org/10.3390/ani11041137
García-Mendívil L, Mediano DR, Hernaiz A, Sanz-Rubio D, Vázquez FJ, Marín B, López-Pérez Ó, Otero A, Badiola JJ, Zaragoza P, et al. Effect of Scrapie Prion Infection in Ovine Bone Marrow-Derived Mesenchymal Stem Cells and Ovine Mesenchymal Stem Cell-Derived Neurons. Animals. 2021; 11(4):1137. https://doi.org/10.3390/ani11041137
Chicago/Turabian StyleGarcía-Mendívil, Laura, Diego R. Mediano, Adelaida Hernaiz, David Sanz-Rubio, Francisco J. Vázquez, Belén Marín, Óscar López-Pérez, Alicia Otero, Juan J. Badiola, Pilar Zaragoza, and et al. 2021. "Effect of Scrapie Prion Infection in Ovine Bone Marrow-Derived Mesenchymal Stem Cells and Ovine Mesenchymal Stem Cell-Derived Neurons" Animals 11, no. 4: 1137. https://doi.org/10.3390/ani11041137
APA StyleGarcía-Mendívil, L., Mediano, D. R., Hernaiz, A., Sanz-Rubio, D., Vázquez, F. J., Marín, B., López-Pérez, Ó., Otero, A., Badiola, J. J., Zaragoza, P., Ordovás, L., Bolea, R., & Martín-Burriel, I. (2021). Effect of Scrapie Prion Infection in Ovine Bone Marrow-Derived Mesenchymal Stem Cells and Ovine Mesenchymal Stem Cell-Derived Neurons. Animals, 11(4), 1137. https://doi.org/10.3390/ani11041137









