Abstract
Fungi produce a variety of volatile organic compounds (VOCs) during their primary and secondary metabolism. In the beverage industry, these volatiles contribute to the the flavor and aroma profile of the final products. We evaluated the fermentation ability and aroma profiles of non-conventional yeasts that have been associated with various food sources. A total of 60 strains were analyzed with regard to their fermentation and flavor profile. Species belonging to the genera Candida, Pichia and Wickerhamomyces separated best from lager yeast strains according to a principal component analysis taking alcohol and ester production into account. The speed of fermentation and sugar utilization were analysed for these strains. Volatile aroma-compound formation was assayed via gas chromatography. Several strains produced substantially higher amounts of aroma alcohols and esters compared to the lager yeast strain Weihenstephan 34/70. Consequently, co-fermentation of this lager yeast strain with a Wickerhamomyces anomalus strain generated an increased fruity-flavour profile. This demonstrates that mixed fermentations utilizing non-Saccharomyces cerevisiae biodiversity can enhance the flavour profiles of fermented beverages.
1. Introduction
Beer is one of the most widely consumed alcoholic beverages in the world. In 2003, worldwide beer production reached around 1.82 billion hectoliters and increased to a volume of 1.93 billion hectoliters in 2013 according to the Kirin Beer University Report of 2014. Production is divided into several beer styles of which ale and lager beers are most prominent. Generally, ale is produced by top-fermenting yeasts at temperatures between 15–30 °C. Ales are known for their fruity aromas which are regarded as a distinctive characteristic of top-fermenting beers. Lager beers, however, are produced by a distinct group of bottom-fermenting yeasts at fermentation temperatures between 10–15 °C. The aroma of lager beers is more neutral compared to ale-type beers as they contain lower amounts of fruity flavors.
Top-fermenting yeasts generally are S. cerevisiae strains. At the end of fermentation, these yeasts rise to the surface of the fermenter, creating a thick cell layer. Bottom-fermenting yeasts belong to two distinct groups of lager yeasts [1]. Lager yeasts are hybrids between S. cerevisiae and S. eubayanus [2]. Lager yeasts can be divided into two groups, group I or Saaz type, and group II or Frohberg type, that are distinguished at the molecular level by ploidy differences, characteristic chromosomal rearrangements and chromosome losses [3,4].
In contrast to lager beers, lambic beers with sometimes exceptional flavor compositions are based on a larger biodiversity, including acetic and lactic acid bacteria and various yeasts, e.g., S. cerevisiae, S. pastorianus and Brettanomyces bruxellensis [5].
Beer is a complex product consisting of volatile and non-volatile components that form the final aroma. The contribution of ale yeasts to the final flavor bouquet is generally higher than that of lager yeasts. This has been attributed to the greater diversity of ale yeasts compared to the limited diversity of the two groups of lager yeast [6]. Other process parameters also influence volatile-compound formation. Specifically, sugar concentration of the wort and different aeration regimes influence the production of flavor-active esters. These esters mainly contribute to the fruitiness of the product. Dominant esters are acetate esters such as ethyl acetate (fruity), isoamyl acetate (banana) and 2-phenyl acetate (rose) and ethyl or medium-chain fatty acid esters such as ethyl hexanoate, ethyl octanoate and ethyl decanoate, which provide a fruity apple- or wine-like flavor to the beer [7]. Among the higher alcohols, n-propanol, isobutanol, 2-phenylethanol and isoamyl alcohol are most abundant. Higher alcohols such as isobutanol can contribute a rum-like aroma which gives a warm mouth feeling, while 2-phenylethanol and isoamyl alcohol are prevalent for their sweet/rose and fruity/banana-like aromas, respectively [8].
Not all flavors are desirable. Strecker aldehydes (aged flavour), aldehydes of the Maillard reaction, e.g., furfural, and aldehydes of fatty acid oxidation, e.g., trans-2-nonenal, are regarded as off-flavors in beer [9]. The ketone diacetyl (2,3-butanedione) is monitored during the lager beer brewing process, in particular, as it imparts an undesirable buttery flavor with a low flavour threshold [10]. Given the low concentration and volatile nature of these aroma compounds, gas chromatography coupled with mass spectrometry (GC/MS) offers an optimal technique to analyze the flavor profile of beer.
