Evolution of the Jawed Vertebrate (Gnathostomata) Stomach Through Gene Repertoire Loss: Findings from Agastric Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Sequence Retrieval and Selection of Gastric Genes
2.2. Gene-Loss Analysis
2.3. Sequence Alignments and Phylogenies
2.4. Selection Analysis (Ka/Ks Ratios)
3. Results
3.1. Conservation and Loss of the Gastrin Release Pathway and Novel Gastric Gene Repertoire in Gastric and Agastric Jawed Vertebrates (Gnathostomata)
3.2. Sequence Evolution and Selection Pressures in the Gastrin Release Pathway Between Gastric and Agastric Jawed Vertebrates (Gnathostomata)
4. Discussion
4.1. Gastrin Physiological Pathway: Correlations with Gastric Phenotype, Known Functions and Genetic Dispensability
4.2. Evolution of Gastric Genes Implicated in the Secondary Loss of Gastric Phenotype in Jawed Vertebrates (Gnathostomata)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gonçalves, O.; Freitas, R.; Ferreira, P.; Araujo, M.; Zhang, G.; Mazan, S.; Cohn, M.J.; Castro, L.F.C.; Wilson, J.M. Molecular ontogeny of the stomach in the castshark Scyliorhinus canicular. Sci. Rep. 2019, 9, 586. [Google Scholar] [CrossRef]
- Nakazawa, K.; Yamazawa, T.; Moriyama, Y.; Ogura, Y.; Kawai, N.; Sasakura, Y.; Saiga, H. Formation of the digestive tract in Ciona intestinalis includes two distinct morphogenic processes between its anterior and posterior parts. Dev. Dyn. 2013, 242, 1172–1183. [Google Scholar] [CrossRef]
- Nakayama, S.; Ogasawara, M. Compartmentalized expression patterns of pancreatic- and gastric-related genes in the alimentary canal of the ascidian Ciona intestinalis: Evolutionary insights into the functional regionality of the gastrointestinal tract in Olfactores. Cell Tissue Res. 2017, 370, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Koelz, H.R. Gastric Acid in Vertebrates. Scand. J. Gastroenterol. 1992, 27, 2–6. [Google Scholar] [CrossRef]
