Multiplexed Detection of Salmonella, Escherichia coli, Campylobacter, and Listeria in Raw Poultry
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Chicken Samples and Pathogen Cultures
2.1.1. Chicken Sample Preparation
2.1.2. Pathogen Cultures
2.1.3. Preparation of Chicken Rinse for Standard Curve Experiments
2.1.4. Preparation of Serial Dilutions of Bacterial Cultures
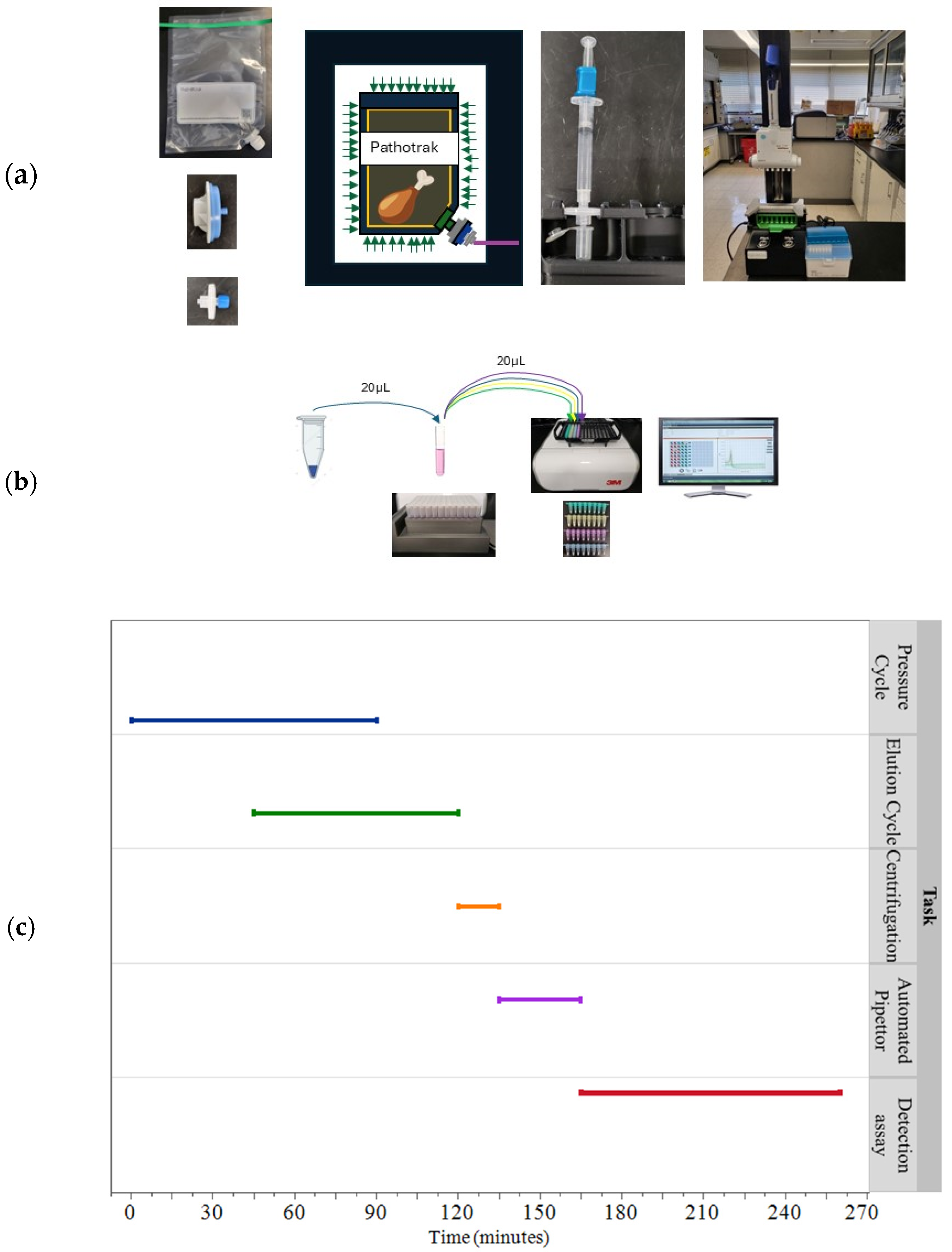
2.2. Preparation for Pathotrak–MDS Detection Experiment
2.3. Pathotrak Process
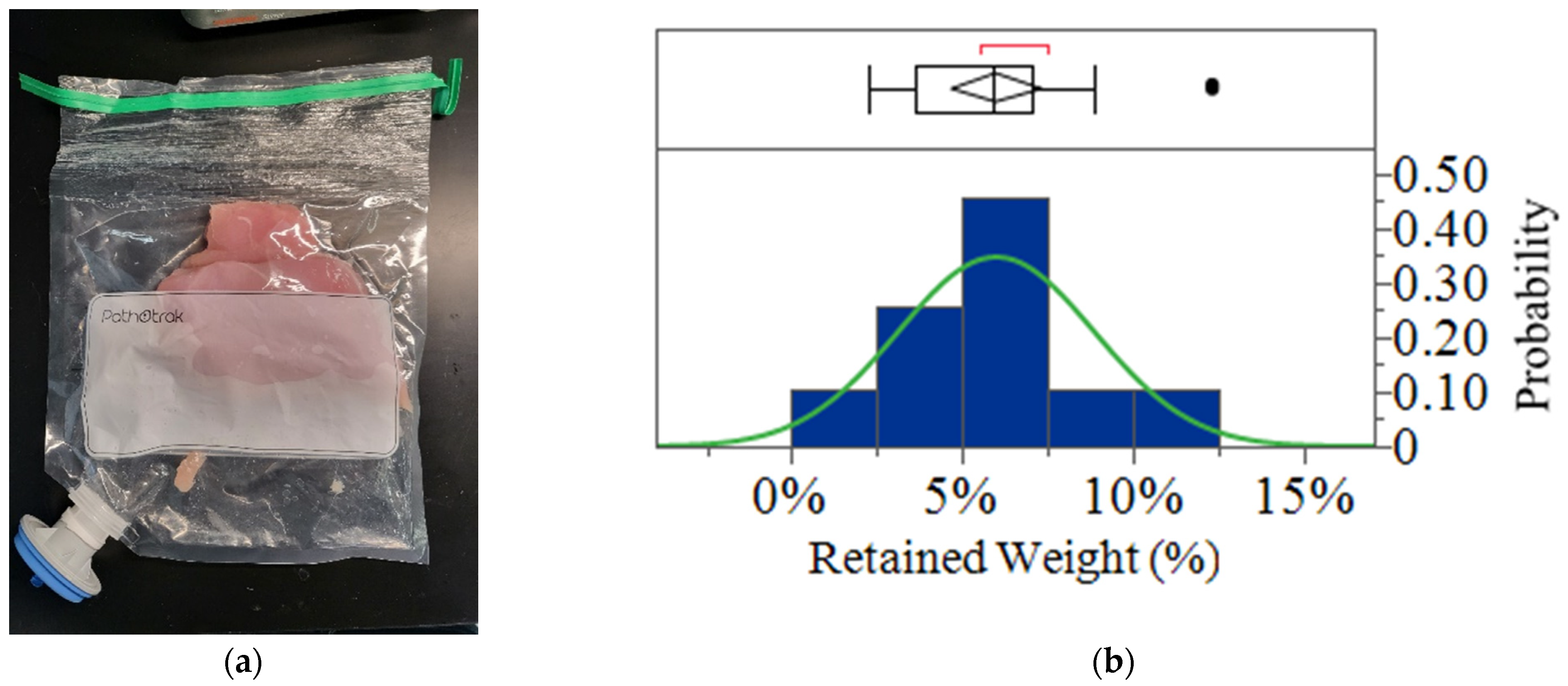
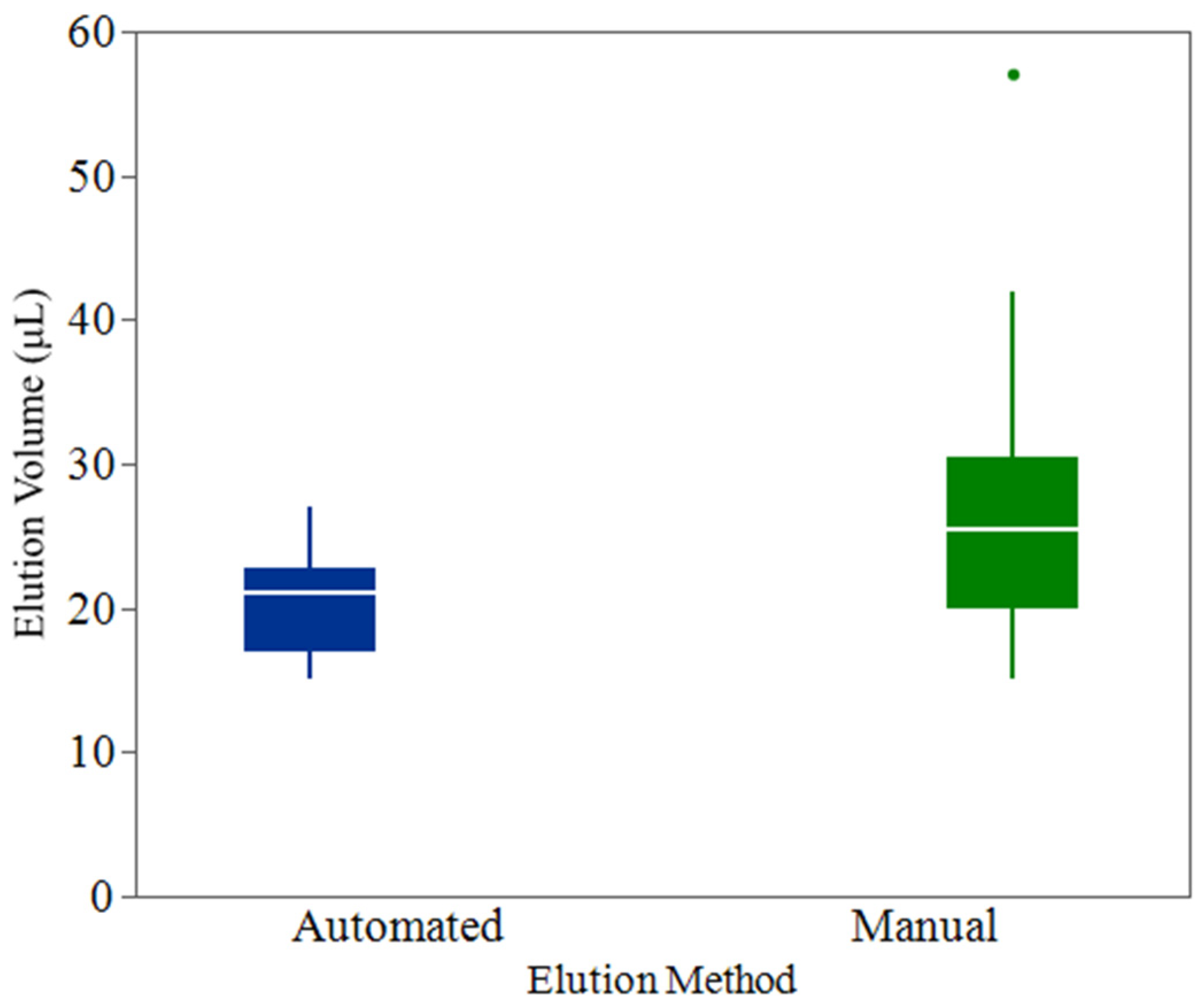
Elution Volume Measurement
2.4. Molecular Detection System (MDS) Assays
2.5. Plating to Determine Standard Curve Concentration
2.6. Data Collection and Analysis
2.7. Analysis of MDS Results
3. Results
3.1. Efficiency of Pressure Cycle
3.2. Evaluation of Automated Pipettor Consistency Versus Manual Elution
3.3. Development of a Standard Curve for Time to Detection (TTD) Using MDS Output
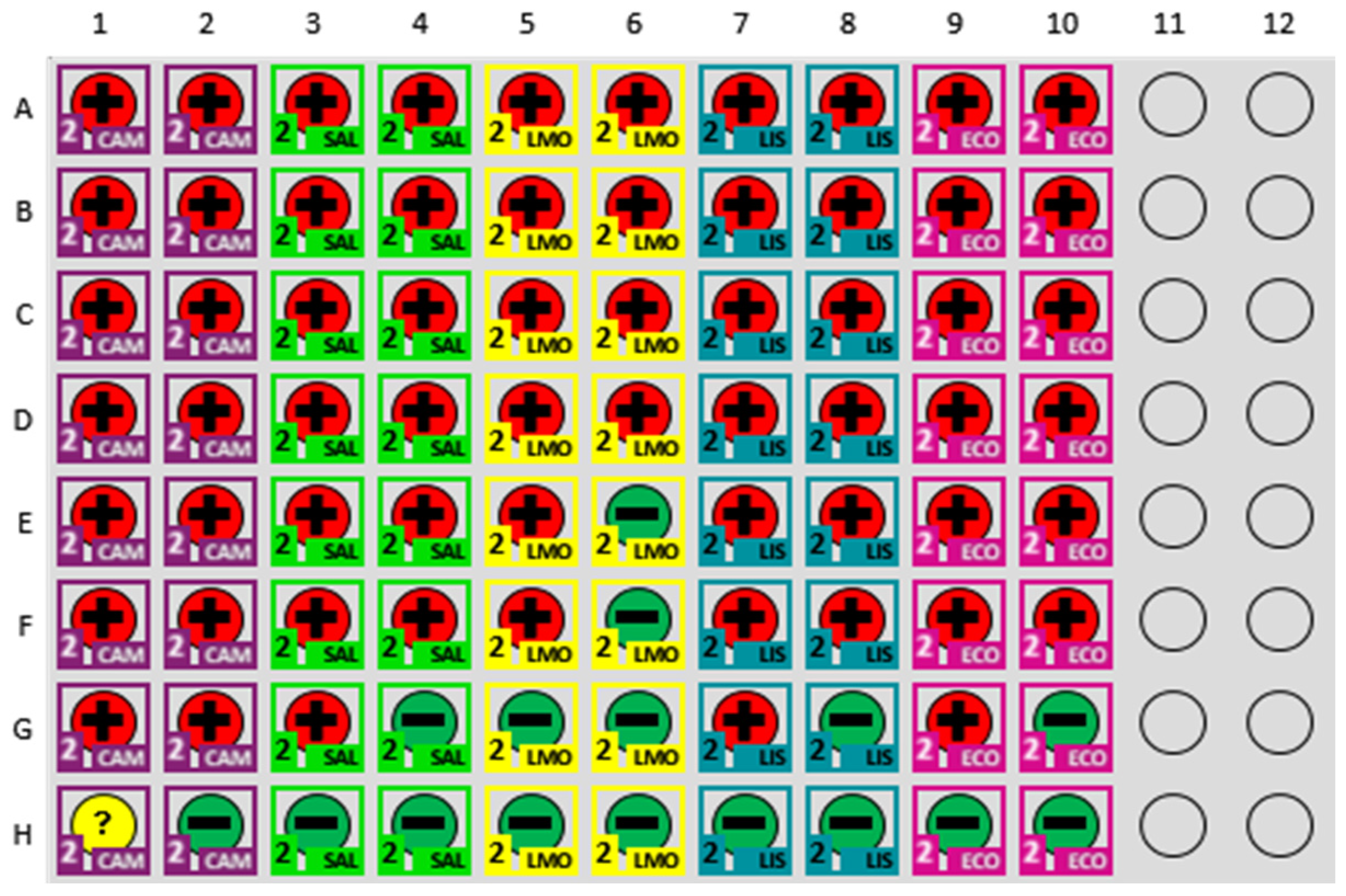
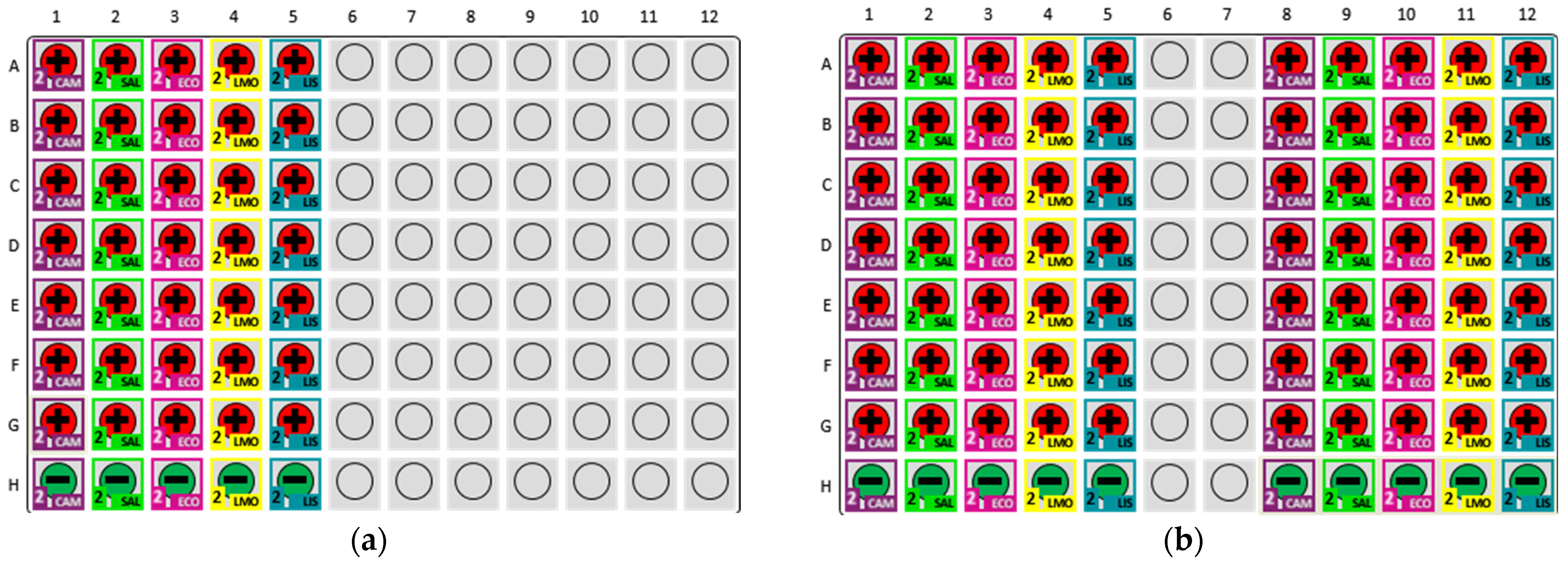
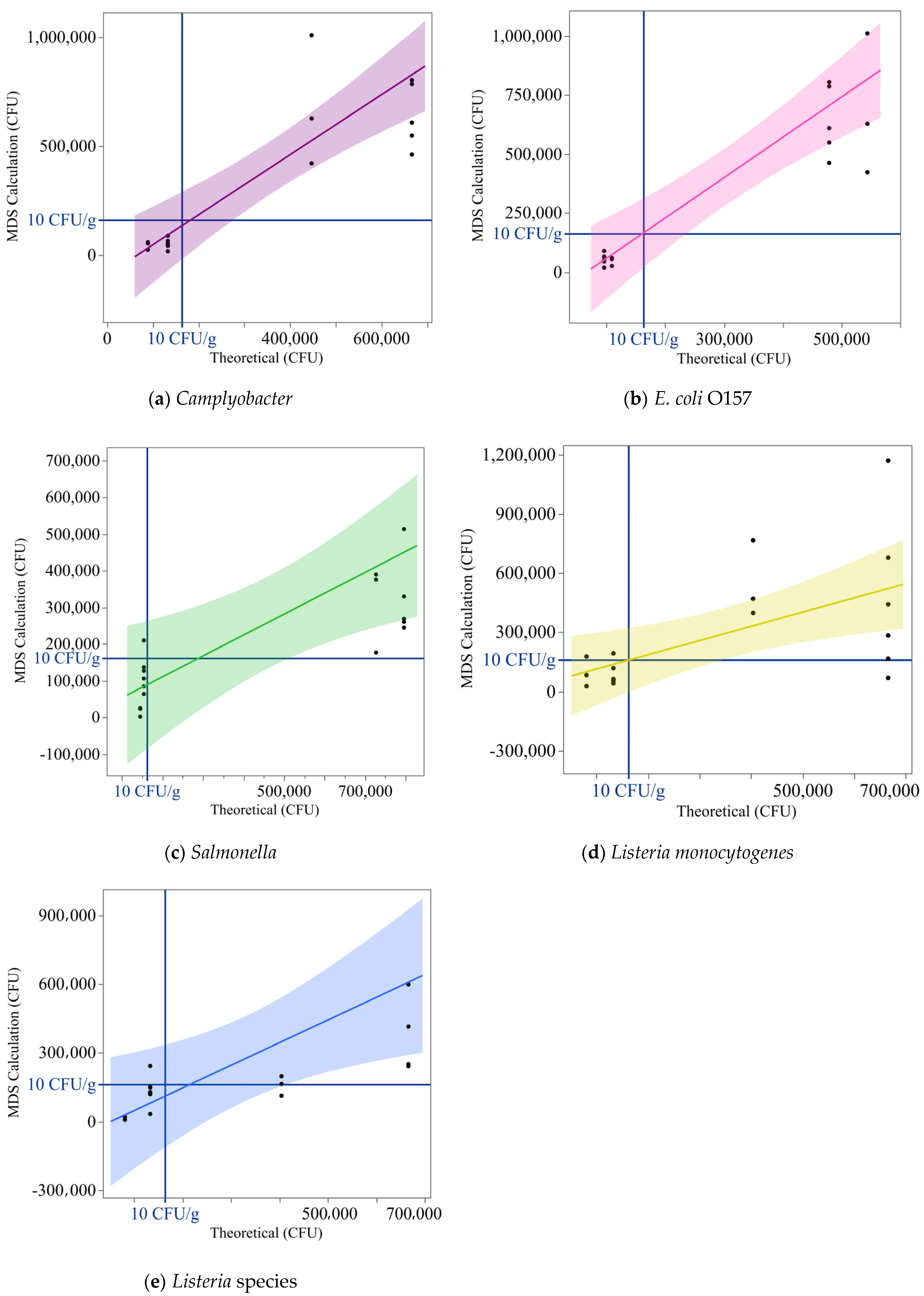
3.4. Semiquantitative Assessment of MDS Output from Artificially Inoculated Chicken Samples
