Drosophila Males Differentially Express Small Proteins Regulating Stem Cell Division Frequency in Response to Mating
Abstract
1. Introduction
2. Methods
2.1. Fly Husbandry
2.2. UAS/Gal4-Expression Studies and Mating Experiments
2.3. Immunofluorescence and Microscopy
2.4. Statistical Analysis of GSc Divisions
2.5. Generation of RNA-Profiles
3. Results
3.1. Mating Caused Differential Gene Expression
3.2. Mating Increased Expression of Secreted Proteins
3.3. Accessory Glands Were Dispensable for the Increase in MIGSC
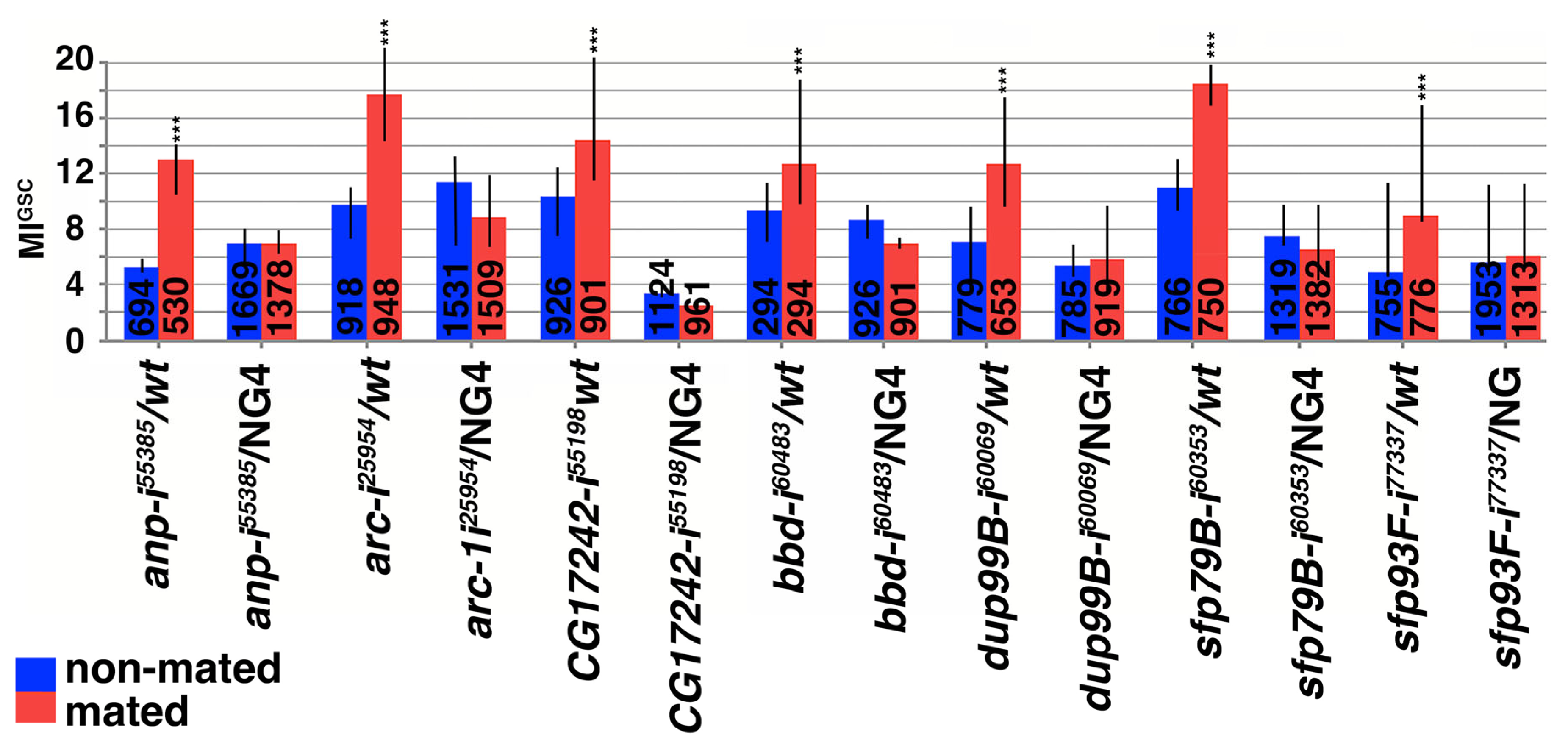
3.4. Small Proteins Were Required for the Increase in MIGSC upon Mating
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, N.W.; Gray, B.; Zuk, M. Acoustic experience shapes alternative mating tactics and reproductive investment in male field crickets. Curr. Biol. 2010, 20, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Dziminski, M.A.; Roberts, J.D.; Beveerdige, M.; Simmons, L.W. Among-population variation between sperm competition and ejaculate expenditure in fropgs. Behav. Ecol. 2010, 21, 322–328. [Google Scholar] [CrossRef]
- Pitcher, T.E.; Dunn, P.O.; Whittingham, L.A. Sperm competition and the evolution of testes size in birds. J. Evol. Biol. 2005, 18, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.W.; Tokuyasu, K.T.; Lindsley, D.L.; Garavito, M. The germinal proliferation center in the testis of Drosophila melanogaster. J. Ultrastruct. Res. 1979, 69, 180–190. [Google Scholar] [CrossRef]
- de Cuevas, M.; Matunis, E.L. The stem cell niche: Lessons from the Drosophila testis. Development 2011, 138, 2861–2869. [Google Scholar] [CrossRef] [PubMed]
- Losick, V.P.; Morris, L.X.; Fox, D.T.; Spradling, A. Drosophila stem cell niches: A decade of discovery suggests a unified view of stem cell regulation. Dev. Cell 2011, 21, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Kiger, A.A.; Jones, D.L.; Schulz, C.; Rogers, M.B.; Fuller, M.T. Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 2001, 294, 2542–2545. [Google Scholar] [CrossRef]
- Kawase, E.; Wong, M.D.; Ding, B.C.; Xie, T. Gbb/Bmp signaling is essential for maintaining germline stem cells and for repressing bam transcription in the Drosophila testis. Development 2004, 131, 1365–1375. [Google Scholar] [CrossRef]
- Shivdasani, A.A.; Ingham, P.W. Regulation of stem cell maintenance and transit amplifying cell proliferation by tgf-beta signaling in Drosophila spermatogenesis. Curr. Biol. 2003, 13, 2065–2072. [Google Scholar] [CrossRef]
- Schulz, C.; Kiger, A.A.; Tazuke, S.I.; Yamashita, Y.M.; Pantalena-Filho, L.C.; Jones, D.L.; Wood, C.G.; Fuller, M.T. A misexpression screen reveals effects of bag-of-marbles and TGF beta class signaling on the Drosophila male germ-line stem cell lineage. Genetics 2004, 167, 707–723. [Google Scholar] [CrossRef]
