Journal Description
Journal of Developmental Biology
Journal of Developmental Biology
is an international, peer-reviewed, open access journal on the development of multicellular organisms at the molecule, cell, tissue, organ and whole organism levels published quarterly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, ESCI (Web of Science), PubMed, PMC, PubAg, CAPlus / SciFinder, and other databases.
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 26.2 days after submission; acceptance to publication is undertaken in 5.9 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Testimonials: See what our editors and authors say about Journal of Developmental Biology.
Impact Factor:
2.5 (2024);
5-Year Impact Factor:
2.8 (2024)
Latest Articles
Exploring the Regulation of Tmem182 Gene Expression in the Context of Retinoid X Receptor Signaling
J. Dev. Biol. 2025, 13(4), 34; https://doi.org/10.3390/jdb13040034 - 24 Sep 2025
Abstract
►
Show Figures
We have previously established that bexarotene, a clinically approved agonist of retinoid X receptor (RXR), promotes the differentiation and fusion of skeletal myoblasts. We have also analyzed the genomic programs underlying rexinoid-enhanced myogenic differentiation to identify novel regulatory pathways. As such, we observed
[...] Read more.
We have previously established that bexarotene, a clinically approved agonist of retinoid X receptor (RXR), promotes the differentiation and fusion of skeletal myoblasts. We have also analyzed the genomic programs underlying rexinoid-enhanced myogenic differentiation to identify novel regulatory pathways. As such, we observed a significant upregulation of a transcript encoding a predicted transmembrane protein, Tmem182, during C2C12 myoblast differentiation. Despite the documentation of Tmem182 expression in skeletal muscles, its regulation had yet to be explored. Here, we show that Tmem182 gene expression is markedly augmented in early myoblast differentiation and further enhanced by RXR signaling. In addition, Tmem182 expression is specific to muscle tissues and related to muscle master regulator MyoD. We found that MyoD and histone acetyltransferase p300 are bound to the Tmem182 promoter, and Tmem182 expression is p300-dependent. Thus, our data display a putative epigenetic signature associated with p300 and histone acetylation in rexinoid-responsive locus activation and transcription of myogenic targets.
Full article
Open AccessReview
Signaling Pathways in Human Blastocyst Development: From Molecular Mechanisms to In Vitro Optimization
by
Yan Jiao, Jiapeng Liu, Congge Li, Yuexin Hu and Sanjun Zhao
J. Dev. Biol. 2025, 13(3), 33; https://doi.org/10.3390/jdb13030033 - 9 Sep 2025
Abstract
In recent years, assisted reproductive technology (ART) has developed rapidly with the delay in reproductive age and the rise in infertility rates. During ART, blastocyst quality is a key factor affecting the rate of implantation and clinical pregnancy, and blastocyst formation is dependent
[...] Read more.
In recent years, assisted reproductive technology (ART) has developed rapidly with the delay in reproductive age and the rise in infertility rates. During ART, blastocyst quality is a key factor affecting the rate of implantation and clinical pregnancy, and blastocyst formation is dependent on the precise regulation of multiple signaling pathways in preimplantation embryo development. In this review, we systematically analyze the molecular mechanisms of the core pathways, including Hippo, Wnt/β-catenin, FGF, Nodal, and BMP, in blastocyst lineage differentiation and morphogenesis, and assess the feasibility of optimizing in vitro culture by targeting key signaling nodes, as well as provide theoretical support for constructing research models of preimplantation embryos.
Full article
(This article belongs to the Collection Hedgehog Signaling in Embryogenesis)
►▼
Show Figures

Figure 1
Open AccessArticle
Zebrafish Unga Is Required for Genomic Maintenance upon Genotoxic Stress and Male Fertility
by
Latifa Kazzazy, Flóra Huba, Bálint Lóránt Hausz, Dávid Mező, Viktória Perey-Simon, Bálint Jezsó, Abdulrahman Seddik, Zoran Marinović, Judit Tóth, Angéla Békési, Beáta G. Vértessy and Máté Varga
J. Dev. Biol. 2025, 13(3), 32; https://doi.org/10.3390/jdb13030032 - 2 Sep 2025
Abstract
DNA repair is a multifaceted biological process that involves multiple pathways to counter the types of damage the genome encounters throughout life. In the past decade zebrafish became a popular model organism to study various aspects of vertebrate DNA repair, and the characterization
[...] Read more.
DNA repair is a multifaceted biological process that involves multiple pathways to counter the types of damage the genome encounters throughout life. In the past decade zebrafish became a popular model organism to study various aspects of vertebrate DNA repair, and the characterization of several mutant lines deficient in key players of the repair pathways has significantly contributed to our understanding of the roles the corresponding proteins play in the maintenance of genomic integrity. Interestingly, the base-excision repair (BER) pathway remained one of the less characterized DNA repair processes in fish. Here we provide a detailed characterization of zebrafish deficient in one of the key components of BER, the uracil-DNA glycosylase Unga. We show that while these fish are viable, they display an altered response to genotoxic stress and unga mutant males show an interesting form of subfertility.
Full article
(This article belongs to the Special Issue Zebrafish—a Model System for Developmental Biology Study III)
►▼
Show Figures