Recently, non-conventional yeasts or non-cerevisiae yeasts have gained importance for fermented alcoholic beverages [11,12]. They produce various mixtures of volatile compounds and so contribute to the aroma profile of beverages [6,13]. Mixed fermentations using S. cerevisiae in combination with a non-conventional yeast strain e.g., belonging to the genera Lachancea, Pichia or Hanseniaspora, could provide novel beverages with improved ester profiles [14,15]. On the other hand, the synergistic effects on aromatic compound production were observed in co-cultures with Metschnikowia pulcherrima and S. cerevisiae [16]. Non-conventional yeasts could contribute to satisfying the demand of novel and distinctive, yet natural, flavors in fermented beverages [17].
A vast number of non-conventional yeast strains has been isolated from various food sources and deposited in culture collection such as the CBS yeast collection of the Westerdijk Fungal Biodiversity Institute (formerly, CBS, Centraalbureau voor Schimmelcultures, Utrecht, Netherlands). In this study, we aimed at covering a broad spectrum of species isolated from different substrates like berries, fruits, cheese, fruit flies or even soil and spanning a broad evolutionary distance within the Saccharomycotina in order to identify species that could contribute with their particular flavour to lager beer fermentations.
2. Materials and Methods
2.1. Strains and Media
Yeast strains used in this study are shown in Table 1, including their CBS reference number. Each strain was coded based on its coordinates in a 96-well plate. Yeast strains were subcultured in YPD (1% yeast extract, 2% peptone, 2% glucose) at room temperature overnight.

Table 1.
Strains used in this study. Each selected strain was assigned to a code based on its coordinates on a 96-well plate.
To evaluate growth at different temperatures or the utilization of maltose, cells were spotted on solid media plates in 10-fold dilution series and incubated for 5 days before evaluation. Growth at the different dilutions was 10-color coded and is presented as a heat map.
2.2. Fermentation Conditions
Lab-scale fermentations were carried out in 50 mL tubes filled with 40 mL nutrient-rich YPD with the glucose concentration adjusted to 16 °Plato at 20 °C. Each fermentation (300 rpm, using a triangular magnetic stirrer) was started with a cell density corresponding to OD600 = 0.2. For co-fermentations, the amounts of cells used equaled OD600 = 0.1. The fermentation progress was monitored for up to 14 days by daily measurement of CO2 release. Sugar content was measured by a DMA 35 Anton Paar densitometer (medium gravity in °P). Plato measurements were taken at the beginning and at the end of each fermentation process. The fermentation was defined as finished when the CO2 loss did not increase any further and the residual sugar concentration remained constant for 2 days. Ethanol was measured after the end of fermentation using an Anton Paar DMA 4500 M Alcolyzer. Aroma profiles were analysed by headspace gas chromatography.
2.3. Sample Preparation for Solid-Phase MicroExtraction–Gas Chromatography/Mass Spectrometry (SPME-GC/MS) Analysis
2.5 mL of the samples were put in 20 mL vials with the addition of sodium chloride (final concentration 40 mg/ml), 50 µL NaN3 0.1%, 25 µL of the internal standard (2-octanol final concentration of 200 µg/L) and ascorbic acid (final concentration of 20 mg/mL).
All samples were incubated for 10 min at 40 °C, then the volatile compounds were collected on a divinylbenzene/carboxen/polydimethylsiloxane fiber (DVB-CAR-PDMS) coating 50/30 μm, and 2-cm length SPME fibre purchased from Supelco (Sigma-Aldrich, Milan, Italy) for 40 min.
2.4. Analytical Methods for GC/MS
GC analysis was performed on a Trace GC Ultra gas chromatograph coupled with a TSQ Quantum Tandem mass spectrometer (Thermo Electron Corporation, Waltham, MA USA), with adaptations as described in Ravasio et al. [18]. GC separation was performed on a 30 m Solgelwax PEG capillary column with an internal diameter of 0.25 mm and a film thickness of 0.25 m (SGE Analytical Science, Melbourne, Australia). The GC oven was kept at 40 °C for 4 min and then increased by 6 °C/min to 250 °C and kept at the final temperature for 5 min. The injector and transfer-line temperatures were kept at 250 °C as well. Helium was used as the carrier gas with a flow rate of 1.2 mL/min. The time for thermal desorption of analytes was 4 min. The MS detector was operated in full scan mode at 70 eV with a scan range from 35 to 350 m/z. Data analysis was performed using the software ThermoXcalibur (Version 2.2 SP1.48, Thermo scientific, Waltham, MA, USA). Identification of compounds was based on comparison with a mass spectral database (NIST version 2.0, Gaithersburg, MD, USA) and with reference standards when available. The relative amount of each volatile was expressed as µg/L of 2-octanol.
