Abstract
Non-Saccharomyces yeasts have aroused interest in brewing science as an innovative and seminal way of creating new beer flavors. A screening system for potential brewing strains of non-Saccharomyces yeasts was set up to investigate the yeast’s utilization of wort sugars and to examine the effect of hop acids as well as ethanol on the growth of different yeast strains. Additionally, phenolic off-flavor (POF) and sensory odor tests of fermented wort samples were performed. The promising strains were further investigated for their propagation ability and for following fermentation trials. The produced beers were analyzed for secondary metabolites, ethanol content and judged by trained panelists. Subsequently to the screening, it was discovered that among the 110 screened yeast strains, approx. 10 strains of the species Saccharomycopsis fibuligera, Schizosaccharomyces pombe and Zygosaccharomyces rouxii generate promising fruity flavors during fermentation and were able to metabolize maltose and maltotriose as a prerequisite for the production of alcoholic beers. Consequently, the screening method described in this study makes it possible to investigate a tremendous number of different non-Saccharomyces yeasts and to test their brewing ability in a relatively short period of time.
1. Introduction
Known to most brewers as spoilage yeasts or as co-fermenters in mixed fermentations, non-Saccharomyces yeasts have received very little attention since the introduction of what are referred to as high-performance Saccharomyces brewing yeasts [1,2]. With the rise in craft brewing and breweries seeking greater individualization, the use of such unconventional yeasts might be a solution [3]. Michel et al. [4] and Basso et al. [5] emphasized the great potential of non-Saccharomyces yeasts to develop beers with different alcohol contents and a broad range of flavors. They highlighted the varying abilities of unconventional yeasts to metabolize desirable aroma-active substances such as fruity esters, monoterpenes, higher alcohols, phenols and acids. Moreover, the fermentation ability of brewer’s wort carbohydrates was discussed with the resulting alcohol content. Both reviews were, amongst others, focusing on species such as Torulaspora delbrueckii, Dekkera/Brettanomyces and Pichia kluyveri.
A screening system for non-Saccharomyces brewing strains was published by Michel et al. in 2016 [6]. A variety of different tests (e.g., wort carbohydrate consumption, hop and ethanol sensitivity, fermentation potential) was introduced to predict the potential of ten T. delbrueckii strains to ferment brewer’s wort to a desirable beer. Ravasio et al. also evaluated the fermentation and aroma profile of 60 different non-Saccharomyces strains in 2018. The applied yeasts were cultured in a medium based on glucose and the resulting volatile compounds were detected by gas chromatography/mass spectrometry (GC/MS) measurement. Only the promising species were additionally analyzed for their maltose utilization on serial-dilution plate assays [7].
The study presented here includes screening the brewing ability of 110 non-Saccharomyces strains with optimized screening conditions as the metabolism of the full range of main wort carbohydrates (glucose, fructose, sucrose, maltose and maltotriose [8]) were taken into account. As maltose and maltotriose represent more than 80% of the total carbohydrates in brewer’s wort [9], the ability to ferment these two substances is essential for a fast, complete and predictable fermentation [10]. Melibiose was additionally included into the screening, as a study of Wickerham indicated that bottom-fermenting yeasts are able to metabolize this type of sugar whereas top-fermenting yeasts do not [11]. Furthermore, part of the first screening step looked at the effect of hop iso-α- and β-acids as well as ethanol on the growth of the yeast strains to determine whether there are any existing resistances at certain concentrations that would restrict the production of a conventional beer. As hop acids have antimicrobial properties and β-acids, in particular, were reported to have an even stronger antimicrobial effect than iso-α-acids [12,13], it is necessary to test the yeast’s tolerance to these acids. Although a conventional Pils has up to 38 IBU, which is approximately comparable to 38 mg iso-α-acids/L, some IPAs can reach 100 IBU [14]. During fermentation, the increasing ethanol concentration is one of the greatest stress factors of the yeast cells, so there is a need to examine whether the yeast can adapt to the ethanol influence [15]. Additionally, the pH of all nutrient solutions was adjusted to 5.2 to map the pH value of a standard wort, which is between 5.0 to 5.7 [16]. To obtain an initial sensory impression of the individual strains, odor tests were performed by transferring the yeasts into brewer’s wort. In addition, a POF test was accomplished to investigate the potential of the different strains to produce 4-vinylphenol, 4-vinylguaiacol or 4-vinylbenzol from precursors [17,18,19].
The non-Saccharomyces yeasts screened in this investigation were of the genera Cyberlindnera, Debaryomyces, Hanseniaspora, Kazachstania, Kluyveromyces, Lachancea, Metschnikowia, Nakazawaea, Pichia, Saccharomycopsis, Schizosaccharomyces, Torulaspora, Wickerhamomyces, Zygosaccharomyces and Zygotorulaspora. They were chosen because of their positive influence on the aroma profile of fermented foods by contributing high amounts of esters, higher alcohols and other volatile flavor compounds [20,21,22,23]. As esters are volatile flavor compounds which mainly contribute to the aroma of beer, these strains might also be interesting as contributors to beer aroma [24,25,26]. According to Verstrepen et al., the esters that mainly convey a fruity sensory impression are ethyl acetate, isoamyl acetate, phenylethyl acetate, ethyl caproate, ethyl caprylate and ethyl decanoate [27]. In addition to esters, higher alcohols such as 2-phenyl ethanol, isobutyl alcohol and isoamyl alcohols also positively affect the aroma profile of beer as long as they stay below 300 mg/L [28]. In contrast, aldehydes and vicinal diketones such as diacetyl or 2,3-pentanedione, which cause an unpleasant buttery flavor, can negatively alter the quality of a beer once their sensory threshold is exceeded [7,29,30]. The organoleptic thresholds of volatile compounds mainly found in beer were summarized by Sannino et al. [31]. In addition, short-chain fatty acids such as isovaleric, hexanoic, octanoic and decanoic acids produced by yeasts during fermentation can create unpleasant, rancid flavor characteristics [25,32].
The aim of this study was to investigate yeast strains for their brewing ability and to test the promising strains in small brewing trials. Yeast strains that cannot metabolize maltose and those that release phenolic off-flavors were excluded from further investigation as the main objective of this study was to discover strains that produce less well known wheat beer flavors and secondary metabolites that create pleasant and novel aroma impressions.
2. Materials and Methods
2.1. Yeast Strains and Nutrient Media
Table 1 shows the yeast strains with their given abbreviations (abbr.) that were investigated in this study. They were grown in YM bouillon (malt extract 0.3%, yeast extract 0.3%, peptone 0.5%, glucose anhydrous 1.0%, double distilled water 97.9%) for 72 h at 20 °C on a WiseShake orbital shaker at an orbital agitation of 80 rpm (Witeg Labortechnik GmbH, Wertheim, Germany) before they could be used for the 96-well plate tests.

Table 1.
Yeast strains applied to 96-well plate tests.
The individual nutrient solutions were prepared according to the recipes as shown in Table 2, filled up to 1000 mL with deionized water, adjusted to a pH of 5.2 and were sterile filtered with 0.2 µm pore sized WhatmanTM sterile filters (Sigma-Aldrich, St. Louis, MO, USA) once the components were completely dissolved. For each nutrient or inhibiting medium, 7 g/L of Difco Yeast Nitrogen Base (BD Biosciences, San Jose, CA, USA) and Y3627 Sigma Yeast Carbon Base (Sigma-Aldrich, St. Louis, MO, USA), according to the Table 2, were used as nitrogen and carbon sources. The individual carbohydrate concentrations were adjusted to approximately those of a standard wort.

Table 2.
Utilized nutrients and inhibiting substances and their compositions for the 96-well microtiter plate tests.
2.2. Yeast Sample Preparation
Yeast cells grown in YM bouillon were washed prior to being applied to the nutrient media in the 96-well plates to eliminate the influence of the previous growth medium. For the washing step, 40 g of each yeast suspension was added to 50 mL FalconTM centrifuge tubes (Sarstedt, Nümbrecht, Germany). The suspension was centrifuged (centrifuge Z 366 K, HERMLE, Wehingen, Germany) for 10 min at 750 g before the supernatant of nutrient solution was discarded. After resuspending the yeast with sterile water, the washing procedure was repeated twice to ensure that there were no residues of the nutrient medium. To ensure direct comparability of the yeast growth, the cells of each strain were counted using the Cellometer® Vision (Nexcelom Bioscience LLC, Lawrence, MA, USA) and the corresponding yeast amounts were calculated to start the 96-well plate tests with an incubation of 100,000 yeast cells for each well, resulting in 500,000 cfu/mL.