- Tulina, N.; Matunis, E. Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 2001, 294, 2546–2549. [Google Scholar] [CrossRef] [PubMed]
- Leatherman, J.L.; Dinardo, S. Zfh-1 controls somatic stem cell self-renewal in the Drosophila testis and nonautonomously influences germline stem cell self-renewal. Cell Stem Cell 2008, 3, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Amoyel, M.; Sanny, J.; Burel, M.; Bach, E.A. Hedgehog is required for CySC self-renewal but does not contribute to the GSC niche in the Drosophila testis. Development 2013, 140, 56–65. [Google Scholar] [CrossRef]
- Yamashita, Y.M.; Jones, D.L.; Fuller, M.T. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science 2003, 301, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Wood, C.G.; Jones, D.L.; Tazuke, S.I.; Fuller, M.T. Signaling from germ cells mediated by the rhomboid homolog stet organizes encapsulation by somatic support cells. Development 2002, 129, 4523–4534. [Google Scholar] [CrossRef]
- Sarkar, A.; Parikh, N.; Hearn, S.A.; Fuller, M.T.; Tazuke, S.I.; Schulz, C. Antagonistic roles of Rac and Rho in organizing the germ cell microenvironment. Curr. Biol. 2007, 17, 1253–1258. [Google Scholar] [CrossRef]
- Fuller, M.T. Spermatogenesis in Drosophila. In The Development of Drosophila melanogaster; Bate, M., Martinez Arias, A., Eds.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 1993; pp. 71–148. [Google Scholar]
- Zoller, R.; Schulz, C. The Drosophila cyst stem cell lineage: Partners behind the scenes? Spermatogenesis 2012, 2, 145–157. [Google Scholar] [CrossRef]
- Fabrizio, J.J.; Boyle, M.; DiNardo, S. A somatic role for eyes absent (eya) and sine oculis (so) in Drosophila spermatocyte development. Dev. Biol. 2003, 258, 117–128. [Google Scholar] [CrossRef]
- Hudson, A.G.; Parrott, B.B.; Qian, Y.; Schulz, C. A temporal signature of epidermal growth factor signaling regulates the differentiation of germline cells in testes of Drosophila melanogaster. PLoS ONE 2013, 8, e70678. [Google Scholar] [CrossRef]
- Qian, Y.; Dominado, N.; Zoller, R.; Ng, C.; Kudyba, K.; Siddall, N.A.; Hime, G.R.; Schulz, C. Ecdysone signaling opposes epidermal growth factor signaling in regulating cyst differentiation in the male gonad of Drosophila melanogaster. Dev. Biol. 2014, 394, 217–227. [Google Scholar] [CrossRef]
- Parrott, B.B.; Hudson, A.; Brady, R.; Schulz, C. Control of germline stem cell division frequency–A novel, developmentally regulated role for epidermal growth factor signaling. PLoS ONE 2012, 7, e36460. [Google Scholar] [CrossRef] [PubMed]
- McLeod, C.J.; Wang, L.; Wong, C.; Jones, D.L. Stem cell dynamics in response to nutrient availability. Curr. Biol. 2010, 20, 2100–2105. [Google Scholar] [CrossRef]
