A Comparative Study of Canine Mesenchymal Stem Cells Isolated from Different Sources
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of MSC from Bone Marrow
2.2. Isolation of MSCs from Adipose Tissue
2.3. Isolation of MSCs from Amniotic Tissue
2.4. Passaging Cells
2.5. Expression of Surface Markers
2.6. Multilineage Potential
2.7. Proliferation Activity of MSC
2.8. Freezing Protocol
3. Results
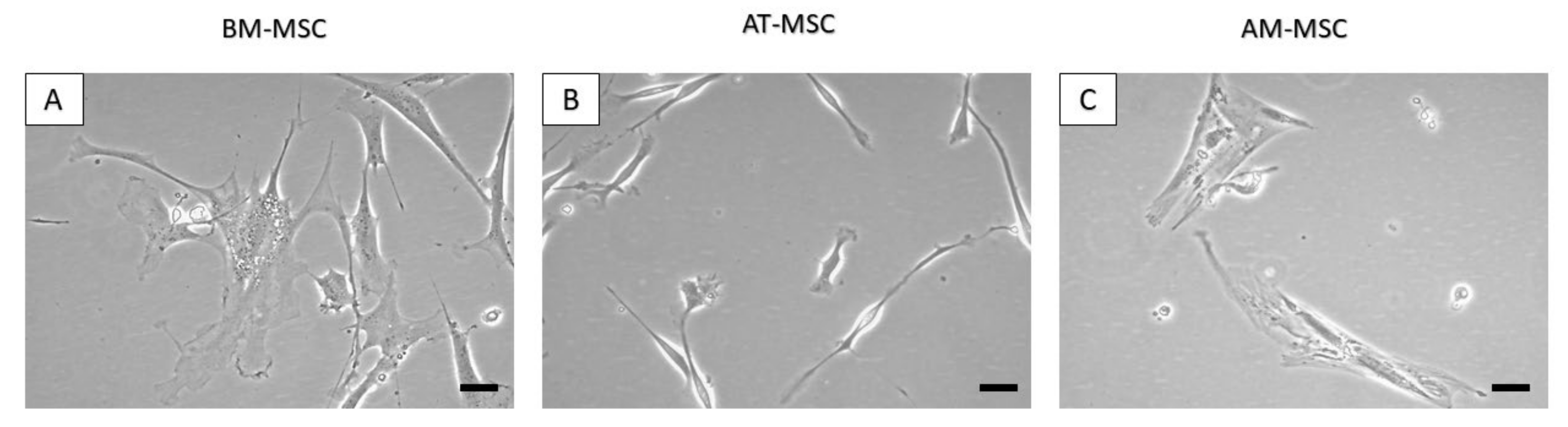
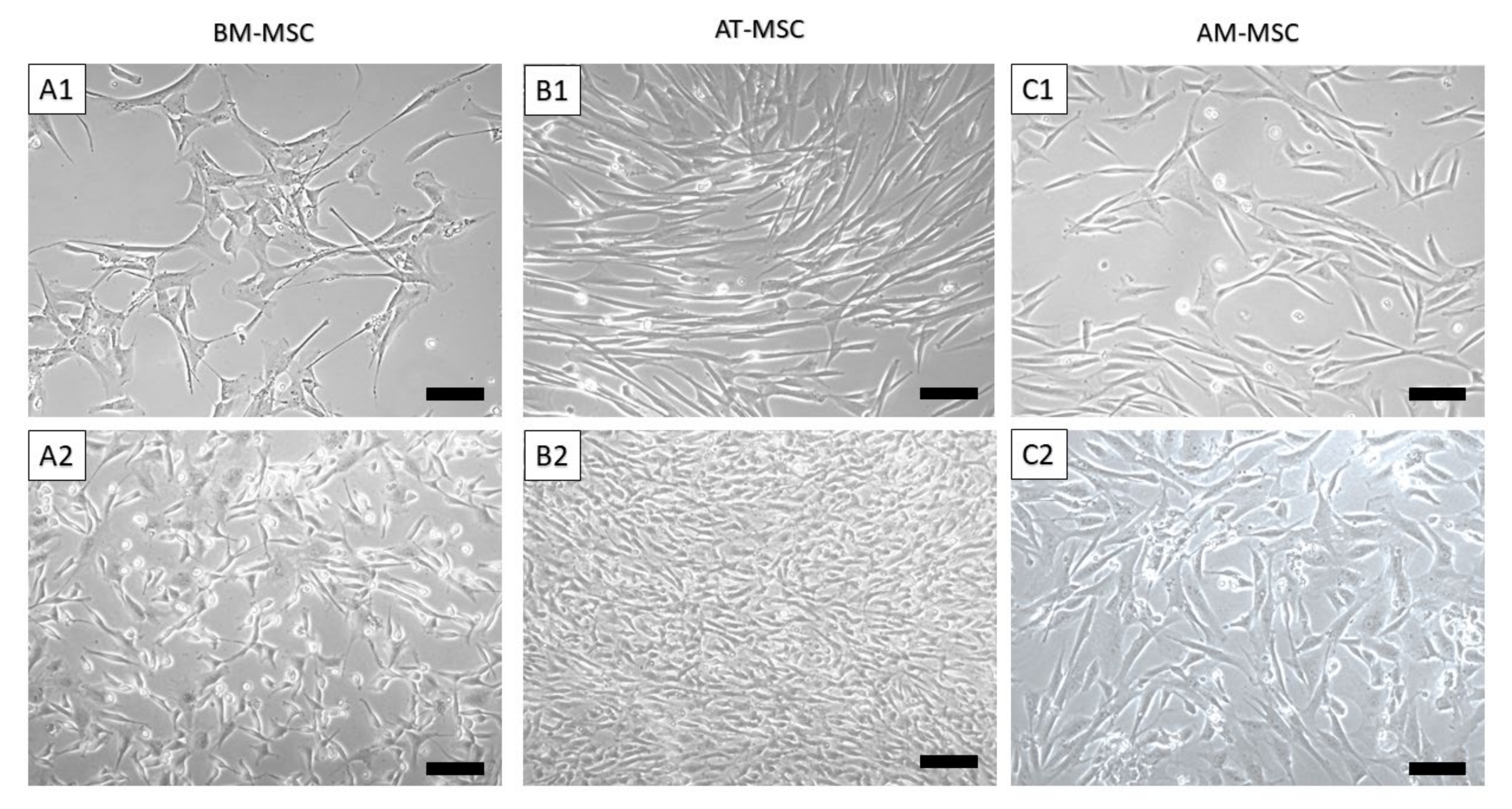
3.1. Isolation of Canine MSCs from Bone Marrow, Adipose Tissue and Amnion
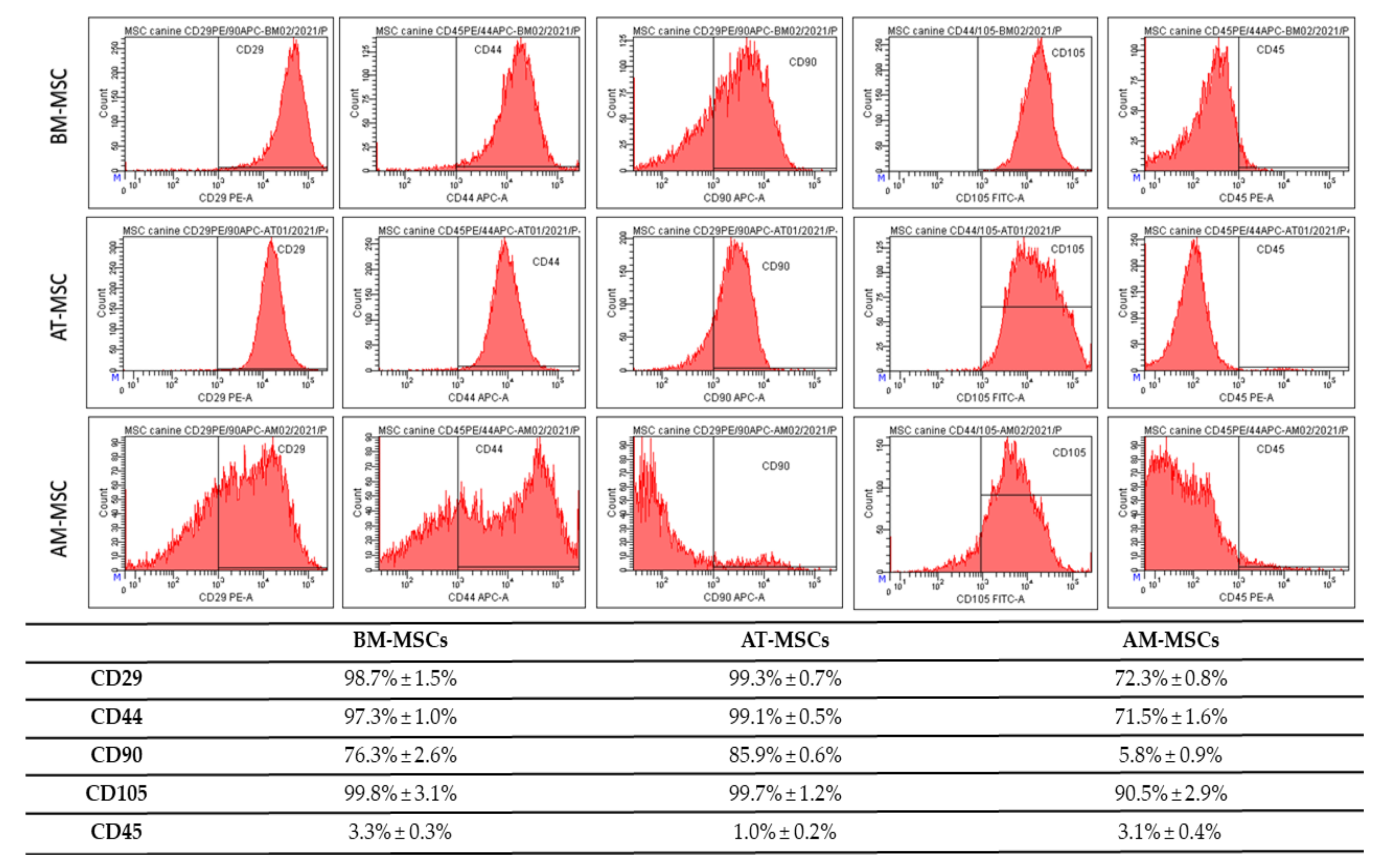
3.2. CD Characterization of Canine MSC
3.3. Multilineage Potential
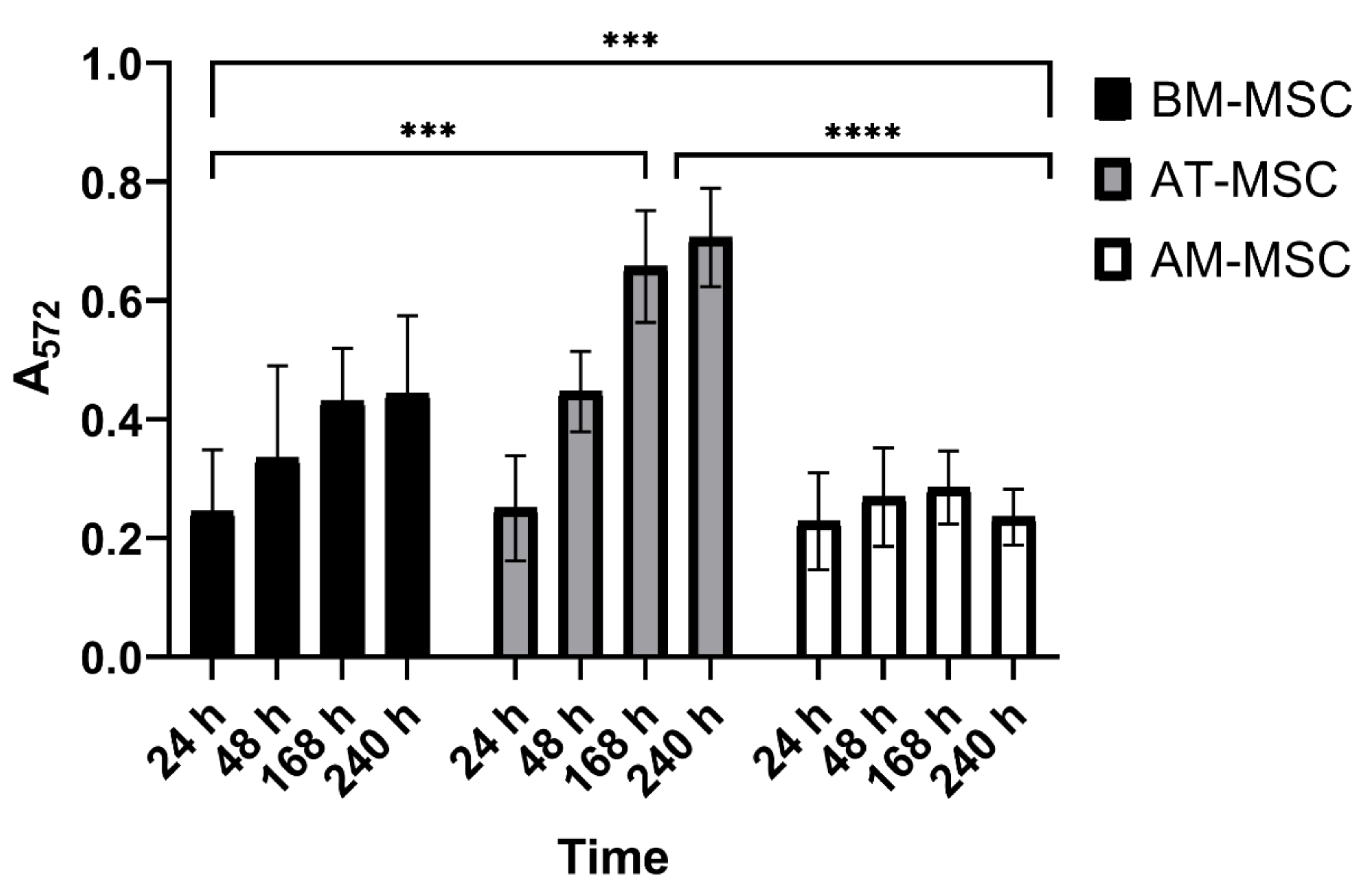
3.4. MTT Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human Mesenchymal Stem Cells—Current Trends and Future Prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Stzepourginski, I.; Nigro, G.; Jacob, J.-M.; Dulauroy, S.; Sansonetti, P.J.; Eberl, G.; Peduto, L. CD34+ Mesenchymal Cells Are a Major Component of the Intestinal Stem Cells Niche at Homeostasis and after Injury. Proc. Natl. Acad. Sci. USA 2017, 114, E506–E513. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and Haematopoietic Stem Cells Form a Unique Bone Marrow Niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, Y.; Bruns, I.; Scheiermann, C.; Ahmed, J.; Pinho, S.; Zhang, D.; Mizoguchi, T.; Wei, Q.; Lucas, D.; Ito, K.; et al. Arteriolar Niches Maintain Haematopoietic Stem Cell Quiescence. Nature 2013, 502, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Neupane, M.; Chang, C.-C.; Kiupel, M.; Yuzbasiyan-Gurkan, V. Isolation and Characterization of Canine Adipose-Derived Mesenchymal Stem Cells. Tissue Eng. Part A 2008, 14, 1007–1015. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.-W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A Perivascular Origin for Mesenchymal Stem Cells in Multiple Human Organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef]
