Abstract
Fermented sausages are popular worldwide due to their sensory and nutritional characteristics, as well as their convenience for storage and consumption. The production and consumption of meat products are associated with negative impacts from the risks of high sodium intake, such as cardiovascular disease and hypertension. Salt (NaCl) plays an important role in the preservation, water loss during drying, reduction in water activity, and sensory characteristics of meat and other fermented food products. NaCl reduction is considered a challenge because it affects the sensory properties of meat and can compromise the safety and microbiological parameters related to the spoilage of the fermented meat product. The use of microorganisms, such as LAB, has been studied as an innovative way to substitute traditional preservatives. They produce various metabolites, including bioactive and antimicrobial substances that are actively involved in health benefits and guarantee the safety of meat products. These natural substances produced by bacteria extend shelf life by inhibiting spoilage and pathogenic microorganisms. This review discusses the potential application of lactic acid bacteria in the reformulation of fermented sausages, challenges, and beneficial effects on sensorial, safety, and health properties.
1. Introduction
The food industry is an iconic example of the relationship between the development of fundamental science and the needs of society. In the post-war periods, the objective of the food industry was to provide sufficient food to the population, and this was related to the development of different preservation techniques. However, with time, due to the increasing consumer demand for healthier products with fewer additives (clean-label products), researchers and development team members of the food (including meat production and processing) industries have been challenged to explore some alternatives to innovate these products through product reformulations. One of the principal tasks was to reduce the use of NaCl as one of the principal preservatives for numerous food products after being used for millennia. The reduction in NaCl levels can be considered one of the principal challenges in reforming meat products. This can influence the water activity (aw) and be directly and indirectly related not only to potential spoilage and pathogen growth but also to the loss of technological and sensory qualities of the final product [1].
Salt is a crucial ingredient in sausage fermentation, significantly influencing sensory characteristics such as color, flavor, and texture. It influences moisture, which affects the juiciness and tenderness of the final product. In addition, different types of salts (including NaCl) act as a preservative, contributing to the extension of the shelf life of food products [2]. In contrast, moderate NaCl consumption (2 g daily) is essential for the normal functioning of the human body and contributes to maintaining good health [3,4].
The reduction of aw through the addition of salts and solutes helps reduce or inhibit the activity of microorganisms and enzymes, thereby improving product preservation. Meat products with reduced NaCl content exhibit a shorter shelf life because NaCl plays a critical role in preservation by inhibiting spoilage and pathogenic microorganisms through its bacteriostatic effect, primarily by lowering aw [5]. In addition, it can be combined with other preservation methods, such as temperature control, acidification, and fermentation. Various antimicrobial compounds produced by LAB during fermentation have been studied with promising results for potential future applications in meat products [6].
The use of strains capable of producing beneficial metabolites, exhibiting compatible growth characteristics, and having distinct advantages in metabolic performance can significantly enhance the safety and quality of low-sodium fermented foods. The beneficial effects of different strains in low-sodium fermented foods extend beyond simple additive interactions. The complex interactions between strains and their environment can play a crucial role in determining the final product’s overall quality [7].
Considering the increasing concern about excessive NaCl intake in the diet, the food industry and researchers in the field are exploring strategies to reduce the NaCl content in traditional meat products. This can be achieved by replacing some amount of salt with lower NaCl alternatives or by employing processing technologies that diminish the reliance on salt for preservation [8]. However, any NaCl reduction must be evaluated in advance to ensure that the product meets food safety requirements and maintains sensory characteristics [9].
Biopreservation by LAB fermentation has been increasing, especially in animal-origin products, with a significant presence in dairy products (yogurt, cheese, fermented milk) and in fermented sausages in recent years [10,11]. The biopreservation method is based on the utilization of natural substances derived from bacteria. Fermentation is one of the most common methods, in which beneficial microorganisms are cultivated on food under controlled conditions to inhibit the growth of spoilage-causing microorganisms [12,13].
LAB are beneficial microorganisms that contribute to improving the quality and safety of meat products [14]. They facilitate rapid acidification, resulting in lower pH values that define the fermentation endpoint. This enhances microbial stability by inhibiting the activity of spoilage organisms and pathogenic bacteria. Moreover, the acidic environment accelerates nitrite reduction, promoting faster color development and stabilization. LAB produces a range of compounds that contribute to flavor development and exhibit antimicrobial activity. These processes trigger physical, chemical, and biochemical changes that shape the products’ distinctive sensory attributes throughout fermentation and ripening [15,16].
Although several factors (such as low pH and aw, salt and nitrites, chemical preservatives, and starter cultures) collaborate to ensure that the meat product does not pose a risk to safe consumption, certain pathogenic microorganisms, such as Listeria monocytogenes, can adapt to the environment of fermented sausages, compromising the product’s safety. Therefore, additional barriers, such as the use of biopreservatives, different packaging technologies, and a reduction in aw, may be necessary [17].
In biopreservation processes, LAB contributes to inhibiting the growth of Listeria spp. (especially L. monocytogenes) and other spoilage and food-borne pathogens by producing lactic acid, synthesizing inhibitory compounds, competing for nutrients, and reducing aw [18]. The inhibitory influence of the representatives from the former Lactobacillus genus, reclassified into 23 new genera in 2020 [19,20], on the growth of L. monocytogenes and food safety is well documented. However, understanding that using biopreservative cultures is not a panacea for controlling spoilage and food-borne pathogens is crucial. Instead, it is an integral part of a broader set of microbial growth management strategies [21].
In the last few decades, the improvement of quality of life, development of all branches of the fundamental and applied sciences, revolution in communication and information sharing, and consumer demand for healthier products have become key factors in the development of new strategies in the formulation of food products, including the rediscovery of the beneficial roles of LAB in improving food safety. in fermented sausages is the reduction of sodium chloride and/or nitrite, driven by consumer demand for healthier options, without compromising sensory quality or microbial safety. Concurrently, the application of probiotics has highlighted the potential of LAB to confer health benefits to consumers. Despite these advances, there is limited understanding of how specific LAB strains can simultaneously improve safety, sensory attributes, and functional properties in reduced-sodium fermented sausages. Therefore, this review aims to critically examine the technological, antimicrobial, and potential probiotic functions of LAB in sodium-reduced fermented sausages, highlighting challenges and future research directions [22].