- Malpe, M.S.; McSwain, L.F.; Kudyba, K.; Ng, C.L.; Nicholson, J.; Brady, M.; Qian, Y.; Choksi, V.; Hudson, A.G.; Parrott, B.B.; et al. G-protein signaling is required for increasing germline stem cell division frequency in response to mating in Drosophila males. Sci. Rep. 2020, 10, 3888. [Google Scholar] [CrossRef] [PubMed]
- Aston, H.M.; Schulz, C. Drosophila male germline stem cells and their transit amplifying daughters depend on G-protein signaling for increasing their mitotic indices in response to mating. Arch. Stem Cells Ther. 2020, 1, 19–22. [Google Scholar]
- Storz, G.; Wolf, Y.I.; Ramamurthi, K.S. Small proteins can no longer be ignored. Annu. Rev. Biochem. 2014, 83, 753–777. [Google Scholar] [CrossRef]
- Ozturk-Colak, A.; Marygold, S.J.; Antonazzo, G.; Attrill, H.; Goutte-Gattat, D.; Jenkins, V.K.; Matthews, B.B.; Millburn, G.; Dos Santos, G.; Tabone, C.J.; et al. FlyBase: Updates to the Drosophila genes and genomes database. Genetics 2024, 227, iyad211. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef]
- von Heijne, G. Signal sequences. The limits of variation. J. Mol. Biol. 1985, 184, 99–105. [Google Scholar] [CrossRef]
- Nagai, K.; Oubridge, C.; Kuglstatter, A.; Menichelli, E.; Isel, C.; Jovine, L. Structure, function and evolution of the signal recognition particle. EMBO J. 2003, 22, 3479–3485. [Google Scholar] [CrossRef]
- Akopian, D.; Shen, K.; Zhang, X.; Shan, S.O. Signal recognition particle: An essential protein-targeting machine. Annu. Rev. Biochem. 2013, 82, 693–721. [Google Scholar] [CrossRef]
- Janda, C.Y.; Li, J.; Oubridge, C.; Hernandez, H.; Robinson, C.V.; Nagai, K. Recognition of a signal peptide by the signal recognition particle. Nature 2010, 465, 507–510. [Google Scholar] [CrossRef]
- Phelps, C.B.; Brand, A.H. Ectopic gene expression in Drosophila using GAL4 system. Methods 1998, 14, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Van Doren, M.; Williamson, A.L.; Lehmann, R. Regulation of zygotic gene expression in Drosophila primordial germ cells. Curr. Biol. 1998, 8, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Lyford, G.L.; Yamagata, K.; Kaufmann, W.E.; Barnes, C.A.; Sanders, L.K.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Lanahan, A.A.; Worley, P.F. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron 1995, 14, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Saraswati, S.; Adolfsen, B.; Littleton, J.T. Genome-wide transcriptional changes associated with enhanced activity in the Drosophila nervous system. Neuron 2005, 48, 91–107. [Google Scholar] [CrossRef]
- Mattaliano, M.D.; Montana, E.S.; Parisky, K.M.; Littleton, J.T.; Griffith, L.C. The Drosophila ARC homolog regulates behavioral responses to starvation. Mol. Cell. Neurosci. 2007, 36, 211–221. [Google Scholar] [CrossRef]