4. Discussion
4.1. Standard Curve
4.2. Semiquantitative Detection of Pathogens in Inoculated Chicken Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Graph Digitizer
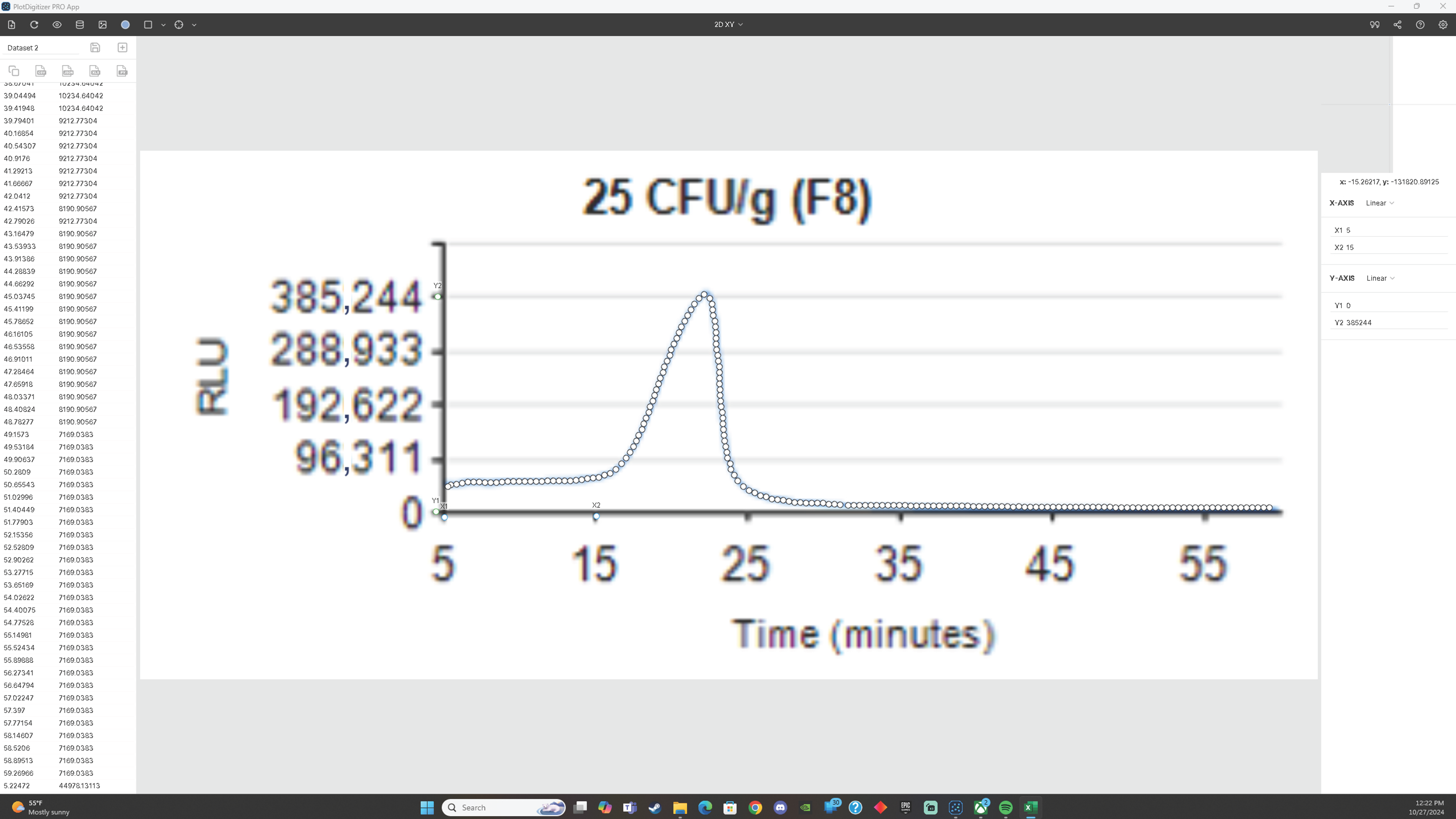
Appendix B
Appendix B.1. Standard and Detection Curves for Campylobacter
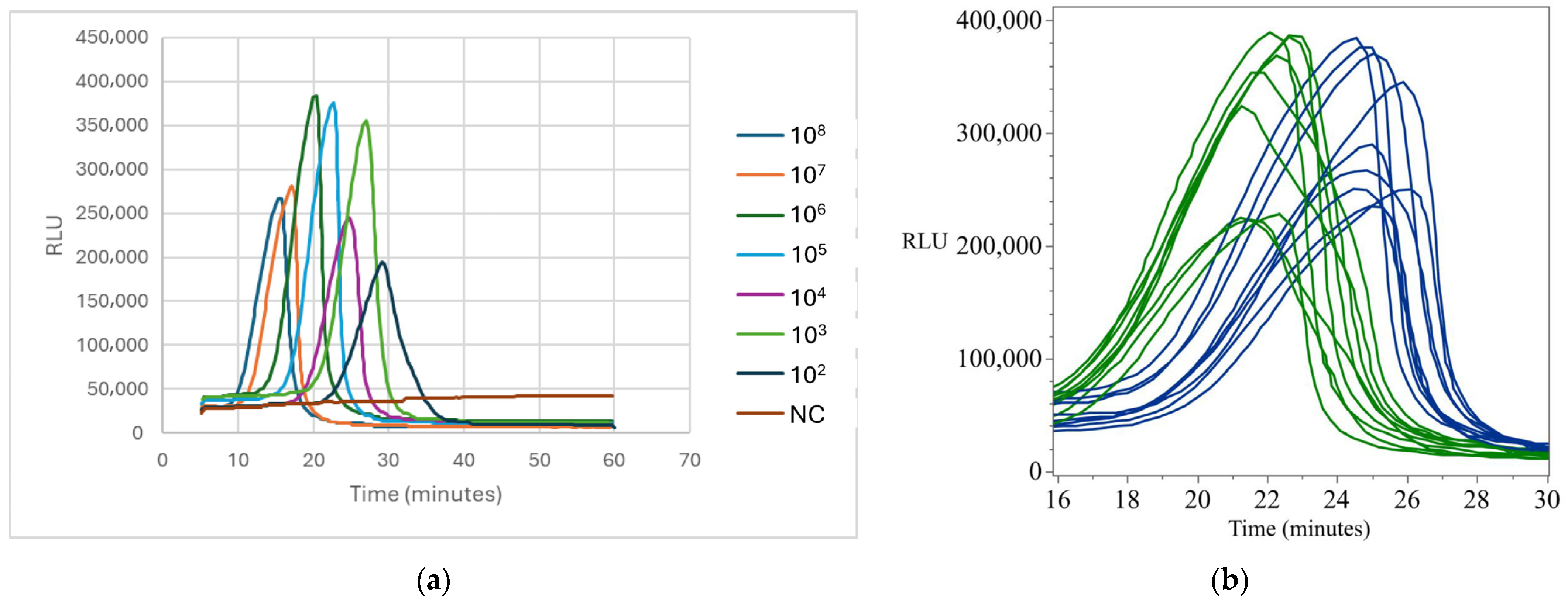
Appendix B.2. Standard and Detection Curves for Salmonella
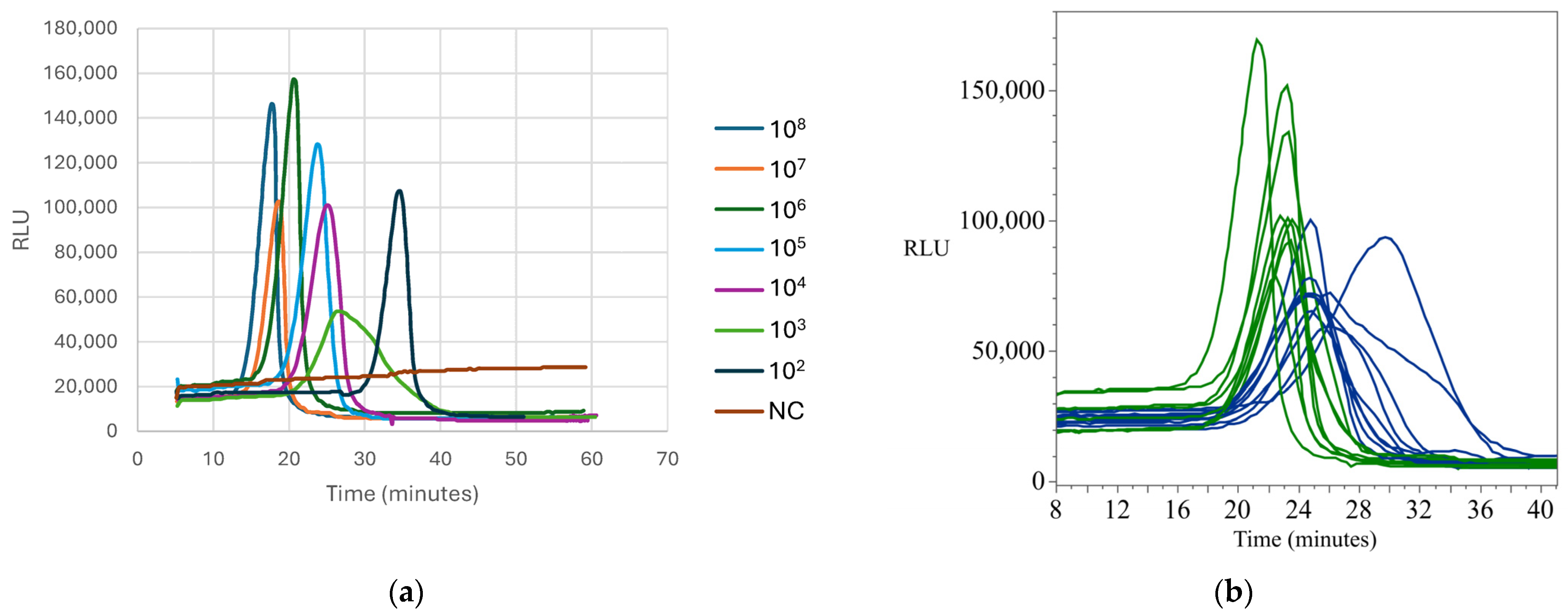
Appendix B.3. Standard and Detection Curves for E. coli
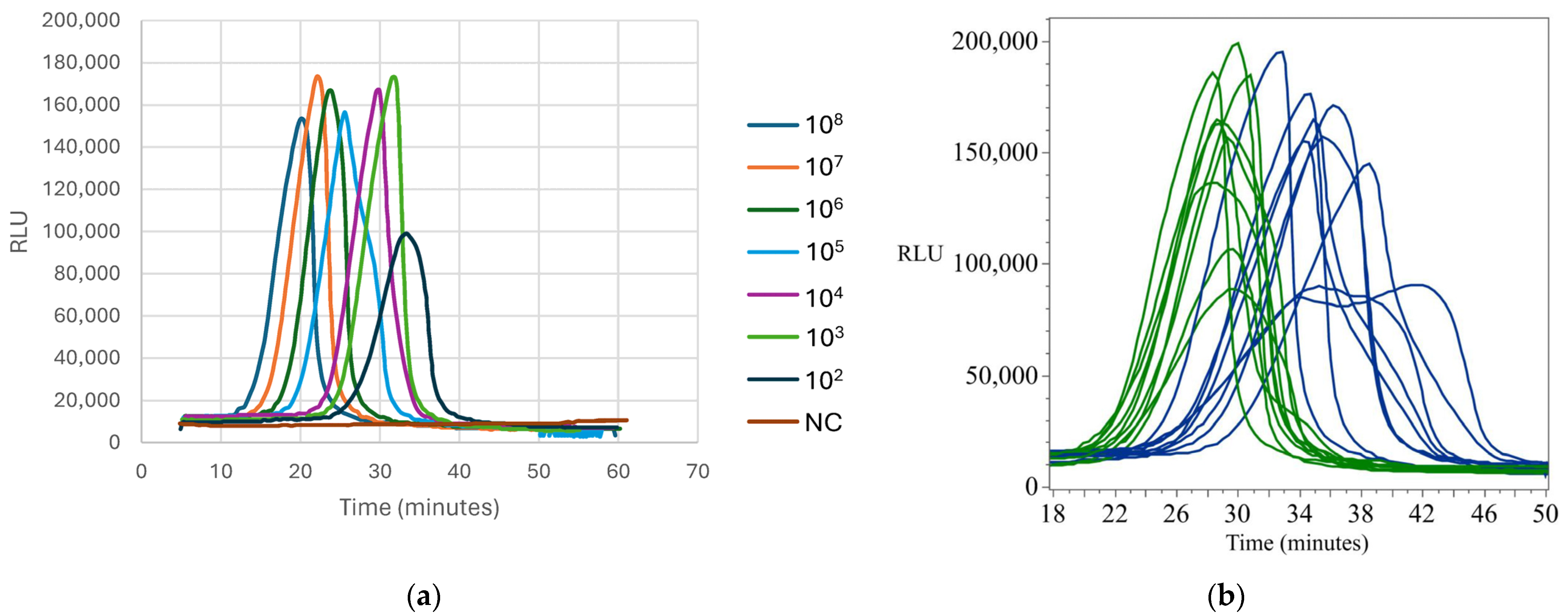
Appendix B.4. Standard and Detection Curves for Listeria Species
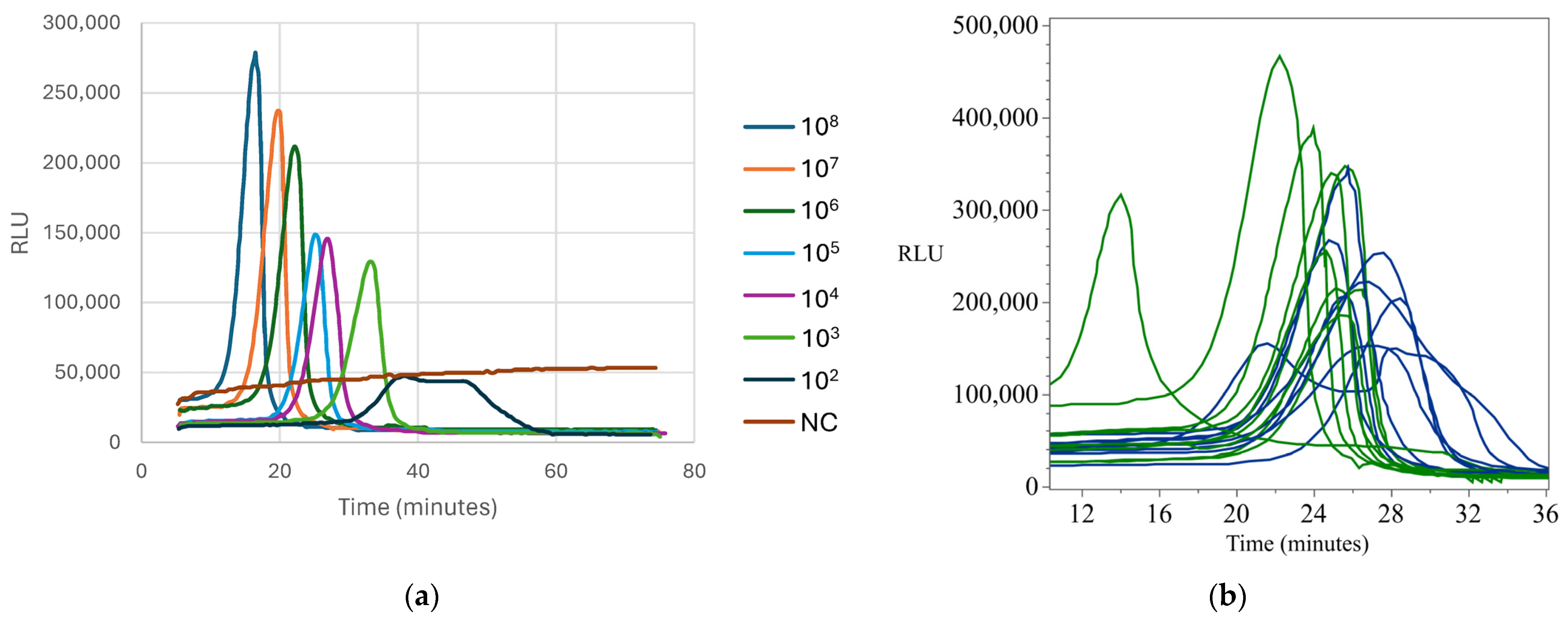
Appendix B.5. Standard and Detection Curves for Listeria monocytogenes
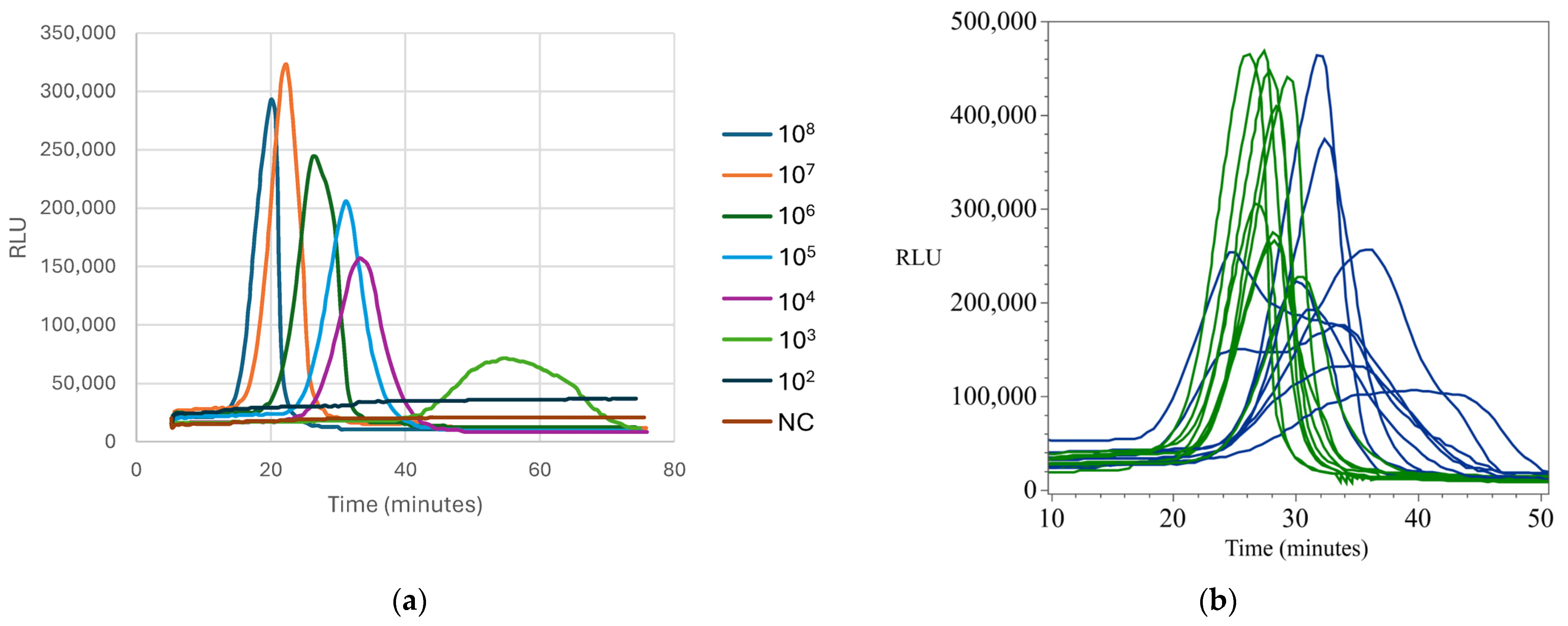
References
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States--major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef]
- Ferone, M.; Gowen, A.; Fanning, S.; Scannell, A.G.M. Microbial detection and identification methods: Bench top assays to omics approaches. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3106–3129. [Google Scholar] [CrossRef] [PubMed]