2. Innovative Reformulation of Fermented Meat Sausages
2.1. Sodium Chloride Reduction Strategies
Fermented sausages constitute a diverse category of meat-based foods that undergo controlled microbial fermentation to enhance preservation, safety, and sensory attributes. This category encompasses various items, including dry-cured hams, fermented sausages, and other regional or traditional products. Fermented meat sausages, a specific subset within this category, are characterized by the comminution and mixing of meat and fat, combined with salt, curing agents, spices, and starter cultures, followed by regulated fermentation, drying, and ripening processes. These processes induce complex physicochemical and microbiological changes that result in distinct texture, flavor development, and microbial stability. Distinguishing fermented meat sausages from the broader class of fermented sausages is important, as the former represents a specific product type with unique processing parameters and quality attributes. In recent years, the food industry has endeavored to enhance the appeal of these meat products to consumers, making them healthier without compromising their sensory characteristics [23]. However, this reformulation poses a challenge as it seeks to preserve technological and sensory characteristics, including texture, flavor, color, aroma, and microbiological quality. These aspects directly influence the shelf life of the product [24]. The reformulation of fermented meat sausages is summarized in Figure 1, which schematically illustrates the main technological and compositional adjustments involved in developing healthier products, including sodium and nitrite reduction strategies and their effects on product quality and safety.
Figure 1.
Reformulation of fermented sausages.
The addition of NaCl to fermented sausage formulation acts as a preservative agent by reducing the aw in meat, creating a less favorable environment for spoilage microorganism growth. It also enhances the flavor and preserves the meat texture [25]. Salt acts on the osmotic balance, promoting water exchange between meat cells and the surrounding environment. This influences water loss from the meat cells to the outside, promoting controlled dehydration during drying [26]. Fresh meats and uncured sausages have high aw, very close to 1.0, which supports microbial growth. In contrast, products such as ham, bacon, and cured sausages (salami) exhibit moderate aw (0.85–0.95), reduced by the addition of salt, nitrites, and nitrates, which helps to inhibit microbial development. Products such as salami, pepperoni, and beef jerky have low aw (typically below 0.85) due to moisture loss during drying. This significant reduction in aw directly impacts meat product quality, safety, stability, and shelf life. Most pathogenic bacteria, including Salmonella spp., Listeria spp., and Escherichia coli, require aw levels of >0.90 for growth and are inhibited at lower aw levels. Yeasts can proliferate at lower aw levels (0.85), whereas fungi and molds can thrive in even drier conditions (0.70) [27].
Reformulation by reducing NaCl meets the demand for healthier products. Approximately 20–30% of an individual’s dietary sodium intake is derived from meat products. Sodium, when consumed in large quantities exceeding 5 g per day according to the World Health Organization (WHO), can cause health risks such as cardiovascular problems and hypertension [28,29]. NaCl facilitates the extraction and solubilization of myofibrillar proteins, which are essential for water retention within the matrix of meat products, directly affecting their texture and yield. Thus, a reduction in NaCl concentration leads to a decrease in technological and sensorial properties and can compromise food safety [30].
There are various strategies for reducing the NaCl content of fermented sausages, including direct reduction. Substituting NaCl with metallic salts, such as potassium chloride, calcium chloride, and magnesium chloride, along with the incorporation of flavor enhancers, such as monosodium glutamate and nucleotides, can aid in lowering the NaCl content in meat products while preserving their sensory quality and safety. The use of potassium chloride is the most employed. However, the heightened use of metallic salts may lead to undesirable side effects, including a bitter or metallic taste and texture degradation [31].
Many studies on NaCl reduction in meat products are available in the literature. Corral [32] and Chen [33] have reported a 30–50% reduction in NaCl in fermented sausages while preserving their sensory characteristics. However, the interaction between the product matrix and the peptides, as well as processing conditions such as temperature and pH, can significantly impact the ability of the peptides to modulate the salty taste. The mechanism underlying this perception involves interactions with taste receptors, modulation of salt sensation, effects on salt release, and flavor potentiation. Peptides can influence the overall flavor profile of the product by balancing saltiness with other taste sensations, such as umami. This interplay can alter the perceived salty taste intensity [34].
2.2. Nitrate and Nitrite Reduction Strategies
The addition of sodium nitrite and sodium nitrate to fermented meat sausages is responsible for many functions, including color enhancement, providing an antimicrobial effect by inhibiting the growth of pathogenic and spoilage bacteria, imparting antioxidant properties, and contributing to the formation of cured product characteristics [35]. However, despite the numerous positive aspects of their application, the use of nitrite and nitrate has raised several consumer health concerns due to the formation of carcinogenic N-nitrous compounds, specifically nitrosamines. These compounds can be produced in both food matrices and the human body [36]. One of the reasons for attributing these risks is heat treatment, which enhances these compounds’ formation. However, this concern does not apply to fermented meat sausages, as they do not require heat treatment [37].
The use of these preservatives is highly regulated in the food industry, with well-defined limits for their incorporation in product preparation. These limitations aim to prevent the consumption of excessive amounts of nitrite and nitrate [38]. Additionally, natural food preservation methods, such as fermentation, are considered favorable because they do not negatively affect consumers’ health and may have a smaller impact on nutritional and sensory properties.
For instance, Chen [39] used Lactiplantibacillus plantarum P2 isolated from fermented sauerkraut and observed its potential to efficiently eradicate peroxide free radicals and degrade sodium nitrite. Reducing the nitrite content in the product using LAB is emphasized as an important production strategy. Moreover, selenium supplementation displayed antioxidant capacity, showing its potential application in fermentation process and new opportunities for producing functional foods.
Zhu [40] investigated the partial replacement of nitrite in Chinese fermented sausage by Lpb. plantarum. LAB enhanced the gel-forming properties and increased viscoelasticity. Additionally, Lpb. plantarum application enhanced the reduction in the risk of biogenic amines, attributed to a decrease in tyramine levels. LAB played a role in improving the color and gel properties and reducing the levels of nitrate and biogenic amines.
