Platelet Lysate for Mesenchymal Stromal Cell Culture in the Canine and Equine Species: Analogous but Not the Same
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood Collection
2.2. Platelet Concentrate and Lysate Preparation
2.3. Microbiological Assessment
2.4. Platelet and Leukocyte Counts
2.5. Growth Factor Quantification and Chemical Analyses
2.6. MSC Culture with FBS and PL Media Supplements
2.7. Cell Proliferation and Metabolic Activity
2.8. Trilineage Differentiation
2.9. Apoptosis, Necrosis, and Senescence Assays
2.10. Fluorescence In Situ Hybridisation (FISH) Analyses
2.11. Statistical Analyses
3. Results
3.1. Canine Platelet Lysate Production
3.1.1. Absence of Pathogen Contamination
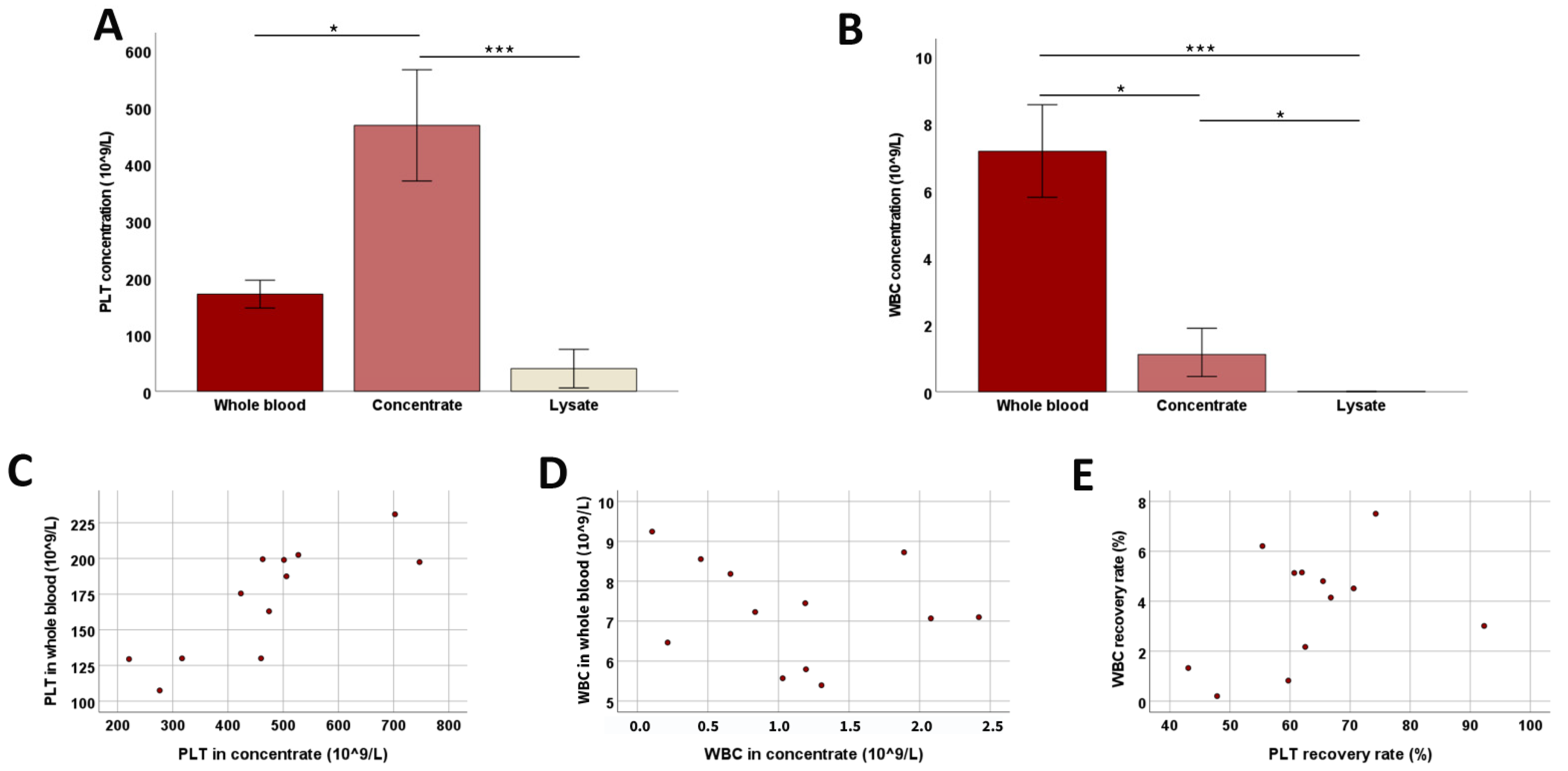
3.1.2. Platelet Concentration and WBC Removal
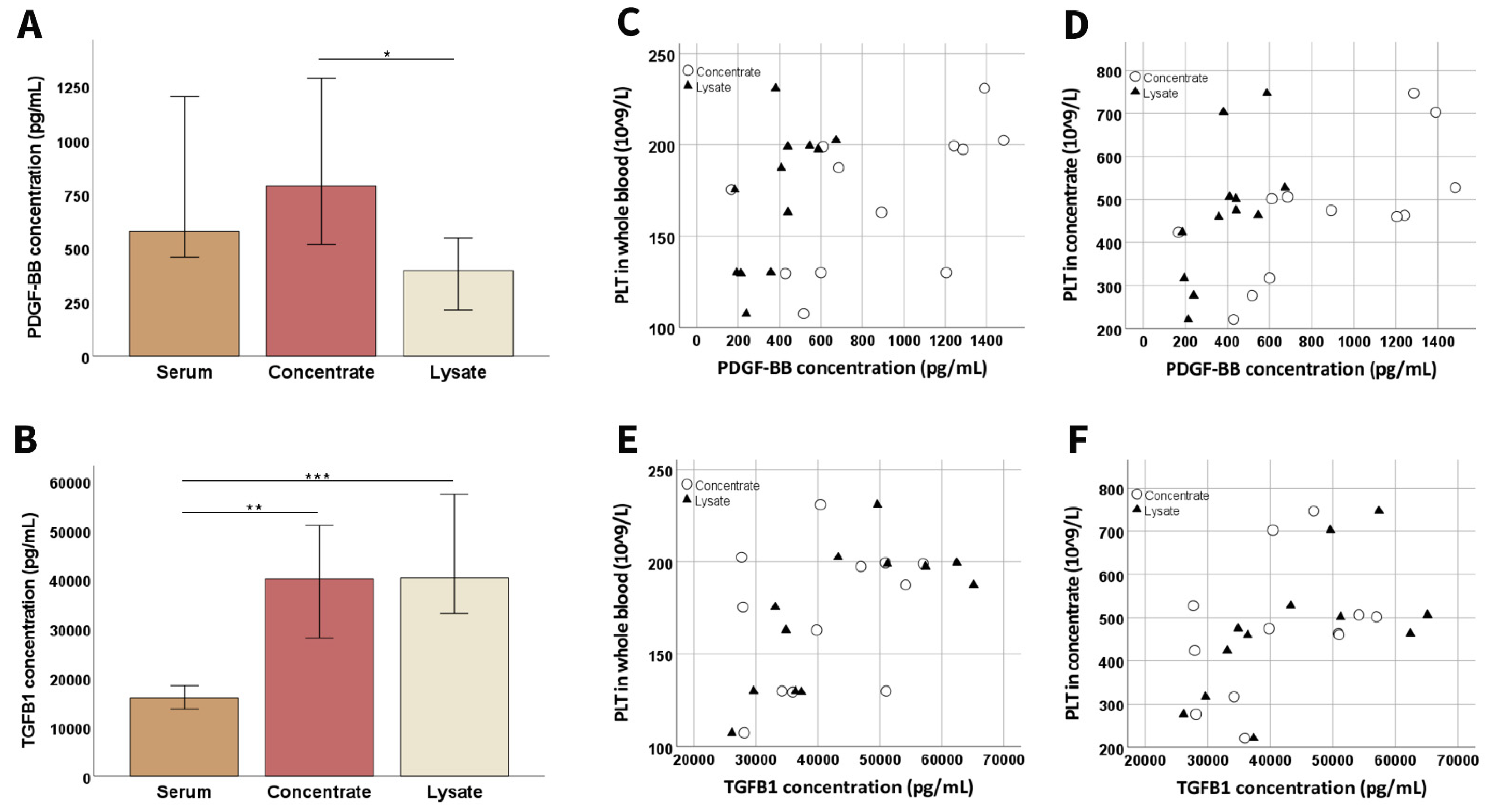
3.1.3. Growth Factor Concentrations and Chemical Analyses
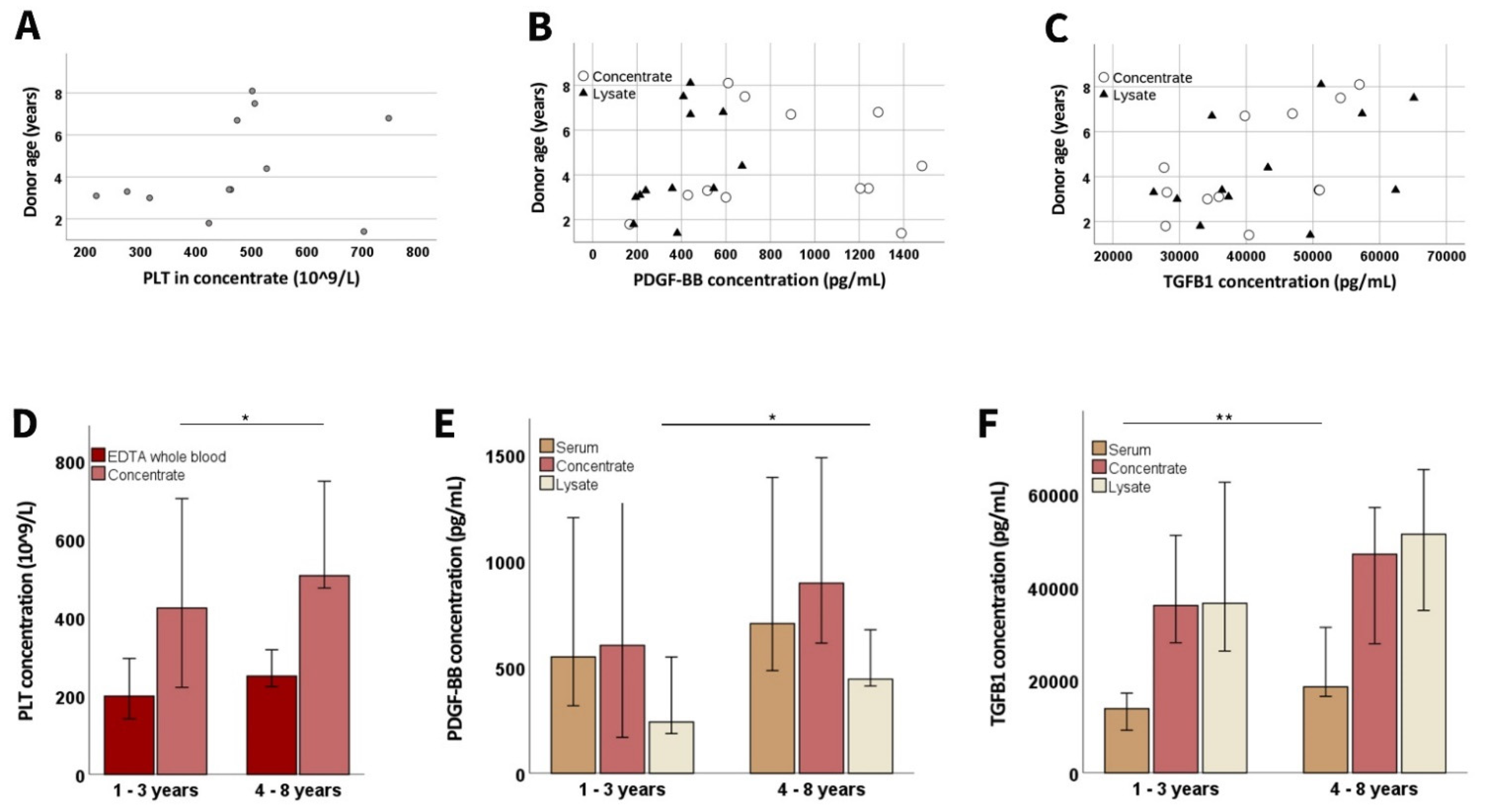
3.1.4. Donor-Related Parameters
3.2. Growth Factor Concentrations and Chemical Compositions in the Cell Culture Supplements
3.3. Platelet Lysate in MSC Culture
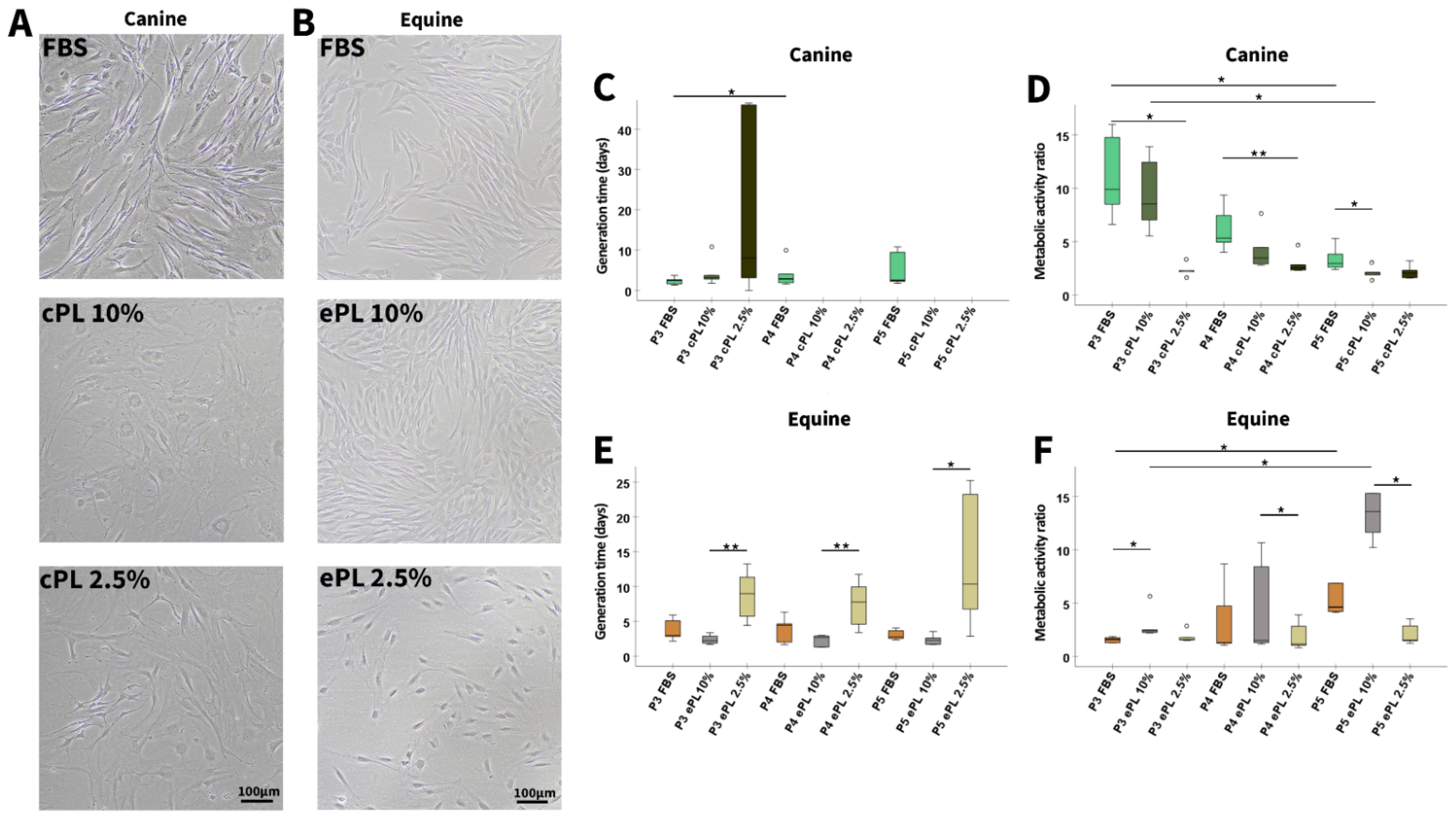
3.3.1. Canine and Equine MSC Morphology, Proliferation, and Metabolic Activity
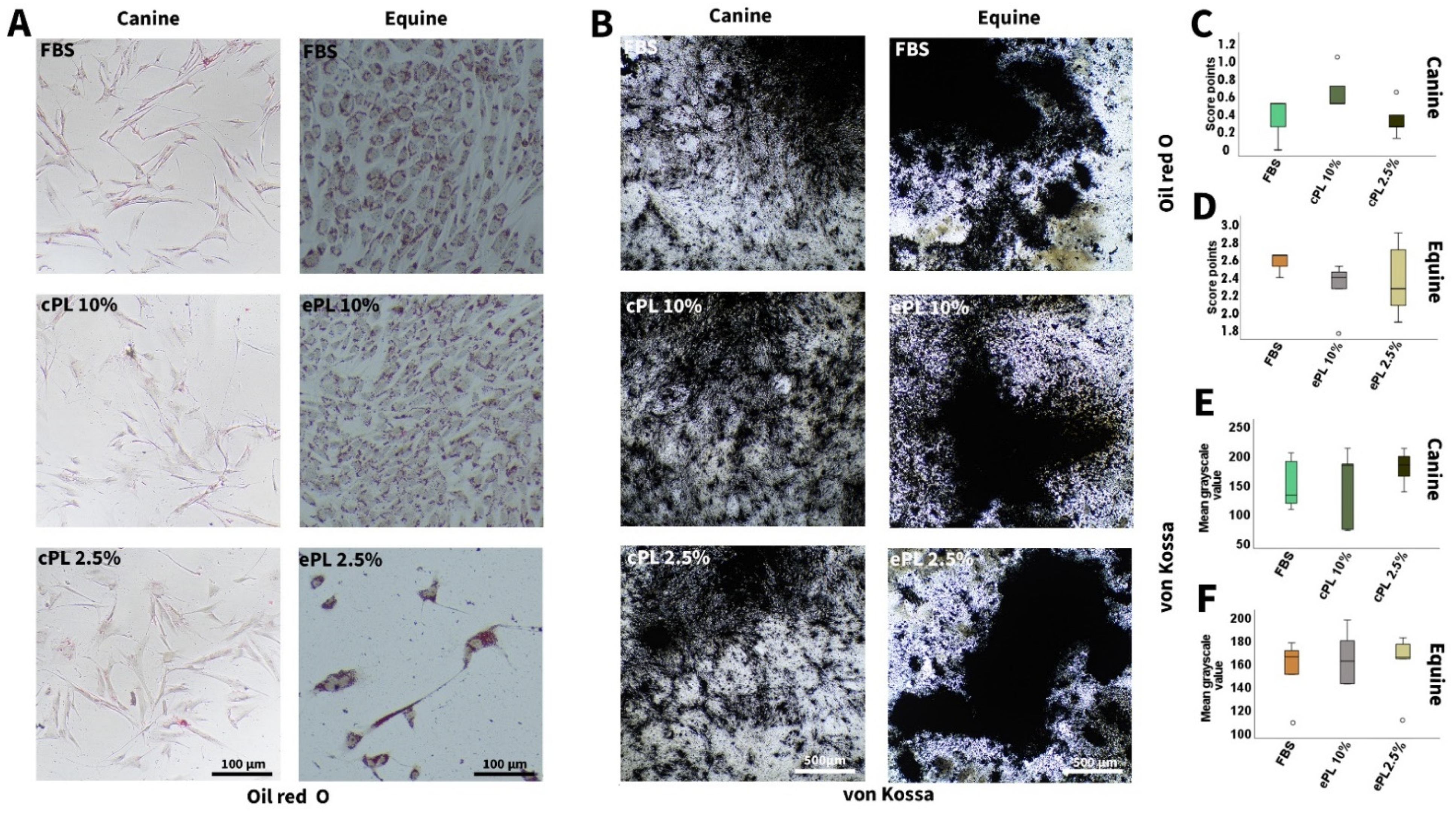
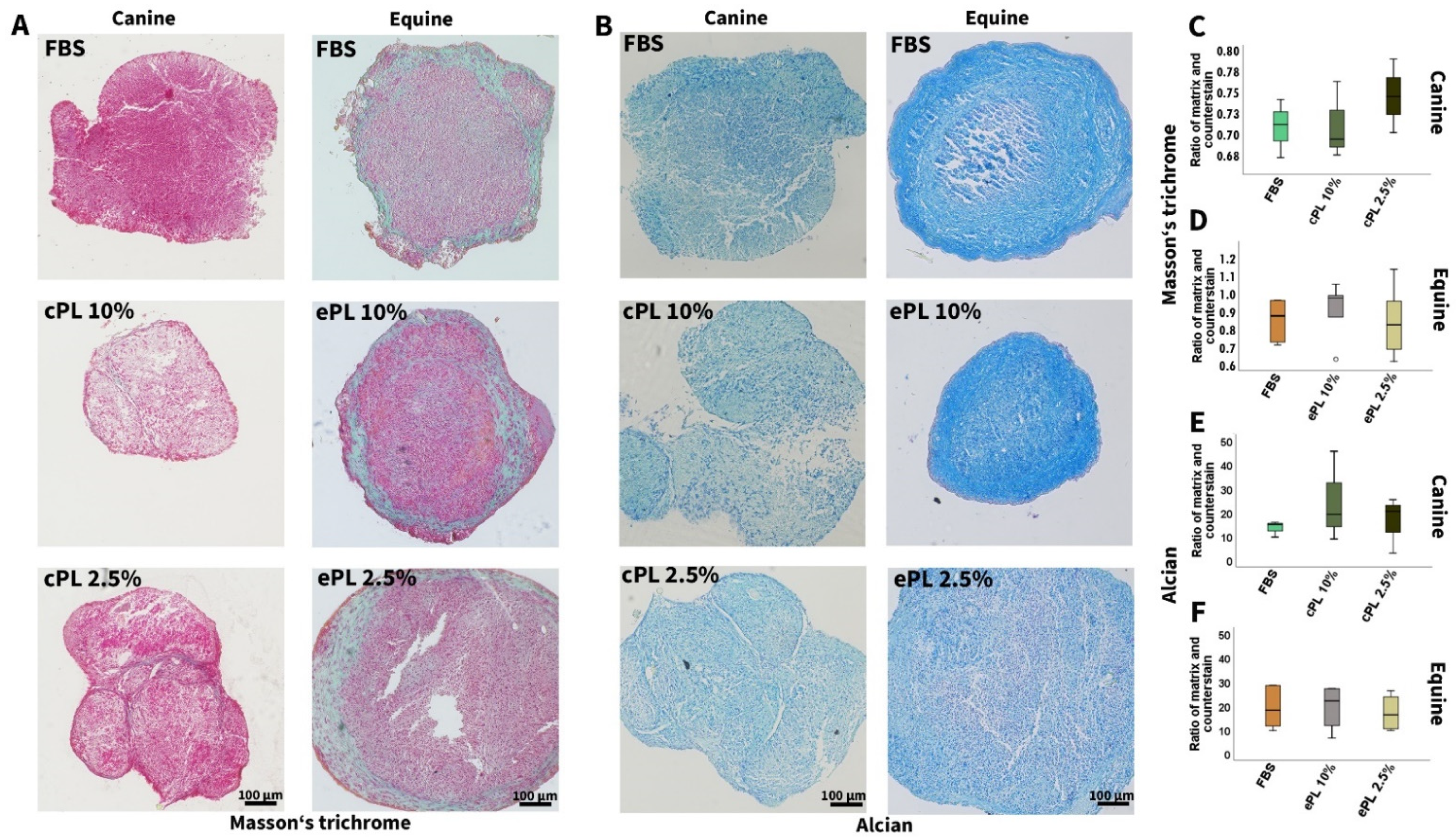
3.3.2. Canine and Equine MSC Trilineage Differentiation
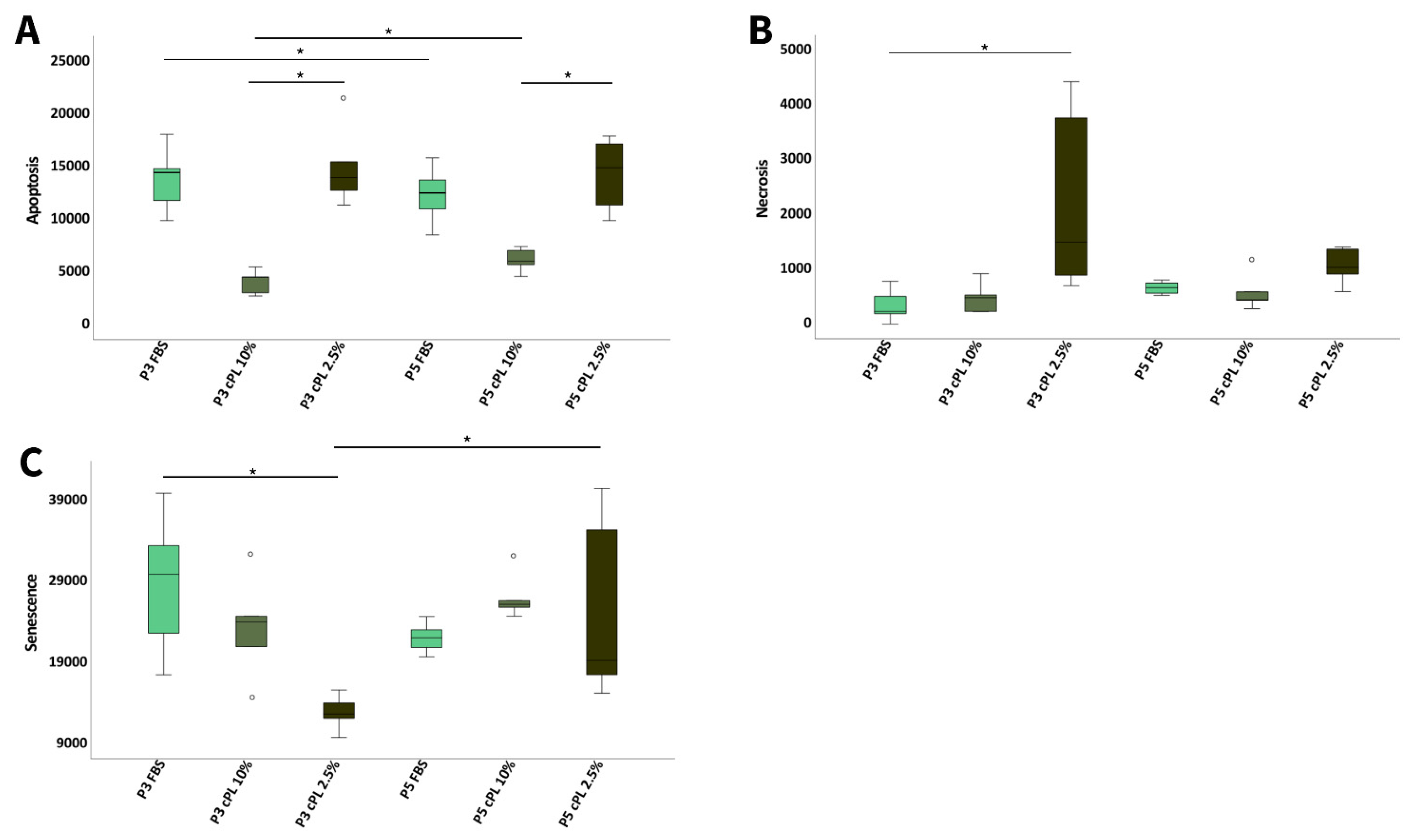
3.3.3. Canine MSC Apoptosis, Necrosis, and Senescence
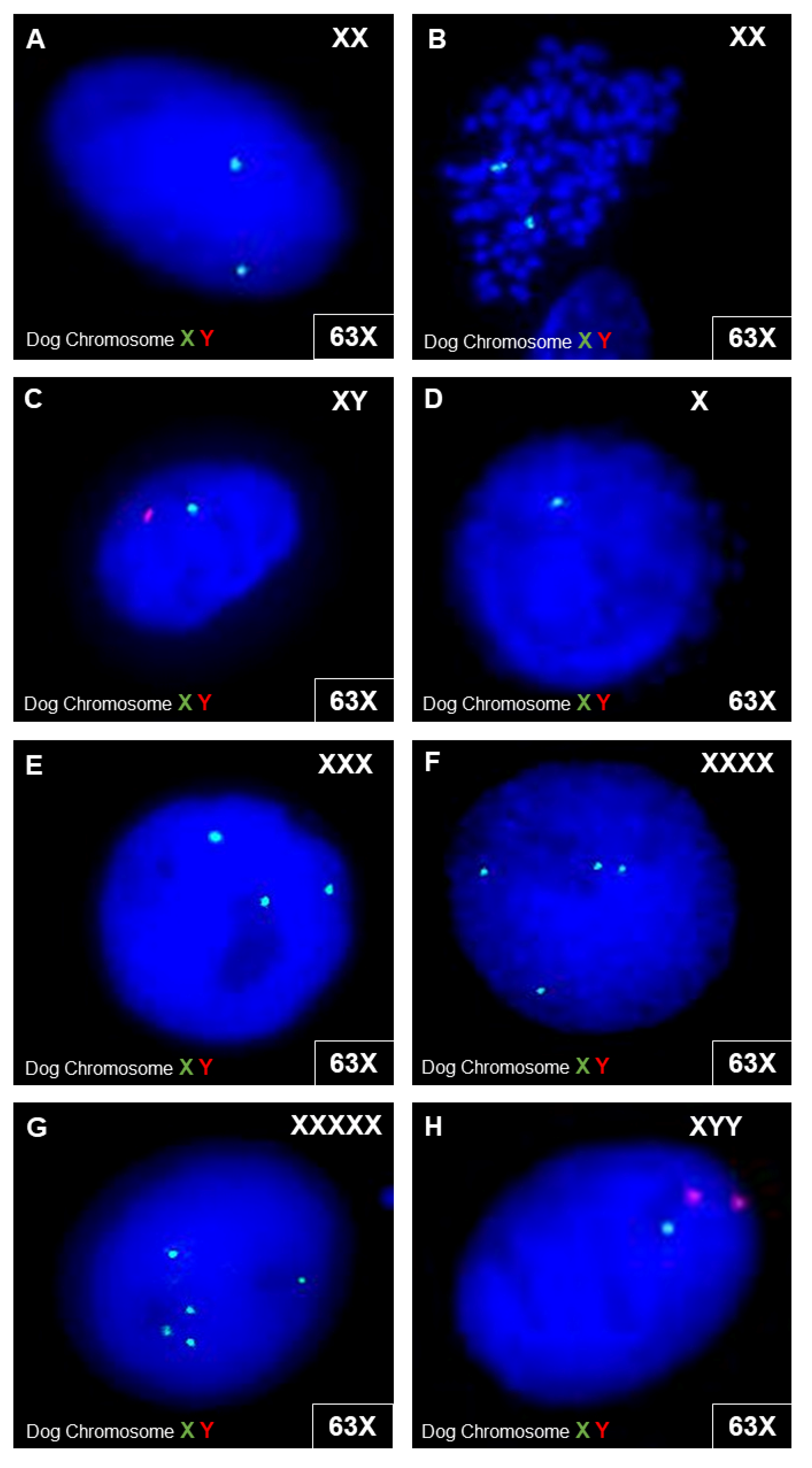
3.3.4. Canine MSC Genetic Stability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pacini, S.; Spinabella, S.; Trombi, L.; Fazzi, R.; Galimberti, S.; Dini, F.; Carlucci, F.; Petrini, M. Suspension of bone marrow-derived undifferentiated mesenchymal stromal cells for repair of superficial digital flexor tendon in race horses. Tissue Eng. 2007, 13, 2949–2955. [Google Scholar] [CrossRef]
- Godwin, E.E.; Young, N.J.; Dudhia, J.; Beamish, I.C.; Smith, R.K.W. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet. J. 2012, 44, 25–32. [Google Scholar] [CrossRef]
- Renzi, S.; Riccò, S.; Dotti, S.; Sesso, L.; Grolli, S.; Cornali, M.; Carlin, S.; Patruno, M.; Cinotti, S.; Ferrari, M. Autologous bone marrow mesenchymal stromal cells for regeneration of injured equine ligaments and tendons: A clinical report. Res. Vet. Sci. 2013, 95, 272–277. [Google Scholar] [CrossRef]
- Smith, R.K.W.; Werling, N.J.; Dakin, S.G.; Alam, R.; Goodship, A.E.; Dudhia, J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS ONE 2013, 8, e75697. [Google Scholar] [CrossRef]