- Hurtado, J.; Almeida, F.C.; Belliard, S.A.; Revale, S.; Hasson, E. Research gaps and new insights in the evolution of Drosophila seminal fluid proteins. Insect Mol. Biol. 2022, 31, 139–158. [Google Scholar] [CrossRef]
- Wolfner, M.F. Tokens of love: Functions and regulation of Drosophila male accessory gland products. Insect Biochem. Mol. Biol. 1997, 27, 179–192. [Google Scholar] [CrossRef]
- Date-Ito, A.; Kasahara, K.; Sawai, H.; Chigusa, S.I. Rapid evolution of the male-specific antibacterial protein andropin gene in Drosophila. J. Mol. Evol. 2002, 54, 665–670. [Google Scholar] [CrossRef]
- Findlay, G.D.; MacCoss, M.J.; Swanson, W.J. Proteomic discovery of previously unannotated, rapidly evolving seminal fluid genes in Drosophila. Genome Res. 2009, 19, 886–896. [Google Scholar] [CrossRef]
- Lin, S.J.H.; Fulzele, A.; Cohen, L.B.; Bennett, E.J.; Wasserman, S.A. Bombardier Enables Delivery of Short-Form Bomanins in the Drosophila Toll Response. Front. Immunol. 2019, 10, 3040. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.; Jiang, H.; Kanost, M.R.; Wang, Y. Serine proteases and their homologs in the Drosophila melanogaster genome: An initial analysis of sequence conservation and phylogenetic relationships. Gene 2003, 304, 117–131. [Google Scholar] [CrossRef]
- Findlay, G.D.; Yi, X.; Maccoss, M.J.; Swanson, W.J. Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol. 2008, 6, e178. [Google Scholar] [CrossRef] [PubMed]
- Avila, F.W.; Cohen, A.B.; Ameerudeen, F.S.; Duneau, D.; Suresh, S.; Mattei, A.L.; Wolfner, M.F. Retention of Ejaculate by Drosophila melanogaster Females Requires the Male-Derived Mating Plug Protein PEBme. Genetics 2015, 200, 1171–1179. [Google Scholar] [CrossRef]
- Rexhepaj, A.; Liu, H.; Peng, J.; Choffat, Y.; Kubli, E. The sex-peptide DUP99B is expressed in the male ejaculatory duct and in the cardia of both sexes. Eur. J. Biochem. 2003, 270, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Richmond, R.C.; Gilbert, D.G.; Sheehan, K.B.; Gromko, M.H.; Butterworth, F.M. Esterase 6 and reproduction in Drosophila melanogaster. Science 1980, 207, 1483–1485. [Google Scholar] [CrossRef]
- Dafforn, T.R.; Della, M.; Miller, A.D. The molecular interactions of heat shock protein 47 (Hsp47) and their implications for collagen biosynthesis. J. Biol. Chem. 2001, 276, 49310–49319. [Google Scholar] [CrossRef]
- Zhou, A.; Wei, Z.; Read, R.J.; Carrell, R.W. Structural mechanism for the carriage and release of thyroxine in the blood. Proc. Natl. Acad. Sci. USA 2006, 103, 13321–13326. [Google Scholar] [CrossRef]
- Jenkins, V.K.; Larkin, A.; Thurmond, J.; FlyBase, C. Using FlyBase: A Database of Drosophila Genes and Genetics. Methods Mol. Biol. 2022, 2540, 1–34. [Google Scholar] [CrossRef]