- Aladhadh, M. A review of modern methods for the detection of foodborne pathogens. Microorganisms 2023, 11, 1111. [Google Scholar] [CrossRef]
- Rohde, A.; Hammerl, J.A.; Boone, I.; Jansen, W.; Fohler, S.; Klein, G.; Dieckmann, R.; Al Dahouk, S. Overview of validated alternative methods for the detection of foodborne bacterial pathogens. Trends Food Sci. Tech. 2017, 62, 113–118. [Google Scholar] [CrossRef]
- Quintela, I.A.; Vasse, T.; Lin, C.S.; Wu, V.C.H. Advances, applications, and limitations of portable and rapid detection technologies for routinely encountered foodborne pathogens. Front. Microbiol. 2022, 13, 1054782. [Google Scholar] [CrossRef]
- United States Department of Agriculture, Food Safety and Inspection Service. MLG 4.15 Isolation and Identification of Salmonella from Meat, Poultry, Pasteurized Egg, Siluriformes (Fish) Products and Carcass and Environmental Sponges; Office of Public Health Science, Ed.; 2024. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/documents/MLG_4.15.pdf (accessed on 21 March 2025).
- United States Department of Agriculture, Food Safety and Inspection Service. MLG 8.15 Isolation and Identification of Listeria Monocytogenes from Ready-to-Eat Meat, Poultry, Siluriformes (Fish), Egg Products, and Environmental Samples; Office of Public Health Science, Ed.; 2024. Available online: https://www.fsis.usda.gov/sites/default/files/media_file/documents/MLG-8.15.pdf (accessed on 21 March 2025).
- Busse, M. Media for Salmonella. Int. J. Food Microbiol. 1995, 26, 117–131. [Google Scholar] [CrossRef] [PubMed]
- D’Aoust, J.Y. Update on Preenrichment and Selective Enrichment Conditions for Detection of Salmonella in Foods. J. Food Prot. 1981, 44, 369–374. [Google Scholar] [CrossRef]
- Sakazume, N.; Hara, K.; Fujihara, M. Comparison of enrichment media for recovery of Salmonella enterica after storage at various temperatures and alkaline stress exposure. Jpn. J. Vet. Res. 2024, 72, 36–40. [Google Scholar] [CrossRef]
- Gorski, L.; Shariat, N.W.; Richards, A.K.; Siceloff, A.T.; Aviles Noriega, A.; Harhay, D.M. Growth assessment of Salmonella enterica multi-serovar populations in poultry rinsates with commonly used enrichment and plating media. Food Microbiol. 2024, 119, 104431. [Google Scholar] [CrossRef]