Figure 1
Open AccessReview
Profilin and Non-Canonical Wnt Signaling: Coordinating Cytoskeletal Dynamics from Development to Disease
by
Samira Alam, Danielle Duncan and Sharmin Hasan
J. Dev. Biol. 2025, 13(3), 31; https://doi.org/10.3390/jdb13030031 - 1 Sep 2025
Abstract
►▼
Show Figures
Vertebrate embryonic development relies on tightly regulated signaling pathways that guide morphogenesis, cell fate specification, and tissue organization. Among these, the Wnt signaling pathway plays a central role, orchestrating key developmental events. The non-canonical Wnt pathways, including the Planar Cell Polarity and Wnt/Ca
[...] Read more.
Vertebrate embryonic development relies on tightly regulated signaling pathways that guide morphogenesis, cell fate specification, and tissue organization. Among these, the Wnt signaling pathway plays a central role, orchestrating key developmental events. The non-canonical Wnt pathways, including the Planar Cell Polarity and Wnt/Ca2+ branches, are especially critical for regulating cytoskeletal dynamics during gastrulation. Recent studies highlight that these pathways interface with cytoskeletal effectors to control actin remodeling in response to extracellular cues. One such effector is Profilin, a small, evolutionarily conserved actin-binding protein that modulates actin polymerization and cellular architecture. Profilins, particularly Profilin1 and 2, are known to interact with Daam1, a formin protein downstream of PCP signaling, thereby linking Wnt signals to actin cytoskeletal regulation. Emerging evidence suggests that Profilins are active signaling intermediates that contribute to morphogenetic processes. Their context-dependent interactions and differential expression across species also suggest that they play specialized roles in development and disease. This review synthesizes the current understanding of Profilin’s role in non-canonical Wnt signaling, examining its molecular interactions and contributions to cytoskeletal control during development. By integrating data across model systems, we aim to clarify how Profilins function at the intersection of signaling and cytoskeletal dynamics, with implications for both developmental biology and disease pathogenesis.
Full article

Figure 1
Open AccessArticle
Drosophila COMPASS Complex Subunits Set1 and Ash2 Are Required for Oocyte Determination and Maintenance of the Synaptonemal Complex
by
Brigite Cabrita, Mary Enyioko and Rui Gonçalo Martinho
J. Dev. Biol. 2025, 13(3), 30; https://doi.org/10.3390/jdb13030030 - 19 Aug 2025
Abstract
Female gametogenesis is orchestrated by dynamic epigenetic modifications. In mammals, SETDB1, a histone H3K9 methyltransferase, is required for proper meiotic progression and early embryonic development. In Drosophila, the ortholog of SETDB1 plays a critical role in germ cell differentiation, transposon silencing, and
[...] Read more.
Female gametogenesis is orchestrated by dynamic epigenetic modifications. In mammals, SETDB1, a histone H3K9 methyltransferase, is required for proper meiotic progression and early embryonic development. In Drosophila, the ortholog of SETDB1 plays a critical role in germ cell differentiation, transposon silencing, and the transcriptional repression of specific germline genes during oocyte fate determination. Moreover, Polycomb group (PcG) proteins in both mammals and Drosophila are essential for primary oocyte viability and meiosis, functioning through the silencing of early prophase I genes during later stages of prophase. While the repressive roles of epigenetic regulators in both Drosophila and mammalian oogenesis are well characterized, the functions of epigenetic activators remain less defined. Gene expression is controlled by the opposing activities of PcG and Trithorax group (TrxG) proteins, with the latter constituting a diverse family of chromatin remodelling factors that include H3K4 methyltransferases. In Drosophila, SET domain containing 1 (Set1)—the ortholog of mammalian SETD1A/B—acts as the primary regulator of global H3K4me2/3 levels. Set1 is critical for germline stem cell (GSC) self-renewal, functioning through both cell-autonomous and non-cell-autonomous mechanisms, with its depletion in the germline resulting in a progressive loss of GSC. More recently, Set1 has been implicated in germline cyst differentiation, although the mechanisms underlying this role remain poorly understood due to the complexity of the observed phenotypes. To investigate this, we analyzed ovaries from recently eclosed females in which Set1 and its highly conserved COMPASS partner, absent, small, or homeotic discs 2 (Ash2), were depleted—thus minimizing the confounding effects from GSC loss. We observed striking defects in both oocyte determination and Synaptonemal Complex (SC) integrity in one- to two-day-old females, within otherwise normal egg chambers. Interestingly, while defects in oocyte fate and oocyte–chromatin architecture were partially recovered in older egg chambers, SC integrity remained compromised. These findings suggest a critical window for SC assembly during germline cyst differentiation, after which this assembly cannot occur.
Full article
(This article belongs to the Special Issue Feature Papers in Journal of Developmental Biology 2025)
►▼
Show Figures

Figure 1
Open AccessArticle
GLP-1-Mediated Pregnancy and Neonatal Complications in Mice
by
Rajalakshmi Ramamoorthy, Arianna K. Carden, Hussain Hussain, Brian Z. Druyan, Ping Ping Chen, Rima Hajjar, Carmen Fernandez, Nila Elumalai, Amirah B. Rashed, Karen Young, Anna Rosa Speciale, Emily M. West, Staci Marbin, Bradley Safro, Ian J. Bishop, Arumugam R. Jayakumar, Luis Sanchez-Ramos and Michael J. Paidas
J. Dev. Biol. 2025, 13(3), 29; https://doi.org/10.3390/jdb13030029 - 15 Aug 2025
Abstract
Glucagon-like peptide 1 (GLP-1), a hormone derived from the proglucagon gene, regulates various physiological processes; however, its impact on pregnancy outcomes remains poorly understood. Assessing the effects of GLP-1 on neonates is vital as GLP-1 is increasingly administered during pregnancy. This study evaluates
[...] Read more.
Glucagon-like peptide 1 (GLP-1), a hormone derived from the proglucagon gene, regulates various physiological processes; however, its impact on pregnancy outcomes remains poorly understood. Assessing the effects of GLP-1 on neonates is vital as GLP-1 is increasingly administered during pregnancy. This study evaluates the effect of GLP-1 exposure on maternal complications and neonatal defects in mice. Pregnant female A/J mice received subcutaneous injections of recombinant GLP-1 (rGLP-1; 1000 nmol/kg) on embryonic day 1 (EP, early pregnancy) or day 15 (E15, late pregnancy). Maternal and neonatal body weights, morphology, and mortality were recorded, and mRNA sequencing was conducted to analyze gene expression in neonatal tissues. Maternal body weight decreased following rGLP-1 exposure, and pups born to both the early and late exposure groups experienced significant weight loss. Pups in the late exposure group exhibited uniform skin detachment and a dramatically higher mortality rate than those born to the early exposure group. Further, RT-PCR analysis confirms the significantly increased expression of selected genes in the skin and associated pathogenesis. RNA sequencing of pups’ skin, brain, lung, and liver tissues from the late exposure group showed altered gene expression. Since maternal weight loss, increased neonatal mortality, and altered gene expression have been observed, GLP-1 receptor agonists (GLP-1RAs) should be avoided during pregnancy.
Full article
(This article belongs to the Special Issue Embryonic Development and Regenerative Medicine)
►▼
Show Figures