- Hatfield, S.D.; Shcherbata, H.R.; Fischer, K.A.; Nakahara, K.; Carthew, R.W.; Ruohola-Baker, H. Stem cell division is regulated by the microRNA pathway. Nature 2005, 435, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Noll, M. Dual role of the Pax gene paired in accessory gland development of Drosophila. Development 2002, 129, 339–346. [Google Scholar] [CrossRef]
- Hsu, H.J.; LaFever, L.; Drummond-Barbosa, D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev. Biol. 2008, 313, 700–712. [Google Scholar] [CrossRef]
- Poiani, A. Complexity of the seminal fluid: A review. Behav. Ecol. Sociobiol. 2006, 60, 289–310. [Google Scholar] [CrossRef]
- Tram, U.; Wolfner, M.F. Male seminal fluid proteins are essential for sperm storage in Drosophila melanogaster. Genetics 1999, 153, 837–844. [Google Scholar] [CrossRef]
- Xue, L.; Noll, M. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proc. Natl. Acad. Sci. USA 2000, 97, 3272–3275. [Google Scholar] [CrossRef] [PubMed]
- Wolfner, M.F. The gifts that keep on giving: Physiological functions and evolutionary dynamics of male seminal proteins in Drosophila. Heredity 2002, 88, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Heifetz, Y.; Tram, U.; Wolfner, M.F. Male contributions to egg production: The role of accessory gland products and sperm in Drosophila melanogaster. Proc. Biol. Sci. 2001, 268, 175–180. [Google Scholar] [CrossRef]
- Lung, O.; Kuo, L.; Wolfner, M.F. Drosophila males transfer antibacterial proteins from their accessory gland and ejaculatory duct to their mates. J. Insect Physiol. 2001, 47, 617–622. [Google Scholar] [CrossRef]
- Galindo, M.I.; Pueyo, J.I.; Fouix, S.; Bishop, S.A.; Couso, J.P. Peptides encoded by short ORFs control development and define a new eukaryotic gene family. PLoS Biol. 2007, 5, e106. [Google Scholar] [CrossRef]
- Pueyo, J.I.; Couso, J.P. Tarsal-less peptides control Notch signalling through the Shavenbaby transcription factor. Dev. Biol. 2011, 355, 183–193. [Google Scholar] [CrossRef]
- Kondo, T.; Plaza, S.; Zanet, J.; Benrabah, E.; Valenti, P.; Hashimoto, Y.; Kobayashi, S.; Payre, F.; Kageyama, Y. Small peptides switch the transcriptional activity of Shavenbaby during Drosophila embryogenesis. Science 2010, 329, 336–339. [Google Scholar] [CrossRef]
- Somssich, M.; Je, B.I.; Simon, R.; Jackson, D. CLAVATA-WUSCHEL signaling in the shoot meristem. Development 2016, 143, 3238–3248. [Google Scholar] [CrossRef]
- Deng, C.; Chen, H.; Yang, N.; Feng, Y.; Hsueh, A.J. Apela Regulates Fluid Homeostasis by Binding to the APJ Receptor to Activate Gi Signaling. J. Biol. Chem. 2015, 290, 18261–18268. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Q.; Yan, H.; Huang, J.; Wang, Z. Apela improves cardiac and renal function in mice with acute myocardial infarction. J. Cell. Mol. Med. 2020, 24, 10382–10390. [Google Scholar] [CrossRef]