- Broeckx, S.; Zimmerman, M.; Crocetti, S.; Suls, M.; Mariën, T.; Ferguson, S.J.; Chiers, K.; Duchateau, L.; Franco-Obregón, A.; Wuertz, K.; et al. Regenerative therapies for equine degenerative joint disease: A preliminary study. PLoS ONE 2014, 9, e85917. [Google Scholar] [CrossRef]
- Ferris, D.J.; Frisbie, D.D.; Kisiday, J.D.; Mcllwraith, C.W.; Hague, B.A.; Major, M.D.; Schneider, R.K.; Zubrod, C.J.; Kawcak, C.E.; Goodrich, L.R. Clinical outcome after intra-articular administration of bone marrow derived mesenchymal stem cells in 33 horses with stifle injury. Vet. Surg. 2014, 43, 255–265. [Google Scholar] [CrossRef]
- Broeckx, S.Y.; Seys, B.; Suls, M.; Vandenberghe, A.; Mariën, T.; Adriaensen, E.; Declercq, J.; Van Hecke, L.; Braun, G.; Hellmann, K.; et al. Equine Allogeneic Chondrogenic Induced Mesenchymal Stem Cells Are an Effective Treatment for Degenerative Joint Disease in Horses. Stem Cells Dev. 2019, 28, 410–422. [Google Scholar] [CrossRef]
- Shah, K.; Drury, T.; Roic, I.; Hansen, P.; Malin, M.; Boyd, R.; Sumer, H.; Ferguson, R. Outcome of Allogeneic Adult Stem Cell Therapy in Dogs Suffering from Osteoarthritis and Other Joint Defects. Stem Cells Int. 2018, 2018, 7309201. [Google Scholar] [CrossRef]
- Lima, V.P.; Tobin, G.C.; Jesus Pereira MR de Silveira, M.D.; Witz, M.I.; Nardi, N.B. Chondrogenic effect of liquid and gelled platelet lysate on canine adipose-derived mesenchymal stromal cells. Res. Vet. Sci. 2019, 124, 393–398. [Google Scholar] [CrossRef]
- Maki, C.B.; Beck, A.; Wallis, C.-B.C.C.; Choo, J.; Ramos, T.; Tong, R.; Borjesson, D.L.; Izadyar, F. Intra-articular Administration of Allogeneic Adipose Derived MSCs Reduces Pain and Lameness in Dogs With Hip Osteoarthritis: A Double Blinded, Randomized, Placebo Controlled Pilot Study. Front. Vet. Sci. 2020, 7, 570. [Google Scholar] [CrossRef] [PubMed]
- Klinger, C. Mesenchymal stem cells: A potential therapy for canine atopic dermatitis? Vet. Rec. 2018, 183, 651. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, A.J.; Hermida-Prieto, M.; Fernández, V.; Fariñas, F.; Alcoholado, C.; Rodríguez-García, M.I.; Mariñas-Pardo, L.; Becerra, J. Allogeneic adipose-derived mesenchymal stem cell therapy in dogs with refractory atopic dermatitis: Clinical efficacy and safety. Vet. Rec. 2018, 183, 654. [Google Scholar] [CrossRef]