3. Role of Lactic Acid Bacteria in Fermented Sausage
Lactic acid bacteria are present in diverse niches, including food of both animal and plant origin, human microbiota, and the environment. LAB are described as Gram-positive microorganisms that have a rod or coccobacilli shape, are devoid of cytochromes, are non-spore-forming microorganisms, and are catalase negative, with noted exceptions (pseudo catalase activity). The oxygen requirements are very diverse, and the bacteria can be classified as anaerobic or facultative anaerobic [41]. Carbohydrate metabolism can be either homofermentative, resulting in the production of organic acids, mainly lactic acid, or heterofermentative, resulting in the production of organic acids, such as lactic acid, carbon dioxide, and other fermentation products, such as alcohol and carbonyl compounds, such as diacetyl, acetaldehyde, acetoin, and 2-butanone [42].
In fermented meat sausage, glucose or other sugars present in muscle tissues are utilized as substrates for bacterial energy production. Moreover, depending on the metabolite properties of the applied species/strains, a variety of organic acids (such as lactic, acetic, and propionic acids) can be produced, which contribute to the acidification of the environment. LAB also exerts a range of adverse effects on other microorganisms, including competition for nutrients and production of inhibitory compounds. Additionally, LAB are known to produce an extended spectrum of other substances, including organic metabolites such as polymers, sweeteners, nutraceuticals, aromatic compounds, various enzymes, and antimicrobial compounds [43,44,45]. Fermentation processes not only influence the physicochemical parameters of meat products but also serve as the foundation for the functional activities of LAB.
3.1. Biopreservative Compounds Produced by LAB
Beyond their fundamental role in acidification and fermentation, LAB also synthesize a variety of biopreservative compounds such as bacteriocins, organic acids, and hydrogen peroxide that contribute significantly to microbial control and food safety. Biopreservative compounds, including organic acids, hydrogen peroxide, antifungal compounds, bacteriocins, and bacteriocin-like inhibitory substances (BLIS), inhibit the growth of food-borne pathogenic and spoilage microorganisms, thereby enhancing product safety [40,46]. For instance, biopreservative compounds can also play a role in a distinct approach to barrier technology, where they are strategically combined with other barriers to address food spoilage without compromising starter or adjunct cultures. This approach ensures that the desired fermentation process proceeds, maintaining both the quality and safety of the food product. The rationale behind adopting this integrated approach is the potential to subject unwanted microorganisms to multiple obstacles that inhibit their growth and survival. Moreover, if there is synergy, using smaller quantities of preserving agents and/or reduced levels of technological treatment becomes feasible [47]. The application LAB that produces biopreservatives meets consumer demand for healthier products with a reduction in chemical products.
Currently, the importance of the quality and safety of fermented sausages is becoming increasingly evident, being a topic of prominent studies. Additionally, several challenges must be addressed for the effective application of bacteriocins, including their stability in different formulations, the influence of environmental factors such as pH and substrate availability, and the need for rigorous validation in vivo and in vitro testing [48,49].
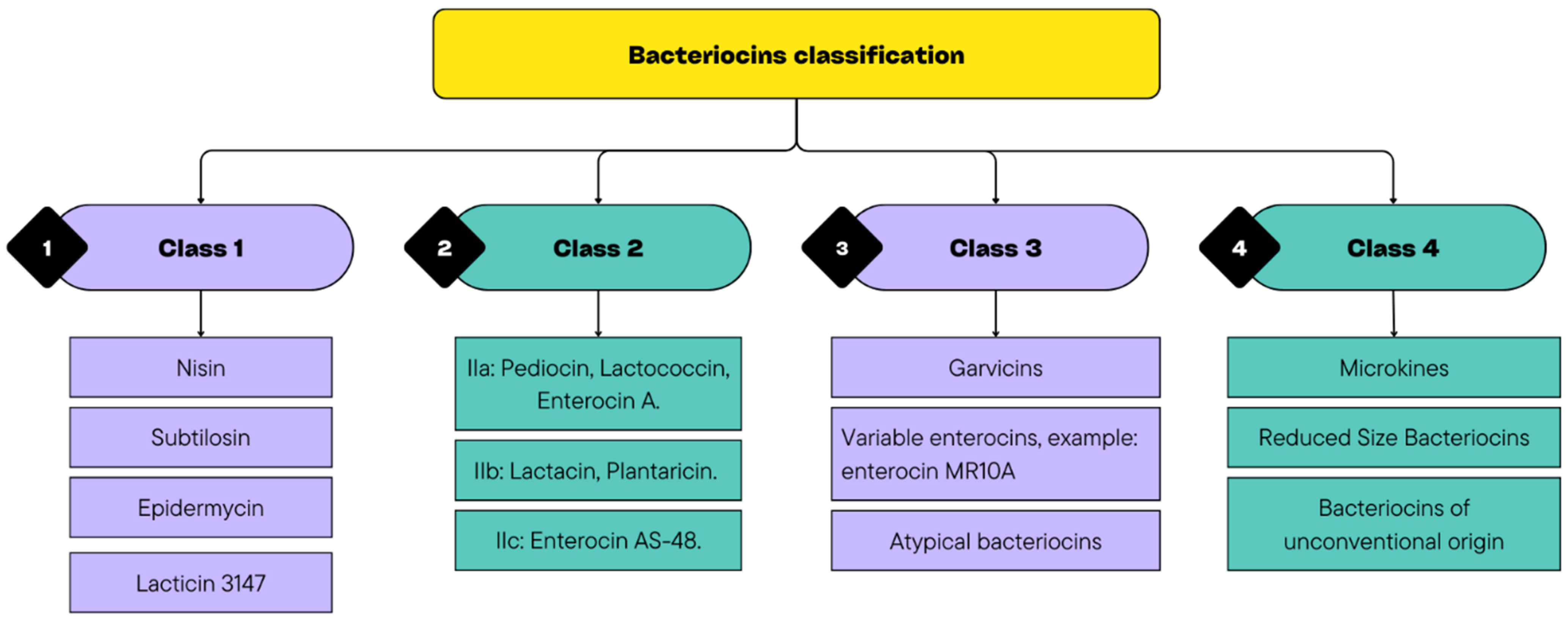
Bacteriocins can help overcome these obstacles through their broad-spectrum antimicrobial activity, resistance to proteolytic degradation under certain conditions, and synergistic interactions with other preservation strategies. They consist of antimicrobial peptides that can inhibit spoilage and pathogenic bacterial species. Moreover, in recent decades, it was suggested that bacteriocins may have activity even against some yeasts and viruses [50]. They are classified into four main classes, with Classes 1 and 2 being the most relevant in food applications (Figure 2). Class 1 includes antibiotics, whereas Class 2 consists of low-molecular-weight non-lantibiotic bacteriocins, which are further subdivided into subgroups (IIa, IIb, and IIc). In addition, Class 3 comprises unmodified low-molecular-weight peptides, such as microcins, and Class 4 encompasses bacteriocins produced by E. coli, which have distinct characteristics that do not align with those of the previous classes, such as colicin [51,52].