- Samakovlis, C.; Kylsten, P.; Kimbrell, D.A.; Engstrom, A.; Hultmark, D. The andropin gene and its product, a male-specific antibacterial peptide in Drosophila melanogaster. EMBO J. 1991, 10, 163–169. [Google Scholar] [CrossRef]
- Ashley, J.; Cordy, B.; Lucia, D.; Fradkin, L.G.; Budnik, V.; Thomson, T. Retrovirus-like Gag Protein Arc1 Binds RNA and Traffics across Synaptic Boutons. Cell 2018, 172, 262–274.e211. [Google Scholar] [CrossRef]
- Erlendsson, S.; Morado, D.R.; Cullen, H.B.; Feschotte, C.; Shepherd, J.D.; Briggs, J.A.G. Structures of virus-like capsids formed by the Drosophila neuronal Arc proteins. Nat. Neurosci. 2020, 23, 172–175. [Google Scholar] [CrossRef]
- Saudan, P.; Hauck, K.; Soller, M.; Choffat, Y.; Ottiger, M.; Sporri, M.; Ding, Z.; Hess, D.; Gehrig, P.M.; Klauser, S.; et al. Ductus ejaculatorius peptide 99B (DUP99B), a novel Drosophila melanogaster sex-peptide pheromone. Eur. J. Biochem. 2002, 269, 989–997. [Google Scholar] [CrossRef]
- Yuan, L.L.; Chen, X.; Zong, Q.; Zhao, T.; Wang, J.L.; Zheng, Y.; Zhang, M.; Wang, Z.; Brownlie, J.C.; Yang, F.; et al. Quantitative Proteomic Analyses of Molecular Mechanisms Associated with Cytoplasmic Incompatibility in Drosophila melanogaster Induced by Wolbachia. J. Proteome Res. 2015, 14, 3835–3847. [Google Scholar] [CrossRef]
- Ueda, M.; Sato, T.; Ohkawa, Y.; Inoue, Y.H. Identification of miR-305, a microRNA that promotes aging, and its target mRNAs in Drosophila. Genes. Cells Devoted Mol. Cell. Mech. 2018, 23, 80–93. [Google Scholar] [CrossRef]
- MacMillan, H.A.; Knee, J.M.; Dennis, A.B.; Udaka, H.; Marshall, K.E.; Merritt, T.J.; Sinclair, B.J. Cold acclimation wholly reorganizes the Drosophila melanogaster transcriptome and metabolome. Sci. Rep. 2016, 6, 28999. [Google Scholar] [CrossRef]
- Mohorianu, I.; Bretman, A.; Smith, D.T.; Fowler, E.K.; Dalmay, T.; Chapman, T. Genomic responses to the socio-sexual environment in male Drosophila melanogaster exposed to conspecific rivals. RNA 2017, 23, 1048–1059. [Google Scholar] [CrossRef]


| Gene ID | Length | Biological/Molecular Process | FlyBase Predicted Location | Potential Secretion Signal Sequence |
|---|---|---|---|---|
| activity-regulated cytoskeleton associated proteins arc1/3 | 254 aa 124 aa | Metabolic stress [36,37,38] | Cytoplasm | N/A |
| acessory gland protein (acp) 54A1 | 45 aa | Sexual reproduction [39] | MLINRHSCSKLLSLMVLLCLAFDLKPVSAM | |
| acessory gland protein (acp) 76A | 388 aa | Non-inhibitory Serpin [40] | extra-cellular | MGNHQVIFLVLCTSLLFQNTIQQNVSFQLI |
| andropin (anp) | 57 aa | Immune response [41,42] | extra-cellular | MKYFVVLVVLALILAISVGPSDAVFIDILD |