- Karasov, W.H.; Douglas, A.E.; Ronald, T. Comparative Digestive Physiology. Compr. Physiol. 2013, 3, 741–783. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Castro, L.F.C. The Multifunctional Gut of Fish; Academic Press: London, UK, 2010. [Google Scholar]
- Castro, L.F.C.; Gonçalves, O.; Mazan, S.; Tay, B.H.; Venkatesh, B.; Wilson, J.M. Recurrent gene loss correlates with the evolution of stomach phenotypes in gnathostome history. Proc. R. Soc. B 2014, 281, 20132669. [Google Scholar] [CrossRef]
- Griffiths, M. The Biology of Monotremes; Academic Press: New York, NY, USA, 1978; pp. 77–81. [Google Scholar]
- Ordoñez, G.R.; Hillier, L.D.W.; Warren, W.C.; Grützner, F.; López-Otín, C.; Puente, X.S. Loss of genes implicated in gastric function during platypus evolution. Genome Biol. 2008, 9, R81. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.L.; Peura, D.A. Control of Gastric Acid Secretion in Health and Disease. Gastroenterology 2008, 134, 1842–1860. [Google Scholar] [CrossRef]
- Zhou, Y.; Shearwin-Whyatt, L.; Li, J.; Song, Z.; Hayakawa, T.; Stevens, D.; Fenelon, J.C.; Peel, E.; Cheng, Y.; Pajpach, F.; et al. Platypus and echidna genomes reveal mammalian biology and evolution. Nature 2021, 592, 756–762. [Google Scholar] [CrossRef]
- Duan, S.; Rico, K.; Merchant, J.L. Gastrin: From Physiology to Gastrointestinal Malignancies. Function 2022, 3, zqab062. [Google Scholar] [CrossRef]
- Kim, T.H.; Shivdasani, R.A. Stomach development, stem cells and disease. Development 2016, 143, 554–565. [Google Scholar] [CrossRef]
- McCracken, K.W.; Wells, J.M. Mechanisms of embryonic stomach development. Semin. Cell Dev. Biol. 2017, 66, 36–42. [Google Scholar] [CrossRef]
- Agace, W.W.; McCoy, K.D. Regionalized Development and Maintenance of the Intestinal Adaptive Immune Landscape. Immunity 2017, 46, 532–548. [Google Scholar] [CrossRef] [PubMed]
- Menheniott, T.R.; Kurklu, B.; Giraud, A.S. Gastrokines: Stomach-specific proteins with putative homeostatic and tumor suppressor roles. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G109–G121. [Google Scholar] [CrossRef]
