Investigating Psychopharmaceutical Effects on Early Vertebrate Development Using a Zebrafish Model System
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
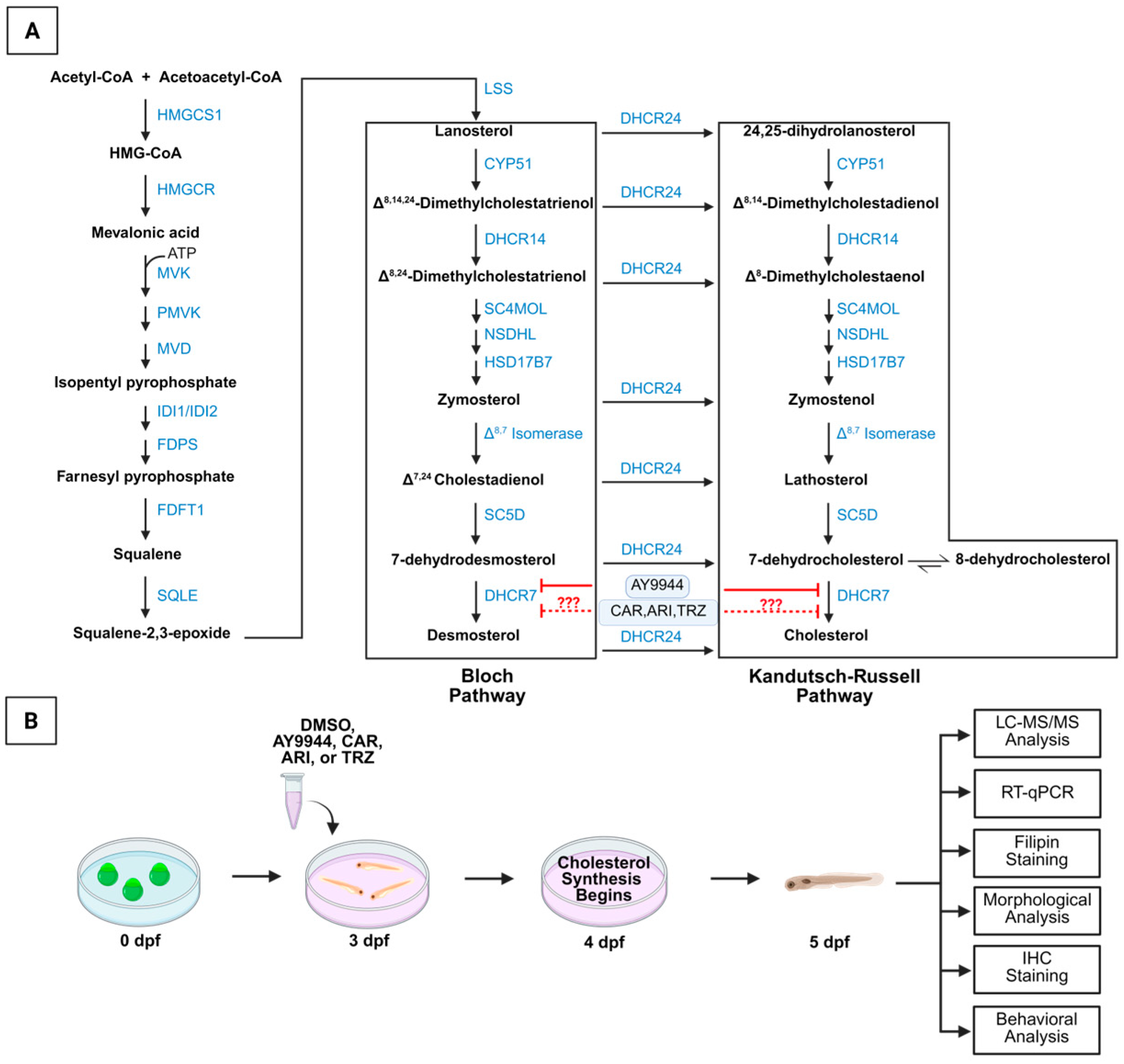
2.2. Psychopharmaceutical Treatment of Zebrafish Embryos
2.3. Liquid Chromatography with Tandem Mass Spectrometry (LC-MS/MS)
2.4. Filipin Staining
2.5. RNA Isolation
2.6. Reverse Transcriptase–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. Morphology
2.8. Immunohistochemistry
2.9. ZebraLab Zebrabox® Viewpoint Behavioral Analyses
2.10. Statistical Analyses
3. Results
3.1. Development of Zebrafish Model System to Assess the Effects of Psychopharmaceuticals on the Cholesterol Synthesis Pathway and Vertebrate Development
3.2. Effect of Psychopharmaceuticals on Key Cholesterol Precursors and Cholesterol Levels
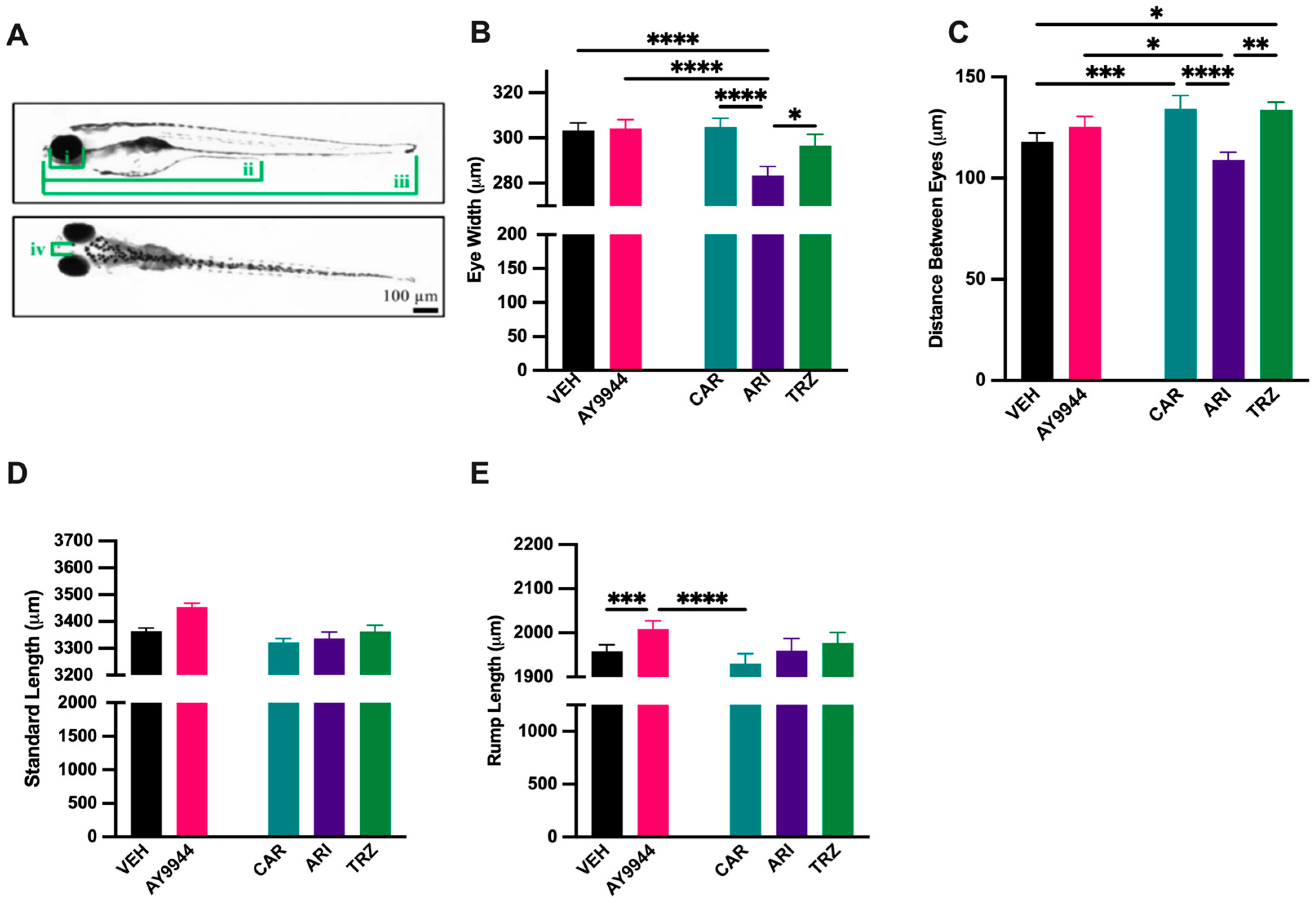
3.3. Effect of AY9944 and Psychopharmaceuticals on Morphological Development
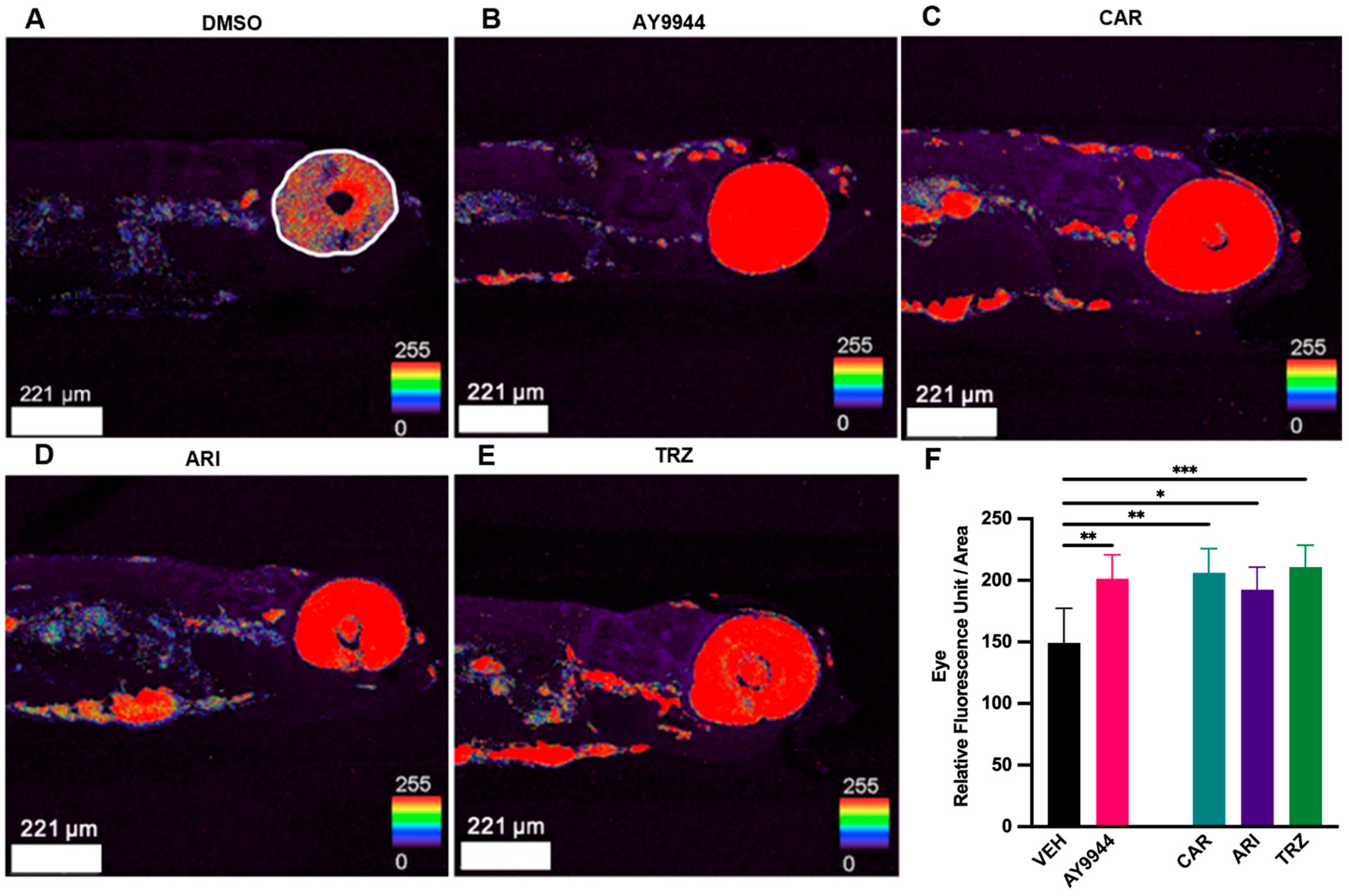
3.4. Effect of Psychopharmaceuticals on Cholesterol Deposition
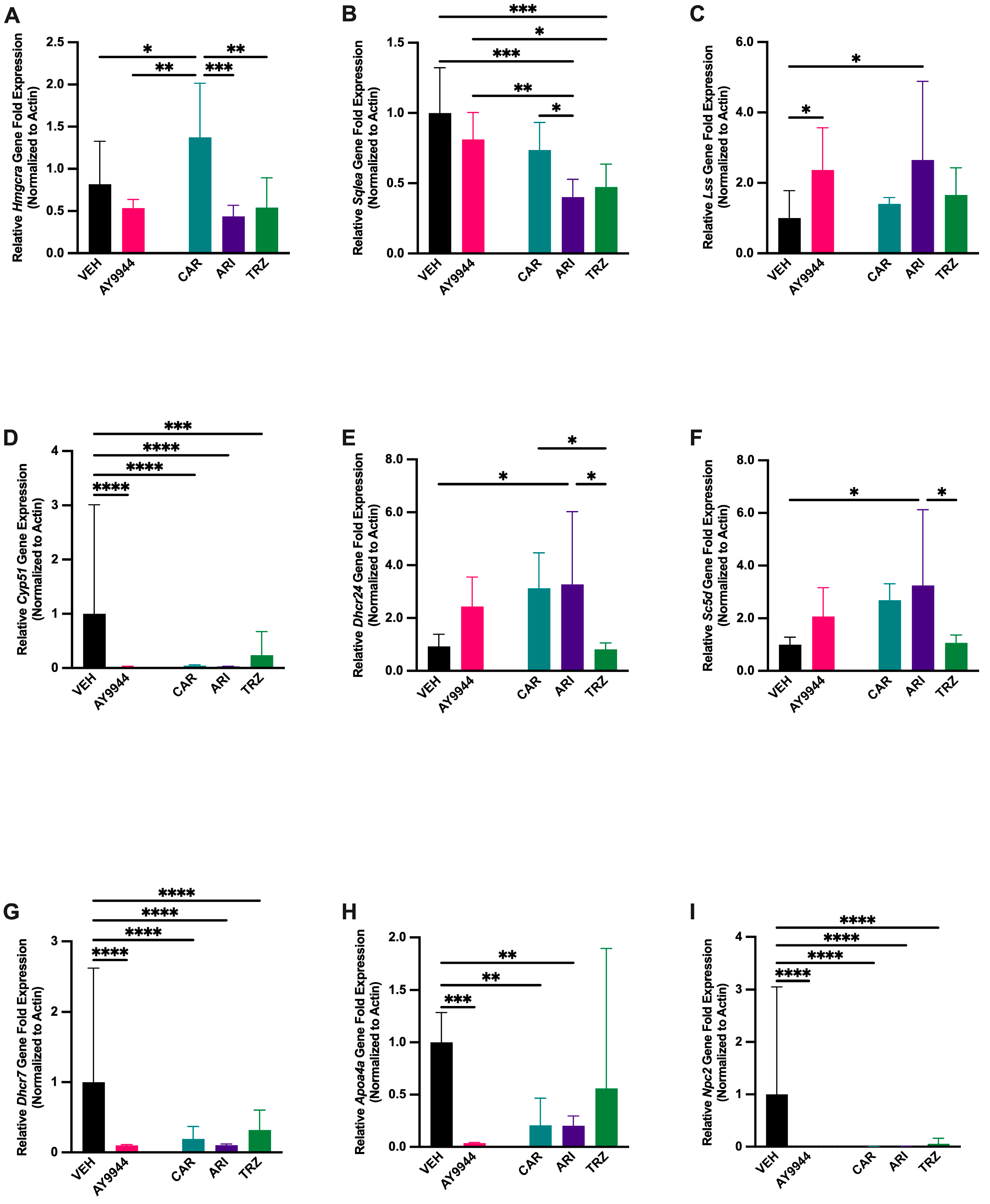
3.5. Effect of AY9944 and Psychopharmaceuticals on Transcription of Cholesterol Synthesis and Transport Genes
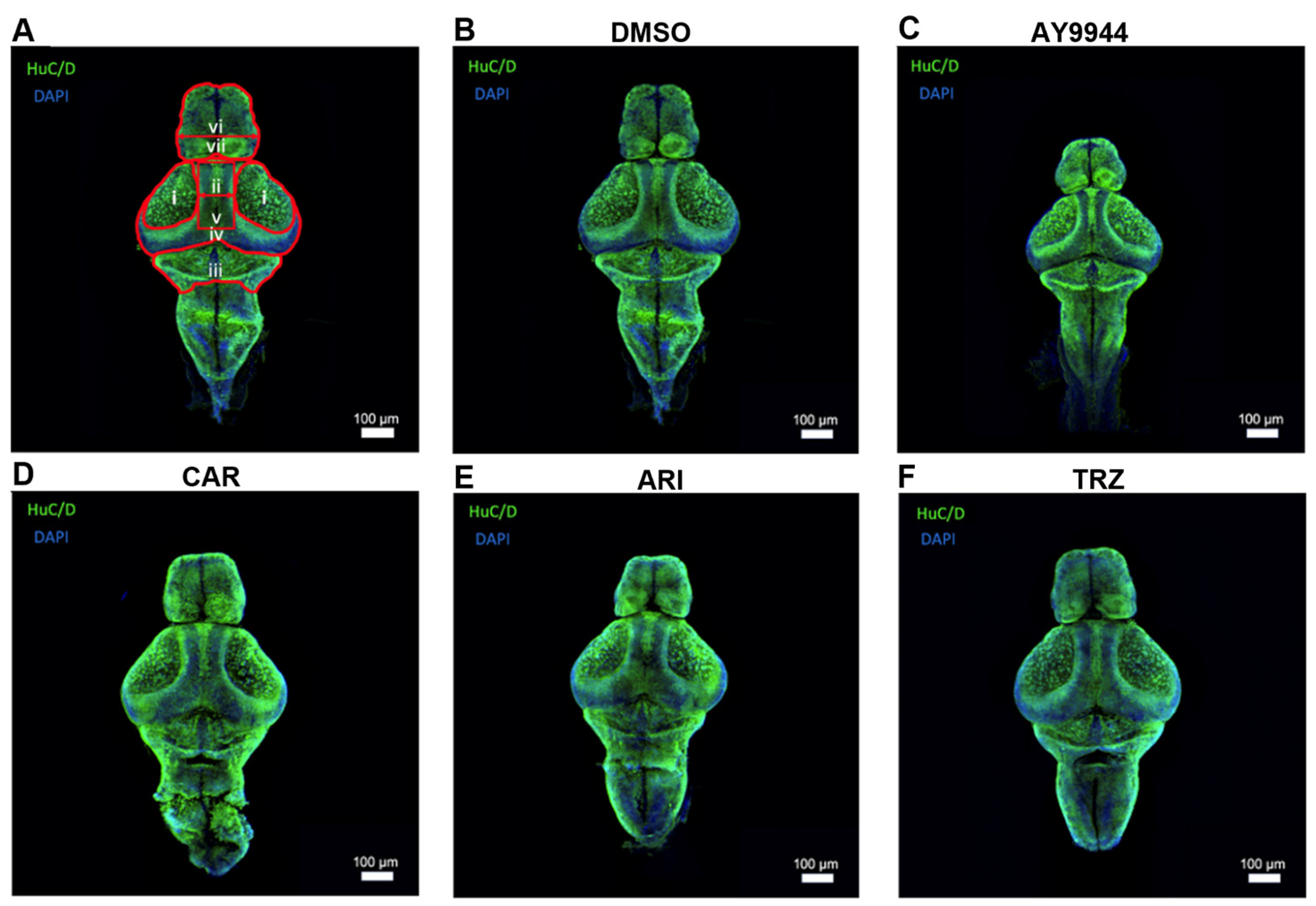
3.6. Huc/D Immnunoreactivity to Identify the Development of Brain Regions Following AY9944 and Psychopharmaceutical Treatments
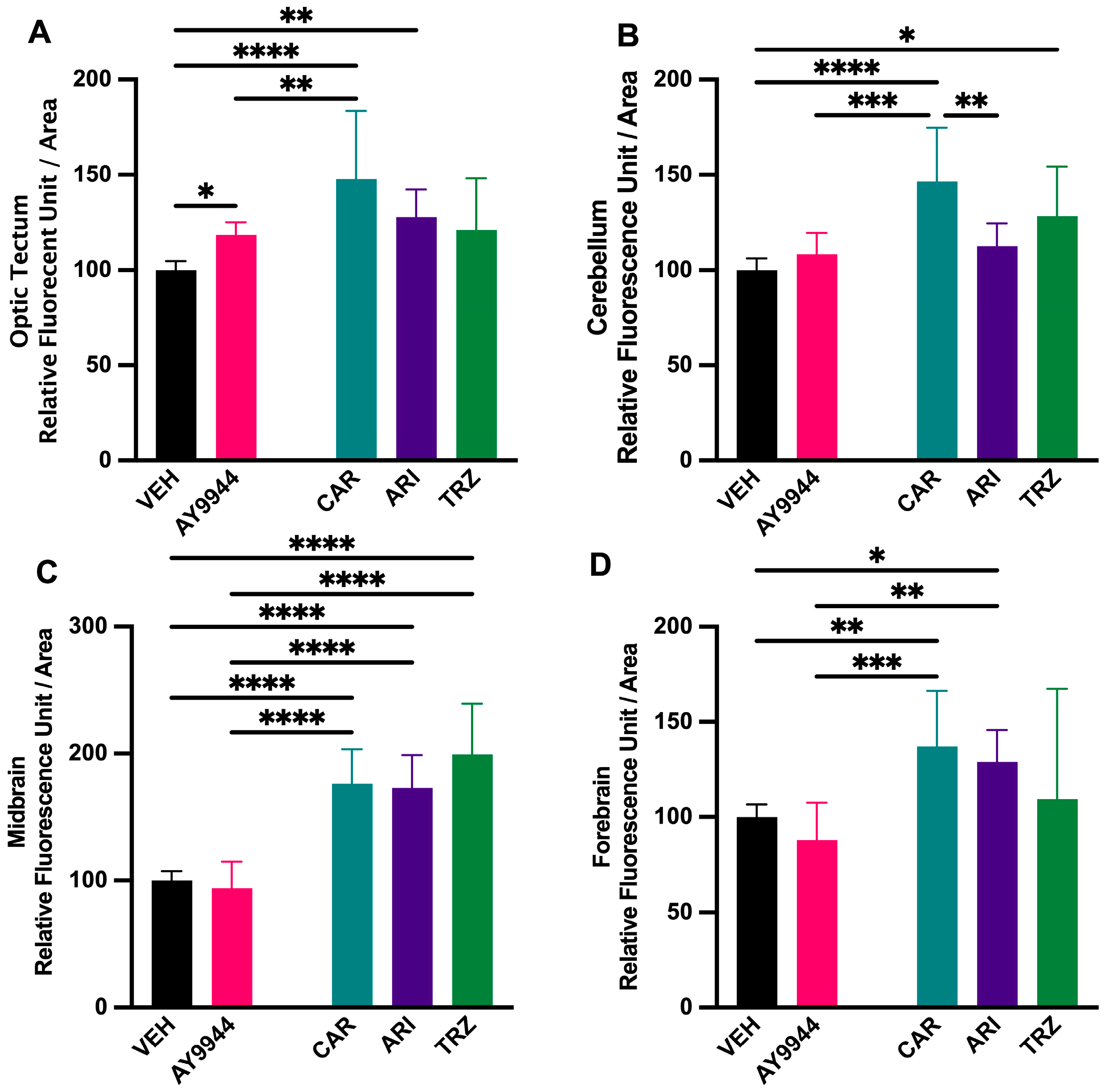
3.7. Effect of AY9944 and Psychopharmaceutical Treatments on the Development of Brain Regions

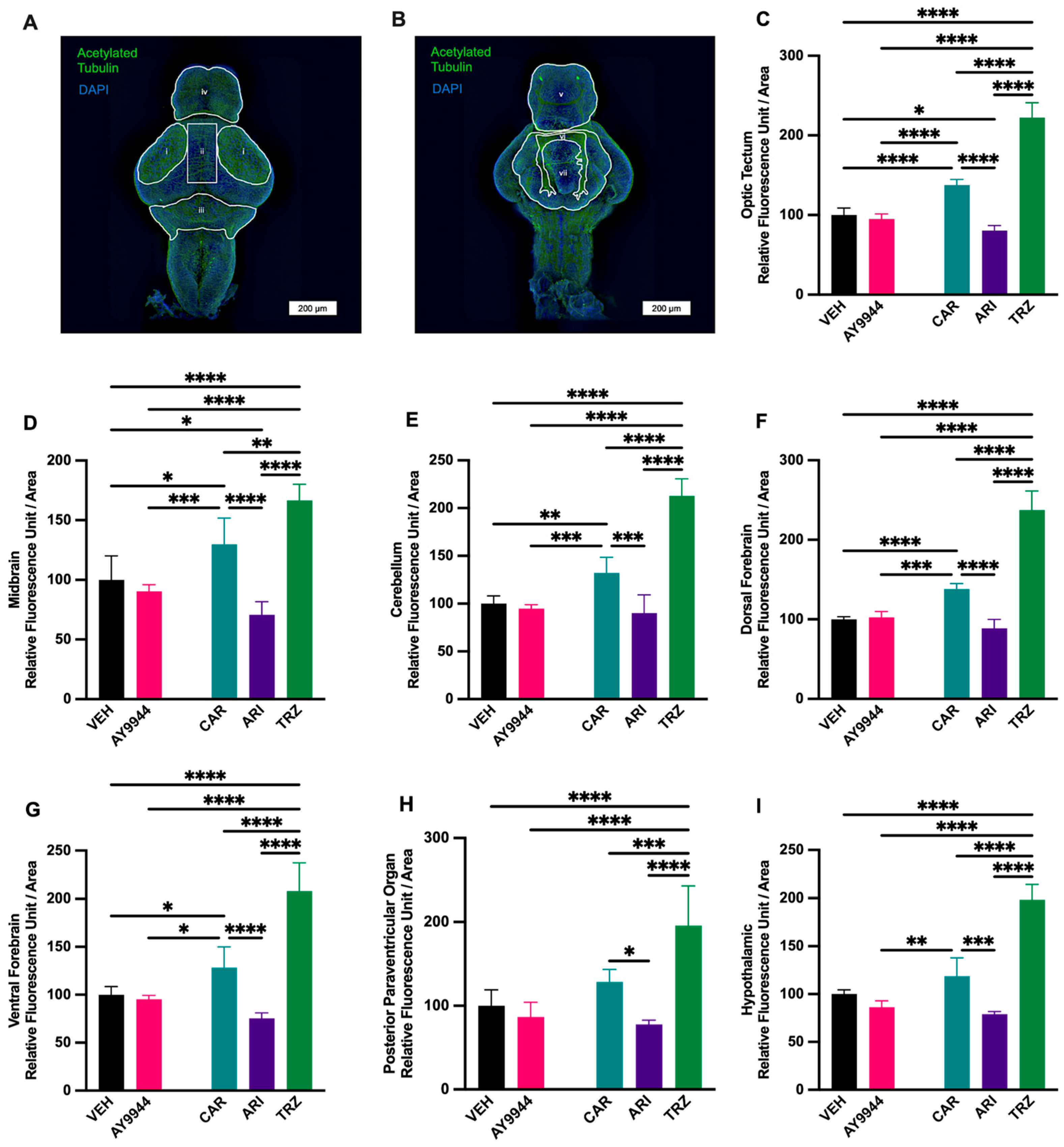
3.8. Effect of AY9944 and Psychopharmaceuticals on Neuronal Density in Developing Brain Regions
3.9. Effect of AY9944 and Psychopharmaceuticals on Neurite Outgrowth
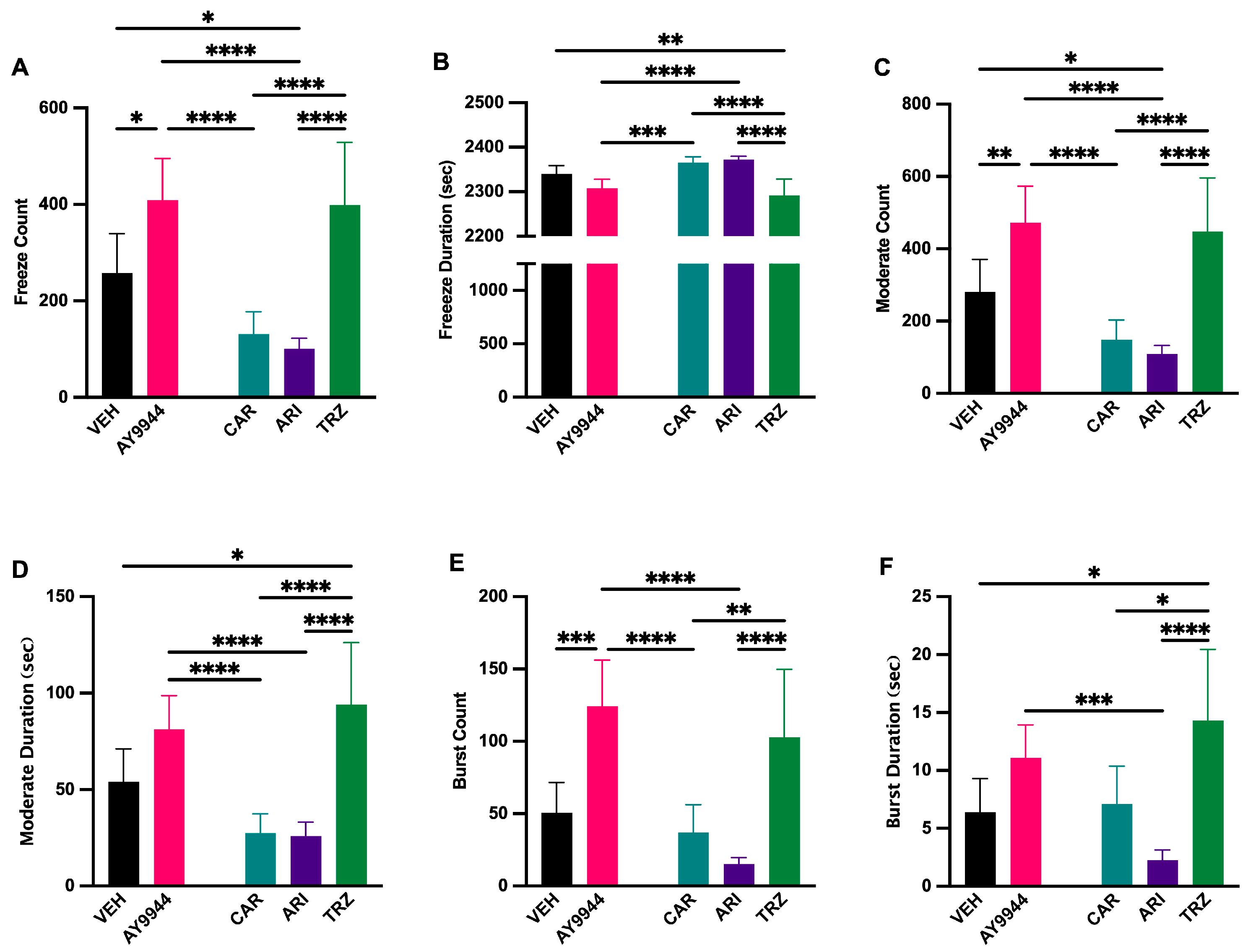
3.10. Effect of AY9944 and Psychopharmaceuticals on Zebrafish Swimming Behavior
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DHCR7 | 7-dehydrocholesterol reductase |
| 7-DHC | 7-dehydrocholesterol |
| SLOS | Smith–Lemli–Opitz syndrome |
| DHCR24 | 24-dehydrocholesterol reductase |
| ARI | Aripiprazole |
| TRZ | Trazadone |
| CAR | Cariprazine |
| CHILD | Congenital hemidysplasia with ichthyosoiform erythroderma and limb defects |
| Dpf | Days post-fertilization |
| VEH | Vehicle |
| SRM | Selective Reaction Monitoring |
| PFA | Paraformaldehyde |
| PBS | Phosphate-buffered saline |
| RT | Room temperature |
| LC-MS/MS | Liquid Chromatography–Tandem Mass Spectroscopy |
| RFU | Relative fluorescent units |
| 2,3-DCPP | 2,3-Dichlorophenylpiperazine |
| D2 | Dopamine-2 |
| 5-HT1A | Serotonin-1A |
| 5-HT2 | Serotonin-2A |
Appendix A
Appendix A.1. Reagent List
| Reagent | Product Information | Product Number |
|---|---|---|
| 4′,6-diamidino-2-phenylindole (DAPI) | ThermoFisher (Thermo Fisher Scientific, MA, USA) | D1306 |