2.5. Multivariate Data Analysis
Multivariate data analysis was performed using StatSoft, Inc. STATISTICA version 8.0 (data analysis software system, 2007, StatSoft (Europe) GmbH, Berikon, Switzerland). Principal component analysis was employed to simplify data interpretation. The matrix initially contained the 60 strains considered in this study and the average of the relative 62 VOCs detected and was later reduced to the sub-selection of 19 strains studied further.
3. Results
3.1. Strain Selection and Identification of Representative Isolates
In order to cover a wide range of the biodiversity of non-conventional yeasts, we selected 60 strains from 48 different species which were obtained from the strain collection of the Westerdijk Institute. The focus was on strains that were previously isolated from various fermentations, e.g., from fermented liquids, fruits, vegetables, or meat. These strains, therefore, may have evolved superior or desirable features, e.g., the production of high levels of ethanol or may have been recognized based on their contribution to flavors. All strains were run in lab-scale fermentation trials in nutrient-rich broth (YPD with adjusted glucose content at 16 °Plato) to assess their aroma production. Flavor profiles were analyzed using GC/MS and compared to a set of brewing and wine yeast strains (Supplementary Table S1).
3.2. Aroma Profiles of Fermentations
In total, we identified 62 different volatiles in the samples of all strains (Table 2). Major volatile-aroma compounds that were detected included esters, alcohols, aldehydes, ketones and acids. Most species harbor the ability to produce a large variety of flavors. Thus, we did not identify single species that produced only very few compounds or species with a very high diversity, as shown in Figure 1 for a selection of species. However, there was an enormous difference in the amounts of specific volatiles produced. Esters were the most prominent group of volatiles. In total, 22 different esters could be identified and within this group ethyl-esters dominated, such as ethyl hexanoate and ethyl acetate, associated with fruity wine/apple-like and sweet pear drop flavors, respectively. Alcohols comprised the second major group of compounds. Besides ethanol, we identified 14 different alcohols. Yet, only two compounds, 2-phenylethanol, perceived as rose flavor, and isoamyl alcohol, a banana-like flavor, were produced by all strains analyzed. During anaerobic fermentations the formation of aroma alcohols is favored over the production of aroma acids [19]. In line with this, we identified only six acids, of which acetic acid and butyric acid were prominent.

Table 2.
List of 62 volatiles that were detected by GC/MS measurement.
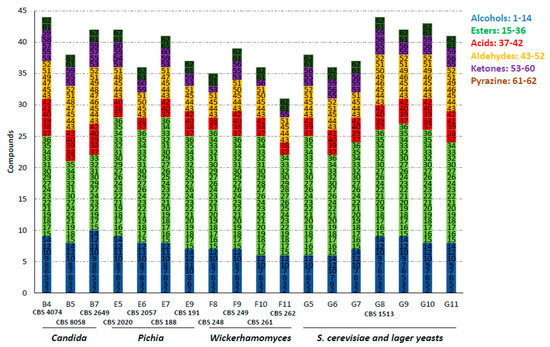
We were most interested in species that separated well from S. cerevisiae or lager yeast strains. Therefore, we narrowed down the selection of strains to those that showed clear separation in their flavour profiles to the set of S. cerevisiae and lager yeast strains based on principal component analysis (Figure 2). This identified species belonging to the genera Candida, Pichia and Wickerhamomyces.
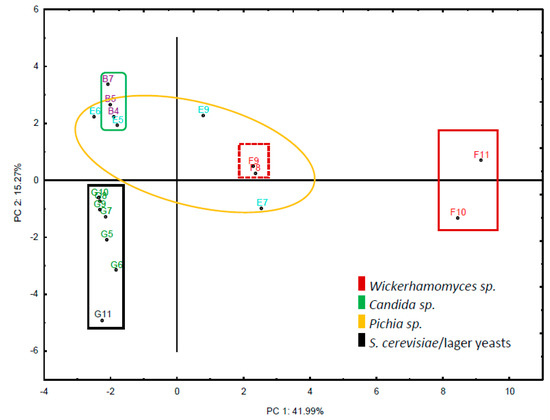
Figure 2.