For the 96-well microtiter plate CorningTM CostarTM (Sigma-Aldrich, St. Louis, MO, USA) tests, each plate was prepared under sterile conditions in the sterile bench Uniflow UVUB 1200 (UniEquip, Planegg, Germany) to avoid contamination. The pipetting scheme was structured in such a way that 200 µL of the 17 different nutrient solutions listed in Table 2 were pipetted in each case to four wells. The yeast cells were inoculated in triplicate with a cell count of 100,000 in columns 2 to 4, 6 to 8, and 10 to 12, leaving one blank value for each media in columns 1, 5 and 9. Finally, each plate was sealed with a cover sheet SealPlate® 100-SEAL-PLT (Excel Scientific, Victorville, CA, USA) to protect against contamination. The extinctions of the suspensions of the 96-well microtiter plates were recorded in triplicate using the Photometer Synergy 2™ Multi-Mode Detection Microplate Reader (BioTek®, Winooski, VT, USA), subsequently, at a wavelength of 600 nm and 25 °C. The results were recorded using the BioTek Gen5™ software. Before each measurement, the plate was automatically shaken for 30 seconds by the device to ensure that the suspensions were thoroughly mixed. As the inoculated plates were incubated in a tempered room at 28 °C, they were kept in a styrofoam box to avoid condensate forming on the cover sheets. Every 24 h, the measurement of the extinction was repeated for four consecutive days. This measurement method over 72 hours allowed the complex screening of a multitude of strains in a manageable time.
2.3. Phenolic Off-Flavor Test (POF Test)
Three stock solutions were prepared for the POF test. Therefore, 1 g of each trans-ferulic and trans-cinnamic acid was diluted in 20 mL of 96% (v/v) ethanol while 0.2 g p-coumaric acid was added to 20 mL of 96% (v/v) ethanol. The ferulic and cinnamic acids were both dosed at 1% into 45–50 °C tempered YM agar (malt extract 0.3%, yeast extract 0.3%, peptone 0.5%, glucose anhydrous 1.0%, agar 2.0%, double distilled water 95.9%) while the coumaric acid was added at 0.2%. Immediately thereafter, the POF agar plates were poured out under sterile conditions and allowed to cool. The yeast strains to be examined were then removed from wort agar slopes with an inoculation loop and spread on three plates, each containing the different acids. A positive control with the strain LeoBavaricus-TUM 68® (Research Center Weihenstephan for Brewing and Food Quality, Freising, Germany) and a negative control with the strain Frisinga-TUM 34/70® (Research Center Weihenstephan for Brewing and Food Quality, Freising, Germany) were also prepared. After an incubation time of three days at 28 °C, the POF plates were evaluated.
2.4. Sensory Odor Test
In order to perform the sensory odor test, unhopped wort was first prepared from unhopped liquid Bavarian Pilsner malt extract (extract anhydrous 72–79%, Weyermann®, Bamberg, Germany) by re-diluting the extract with hot water to 7 °P and 12 °P wort. Since standard alcoholic beers generally have an original gravity of about 12 °P, whereas maltose- and maltotriose-negative yeasts require an original gravity of about 7 °P to avoid exceeding the limit of 0.5% (v/v) alcohol in the final beer, the original gravity was adjusted accordingly in the experiments. The use of malt extract ensured a standardized wort quality for all the trials to be able to compare the different yeast strains. In each case, 75 mL of wort was filled into sterile flasks and cooled down to room temperature at 20 °C. The different yeast strains were inoculated with a sterile loop from wort agar slopes into each of the 7 °P and 12 °P wort batches and incubated for 72 h. Thereafter, the individual samples were evaluated by a sensory panel of ten panelists, describing the odor impressions as well as rating them as positive, negative or neutral.
2.5. Yeast Propagation and Wort Analysis
The promising yeast strains not showing phenolic off-flavors but desired results from the sensory odor test and with the ability to at least ferment glucose, fructose and maltose to produce a beer with a reduced or even a standard alcohol content of approx. 5% (v/v) were investigated for their propagation ability. If found to grow in high cell numbers, they were pitched from wort agar slopes under sterile conditions into a 500 mL flask containing 250 mL of unhopped brewer’s wort (12.5 °P, pH 5.4) diluted from Bavarian Pilsner malt extract (Weyermann®, Bamberg, Germany). The malt extract was chosen as the composition is always the same and the results are highly comparable. The composition of the wort used for the yeast propagation and fermentation trials is shown in Table 3.

Table 3.
Sugar composition of the wort used for the fermentation trials.
After 72 h of propagation at 20 °C and orbital shaking at 80 rpm on a WiseShake orbital shaker (Witeg Labortechnik GmbH, Wertheim, Germany), the yeast suspensions were transferred to sterile 2500 mL flasks filled with 2000 mL unhopped wort and propagated for a further 72 h. Following the propagation, the cell count was performed using the Cellometer® Vision (Nexcelom Bioscience LLC, Lawrence, MA, USA).
2.6. Fermentation Trials
The pitching rate was chosen at 30 × 106 cells/mL (±σ = 3 × 106 cells/mL) as many non-Saccharomyces cells are much smaller than usual brewer’s yeast cells and therefore, show differing fermentation speeds [33]. The respective amounts of propagated yeast suspensions were inoculated in triplicate into 1400 mL of sterilized wort (wort attributes can be viewed in Table 3) in 2000 mL sterile Duran glass bottles (Schott AG, Mainz, Germany) with glass fermentation blocks on top. The samples were stored at 27 °C for the main fermentation. The fermentation progress was checked every 24 h by recording the weight loss (mainly due to escaping carbon dioxide) of the samples. The fermentation was considered to be completed either after 336 h or as soon as the samples had lost 50 g. Starting at 1400 mL of 12.5 °P wort, the weight loss was set at 50 g, since over 60% of the fermentable extract is spent once this limit is reached. This is based on the assumption of Balling that during fermentation, an average of 2.0665 g of extract is converted to 1 g of alcohol, 0.9565 g of carbon dioxide and 0.11 g of yeast [34]. Following the main fermentation, the samples were sealed with sterile screw caps and the young beers were ripened for another 168 h at 27 °C to continue fermentation of the remaining extract while the carbon dioxide produced during the secondary fermentation could be enriched in the beers under pressure. After maturation, the young beers were stored in the sealed bottles at 2 °C for a further 168 h.
2.7. Analysis of Produced Beers
The final beer samples from five selected promising strains were analyzed for the parameters shown in Table 4.

Table 4.
Examinations of the final beers after MEBAK 1.
Additionally, glycerol was analyzed using the glycerol UV-test by Boehringer Mannheim/R-Biopharm, Germany, according to the manufacturer’s instructions.
2.8. Sensory Evaluation
Finally, the beer samples were profiled at 20 °C room temperature by a sensory panel of ten DLG (Deutsche Landwirtschafts-Gesellschaft e.V.)-certified assessors to determine the main flavor components and the acceptance of the individual beers. Based on the sensory profiling, a sensory analysis was developed to obtain a consistent rating which was not differentiated into smell and taste.
3. Results
Primarily, yeast species were selected that were already used in food fermentations and are known for their aroma contribution, as described in the introduction. The heat map displayed in Figure 1 summarizes the results of the 96-well microtiter plate tests and gives an overview of the brewing ability of the selected 110 yeast strains from 18 yeast species with regard to the key parameters of wort sugar utilization, the ability to grow in hopped wort and ethanol resistance. Additionally, POF and odor tests were taken into consideration as the aim of this study was to identify yeast strains that are able to produce pleasant novel flavors during the wort fermentation, however, excluding POF positive yeast strains. Although non-alcoholic beers are as promising as those with an average alcohol content, this study first focused on the yeasts strains which are able to metabolize maltose to produce a standard or at least an alcohol-reduced beer.
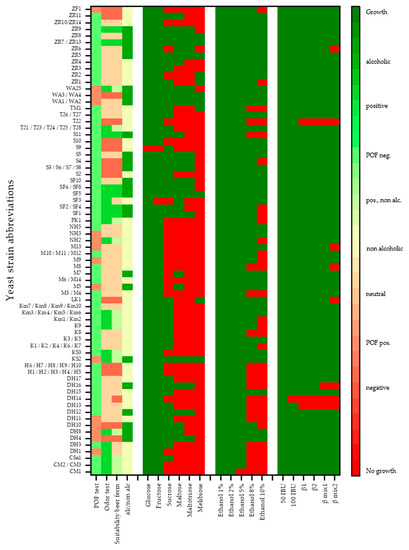
Figure 1.
Heat map indicating carbohydrate utilization, ethanol and hop resistance (iso-α-acids 50 IBU/100 IBU = 50 and 100 international bitterness units; β1/β2 = β-acids in different concentrations; βmix1/βmix2 = mix from iso-α-acids und β-acids in different concentrations) as well as POF and odor test results of the yeast strains screened in the 96-well plate test (ferm. = fermentation; alc. = alcoholic; neg. = negative; pos. = positive).