| Anatech Ltd. Prefer Fixative | ThermoFisher (Thermo Fisher Scientific, MA, USA) | NC9053360 |
| Anatech Ltd. Prefer Fixative | ThermoFisher (Thermo Fisher Scientific, MA, USA) | NC9053360 |
| Anti-Acetylated Tubulin Monoclonal Antibody | Millipore Sigma (Merck KGaA, Darmstadt, Germany) | T7451 |
| Anti-HuC/HuD Antibody | Abcam (Celgene Corporation, Cambridge, UK) | ab210554 |
| Aripiprazole | Selleckchem (Selleck Chemicals, Houston, TX, USA) | S1975 |
| Bovine Serum Albumin | ThermoFisher (Thermo Fisher Scientific, MA, USA) | BP1600-100 |
| Cariprazine | Selleckchem (Selleck Chemicals, Houston, TX, USA) | S7236 |
| Chloroform | ThermoFisher (Thermo Fisher Scientific, MA, USA) | J67241.AP |
| Cholesterol Detection Filipin III | Cayman Chemical (Cayman Chemical Company, Ann Arbor, MI, USA) | 70440 |
| Cholesterol Detection Filipin III | Cayman Chemical (Cayman Chemical Company, Ann Arbor, MI, USA) | 10009867 |
| Dimethylsulfoxide (DMSO) | Corning (Corning Inc., Corning, NY, USA) | 25-950-CQC |
| Ethanol | Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) | E7023 |
| Glycerol | ThermoFisher (Thermo Fisher Scientific, MA, USA) | G33-500 |
| Goat Anti-Mouse IgG H&L (Alexa FluorTM 488) | Invitrogen (Thermo Fisher Scientific, MA, USA) | A-11001 |
| Goat Anti-Rabbit IgG H&L (Alexa FluorTM 488) | Abcam (Celgene Corporation, Cambridge, UK) | ab150077 |
| Goat Serum | Gibco (Thermo Fisher Scientific, MA, USA) | 16210064 |
| Hydrogen Peroxide Solution | Millipore Sigma (Merck KGaA, Darmstadt, Germany) | H1009 |
| iScript | Bio-Rad (Bio-Rad Laboratories, CA, USA) | 1708841 |
| Isopropanol | ThermoFisher (Thermo Fisher Scientific, MA, USA) | T036181000 |
| Paraformaldehyde | ThermoFisher (Thermo Fisher Scientific, MA, USA) | 416785000 |
| Phosphate-Buffered Saline | ThermoFisher (Thermo Fisher Scientific, MA, USA) | BP399-1 |
| Potassium Hydroxide | Millipore Sigma (Merck KGaA, Darmstadt, Germany) | 221473 |
| SsoAdvanced® Universal SYBR Green Supermix | Bio-Rad (Bio-Rad Laboratories, CA, USA) | 1725271 |
| Trans-1,4-bis(2-chlorobenzylaminomethyl) cyclohexane dihydrochloride (AY9944) | Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) | 366-93-8 |
| Trazodone | Selleckchem (Selleck Chemicals, Houston, TX, USA) | S5857 |
| TRI Reagen™ Solution | Invitrogen (Thermo Fisher Scientific, MA, USA) | AM9738 |
| Triton X-100 | Cayman Chemical (Cayman Chemical Company, Ann Arbor, MI, USA) | 600217 |
| Tween 20 | ThermoFisher (Thermo Fisher Scientific, MA, USA) | BP337-500 |
| UltraPure Agarose 1000 | Invitrogen (Thermo Fisher Scientific, MA, USA) | 16550-100 |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Abca1a | 5′-CCTCAGCATCTCCAGCTACG-3′ | 5′-GAATGCATGTCTGCGGTTCC-3′ |
| Actin | 5′-TCACACCTTCTACAACGAGCTGCG-3′ | 5′-GAAGCTGTAGCCTCTCTCGGTCAG-3′ |
| Apoa4a | 5′-CGCAGATACCCTCGCACTTA-3 | 5′-AGAGAAAGCTGCGATGACGA-3′ |