- Hoffmann, W. Trefoil factor family (TFF) peptides and their diverse molecular functions in mucus barrier protection and more: Changing the paradigm. Int. J. Mol. Sci. 2020, 21, 4535. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef]
- Lu, S.H.; Chen, Y.L.; Shun, C.T.; Lai, J.N.; Peng, S.Y.; Lai, P.L.; Hsu, H.C. Expression and prognostic significance of gastric-specific annexin A10 in diffuse- and intestinal-type gastric carcinoma. J. Gastroenterol. Hepatol. 2011, 26, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Oidovsambuu, O.; Nyamsuren, G.; Liu, S.; Goring, W.; Engel, W.; Adham, I.M.; Gires, O. Adhesion protein VSIG1 is required for the proper differentiation of glandular gastric epithelia. PLoS ONE 2011, 6, e25908. [Google Scholar] [CrossRef]
- Kato, A.; Pipil, S.; Ota, C.; Kusakabe, M.; Watanabe, T.; Nagashima, A.; Chen, A.P.; Islam, Z.; Hayashi, N.; Wong, M.K.S.; et al. Convergent gene losses and pseudogenizations in multiple lineages of stomachless fishes. Commun. Biol. 2024, 7, 408. [Google Scholar] [CrossRef]
- Jiang, Z.; Lossie, A.C.; Applegate, T.J. Evolution of Trefoil Factor(s): Genetic and Spatio-Temporal Expression of Trefoil Factor 2 in the Chicken (Gallus Gallus Domesticus). PLoS ONE 2011, 6, e22691. [Google Scholar] [CrossRef]
- Geahlen, J.H.; Lapid, C.; Thorell, K.; Nikolskiy, I.; Huh, W.J.; Oates, E.L.; Lennerz, J.K.M.; Tian, X.; Weis, V.G.; Khurana, S.S.; et al. Evolution of the human gastrokine locus and confounding factors regarding the pseudogenicity of GKN3. Physiol. Genom. 2013, 45, 667–683. [Google Scholar] [CrossRef]