- Kerkis, I.; Kerkis, A.; Dozortsev, D.; Stukart-Parsons, G.C.; Gomes Massironi, S.M.; Pereira, L.V.; Caplan, A.I.; Cerruti, H.F. Isolation and Characterization of a Population of Immature Dental Pulp Stem Cells Expressing OCT-4 and Other Embryonic Stem Cell Markers. Cells Tissues Organs 2006, 184, 105–116. [Google Scholar] [CrossRef]
- Song, L.; Tuan, R.S. Transdifferentiation Potential of Human Mesenchymal Stem Cells Derived from Bone Marrow. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2004, 18, 980–982. [Google Scholar] [CrossRef]
- Pezzanite, L.M.; Fortier, L.A.; Antczak, D.F.; Cassano, J.M.; Brosnahan, M.M.; Miller, D.; Schnabel, L.V. Equine Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Elicit Antibody Responses in Vivo. Stem Cell Res. Ther. 2015, 6, 54. [Google Scholar] [CrossRef]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal Stem Cells: Amazing Remedies for Bone and Cartilage Defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef]
- Miao, C.; Lei, M.; Hu, W.; Han, S.; Wang, Q. A Brief Review: The Therapeutic Potential of Bone Marrow Mesenchymal Stem Cells in Myocardial Infarction. Stem Cell Res. Ther. 2017, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Cubinkova, V.; Smolek, T.; Murgoci, A.-N.; Danko, J.; Vdoviakova, K.; Humenik, F.; Cizek, M.; Quanico, J.; Fournier, I.; et al. Localized Intrathecal Delivery of Mesenchymal Stromal Cells Conditioned Medium Improves Functional Recovery in a Rat Model of Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 870. [Google Scholar] [CrossRef]
- The Secretion Profile of Mesenchymal Stem Cells and Potential Applications in Treating Human Diseases|Signal Transduction and Targeted Therapy. Available online: https://www.nature.com/articles/s41392-022-00932-0 (accessed on 2 May 2022).