| Dhcr7 | 5′-GGGGTAAGAAGCCCACCTTC-3′ | 5′-TCAAGTCGCCCGTGTAGTTC-3′ |
| Dhcr24 | 5′-TCACACCTTCTACAACGAGCTGCG-3′ | 5′-GAAGCTGTAGCCTCTCTCGGTCAG-3′ |
| Fdft1 | 5′-TGGGAGGATGTCGAGCTGTC-3′ | 5′-GGTTCAGATAGGCGTAGCAGG-3′ |
| Hmgcs1 | 5′-CTCTCCGAATCTAACCGTCCG-3′ | 5′-CACATGGACTCGCAAATCGC-3′ |
| Hmgcra | 5′-GGAACATGCACTAAGCAGGC-3′ | 5′-AGAGAAGAAGGGATCGGTTGC-3′ |
| Lss | 5′-GGACCAACTTCACCTGCCTT-3′ | 5′-GGGCTTGTGTAAGGCACTCT-3′ |
| Npc2 | 5′-CATTCATTTGAACCATGGACTACAACG-3′ | 5′-CTACTTTTCCGTCTACCGAGCCTG-3′ |
| Nsdhl | 5′-CTACAGCGCTCCACGATACC-3′ | 5′-ATCAGCGGTCGCAGTATGAG-3′ |
| Sc5d | 5′-CTGCCGTACCACATCTACCC-3′ | 5′-TACACGATAGTCCCCGTCGT-3′ |
| Sqlea | 5′-GAAAGACACCGGGATGCTCA-3′ | 5′-CTGCATGGTGGGCTTTGAAC-3′ |
References
- Griffiths, W.J.; Abdel-Khalik, J.; Yutuc, E.; Morgan, A.H.; Gilmore, I.; Hearn, T.; Wang, Y. Cholesterolomics: An update. Anal Biochem. 2017, 524, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, N.M.; Oliveira, E.F.; Gesto, D.S.; Santos-Martins, D.; Moreira, C.; Moorthy, H.N.; Ramos, M.J.; Fernandes, P.A. Cholesterol Biosynthesis: A Mechanistic Overview. Biochemistry. 2016, 55, 5483–5506. [Google Scholar] [CrossRef]
- Goicoechea, L.; Conde de la Rosa, L.; Torres, S.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial cholesterol: Metabolism and impact on redox biology and disease. Redox Biol. 2023, 61, 102643. [Google Scholar] [CrossRef]
- Barres, B.A.; Smith, S.J. Neurobiology. Cholesterol-making or breaking the synapse. Science 2001, 294, 1296–1297. [Google Scholar] [CrossRef]
- Dietschy, J.M.; Turley, S.D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001, 12, 105–112. [Google Scholar] [CrossRef]
- Pfrieger, F.W. Cholesterol homeostasis and function in neurons of the central nervous system. Cell. Mol. Life Sci. 2003, 60, 1158–1171. [Google Scholar] [CrossRef]
- Li, D.; Zhang, J.; Liu, Q. Brain cell type-specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022, 45, 401–414. [Google Scholar] [CrossRef]
- Tint, G.S.; Irons, M.; Elias, E.R.; Batta, A.K.; Frieden, R.; Chen, T.S.; Salen, G. Defective cholesterol biosynthesis associated with the Smith-Lemli-Opitz syndrome. N. Engl. J. Med. 1994, 330, 107–113. [Google Scholar] [CrossRef]
- Yu, H.; Patel, S.B. Recent insights into the Smith-Lemli-Opitz syndrome. Clin. Genet. 2005, 68, 383–391. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Abdel-Khalik, J.; Crick, P.J.; Ogundare, M.; Shackleton, C.H.; Tuschl, K.; Kwok, M.K.; Bigger, B.W.; Morris, A.A.; Honda, A.; et al. Sterols and oxysterols in plasma from Smith-Lemli-Opitz syndrome patients. J. Steroid Biochem. Mol. Biol. 2017, 169, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kritzer, A.; Dutta, R.; Pramparo, T.; Terner-Rosenthal, J.; Vig, P.; Steiner, R.D. Smith-Lemli-Opitz Syndrome: Clinical, Biochemical, and Genetic Insights With Emerging Treatment Opportunities. Genet. Med. 2025, 27, 101450. [Google Scholar] [CrossRef] [PubMed]
