Isolation of Vascular Wall Mesenchymal Stem Cells from the Thoracic Aorta of Adult Göttingen Minipigs: A New Protocol for the Simultaneous Endothelial Cell Collection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
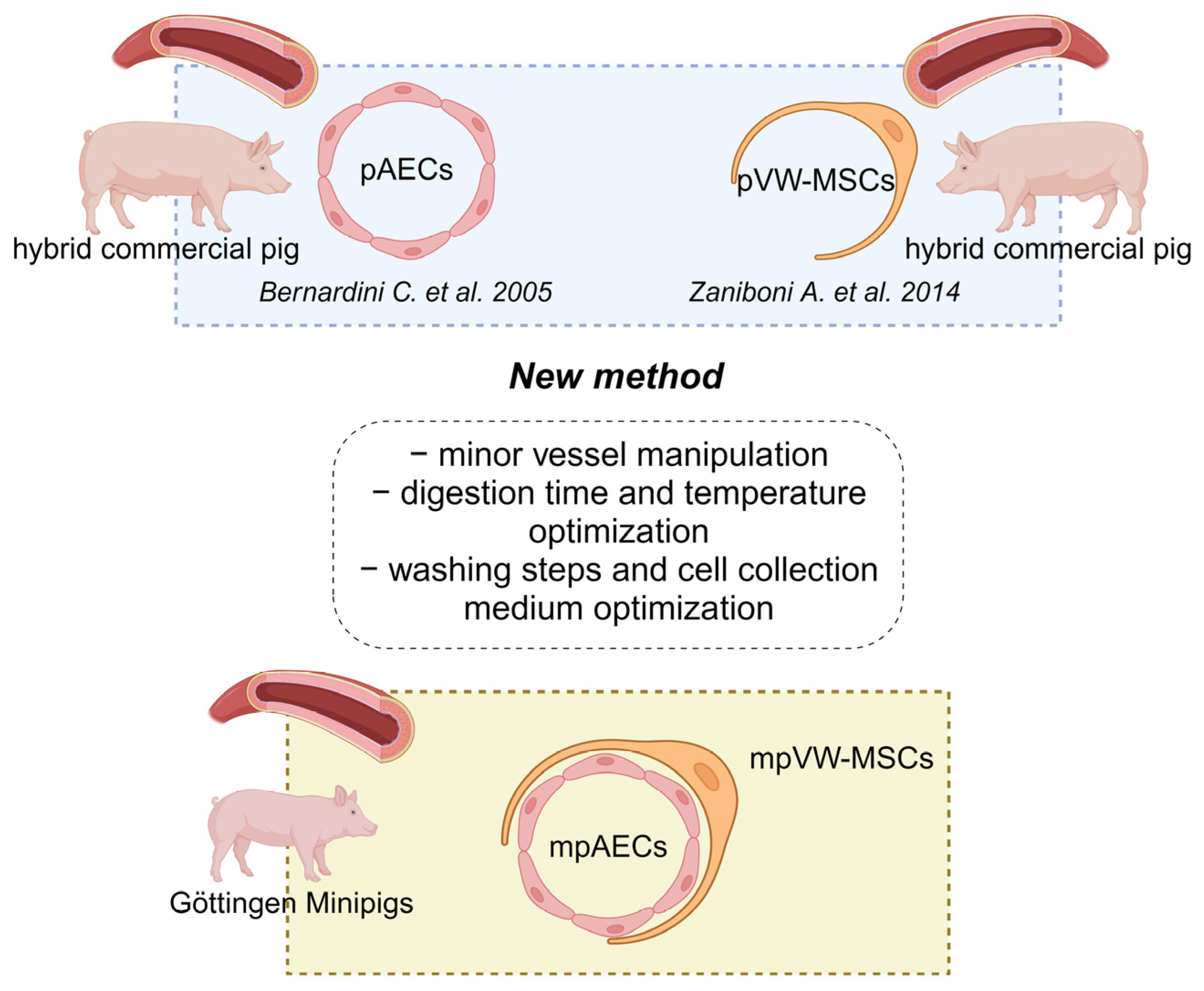
2.2. Animal Description
2.3. Histological Examination
2.4. Cell Isolation
2.5. Cell Expansion
2.6. Cell Characterization by Flow Cytometry Analysis
2.7. mpAEC In Vitro Angiogenesis Assay
2.8. mpVW-MSC Cell Adhesion Assay
2.9. Mesenchymal Trilineage Differentiation Potential
2.10. Statistical Analysis
3. Results
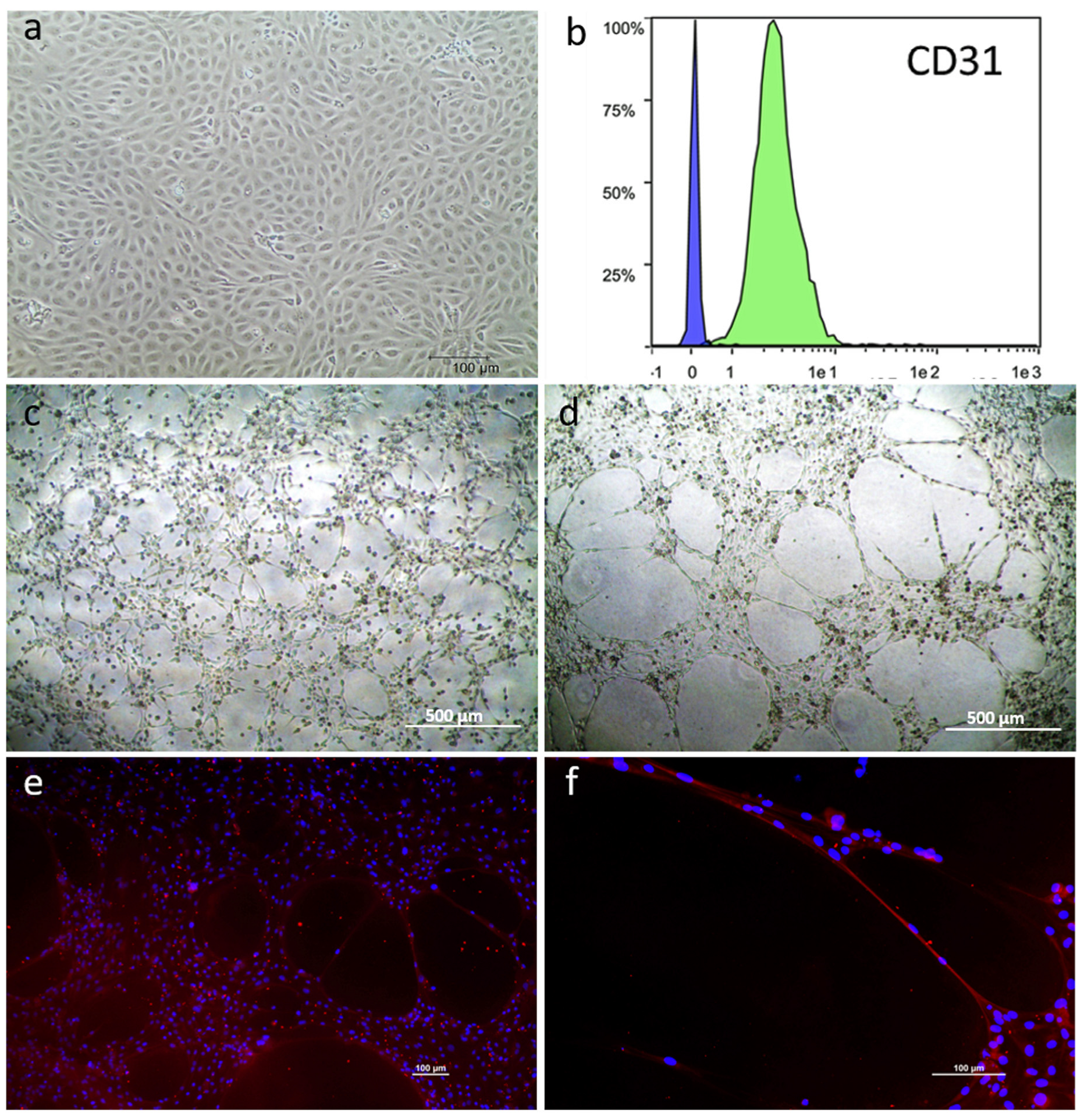
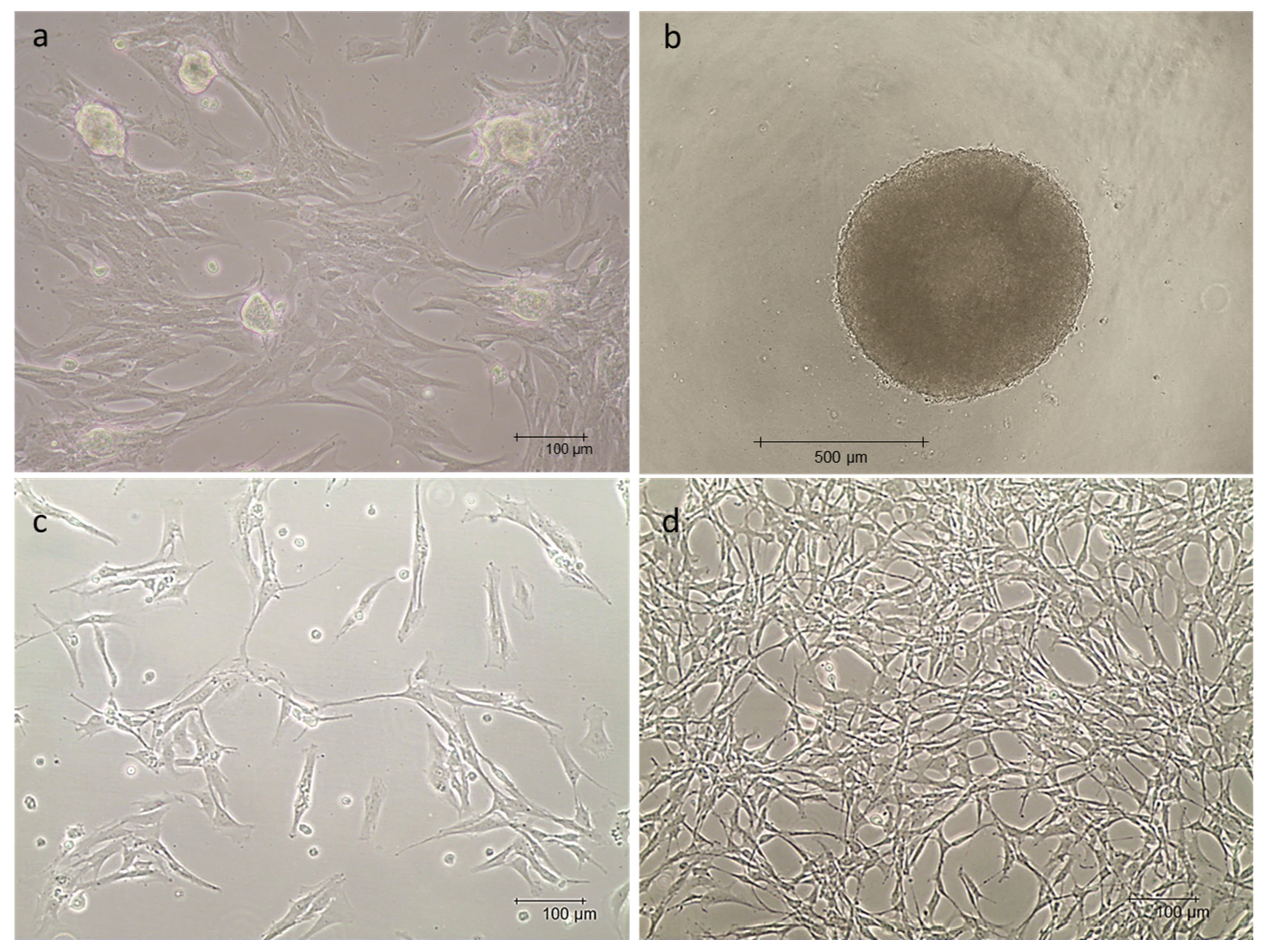
3.1. mpAECs and mpVW-MSCs Isolation
3.2. mpAEC and mpVW-MSC Characterization
3.3. mpVW-MSC Trilieaneage Potential
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alessandri, G.; Girelli, M.; Taccagni, G.; Colombo, A.; Nicosia, R.; Caruso, A.; Baronio, M.; Pagano, S.; Cova, L.; Parati, E. Human Vasculogenesis Ex Vivo: Embryonal Aorta as a Tool for Isolation of Endothelial Cell Progenitors. Lab. Investig. 2001, 81, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Tintut, Y.; Alfonso, Z.; Saini, T.; Radcliff, K.; Watson, K.; Boström, K.; Demer, L.L. Multilineage Potential of Cells from the Artery Wall. Circulation 2003, 108, 2505–2510. [Google Scholar] [CrossRef] [PubMed]
- Majka, S.M.; Jackson, K.A.; Kienstra, K.A.; Majesky, M.W.; Goodell, M.A.; Hirschi, K.K. Distinct Progenitor Populations in Skeletal Muscle Are Bone Marrow Derived and Exhibit Different Cell Fates during Vascular Regeneration. J. Clin. Investig. 2003, 111, 71–79. [Google Scholar] [CrossRef]
- Bautch, V.L. Stem Cells and the Vasculature. Nat. Med. 2011, 17, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Psaltis, P.J.; Harbuzariu, A.; Delacroix, S.; Holroyd, E.W.; Simari, R.D. Resident Vascular Progenitor Cells—Diverse Origins, Phenotype, and Function. J. Cardiovasc. Transl. Res. 2011, 4, 161–176. [Google Scholar] [CrossRef]
- Tang, Z.; Wang, A.; Yuan, F.; Yan, Z.; Liu, B.; Chu, J.S.; Helms, J.A.; Li, S. Differentiation of Multipotent Vascular Stem Cells Contributes to Vascular Diseases. Nat. Commun. 2012, 3, 875. [Google Scholar] [CrossRef]
- Lin, C.-S.; Lue, T.F. Defining Vascular Stem Cells. Stem Cells Dev. 2013, 22, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Murray, I.R.; West, C.C.; Hardy, W.R.; James, A.W.; Park, T.S.; Nguyen, A.; Tawonsawatruk, T.; Lazzari, L.; Soo, C.; Péault, B. Natural History of Mesenchymal Stem Cells, from Vessel Walls to Culture Vessels. Cell Mol. Life Sci. 2014, 71, 1353–1374. [Google Scholar] [CrossRef]
- Zhang, L.; Issa Bhaloo, S.; Chen, T.; Zhou, B.; Xu, Q. Role of Resident Stem Cells in Vessel Formation and Arteriosclerosis. Circ. Res. 2018, 122, 1608–1624. [Google Scholar] [CrossRef]