As shown in Figure 1 and to enable a simple evaluation, the carbohydrate utilization, ethanol and hop resistance were evaluated according to whether there is growth or no growth. The limit above which growth can be detected was set at an extinction of 0.4 as distinct growth can be determined from this value. Values equal to or above 0.4 are displayed in green in the heat map, while values smaller than 0.4 are marked in red. The POF test and odor test provide initial information about the aroma profile the yeasts can produce during the fermentation of wort. The alc./non alc. column summarizes the sugar utilization of the individual yeasts and is therefore an indicator of whether the yeast is suitable for the production of an alcohol-free beer or beer with an average alcohol content. The yeast’s suitability for beer fermentation outlines all the described aspects and already represents a selection of the screened yeasts for further fermentation trials.
3.1. Overall Results
According to the results in Figure 1, the hop as well as ethanol resistance of the screened yeasts is generally high enough to be considered for the production of a standard beer. Neither a concentration of iso-α-acids of up to 100 ppm, a concentration of β-acids of up to 200 ppm, nor a mixture of both acids inhibit the growth of most yeasts. Only 3% of the total screened yeasts, namely two yeast strains of the species D. hansenii (DH13, DH14) and one T. delbrueckii strain (T22) do not grow at a β-acid concentration of 200 ppm in the medium. DH14 was the only yeast significantly inhibited even at an iso-α-acid concentration of 100 ppm, so that no more growth was recorded. Moreover, the growth of individual yeast strains was restricted as soon as higher concentrations of iso-α- (100 IBU) and β-acids (200 ppm) in the form of βmix2 were used, which affected approx. 7% of the yeasts, including one L. kluyveri strain (LK1), two yeasts of the species Metschnikowia (M8, M13), one strain of the yeast Z. rouxii (ZR6) and one strain of the species D. hansenii (DH16) besides the above-mentioned strains DH13, DH16 and T22. In addition, the βmix1 containing 50 IBU iso-α-acids and 100 ppm β-acids inhibited growth in nearly 4% of the yeasts. While only 3% of them were inhibited at a concentration of 200 ppm β-acids, this shows that the combination of both hop acids seems to lead to an enhanced inhibitory effect which suggests that both hop acids have an antimicrobial effect. However, since a standard beer does not contain more than 50 ppm iso-α-acids and the β-acids only become relevant after an additional cold hopping, all yeasts have a sufficient hop tolerance for standard beer brewing. In contrast to iso-α-acids, β-acids are poorly soluble in beer. They are not isomerized during wort boiling and are not conveyed into beer [35].
With regard to the ethanol tolerance, approx. 25% of the screened yeasts show no growth at an ethanol concentration of 8% (v/v), especially yeasts of the species Cyberlindnera, D. hansenii and H. uvarum. For only one of the 110 screened yeasts, namely one strain of the species C. misumaiensis (CM1), growth was significantly inhibited at an ethanol concentration of 5% (v/v). Consequently, apart from strain CM1, all the screened yeasts have a sufficient ethanol tolerance to produce a standard beer with 5% (v/v) ethanol concentration. However, since the yeast CM1 can only be used to produce non-alcoholic beers or possibly mixed fermentations because of its carbohydrate utilization (only glucose and fructose), its lower ethanol tolerance is negligible.
Sugar utilization is variable; however, it is striking that all the yeast strains, with the exception of S. pombe S9, can metabolize glucose. S. pombe S9 does not ferment fructose either, similarly to the strain S. fibuligera SF3. Since there are literature sources that state that S. pombe can metabolize glucose and S. fibuligera utilizes fructose [36,37,38], the lack of growth in this case can be considered to be an outlier. In terms of the disaccharides sucrose, maltose and melibiose, the ability to ferment these carbohydrate sources decreases. Yet, while 65% of the yeasts still metabolize sucrose, maltose can only be utilized by approx. 30% of the yeasts, whereas melibiose is utilized by 14%. The trisaccharide maltotriose is metabolized by merely 25% of the yeast strains. Therefore, only about one third of the screened yeasts can be considered for the production of alcoholic beers and these belong to the species D. hansenii, K. servazzii, S. fibuligera, S. pombe, W. anomalus, Z. rouxii and Metschnikowia. In order to further limit the selection of suitable yeasts for the production of beers with novel flavor properties, all yeasts with POF-positive results or neutral or negative odor outcomes, such as D. hansenii, K. servazzii, Metschnikowia and W. anomalus, were excluded.
As a result, six strains of the species S. fibuligera (SF1, SF2, SF4, SF5, SF6, SF8), one S. pombe strain (S11) and three strains of the species Z. rouxii (ZR7, ZR9, ZR13) were found to be POF-negative and also had a positive odor impression. In order to ensure a manageable experimental framework in this study, half of the promising yeast strains were further tested for the following fermentation trials, namely SF2, SF4, SF8, S11 und ZR9. Although several other yeasts species, such as C. misumaiensis, C. saturnus, K. marxianus and T. delbrueckii, revealed pleasant sensory odor impressions and POF-negative outcomes, they were not further considered for this study as they can be considered for the production of non-alcoholic beers.
3.2. Exemplary Evaluation of Carbohydrate Utilisation, Ethanol and Hop Resistance
The diagrams (Figure 2, Figure 3 and Figure 4) represent the extinction curves at 600 nm of carbohydrate utilization, ethanol and hop tolerance using the yeast T. delbrueckii T21 as an example over a measurement period of 72 h and an incubation temperature of 28 °C. The yeast T. delbrueckii T21 was selected as it is maltose-negative and therefore, represents about 70% of the screened yeasts. The extinction threshold of whether the yeast has grown in the nutrient media was marked at 0.4 in the diagrams. Values above an extinction of 0.4 indicate significant growth, while values below 0.4 indicate that the yeast has not grown in the corresponding nutrient solution.
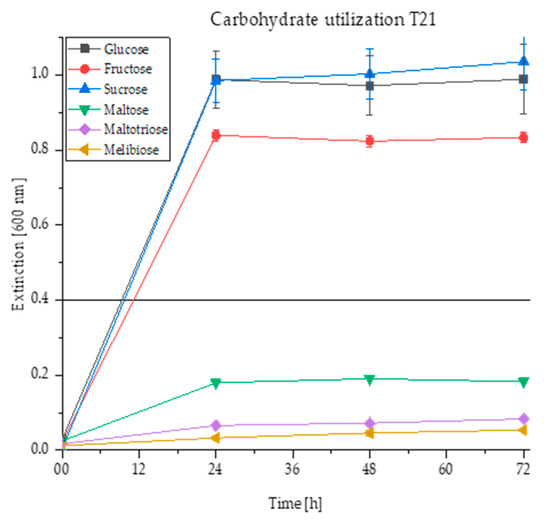
Figure 2.
Yeast growth of the strain T. delbrueckii T21 measured at an extinction of 600 nm in nutrient solutions with the carbohydrate additives glucose, fructose, sucrose, maltose, maltotriose and melibiose in the 96-well microtiter plates at 28 °C for four days and the respective standard deviations per measurement day (displayed as error bars). The growth threshold is set at an extinction of 0.4.
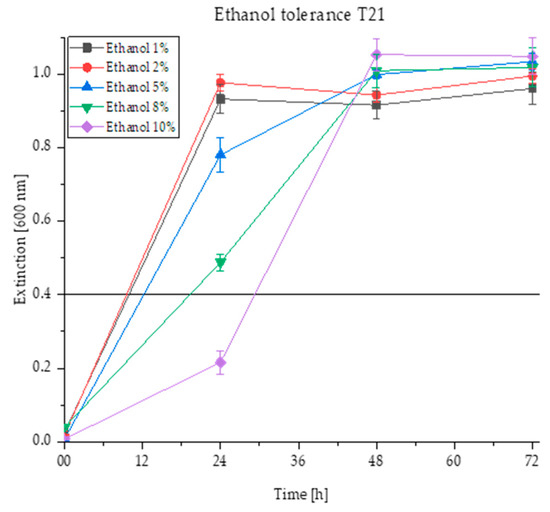
Figure 3.
Ethanol tolerance of the strain T. delbrueckii T21 shown as growth at an extinction of 600 nm in nutrient solutions with different ethanol concentrations of 1% (v/v), 2% (v/v), 5% (v/v), 8% (v/v) and 10% (v/v) in the 96-well microtiter plates at 28 °C for four days and the respective standard deviations per measurement day (displayed as error bars). The growth threshold is set at an extinction of 0.4.
