Isolation and Characterization of Cat Olfactory Ecto-Mesenchymal Stem Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Biopsy of Olfactory Mucosa and Isolation and Expansion of OE-MSCs
2.3. Generation of Spheres
2.4. In Vitro Neural Lineage Differentiation Assays
2.5. Expression of Nestin
2.6. Clonal Efficiency Assay
2.7. In Vitro Proliferation Assay
2.8. In Vitro Mesodermal Differentiation Assays
2.9. Immunocytochemistry
2.10. Image Acquisition
3. Results
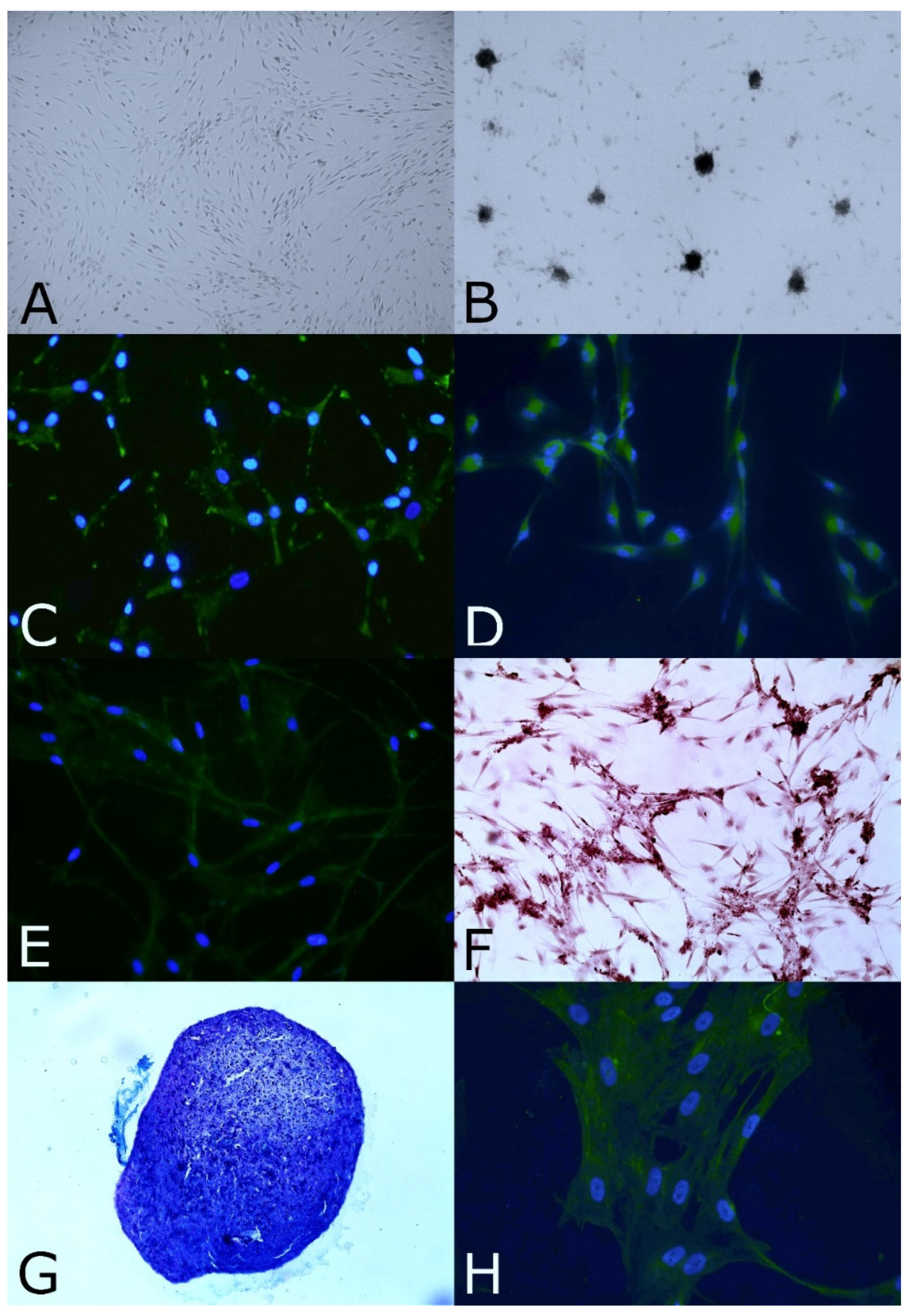
3.1. Biopsy of Olfactory Mucosa and Isolation and Expansion of OE-MSCs
3.2. Stemness and Immature Features
3.3. Clonal Efficiency Assay
3.4. In Vitro Proliferation Assay
3.5. In Vitro Neural and Mesodermal Differentiation Assays
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Hmadcha, A.; Martin-Montalvo, A.; Gauthier, B.R.; Soria, B.; Capilla-Gonzalez, V. Therapeutic Potential of Mesenchymal Stem Cells for Cancer Therapy. Front. Bioeng. Biotechnol. 2020, 8, 43. [Google Scholar] [CrossRef]
- Willing, A.E.; Das, M.; Howell, M.; Mohapatra, S.S.; Mohapatra, S. Potential of mesenchymal stem cells alone, or in combination, to treat traumatic brain injury. CNS Neurosci. Epub. 2020, 26, 616–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriakidis, T.; Iosifidis, M.; Michalopoulos, E.; Melas, I.; Stavropoulos-Giokas, C.; Verdonk, R. Good mid-term outcomes after adipose-derived culture-expanded mesenchymal stem cells implantation in knee focal cartilage defects. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 502–508. [Google Scholar] [CrossRef]
- Goradel, N.H.; Ghiyami-Hour, F.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem Cell Therapy: A New Therapeutic Option for Cardiovascular Diseases. J. Cell Biochem. 2018, 119, 95–104. [Google Scholar] [CrossRef]
- Rajabzadeh, N.; Fathi, E.; Farahzad, R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019, 6, 19. [Google Scholar] [CrossRef]
- Viktorova, V.; Savchenkova, I.P. Multipotent mesenchymal stem cells in clinical veterinary practice. IOP Conf. Ser. Earth Environ. Sci. 2020, 548, 072072. [Google Scholar] [CrossRef]
- Vikartovska, Z.; Humenik, F.; Maloveska, M.; Farbakova, J.; Hornakova, L.; Murgoci, A.N.; Cizkova, D. Adult Stem Cells Based Therapies in Veterinary Medicine. Arch. Vet. Sci. Med. 2020, 3, 40–50. [Google Scholar] [CrossRef]
- Dias, I.E.; Pinto, P.O.; Barros, L.C.; Viegas, C.A.; Isabel Ribeiro Dias, I.R.; Carvalho, P.P. Mesenchymal stem cells therapy in companion animals: Useful for immune-mediated diseases? BMC Vet. Res. 2019, 15, 358. [Google Scholar] [CrossRef] [Green Version]
- Martin, D.R.; Cox, N.R.; Hathcock, T.L.; Niemeyer, G.P.; Baker, H.J. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp. Hematol. 2002, 30, 879–886. [Google Scholar] [CrossRef]