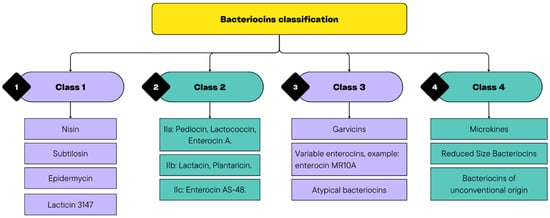
Figure 2.
Diversity of Bacteriocin Classes.
Their mechanisms include cell wall permeabilization, vital protein inhibition, pore formation, interference with DNA replication, and immune system modulation. It is noteworthy that the bacteria that produce these bacteriocins possess specific immune mechanisms that protect them from their effects. These substances are widely recognized as safe, exhibiting no cytotoxic activity on eukaryotic cells (such as nisin in most of the studied cases), being inactivated by digestive enzymes such as proteases, and having minimal impact on the intestinal microbiota [42,53]. The ideal characteristic of a biopreservative agent is to exhibit specific antimicrobial activity solely against the target pathogenic or spoilage microorganism without adversely affecting the product and the microbiota of consumers [54].
BLIS refers to a heterogeneous group of antimicrobial peptides produced by bacteria, primarily LAB, that exhibit inhibitory activity against spoilage microorganisms and foodborne pathogens [55]. Unlike well-characterized bacteriocins, BLIS have not been fully defined in terms of their amino acid composition and biochemical properties, which distinguishes them in both definition and applicability. Despite these limitations, BLIS shows potential as a natural biopreservative, displaying bactericidal or bacteriostatic effects against Gram-positive and, in some cases, Gram-negative bacteria [56]. Interactions with food matrix components such as lipids and proteins, as well as susceptibility to proteolytic degradation, may hinder their antimicrobial effectiveness [57,58]. However, combinatory strategies such as the co-application of BLIS with bacteriocins or other antimicrobial metabolites have proven effective in enhancing their activity, particularly in high-fat meat products where isolated bacteriocins often exhibit reduced efficacy. Therefore, BLIS represents a promising alternative within the concept of postbiotics, contributing to food microbiological safety through a natural and technologically viable approach [59].
As examples, pediocin from P. acidilactici, nisin from L. lactis, and carnobacteriocin BM1, carnocyclin A, and piscicolin 126 from C. maltaromaticum are some of the few commercial bacteriocins approved by the American Food and Drug Administration (FDA). These bacteriocins are classified as Generally Recognized as Safe for use as food preservatives or additives. Moreover, their antimicrobial activity and specificity against pathogens can be enhanced through bioengineering. Because of their relatively simple biosynthetic pathways, their genetic determinants can be manipulated with minimal challenges [60]. These combined mechanisms effectively hinder the growth of undesirable microorganisms in fermented foods, thereby contributing to their preservation and safety [61]. Various strains of LAB, including L. sakei, Lpb. plantarum, L. animalis, and L. curvatus, can function as effective bioprotective agents in meat and meat products [62].
Bacteriocins are classified into three major classes based on their structural, biochemical, and genetic characteristics: class I (lantibiotics), class II (unmodified heat-stable peptides), and class III (large heat-labile peptides). Class I bacteriocins, such as nisin, are peptides smaller than 5 kDa that contain unusual amino acids, such as lanthionine and methyl-lanthionine, which confer high thermal stability and resistance to proteolytic enzymes. Class II bacteriocins, exemplified by pediocin PA-1 (class IIa), are small (<10 kDa), cationic, hydrophobic, and heat-stable peptides that form pores in the target cell membrane and exhibit strong antilisterial activity. In contrast, class III bacteriocins, such as helveticin J, are larger molecules (>30 kDa) and are generally thermolabile, exerting their antibacterial effects primarily through enzymatic degradation of the bacterial cell wall. In meat products, class I and II bacteriocins have demonstrated greater efficacy against Gram-positive pathogens, such as Lactobacillus monocytogenes and Clostridium botulinum, particularly when used in combination with other hurdle technologies. Nisin is widely used in processed meat products because of its broad-spectrum antimicrobial activity and stability under adverse conditions. Meanwhile, pediocin PA-1 is especially effective in fermented sausages because of its high specificity against Listeria species [63,64].
Lactocin MM4, a novel bacteriocin produced by Companilactobacillus alimentarius FM-MM4, showed a molecular mass and N-terminal sequence of 1104.58 Da (QGVGPLGQGHR) and low homology with known class II bacteriocins. This bacteriocin exhibited broad-spectrum antimicrobial activity against Gram-positive and -negative pathogens, as well as several yeasts. It demonstrated high thermal stability, retaining 84.7% of its antimicrobial activity after exposure to 121 °C for 15 min, and remained effective at acidic pH (2–5). Hu [65] reported that proteolytic enzymes completely inactivated lactocin MM4, whereas lipase and amylase had no effect, reinforcing its potential application in food preservation.
Due to the non-sterility of the raw materials and ingredients used in the production of fermented sausages, there exists the potential for contamination by food-borne pathogens. This allows these microorganisms to proliferate during the fermentation process. An alarming example of a foodborne pathogen is L. monocytogenes, which poses a potentially fatal threat to public health [63]. While acidification plays a central role in stabilizing fermented sausages, the bioprotective capacity of LAB extends far beyond pH reduction, involving the production of metabolites with antimicrobial activity against spoilage and pathogenic microorganisms.