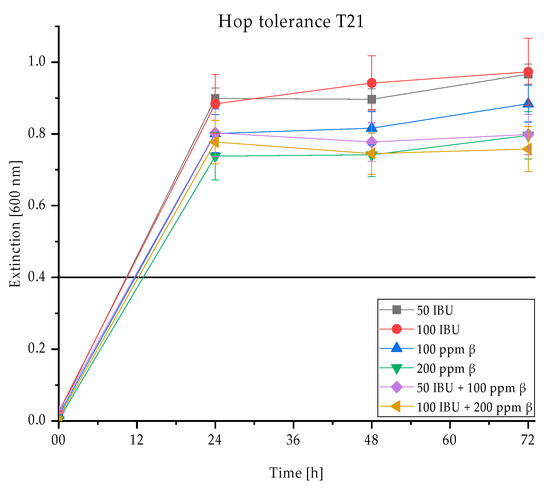
Figure 4.
Hop tolerance of the strain T. delbrueckii T21 shown as growth at an extinction of 600 nm in nutrient solutions with different hop acid additives 50 and 100 IBU, 100 and 200 ppm β-acids, 50 IBU + 100 ppm β-acids and 100 IBU + 200 ppm β-acids in the 96-well microtiter plates at 28 °C for four days and the respective standard deviations per measurement day (displayed as error bars). The growth threshold is set at an extinction of 0.4.
3.2.1. Carbohydrate Utilization
Figure 2 depicts the increase of the extinctions of the yeast strain T21 in different sugar solutions over 72 h. After 24 h incubation at 28 °C, it can be observed that the wells containing glucose, fructose and sucrose solutions clearly exceed the growth threshold of 0.4 so they already show distinct turbidity. Taking the standard deviation into account, the curves for glucose and sucrose are similar, whereas the yeast’s metabolism of fructose is slightly lower. Although the extinction curve for maltose increases slightly within the first 24 h, it then stagnates at an extinction of approx. 0.2 and does not show a significant growth accordingly. The values for the wort sugars maltotriose and melibiose hardly increase. Therefore, the sugars maltose, maltotriose and melibiose cannot be utilized by the yeast T21.
While the growth of the yeast T21 in Figure 2 stagnates after the first measuring day, the growth of other yeasts could be partially detected first after 48 or 72 h of incubation by reaching the growth threshold of 0.4. All the yeast strains presented in the heat map in Figure 1 and evaluated as “non-alcoholic” show similar curves at least for maltose and maltotriose, as demonstrated in Figure 2 remaining below the extinction threshold of 0.4.
3.2.2. Ethanol Tolerance
From Figure 3, in which the ethanol tolerance of the yeast strain T21 was investigated, it can be seen that the extinction curves for all concentrations of 1 to 10% (v/v) ethanol far exceed the threshold of 0.4. It is noticeable that the yeast growth within the first 24 h differs depending on the ethanol concentration. The higher the ethanol concentration in the nutrient solution, the slower the growth. This is shown by the decreasing turbidity at increasing ethanol concentrations. Nevertheless, there is significant growth at an ethanol concentration of up to 8% (v/v) after 24 h, while the growth in the 10% (v/v) solution remains below the 0.4 extinction threshold. During the second measuring day, all extinction values converge again and the yeast T21 also shows a significant growth at 10% (v/v) after 48 h of incubation. Taking the standard deviations into account, the extinction values all remain similar at approx. 1.0 after 48 h.
3.2.3. Hop Tolerance
With regard to the yeast’s hop tolerance, Figure 4 demonstrates that the yeast T21 grows within 24 h of incubation at 28 °C in nutrient solutions with different hop acid additives as the curves already exceed the extinction limit of 0.4 during 24 h of incubation. While the curves of all nutrient solutions look similar and the yeast stops growing after 24 h in all cases, it can nevertheless be observed that the growth of the yeast T21 is the strongest at 50 and 100 IBU iso-α-acids. As soon as β-acids were added into the nutrient solutions, the extinctions and the corresponding growth of the yeast are slightly lower.
3.3. Results of the Sensory Odor Test
In addition to the general evaluation of the odor tests, which are divided into the categories “positive”, “neutral” and “negative” within the heat map (cf. Figure 1), a descriptive evaluation was performed by ten panelists. The odor descriptions are listed in Table 5.

Table 5.
Evaluation of the descriptive sensory odor impressions of the screened yeast strains after fermentation in 7 °P wort as well as 12 °P wort.
In general, the results in Table 5 show that the sensory differences of the fermented wort samples do not differ between 7 °P and 12 °P in most cases and that a great variety of the yeasts produce fruity flavors. Only the wort samples fermented with Metschnikowia sp. (M3 to M14) and T. microellipsoides (TM1) were found to be mainly neutral with regard to their flavor perception while e.g. Z. florentina (ZF1) causes a fruity, but also an unpleasant musty smell. Although K. lactis (K1 to K9) and S. pombe (S2 to S11) partly produce disagreeable sulfurous and musty flavors, they also reveal fruity odor impressions. The wort fermented with L. kluyveri (LK1) and H. uvarum (H1 to H10) shows neutral to fruity flavors as well, however, the samples are unacceptable due to their acidic smell. The yeasts D. hansenii (DH1 to DH17) and W. anomalus (WA1 to WA4, WA25) turned out to be the strains with the greatest diversity. While all screened W. anomalus strains are POF positive, this varies between the investigated D. hansenii strains. The olfactory impression of the W. anomalus samples clearly reflects the POF character, although their acceptance varies strongly. The wort samples fermented with D. hansenii range from neutral to sulfurous to an exotic fruity and POF character and differ widely in their sensory acceptance. The samples fermented with N. holstii (NH2 to NH5) and Z. rouxii (ZR1 to ZR14) are partly found to be positive due to their fruity properties, yet some strains produce dominating POF and diacetyl odors. Generally, Cyberlindnera (CM1 to CM3, CSa1), P. kluyveri (PK1), K. servazzii (KS2, KS3), K. marxianus (Km1 to Km10), S. fibuligera (SF1 to SF10) and T. delbrueckii (T21 to T28) produce the purest fruity flavors and are perceived as the most pleasing. S. fibuligera is characterized by its ability to generate particularly plum-like aromas, while Cyberlindera and P. kluyveri reveal noticeable amounts of isoamyl acetate.
3.4. Fermentation Trials
As previously mentioned in Chapter 3.1, the promising yeast strains S. fibuligera SF2, SF4, SF8, S. pombe S11 and Z. rouxii ZR9 were further tested in brewing trials.
3.4.1. Fermentation Process
In Figure 5, the fermentation process of the five selected yeast strains is illustrated by a total weight loss in grams of the fermentation samples due to the extract degradation of the yeast strains over a period of 336 h at a fermentation temperature of 27 °C. The yeast strain S11 shows by far the highest weight loss over this period and the extract degradation by this yeast occurs faster compared to the other yeasts. After 240 h (10 days), a weight loss of more than 50 g of the original pitching quantity of 1400 mL wort is reached. With an average original wort content of 12.5 °P, more than 60% of the extract is therefore fermented and the secondary fermentation can be started. The fermentation process of the other four yeasts shows that they ferment much slower than the strain S11 and have a significantly lower fermentation activity. None of the four yeast strains reached the specified weight loss of 50 g within 14 days. While ZR9 and SF4 show a similar fermentation activity, followed by SF2, and even continue to ferment slowly during the second week, SF8 degraded the least extract and did not even reach a weight loss of 20 g after 336 h. However, it should be noted that the yeasts of the species S. fibuligera formed distinct lumps during their growth phase, which means that counting the cells results in a significantly increased standard deviation compared with conventional yeasts. Accordingly, the number of 30 × 106 cells/mL could possibly be imprecise, which may have led to a lower cell number of the samples fermented with SF8.
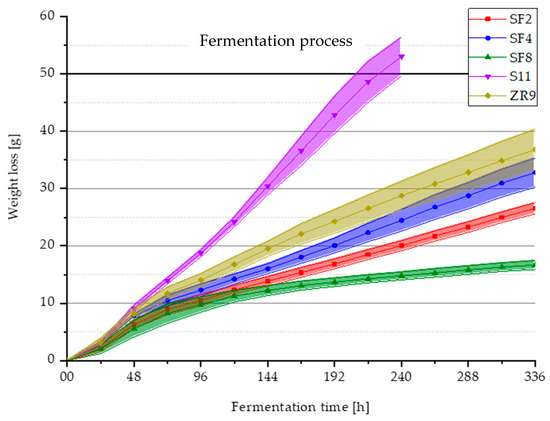
Figure 5.
Mean values (n = 3) with standard deviations of the fermentation samples’ total weight loss in grams of the yeast strains S. fibuligera SF2, SF4, SF8, S. pombe S11 and Z. rouxii ZR9 during the fermentation process over a fermentation time period of 336 hours (14 days) at 27 °C.
3.4.2. Wort Sugar Utilization
Figure 6 and Table 6 provide a more detailed insight of the fermentation activity of the five selected yeast strains.