- Wright, A.; Arthaud-Day, M.L.; Weiss, M.L. Therapeutic Use of Mesenchymal Stromal Cells: The Need for Inclusive Characterization Guidelines to Accommodate All Tissue Sources and Species. Front. Cell Dev. Biol. 2021, 9, 632717. [Google Scholar] [CrossRef]
- Frontiers|A Set of Grand Challenges for Veterinary Regenerative Medicine|Veterinary Science. Available online: https://www.frontiersin.org/articles/10.3389/fvets.2016.00020/full (accessed on 2 May 2022).
- Rizk, M.; Monaghan, M.; Shorr, R.; Kekre, N.; Bredeson, C.N.; Allan, D.S. Heterogeneity in Studies of Mesenchymal Stromal Cells to Treat or Prevent Graft-versus-Host Disease: A Scoping Review of the Evidence. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 22, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, E1852. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Munoz-Perez, E.; Gonzalez-Pujana, A.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Mesenchymal Stromal Cell Secretome for the Treatment of Immune-Mediated Inflammatory Diseases: Latest Trends in Isolation, Content Optimization and Delivery Avenues. Pharmaceutics 2021, 13, 1802. [Google Scholar] [CrossRef]
- Sasaki, A.; Mizuno, M.; Ozeki, N.; Katano, H.; Otabe, K.; Tsuji, K.; Koga, H.; Mochizuki, M.; Sekiya, I. Canine Mesenchymal Stem Cells from Synovium Have a Higher Chondrogenic Potential than Those from Infrapatellar Fat Pad, Adipose Tissue, and Bone Marrow. PLoS ONE 2018, 13, e0202922. [Google Scholar] [CrossRef]
- Rashid, U.; Yousaf, A.; Yaqoob, M.; Saba, E.; Moaeen-ud-Din, M.; Waseem, S.; Becker, S.K.; Sponder, G.; Aschenbach, J.R.; Sandhu, M.A. Characterization and Differentiation Potential of Mesenchymal Stem Cells Isolated from Multiple Canine Adipose Tissue Sources. BMC Vet. Res. 2021, 17, 388. [Google