3.2. LAB for the Biopreservation of Fermented Sausages
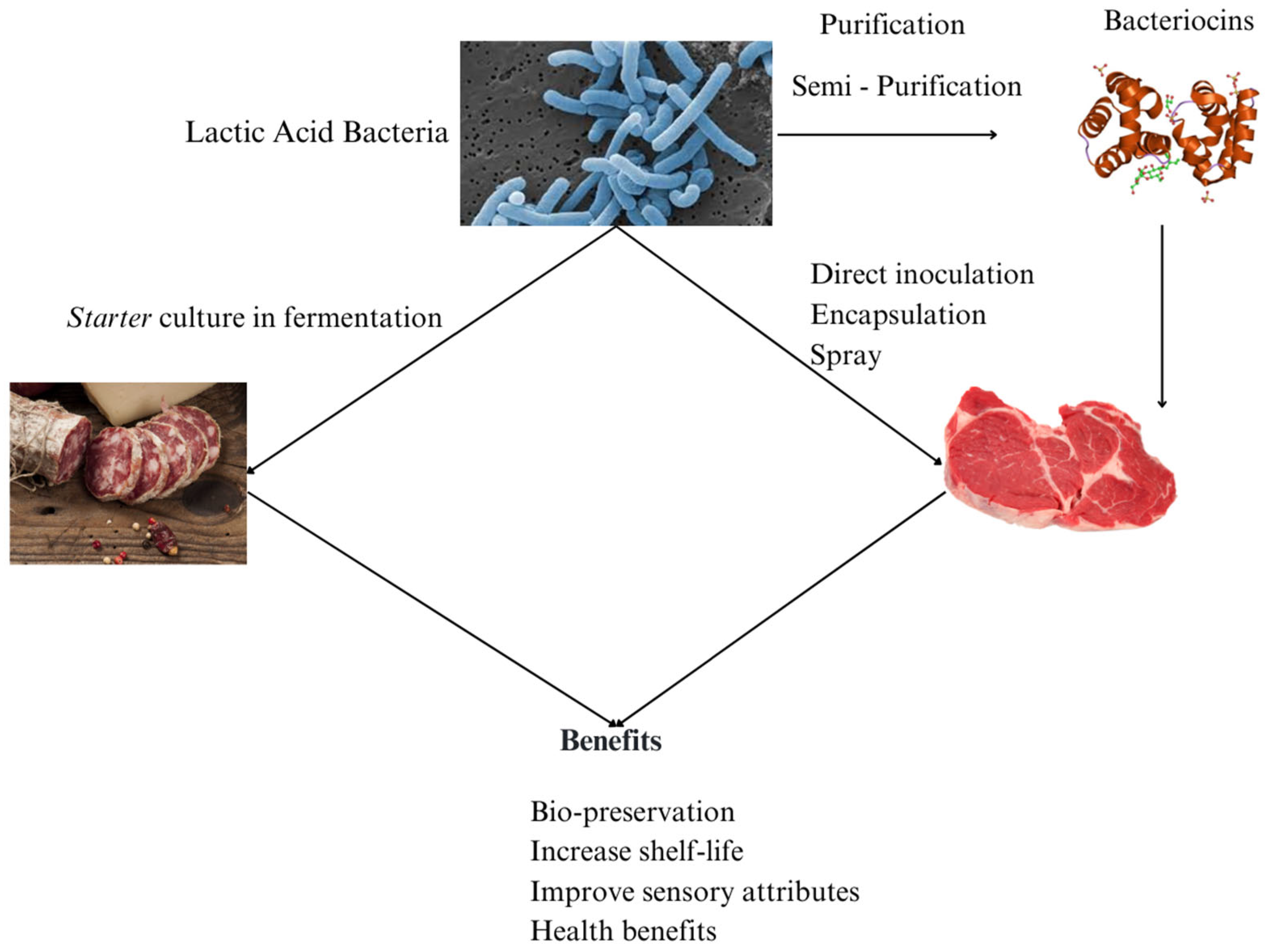
In addition to lowering pH and promoting fermentation stability, LAB produce a wide range of antimicrobial substances that enhance food safety and extend shelf life. Certain compounds are particularly noteworthy to the food industry because of their capacity to minimize lipid oxidation through a combination of effects, including acidification of the environment, oxygen consumption, production of antioxidant substances, and modulation of enzymes. These mechanisms collectively contribute to product quality preservation and shelf life extension [64]. Figure 3 provides a schematic overview of the different approaches for applying lactic acid bacteria in meat products, highlighting their multifunctional roles as starter, protective, and probiotic cultures contributing to safety, shelf life, and sensory quality.
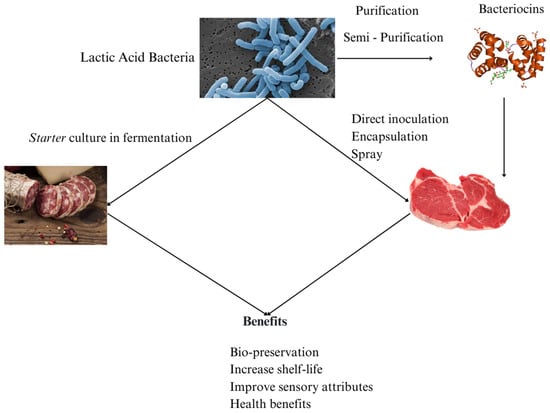
Figure 3.
Lactic acid bacteria application in meat products.
The application of different LAB species in fermented meat sausages—Lpb. plantarum, L. casei, L. paracasei, Ltb. sakei, and L. rhamnosus—has been evaluated as a protective culture. These LAB strains produce a wide range of antimicrobial compounds, including organic acids (e.g., lactic acid and acetic acid), bacteriocins, hydrogen peroxide, and enzymes, which contribute to their bioprotective effects. This biopreservation strategy harnesses the natural metabolic activities of LAB to suppress the growth of spoilage microorganisms and pathogenic bacteria, such as Listeria monocytogenes, Escherichia coli, and Salmonella, thereby extending the shelf life and safety of meat products [65,66].
Several studies [67,68,69] have demonstrated the capability of L. monocytogenes to grow and proliferate in “stress” conditions, such as low temperatures, high salinity levels, and low pH, and the ability to form biofilms, among other challenging situations that typically inhibit microbiological growth. Consequently, L. monocytogenes may be present in food processing environments, posing a potential contamination risk. Numerous studies have associated LAB in meat products with enhanced fermentation, product characteristics, and preservation (Table 1).
The positive results indicate that the integration of microorganisms into fermented meat sausages enhances their ability to extend the shelf life of foods. The applications of microorganisms influence technological characteristics such as pH, water activity, and inhibition of pathogenic and spoilage microorganisms [70]. Several studies have focused on the application of LAB to fermented sausages. The variability in the technological and bioprotective performance of LAB strains can be attributed to their strain specific metabolic profiles, including differences in acidification kinetics, proteolytic and lipolytic systems, and bacteriocin production capacity are demonstrated in Table 1, while bacteriocin production capacity is presented in Table 2.