Figure 1
Open AccessReview
The Congenital Malformation of the Interatrial Septum—A Review of Its Development and Embryology with Clinical Implications
by
Rui Caetano Oliveira, Paula Martins and Maria de Fátima Martins
J. Dev. Biol. 2025, 13(3), 28; https://doi.org/10.3390/jdb13030028 - 5 Aug 2025
Abstract
►▼
Show Figures
The development process of the heart and cardiovascular system is fundamental in human development and highly regulated by genetic factors. This process needs to be highly regulated to prevent malformations. Nevertheless, some heart defects may be identified, especially with modern imaging methodology. Atrial
[...] Read more.
The development process of the heart and cardiovascular system is fundamental in human development and highly regulated by genetic factors. This process needs to be highly regulated to prevent malformations. Nevertheless, some heart defects may be identified, especially with modern imaging methodology. Atrial septal defects (ASDs) are particularly common. Understanding the mechanisms involved in ASD formation is fundamental for developing new treatment strategies. In this article, we explore cardiac development and embryology, with a focus on atrial septal defects and their clinical implications.
Full article

Figure 1
Open AccessArticle
Evolution of the Jawed Vertebrate (Gnathostomata) Stomach Through Gene Repertoire Loss: Findings from Agastric Species
by
Jackson Dann and Frank Grützner
J. Dev. Biol. 2025, 13(3), 27; https://doi.org/10.3390/jdb13030027 - 5 Aug 2025
Abstract
►▼
Show Figures
The stomach has been a highly conserved organ throughout vertebrate evolution; however, there are now over 20 lineages composed of monotremes, lungfish and teleost fish displaying a secondary loss of stomach function and morphology. This “agastric phenotype” has evolved convergently and is typified
[...] Read more.
The stomach has been a highly conserved organ throughout vertebrate evolution; however, there are now over 20 lineages composed of monotremes, lungfish and teleost fish displaying a secondary loss of stomach function and morphology. This “agastric phenotype” has evolved convergently and is typified by a loss of gastric glands and gastric acid secretion and a near-to-complete loss of storage capacity of the stomach. All agastric species have lost the genes for gastric enzymes (Pga and Pgc) and proton pump subunits (Atp4a and Atp4b), and gastrin (Gast) has been lost in monotremes. As a key gastric hormone, the conservation of gastrin has not yet been investigated in the lungfish or agastric teleosts, and it is unclear how the loss of gastrin affects the evolution and selection of the native receptor (Cckbr), gastrin-releasing peptide (Grp) and gastrin-releasing peptide receptor (Grpr) in vertebrates. Furthermore, there are still many genes implicated in gastric development and function which have yet to be associated with the agastric phenotype. We analysed the evolution, selection and conservation of the gastrin pathway and a novel gastric gene repertoire (Gkn1, Gkn2, Tff1, Tff2, Vsig1 and Anxa10) to determine the correlation with the agastric phenotype. We found that the loss of gastrin or its associated genes does not correlate with the agastric phenotype, and their conservation is due to multiple pleiotropic roles throughout vertebrate evolution. We found a loss of the gastric gene repertoire in the agastric phenotype, except in the echidna, which retained several genes (Gkn1, Tff2 and Vsig1). Our findings suggest that the gastrin physiological pathway evolved differently in pleiotropic roles throughout vertebrate evolution and support the convergent evolution of the agastric phenotype through shared independent gene-loss events.
Full article

Figure 1
Open AccessArticle
Identification and Characterization of Static Craniofacial Defects in Pre-Metamorphic Xenopus laevis Tadpoles
by
Emilie Jones, Jay Miguel Fonticella and Kelly A. McLaughlin
J. Dev. Biol. 2025, 13(3), 26; https://doi.org/10.3390/jdb13030026 - 25 Jul 2025
Abstract
►▼
Show Figures
Craniofacial development is a complex, highly conserved process involving multiple tissue types and molecular pathways, with perturbations resulting in congenital defects that often require invasive surgical interventions to correct. Remarkably, some species, such as Xenopus laevis, can correct some craniofacial abnormalities during
[...] Read more.
Craniofacial development is a complex, highly conserved process involving multiple tissue types and molecular pathways, with perturbations resulting in congenital defects that often require invasive surgical interventions to correct. Remarkably, some species, such as Xenopus laevis, can correct some craniofacial abnormalities during pre-metamorphic stages through thyroid hormone-independent mechanisms. However, the full scope of factors mediating remodeling initiation and coordination remain unclear. This study explores the differential remodeling responses of craniofacial defects by comparing the effects of two pharmacological agents, thioridazine-hydrochloride (thio) and ivermectin (IVM), on craniofacial morphology in X. laevis. Thio-exposure reliably induces a craniofacial defect that can remodel in pre-metamorphic animals, while IVM induces a permanent, non-correcting phenotype. We examined developmental changes from feeding stages to hindlimb bud stages and mapped the effects of each agent on the patterning of craniofacial tissue types including: cartilage, muscle, and nerves. Our findings reveal that thio-induced craniofacial defects exhibit significant consistent remodeling, particularly in muscle, with gene expression analysis revealing upregulation of key remodeling genes, matrix metalloproteinases 1 and 13, as well as their regulator, prolactin.2. In contrast, IVM-induced defects show no significant remodeling, highlighting the importance of specific molecular and cellular factors in pre-metamorphic craniofacial correction. Additionally, unique neuronal profiles suggest a previously underappreciated role for the nervous system in tissue remodeling. This study provides novel insights into the molecular and cellular mechanisms underlying craniofacial defect remodeling and lays the groundwork for future investigations into tissue repair in vertebrates.
Full article