- Corselli, M.; Crisan, M.; Murray, I.R.; West, C.C.; Scholes, J.; Codrea, F.; Khan, N.; Péault, B. Identification of Perivascular Mesenchymal Stromal/Stem Cells by Flow Cytometry. Cytom. A 2013, 83, 714–720. [Google Scholar] [CrossRef]
- Craig, D.J.; James, A.W.; Wang, Y.; Tavian, M.; Crisan, M.; Péault, B.M. Blood Vessel Resident Human Stem Cells in Health and Disease. Stem Cells Transl. Med. 2022, 11, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Howson, K.M.; Aplin, A.C.; Gelati, M.; Alessandri, G.; Parati, E.A.; Nicosia, R.F. The Postnatal Rat Aorta Contains Pericyte Progenitor Cells That Form Spheroidal Colonies in Suspension Culture. Am. J. Physiol. Cell Physiol. 2005, 289, C1396–C1407. [Google Scholar] [CrossRef]
- Iurlaro, M.; Scatena, M.; Zhu, W.-H.; Fogel, E.; Wieting, S.L.; Nicosia, R.F. Rat Aorta-Derived Mural Precursor Cells Express the Tie2 Receptor and Respond Directly to Stimulation by Angiopoietins. J. Cell Sci. 2003, 116, 3635–3643. [Google Scholar] [CrossRef] [PubMed]
- Invernici, G.; Emanueli, C.; Madeddu, P.; Cristini, S.; Gadau, S.; Benetti, A.; Ciusani, E.; Stassi, G.; Siragusa, M.; Nicosia, R.; et al. Human Fetal Aorta Contains Vascular Progenitor Cells Capable of Inducing Vasculogenesis, Angiogenesis, and Myogenesis in Vitro and in a Murine Model of Peripheral Ischemia. Am. J. Pathol. 2007, 170, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Psaltis, P.J.; Simari, R.D. Vascular Wall Progenitor Cells in Health and Disease. Circ. Res. 2015, 116, 1392–1412. [Google Scholar] [CrossRef]
- Klein, D. Vascular Wall-Resident Multipotent Stem Cells of Mesenchymal Nature within the Process of Vascular Remodeling: Cellular Basis, Clinical Relevance, and Implications for Stem Cell Therapy. Stem Cells Int. 2016, 2016, 1905846. [Google Scholar] [CrossRef]
- Feng, R.; Ullah, M.; Chen, K.; Ali, Q.; Lin, Y.; Sun, Z. Stem Cell-Derived Extracellular Vesicles Mitigate Ageing-Associated Arterial Stiffness and Hypertension. J. Extracell. Vesicles 2020, 9, 1783869. [Google Scholar] [CrossRef]
- Nassiri, S.M.; Rahbarghazi, R. Interactions of Mesenchymal Stem Cells with Endothelial Cells. Stem Cells Dev. 2014, 23, 319–332. [Google Scholar] [CrossRef]
- Pasquinelli, G.; Tazzari, P.L.; Vaselli, C.; Foroni, L.; Buzzi, M.; Storci, G.; Alviano, F.; Ricci, F.; Bonafè, M.; Orrico, C.; et al. Thoracic Aortas from Multiorgan Donors Are Suitable for Obtaining Resident Angiogenic Mesenchymal Stromal Cells. Stem Cells 2007, 25, 1627–1634. [Google Scholar] [CrossRef]
- Da Silva Meirelles, L.; Chagastelles, P.C.; Nardi, N.B. Mesenchymal Stem Cells Reside in Virtually All Post-Natal Organs and Tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Pacilli, A.; Pasquinelli, G. Vascular Wall Resident Progenitor Cells: A Review. Exp. Cell Res. 2009, 315, 901–914. [Google Scholar] [CrossRef]