- Quimby, J.M.; Borjesson, D.L. Mesenchymal stem cell therapy in cats: Current knowledge and future potential. J. Feline Med. Surg. 2018, 20, 208–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gugjoo, M.B.; Pal, A. Cat Mesenchymal Stem Cell Characteristics and Potential Applications. In Mesenchymal Stem Cell in Veterinary Sciences; Gugjoo, M.B., Pal, A., Eds.; Springer: Singapore, 2020; pp. 197–212. [Google Scholar] [CrossRef]
- Arzi, B.; Mills-Ko, E.; Verstraete, F.J.; Kol, A.; Walker, N.J.; Badgley, M.R.; Fazel, N.; Murphy, W.J.; Vapniarsky, N.; Borjesson, D.L. Therapeutic efficacy of fresh, autologous mesenchymal stem cells for severe refractory gingivostomatitis in cats. Stem Cells Transl. Med. 2016, 5, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arzi, B.; Kol, A.; Murphy, B.; Walker, N.J.; Wood, J.A.; Clark, K.; Verstraete, F.J.; Borjesson, D.L. Feline foamy virus adversely affects feline mesenchymal stem cell culture and expansion: Implications for animal model development. Stem Cells Dev. 2015, 24, 814–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzil, J.E.; Masseau, I.; Webb, T.L.; Chang, C.H.; Dodam, J.R.; Cohn, L.A.; Liu, H.; Quimby, J.M.; Dow, S.W.; Rejnero, C.R. Long-term evaluation of mesenchymal stem cell therapy in a feline model of chronic allergic asthma. Clin. Exp. Allergy 2014, 44, 1546–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trzil, J.E.; Masseau, I.; Webb, T.L.; Chang, C.H.; Dodam, J.R.; Liu, H.; Quimby, J.M.; Dow, S.W.; Rejnero, C.R. Intravenous adipose-derived mesenchymal stem cell therapy for the treatment of feline asthma: A pilot study. J. Feline Med. Surg. 2016, 18, 981–990. [Google Scholar] [CrossRef]
- Webb, T.L.; Webb, C.B. Stem cell therapy in cats with chronic enteropathy: A proof-of-concept study. J. Feline Med. Surg. 2015, 17, 901–908. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Gibbons, D.S.; Dow, S.W. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: A pilot study. J. Feline Med. Surg. 2011, 13, 418–426. [Google Scholar] [CrossRef]
- Quimby, J.M.; Webb, T.L.; Habenicht, L.M.; Dow, S.W. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Res. Ther. 2013, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Vidane, A.S.; Pinheiro, A.O.; Casals, J.B.; Passarelli, D.; Hage, M.; Bueno, R.S.; Martins, D.S.; Ambrosio, C.E. Transplantation of amniotic membranederived multipotent cells ameliorates and delays the progression of chronic kidney disease in cats. Reprod. Domest. Anim. 2017, 52 (Suppl. 2), 316–326. [Google Scholar] [CrossRef] [Green Version]
- Quimby, J.M.; Webb, T.L.; Randall, E.; Marolf, A.; Martinez, A.V.; Dow, S.W. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: A randomized, placebo-controlled clinical trial in eight cats. J. Feline Med. Surg. 2016, 18, 165–171. [Google Scholar] [CrossRef]