Scholar] [CrossRef]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-Vitro Characterization of Canine Multipotent Stromal Cells Isolated from Synovium, Bone Marrow, and Adipose Tissue: A Donor-Matched Comparative Study. Stem Cell Res. Ther. 2017, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Kisiel, A.H.; McDuffee, L.A.; Masaoud, E.; Bailey, T.R.; Esparza Gonzalez, B.P.; Nino-Fong, R. Isolation, Characterization, and in Vitro Proliferation of Canine Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, Muscle, and Periosteum. Am. J. Vet. Res. 2012, 73, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, Cultivation, and Characterization of Human Mesenchymal Stem Cells. Cytometry A 2018, 93, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Böhm, S.; Jacobs, R. Different Populations and Sources of Human Mesenchymal Stem Cells (MSC): A Comparison of Adult and Neonatal Tissue-Derived MSC. Cell Commun. Signal. CCS 2011, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, Y.; Vackova, I.; Kekulova, K.; Chudickova, M.; Koci, Z.; Turnovcova, K.; Kupcova Skalnikova, H.; Vodicka, P.; Kubinova, S. A Comparative Analysis of Multipotent Mesenchymal Stromal Cells Derived from Different Sources, with a Focus on Neuroregenerative Potential. Sci. Rep. 2020, 10, 4290. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, L.; Huang, Y.; Huang, Y.; Parolini, O.; Zhong, Q.; Tian, X.; Deng, L. Comparison of the Proliferation and Differentiation Potential of Human Urine-, Placenta Decidua Basalis-, and Bone Marrow-Derived Stem Cells. Stem Cells Int. 2018, 2018, e7131532. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, W.; Rubin, J.P.; Marra, K.G. Adipose-Derived Stem Cells: Implications in Tissue Regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef]