- Zaniboni, A.; Bernardini, C.; Alessandri, M.; Mangano, C.; Zannoni, A.; Bianchi, F.; Sarli, G.; Calzà, L.; Bacci, M.L.; Forni, M. Cells Derived from Porcine Aorta Tunica Media Show Mesenchymal Stromal-like Cell Properties in in Vitro Culture. Am. J. Physiol. Cell Physiol. 2014, 306, C322–C333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaniboni, A.; Bernardini, C.; Bertocchi, M.; Zannoni, A.; Bianchi, F.; Avallone, G.; Mangano, C.; Sarli, G.; Calzà, L.; Bacci, M.L.; et al. In Vitro Differentiation of Porcine Aortic Vascular Precursor Cells to Endothelial and Vascular Smooth Muscle Cells. Am. J. Physiol. Cell Physiol. 2015, 309, C320–C331. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernardini, C.; Bertocchi, M.; Zannoni, A.; Salaroli, R.; Tubon, I.; Dothel, G.; Fernandez, M.; Bacci, M.L.; Calzà, L.; Forni, M. Constitutive and LPS-Stimulated Secretome of Porcine Vascular Wall-Mesenchymal Stem Cells Exerts Effects on in Vitro Endothelial Angiogenesis. BMC Vet. Res. 2019, 15, 123. [Google Scholar] [CrossRef] [PubMed]
- Dothel, G.; Bernardini, C.; Zannoni, A.; Spirito, M.R.; Salaroli, R.; Bacci, M.L.; Forni, M.; Ponti, F.D. Ex Vivo Effect of Vascular Wall Stromal Cells Secretome on Enteric Ganglia. World J. Gastroenterol. 2019, 25, 4892–4903. [Google Scholar] [CrossRef]
- Ringe, J.; Kaps, C.; Schmitt, B.; Büscher, K.; Bartel, J.; Smolian, H.; Schultz, O.; Burmester, G.R.; Häupl, T.; Sittinger, M. Porcine Mesenchymal Stem Cells. Induction of Distinct Mesenchymal Cell Lineages. Cell Tissue Res. 2002, 307, 321–327. [Google Scholar] [CrossRef]
- Casado, J.G.; Gomez-Mauricio, G.; Alvarez, V.; Mijares, J.; Tarazona, R.; Bernad, A.; Sanchez-Margallo, F.M. Comparative Phenotypic and Molecular Characterization of Porcine Mesenchymal Stem Cells from Different Sources for Translational Studies in a Large Animal Model. Vet. Immunol. Immunopathol. 2012, 147, 104–112. [Google Scholar] [CrossRef]
- Bharti, D.; Shivakumar, S.B.; Subbarao, R.B.; Rho, G.-J. Research Advancements in Porcine Derived Mesenchymal Stem Cells. Curr. Stem Cell Res. Ther. 2016, 11, 78–93. [Google Scholar] [CrossRef]
- La Mantia, D.; Bernardini, C.; Zannoni, A.; Salaroli, R.; Wang, C.; Bencivenni, S.; Forni, M. Efficacy of Stem Cell Therapy in Large Animal Models of Ischemic Cardiomyopathies: A Systematic Review and Meta-Analysis. Animals 2022, 12, 749. [Google Scholar] [CrossRef]
- Grøgaard, H.K.; Sigurjonsson, O.E.; Brekke, M.; Kløw, N.E.; Landsverk, K.S.; Lyberg, T.; Eriksen, M.; Egeland, T.; Ilebekk, A. Cardiac Accumulation of Bone Marrow Mononuclear Progenitor Cells after Intracoronary or Intravenous Injection in Pigs Subjected to Acute Myocardial Infarction with Subsequent Reperfusion. Cardiovasc. Revasc Med. 2007, 8, 21–27. [Google Scholar] [CrossRef]