- Rhew, S.-Y.; Park, S.-M.; Li, Q.; An, J.-H.; Chae, H.-K.; Lee, J.-H.; Ahn, J.-O.; Song, W.-J.; Youn, H.-Y. Efficacy and safety of allogenic canine adipose tissue-derived mesenchymal stem cell therapy for insulin-dependent diabetes mellitus in four dogs: A pilot study. J. Vet. Med. Sci. 2021, 83, 592–600. [Google Scholar] [CrossRef]
- Pérez-Merino, E.M.; Usón-Casaús, J.M.; Zaragoza-Bayle, C.; Duque-Carrasco, J.; Mariñas-Pardo, L.; Hermida-Prieto, M.; Barrera-Chacón, R.; Gualtieri, M. Safety and efficacy of allogeneic adipose tissue-derived mesenchymal stem cells for treatment of dogs with inflammatory bowel disease: Clinical and laboratory outcomes. Vet. J. 2015, 206, 385–390. [Google Scholar] [CrossRef]
- Sharun, K.; Rawat, T.; Kumar, R.; Chandra, V.; Saxena, A.C.; Pawde, A.M.; Kinjavdekar, P.; Amarpal; Sharma, G.T. Clinical evaluation following the percutaneous transplantation of allogenic bone marrow-derived mesenchymal stem cells (aBM-MSC) in dogs affected by vertebral compression fracture. Vet. Anim. Sci. 2020, 10, 100152. [Google Scholar] [CrossRef]
- Karnieli, O.; Friedner, O.M.; Allickson, J.G.; Zhang, N.; Jung, S.; Fiorentini, D.; Abraham, E.; Eaker, S.S.; Yong, T.K.; Chan, A.; et al. A consensus introduction to serum replacements and serum-free media for cellular therapies. Cytotherapy 2017, 19, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Fahie, M.A.; Ortolano, G.A.; Guercio, V.; Schaffer, J.A.; Johnston, G.; Au, J.; Hettlich, B.A.; Phillips, T.; Allen, M.J.; Bertone, A.L. A randomized controlled trial of the efficacy of autologous platelet therapy for the treatment of osteoarthritis in dogs. J. Am. Vet. Med. Assoc. 2013, 243, 1291–1297. [Google Scholar] [CrossRef]
- Catarino, J.; Carvalho, P.; Santos, S.; Martins, Â.; Requicha, J. Treatment of canine osteoarthritis with allogeneic platelet-rich plasma: Review of five cases. Open Vet. J. 2020, 10, 226–231. [Google Scholar] [CrossRef]
- Venator, K.P.; Frye, C.W.; Gamble, L.-J.; Wakshlag, J.J. Assessment of a Single Intra-Articular Stifle Injection of Pure Platelet Rich Plasma on Symmetry Indices in Dogs with Unilateral or Bilateral Stifle Osteoarthritis from Long-Term Medically Managed Cranial Cruciate Ligament Disease. Vet. Med. 2020, 11, 31–38. [Google Scholar] [CrossRef]
- Sanghani-Kerai, A.; Black, C.; Cheng, S.O.; Collins, L.; Schneider, N.; Blunn, G.; Watson, F.; Fitzpatrick, N. Clinical outcomes following intra-articular injection of autologous adipose-derived mesenchymal stem cells for the treatment of osteoarthritis in dogs characterized by weight-bearing asymmetry. Bone Jt. Res. 2021, 10, 650–658. [Google Scholar] [CrossRef]