Principal component analysis of the set of selected strains and their chemical compounds produced. The matrix is based on the set of 19 strains and the VOCs detected. Strains are presented with their assigned coordinates (see Table 1). Species belonging to the same genus are represented by the same color.
Based on the GC data, we generated relative quantifications of the volatile aroma compounds formed. The set of strains was then compared to the lager yeast S. pastorianus/Weihenstephan to identify strains that produced higher amounts of esters and alcohols (Table 3 and Table 4). One strain of Saccharomyces cerevisiae (CBS 1250; E11) was a very good producer of alcohols, particularly of isoamyl alcohol, by contrast with lager yeast. Yet, this strain only produced moderate amounts of esters, mostly ethyl acetate. Its origin from sherry production suggests that it has been selected as the preferred strain for these fermentations.

Table 3.
Strains with higher aroma alcohol production than the Weihenstephan lager yeast strain.

Table 4.
Strains with higher aroma ester production than the Weihenstephan lager yeast strain.
The Wickerhamomyces anomalus isolates showed a remarkably high amount of both alcohol and ester production. Production of higher alcohols was strongly increased in these W. anomalus strains compared to lager yeast. Interestingly, here mainly isoamyl alcohol and 2-phenyl ethanol production was increased. These represent two desirable flavors associated with banana and rose flavours. The sherry isolate (CBS 1250, E11) was found to produce even more isoamyl alcohol than the W. anomalus strains, but far less 2-phenylethanol than these strains (Table 3). Yet, the W. anomalus strains produced abundant amounts of esters, particularly ethyl acetate, isoamyl acetate and 2-phenylethyl acetate. Overall, these strains produced up to 10-fold more esters than the Weihenstephan lager yeast strain. (Table 4). Another highly aromatic strain identified in this collection of strains was a Pichia kluyveri strain, CBS 188. This strain produced almost fourfold more esters than lager yeast, while its aroma alcohols profile was similar to lager yeast with the exception of fourfold higher 2-phenylethanol production.
3.3. Fermentation Performance
We compared the fermenting capacity of the strains with very strong volatile compound formation to the lager yeast strain S. pastorianus WS34/70 and other S. cerevisiae strains. All strains were adjusted to the same optical density prior to the start of fermentation to allow for comparison of the speed of fermentation between strains. Fermentation rates were followed by measuring the CO2 release daily (Figure 3). All strains were able to ferment the liquid within one week except for the W. anomalus strains CBS 248; CBS 249 and CBS 261. However, in contrast to the other strains, the W. anomalus strains showed an almost linear fermentation curve with equal amounts of CO2 released per day. During fermentation the W. anomalus strains produced oily top layers and biofilms of cells. This oily phase may have reduced the CO2 release as we observed the lowest pH in the W. anomalus fermentations measured among all strains: for strain CBS 261 the pH reached 4.79 and for strain CBS 262 the pH was 4.59, while the liquids fermented by other yeast strains showed a pH of >5. This suggests that the dissolved CO2 contributed to the acidification of the liquid in the W. anomalus fermentations. The most rapid fermentations as represented by fast CO2 loss were observed with the S. cerevisiae and lager beer production strains. Yet, in addition to the Wickerhamomyces strains, several other strains showed prolonged CO2 loss, including P. kluyveri CBS 188 (Figure 3).
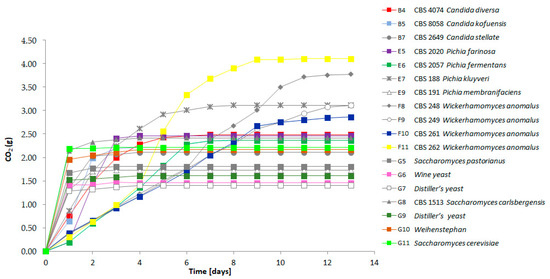
Figure 3.
Fermentation profiles of the selected strains representing the detected flavor diversity. The CO2 release was measured daily for each individual strain (see Table 1 for the nomenclature of strains). Fermentations were followed for 13 days.