- Carbon, S.; Ireland, A.; Mungall, C.J.; Shu, S.; Marshall, B.; Lewis, S.; AmiGO Hub; Web Presence Working Group. AmiGO: Online access to ontology and annotation data. Bioinformatics 2009, 25, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 1, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Hecker, N.; Roscito, J.G.; Foerster, L.; Langer, B.; Hiller, M. A genomics approach reveals insights into the importance of gene losses for mammalian adaptations. Nat. Commun. 2018, 9, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Marín, B.; Rakijas, J.B.; Tyagi, A.; Pandey, A.; Hanschen, E.R.; Anderson, J.; Heffel, M.G.; Platt, T.G.; Olson, B.J.S.C. Gene loss during a transition to multicellularity. Sci. Rep. 2023, 13, 5268. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 12, 421. [Google Scholar] [CrossRef]
- Katz, K.; Shutov, O.; Lapoint, R.; Kimelman, M.; Brister, J.R.; O’Sullivan, C. The Sequence Read Archive: A decade of more explosive growth. Nucleic Acids Res. 2022, 50, D387–D390. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haasler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Mikita, S.; Torrents, D.; Bork, P. PAL2NAL: Robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 2006, 34, W609–W612. [Google Scholar] [CrossRef]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, A.H. Phylogeny of the Cholecystokinin/Gastrin Family. Front. Neuroendocr. 1998, 19, 73–99. [Google Scholar] [CrossRef]
- Cui, T.; Wang, J.; Hu, Z.; Chen, X. Expression of gastrin and cholecystokinin B receptor in Lateolabrax maculatus. Aquac. Fish. 2023, 8, 492–497. [Google Scholar] [CrossRef]
- Nässel, D.R.; Wu, S.F. Cholecystokinin/sulfakinin peptide signaling: Conserved roles at the intersection between feeding, mating and aggression. Cell. Mol. Life Sci. 2022, 79, 188. [Google Scholar] [CrossRef]
- Dimaline, R.; Varro, A. Novel roles of gastrin. J. Physiol. 2014, 592, 2951–2958. [Google Scholar] [CrossRef]
- Friis-Hansen, L. Lessons from the gastrin knockout mice. Regul. Pept. 2007, 139, 5–22. [Google Scholar] [CrossRef]