- Hernández-Guerra, Á.M.; Carrillo, J.M.; Sopena, J.J.; Vilar, J.M.; Peláez, P.; Cuervo, B.; Santana, A.; Rubio, M. Platelet-Rich Plasma for the Treatment of Degenerative Lumbosacral Stenosis: A Study with Retired Working Dogs. Animals 2021, 11, 2965. [Google Scholar] [CrossRef]
- Iacopetti, I.; Patruno, M.; Melotti, L.; Martinello, T.; Bedin, S.; Badon, T.; Righetto, E.M.; Perazzi, A. Autologous Platelet-Rich Plasma Enhances the Healing of Large Cutaneous Wounds in Dogs. Front. Vet. Sci. 2020, 7, 575449. [Google Scholar] [CrossRef]
- Berni, P.; Leonardi, F.; Conti, V.; Ramoni, R.; Grolli, S.; Mattioli, G. Case Report: A Novel Ventilated Thermoplastic Mesh Bandage for Post-operative Management of Large Soft Tissue Defects: A Case Series of Three Dogs Treated With Autologous Platelet Concentrates. Front. Vet. Sci. 2021, 8, 704567. [Google Scholar] [CrossRef]
- Farghali, H.A.; AbdElKader, N.A.; AbuBakr, H.O.; Ramadan, E.S.; Khattab, M.S.; Salem, N.Y.; Emam, I.A. Corneal Ulcer in Dogs and Cats: Novel Clinical Application of Regenerative Therapy Using Subconjunctival Injection of Autologous Platelet-Rich Plasma. Front. Vet. Sci. 2021, 8, 641265. [Google Scholar] [CrossRef]
- Perego, R.; Spada, E.; Moneta, E.; Baggiani, L.; Proverbio, D. Use of Autologous Leucocyte- and Platelet-Rich Plasma (L-PRP) in the Treatment of Aural Hematoma in Dogs. Vet. Sci. 2021, 8, 172. [Google Scholar] [CrossRef]
- Zamani, M.; Yaghoubi, Y.; Movassaghpour, A.; Shakouri, K.; Mehdizadeh, A.; Pishgahi, A.; Yousefi, M. Novel therapeutic approaches in utilizing platelet lysate in regenerative medicine: Are we ready for clinical use? J. Cell Physiol. 2019, 234, 17172–17186. [Google Scholar] [CrossRef]
- Carr, B.J.; Canapp, S.O.; Mason, D.R.; Cox, C.; Hess, T. Canine Platelet-Rich Plasma Systems: A Prospective Analysis. Front. Vet. Sci. 2015, 2, 73. [Google Scholar] [CrossRef]
- Franklin, S.P.; Birdwhistell, K.E.; Strelchik, A.; Garner, B.C.; Brainard, B.M. Influence of Cellular Composition and Exogenous Activation on Growth Factor and Cytokine Concentrations in Canine Platelet-Rich Plasmas. Front. Vet. Sci. 2017, 4, 40. [Google Scholar] [CrossRef]
- Hagen, A.; Lehmann, H.; Aurich, S.; Bauer, N.; Melzer, M.; Moellerberndt, J.; Patané, V.; Schnabel, C.L.; Burk, J. Scalable Production of Equine Platelet Lysate for Multipotent Mesenchymal Stromal Cell Culture. Front. Bioeng. Biotechnol. 2020, 8, 613621. [Google Scholar] [CrossRef]