Figure 1
Open AccessArticle
Deletion of Ptpmt1 by αMHC-Cre in Mice Results in Left Ventricular Non-Compaction
by
Lei Huang, Maowu Cao, Xiangbin Zhu, Na Li, Can Huang, Kunfu Ouyang and Ze'e Chen
J. Dev. Biol. 2025, 13(3), 25; https://doi.org/10.3390/jdb13030025 - 18 Jul 2025
Abstract
►▼
Show Figures
Background: Left ventricular non-compaction cardiomyopathy (LVNC) is a congenital heart disease characterized by abnormal prenatal development of the left ventricle that has an aberrantly thick trabecular layer and a thinner compacted myocardial layer. However, the underlying molecular mechanisms of LVNC regulated by mitochondrial
[...] Read more.
Background: Left ventricular non-compaction cardiomyopathy (LVNC) is a congenital heart disease characterized by abnormal prenatal development of the left ventricle that has an aberrantly thick trabecular layer and a thinner compacted myocardial layer. However, the underlying molecular mechanisms of LVNC regulated by mitochondrial phosphatase genes remain largely unresolved. Methods: We generated a mouse model with cardiac-specific deletion (CKO) of Ptpmt1, a type of mitochondrial phosphatase gene, using the αMHC-Cre, and investigated the effects of cardiac-specific Ptpmt1 deficiency on cardiac development. Morphological, histological, and immunofluorescent analyses were conducted in Ptpmt1 CKO and littermate controls. A transcriptional atlas was identified by RNA sequencing (RNA-seq) analysis. Results: We found that CKO mice were born at the Mendelian ratio with normal body weights. However, most of the CKO mice died within 24 h after birth, developing spontaneous ventricular tachycardia. Morphological and histological analysis further revealed that newborn CKO mice developed an LVNC phenotype, evidenced by a thicker trabecular layer and a thinner myocardium layer, when compared with the littermate control. We then examined the embryonic hearts and found that such an LVNC phenotype could also be observed in CKO hearts at E15.5 but not at E13.5. We also performed the EdU incorporation assay and demonstrated that cardiac cell proliferation in both myocardium and trabecular layers was significantly reduced in CKO hearts at E15.5, which is also consistent with the dysregulation of genes associated with heart development and cardiomyocyte proliferation in CKO hearts at the same stage, as revealed by both the transcriptome analysis and the quantitative real-time PCR. Deletion of Ptpmt1 in mouse cardiomyocytes also induced an increase in phosphorylated eIF2α and ATF4 levels, indicating a mitochondrial stress response in CKO hearts. Conclusions: Our results demonstrated that Ptpmt1 may play an essential role in regulating left ventricular compaction during mouse heart development.
Full article

Figure 1
Open AccessReview
Is Hydra Axis Definition a Fluctuation-Based Process Picking Up External Cues?
by
Mikhail A. Zhukovsky, Si-Eun Sung and Albrecht Ott
J. Dev. Biol. 2025, 13(3), 24; https://doi.org/10.3390/jdb13030024 - 17 Jul 2025
Abstract
Axis definition plays a key role in the establishment of animal body plans, both in normal development and regeneration. The cnidarian Hydra can re-establish its simple body plan when regenerating from a random cell aggregate or a sufficiently small tissue fragment. At the
[...] Read more.
Axis definition plays a key role in the establishment of animal body plans, both in normal development and regeneration. The cnidarian Hydra can re-establish its simple body plan when regenerating from a random cell aggregate or a sufficiently small tissue fragment. At the beginning of regeneration, a hollow cellular spheroid forms, which then undergoes symmetry breaking and de novo body axis definition. In the past, we have published related work in a physics journal, which is difficult to read for scientists from other disciplines. Here, we review our work for readers not so familiar with this type of approach at a level that requires very little knowledge in mathematics. At the same time, we present a few aspects of Hydra biology that we believe to be linked to our work. These biological aspects may be of interest to physicists or members of related disciplines to better understand our approach. The proposed theoretical model is based on fluctuations of gene expression that are triggered by mechanical signaling, leading to increasingly large groups of cells acting in sync. With a single free parameter, the model quantitatively reproduces the experimentally observed expression pattern of the gene ks1, a marker for ‘head forming potential’. We observed that Hydra positions its axis as a function of a weak temperature gradient, but in a non-intuitive way. Supposing that a large fluctuation including ks1 expression is locked to define the head position, the model reproduces this behavior as well—without further changes. We explain why we believe that the proposed fluctuation-based symmetry breaking process agrees well with recent experimental findings where actin filament organization or anisotropic mechanical stimulation act as axis-positioning events. The model suggests that the Hydra spheroid exhibits huge sensitivity to external perturbations that will eventually position the axis.
Full article
(This article belongs to the Special Issue Feature Papers in Journal of Developmental Biology 2025)
►▼
Show Figures