- Krause, U.; Harter, C.; Seckinger, A.; Wolf, D.; Reinhard, A.; Bea, F.; Dengler, T.; Hardt, S.; Ho, A.; Katus, H.A.; et al. Intravenous Delivery of Autologous Mesenchymal Stem Cells Limits Infarct Size and Improves Left Ventricular Function in the Infarcted Porcine Heart. Stem Cells Dev. 2007, 16, 31–37. [Google Scholar] [CrossRef]
- Halkos, M.E.; Zhao, Z.-Q.; Kerendi, F.; Wang, N.-P.; Jiang, R.; Schmarkey, L.S.; Martin, B.J.; Quyyumi, A.A.; Few, W.L.; Kin, H.; et al. Intravenous Infusion of Mesenchymal Stem Cells Enhances Regional Perfusion and Improves Ventricular Function in a Porcine Model of Myocardial Infarction. Basic Res. Cardiol. 2008, 103, 525–536. [Google Scholar] [CrossRef]
- Liao, S.; Zhang, Y.; Ting, S.; Zhen, Z.; Luo, F.; Zhu, Z.; Jiang, Y.; Sun, S.; Lai, W.-H.; Lian, Q.; et al. Potent Immunomodulation and Angiogenic Effects of Mesenchymal Stem Cells versus Cardiomyocytes Derived from Pluripotent Stem Cells for Treatment of Heart Failure. Stem Cell Res. Ther. 2019, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Charles, C.J.; Li, R.R.; Yeung, T.; Mazlan, S.M.I.; Lai, R.C.; de Kleijn, D.P.V.; Lim, S.K.; Richards, A.M. Systemic Mesenchymal Stem Cell-Derived Exosomes Reduce Myocardial Infarct Size: Characterization With MRI in a Porcine Model. Front. Cardiovasc. Med. 2020, 7, 601990. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, M.D.; West, M.E.D.; Lam, G.C.; Sefton, M.V. In Vivo Remodelling of Vascularizing Engineered Tissues. Ann. Biomed. Eng. 2015, 43, 1189–1200. [Google Scholar] [CrossRef]
- Bollen, P.; Ellegaard, L. The Göttingen Minipig in Pharmacology and Toxicology. Pharmacol. Toxicol. 1997, 80 (Suppl. S2), 3–4. [Google Scholar] [CrossRef] [PubMed]
- Simianer, H.; Köhn, F. Genetic Management of the Göttingen Minipig Population. J. Pharmacol. Toxicol. Methods 2010, 62, 221–226. [Google Scholar] [CrossRef]
- Flisikowska, T.; Egli, J.; Flisikowski, K.; Stumbaum, M.; Küng, E.; Ebeling, M.; Schmucki, R.; Georges, G.; Singer, T.; Kurome, M.; et al. A Humanized Minipig Model for the Toxicological Testing of Therapeutic Recombinant Antibodies. Nat. Biomed. Eng. 2022, 6, 1248–1256. [Google Scholar] [CrossRef]
- Brenner, G.B.; Giricz, Z.; Garamvölgyi, R.; Makkos, A.; Onódi, Z.; Sayour, N.V.; Gergely, T.G.; Baranyai, T.; Petneházy, Ö.; Kőrösi, D.; et al. Post-Myocardial Infarction Heart Failure in Closed-Chest Coronary Occlusion/Reperfusion Model in Göttingen Minipigs and Landrace Pigs. J. Vis. Exp. 2021, 170, e61901. [Google Scholar] [CrossRef]
- Miyagawa, S.; Mizoguchi, H.; Fukushima, S.; Imanishi, Y.; Watabe, T.; Harada, A.; Sakai, Y.; Sawa, Y. New Regional Drug Delivery System by Direct Epicardial Placement of Slow-Release Prostacyclin Agonist Promise Therapeutic Angiogenesis in a Porcine Chronic Myocardial Infarction. J. Artif. Organs 2021, 24, 465–472. [Google Scholar] [CrossRef]