Table 1.
Application of lactic acid bacteria in reformulated fermented sausages and their challenges.
Table 1.
Application of lactic acid bacteria in reformulated fermented sausages and their challenges.
| Lactic Acid Bacteria | Objective | Results | Reference |
|---|---|---|---|
| Nitrite reduction/free | |||
| Lactobacillus lactis MP11, P. acidilactici MP14, L. salivarius MP02c, and P. acidilactici B-LC-20 | Anti-listerial activity of selected bacteriocin-producing LAB in vitro and in a fermented sausage model developed with or without small sodium nitrite concentrations | P. acidilactici MP14 reduced Listeria counts in a nitrite-reduced environment. The reduction observed was similar to that caused by the commercial strain P. acidilactici B-LC-20, both in vitro and in the meat model. | [71] |
| Weissella cibaria X31 and Weissella confusa L2 | The effects of the Weissella species as a starter on the physicochemical and proteolytic properties of low-nitrite dry-fermented sausage were evaluated. | W. cibaria X31 and W. confusa L2 resulted in high redness values in the final product. Residual nitrite levels were reduced by 95–97%. Both strains suppressed the growth of S. enterica. | [72] |
| Lpb. plantarum | The positive effect of Lpb. plantarum on the reduction of nitrate and biogenic amine content, color, and gel property of fermented sausages | In Chinese fermented sausages, the combination of low levels of sodium nitrite and Lpb. plantarum resulted in reduced residual nitrite and biogenic amine levels, and increased color and gel properties. | [40] |
| Mammaliicoccus sciuri IMDO-S72 and Ltb. sakei CTC 494 | Monitoring the growth of C. botulinum-like strains in group I during the production of fermented sausages without added nitrate and nitrite salts | The addition of M. sciuri IMDO-S72 as an anticlostridial starter culture did not result in any additional antibacterial effect. | [73] |
| Lactobacillus MPKL03 and MPKL04 | The ability of nitrite-reducing performance under different production and processing conditions of two LABs isolated from Sichuan traditional sausage | MPKL03 and MPKL04 reduced residual nitrite levels and influenced microbial counts in traditional Sichuan sausages. | [74] |
| Low sodium | |||
| Ltb. curvatus, Ltb. sakei, Weissella hellenica, and Lpb. plantarum. | The potential taste-compensating role of these LAB strains in reduced-salt dry sausage was evaluated. | Free amino acids (FAAs) and organic acids were detected in reduced-salt dry sausage, influencing measurable taste-related parameters. | [75] |
| Ltb. curvatus SYS29, Ltb. sakei HRB10, W. hellenica HRB6, and Lpb. plantarum MDJ2 | investigate the compensative role of four autochthonous LAB strains in the physicochemical properties and taste profiles of dry sausages substituted with NaCl | In dry sausages with 40% NaCl substituted by KCl, inoculation with LAB starter cultures resulted in faster acidification, increased water loss, and higher levels of FAAs and organic acids. | [76] |
| Lpb. plantarum LPL-1 | Potential application of the strain as a starter culture for low-salt fermented sausage production | LPL-1 reduced microbial counts associated with spoilage and pathogens in low-salt fermented sausages. | [77] |
| Lpb. plantarum CRL681, Ltb. curvatus CRL705, Ltb. sakei CRL1862 and Enterococcus mundtii CRL35 | Biochemical analysis of the production of small peptides and free amino acids by different LAB strains in a sausage model with reduced sodium content | The starter combination E. mundtii CRL35 + S. vitulinus GV318 showed the highest levels of peptides. L. sakei CRL1862 + S. vitulinus GV318 resulted in increased amino acid production. | [78] |
| Bifidobacterium animalis ssp. lactic BB-12 | Produce Italian salami with encapsulated probiotic microparticles and reduced curing salt, and evaluate the product’s physicochemical and sensory characteristics and probiotic viability. | The addition of microparticles containing Bifidobacterium BB-12 combined with reduced curing salt did not significantly change physicochemical parameters, lipid oxidation, color parameters (a * and b *), texture profile, fatty acid profile, or organic acid content. In sensory evaluation, treatment B2 (curing salt reduction + encapsulated BB-12) received the highest acceptance scores. | [79] |

Table 2.
Bacteriocin-producing lactic acid bacteria in fermented sausages.
Table 2.
Bacteriocin-producing lactic acid bacteria in fermented sausages.