- Schallmoser, K.; Henschler, R.; Gabriel, C.; Koh, M.B.C.; Burnouf, T. Production and Quality Requirements of Human Platelet Lysate: A Position Statement from the Working Party on Cellular Therapies of the International Society of Blood Transfusion. Trends Biotechnol. 2020, 38, 13–23. [Google Scholar] [CrossRef]
- Tyrnenopoulou, P.; Diakakis, N.; Karayannopoulou, M.; Savvas, I.; Koliakos, G. Evaluation of intra-articular injection of autologous platelet lysate (PL) in horses with osteoarthritis of the distal interphalangeal joint. Vet. Q. 2016, 36, 56–62. [Google Scholar] [CrossRef]
- Mojica-Henshaw, M.P.; Jacobson, P.; Morris, J.; Kelley, L.; Pierce, J.; Boyer, M.; Reems, J.-A. Serum-converted platelet lysate can substitute for fetal bovine serum in human mesenchymal stromal cell cultures. Cytotherapy 2013, 15, 1458–1468. [Google Scholar] [CrossRef]
- Becherucci, V.; Piccini, L.; Casamassima, S.; Bisin, S.; Gori, V.; Gentile, F.; Ceccantini, R.; De Rienzo, E.; Bindi, B.; Pavan, P.; et al. Human platelet lysate in mesenchymal stromal cell expansion according to a GMP grade protocol: A cell factory experience. Stem Cell Res. Ther. 2018, 9, 124. [Google Scholar] [CrossRef]
- Del Bue, M.; Riccò, S.; Conti, V.; Merli, E.; Ramoni, R.; Grolli, S. Platelet lysate promotes in vitro proliferation of equine mesenchymal stem cells and tenocytes. Vet. Res. Commun. 2007, 31 (Suppl. S1), 289–292. [Google Scholar] [CrossRef]
- Seo, J.; Tsuzuki, N.; Haneda, S.; Yamada, K.; Furuoka, H.; Tabata, Y.; Sasaki, N. Comparison of allogeneic platelet lysate and fetal bovine serum for in vitro expansion of equine bone marrow-derived mesenchymal stem cells. Res. Vet. Sci. 2013, 95, 693–698. [Google Scholar] [CrossRef]
- Russell, K.A.; Koch, T.G. Equine platelet lysate as an alternative to fetal bovine serum in equine mesenchymal stromal cell culture—Too much of a good thing? Equine Vet. J. 2016, 48, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Gilbertie, J.M.; Long, J.M.; Schubert, A.G.; Berglund, A.K.; Schaer, T.P.; Schnabel, L.V. Pooled Platelet-Rich Plasma Lysate Therapy Increases Synoviocyte Proliferation and Hyaluronic Acid Production While Protecting Chondrocytes From Synoviocyte-Derived Inflammatory Mediators. Front. Vet. Sci. 2018, 5, 150. [Google Scholar] [CrossRef]