- Dann, J.; Qu, Z.; Shearwin-Whyatt, L.; van der Ploeg, R.; Grützner, F. Pseudogenization of NK3 homeobox 2 (Nkx3.2) in monotremes provides insight into unique gastric anatomy and physiology. Open Biol. 2024, 14, 240071. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.R.; Xu, S.H.; Tan, Z.L.; Yin, X.F.; He, Q.Y. Proteomics characterization of gastrokine 1-induced growth inhibition of gastric cancer cells. Proteomics 2011, 11, 3657–3664. [Google Scholar] [CrossRef]
- Kim, O.; Yoon, J.H.; Choi, W.S.; Ashktorab, H.; Smoot, D.T.; Nam, S.W.; Lee, J.Y.; Park, W.S. GKN2 Contributes to the Homeostasis of Gastric Mucosa by Inhibiting GKN1 Activity. J. Cell. Physiol. 2013, 229, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W. TFF2, a MUC6-binding lectin stabilizing the gastric mucus barrier and more (Review). Int. J. Oncol. 2015, 47, 806–816. [Google Scholar] [CrossRef]
- Jahan, R.; Shah, A.; Kisling, S.G.; Macha, M.A.; Thayer, S.; Batra, S.K.; Sukhwinder, K. Odyssey of trefoil factors in cancer: Diagnostic and therapeutic implications. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188362. [Google Scholar] [CrossRef]
- Soutto, M.; Belkhiri, A.; Piazuelo, M.B.; Blanca, M.; Schneider, B.G.; Peng, D.; Jiang, A.; Washington, M.K.; Kokoye, Y.; Crowe, S.E.; et al. Loss of TFF1 is associated with activation of NF-κB-mediated inflammation and gastric neoplasia in mice and humans. J. Clin. Investig. 2011, 121, 1753–1767. [Google Scholar] [CrossRef]
- Moss, S.E.; Morgan, R.O. The annexins. Genome Biol. 2004, 5, 219–735. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, P.J.; Jung, K.H.; Noh, J.H.; Eun, J.W.; Bae, H.J.; Xie, H.J.; Shan, J.M.; Ping, W.Y.; Park, W.S.; et al. Decreased expression of Annexin A10 in gastric cancer and its overexpression in tumor cell growth expression. Oncol. Rep. 2010, 24, 607–612. [Google Scholar] [PubMed]
- Galura, G.M.; Chavez, L.O.; Robles, A.; McCallum, R. Gastroduodenal Injury: Role of Protective Factors. Curr. Gastroenterol. Rep. 2019, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Lin, Y.L.; Cheng, Y.C.; Chen, C.C.; Lin, L.I.; Tseng, L.H.; Cheng, M.L.; Liau, J.Y.; Jeng, Y.M. Aberrant expression of annexin A10 is closely related to gastric phenotype in serrated pathway to colorectal carcinoma. Mod. Pathol. 2015, 28, 268–278. [Google Scholar] [CrossRef] [PubMed]