| Bacteriocin-Producing Strain | Objective | Results | Reference |
|---|---|---|---|
| P. acidilactici (B-L20SafePro®, Hansen). | Bioprotection cultures for dry-fermented salami to control L. monocytogenes growth during the manufacturing process | The bioprotection culture inhibited the growth of L. monocytogenes in dry fermented sausages throughout the ripening/drying stage. | [80] |
| Lactiplantibacillus paraplantarum BPF2 and P. acidilactici ST6. | Starter cultures for the production of salchichones | The two autochthonous strains reduced L. monocytogenes counts in the samples. | [81] |
| P. acidilactici 13. | Evaluate the potential antilisterial effect when used as a starter culture. | The strain inhibited L. monocytogenes during sucuk fermentation. | [82] |
| Ltb. curvatus 54M16. | LAB isolated from fermented sausages for novel antimicrobial substances, producing bacteriocin(s) that are active against L. monocytogenes. | Ltb. curvatus 54M16 reduced microbial counts associated with spoilage and pathogens in traditional fermented sausages prepared without antimicrobial additives. | [83] |
| Ltb. curvatus MBSa2 and MBSa3 | Isolation of LAB with anti-Listeria activity from Italian salami produced in Brazil | Strains isolated from Italian-type salami produced two bacteriocins, sakacin P and sakacin X, which were stable under heat, pH, and NaCl conditions, and inhibited L. monocytogenes. | [84] |
| P. acidilactici strain HA-6111-2 | The combined effect of mild high-pressure processing (300 MPa) with phage P100 and P. acidilactici HA6111-2 as a novel decontamination method to inactivate L. monocytogenes in fermented meat sausages was evaluated. | In the Alheira model, the combination of mild high hydrostatic pressure, phage P100, and bacteriocinogenic P. acidilactici resulted in no detectable L. monocytogenes immediately after processing. | [85] |
| Ltb. curvatus MBSa2 | The application of the free and entrapped strain in calcium alginate was tested for activity against L. monocytogenes AL602/08, a strain isolated from a meat product. | Entrapment of Lb. curvatus MBSa2 in calcium alginate did not affect bacteriocin production in salami. | [86] |
| Ltb. sakei ST22Ch, ST153Ch, and ST154Ch | Bacteriocins ST22Ch, ST153Ch, and ST154Ch produced by L. sakei strains ST22Ch, ST153Ch, and ST154Ch isolated from Salpicao were characterized to use these strains as co-starter bioprotective cultures in meat fermentation. | The strains exhibited antibacterial activity against multiple target microorganisms. | [87] |
| Cob. alimentarius FM-MM4 | Purification and characterization of a novel bacteriocin produced by the bioactive strain FM-MM4 | Lactocin MM4 was resistant to heat and a wide range of pH values. It was inactivated by proteolytic enzymes but remained active after treatment with lipase and amylase. | [65] |
| P. acidilactici HA-6111-2 | Assess the combined effect of pediocin bacHA-6111-2 and mild hydrostatic pressure to control L. innocua. | The combination of pediocin bacHA-6111-2 and mild pressure treatments reduced L. innocua counts in Alheiras fermented sausages. | [88] |
Overall, strain dependent variability among LAB can be explained by differences in metabolic pathways, stress tolerance, and substrate utilization. Such heterogeneity directly influences their acidification rate, bacteriocin synthesis, proteolytic capacity, and ability to dominate native microbiota under reduced-sodium conditions. The incorporation of LAB into fermented meat sausages has a positive impact on the reduction in synthetic or chemical additives, and these microorganisms have played a satisfactory role in the development of antioxidants [89]. Fernandez [71] used LAB as an alternative to inhibit the growth of Listeria spp. in fermented meat sausage by reducing the amount of sodium nitrite. The strains P. acidilactici MP14 and the commercial P. acidilactici B-LC-20 demonstrated efficacy in reducing Listeria spp., reinforcing their potential as a protective culture in nitrite-free fermented meat sausage. In addition to their direct antimicrobial activity, LAB can play a crucial role in inhibiting stress-adapted L. monocytogenes strains that have developed resistance to acidic or osmotic pressure environments. Given that stress adaptation enhances the survival of pathogenic bacteria, LAB can create a more restrictive environment, reducing the likelihood of persistent contamination in food matrices.
Bertuci [10] used four different strains of Lacticaseibacillus spp. as a biopreservative in fermented sausages. The initial assessment focused on their ability to inhibit pathogenic and spoilage microorganisms, specifically L. monocytogenes, Bacillus cereus, Staphylococcus aureus, and E. coli. Subsequently, the strains were tested for parameters such as pH and additives relevant to fermented sausages, enabling their application in a food matrix. The authors emphasized the notable ability of two commercial strains, Lbs. rhamnosus GG and Lbs. paracasei BGP1, to inhibit microorganisms and improve the product’s technological qualities.
Liu [72] evaluated the impact of Weissella species isolated from Sichuan dry sausage on the physicochemical, proteolytic, volatile, microbiological, and sensory properties of nitrite-reduced fermented sausages. Both W. cibaria X31 and W. confusa L2 demonstrated promising results, reducing the amount of nitrite (95–97%) while simultaneously ensuring microbiological quality and favorable sensory acceptance. This study revealed that these strains contributed to the “reddish” coloration of the fermented product, enhancing a desired characteristic.
In another study, given the increasing demand for clean-label alternatives, Van der Veken [73] evaluated the response of a cocktail of nontoxigenic group I C. botulinum to the removal of nitrate and nitrite salts in fermented sausages produced under different acidification conditions and starter culture formulations, including the application of an anticlostridial Mammaliicoccus sciuri strain. The results indicated limited C. botulinum outgrowth, even in the absence of acidification, while the anticlostridial starter culture did not enhance the inhibitory effect.
The selection of LAB from fermented products is a methodological approach designed to isolate and identify strains exhibiting specific phenotypic and genotypic traits, tailored for targeted biotechnological or industrial applications. Ji [74] isolated two strains, L. MPKL03 and L. MPKL04, which were identified as P. pentosaceus and Leu. mesenteroides, demonstrating the ability to reduce nitrite levels. The strains were observed to enhance the technological profile of the product by influencing parameters such as pH, water activity, humidity, color, and nitrite content. The study, incorporating both microorganisms, highlighted the potential of nitrite reduction and food safety enhancement.
Hu [75] conducted a prior study on 37 strains of LAB, including Ltb. curvatus, Ltb. sakei, Weissella hellenica, and Lpb. plantarum, isolated from fermented sausages, assessing growth, acidification, and antimicrobial activity. In a subsequent study [90], the most promising strains were employed to evaluate the taste potential of salt-reduced fermented sausages. W. hellenica and Lpb. plantarum exhibited satisfactory results when utilized as a starter culture, contributing to the taste enhancement of salt-reduced fermented sausages. This study investigated the compensatory role of four autochthonous LAB species—Ltb. curvatus, Ltb. sakei, W. hellenica, and Lpb. plantarum—in the physicochemical properties and taste profiles of dry sausages with 40% NaCl replaced by KCl. LAB inoculation significantly reduced pH, moisture content, and water activity while increasing organic acid and certain free amino acid (FAA) concentrations. Ltb. curvatus- and Ltb. sakei-inoculated sausages exhibited higher total acid contents. The inclusion of Ltb. sakei led to a 36.90% increase in sweet FAA content and a 18.18% reduction in bitter FAA content. Furthermore, ETA indicated a 5.7% reduction in bitterness response in Llb. sakei-inoculated sausages, which also demonstrated the most similar taste profile to traditional formulations. Li [76] highlighted the potential of Ltb. sakei as a starter culture to improve physicochemical parameters and mitigate flavor deficiencies in NaCl-reduced dry sausages. In this study, four LAB strains—Llb. curvatus, Ltb. sakei, W. hellenica, and Lpb. plantarum—were individually inoculated into low-sodium formulations with 40% NaCl substitution by KCl. Among these, Ltb. sakei was the most effective in accelerating acidification and water loss, increasing the content of sweet FAAs, and reducing bitterness, thereby compensating for taste defects associated with salt reduction. These results reinforce the applicability of Ltb. sakei in the development of healthier fermented sausages without compromising sensory and technological quality.