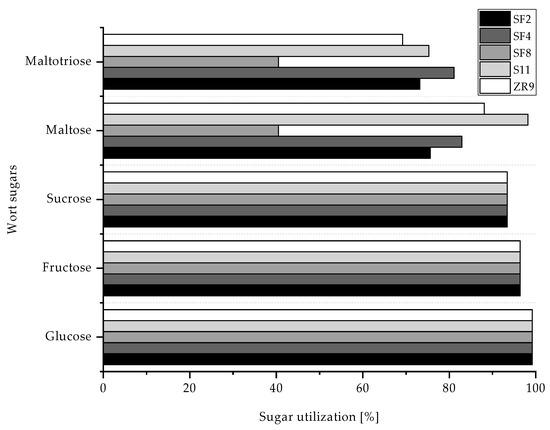
Figure 6.
Wort sugar utilization in % of yeast strains S. fibuligera SF2, SF4, SF8, S. pombe S11 and Z. rouxii ZR9 during fermentation from wort to beers.

Table 6.
Original wort [%], apparent attenuation [%], ethanol content [% v/v], pH and glycerol [g/L] values in the final beers fermented with the yeast strains S. fibuligera SF2, SF4, SF8, S. pombe S11 and Z. rouxii ZR9.
All experimental yeasts almost completely metabolize glucose, fructose and sucrose. The yeast strain S11 can metabolize over 98% maltose and almost 80% maltotriose, which explains the high degree of fermentation and the beer’s final ethanol concentration of around 5.7% (v/v). This is followed by yeasts ZR9 and SF4, which ferment about 80% of each maltose and maltotriose on average, followed by SF2, which still ferments over 70% maltose and maltotriose. Consequently, the ethanol content of the beers is between 2.6 and 4% (v/v). The strain SF8 shows the weakest fermentation performance and only metabolizes half the maltose and maltotriose compared with SF4, which results in lower fermentation and an ethanol concentration of only 1.5% (v/v) in the final beer. As a result of the 96-well plate screening, it was expected that S11, SF2 and SF4 would not metabolize maltotriose and therefore, SF8 and ZR9 had a significantly higher sugar utilization. In fact, SF8 and ZR9 were the worst metabolizers of maltotriose in comparison with the other three yeast strains (cf. Figure 1). However, as maltotriose cannot be fully utilized by the yeasts, this may have led to deviations in the screening. Still, all yeasts without exception can cause a pH drop to between 4.3 and 4.5. The glycerol content of the final beers was also measured. All beers have low glycerol levels between 0.24 and 0.47 g/L, while the average value in beer lies between 1 and 3 g/L with a threshold of 10 g/L [39]. Therefore, glycerol does not noticeably influence the mouthfeel of the beers.
3.4.3. Volatile Compounds in Final Beers
In the final beers, esters, organic acids, higher alcohols, acetaldehyde and ketones were analyzed by GC measurement. In order to ensure comparability between the individual compounds, the analytical results are expressed as a percentage and are listed in Figure 7.
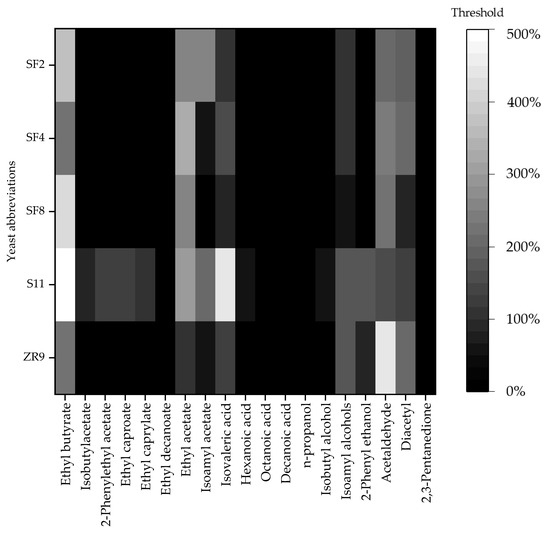
Figure 7.
Heat map of volatile compounds in final beers produced by the yeast strains S. fibuligera SF2, SF4, SF8, S. pombe S11 and Z. rouxii ZR9 during fermentation of brewer’s wort at 27 °C and detected by headspace gas chromatography measurement. The 100% value represents the threshold of volatile compounds in beer, which is based on relative values from Meilgaard and Sannino et al. [29,31].
By evaluating the different esters measured in the beers, Figure 7 shows that all the yeasts produce ethyl butyrate in concentrations above the threshold. Except for the beer fermented with ZR9, the other beers contain considerable amounts of ethyl acetate. Isoamyl acetate is above the threshold in the beers produced with SF2 and S11. While the organic acids are only significantly above the threshold in the form of isovaleric acid in the sample fermented with S11, higher alcohols are not significantly increased. In contrast, all five yeast strains are responsible for the acetaldehyde values being above the threshold. While 2,3-pentanedione has no influence as a ketone, diacetyl is increased in the beers fermented with SF2, SF4 and ZR9.
3.4.4. Aroma Profiles of Final Beers
The tasting results in Figure 8 give an overview of the variety of aromas produced by the different yeast strains. Evaluation levels range from 0 to 10, where 0 is considered to be the weakest and 10 the strongest intensity for the flavor impressions “fruity”, “plum”, “grape”, “red wine”, “honey”, “floral”, “clove”, “sulfurous”, “wheat beer” and “strawberry”.
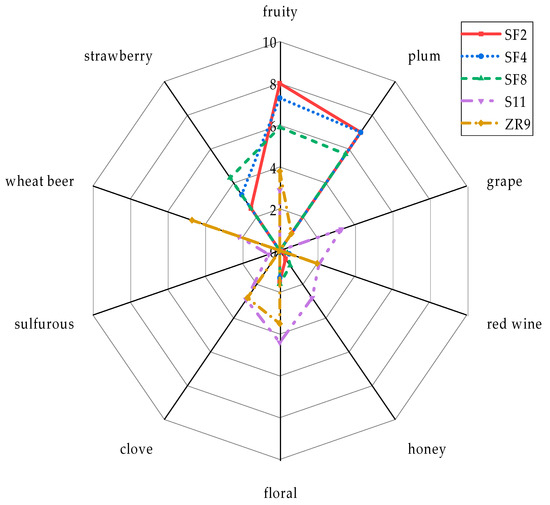
Figure 8.
Aroma profiles of the beers fermented with the yeast strains S. fibuligera SF2, SF4, SF8, S. pombe S11 and Z. rouxii ZR9.
By comparing the aroma profiles of the beers from the five different yeast strains, it is obvious that the yeasts SF2, SF4 and SF8 have a similar profile with distinct fruity notes that reach towards plum and strawberry. Only the flavor intensity of the strain SF8 is weaker. Additionally, some slightly floral aromas are noticeable. The aroma profile of the yeast strain S11 goes more towards the direction of wheat beer. Although there are fruity notes that are described as grape, the aroma spectrum is quite broad. Dominating floral impressions are supported by clove, honey and red wine aromas. The beer fermented with ZR9 exhibits a remarkable clove-like wheat beer character as well as floral, fruity and less intense red wine notes. Finally, the acceptance of the five beers was compared. The beer fermented with the yeast SF2 scores best with 8 out of 10 points, closely followed by the beer produced with SF4 (7.8 points). The strain SF8 with 7.4 points and S11 with 7.3 points are in the midfield, while beer from yeast ZR9 with 6.7 points is rated the worst in sensory terms.
Although the tasting samples were spot checks, the results largely reflect the flavor results from the GC analyses. The three screened S. fibuligera strains produce significant ester amounts of ethyl butyrate and ethyl acetate, which lead to sweet and fruity aroma impressions in taste and smell. SF2 further releases isoamyl acetate which underlines the pleasant fruity character [31]. Despite diacetyl being present in the beers fermented with SF2 and SF4, a buttery off-flavor was not noticed [40]. As both beers are rated highest, diacetyl cannot be considered to be an off-flavor in this case. Strawberry and plum flavors are not obviously apparent in the GC results, so further analyses may be necessary. The yeast strain S11 generally forms high amounts of ester compounds with a total concentration of 86 mg/L, which even exceeds the usual value of 70 mg/L for top-fermented beers, and leads to the fruity aroma character [41]. The significant amount of isoamyl acetate in conjunction with isobutyl acetate causes the wheat beer impression, which is supported by the increased amount of isoamyl alcohols. The synergistic effect of isoamyl acetate and isobutyl acetate was already published by Meilgaard in 1975 [29]. The floral aroma may be attributed to the threshold of 2-phenyl ethanol being exceeded. Although 2-phenylethyl acetate stays below the threshold, the concentration is still relatively high and can be traced back to the honey-like flavor. The overall increased values of esters, acids and higher alcohols trigger the perceptible red wine aroma. It was expected that the high amount of isovaleric acid would produce a rancid, cheesy aroma [29], however, this was not criticized by the sensory panel. The beer fermented with the yeast strain ZR9 has a remarkable wheat beer character and is most likely caused by the increased values of isoamyl alcohols, whereas the fruity perception can be mainly attributed to ethyl butyrate. Despite the fact that acetaldehyde and diacetyl are above the threshold, there is no remarkable flavor influence. Only the citrus note from the sensory odor test (cf. Table 5) may originate from acetaldehyde. In addition, the odor impressions of the sensory odor tests presented in Table 5 correspond basically to the results of the GC analysis and the tastings of the sensory panel. In some cases, the beers contain aromas which are not directly attributed to a single precise volatile compound, however, the flavor is a result of the synergistic interaction of different substances [25].