For most of the strains we could observe a correlation between fermentation speed, residual sugar concentration and ethanol production. The S. cerevisiae and lager yeast strains left 2–2.5% of sugar in the medium, while the residual sugar in Candida and Pichia strains was between 3–3.5%. Wickerhamomyces anomalus strains left 4.5–5% of sugars unfermented (Figure 4). Concomitantly, S. cerevisiae and lager yeast strains produced the highest amount of alcohol (up to 8%) and the W. anomalus strains only 4%.

Figure 4.
Final sugar content of the selected strains at the end of fermentation. Sugar concentration was measured in °P after 13 days of fermentation.
3.4. Growth on Other Carbon Sources and Temperatures
The selected species were analysed for growth at low (10 °C) and high (37 °C) temperatures as well as for their ability to utilize maltose using serial-dilution plate assays. Growth at elevated temperatures was mainly restricted to Saccharomyces and Zygosaccharomyces strains, while growth at lower temperatures was often better in non-cerevisiae strains. Typically, all strains grew well at intermediate temperatures. Lager beer fermentation relies on maltose utilization. However, in this screening, good maltose utilization was restricted to lager yeast strains, with the exception of Candida diversa CBS 4074 (Figure 5), which also showed very good growth on maltose plates and Clavispora lusitaniae CBS 6936.
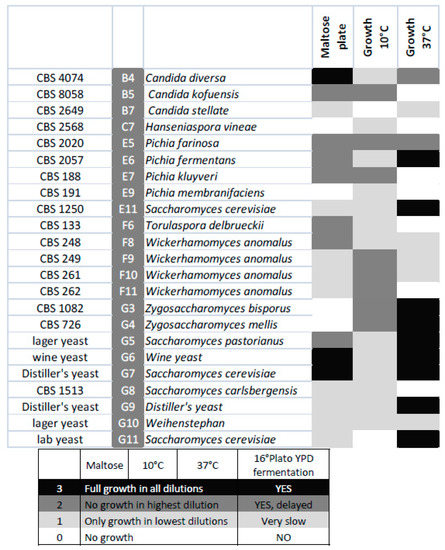
Figure 5.
Heat map displaying maltose utilization and growth at the indicated temperatures of the selected strains.
3.5. Utilization of Mixed Fermentations for Flavor Improvement
Co-fermentations are one way to improve the flavour composition of fermented beverages. We plotted volatile compound formation of several candidate strains against lager yeast (Figure 6a). This demonstrated the superior flavour-generation capacity of several strains, most notably of W. anomalus. Therefore, we used the same nutrient-rich high Plato fermentation broth and inoculated the Weihenstephan lager yeast WS34/70 in a 1:1 ratio with W. anomalus CBS 261. Volatiles of this mixed fermentation were determined at the end of fermentation. This clearly demonstrated an enhancement of ethyl hexadecanoate, isoamyl alcohol and 2-phenyl ethanol, improving the fruity flavour perception of the fermented liquid (Figure 6b). W. anomalus CBS 261 is a very strong producer of ethyl acetate, which was also pronounced in the co-fermentation.
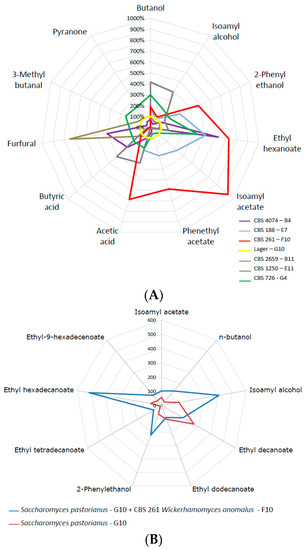
Figure 6.
(A) Comparisons of selected volatiles of selected strains that produced exceptionally high concentrations of organic volatiles with the lager yeast reference. Strains used were: Candida diversa (CBS 4074, B4); Debaryomyces subglobosus (CBS 2659, B11); Pichia kluyveri (CBS 188, E7); Saccharomyces cerevisiae (CBS 1250; E11); Wickerhamomyces anomalus (CBS 261, F10); Zygosaccharomyces mellis (CBS 726, G4); Saccharomyces pastorianus (G10, reference). (B) Concentration of volatiles at the end of a co-fermentation using Wickerhamomyces anomalus (CBS 261, F10) and the Weihenstephan lager yeast (WS, Saccharomyces pastorianus G10, reference).