- Naskou, M.C.; Sumner, S.M.; Chocallo, A.; Kemelmakher, H.; Thoresen, M.; Copland, I.; Galipeau, J.; Peroni, J.F. Platelet lysate as a novel serum-free media supplement for the culture of equine bone marrow-derived mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 75. [Google Scholar] [CrossRef]
- Yaneselli, K.; Barrachina, L.; Remacha, A.R.; Algorta, A.; Vitoria, A.; Cequier, A.; Romero, A.; Vázquez, F.J.; Maisonnave, J.; Rodellar, C. Effect of allogeneic platelet lysate on equine bone marrow derived mesenchymal stem cell characteristics, including immunogenic and immunomodulatory gene expression profile. Vet. Immunol. Immunopathol. 2019, 217, 109944. [Google Scholar] [CrossRef]
- Russell, K.A.; Gibson, T.W.G.; Chong, A.; Co, C.; Koch, T.G. Canine Platelet Lysate Is Inferior to Fetal Bovine Serum for the Isolation and Propagation of Canine Adipose Tissue- and Bone Marrow-Derived Mesenchymal Stromal Cells. PLoS ONE 2015, 10, e0136621. [Google Scholar] [CrossRef]
- Van Kuppeveld, F.J.; van der Logt, J.T.; Angulo, A.F.; van Zoest, M.J.; Quint, W.G.; Niesters, H.G.; Galama, J.M.; Melchers, W.J. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl. Environ. Microbiol. 1992, 58, 2606–2615. [Google Scholar] [CrossRef]
- Gittel, C.; Brehm, W.; Burk, J.; Juelke, H.; Staszyk, C.; Ribitsch, I. Isolation of equine multipotent mesenchymal stromal cells by enzymatic tissue digestion or explant technique: Comparison of cellular properties. BMC Vet. Res. 2013, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Ruifrok, A.C.; Johnston, D.A. Quantification of Histochemical Staining by Color Deconvolution. Anal. Quant. Cytol. Histol. 2001, 23, 291–299. [Google Scholar] [PubMed]
- Seabright, M. The use of proteolytic enzymes for the mapping of structural rearrangements in the chromosomes of man. Chromosoma 1972, 36, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Seabright, M. Human chromosome banding. Lancet 1972, 299, 967. [Google Scholar] [CrossRef]
- Gulliksson, H. Platelets from platelet-rich-plasma versus buffy-coat-derived platelets: What is the difference? Rev. Bras. Hematol. Hemoter. 2012, 34, 76–77. [Google Scholar] [CrossRef]
- Burnouf, T.; Strunk, D.; Koh, M.B.C.; Schallmoser, K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials 2016, 76, 371–387. [Google Scholar] [CrossRef]
- Bozorgmanesh, R.; Magdesian, K.G.; Sutton-Burges, J.W.; Owens, S.D.; Tablin, F. Equine platelet concentrate preparation and validation. J. Vet. Intern. Med. 2019, 33, 1500–1506. [Google Scholar] [CrossRef]