- Waterham, H.R.; Koster, J.; Romeijn, G.J.; Hennekam, R.C.; Vreken, P.; Andersson, H.C.; FitzPatrick, D.R.; Kelley, R.I.; Wanders, R.J. Mutations in the 3beta-hydroxysterol Delta24-reductase gene cause desmosterolosis, an autosomal recessive disorder of cholesterol biosynthesis. Am. J. Hum. Genet. 2001, 69, 685–694. [Google Scholar] [CrossRef]
- Yaplito-Lee, J.; Pai, G.; Hardikar, W.; Hong, K.M.; Pitt, J.; Marum, J.; Amor, D.J. Successful treatment of lathosterolosis: A rare defect in cholesterol biosynthesis-A case report and review of literature. JIMD Rep. 2020, 56, 14–19. [Google Scholar] [CrossRef]
- Ramphul, K.; Kota, V.; Sathe, N.C.; Mejias, S.G. CHILD Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Porter, F.D. Human malformation syndromes due to inborn errors of cholesterol synthesis. Curr. Opin. Pediatr. 2003, 15, 607–613. [Google Scholar] [CrossRef]
- Keeler, R.F. Cyclopamine and related steroidal alkaloid teratogens: Their occurrence, structural relationship, and biologic effects. Lipids 1978, 13, 708–715. [Google Scholar] [CrossRef]
- Siebold, C.; Rohatgi, R. The Inseparable Relationship Between Cholesterol and Hedgehog Signaling. Annu. Rev. Biochem. 2023, 92, 273–298. [Google Scholar] [CrossRef]
- Abramyan, J. Hedgehog Signaling and Embryonic Craniofacial Disorders. J. Dev. Biol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Hall, P.; Michels, V.; Gavrilov, D.; Matern, D.; Oglesbee, D.; Raymond, K.; Rinaldo, P.; Tortorelli, S. Aripiprazole and trazodone cause elevations of 7-dehydrocholesterol in the absence of Smith-Lemli-Opitz Syndrome. Mol. Genet. Metab. 2013, 110, 176–178. [Google Scholar] [CrossRef]
- Korade, Z.; Heffer, M.; Mirnics, K. Psychopharmaceutical effects on developmental sterol biosynthesis. Mol. Psychiatry 2022, 27, 490–501. [Google Scholar] [CrossRef]
- Tallman, K.A.; Allen, L.B.; Klingelsmith, K.B.; Anderson, A.; Genaro-Mattos, T.C.; Mirnics, K.; Porter, N.A.; Korade, Z. Prescription Medications Alter Neuronal and Glial Cholesterol Synthesis. ACS Chem Neurosci. 2021, 12, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Son, S.; Park, J.; Kim, C.Y.; Kim, J. Antidepressant aripiprazole induces adverse effects on neural development during cortex organoid generation. Reprod Toxicol. 2025, 133, 108862. [Google Scholar] [CrossRef] [PubMed]
- Genaro-Mattos, T.C.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Maternal aripiprazole exposure interacts with 7-dehydrocholesterol reductase mutations and alters embryonic neurodevelopment. Mol. Psychiatry 2019, 24, 491–500. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Anderson, A.; Allen, L.B.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Maternal cariprazine exposure inhibits embryonic and postnatal brain cholesterol biosynthesis. Mol. Psychiatry 2020, 25, 2685–2694. [Google Scholar] [CrossRef]