4. Discussion
In our study we have screened non-Saccharomyces cerevisiae yeast biodiversity in order to identify strains with more pronounced volatile compound formation than present in lager yeast strains. Pronounced differences in aroma alcohol production were identified between S. cerevisiae and lager yeast strains, as expected from a clean pilsner beer produced by lager yeasts versus the more complex flavors produced by ale and wine yeasts. In our screening, we identified W. anomalus strains as the most dominant flavor producers, which also included production of substantial amounts of acetic acid. The formation of floating cell layers such as seen for the W. anomalus strains has been reported as a typical phenomenon in stored wines [20]. Yeast biodiversity holds a plethora of strains that show useful characteristics such as ethanol production and flavor formation. This requires a detailed evaluation of the initially identified favorable strains under different conditions, particularly in co-fermentation regimes [21]. It will be challenging to identify the most suitable co-fermentation setups, as flavor profiles will certainly be influenced by different ratios of non-conventional yeasts versus standard brewing strains.
In earlier studies, W. anomalus strains have been isolated from a range of cereal-based sources. It has been reported from sourdoughs and was found as the dominating yeast in sourdough microbial ecosystems next to S. cerevisiae. The prevalence of the fungus was associated with its osmotolerance and increased acid tolerance in comparison to S. cerevisiae [22]. Furthermore, it was shown that W. anomalus provides some antimicrobial activity, e.g., mycocin production, that can be used to prolong the shelf life of bread [23,24]. Other Wickerhamomyces species have been put to use in baking using microbread baking platforms. Specifically, bread obtained with Kazachstania gamospora and Wickerhamomyces subpelliculosus provided added broader aroma profiles compared to control baker's yeast [25].
P. kluyveri CBS 188 produced a total of 41 volatiles during our fermentations. Yet, it was outstanding in its ester profile, e.g., ethyl acetate, isoamyl acetate or phenethyl acetate that was several folds higher than in the reference lager yeast strain WS34/70. P. kluyveri strains are found in “wild ferments” of wine but it is also commercially available to boost flavour production through sequential fermentations. P. kluyveri strains together with strains of K. marxianus were also presented as potential starter yeasts for controlled cocoa fermentation [26].
Based on increased demand for natural flavors produced from yeasts, the use of non-conventional yeasts as platform strains for the production of aroma volatile has gained attention. Hence, K. marxianus was suggested as a cell factory for flavor and fragrance production based on several advantageous traits, e.g., thermotolerance and the wide array of volatile molecules it produces [27]. We had one strain of K. marxianus (CBS 1557) in our collection. However, this strain was as low in aroma-compound production as our lager yeast reference. This indicates the need to obtain a larger collection of strains of one species and also to analyse volatile compound formation under different nutritional regimes and fermentation conditions [28]. It further requires the implementation of high-throughput screening tools and assays to identify suitable strains, which was actually successfully shown for K. marxianus strains producing ethyl acetate [29]. Additionally, it requires more effort to acquire genomic, transcriptomic and metabolomic datasets of non-conventional yeasts in order to bridge the knowledge gap about S. cerevisiae [12,30]. That said, our approach to screening non-conventional yeasts in non-industrial fermentation broths only provides an initial glance of the volatile production capabilities of the tested strains. These capabilities will certainly vary with fermentation and process conditions, particularly at larger scales.
The strong interest in volatile-compound formation by non-conventional yeasts may also open a new perspective on yeast ecology. Yeasts occur in diverse niches and interact with other microbes, insects and plants. These interactions may present selective forces for the production of specific volatile compounds. Interestingly, strains of Debaryomyces hansenii were shown to produce methyl salicylate (MeS) as a major compound under very specific conditions using pine weevil (Hylobius abietis) frass broth [31]. This compound essentially acts as a deterrent for pine weevil. In a follow-up case, 2-phenylethanol was identified as a strong anti-feedant compound against the pine weevil [32]. Several of the strains we analyzed produced large amounts of 2-phenylethanol, including strains from S. cerevisiae but most prominently the W. anomalus strains. W. anomalus strains are often isolated from tree habitats, insects and insect frass [33]. Elucidating this fascinating interplay of yeasts, insects and plants will provide substantial insight into yeast biology and ecology in the future.
The results obtained in this study indicate that yeast biodiversity harbors a large variety of strains that could enter diverse beverage production pipelines and provide additional all-natural flavor variants to improve the taste and sensory perception of lager beers and, beyond that, other fermented beverages. We used a non-industrial platform to assay strains. In future research, more detailed fermentations using specific industrial fermentation broths, e.g., wort, grape must, and other juices, should be explored with non-conventional yeasts.