4. Discussion
The results described in this study reveal that the applied screening method provides a useful first overview to investigate a great number of yeast strains for their brewing ability in a short period of time. Still, it should be remarked that extinction curves may not have increased or slightly decreased over the measurement period. The reason for this is the yeast growth within the single wells. A few yeast strains tended to sediment on the walls of the wells, causing decreased turbidity at the bottom and in the liquid of the wells. Although the well plate was shaken by the photometer prior to measurement, the cells stayed in place. Other yeasts formed small clumps during their growth phase, for example, S. fibuligera, which also caused non-homogeneous distribution in the wells. As a result, the light from the photometer was directed through a medium with apparently less turbidity resulting in a lower extinction value, which could lead to measurement inaccuracies.
The screening of the utilization of different wort carbohydrates was a main criterion for the later selection of fermentation trials. Although there are reliable sources on the fermentation ability of glucose, sucrose and maltose by various yeast species published by Kurtzman [36], it often varies within the species as to the kind of carbohydrates they utilize. For instance, within the species S. pombe, T. delbrueckii, W. anomalus, Z. rouxii and K. lactis, the sugar utilization differs significantly between the single yeast strains [36]. With the exception of W. anomalus, the same observations were made during the screening. While W. anomalus strains consistently metabolized all carbohydrates used in the test, this varied within the other strains mentioned. However, it should be noted that only five W. anomalus strains were screened.
Furthermore, it was of interest to investigate the yeasts’ assimilation of fructose, maltotriose and melibiose. Since non-Saccharomyces yeasts are classified as top-fermenting yeasts, it is to be expected that they cannot metabolize melibiose based on literature [11]. However, since the comparison with regard to the melibiose utilization of Wickerham is only made between S. cerevisiae as a top-fermenting yeast and S. pastorianus as a bottom-fermenting yeast, it is assumed that individual top-fermenting yeast strains also partly utilize melibiose. Whether melibiose is metabolized depends on the presence of the enzyme alpha galactosidase in the yeast cell. This enzyme catalyzes the hydrolysis from melibiose to galactose and glucose [42]. The screening reveals that W. anomalus species, in particular, ferment melibiose. As a result of this study, some strains of the species Z. rouxii, S. fibuligera, K. servazzii, L. kluyveri and T. microellipsoides also have the ability to assimilate melibiose. Nevertheless, only 14% of the 110 screened yeasts were able to utilize this kind of sugar.
Some of the screened yeast species, such as P. kluyveri, are already known from beer fermentations for which there is already a patent for the production of low-alcohol or alcohol-free beer [43]. S. pombe was first isolated from an ancient beer called “pombe” by Lindner in 1893 [44] and is still one of the main aroma-contributing species today in many mixed beer fermentations of traditional African beers [45]. T. delbrueckii was first assumed to be a suitable yeast strain for beer brewing by King and Dickinson in 2000 [46] and W. anomalus, Z. rouxii as well as Z. florentina were investigated in relation to wort fermentations. The yeasts have a common ability to release a high concentration of volatile flavor metabolites such as esters by fermenting brewer’s wort, which results in fruity and floral sensory impressions [47,48]. The fruity and floral flavors can be confirmed in this study for the yeast strains S. pombe (S11) and Z. rouxii (ZR9). The strain S11 releases even greater amounts of esters than typical wheat beer yeast strains do. In addition, further T. delbrueckii strains with promising flavor impressions were discovered following the investigations of Michel et al. [6], whereby none of the screened strains could utilize maltose. According to several existing studies, W. anomalus releases significant amounts of ethyl butyrate and ethyl acetate leading to fruity flavor impressions. P. kluyveri and Z. florentina produce considerable amounts of pleasant esters, such as isoamyl acetate, during the fermentation process of all-malt wort, which can be attributed to the fruity banana-like sensory perception [5,7,43,47]. While the W. anomalus (WA1 to WA4, WA25) strains investigated in this study revealed a broad range of different positive to negative sensory odor impressions, P. kluyveri (PK1) actually produced remarkable amounts of pleasant isoamyl acetate. In contrast, the Z. florentina strain (ZF1) caused a musty smell next to the fruity flavor impression. However, since only a single yeast strain was tested, it does not represent the entire species. As per previous investigations, Z. rouxii strains release higher amounts of esters causing fruity, floral and solvent-like aromas. However, its diacetyl content is described to be above the threshold which negatively influences the sensory perception [48]. The analysis of the strain ZR9 confirms that the yeast produces an increased amount of diacetyl. Although this could not be tasted in the final beer, one Z. rouxii (ZR1) strain was found to be responsible for noticeable amounts of diacetyl in the sensory odor test. The floral and fruity aroma components are also confirmed by the sensory panelists and the fruity compounds, in particular, were detected during the GC measurement for the strain ZR9. A solvent-like aroma could not be observed explicitly. Nevertheless, the beer fermented with ZR9 had a distinct wheat beer character and a significant amount of ethyl acetate was analyzed. Even though ZR9 and S11 were described as wheat beer types by the panelists and were described as clove-like, they were evaluated as POF-negative. As part of the POF test, the yeasts obviously did not convert the ferulic acid to 4-vinylguaiacol, which would have led to a clove-like odor. Despite the POF-negative results, it can be assumed that the clove-like flavor either came from eugenol, which was not analyzed in this study [49], or that the complex interaction of the flavors in the beer triggered the clove-like wheat beer character. Neither strains of the species Z. rouxii nor S. pombe are known to convert ferulic acid to 4-VG in the literature, which is confirmed by the POF tests.
Most of the investigated yeast species i.e. C. saturnus, C. misumaiensis, D. hansenii, H. uvarum, L. kluyveri, Metschnikowia sp., N. holstii, P. kluyveri, S. fibuligera, S. pombe, T. delbrueckii, W. anomalus are known for their positive fruity aroma impact caused by releasing volatile flavor compounds during wine fermentation [50,51,52,53,54] and C. misumaiensis, H. uvarum and W. anomalus also during cider fermentation [20,55,56,57]. Various studies state that H. uvarum, S. pombe, T. delbrueckii and W. anomalus improve the aromatic quality and the flavor complexity of wine [58,59,60]. The release of pleasant fruity aroma components during wine production by the yeasts can only be partially transferred to the fermentation of brewer’s wort as the H. uvarum and L. kluyveri strains screened in this study released rather undesired flavors. While C. misumaiensis, C. saturnus, P. kluyveri, S. fibuligera and T. delbrueckii almost all developed positive aroma impressions in wort (cf. Table 5), the aroma impression of D. hansenii, N. holstii, S. pombe and W. anomalus varied within the species. Therefore, a direct transfer from wine to beer aromas cannot be assumed and differences could be based on the different fermentation medium (e.g. due to a varying carbohydrate composition) and fermentation conditions. Nevertheless, the diversity of flavors within one species is so extensive that individual strains do not necessarily provide information on the entire species, a fact proven by the species S. pombe in this study. Only strain S11 shows a positive aroma impression in the odor test, while the nine other strains mainly released negative flavor components (cf. Figure 1, Table 5). Therefore, although general statements can be made about the aroma profile of one yeast species, individual yeast strains may deviate from the norm and produce exceptionally positive or negative aromas.
C. saturnus in particular is known for its remarkably high synthesis of isoamyl acetate during wine fermentation which, as previously mentioned, causes a banana-like flavor and ethyl acetate, which leads to a fruity flavor [5,61,62,63]. Although the yeast strain CSa1 was not explicitly analyzed for isoamyl acetate, the olfactory tests clearly revealed that perceptible amounts of this compound were produced. In terms of the fermentation of rice wine with S. fibuligera, it can be highlighted that recent studies revealed the distribution of higher alcohols and acetate esters [64]. In fact, the investigated strains of the species S. fibuligera (SF2, SF4 and SF8) mainly produced large amounts of ethyl acetate, and SF2 additionally produced isoamyl acetate esters from brewer’s wort. Larger amounts of higher alcohols were only released in the form of isoamyl alcohols, which did not exceed the threshold of 65 mg/L (cf. Figure 7) [29].