- Harvey, R.W.; Price, T.H. The examination of samples infected with multiple salmonella serotypes. J. Hyg. 1967, 65, 423–434. [Google Scholar] [CrossRef]
- Singer, R.S.; Mayer, A.E.; Hanson, T.E.; Isaacson, R.E. Do microbial interactions and cultivation media decrease the accuracy of Salmonella surveillance systems and outbreak investigations? J. Food Prot. 2009, 72, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Gorski, L. Selective enrichment media bias the types of Salmonella enterica strains isolated from mixed strain cultures and complex enrichment broths. PLoS ONE 2012, 7, e34722. [Google Scholar] [CrossRef]
- Ripolles-Avila, C.; Martinez-Garcia, M.; Capellas, M.; Yuste, J.; Fung, D.Y.C.; Rodriguez-Jerez, J.J. From hazard analysis to risk control using rapid methods in microbiology: A practical approach for the food industry. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1877–1907. [Google Scholar] [CrossRef]
- Kim, H.E.; Lee, J.J.; Lee, M.J.; Kim, B.S. Analysis of microbiome in raw chicken meat from butcher shops and packaged products in South Korea to detect the potential risk of foodborne illness. Food Res. Int. 2019, 122, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Marmion, M.; Ferone, M.T.; Whyte, P.; Scannell, A.G.M. The changing microbiome of poultry meat; from farm to fridge. Food Microbiol. 2021, 99, 103823. [Google Scholar] [CrossRef]