- Brozovich, A.; Sinicrope, B.J.; Bauza, G.; Niclot, F.B.; Lintner, D.; Taraballi, F.; McCulloch, P.C. High Variability of Mesenchymal Stem Cells Obtained via Bone Marrow Aspirate Concentrate Compared with Traditional Bone Marrow Aspiration Technique. Orthop. J. Sports Med. 2021, 9, 23259671211058460. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative Analysis of Mesenchymal Stem Cells from Bone Marrow, Umbilical Cord Blood, or Adipose Tissue. Stem Cells Dayt. Ohio 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and Differences between Mesenchymal Stem/Progenitor Cells Derived from Various Human Tissues. World J. Stem Cells 2019, 11, 347–374. [Google Scholar] [CrossRef]
- Zhang, J.; Nuebel, E.; Daley, G.Q.; Koehler, C.M.; Teitell, M.A. Metabolic Regulation in Pluripotent Stem Cells during Reprogramming and Self-Renewal. Cell Stem Cell 2012, 11, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-Z.; Lee, J.H. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin. Orthop. Surg. 2018, 10, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Ojima, M.; Otabe, K.; Horie, M.; Koga, H.; Sekiya, I.; Muneta, T. Effects of Different Cell-Detaching Methods on the Viability and Cell Surface Antigen Expression of Synovial Mesenchymal Stem Cells. Cell Transplant. 2017, 26, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Krešić, N.; Prišlin, M.; Vlahović, D.; Kostešić, P.; Ljolje, I.; Brnić, D.; Turk, N.; Musulin, A.; Habrun, B. The Expression Pattern of Surface Markers in Canine Adipose-Derived Mesenchymal Stem Cells. Int. J. Mol. Sci. 2021, 22, 7476. [Google Scholar] [CrossRef]
- Fan, W.; Li, J.; Wang, Y.; Pan, J.; Li, S.; Zhu, L.; Guo, C.; Yan, Z. CD105 Promotes Chondrogenesis of Synovium-Derived Mesenchymal Stem Cells through Smad2 Signaling. Biochem. Biophys. Res. Commun. 2016, 474, 338–344. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.-Q.; Wang, A.-T.; Yu, C.-Y.; Luo, Y.; Liu, R.-M.; Zhao, Y.-J.; Xiao, J.-H. Effect of CD44 on Differentiation of Human Amniotic Mesenchymal Stem Cells into Chondrocytes via Smad and ERK Signaling Pathways. Mol. Med. Rep. 2020, 21, 2357–2366. [Google Scholar] [CrossRef]