- Atiq, F.; Van de Wouw, J.; Sorop, O.; Heinonen, I.; De Maat, M.P.M.; Merkus, D.; Duncker, D.J.; Leebeek, F.W.G. Endothelial Dysfunction, Atherosclerosis, and Increase of von Willebrand Factor and Factor VIII: A Randomized Controlled Trial in Swine. Thromb. Haemost. 2021, 121, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Emerging Role of Precision Medicine in Cardiovascular Disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, C.; Zannoni, A.; Turba, M.E.; Fantinati, P.; Tamanini, C.; Bacci, M.L.; Forni, M. Heat Shock Protein 70, Heat Shock Protein 32, and Vascular Endothelial Growth Factor Production and Their Effects on Lipopolysaccharide-Induced Apoptosis in Porcine Aortic Endothelial Cells. Cell Stress. Chaperones 2005, 10, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, C.; La Mantia, D.; Salaroli, R.; Zannoni, A.; Nauwelaerts, N.; Deferm, N.; Ventrella, D.; Bacci, M.L.; Sarli, G.; Bouisset, M.; et al. Development of a Pig Mammary Epithelial Cell Culture Model as a Non-Clinical Tool for Studying Epithelial Barrier—A Contribution from the IMI-ConcePTION Project. Animals 2021, 21, 2012. [Google Scholar] [CrossRef]
- Cesarz, Z.; Tamama, K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016, 2016, 9176357. [Google Scholar] [CrossRef]
- Segal, J.B.; McNamara, R.L.; Miller, M.R.; Kim, N.; Goodman, S.N.; Powe, N.R.; Robinson, K.; Yu, D.; Bass, E.B. The Evidence Regarding the Drugs Used for Ventricular Rate Control. J. Fam. Pract. 2000, 49, 47–59. [Google Scholar]
- Popov, A.F.; Dorge, H.; Hinz, J.; Schmitto, J.D.; Stojanovic, T.; Seipelt, R.; Didilis, V.; Schoendube, F.A. Accelerated Intimal Hyperplasia in Aortocoronary Internal Mammary Vein Grafts in Minipigs. J. Cardiothorac. Surg. 2008, 3, 20. [Google Scholar] [CrossRef]
- Schuleri, K.H.; Boyle, A.J.; Centola, M.; Amado, L.C.; Evers, R.; Zimmet, J.M.; Evers, K.S.; Ostbye, K.M.; Scorpio, D.G.; Hare, J.M.; et al. The Adult Göttingen Minipig as a Model for Chronic Heart Failure after Myocardial Infarction: Focus on Cardiovascular Imaging and Regenerative Therapies. Comp. Med. 2008, 58, 568–579. [Google Scholar]
- Roh, J.; Hill, J.A.; Singh, A.; Valero-Muñoz, M.; Sam, F. Heart Failure With Preserved Ejection Fraction: Heterogeneous Syndrome, Diverse Preclinical Models. Circ. Res. 2022, 130, 1906–1925. [Google Scholar] [CrossRef]
- Correia, C.; Wang, Q.-D.; Linhardt, G.; Carlsson, L.G.; Ulfenborg, B.; Walentinsson, A.; Rydén-Markinhutha, K.; Behrendt, M.; Wikström, J.; Sartipy, P.; et al. Unraveling the Metabolic Derangements Occurring in Non-Infarcted Areas of Pig Hearts With Chronic Heart Failure. Front. Cardiovasc. Med. 2021, 8, 753470. [Google Scholar] [CrossRef]