- Hedman, J.; Lövenklev, M.; Wolffs, P.; Löfström, C.; Knutsson, R.; Rådström, P. Pre-PCR processing strategies. In PCR Technology: Current Innovations; Taylor & Francis: Abingdon, UK, 2013; pp. 3–18. [Google Scholar]
- Schrader, C.; Schielke, A.; Ellerbroek, L.; Johne, R. PCR inhibitors—Occurrence, properties and removal. J. Appl. Microbiol. 2012, 113, 1014–1026. [Google Scholar] [CrossRef]
- Sidstedt, M.; Rådström, P.; Hedman, J. PCR inhibition in qPCR, dPCR and MPS-mechanisms and solutions. Anal. Bioanal. Chem. 2020, 412, 2009–2023. [Google Scholar] [CrossRef]
- Nwe, M.K.; Jangpromma, N.; Taemaitree, L. Evaluation of molecular inhibitors of loop-mediated isothermal amplification (LAMP). Sci. Rep. 2024, 14, 5916. [Google Scholar] [CrossRef]
- Shi, X.; Liu, G.; Shi, L.; Chen, M.; Wu, X.; Zhao, J.; Lu, Y. The detection efficiency of digital PCR for the virulence genes of waterborne pathogenic bacteria. Water Supply 2021, 21, 2285–2297. [Google Scholar] [CrossRef]