- Park, Y.; Huybrechts, K.F.; Cohen, J.M.; Bateman, B.T.; Desai, R.J.; Patorno, E.; Mogun, H.; Cohen, L.S.; Hernandez-Diaz, S. Antipsychotic Medication Use Among Publicly Insured Pregnant Women in the United States. Psychiatr Serv. 2017, 68, 1112–1119. [Google Scholar] [CrossRef]
- Boland, M.R.; Tatonetti, N.P. Investigation of 7-dehydrocholesterol reductase pathway to elucidate off-target prenatal effects of pharmaceuticals: A systematic review. Pharmacogenomics J. 2016, 16, 411–429. [Google Scholar] [CrossRef]
- Genaro-Mattos, T.C.; Klingelsmith, K.B.; Allen, L.B.; Anderson, A.; Tallman, K.A.; Porter, N.A.; Korade, Z.; Mirnics, K. Sterol Biosynthesis Inhibition in Pregnant Women Taking Prescription Medications. ACS Pharmacol. Transl. Sci. 2021, 4, 848–857. [Google Scholar] [CrossRef]
- Creeley, C.E.; Denton, L.K. Use of Prescribed Psychotropics during Pregnancy: A Systematic Review of Pregnancy, Neonatal, and Childhood Outcomes. Brain Sci. 2019, 9, 235. [Google Scholar] [CrossRef]
- Lieschke, G.J.; Currie, P.D. Animal models of human disease: Zebrafish swim into view. Nat. Rev. Genet. 2007, 8, 353–367. [Google Scholar] [CrossRef]
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Milan, D.J.; Peterson, T.A.; Ruskin, J.N.; Peterson, R.T.; MacRae, C.A. Drugs that induce repolarization abnormalities cause bradycardia in zebrafish. Circulation 2003, 107, 1355–1358. [Google Scholar] [CrossRef] [PubMed]
- Quinlivan, V.H.; Farber, S.A. Lipid Uptake, Metabolism, and Transport in the Larval Zebrafish. Front. Endocrinol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Ka, J.; Jin, S. Zebrafish as an Emerging Model for Dyslipidemia and Associated Diseases. J. Lipid Atheroscler. 2021, 10, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.K.; Ma, J.; Chen, Z.; Li, Z.; Vitery, M.D.C.; Kelley, M.L.; Peterson, R.T.; Gerszten, R.E.; Yeh, J.J. Genetic deletion of gpr27 alters acylcarnitine metabolism, insulin sensitivity, and glucose homeostasis in zebrafish. FASEB J. 2020, 34, 1546–1557. [Google Scholar] [CrossRef]
- O’Hare, E.A.; Wang, X.; Montasser, M.E.; Chang, Y.C.; Mitchell, B.D.; Zaghloul, N.A. Disruption of ldlr causes increased LDL-c and vascular lipid accumulation in a zebrafish model of hypercholesterolemia. J. Lipid Res. 2014, 55, 2242–2253. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef]
- Tseng, W.; Loeb, H.E.; Pei, W.; Tsai-Morris, C.; Xu, L.; Cluzeau, C.V.; Wassif, C.A.; Feldman, B.; Burgess, S.M.; Pavan, W.J.; et al. Modeling Niemann-Pick disease type C1 in zebrafish: A robust platform for in vivo screening of candidate therapeutic compounds. Dis. Model. Mech. 2018, 11, dmm034165. [Google Scholar] [CrossRef]