- Sumner, S.M.; Naskou, M.C.; Thoresen, M.; Copland, I.; Peroni, J.F. Platelet lysate obtained via plateletpheresis performed in standing and awake equine donors. Transfusion 2017, 57, 1755–1762. [Google Scholar] [CrossRef]
- Abrams-Ogg, A.C.; Kruth, S.A.; Carter, R.F.; Valli, V.E.; Kamel-Reid, S.; Dubé, I.D. Preparation and transfusion of canine platelet concentrates. Am. J. Vet. Res. 1993, 54, 635–642. [Google Scholar]
- Van der Meer, P.; Pietersz, R.; Hinloopen, B.; Dekker, W.; Reesink, H. Automated separation of whole blood in top and bottom bags into components using the Compomat G4. Vox Sanguinis 1999, 76, 90–99. [Google Scholar] [CrossRef]
- Bowen-Pope, D.F.; Hart, C.E.; Seifert, R.A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J. Biol. Chem. 1989, 264, 2502–2508. [Google Scholar] [CrossRef]
- Stief, M.; Gottschalk, J.; Ionita, J.-C.; Einspanier, A.; Oechtering, G.; Böttcher, P. Concentration of platelets and growth factors in canine autologous conditioned plasma. Vet. Comp. Orthop. Traumatol. 2011, 24, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, I.; Marukawa, E.; Takahashi, Y.; Omura, K. Effects of platelet-poor plasma, platelet-rich plasma, and platelet-rich fibrin on healing of extraction sockets with buccal dehiscence in dogs. Tissue Eng. Part A 2014, 20, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Perego, R.; Spada, E.; Baggiani, L.; Martino, P.A.; Proverbio, D. Efficacy of a Semi Automated Commercial Closed System for Autologous Leukocyte- and Platelet-Rich Plasma (l-prp) Production in Dogs: A Preliminary Study. Animals 2020, 10, 1342. [Google Scholar] [CrossRef] [PubMed]
- Haines, J.M.; Hwang, J.K.; Wardrop, K.J. The effects of additive solutions on the development of storage lesions in stored canine platelet concentrates. J. Vet. Emerg. Crit. Care 2021, 31, 247–255. [Google Scholar] [CrossRef]
- Giraldo, C.E.; López, C.; Álvarez, M.E.; Samudio, I.J.; Prades, M.; Carmona, J.U. Effects of the breed, sex and age on cellular content and growth factor release from equine pure-platelet rich plasma and pure-platelet rich gel. BMC Vet. Res. 2013, 9, 29. [Google Scholar] [CrossRef]
- European Medicines Agency, London Committee for Medicinal Products for Human Use (CHMP) (Ed.) Guideline on the Use of Bovine Serum in the Manufacture of Human Biological Medicinal Products. 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-use-bovine-serum-manufacture-human-biological-medicinal-products_en.pdf (accessed on 12 January 2022).