- Davies, O.G.; Cooper, P.R.; Shelton, R.M.; Smith, A.J.; Scheven, B.A. Isolation of Adipose and Bone Marrow Mesenchymal Stem Cells Using CD29 and CD90 Modifies Their Capacity for Osteogenic and Adipogenic Differentiation. J. Tissue Eng. 2015, 6, 2041731415592356. [Google Scholar] [CrossRef]
- Campioni, D.; Rizzo, R.; Stignani, M.; Melchiorri, L.; Ferrari, L.; Moretti, S.; Russo, A.; Bagnara, G.P.; Bonsi, L.; Alviano, F.; et al. A Decreased Positivity for CD90 on Human Mesenchymal Stromal Cells (MSCs) Is Associated with a Loss of Immunosuppressive Activity by MSCs. Cytometry B Clin. Cytom. 2009, 76B, 225–230. [Google Scholar] [CrossRef]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A Reduction in CD90 (THY-1) Expression Results in Increased Differentiation of Mesenchymal Stromal Cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef]
- Maleki, M.; Ghanbarvand, F.; Reza Behvarz, M.; Ejtemaei, M.; Ghadirkhomi, E. Comparison of Mesenchymal Stem Cell Markers in Multiple Human Adult Stem Cells. Int. J. Stem Cells 2014, 7, 118–126. [Google Scholar] [CrossRef]
- Schachtele, S. Markers and Methods to Verify Mesenchymal Stem Cell Identity, Potency, and Quality. 13. Available online: https://resources.rndsystems.com/images/site/wp-msc-13763.pdf?_ga=2.169946848.1123720049.1654517715-902278695.1654517715 (accessed on 4 June 2022).
- Ahmed, N.; Vogel, B.; Rohde, E.; Strunk, D.; Grifka, J.; Schulz, M.B.; Grässel, S. CD45-Positive Cells of Haematopoietic Origin Enhance Chondrogenic Marker Gene Expression in Rat Marrow Stromal Cells. Int. J. Mol. Med. 2006, 18, 233–240. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bertolo, A.; Baur, M.; Guerrero, J.; Pötzel, T.; Stoyanov, J. Autofluorescence Is a Reliable in Vitro Marker of Cellular Senescence in Human Mesenchymal Stromal Cells. Sci. Rep. 2019, 9, 2074. [Google Scholar] [CrossRef] [PubMed]