- Mathews, E.S.; Appel, B. Cholesterol Biosynthesis Supports Myelin Gene Expression and Axon Ensheathment through Modulation of P13K/Akt/mTor Signaling. J. Neurosci. 2016, 36, 7628–7639. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Lai, K.; Zhong, Q.; Demin, K.A.; Kalueff, A.V.; Song, C. High-glucose/high-cholesterol diet in zebrafish evokes diabetic and affective pathogenesis: The role of peripheral and central inflammation, microglia and apoptosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 96, 109752. [Google Scholar] [CrossRef]
- Cook, S.R.; Bladen, C.; Smith, J.; Maguire, E.; Copner, J.; Fenn, G.D.; Wager, K.; Waller-Evans, H.; Lloyd-Evans, E. Visualisation of cholesterol and ganglioside GM1 in zebrafish models of Niemann-Pick type C disease and Smith-Lemli-Opitz syndrome using light sheet microscopy. Histochem. Cell Biol. 2020, 154, 565–578. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Lamberson, C.; Haas, D.; Korade, Z.; Porter, N.A. A highly sensitive method for analysis of 7-dehydrocholesterol for the study of Smith-Lemli-Opitz syndrome. J. Lipid Res. 2014, 55, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Unlu, G.; Qi, X.; Gamazon, E.R.; Melville, D.B.; Patel, N.; Rushing, A.R.; Hashem, M.; Al-Faifi, A.; Chen, R.; Li, B.; et al. Phenome-based approach identifies RIC1-linked Mendelian syndrome through zebrafish models, biobank associations and clinical studies. Nat. Med. 2020, 26, 98–109. [Google Scholar] [CrossRef]
- Teixido, E.; Kiessling, T.R.; Krupp, E.; Quevedo, C.; Muriana, A.; Scholz, S. Automated Morphological Feature Assessment for Zebrafish Embryo Developmental Toxicity Screens. Toxicol. Sci. 2019, 167, 438–449. [Google Scholar] [CrossRef]
- Correa-Cerro, L.S.; Porter, F.D. 3β-Hydroxysterol Δ7-reductase and the Smith–Lemli–Opitz syndrome. Mol. Genet. Metab. 2005, 84, 112–126. [Google Scholar] [CrossRef]
- Peeples, E.S.; Mirnics, K.; Korade, Z. Chemical Inhibition of Sterol Biosynthesis. Biomolecules 2024, 14, 410. [Google Scholar] [CrossRef]
- Kanungo, S.; Soares, N.; He, M.; Steiner, R.D. Sterol metabolism disorders and neurodevelopment-an update. Dev. Disabil. Res. Rev. 2013, 17, 197–210. [Google Scholar] [CrossRef]
- Pillinger, T.; McCutcheon, R.A.; Vano, L.; Mizuno, Y.; Arumuham, A.; Hindley, G.; Beck, K.; Natesan, S.; Efthimiou, O.; Cipriani, A.; et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 64–77. [Google Scholar] [CrossRef]
- Gunda, V.; Genaro-Mattos, T.C.; Kaushal, J.B.; Chirravuri-Venkata, R.; Natarajan, G.; Mallya, K.; Grandgenett, P.M.; Mirnics, K.; Batra, S.K.; Korade, Z.; et al. Ubiquitous Aberration in Cholesterol Metabolism across Pancreatic Ductal Adenocarcinoma. Metabolites 2022, 12, 47. [Google Scholar] [CrossRef]