- Dickson, J.S.; Koohmaraie, M. Cell surface charge characteristics and their relationship to bacterial attachment to meat surfaces. Appl. Environ. Microbiol. 1989, 55, 832–836. [Google Scholar] [CrossRef]
- Ukuku, D.O.; Fett, W.F. Relationship of cell surface charge and hydrophobicity to strength of attachment of bacteria to cantaloupe rind. J. Food Prot. 2002, 65, 1093–1099. [Google Scholar] [CrossRef]
- Selgas, D.; Marin, M.; Pin, C.; Casas, C. Attachment of bacteria to meat surfaces: A review. Meat Sci. 1993, 34, 265–273. [Google Scholar] [CrossRef]
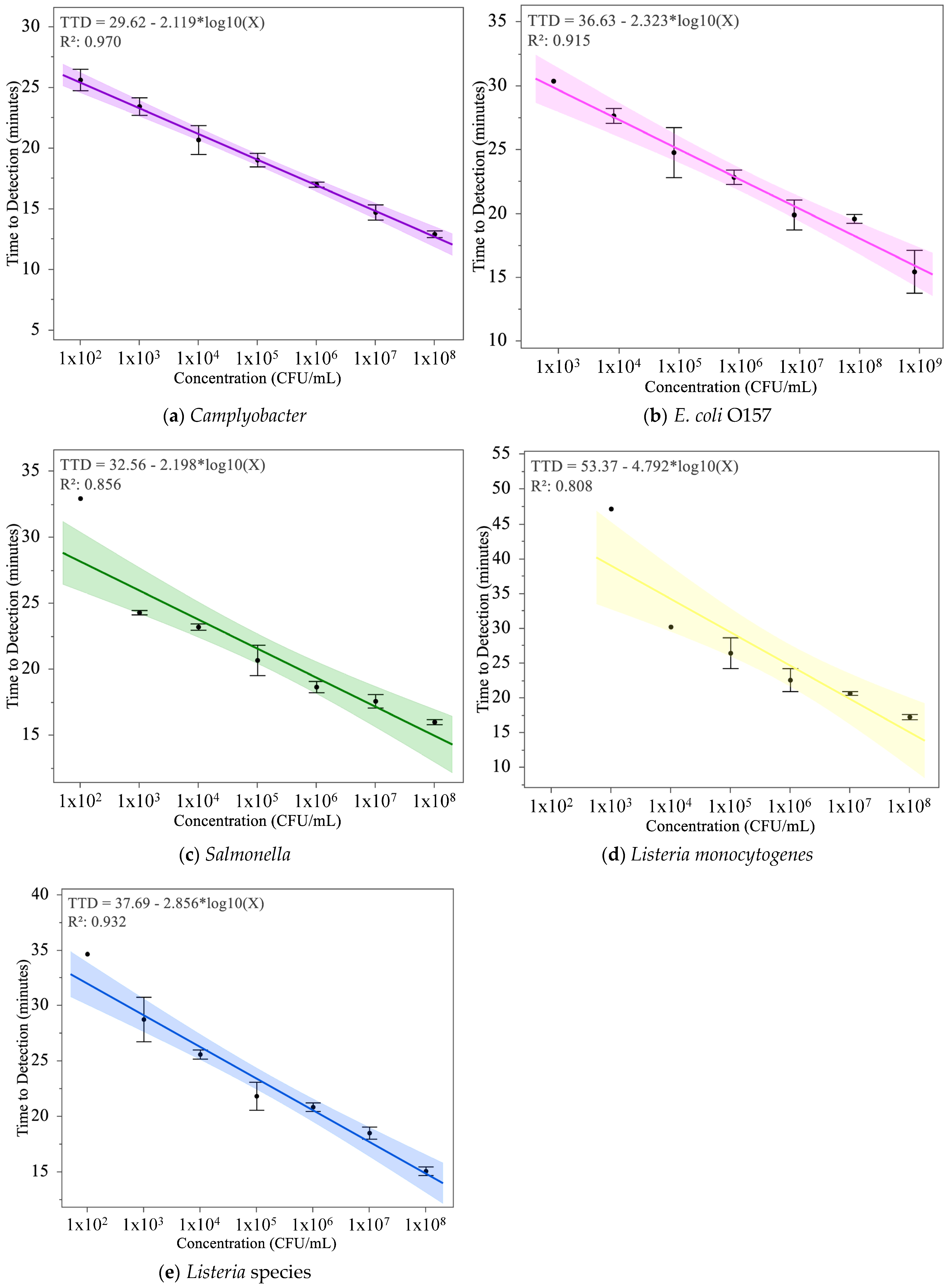
| Pathogen | R-Squared | RMSE | Slope | Intercept | False Negative Concentration (CFU/mL) |
|---|---|---|---|---|---|
| Campylobacter | 0.970 | 0.808 | −2.119 | 29.62 | None |
| Salmonella | 0.856 | 1.853 | −2.198 | 32.56 | 102 |
| E. coli | 0.915 | 1.452 | −2.323 | 34.51 | 102 |
| Listeria species | 0.932 | 1.583 | −2.856 | 37.69 | 102 |
| Listeria monocytogenes | 0.808 | 4.118 | −4.792 | 53.37 | 104, 103, 102 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-Y.; Armstrong, C.M.; He, Y.; Counihan, K.; Atencia, J.; Lee, J.; Dykes, G.; Johnson, K.; Koppenhofer, H.; Tilman, S.; et