- Rosselli, D.D.; Mumaw, J.L.; Dickerson, V.; Brown, C.A.; Brown, S.A.; Schmiedt, C.W. Efficacy of allogeneic mesenchymal stem cell administration in a model of acute ischemic kidney injury in cats. Res. Vet Sci. 2016, 108, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Delorme, B.; Nivet, E.; Gaillard, J.; Häupl, T.; Ringe, J.; Devèze, A.; Magnan, J.; Sohier, J.; Khrestchatisky, M.; Roman, F.S.; et al. The human nose harbors a niche of olfactory ectomesenchymal stem cells displaying neurogenic and osteogenic properties. Stem Cells Dev. 2010, 19, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.D.; Devéze, A.; Nivet, E.; Gepner, B.; Roman, F.S.; Féron, F. Isolating nasal olfactory stem cells from rodents or humans. J. Vis. Exp. 2011, 54, 2762. [Google Scholar] [CrossRef] [PubMed]
- Veron, A.D.; Bienboire-Frosini, C.; Feron, F.; Codecasa, E.; Deveze, A.; Royer, D.; Watelet, P.; Asproni, P.; Sadelli, K.; Chabaud, C.; et al. Isolation and characterization of olfactory ecto-mesenchymal stem cells from eight mammalian genera. BMC Vet. Res. 2018, 14, 17. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Westerberg, B.; Yanai, A.; Evans, K.G. The olfactory mucosa: A potential source of stem cells for hearing regeneration. Regen. Med. 2018, 13, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Veron, A.D.; Bienboire-Frosini, C.; Girard, S.D.; Sadelli, K.; Stamegna, J.C.; Khrestchatisky, M.; Alexis, J.; Pageat, P.; Asproni, P.; Mengoli, M.; et al. Syngeneic Transplantation of Olfactory Ectomesenchymal Stem Cells Restores Learning and Memory Abilities in a Rat Model of Global Cerebral Ischemia. Stem Cells Int. 2018, 2018, 2683969. [Google Scholar] [CrossRef] [Green Version]
- Murrell, W.; Wetzig, A.; Donnellan, M.; Féron, F.; Burne, T.; Meedeniya, A.; Kesby, J.; Bianco, J.; Perry, C.; Silburn, P.; et al. Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson’s disease. Stem Cells 2008, 26, 2183–2192. [Google Scholar] [CrossRef]
- Ohnishi, Y.I.; Iwatsuki, K.; Ishihara, M.; Shikina, T.; Shinzawa, K.; Moriwaki, T.; Ninomiya, K.; Ohkawa, T.; Umegaki, M.; Kishima, H.; et al. Adult olfactory sphere cells are a source of oligodendrocyte and Schwann cell progenitors. Stem Cell Res. 2013, 11, 1178–1190. [Google Scholar] [CrossRef]
- Chu, T.; Zhou, H.; Wang, T.; Lu, L.; Li, F.; Liu, B.; Kong, X.; Feng, S. In Vitro characteristics of valproic acid and all-trans-retinoic acid and their combined use in promoting neuronal differentiation while suppressing astrocytic differentiation in neural stem cells. Brain Res. 2015, 1596, 31–47. [Google Scholar] [CrossRef]
- Maciel, B.B.; Rebelatto, C.L.K.; Brofman, P.R.S.; Brito, H.F.V.; Patricio, L.F.L.; Cruz, M.A.; Locatelli-Dittrich, R. Morphology and morphometry of feline bone marrow-derived mesenchymal stem cells in culture. Pesq. Vet. Bras. 2014, 34, 1127–1134. [Google Scholar] [CrossRef] [Green Version]