Figure 1
Open AccessArticle
HP1-Mediated Silencing of the Doublesex1 Gene for Female Determination in the Crustacean Daphnia magna
by
Junya Leim, Nikko Adhitama, Quang Dang Nong, Pijar Religia, Yasuhiko Kato and Hajime Watanabe
J. Dev. Biol. 2025, 13(3), 23; https://doi.org/10.3390/jdb13030023 - 3 Jul 2025
Abstract
►▼
Show Figures
The crustacean Daphnia magna produces genetically identical females and males by parthenogenesis. Males are produced in response to environmental cues including crowding and lack of food. For male development, the DM-domain containing transcription factor Doublesex1 (DSX1) is expressed spatiotemporally in male-specific traits and
[...] Read more.
The crustacean Daphnia magna produces genetically identical females and males by parthenogenesis. Males are produced in response to environmental cues including crowding and lack of food. For male development, the DM-domain containing transcription factor Doublesex1 (DSX1) is expressed spatiotemporally in male-specific traits and orchestrates male trait formation in both somatic and gonadal tissues. However, it remains unknown how the dsx1 gene is silenced in females to avoid male trait development. Heterochromatin Protein 1 (HP1) plays a crucial role in epigenetic gene silencing during developmental processes. Here we report the identification of four HP1 orthologs in D. magna. None of these orthologs exhibited sexually dimorphic expression, and among them, HP1-1 was most abundantly expressed during embryogenesis. The knock-down of HP1-1 in female embryos led to the derepression of dsx1 in the male-specific traits, resulting in the development of male characteristics, such as the elongation of the first antennae. These results suggest that HP1-1 silences dsx1 for female development while environmental cues unlock this silencing to induce male production. We infer the HP1-dependent formation of a sex-specific chromatin structure on the dsx1 locus is a key process in the environmental sex determination of D. magna.
Full article

Figure 1
Open AccessArticle
Investigating Psychopharmaceutical Effects on Early Vertebrate Development Using a Zebrafish Model System
by
Nathan Zimmerman, Aaron Marta, Carly Baker, Zeljka Korade, Károly Mirnics and Annemarie Shibata
J. Dev. Biol. 2025, 13(3), 22; https://doi.org/10.3390/jdb13030022 - 27 Jun 2025
Abstract
►▼
Show Figures
Cholesterol homeostasis is necessary for normal vertebrate development. The disruption of cholesterol homeostasis can cause abnormal body and nervous system development and lead to dysfunctional behavior and increased mortality. Commonly prescribed psychopharmaceuticals can alter cholesterol synthesis and may disrupt early vertebrate development. A
[...] Read more.
Cholesterol homeostasis is necessary for normal vertebrate development. The disruption of cholesterol homeostasis can cause abnormal body and nervous system development and lead to dysfunctional behavior and increased mortality. Commonly prescribed psychopharmaceuticals can alter cholesterol synthesis and may disrupt early vertebrate development. A high-throughput vertebrate zebrafish model system was used to test the hypothesis that exposure to psychopharmaceutical medications alters cholesterol biosynthesis and disrupts gene transcription, early whole-body and brain development, and nervous system function, resulting in abnormal behavior. Exposure to cariprazine, aripiprazole, trazodone, and AY9944 increased 7-dehydrocholesterol levels compared to vehicle-treated zebrafish. Significant differences in disease-associated gene expression, brain structure, and functional behaviors were observed in psychopharmaceutical and AY9944-treated zebrafish compared to controls. These data reveal that the high-throughput zebrafish model system can discern psychopharmaceutical effects on cholesterol synthesis, gene transcription, and key features of early vertebrate development that influences behavior.
Full article