Supplementary Materials
The following are available online at http://www.mdpi.com/2311-5637/4/1/15/s1.
Acknowledgments
This research was supported in part by the European Union Marie Curie Initial Training Networks Cornucopia 264717 (http://www.yeast-cornucopia.se/) and Aromagenesis 764364. Yeast strains were obtained from the Westerdijk Fungal Biodiversity Institute, Utrecht, The Netherlands.
Author Contributions
Davide Ravasio, Andrea Walther, Marizeth Groenewald, Teun Boekhout and Jürgen Wendland conceived and designed the experiments; Davide Ravasio, Silvia Carlin, Urska Vrhovsek and Andrea Walther performed the experiments; Davide Ravasio, Silvia Carlin, Urska Vrhovsek, Andrea Walther and Jürgen Wendland analyzed the data; all authors contributed to writing the paper.
Conflicts of Interest
The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- Dunn, B.; Sherlock, G. Reconstruction of the genome origins and evolution of the hybrid lager yeast Saccharomyces pastorianus. Genome Res. 2008, 18, 1610–1623. [Google Scholar] [CrossRef] [PubMed]
- Libkind, D.; Hittinger, C.T.; Valerio, E.; Goncalves, C.; Dover, J.; Johnston, M.; Goncalves, P.; Sampaio, J.P. Microbe domestication and the identification of the wild genetic stock of lager-brewing yeast. Proc. Natl. Acad. Sci. USA 2011, 108, 14539–14544. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Kanamori, T.; Itoh, T.; Kodama, Y.; Rainieri, S.; Nakamura, N.; Shimonaga, T.; Hattori, M.; Ashikari, T. Genome sequence of the lager brewing yeast, an interspecies hybrid. DNA Res. 2009, 16, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Hesselbart, A.; Wendland, J. Genome sequence of Saccharomyces carlsbergensis, the world's first pure culture lager yeast. G3 (Bethesda) 2014, 4, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Spitaels, F.; Wieme, A.D.; Janssens, M.; Aerts, M.; Daniel, H.M.; Van Landschoot, A.; De Vuyst, L.; Vandamme, P. The microbial diversity of traditional spontaneously fermented lambic beer. PLoS ONE 2014, 9, e95384. [Google Scholar] [CrossRef] [PubMed]
- Mertens, S.; Steensels, J.; Saels, V.; De Rouck, G.; Aerts, G.; Verstrepen, K.J. A large set of newly created interspecific Saccharomyces hybrids increases aromatic diversity in lager beers. Appl. Environ. Microbiol. 2015, 81, 8202–8214. [Google Scholar] [CrossRef] [PubMed]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. Flavor-active esters: Adding fruitiness to beer. J. Biosci. Bioeng. 2003, 96, 110–118. [Google Scholar] [CrossRef]
- Pires, E.J.; Teixeira, J.A.; Branyik, T.; Vicente, A.A. Yeast: The soul of beer's aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Vanderhaegen, B.; Neven, H.; Coghe, S.; Verstrepen, K.J.; Verachtert, H.; Derdelinckx, G. Evolution of chemical and sensory properties during aging of top-fermented beer. J. Agric. Food Chem. 2003, 51, 6782–6790. [Google Scholar] [CrossRef] [PubMed]
- Duong, C.T.; Strack, L.; Futschik, M.; Katou, Y.; Nakao, Y.; Fujimura, T.; Shirahige, K.; Kodama, Y.; Nevoigt, E. Identification of sc-type ilv6 as a target to reduce diacetyl formation in lager brewers' yeast. Metab. Eng. 2011, 13, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-throughput screening of a large collection of non-conventional yeasts reveals their potential for aroma formation in food fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Varela, C. The impact of non-saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef] [PubMed]