- Panasophonkul, S.; Samart, P.; Kongon, K.; Sathanawongs, A. Phenotypic characteristics of feline adipose-derived stem cells affected by cell passage number. Pol. J. Vet. Sci. 2017, 20, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Lee, J.; Byeon, J.S.; Gu, N.Y.; Lee, J.; Cho, I.S.; Cha, S.H. Extensive characterization of feline intra-abdominal adipose-derived mesenchymal stem cells. J. Vet. Sci. 2017, 18, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Li, Q.; Song, W.J.; Chae, H.K.; Kweon, K.; Ahn, J.O.; Youn, H.Y. Altered properties of feline adipose-derived mesenchymal stem cells during continuous in vitro cultivation. J. Vet. Med. Sci. 2018, 80, 930–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Yamawaki-Ogata, A.; Kanemoto, I.; Usui, A.; Narita, Y. Isolation and characterisation of peripheral blood-derived feline mesenchymal stem cells. Vet. J. 2016, 216, 183–188. [Google Scholar] [CrossRef]
- Veron, A.D.; Mengoli, M. Olfactory Stem Cell Therapy in Canine Age-related Disorders Treatment: A Controlled Study. In Proceedings of the 11th International Veterinary Behaviour Meeting, Samorin, Slovakia, 14–16 September 2017; Denenberg, S., Ed.; Langford Vets: Bristol, UK Chapter 43. ; p. 118. [Google Scholar]
- Smith, R.K.W.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS ONE 2013, 8, e75697. [Google Scholar] [CrossRef]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K.W. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef]
- Perez, J.R.; Kouroupis, D.; Li, D.J.; Best, T.M.; Kaplan, L.; Correa, D. Tissue Engineering and Cell-Based Therapies for Fractures and Bone Defects. Front. Bioeng. Biotechnol. 2018, 6, 105. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, A.; Mizuno, M.; Mochizuki, M.; Sekiya, I. Mesenchymal stem cells for cartilage regeneration in dogs. World J. Stem Cells 2019, 11, 254–269. [Google Scholar] [CrossRef]
| Antibody | Target | Host | Supplier | Reference | Dilution | Secondary Antibody |
|---|---|---|---|---|---|---|
| Anti-nestin | Stemness marker | Rabbit | Abcam | ab7659 | 1/500 | Alexa Fluor 488 |
| Anti-GFAP | Neural marker | Chicken | Abcam | ab4674 | 1/500 | Alexa Fluor 488 |
| Anti-MAP2 | Neural marker | Chicken | Abcam | ab5392 | 1/500 | Alexa Fluor 488 |
| Anti-tenomodulin | Tenoblast marker | Rabbit | Abcam | ab81328 | 1/250 | Alexa Fluor 488 |
| Anti-scleraxis | Tenoblast marker | Rabbit | Abcam | ab58655 | 1/250 | Alexa Fluor 488 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mollichella, M.-L.; Mechin, V.; Royer, D.; Pageat, P.; Asproni, P. Isolation and Characterization of Cat Olfactory Ecto-Mesenchymal Stem Cells. Animals 2022, 12, 1284. https://doi.org/10.3390/ani12101284
Mollichella M-L, Mechin V, Royer D, Pageat P, Asproni P. Isolation and Characterization of Cat Olfactory Ecto-Mesenchymal Stem Cells. Animals. 2022; 12(10):1284. https://doi.org/10.3390/ani12101284
Chicago/Turabian StyleMollichella, Marie-Laure, Violaine Mechin, Dany Royer, Patrick Pageat, and Pietro Asproni. 2022. "Isolation and Characterization of Cat Olfactory Ecto-Mesenchymal Stem Cells" Animals 12, no. 10: 1284. https://doi.org/10.3390/ani12101284
APA StyleMollichella, M.-L., Mechin, V., Royer, D., Pageat, P., & Asproni, P. (2022). Isolation and Characterization of Cat Olfactory Ecto-Mesenchymal Stem Cells. Animals, 12(10), 1284. https://doi.org/10.3390/ani12101284