Figure 1
Open AccessArticle
Drosophila Males Differentially Express Small Proteins Regulating Stem Cell Division Frequency in Response to Mating
by
Manashree S. Malpe, Leon F. McSwain, Heath M. Aston, Karl A. Kudyba, Chun Ng, Megan P. Wright and Cordula Schulz
J. Dev. Biol. 2025, 13(3), 21; https://doi.org/10.3390/jdb13030021 - 23 Jun 2025
Abstract
The germline stem cells (GSCs) in the male gonad of Drosophila can increase their division frequency in response to a demand for more sperm caused by repeated mating. However, the molecules and mechanisms regulating and mediating this response have yet to be fully
[...] Read more.
The germline stem cells (GSCs) in the male gonad of Drosophila can increase their division frequency in response to a demand for more sperm caused by repeated mating. However, the molecules and mechanisms regulating and mediating this response have yet to be fully explored. Here, we present the results of a transcriptome analysis comparing expression from the testis tips from non-mated and mated males. An overlapping set of 18 differentially expressed genes (DEGs) from two independent wild-type (wt) strains revealed that the majority of the DEGs encode secreted proteins, which suggests roles for them in cell–cell interactions. Consistent with a role for secretion in regulating GSC divisions, knocking down Signal Recognition Particle (SRP) components within the germline cells using RNA Interference (RNAi), prevented the increase in GSC division frequency in response to mating. The major class of DEGs encodes polypeptides below the size of 250 amino acids, also known as small proteins. Upon reducing germline expression of small proteins, males no longer increased GSC division frequency after repeated mating. We hypothesize that mating induces cellular interactions via small proteins to ensure continued GSC divisions for the production of sperm.
Full article
(This article belongs to the Special Issue Drosophila in Developmental Biology—Past, Present and Future)
►▼
Show Figures
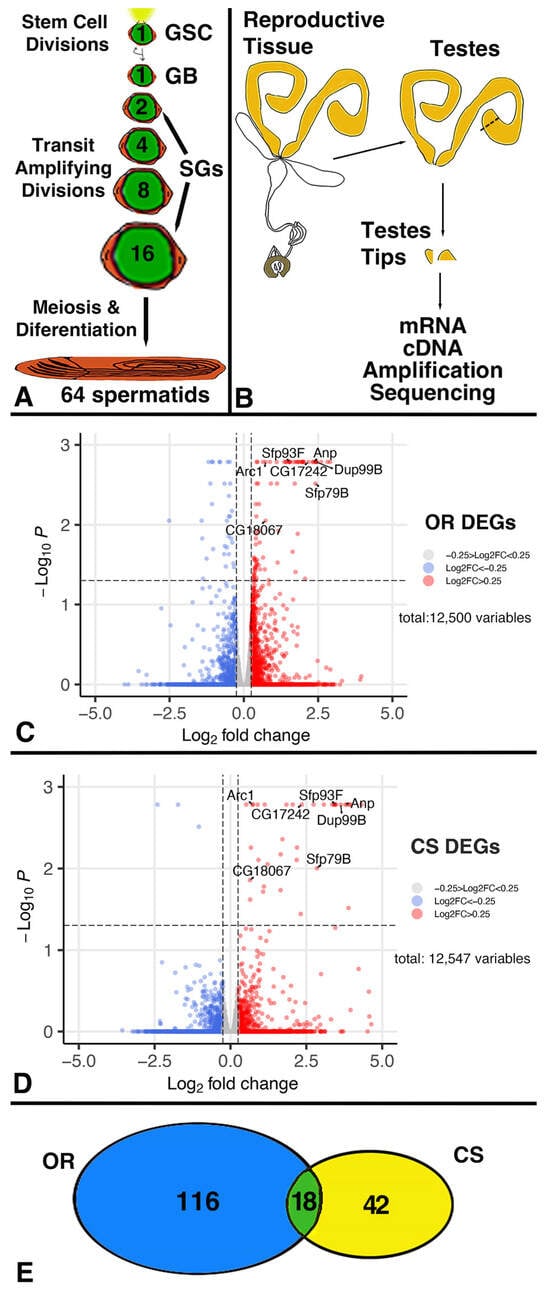
Figure 1
Open AccessArticle
Cannabinoid Receptor 1 Regulates Zebrafish Renal Multiciliated Cell Development via cAMP Signaling
by
Thanh Khoa Nguyen, Sophia Baker, Julienne Angtuaco, Liana Arceri, Samuel Kaczor, Bram Fitzsimonds, Matthew R. Hawkins and Rebecca A. Wingert
J. Dev. Biol. 2025, 13(2), 20; https://doi.org/10.3390/jdb13020020 - 17 Jun 2025
Abstract
Endocannabinoid signaling plays a significant role in neurogenesis and nervous system physiology, but its roles in the development of other tissues are just beginning to be appreciated. Previous reports have shown the presence of the key endocannabinoid receptor Cannabinoid receptor 1 (CB1 or
[...] Read more.
Endocannabinoid signaling plays a significant role in neurogenesis and nervous system physiology, but its roles in the development of other tissues are just beginning to be appreciated. Previous reports have shown the presence of the key endocannabinoid receptor Cannabinoid receptor 1 (CB1 or Cnr1) in multiciliated (MCC) tissues and its upregulation in kidney diseases, yet the relationship between Cnr1 and renal MCC development is unknown. Here, we report that Cnr1 is essential for cilia development across tissues and regulates renal MCCs via cyclic AMP (cAMP) signaling during zebrafish embryogenesis. Using a combination of genetic and pharmacological studies, we found that the loss of function, agonism and antagonism of cnr1 all lead to reduced mature renal MCC populations. cnr1 deficiency also led to reduced cilia development across tissues, including the pronephros, ear, Kupffer’s vesicle (KV), and nasal placode. Interestingly, treatment with the cAMP activator Forskolin (FSK) restored renal MCC defects in agonist-treated embryos, suggesting that cnr1 mediates cAMP signaling in renal MCC development. Meanwhile, treatment with the cAMP inhibitor SQ-22536 alone or with cnr1 deficiency led to reduced MCC populations, suggesting that cnr1 also mediates renal MCC development independently of cAMP signaling. Our findings indicate that cnr1 has a critical role in controlling renal MCC development both via cAMP signaling and an independent pathway, further revealing implications for ciliopathies and renal diseases.
Full article
(This article belongs to the Special Issue Feature Papers from Journal of Developmental Biology Reviewers)
►▼
Show Figures
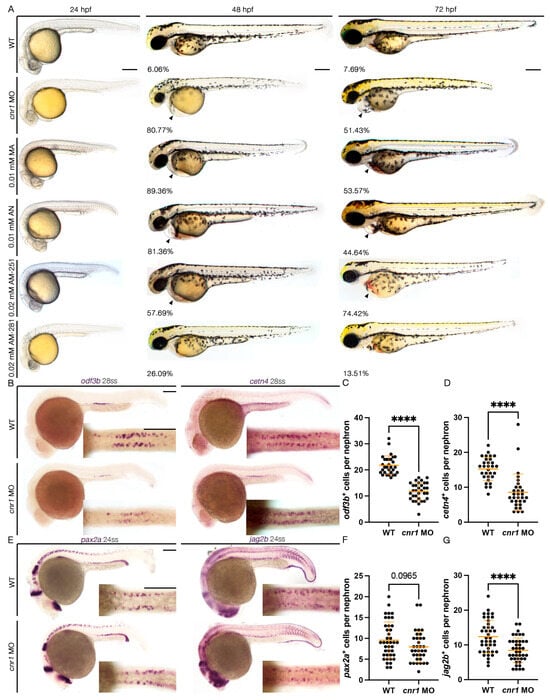
Figure 1
Open AccessArticle
Ribosome Incorporation Transdifferentiates Chick Primary Cells and Induces Their Proliferation by Secreting Growth Factors
by
Shota Inoue, Arif Istiaq, Anamika Datta, Mengxue Lu, Shintaro Nakayama, Kousei Takashi, Nobushige Nakajo, Shigehiko Tamura, Ikko Kawashima and Kunimasa Ohta
J. Dev. Biol. 2025, 13(2), 19; https://doi.org/10.3390/jdb13020019 - 1 Jun 2025
Abstract
►▼
Show Figures
Previously, we reported that mammalian cells, specifically human dermal fibroblasts (HDFs), could be transdifferentiated by lactic acid bacteria (LAB). Later, we observed that HDFs incorporated LAB-derived ribosomes, forming the ribosome-induced cell clusters (RICs) and transdifferentiating into cells derived from all three germ layers.
[...] Read more.
Previously, we reported that mammalian cells, specifically human dermal fibroblasts (HDFs), could be transdifferentiated by lactic acid bacteria (LAB). Later, we observed that HDFs incorporated LAB-derived ribosomes, forming the ribosome-induced cell clusters (RICs) and transdifferentiating into cells derived from all three germ layers. Based on this insight, we hypothesized that incorporating ribosomes into non-mammalian cells could reveal the universality of this mechanism and open the door to commercial applications. Our current study demonstrates that ribosome incorporation can transdifferentiate chick primary muscle-derived cells (CMCs) into adipocytes, osteoblasts, and chondrocytes. Furthermore, the culture medium supernatant from ribosome-incorporated CMCs was found to significantly enhance CMC’s proliferation. RNA-seq analysis revealed that RICs-CMC exhibit increased expression of genes related to multi-lineage cell growth. In addition, we developed a novel technological shift in meat production—the “CulNet System”—which replicates organ interactions within mechanical systems for cell-cultured meat production. While significant efforts are still required to implement this technology in a cost-effective manner, we believe that combining the “CulNet System” with ribosome-incorporated multipotent cells that have prolonged culture capability could substantially improve the scalability and cost-effectiveness of cultured chicken meat production. This report highlights a promising approach for cell-culture-based meat production, offering a sustainable alternative to traditional methods.
Full article