al. Multiplexed Detection of Salmonella, Escherichia coli, Campylobacter, and Listeria in Raw Poultry. Foods 2025, 14, 1137. https://doi.org/10.3390/foods14071137
Chen C-Y, Armstrong CM, He Y, Counihan K, Atencia J, Lee J, Dykes G, Johnson K, Koppenhofer H, Tilman S, et al. Multiplexed Detection of Salmonella, Escherichia coli, Campylobacter, and Listeria in Raw Poultry. Foods. 2025; 14(7):1137. https://doi.org/10.3390/foods14071137
Chicago/Turabian StyleChen, Chin-Yi, Cheryl M. Armstrong, Yiping He, Katrina Counihan, Javier Atencia, Joe Lee, Gretchen Dykes, Kathleen Johnson, Heather Koppenhofer, Shannon Tilman, and et al. 2025. "Multiplexed Detection of Salmonella, Escherichia coli, Campylobacter, and Listeria in Raw Poultry" Foods 14, no. 7: 1137. https://doi.org/10.3390/foods14071137
APA StyleChen, C.-Y., Armstrong, C. M., He, Y., Counihan, K., Atencia, J., Lee, J., Dykes, G., Johnson, K., Koppenhofer, H., Tilman, S., Martinos, S., & Capobianco, J. (2025). Multiplexed Detection of Salmonella, Escherichia coli, Campylobacter, and Listeria in Raw Poultry. Foods, 14(7), 1137. https://doi.org/10.3390/foods14071137