- Zou, J.; Liu, S.; Long, J.; Yan, B. High DHCR7 Expression Predicts Poor Prognosis for Cervical Cancer. Comput. Math. Methods Med. 2022, 2022, 8383885. [Google Scholar] [CrossRef] [PubMed]
- Frankel, J.S.; Schwartz, T.L. Brexpiprazole and cariprazine: Distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther. Adv. Psychopharmacol. 2017, 7, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Khouzam, H.R. A review of trazodone use in psychiatric and medical conditions. Postgrad Med. 2017, 129, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, S.; Shimizu, N.; Miyahara, H.; Teranishi, H.; Umeda, R.; Yano, S.; Shimada, T.; Shiraishi, H.; Komiya, K.; Katoh, A.; et al. DHCR7 links cholesterol synthesis with neuronal development and axonal integrity. Biochem. Biophys. Res. Commun. 2024, 712–713, 149932. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, W.; Zeng, J.; Meng, J.; Shi, L.; Yang, S.; Chang, J.; Wang, C.; Xing, K.; Wen, J.; et al. Recent advances in the screening methods of NPC1L1 inhibitors. Biomed. Pharmacother. 2022, 155, 113732. [Google Scholar] [CrossRef]
- Gimpl, G.; Gehrig-Burger, K. Probes for studying cholesterol binding and cell biology. Steroids 2011, 76, 216–231. [Google Scholar] [CrossRef]
- Gimpl, G. Cholesterol-protein interaction: Methods and cholesterol reporter molecules. Subcell. Biochem. 2010, 51, 1–45. [Google Scholar]
- Fliesler, S.J.; Peachey, N.S.; Richards, M.J.; Nagel, B.A.; Vaughan, D.K. Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: Electrophysiologic, biochemical, and morphologic features. Arch. Ophthalmol. 2004, 122, 1190–1200. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Xu, L. Oxysterols and Retinal Degeneration in a Rat Model of Smith-Lemli-Opitz Syndrome: Implications for an Improved Therapeutic Intervention. Molecules 2018, 23, 2720. [Google Scholar] [CrossRef]
- Zhang, L.; Xiang, L.; Liu, Y.; Venkatraman, P.; Chong, L.; Cho, J.; Bonilla, S.; Jin, Z.; Pang, C.P.; Ko, K.M.; et al. A Naturally-Derived Compound Schisandrin B Enhanced Light Sensation in the pde6c Zebrafish Model of Retinal Degeneration. PLoS ONE 2016, 11, e0149663. [Google Scholar]
- Huang, W.; Wu, T.; Wu, R.; Peng, J.; Zhang, Q.; Shi, X.; Wu, K. Fish to learn: Insights into the effects of environmental chemicals on eye development and visual function in zebrafish. Environ. Sci. Pollut. Res. Int. 2023, 30, 73018–73030. [Google Scholar] [CrossRef] [PubMed]
- Schroepfer, G.J., Jr. Sterol biosynthesis. Annu. Rev. Biochem. 1981, 50, 585–621. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.N.; Paik, Y.K. Cholesterol biosynthesis from lanosterol. A concerted role for Sp1 and NF-Y-binding sites for sterol-mediated regulation of rat 7-dehydrocholesterol reductase gene expression. J. Biol. Chem. 2001, 276, 18153–18160. [Google Scholar] [CrossRef] [PubMed]