Figure 1
Open AccessArticle
Cornified Epithelial Teeth of Jawless Vertebrates Contain Proteins Similar to Keratin-Associated Proteins of Mammalian Skin Appendages
by
Attila Placido Sachslehner, David A. D. Parry and Leopold Eckhart
J. Dev. Biol. 2025, 13(2), 18; https://doi.org/10.3390/jdb13020018 - 19 May 2025
Abstract
Keratins and keratin-associated proteins (KRTAPs) are the main components of mammalian nails and hair. Comparative genomics and gene expression studies have revealed that keratins are conserved in all vertebrates, whereas KRTAPs exist only in mammals. Recently, we found hair keratin-like cysteine-rich keratins in
[...] Read more.
Keratins and keratin-associated proteins (KRTAPs) are the main components of mammalian nails and hair. Comparative genomics and gene expression studies have revealed that keratins are conserved in all vertebrates, whereas KRTAPs exist only in mammals. Recently, we found hair keratin-like cysteine-rich keratins in jawless vertebrates with confirmed expression in the cornified epithelial teeth of the sea lamprey (Petromyzon marinus). Here, we report that KRTAP-like proteins are also present in the horny teeth of the lamprey. Mass spectrometry-based proteomics identified proteins that share features with KRTAPs, such as high contents of cysteine and tyrosine residues, which support intermolecular interactions, and abundant glycine residues, which endow the proteins with flexibility. Genes encoding KRTAP-like proteins are arranged in a cluster in P. marinus, and the presence of at least one KRTAP-like protein is conserved in phylogenetically diverse species of lamprey, including Lampetra fluviatilis, Lethenteron reissneri, Geotria australis, and Mordacia mordax. The KRTAP-like genes of lampreys contain two exons, whereas mammalian KRTAPs have only a single exon. Although KRTAPs and KRTAP-like proteins are products of independent evolution, their common expression in cornified skin appendages suggests that they fulfill similar functions.
Full article
(This article belongs to the Special Issue Feature Papers from Journal of Developmental Biology Reviewers)
►▼
Show Figures

Figure 1
Open AccessArticle
In Vitro Embryo Culture Impacts Heart Mitochondria in Male Adolescent Sheep
by
Reza Amanollahi, Stacey L. Holman, Ashley S. Meakin, Monalisa Padhee, Kimberley J. Botting-Lawford, Song Zhang, Severence M. MacLaughlin, David O. Kleemann, Simon K. Walker, Jennifer M. Kelly, Skye R. Rudiger, I. Caroline McMillen, Michael D. Wiese, Mitchell C. Lock and Janna L. Morrison
J. Dev. Biol. 2025, 13(2), 17; https://doi.org/10.3390/jdb13020017 - 13 May 2025
Abstract
►▼
Show Figures
Assisted reproductive technology (ART)such as in vitro embryo culture (IVC), is widely used in human infertility treatments; however, its long-term effects on the cardiac health of offspring remain unclear. This study aimed to determine whether the effects of IVC on cardiac metabolism and
[...] Read more.
Assisted reproductive technology (ART)such as in vitro embryo culture (IVC), is widely used in human infertility treatments; however, its long-term effects on the cardiac health of offspring remain unclear. This study aimed to determine whether the effects of IVC on cardiac metabolism and associated signaling pathways persist after birth into adolescence. Embryos were either transferred to an intermediate ewe (ET) or cultured in vitro in the absence (IVC) or presence of human serum (IVCHS) with methionine supplementation (IVCHS+M) for 6 days after mating. Naturally mated (NM) ewes were used as controls. Protein expression and hormone concentrations in the left ventricle (LV) were analyzed using Western blot and LC-MS/MS analyses, respectively. IVC was associated with sex-specific alterations in cardiac mitochondria, with males exhibiting reduced mitochondrial abundance. Cardiac protein expression of oxidative phosphorylation (OXPHOS) complexes 1 and 4 was reduced by IVC. Additionally, IVC reduced protein expression of PDK-4 and Mn-SOD in the IVCHS+M group, which may impact energy efficiency and defense against oxidative stress. These changes may predispose IVC offspring to cardiac oxidative stress and mitochondrial dysfunction, particularly in males. This study provides insights into the sex-dependent effects of IVC on cardiac health, emphasizing the importance of evaluating long-term cardiovascular risks associated with IVC protocols.
Full article