- Steensels, J.; Verstrepen, K.J. Taming wild yeast: Potential of conventional and nonconventional yeasts in industrial fermentations. Annu. Rev. Microbiol. 2014, 68, 61–80. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Gil, J.V.; Genoves, S.; Valles, S.; Manzanares, P. Rational selection of non-saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Sadoudi, M.; Tourdot-Marechal, R.; Rousseaux, S.; Steyer, D.; Gallardo-Chacon, J.J.; Ballester, J.; Vichi, S.; Guerin-Schneider, R.; Caixach, J.; Alexandre, H. Yeast-yeast interactions revealed by aromatic profile analysis of sauvignon blanc wine fermented by single or co-culture of non-saccharomyces and Saccharomyces yeasts. Food Microbiol. 2012, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii contribution in mixed brewing fermentations with different saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2017, 259, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Ravasio, D.; Wendland, J.; Walther, A. Major contribution of the Ehrlich pathway for 2-phenylethanol/rose flavor production in Ashbya gossypii. FEMS Yeast Res. 2014, 14, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H. Flor yeasts of Saccharomyces cerevisiae—Their ecology, genetics and metabolism. Int. J. Food Microbiol. 2013, 167, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Belloch, C.; Valles, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae-Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Daniel, H.M.; Moons, M.C.; Huret, S.; Vrancken, G.; De Vuyst, L. Wickerhamomyces anomalus in the sourdough microbial ecosystem. Antonie Van Leeuwenhoek 2011, 99, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Di Cagno, R.; Rizzello, C.G.; Nionelli, L.; Edema, M.O.; Gobbetti, M. Utilization of African grains for sourdough bread making. J. Food Sci. 2011, 76, M329–M335. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.T.; Lim, S.L.; Tan, H.W. Growth inhibition of Candida species by Wickerhamomyces anomalus mycocin and a lactone compound of Aureobasidium pullulans. BMC Complement. Altern. Med. 2014, 14, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Schifferdecker, A.J.; Gamero, A.; Compagno, C.; Boekhout, T.; Piskur, J.; Knecht, W. Kazachstania gamospora and Wickerhamomyces subpelliculosus: Two alternative baker's yeasts in the modern bakery. Int. J. Food Microbiol. 2017, 250, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Crafack, M.; Mikkelsen, M.B.; Saerens, S.; Knudsen, M.; Blennow, A.; Lowor, S.; Takrama, J.; Swiegers, J.H.; Petersen, G.B.; Heimdal, H.; et al. Influencing cocoa flavour using Pichia kluyveri and Kluyveromyces marxianus in a defined mixed starter culture for cocoa fermentation. Int. J. Food Microbiol. 2013, 167, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Morrissey, J.P.; Etschmann, M.M.; Schrader, J.; de Billerbeck, G.M. Cell factory applications of the yeast Kluyveromyces marxianus for the biotechnological production of natural flavour and fragrance molecules. Yeast 2015, 32, 3–16. [Google Scholar] [PubMed]
- Urit, T.; Li, M.; Bley, T.; Loser, C. Growth of Kluyveromyces marxianus and formation of ethyl acetate depending on temperature. Appl. Microbiol. Biotechnol. 2013, 97, 10359–10371. [Google Scholar] [CrossRef] [PubMed]
- Lobs, A.K.; Lin, J.L.; Cook, M.; Wheeldon, I. High throughput, colorimetric screening of microbial ester biosynthesis reveals high ethyl acetate production from Kluyveromyces marxianus on c5, c6, and c12 carbon sources. Biotechnol. J. 2016, 11, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Masneuf-Pomarede, I.; Bely, M.; Marullo, P.; Albertin, W. The genetics of non-conventional wine yeasts: Current knowledge and future challenges. Front. Microbiol. 2015, 6, 1563. [Google Scholar] [CrossRef] [PubMed]
- Azeem, M.; Terenius, O.; Rajarao, G.K.; Nagahama, K.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Chemodiversity and biodiversity of fungi associated with the pine weevil Hylobius abietis. Fungal Biol. 2015, 119, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, K.; Konstanzer, V.; Rajarao, G.K.; Terenius, O.; Seriot, L.; Nordenhem, H.; Nordlander, G.; Borg-Karlson, A.K. Antifeedants produced by bacteria associated with the gut of the pine weevil Hylobius abietis. Microb. Ecol. 2017, 74, 177–184. [Google Scholar] [CrossRef] [PubMed]
- James, S.A.; Barriga, E.J.; Barahona, P.P.; Harrington, T.C.; Lee, C.F.; Bond, C.J.; Roberts, I.N. Wickerhamomyces arborarius f.A.; sp. Nov., an ascomycetous yeast species found in arboreal habitats on three different continents. Int. J. Syst. Evol. Microbiol. 2014, 64, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).