- Debeljak, N.; Fink, M.; Rozman, D. Many facets of mammalian lanosterol 14alpha-demethylase from the evolutionarily conserved cytochrome P450 family CYP51. Arch. Biochem. Biophys. 2003, 409, 159–171. [Google Scholar] [CrossRef]
- Liu, J.P. New functions of cholesterol binding proteins. Mol. Cell. Endocrinol. 2009, 303, 1–6. [Google Scholar] [CrossRef]
- Kim, C.H.; Ueshima, E.; Muraoka, O.; Tanaka, H.; Yeo, S.Y.; Huh, T.L.; Miki, N. Zebrafish elav/HuC homologue as a very early neuronal marker. Neurosci. Lett. 1996, 216, 109–112. [Google Scholar] [CrossRef]
- Janke, C.; Kneussel, M. Tubulin post-translational modifications: Encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 2010, 33, 362–372. [Google Scholar] [CrossRef]
- Boulanger-Weill, J.; Sumbre, G. Functional Integration of Newborn Neurons in the Zebrafish Optic Tectum. Front. Cell. Dev. Biol. 2019, 7, 57. [Google Scholar] [CrossRef]
- Kusumi, I.; Boku, S.; Takahashi, Y. Psychopharmacology of atypical antipsychotic drugs: From the receptor binding profile to neuroprotection and neurogenesis. Psychiatry Clin. Neurosci. 2015, 69, 243–258. [Google Scholar] [CrossRef]
- Ishima, T.; Futamura, T.; Ohgi, Y.; Yoshimi, N.; Kikuchi, T.; Hashimoto, K. Potentiation of neurite outgrowth by brexpiprazole, a novel serotonin-dopamine activity modulator: A role for serotonin 5-HT1A and 5-HT2A receptors. Eur. Neuropsychopharmacol. 2015, 25, 505–511. [Google Scholar] [CrossRef]
- Wiweger, M.; Majewski, L.; Adamek-Urbanska, D.; Wasilewska, I.; Kuznicki, J. Npc2-Deficient Zebrafish Reproduce Neurological and Inflammatory Symptoms of Niemann-Pick Type C Disease. Front. Cell. Neurosci. 2021, 15, 647860. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimmerman, N.; Marta, A.; Baker, C.; Korade, Z.; Mirnics, K.; Shibata, A. Investigating Psychopharmaceutical Effects on Early Vertebrate Development Using a Zebrafish Model System. J. Dev. Biol. 2025, 13, 22. https://doi.org/10.3390/jdb13030022
Zimmerman N, Marta A, Baker C, Korade Z, Mirnics K, Shibata A. Investigating Psychopharmaceutical Effects on Early Vertebrate Development Using a Zebrafish Model System. Journal of Developmental Biology. 2025; 13(3):22. https://doi.org/10.3390/jdb13030022
Chicago/Turabian StyleZimmerman, Nathan, Aaron Marta, Carly Baker, Zeljka Korade, Károly Mirnics, and Annemarie Shibata. 2025. "Investigating Psychopharmaceutical Effects on Early Vertebrate Development Using a Zebrafish Model System" Journal of Developmental Biology 13, no. 3: 22. https://doi.org/10.3390/jdb13030022
APA StyleZimmerman, N., Marta, A., Baker, C., Korade, Z., Mirnics, K., & Shibata, A. (2025). Investigating Psychopharmaceutical Effects on Early Vertebrate Development Using a Zebrafish Model System. Journal of Developmental Biology, 13(3), 22. https://doi.org/10.3390/jdb13030022