Although C. misumaiensis, L. kluyveri and N. holstii are associated with the fermentation of wine or apple products [53,55,56] they have not yet been investigated in literature for their flavor characteristics. This study reveals that it might be promising to investigate specific yeasts of the species C. misumaiensis for their volatile flavor compounds as in some cases they produced sweet, honey-like aromas and consistently pleasant fruity flavors with isoamyl acetate to some extent (cf. Figure 1, Table 5). Referring to wort fermentation, this yeast species could be used for the production of non-alcoholic beers.
Originally related to the production of milk products, K. lactis and K. marxianus were also screened for their brewing ability as the literature points out that both yeasts have the ability to produce aroma compounds that give fruity, floral and honey-like aromas [65,66,67]. While the sensory odor test showed that the yeasts of the species K. lactis produced slightly fruity aromas, some of the yeasts of the species K. marxianus stood out with remarkably pleasant aroma impressions (cf. Figure 1, Table 5). These yeasts could also be considered for the production of alcohol-free beers.
5. Conclusions
In general, it can be summarized that the yeast strains of the species S. fibuligera are fairly interesting for the fermentation of brewer’s wort to produce an alcoholic beer. The strains SF2, SF4 and SF8 release, without exception, desirable fruity flavors reminiscent of plum and berry. Esters such as ethyl butyrate and ethyl acetate result in sweet and fruity aroma impressions. Additionally, significant amounts of isoamyl acetate were analyzed in the beer fermented with SF2, which underlined the pleasant fruity character. In contrast to the yeast strains S. pombe (S11) and Z. rouxii (ZR9), no wheat beer aroma was perceptible in the beers produced with the S. fibuligera yeast strains. Although the POF test results of S11 and ZR9 were negative, both yeasts still produced flavor compounds that gave a phenolic, clove-like character.
Author Contributions
M.M. and Y.M. conceived and designed the experiments; Y.M. performed the experiments and analyzed the data using OriginPro 2019 as statistical software; M.H. and D.M. organized and provided a large amount of yeast strains and M.H. revised the conception; Y.M. wrote the paper; M.M. and F.J. revised the manuscript and agreed the submission.
Funding
This research was funded by the Wifö (Wissenschaftsförderung der Deutschen Brauwirtschaft e.V., Berlin, Germany) in the project AiF 20658 N and by the Ministry of Agriculture of the Czech Republic (RO1918).
Acknowledgments
The isomerized hop extract 30% (iso-α-acid) and the beta-rich hop extract 40% were kindly provided by Hopsteiner, Mainburg, Germany.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Piškur, J.; Rozpędowska, E.; Polakova, S.; Merico, A.; Compagno, C. How did Saccharomyces evolve to become a good brewer? Trends Genet. 2006, 22, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Claussen, N.H. Eine Methode zur Anwendung von Hansens Reinzuchtsystem bei der Herstellung von englischen gelagerten Biersorten. Wochenschr. Brau 1904, 1904, 370–383. [Google Scholar]
- De Estela-Escalante, W.; Rosales-Mendoza, S.; Moscosa-Santillán, M.; González-Ramírez, J.E. Evaluation of the fermentative potential of Candida zemplinina yeasts for craft beer fermentation. J. Inst. Brew. 2016, 122, 530–535. [Google Scholar] [CrossRef]
- Michel, M.; Meier-Dörnberg, T.; Jacob, F.; Methner, F.-J.; Wagner, R.S.; Hutzler, M. Pure non-Saccharomyces starter cultures for beer fermentation with a focus on secondary metabolites and practical applications. J. Inst. Brew. 2016, 122, 569–587. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Michel, M.; Meier-Dörnberg, T.; Zarnkow, M.; Jacob, F.; Hutzler, M. Screening for the Brewing Ability of Non-Saccharomyces Yeast and Optimization of Fermentation Performance of One Torulaspora Delbrueckii Strain Found Suitable for Beer Production. In Proceedings of the World Brewing Congress, Denver, CO, USA, 13–17 August 2016. [Google Scholar]
- Ravasio, D.; Carlin, S.; Boekhout, T.; Groenewald, M.; Vrhovsek, U.; Walther, A.; Wendland, J. Adding Flavor to Beverages with Non-Conventional Yeasts. Fermentation 2018, 4, 15. [Google Scholar] [CrossRef]
- Narziß, L.; Back, W.; Miedaner, H.; Lustig, S. Untersuchung zur Beeinflussung der Geschmacksstabilität durch Variation technologischer Parameter bei der Bierherstellung. Monatsschrift für Brauwissenschaft 1999, 1999, 192–206. [Google Scholar]
- Stewart, G.G. The Horace Brown Medal Lecture: Forty Years of Brewing Research. J. Inst. Brew. 2009, 115, 3–29. [Google Scholar] [CrossRef]
- Vidgren, V.; Multanen, J.-P.; Ruohonen, L.; Londesborough, J. The temperature dependence of maltose transport in ale and lager strains of brewer’s yeast. FEMS Yeast Res. 2010, 10, 402–411. [Google Scholar] [CrossRef]
- Wickerham, L.J. A simple technique for the detection of melibiose-fermenting yeasts1. J. Bacteriol. 1943, 46, 501–505. [Google Scholar]
- Srinivasan, V.; Goldberg, D.; Haas, G.J. Contributions to the Antimicrobial Spectrum of Hop Constituents. Econ. Bot. 2004, 58, S230–S238. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Walsh, M.C.; Pronk, J.T.; Daran, J.-M. Involvement of vacuolar sequestration and active transport in tolerance of Saccharomyces cerevisiae to hop iso-alpha-acids. Appl. Environ. Microbiol. 2010, 76, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Bamforth, C.W. Wort Composition and Beer Quality. In Brewing Yeast Fermentation Performance, 2nd ed.; Smart, K., Ed.; Blackwell Science: Oxford, UK; Malden, MA, USA, 2003; pp. 75–85. ISBN 9780470696040. [Google Scholar]
- Ding, J.; Huang, X.; Zhang, L.; Zhao, N.; Yang, D.; Zhang, K. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2009, 85, 253. [Google Scholar] [CrossRef]
- Narziss, L.; Back, W.; Gastl, M.; Zarnkow, M. Abriss der Bierbrauerei, 8th ed.; John Wiley & Sons Incorporated: Newark, NJ, USA, 2017; ISBN 9783527340361. [Google Scholar]
- Shinohara, T.; Kubodera, S.; Yanagida, F. Distribution of phenolic yeasts and production of phenolic off-flavors in wine fermentation. J. Biosci. Bioeng. 2000, 90, 90–97. [Google Scholar] [CrossRef]
- Montanari, L.; Perretti, G.; Natella, F.; Guidi, A.; Fantozzi, P. Organic and Phenolic Acids in Beer. LWT 1999, 32, 535–539. [Google Scholar] [CrossRef]
- Schwarz, K.J.; Stübner, R.; Methner, F.-J. Formation of styrene dependent on fermentation management during wheat beer production. Food Chem. 2012, 134, 2121–2125. [Google Scholar] [CrossRef]
- De Arruda Moura Pietrowski, G.; dos Santos, C.M.E.; Sauer, E.; Wosiacki, G.; Nogueira, A. Influence of fermentation with Hanseniaspora sp. yeast on the volatile profile of fermented apple. J. Agric. Food Chem. 2012, 60, 9815–9821. [Google Scholar] [CrossRef]
- Benito, S.; Palomero, F.; Morata, A.; Calderón, F.; Suárez-Lepe, J.A. New applications for Schizosaccharomyces pombe in the alcoholic fermentation of red wines. Int. J. Food Sci. Technol. 2012, 47, 2101–2108. [Google Scholar] [CrossRef]
- Amaya-Delgado, L.; Herrera-López, E.J.; Arrizon, J.; Arellano-Plaza, M.; Gschaedler, A. Performance evaluation of Pichia kluyveri, Kluyveromyces marxianus and Saccharomyces cerevisiae in industrial tequila fermentation. World J. Microbiol. Biotechnol. 2013, 29, 875–881. [Google Scholar] [CrossRef]
- Steensels, J.; Verstrepen, K.J. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef]