| Results of the Comparative Study of Canine MSCs | |||
|---|---|---|---|
| BM-MSCs | AT-MSCs | AM-MSCs | |
| Invasiveness of tissue collection | +++ | +++ | – |
| Yield | + | ++ | +++ |
| Homogeneity | + | +++ | + |
| Osteogenic potential | +++ | +++ | +++ |
| Chondrogenic potential | +++ | +++ | +++ |
| Adipogenic potential | – | + | – |
| Proliferation capacity | ++ | +++ | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humenik, F.; Maloveska, M.; Hudakova, N.; Petrouskova, P.; Hornakova, L.; Domaniza, M.; Mudronova, D.; Bodnarova, S.; Cizkova, D. A Comparative Study of Canine Mesenchymal Stem Cells Isolated from Different Sources. Animals 2022, 12, 1502. https://doi.org/10.3390/ani12121502
Humenik F, Maloveska M, Hudakova N, Petrouskova P, Hornakova L, Domaniza M, Mudronova D, Bodnarova S, Cizkova D. A Comparative Study of Canine Mesenchymal Stem Cells Isolated from Different Sources. Animals. 2022; 12(12):1502. https://doi.org/10.3390/ani12121502
Chicago/Turabian StyleHumenik, Filip, Marcela Maloveska, Nikola Hudakova, Patricia Petrouskova, Lubica Hornakova, Michal Domaniza, Dagmar Mudronova, Simona Bodnarova, and Dasa Cizkova. 2022. "A Comparative Study of Canine Mesenchymal Stem Cells Isolated from Different Sources" Animals 12, no. 12: 1502. https://doi.org/10.3390/ani12121502
APA StyleHumenik, F., Maloveska, M., Hudakova, N., Petrouskova, P., Hornakova, L., Domaniza, M., Mudronova, D., Bodnarova, S., & Cizkova, D. (2022). A Comparative Study of Canine Mesenchymal Stem Cells Isolated from Different Sources. Animals, 12(12), 1502. https://doi.org/10.3390/ani12121502