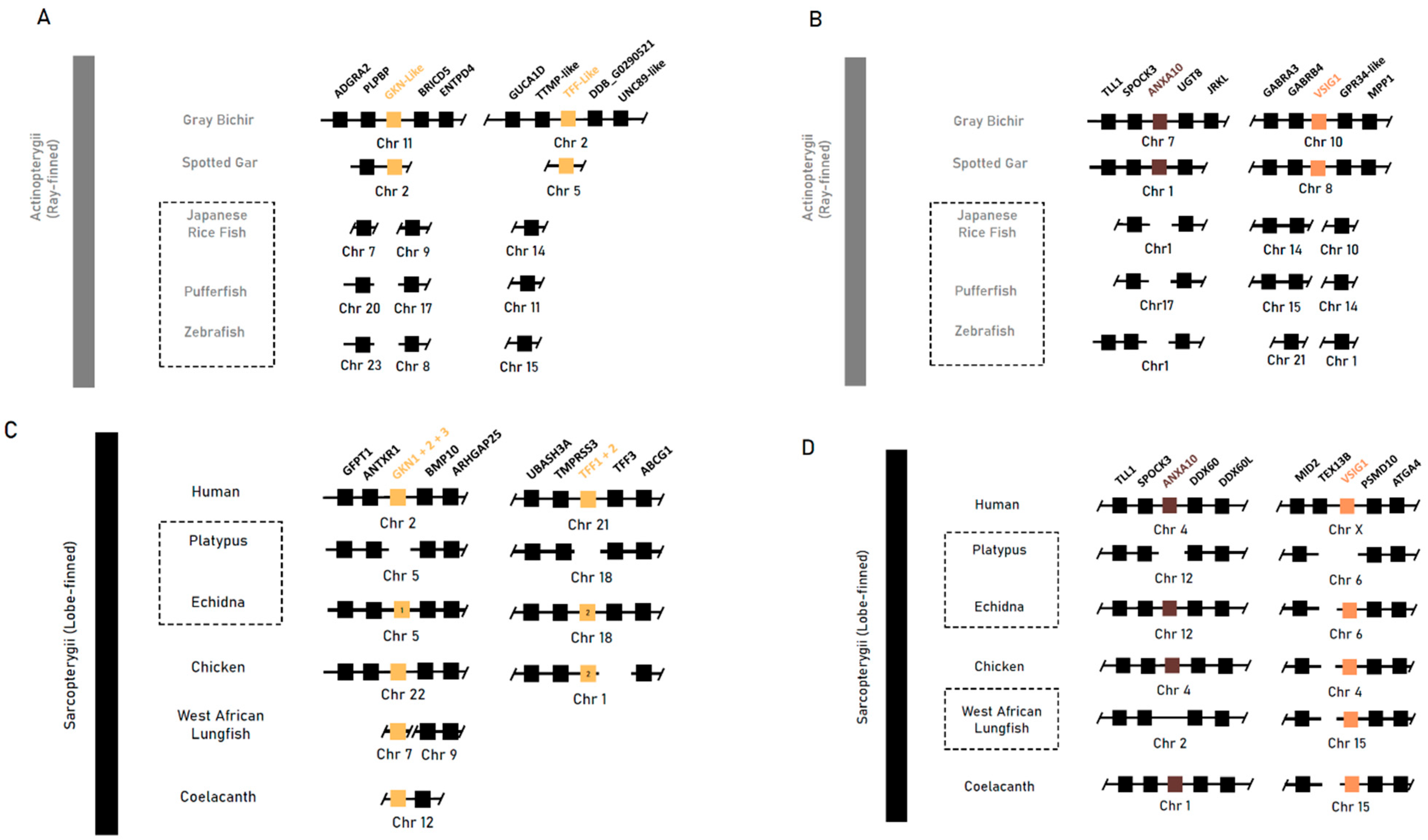
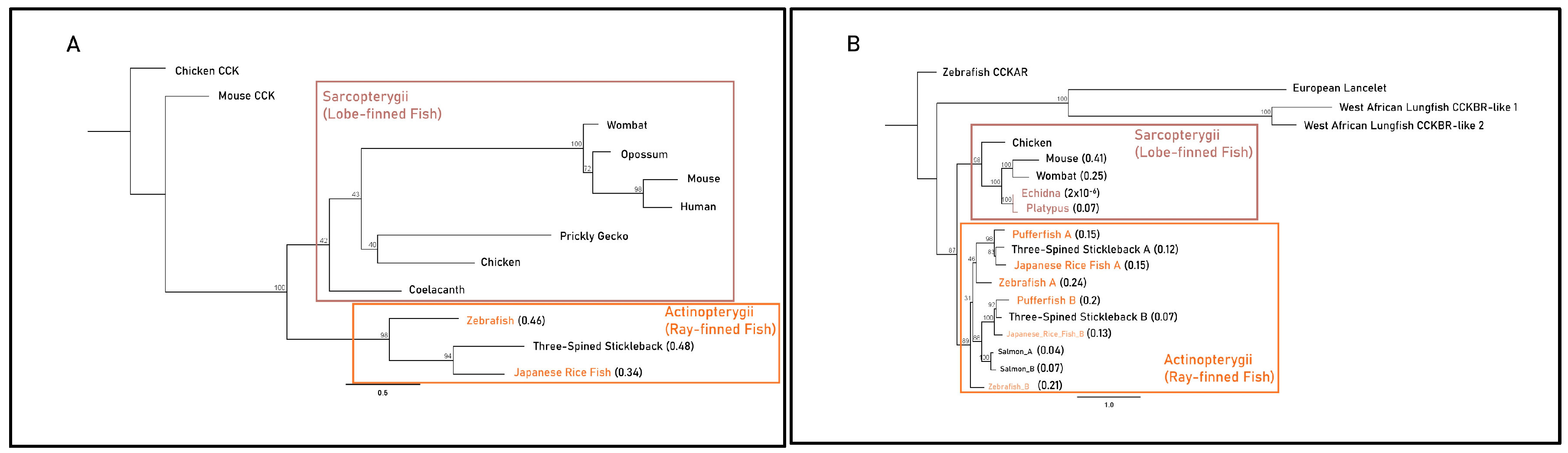

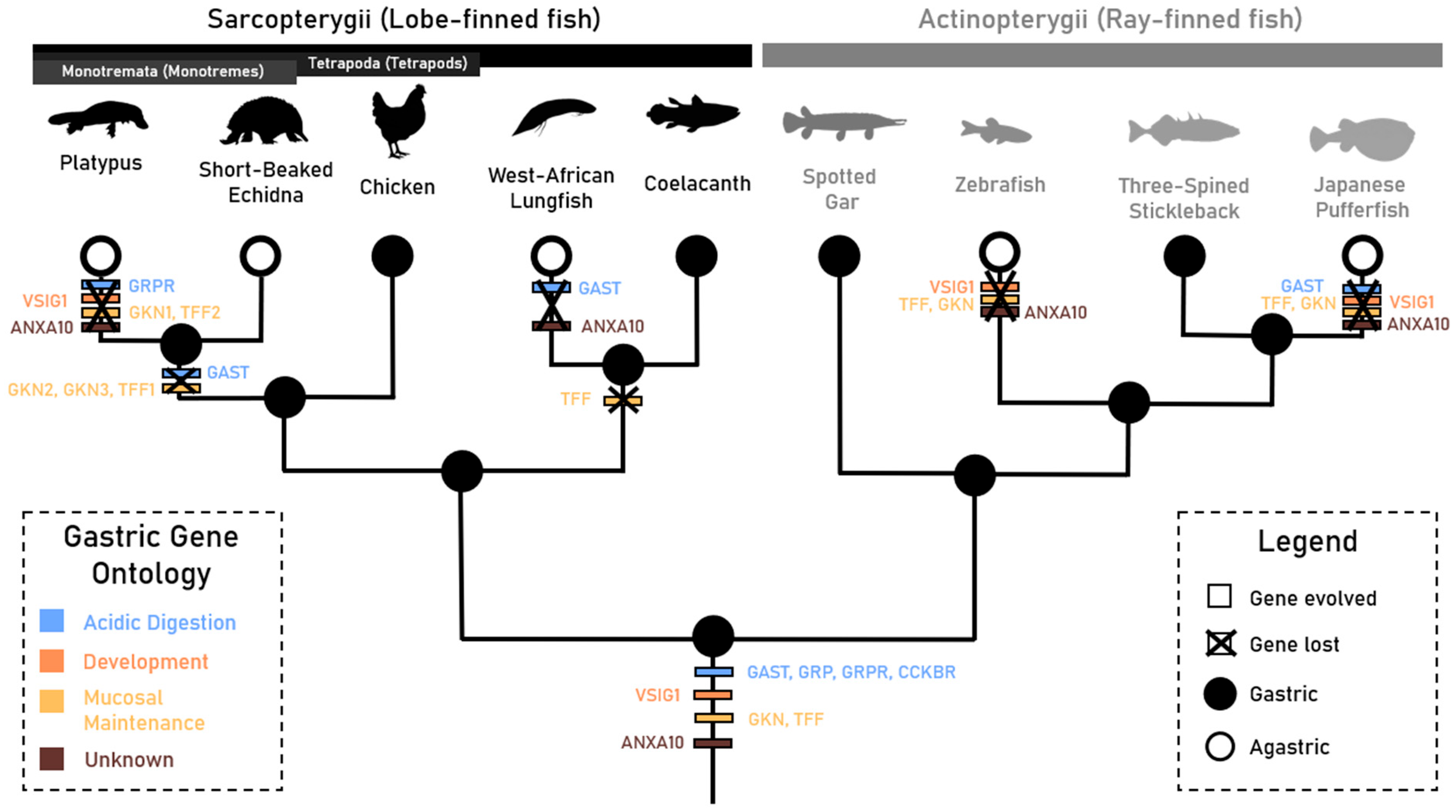
| Gene | Foreground Branch | LRT p Value | Foreground Ka/Ks (ω1) | Background Ka/Ks (ω0) |
|---|---|---|---|---|
| Cckbr | Japanese Rice Fish B, Pufferfish B and Three-Spined Stickleback B | 0.04 | 0.19 | 0.08 |
| Cckbr | Three-Spined Stickleback B | 1.12 × 10−5 | 0.33 | 0.07 |
| Cckbr | Three-Spined Stickleback A | 0.01 | 3 × 10−3 | 0.08 |
| Grp | Tetrapoda (Mammals and Reptiles) | 2.17 × 10−5 | 8.9 × 10−4 | 0.28 |
| Grp | Mammalia | 0.03 | 0.02 | 0.24 |
| Grp | Mouse | 0.04 | 0.06 | 0.24 |
| Grpr | Pufferfish | 0.02 | 0.11 | 0.04 |
| Grpr | Sarcopterygii (Mammals, Reptiles and Lobe-Finned Fish) | 8.93 × 10−7 | 1.94 × 10−3 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dann, J.; Grützner, F. Evolution of the Jawed Vertebrate (Gnathostomata) Stomach Through Gene Repertoire Loss: Findings from Agastric Species. J. Dev. Biol. 2025, 13, 27. https://doi.org/10.3390/jdb13030027
Dann J, Grützner F. Evolution of the Jawed Vertebrate (Gnathostomata) Stomach Through Gene Repertoire Loss: Findings from Agastric Species. Journal of Developmental Biology. 2025; 13(3):27. https://doi.org/10.3390/jdb13030027
Chicago/Turabian StyleDann, Jackson, and Frank Grützner. 2025. "Evolution of the Jawed Vertebrate (Gnathostomata) Stomach Through Gene Repertoire Loss: Findings from Agastric Species" Journal of Developmental Biology 13, no. 3: 27. https://doi.org/10.3390/jdb13030027
APA StyleDann, J., & Grützner, F. (2025). Evolution of the Jawed Vertebrate (Gnathostomata) Stomach Through Gene Repertoire Loss: Findings from Agastric Species. Journal of Developmental Biology, 13(3), 27. https://doi.org/10.3390/jdb13030027