- Hiralal, L.; Olaniran, A.O.; Pillay, B. Aroma-active ester profile of ale beer produced under different fermentation and nutritional conditions. J. Biosci. Bioeng. 2014, 117, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Derdelinckx, G.; Delvaux, F.R. Esters in beer-part 1: The fermentation process: More than ethanol formation. Cerevisia 2003, 28, 41–49. [Google Scholar]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Derdelinckx, G.; Verachtert, H. Bioflavoring and beer refermentation. Appl. Microbiol. Biotechnol. 2003, 62, 140–150. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.-P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Meilgaard, M.C. Flavor Chemistry of Beer: Part II: Flavor and Threshold of 239 Aroma Volatiles. MBAA Tech. Tech. Quart. Master. Brew. Assoc. Am. 1975, 12, 151–168. [Google Scholar]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Sannino, C.; Mezzasoma, A.; Buzzini, P.; Turchetti, B. Non-conventional Yeasts for Producing Alternative Beers. In Non-conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 361–388. ISBN 978-3-030-21109-7. [Google Scholar]
- Olšovská, J.; Vrzal, T.; Štěrba, K.; Slabý, M.; Kubizniaková, P.; Čejka, P. The chemical profiling of fatty acids during the brewing process. J. Sci. Food Agric. 2019, 99, 1772–1779. [Google Scholar] [CrossRef]
- Wahyono, A.; Kang, W.-W.; Park, H.-D. Characterization and application of Torulaspora delbrueckii JK08 and Pichia anomala JK04 as baker’s yeasts. J. Food Nutr. Res. 2015, 54. [Google Scholar]
- Esslinger, H.M. Handbook of Brewing: Processes, Technology, Markets; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 3527623493. [Google Scholar]
- Sakamoto, K.; Konings, W.N. Beer spoilage bacteria and hop resistance. Int. J. Food Microbiol. 2003, 89, 105–124. [Google Scholar] [CrossRef]
- Kurtzman, C.P. (Ed.) The Yeasts. A taxonomic study, 5th ed.; Elsevier: Amsterdam, The Netherland, 2011; ISBN 9780123847072. [Google Scholar]
- Owens, J.D. Indigenous Fermented Foods of Southeast Asia; CRC Press: Boca Raton, FL, USA, 2014; ISBN 0429105916. [Google Scholar]
- Lee, S.M.; Jung, J.H.; Seo, J.-A.; Kim, Y.-S. Bioformation of Volatile and Nonvolatile Metabolites by Saccharomycopsis fibuligera KJJ81 Cultivated under Different Conditions-Carbon Sources and Cultivation Times. Molecules 2018, 23, 2762. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Procopio, S.; Becker, T. Flavor impacts of glycerol in the processing of yeast fermented beverages: A review. J. Food Sci. Technol. 2015, 52, 7588–7598. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.T.; Strack, L.; Futschik, M.; Katou, Y.; Nakao, Y.; Fujimura, T.; Shirahige, K.; Kodama, Y.; Nevoigt, E. Identification of Sc-type ILV6 as a target to reduce diacetyl formation in lager brewers’ yeast. Metab. Eng. 2011, 13, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M. Entstehung und Beeinflussung qualitätsbestimmender Aromastoffe bei der Herstellung von Weißbier; Technische Universität München: Munich, Germany, 2005. [Google Scholar]
- Boulton, C.; Quain, D. Brewing Yeast and Fermentation; John Wiley & Sons: Hoboken, NJ, USA, 2008; ISBN 0470999403. [Google Scholar]
- Saerens, S.; Swiegers, J.H. Production of Low-Alcohol or Alcohol-Free beer with Pichia Kluyveri Yeast Strains. Google Patents EP2964742A2, 2016. [Google Scholar]
- Lindner, P. Schizosaccharomyces pombe n. sp., ein neuer Gährungserreger. Wochenschrift für Brauerei 1893, 1298–1300. [Google Scholar]
- Sefa-Dedeh, S.; Sanni, A.I.; Tetteh, G.; Sakyi-Dawson, E. Yeasts in the traditional brewing of pito in Ghana. World J. Microbiol. Biotechnol. 1999, 15, 593–597. [Google Scholar] [CrossRef]
- King, A.; Richard Dickinson, J. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 2000, 16, 499–506. [Google Scholar] [CrossRef]
- Canonico, L.; Galli, E.; Ciani, E.; Comitini, F.; Ciani, M. Exploitation of Three Non-Conventional Yeast Species in the Brewing Process. Microorganisms 2019, 7, 11. [Google Scholar] [CrossRef]
- De Francesco, G.; Turchetti, B.; Sileoni, V.; Marconi, O.; Perretti, G. Screening of new strains of Saccharomycodes ludwigii and Zygosaccharomyces rouxii to produce low-alcohol beer. J. Inst. Brew. 2015, 121, 113–121. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Reid, D.S.; Wyborski, K.A. Reference standards for beer flavor terminology system. J. Am. Soc. Brew. Chem. 1982, 40, 119–128. [Google Scholar] [CrossRef]
- Andorrá, I.; Berradre, M.; Mas, A.; Esteve-Zarzoso, B.; Guillamón, J.M. Effect of mixed culture fermentations on yeast populations and aroma profile. LWT 2012, 49, 8–13. [Google Scholar] [CrossRef]
- Varela, C.; Barker, A.; Tran, T.; Borneman, A.; Curtin, C. Sensory profile and volatile aroma composition of reduced alcohol Merlot wines fermented with Metschnikowia pulcherrima and Saccharomyces uvarum. Int. J. Food Microbiol. 2017, 252, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Díaz, C.; Molina, A.M.; Nähring, J.; Fischer, R. Characterization and dynamic behavior of wild yeast during spontaneous wine fermentation in steel tanks and amphorae. Biomed Res. Int. 2013, 2013, 540465. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Borneman, A.R. Yeasts found in vineyards and wineries. Yeast 2017, 34, 111–128. [Google Scholar] [CrossRef]
- Hutzler, M.; Riedl, R.; Koob, J.; Jacob, F. Fermentation and spoilage yeasts and their relevance for the beverage industry-a review. BrewingScience-Monatsschrift Für Brauwissenschaft 2012, 65, 33–50. [Google Scholar]
- Coton, E.; Coton, M.; Levert, D.; Casaregola, S.; Sohier, D. Yeast ecology in French cider and black olive natural fermentations. Int. J. Food Microbiol. 2006, 108, 130–135. [Google Scholar] [CrossRef]
- Ye, M.; Yue, T.; Yuan, Y. Effects of sequential mixed cultures of Wickerhamomyces anomalus and Saccharomyces cerevisiae on apple cider fermentation. FEMS Yeast Res. 2014, 14, 873–882. [Google Scholar] [CrossRef]
- Cañas, P.M.I.; García-Romero, E.; Manso, J.M.H.; Fernández-González, M. Influence of sequential inoculation of Wickerhamomyces anomalus and Saccharomyces cerevisiae in the quality of red wines. Eur. Food Res. Technol. 2014, 239, 279–286. [Google Scholar] [CrossRef]
- De Benedictis, M.; Bleve, G.; Grieco, F.; Tristezza, M.; Tufariello, M. An optimized procedure for the enological selection of non-Saccharomyces starter cultures. Antonie Van Leeuwenhoek 2011, 99, 189–200. [Google Scholar] [CrossRef]
- Loira, I.; Morata, A.; Comuzzo, P.; Callejo, M.J.; González, C.; Calderón, F.; Suárez-Lepe, J.A. Use of Schizosaccharomyces pombe and Torulaspora delbrueckii strains in mixed and sequential fermentations to improve red wine sensory quality. Food Res. Int. 2015, 76, 325–333. [Google Scholar] [CrossRef]
- Lee, P.-R.; Ong, Y.-L.; Yu, B.; Curran, P.; Liu, S.-Q. Evolution of volatile compounds in papaya wine fermented with three Williopsis saturnus yeasts. Int. J. Food Sci. Technol. 2010, 45, 2032–2041. [Google Scholar] [CrossRef]
- Yilmaztekin, M.; Erten, H.; Cabaroglu, T. Production of Isoamyl Acetate from Sugar Beet Molasses by Williopsis saturnus var. saturnus. J. Inst. Brew. 2008, 114, 34–38. [Google Scholar] [CrossRef]
- Erten, H.; Tanguler, H. Influence of Williopsis saturnus yeasts in combination with Saccharomyces cerevisiae on wine fermentation. Lett. Appl. Microbiol. 2010, 50, 474–479. [Google Scholar] [CrossRef]
- Son, E.Y.; Lee, S.M.; Kim, M.; Seo, J.-A.; Kim, Y.-S. Comparison of volatile and non-volatile metabolites in rice wine fermented by Koji inoculated with Saccharomycopsis fibuligera and Aspergillus oryzae. Food Res. Int. 2018, 109, 596–605. [Google Scholar] [CrossRef]
- Medeiros, A.B.P.; Pandey, A.; Christen, P.; Fontoura, P.S.G.; de Freitas, R.J.S.; Soccol, C.R. Aroma compounds produced by Kluyveromyces marxianus in solid state fermentation on a packed bed column bioreactor. World J. Microbiol. Biotechnol. 2001, 17, 767–771. [Google Scholar] [CrossRef]
- Fabre, C.E.; Duviau, V.J.; Blanc, P.J.; Goma, G. Identification of volatile flavour compounds obtained in culture of Kluyveromyces marxianus. Biotechnol. Lett. 1995, 17, 1207–1212. [Google Scholar] [CrossRef]
- Jiang, J. Identification of flavour volatile compounds produced by Kluyveromyces lactis. Biotechnol. Tech. 1993, 7, 863–866. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).