- Jacobsson, L. Comparison of Experimental Hypercholesterolemia and Atherosclerosis in Male and Female Mini-Pigs of the Göttingen Strain. Artery 1989, 16, 105–117. [Google Scholar] [PubMed]
- Manno, R.A.; Grassetti, A.; Oberto, G.; Nyska, A.; Ramot, Y. The Minipig as a New Model for the Evaluation of Doxorubicin-Induced Chronic Toxicity. J. Appl. Toxicol. 2016, 36, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsen, T.P.; Pedersen, S.F.; Vegge, A.; Ripa, R.S.; Johannesen, H.H.; Hansen, A.E.; Löfgren, J.; Schumacher-Petersen, C.; Kirk, R.K.; Pedersen, H.D.; et al. 18F-FDG PET/MR-Imaging in a Göttingen Minipig Model of Atherosclerosis: Correlations with Histology and Quantitative Gene Expression. Atherosclerosis 2019, 285, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Duvivier, V.; Creusot, S.; Broux, O.; Helbert, A.; Lesage, L.; Moreau, K.; Lesueur, N.; Gerard, L.; Lemaitre, K.; Provost, N.; et al. Characterization and Pharmacological Validation of a Preclinical Model of NASH in Göttingen Minipigs. J. Clin. Exp. Hepatol. 2022, 12, 293–305. [Google Scholar] [CrossRef]
- Johansen, T.; Hansen, H.S.; Richelsen, B.; Malmlöf, R. The Obese Göttingen Minipig as a Model of the Metabolic Syndrome: Dietary Effects on Obesity, Insulin Sensitivity, and Growth Hormone Profile. Comp. Med. 2001, 51, 150–155. [Google Scholar]
- Ludvigsen, T.P.; Kirk, R.K.; Christoffersen, B.Ø.; Pedersen, H.D.; Martinussen, T.; Kildegaard, J.; Heegaard, P.M.H.; Lykkesfeldt, J.; Olsen, L.H. Göttingen Minipig Model of Diet-Induced Atherosclerosis: Influence of Mild Streptozotocin-Induced Diabetes on Lesion Severity and Markers of Inflammation Evaluated in Obese, Obese and Diabetic, and Lean Control Animals. J. Transl. Med. 2015, 13, 312. [Google Scholar] [CrossRef]
- Caplan, A.I. Why Are MSCs Therapeutic? New Data: New Insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef]
- Bosch, P.; Pratt, S.L.; Stice, S.L. Isolation, Characterization, Gene Modification, and Nuclear Reprogramming of Porcine Mesenchymal Stem Cells. Biol. Reprod. 2006, 74, 46–57. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Q.; Wang, Z.; Xu, C.; Wang, X.; Gong, G.; Mansoor, A.; Lee, J.; Hou, M.; Zeng, L.; et al. Autologous Stem Cell Transplantation for Myocardial Repair. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H501–H511. [Google Scholar] [CrossRef]
- Jin, H.-F.; Kumar, B.M.; Kim, J.-G.; Song, H.-J.; Jeong, Y.-J.; Cho, S.-K.; Balasubramanian, S.; Choe, S.-Y.; Rho, G.-J. Enhanced Development of Porcine Embryos Cloned from Bone Marrow Mesenchymal Stem Cells. Int. J. Dev. Biol. 2007, 51, 85–90. [Google Scholar] [CrossRef]