| bombardier (bbd) | 250 aa | Sexual reproduction [42] Anti-microbial response, Toll signaling [43] | extra-cellular | MGSNTGAWILLGLLAGIASLSSAANIQRNE |
| CG17242 | 230 aa | Protease [44] Sexual Reproduction [42] | extra-cellular | MLLKGILLLVSIAQIAADFKSIGIEQAPWQ |
| CG18258 | 468 aa | Lipase [27,39] | MSSSIAVVLVVVLIGISESIKTDSLMMTSS | |
| CG34034 | 135 aa | Sexual reproduction [42,45] | extra-cellular | MSSISTIIGLCLLFFMLSNVDAYGQKCSPV |
| CG42521 | 123 aa | Sexual reproduction [39] | MRTVPILLLICCLGWLHKGQADERKIGAVG | |
| CG42782 | 46 aa | Sexual reproduction [46] | MLISQYSGLKLMLLMVGLGMASSYEIIRQC | |
| CG5402 | 154 aa | Sexual reproduction [42,45] | extra-cellular | MKLLIWLCLLGFLASAYGIFLDKITGRGDS |
| CG5162 | 411 aa | Sexual reproduction [42,45] Lipase [27] | extra-cellular | MGRVPAKMHTLLALLLQLLVASIHAIEWSL |
| ductus ejaculatorius peptide (dup) 99B | 54 aa | Sexual reproduction [42,45,47] | extra-cellular | MKTPLFLLLVVLASLLGLALSQDRNDTEWI |
| esterase-6 (est-6) | 500 aa | Sexual reproduction [45,48] | extra-cellular | MNYVGLGLIIVLSCLWLGSNEADPLIVEIT |
| odorant binding protein (Obp) 51a | 117 aa | Sexual reproduction [42,45] | extra-cellular | MKVFIGLVLLLAVTTLSSALFESEANECAK |
| seminal fluid protein (sfp) 79B | 35 aa | Sexual reproduction [42,45] | extra-cellular | MKLLSAALVLLMSSALAMAQKNTNTNENNIVIGKV |
| seminal fluid protein (sfp) 93F | 53 aa | Sexual reproduction [42] | extra-cellular | MLIARLGFLLCSLGLATAICQPNGQSCKSH |
| serpin77Bb (spn77Bb) | 362 aa | Sexual reproduction [45] Hormone transport and protein folding [49,50] | extra-cellular | MKLGFLGLFGMVLMIMFYEGAEGYTVNELR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malpe, M.S.; McSwain, L.F.; Aston, H.M.; Kudyba, K.A.; Ng, C.; Wright, M.P.; Schulz, C. Drosophila Males Differentially Express Small Proteins Regulating Stem Cell Division Frequency in Response to Mating. J. Dev. Biol. 2025, 13, 21. https://doi.org/10.3390/jdb13030021
Malpe MS, McSwain LF, Aston HM, Kudyba KA, Ng C, Wright MP, Schulz C. Drosophila Males Differentially Express Small Proteins Regulating Stem Cell Division Frequency in Response to Mating. Journal of Developmental Biology. 2025; 13(3):21. https://doi.org/10.3390/jdb13030021
Chicago/Turabian StyleMalpe, Manashree S., Leon F. McSwain, Heath M. Aston, Karl A. Kudyba, Chun Ng, Megan P. Wright, and Cordula Schulz. 2025. "Drosophila Males Differentially Express Small Proteins Regulating Stem Cell Division Frequency in Response to Mating" Journal of Developmental Biology 13, no. 3: 21. https://doi.org/10.3390/jdb13030021
APA StyleMalpe, M. S., McSwain, L. F., Aston, H. M., Kudyba, K. A., Ng, C., Wright, M. P., & Schulz, C. (2025). Drosophila Males Differentially Express Small Proteins Regulating Stem Cell Division Frequency in Response to Mating. Journal of Developmental Biology, 13(3), 21. https://doi.org/10.3390/jdb13030021