Figure 1
Open AccessArticle
Dynamic Changes of Immunoreactive CD34, CD117, and CD41 Hematopoietic Stem Cells in Human Placentas of Different Gestational Ages
by
Sanja Jovicic, Ivan R. Nikolic, Ljiljana Amidžić, Vesna Ljubojevic, Maja Barudzija and Ranko Skrbic
J. Dev. Biol. 2025, 13(2), 16; https://doi.org/10.3390/jdb13020016 - 9 May 2025
Cited by 1
Abstract
►▼
Show Figures
Background: The process of prenatal hematopoiesis occurs in various anatomical locations, including the placenta. The placenta is not merely a temporary hematopoietic reservoir, but it is one of the key sites for the synthesis of hematopoietic stem cells (HSCs). This study aimed
[...] Read more.
Background: The process of prenatal hematopoiesis occurs in various anatomical locations, including the placenta. The placenta is not merely a temporary hematopoietic reservoir, but it is one of the key sites for the synthesis of hematopoietic stem cells (HSCs). This study aimed to investigate the presence, distribution, and immunoprofiles of HSCs in the human placenta during different gestational periods. Materials and Methods: Placental samples of different gestational ages (first, second, and third trimesters) were analyzed using classical hematoxylin and eosin staining and immunohistochemical staining for CD34, CD117, and CD41 markers, with HSC quantification through numerical areal density (NA). Results: Highly immunoreactive CD34 HSCs were present in placentas throughout gestation, while highly immunoreactive CD117 and CD41 HSCs were observed during the first two trimesters. In the first trimester, HSCs were found within the lumen of blood vessels and as individual cells in the mesenchyme of chorionic villi. With advancing gestation, the number of HSCs in the mesenchyme of chorionic villi increased. Conclusions: Immunoreactive CD34, CD117, and CD41 cells are present in significant proportions in various parts of the placenta throughout gestation, indicating that the placenta provides a substantial proportion of HSCs for hematopoiesis.
Full article
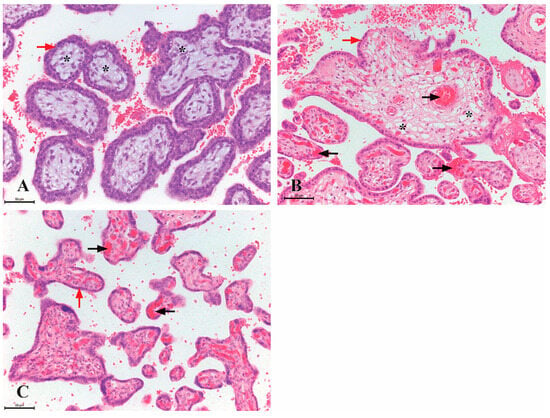
Figure 1
Open AccessArticle
Probe Sequencing Analysis of Regenerating Lizard Tails Indicates Crosstalk Among Osteoclasts, Epidermal Cells, and Fibroblasts
by
Darian J. Gamble, Samantha Lopez, Melody Yazdi, Toni Castro-Torres and Thomas P. Lozito
J. Dev. Biol. 2025, 13(2), 15; https://doi.org/10.3390/jdb13020015 - 3 May 2025
Abstract
Lizards are distinguished as the only amniotes, and closest relatives of mammals, capable of multilineage epimorphic regeneration. Tail blastemas of green anole lizards (Anolis carolinensis) consist of col3a1+ fibroblastic connective tissue cells enclosed in krt5+ wound epidermis (WE), both
[...] Read more.
Lizards are distinguished as the only amniotes, and closest relatives of mammals, capable of multilineage epimorphic regeneration. Tail blastemas of green anole lizards (Anolis carolinensis) consist of col3a1+ fibroblastic connective tissue cells enclosed in krt5+ wound epidermis (WE), both of which are required for regeneration. Blastema and WE formation are known to be closely associated with phagocytic cell populations, including macrophages and osteoclasts. However, it remains unclear what specific phagocytic cell types are required to stimulate regeneration. Here, we explicitly assess the roles of osteoclast activity during blastema and WE formation in regenerating lizard tails. First, probe sequencing was performed at regenerative timepoints on fibroblasts isolated based on col3a1 expression toward establishing pathways involved in stimulating blastema formation and subsequent tail regrowth. Next, treatments with osteoclast inhibitor zoledronic acid (ZA) were used to assess the roles of osteoclast activity in lizard tail regeneration and fibroblast signaling. ZA treatment stunted lizard tail regrowth, suggesting osteoclast activity was required for blastema formation and regeneration. Transcriptomic profiling of fibroblasts isolated from ZA-treated and control lizards linked inhibition of osteoclast activity with limitations in fibroblasts to form pro-regenerative extracellular matrix and support WE formation. These results suggest that crosstalk between osteoclasts and fibroblasts regulates blastema and WE formation during lizard tail regeneration.
Full article
(This article belongs to the Special Issue Skin Wound Healing and Regeneration in Vertebrates)
►▼
Show Figures

Figure 1
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics

Special Issues
Special Issue in
JDB
Zebrafish—a Model System for Developmental Biology Study III
Guest Editor: Lisa MavesDeadline: 15 October 2025
Special Issue in
JDB
Molecular and Cellular Mechanisms of Brain Development and Neurodevelopmental Disorders
Guest Editor: Mojgan RastegarDeadline: 30 November 2025
Special Issue in
JDB
Skin Wound Healing and Regeneration in Vertebrates
Guest Editor: Lorenzo AlibardiDeadline: 25 December 2025
Special Issue in
JDB
Embryonic Development and Regenerative Medicine
Guest Editors: Lon J. van Winkle, Philip Iannaccone, Rebecca Jean RyznarDeadline: 25 December 2025
Topical Collections
Topical Collection in
JDB
Hedgehog Signaling in Embryogenesis
Collection Editors: Henk Roelink, Kay Grobe