- Mitchell, K.E.; Weiss, M.L.; Mitchell, B.M.; Martin, P.; Davis, D.; Morales, L.; Helwig, B.; Beerenstrauch, M.; Abou-Easa, K.; Hildreth, T.; et al. Matrix Cells from Wharton’s Jelly Form Neurons and Glia. Stem Cells 2003, 21, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Carlin, R.; Davis, D.; Weiss, M.; Schultz, B.; Troyer, D. Expression of Early Transcription Factors Oct-4, Sox-2 and Nanog by Porcine Umbilical Cord (PUC) Matrix Cells. Reprod. Biol. Endocrinol. 2006, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Faast, R.; Harrison, S.J.; Beebe, L.F.S.; McIlfatrick, S.M.; Ashman, R.J.; Nottle, M.B. Use of Adult Mesenchymal Stem Cells Isolated from Bone Marrow and Blood for Somatic Cell Nuclear Transfer in Pigs. Cloning Stem Cells 2006, 8, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sartore, S.; Lenzi, M.; Angelini, A.; Chiavegato, A.; Gasparotto, L.; De Coppi, P.; Bianco, R.; Gerosa, G. Amniotic Mesenchymal Cells Autotransplanted in a Porcine Model of Cardiac Ischemia Do Not Differentiate to Cardiogenic Phenotypes. Eur. J. Cardiothorac. Surg. 2005, 28, 677–684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumar, B.M.; Yoo, J.-G.; Ock, S.-A.; Kim, J.-G.; Song, H.-J.; Kang, E.-J.; Cho, S.-K.; Lee, S.-L.; Cho, J.-H.; Balasubramanian, S.; et al. In Vitro Differentiation of Mesenchymal Progenitor Cells Derived from Porcine Umbilical Cord Blood. Mol. Cells 2007, 24, 343–350. [Google Scholar] [PubMed]
- Huang, T.; He, D.; Kleiner, G.; Kuluz, J. Neuron-like Differentiation of Adipose-Derived Stem Cells from Infant Piglets in Vitro. J. Spinal Cord. Med. 2007, 30 (Suppl. S1), S35–S40. [Google Scholar] [CrossRef]
- Agay, D.; Scherthan, H.; Forcheron, F.; Grenier, N.; Hérodin, F.; Meineke, V.; Drouet, M. Multipotent Mesenchymal Stem Cell Grafting to Treat Cutaneous Radiation Syndrome: Development of a New Minipig Model. Exp. Hematol. 2010, 38, 945–956. [Google Scholar] [CrossRef]
- Hanson, S.E.; Kleinbeck, K.R.; Cantu, D.; Kim, J.; Bentz, M.L.; Faucher, L.D.; Kao, W.J.; Hematti, P. Local Delivery of Allogeneic Bone Marrow and Adipose Tissue-Derived Mesenchymal Stromal Cells for Cutaneous Wound Healing in a Porcine Model. J. Tissue Eng. Regen. Med. 2016, 10, E90–E100. [Google Scholar] [CrossRef]
- Mohsin, S.; Troupes, C.D.; Starosta, T.; Sharp, T.E.; Agra, E.J.; Smith, S.; Duran, J.M.; Zalavadia, N.; Zhou, Y.; Kubo, H.; et al. Unique Features of Cortical Bone Stem Cells Associated With Repair of the Injured Heart. Circ. Res. 2015, 117, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Luu, N.T.; McGettrick, H.M.; Buckley, C.D.; Newsome, P.N.; Rainger, G.E.; Frampton, J.; Nash, G.B. Crosstalk between Mesenchymal Stem Cells and Endothelial Cells Leads to Downregulation of Cytokine-Induced Leukocyte Recruitment. Stem Cells 2013, 31, 2690–2702. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Yet, S.-F.; Hsu, Y.-T.; Wang, G.-J.; Hung, S.