- Trento, C.; Bernardo, M.E.; Nagler, A.; Kuçi, S.; Bornhäuser, M.; Köhl, U.; Strunk, D.; Galleu, A.; Sanchez-Guijo, F.; Gaipa, G.; et al. Manufacturing Mesenchymal Stromal Cells for the Treatment of Graft-versus-Host Disease: A Survey among Centers Affiliated with the European Society for Blood and Marrow Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 2365–2370. [Google Scholar] [CrossRef] [PubMed]
- Neupane, M.; Chang, C.-C.; Kiupel, M.; Yuzbasiyan-Gurkan, V. Isolation and characterization of canine adipose-derived mesenchymal stem cells. Tissue Eng. Part A 2008, 14, 1007–1015. [Google Scholar] [CrossRef]
- Volk, S.W.; Wang, Y.; Hankenson, K.D. Effects of donor characteristics and ex vivo expansion on canine mesenchymal stem cell properties: Implications for MSC-based therapies. Cell Transpl. 2012, 21, 2189–2200. [Google Scholar] [CrossRef]
- Bearden, R.N.; Huggins, S.S.; Cummings, K.J.; Smith, R.; Gregory, C.A.; Saunders, W.B. In-vitro characterization of canine multipotent stromal cells isolated from synovium, bone marrow, and adipose tissue: A donor-matched comparative study. Stem Cell Res. Ther. 2017, 8, 218. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated β-galactosidase is lysosomal β-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Wagner, W.; Bork, S.; Lepperdinger, G.; Joussen, S.; Ma, N.; Strunk, D.; Koch, C. How to track cellular aging of mesenchymal stromal cells? Aging 2010, 2, 224–230. [Google Scholar] [CrossRef]
- Bertolo, A.; Guerrero, J.; Stoyanov, J. Autofluorescence-based sorting removes senescent cells from mesenchymal stromal cell cultures. Sci. Rep. 2020, 10, 19084. [Google Scholar] [CrossRef]
- Miettinen, T.P.; Caldez, M.J.; Kaldis, P.; Björklund, M. Cell size control—A mechanism for maintaining fitness and function. Bioessays 2017, 39, 1700058. [Google Scholar] [CrossRef]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Trojahn Kølle, S.-F.; Oliveri, R.S.; Glovinski, P.V.; Kirchhoff, M.; Mathiasen, A.B.; Elberg, J.J.; Andresen, P.S.; Drzewiecki, K.T.; Fischer-Nielsen, A. Pooled human platelet lysate versus fetal bovine serum-investigating the proliferation rate, chromosome stability and angiogenic potential of human adipose tissue-derived stem cells intended for clinical use. Cytotherapy 2013, 15, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
| Sample | pH | Na+ (mmol/L) | K+ (mmol/L) | Ca2+ (mmol/L) | Cl-(mmol/L) | HCO3 (mmol/L) | Glucose (mmol/L) | Lactate (mmol/L) | Total Protein (g/L) | Albumin (g/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| Serum | 7.52 (0.13) | 149.15 (2.75) | 4.87 (0.65) | 1.33 (0.06) | 110.40 (1.63) | 19.60 (2.85) | 3.70 (1.83) | 4.95 (1.90) | 64.35 (5.55) | 30.10 (2.80) |
| Concentrate | 7.19 (0.06) | 156.80 (3.20) | 2.86 (0.26) | 0 | 81.20 (3.07) | 16.65 (1.58) | 26.35 (1.80) | 2.45 (0.45) | 47.25 (2.65) | 24.35 (1.88) |
| Lysate | 7.34 (0.03) | 154.55 (1.50) | 3.29 (0.28) | 0 | 80.45 (2.55) | 13.60 (1.85) | 29.65 (0.82) | 2.75 (0.53) | 49.30 (3.08) | 24.45 (2.33) |
| Parameter | cPL 1 | ePL 1 | FBS |
|---|---|---|---|
| pH | 7.33 | 7.52 | 7.45 |
| Na+ (mmol/L) | 153.70 | 147.80 | 138.60 |
| K+ (mmol/L) | 3.25 | 3.68 | 11.21 |
| Ca2+ (mmol/L) | 0 | <0.10 | 1.268 |
| CL- (mmol/L) | 80.70 | 84.50 | 106.60 |
| HCO3 (mmol/L) | 13.8 | 16.70 | 12.70 |
| Glucose (mmol/L) | >30 | 23.10 | 2.20 |
| Lactate (mmol/L) | 2.70 | 2.90 | 17.70 |
| Total protein (g/L) | 49.20 | 54.10 | 36.80 |
| Albumin (g/L) | 25.50 | 27.90 | 23.00 |
| PDGF-BB concentration (pg/mL) | 307 | 3783 | 547 |
| TGF-β1 concentration (pg/mL) | 36,575 | 3966 | 3379 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, A.; Holland, H.; Brandt, V.-P.; Doll, C.U.; Häußler, T.C.; Melzer, M.; Moellerberndt, J.; Lehmann, H.; Burk, J. Platelet Lysate for Mesenchymal Stromal Cell Culture in the Canine and Equine Species: Analogous but Not the Same. Animals 2022, 12, 189. https://doi.org/10.3390/ani12020189
Hagen A, Holland H, Brandt V-P, Doll CU, Häußler TC, Melzer M, Moellerberndt J, Lehmann H, Burk J. Platelet Lysate for Mesenchymal Stromal Cell Culture in the Canine and Equine Species: Analogous but Not the Same. Animals. 2022; 12(2):189. https://doi.org/10.3390/ani12020189
Chicago/Turabian StyleHagen, Alina, Heidrun Holland, Vivian-Pascal Brandt, Carla U. Doll, Thomas C. Häußler, Michaela Melzer, Julia Moellerberndt, Hendrik Lehmann, and Janina Burk. 2022. "Platelet Lysate for Mesenchymal Stromal Cell Culture in the Canine and Equine Species: Analogous but Not the Same" Animals 12, no. 2: 189. https://doi.org/10.3390/ani12020189
APA StyleHagen, A., Holland, H., Brandt, V.-P., Doll, C. U., Häußler, T. C., Melzer, M., Moellerberndt, J., Lehmann, H., & Burk, J. (2022). Platelet Lysate for Mesenchymal Stromal Cell Culture in the Canine and Equine Species: Analogous but Not the Same. Animals, 12(2), 189. https://doi.org/10.3390/ani12020189