-C. Mesenchymal Stem Cells Ameliorate Atherosclerotic Lesions via Restoring Endothelial Function. Stem Cells Transl. Med. 2015, 4, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Vorwald, C.E.; Joshee, S.; Leach, J.K. Spatial Localization of Endothelial Cells in Heterotypic Spheroids Influences Notch Signaling. J. Mol. Med. 2020, 98, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Bernardini, C.; Zannoni, A.; Bertocchi, M.; Tubon, I.; Fernandez, M.; Forni, M. Water/Ethanol Extract of Cucumis sativus L. Fruit Attenuates Lipopolysaccharide-Induced Inflammatory Response in Endothelial Cells. BMC Complement. Altern. Med. 2018, 18, 194. [Google Scholar] [CrossRef]



| Antibody | P. Number | Species | Supplier | Dilution |
|---|---|---|---|---|
| Primary | ||||
| CD45-APC | K252-1E4 | mouse | AbD Serotec | 10 µL/106 cells |
| CD90-APC | Ab139364 | mouse | Abcam | 10 µL/106 cells |
| CD105-FITC | Ab53318 | mouse | Abcam | 20 µL/106 cells |
| CD56-PE | 304606 | mouse | Biolegend | 10 µL/106 cells |
| CD44-PerPC | 103036 | rat | Biolegend | 10 µL/106 cells |
| CD34 unconjugated | Ab81289 | rabbit | Abcam | 1:60 |
| CD31-unconjugated | MCA1746 | mouse | AbD Serotec | 1:50 |
| VE-Cadherin | MCA1748GA | rat | AbD Serotec | 1:25 |
| Secondary | ||||
| Anti-Rabbit-PE | Ab97070 | goat | Abcam | 1:200 |
| Anti-Mouse-FITC | F4143 | goat | Sigma-Aldrich | 1:100 |
| Anti-rat-FITC | F1763 | rabbit | Sigma-Aldrich | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardini, C.; Mantia, D.L.; Salaroli, R.; Ventrella, D.; Elmi, A.; Zannoni, A.; Forni, M. Isolation of Vascular Wall Mesenchymal Stem Cells from the Thoracic Aorta of Adult Göttingen Minipigs: A New Protocol for the Simultaneous Endothelial Cell Collection. Animals 2023, 13, 2601. https://doi.org/10.3390/ani13162601
Bernardini C, Mantia DL, Salaroli R, Ventrella D, Elmi A, Zannoni A, Forni M. Isolation of Vascular Wall Mesenchymal Stem Cells from the Thoracic Aorta of Adult Göttingen Minipigs: A New Protocol for the Simultaneous Endothelial Cell Collection. Animals. 2023; 13(16):2601. https://doi.org/10.3390/ani13162601
Chicago/Turabian StyleBernardini, Chiara, Debora La Mantia, Roberta Salaroli, Domenico Ventrella, Alberto Elmi, Augusta Zannoni, and Monica Forni. 2023. "Isolation of Vascular Wall Mesenchymal Stem Cells from the Thoracic Aorta of Adult Göttingen Minipigs: A New Protocol for the Simultaneous Endothelial Cell Collection" Animals 13, no. 16: 2601. https://doi.org/10.3390/ani13162601
APA StyleBernardini, C., Mantia, D. L., Salaroli, R., Ventrella, D., Elmi, A., Zannoni, A., & Forni, M. (2023). Isolation of Vascular Wall Mesenchymal Stem Cells from the Thoracic Aorta of Adult Göttingen Minipigs: A New Protocol for the Simultaneous Endothelial Cell Collection. Animals, 13(16), 2601. https://doi.org/10.3390/ani13162601