The Lpb. plantarum LPL-1 strain, investigated in the study by Zhang [77], played a role in decreasing the number of spoilage bacteria from the genera Staphylococcus, Micrococcus, Enterobacteriaceae, and Pseudomonas and species such as L. monocytogenes throughout the salami fermentation and ripening stages. This effect was evident through the reduction in pH values and the levels of tyramine, putrescine, cadaverine, and total biogenic amines in sausages. Consequently, LPL-1 emerges as a strain suitable for application in salt-reduced fermented sausages and can serve as a beneficial starter culture for the fermentation process.
Almeida [78] used models simulating the fermentation of low-sodium meat products using Lpb. plantarum CRL681, Ltb. curvatus CRL705, Ltb. sakei CRL1862, and E. mundtii CRL35. S. vitulinus GV318 was present in all formulations used, acting as a co-culture alongside the lactic acid bacteria strains. Positive outcomes were observed using the proteolytic strains Ltb. curvatus CRL705 and S. vitulinus GV318 under low-chloride conditions, indicating a favorable response in terms of amino acids and flavor-related peptides. Additionally, the combination of strains with low sodium concentrations may impact the cardiovascular system, owing to the limited production of peptides and free amino acids during the fermentation process.
De Oliveira [79] reported that the incorporation of BB-12 microparticles from B. animalis subsp. lactis did not affect the technological characteristics of salt-reduced salami. Lipids, color, texture profile, fatty acids, and organic acids remained unaffected. The study included three treatments: control (C), without lactic acid bacteria; B1, containing encapsulated B. animalis and 0.020% salt; and B2, containing the same strain with 0.010% salt. Notably, the sensory acceptance of the treatments incorporating the encapsulated LAB was also favorable. This illustrates the potential application of this strain, demonstrating its compatibility with technological and sensory parameters, and positioning it as an alternative for the development of functional fermented products with reduced salt content.
Stefan and Predescu [80] investigated the efficacy of bioprotective cultures containing P. acidilactici, a bacteriocin-producing strain known for its antimicrobial activity against L. monocytogenes. This study demonstrated that L. monocytogenes counts decreased by 3.2 log CFU/g over 30 days in the batch inoculated with bioprotective cultures, whereas the reduction in the control batch (without bioprotective cultures) was only 1.03 log CFU/g. These results highlight the potential of bioprotective cultures as a preventive measure to control the growth of L. monocytogenes in dry fermented salami, thereby contributing to enhanced food safety and reducing the risk of human listeriosis.
García-Lopez [81] evaluated the potential of two autochthonous LAB strains Lpb. paraplantarum BPF2 and P. acidilactici ST6 isolated from spontaneously fermented Spanish sausages as starter cultures for salchichón production at a pilot scale. These strains were assessed for their impact on physicochemical parameters, microbial composition, biogenic amine content, and sensory properties compared to a commercial starter (RAP) and a spontaneously fermented control. The results indicated that ST6 exhibited a lower final percentage in the product, whereas both BPF2 and ST6 reduced biogenic amine levels and rancidity. A challenge test confirmed their bacteriocinogenic activity against L. monocytogenes, demonstrating greater inhibitory efficacy than RAP and control samples.
Cosansu [82] investigated the efficacy of P. acidilactici 13, an autochthonous strain isolated from naturally fermented sucuk, in controlling L. monocytogenes during the ripening and storage of sliced turkey breast during the ripening of this dry fermented sausage. When used as a starter culture in sucuk production, P. acidilactici 13 reduced L. monocytogenes counts by 3.32 log CFU/g over an 8-day ripening period, compared with a 1.37 log CFU/g reduction in control samples. Additionally, treatment of turkey breast slices with a partially purified antimicrobial substance from P. acidilactici 13 led to an immediate L. monocytogenes reduction of 1.03 log CFU/cm2. These results that P. acidilactici 13 is a promising protective culture for controlling L. monocytogenes in fermented sausages.
Ltb. curvatus 54M16 isolated from traditional fermented sausages in Italy, exhibited the ability to produce multiple bacteriocins (sakacin X, T, and P) with antimicrobial activity against L. monocytogenes, Bacillus cereus, and Brochothrix thermosphacta. The strain demonstrated stability at pH 4.5 and 4% NaCl, along with strong acidification capacity, nitrate reduction, and high superoxide dismutase activity. When applied as a starter culture, it enhanced the quality and safety of fermented sausages without antimicrobial additives, highlighting its potential for clean-label meat products, as evidenced by the study of Casaburi [83].
Souza Barbosa [84] investigated the behavior of Ltb. curvatus MBSa2, a bacteriocinogenic strain isolated from salami, when encapsulated in calcium alginate. The performance of the strain was assessed in MRS broth and in salami artificially contaminated with L. monocytogenes AL602/08 over a 30-day period, simulating fermentation and maturation conditions. The entrapment process did not impair bacteriocin production, as both free and encapsulated Ltb. curvatus MBSa2 reduced L. monocytogenes counts during salami processing.
In a study by Todorov [87], Ltb. sakei ST22Ch, ST153Ch, and ST154Ch, isolated from traditional pork products in Northwest Portugal, demonstrated broad-spectrum antimicrobial activity against Enterococcus spp., Listeria spp., E. coli, Klebsiella spp., Pseudomonas spp., Staphylococcus spp., and Streptococcus spp. Bacteriocins produced were bactericidal, non-glycosylated, and heat-stable, and activity was maintained after 2 h at 100 °C. Maximum activity was observed in the early stationary phase but declined over time. Beyond their antimicrobial function, LAB also contribute to the technological and sensory development of fermented sausages through proteolysis, lipolysis, and the generation of volatile compounds.
3.3. Bacteriocins in Fermented Sausage Meat
The technological properties of LAB are closely related to their metabolic versatility, which influences texture, flavor, and color development in fermented sausages. The application of bacteriocins in fermented sausages provides several advantages, particularly in terms of food safety. Bacteriocins produced by LAB effectively inhibit pathogens such as L. monocytogenes. Notably, LAB-derived bacteriocins can also exhibit inhibitory effects against some Gram-negative pathogens and microorganisms that spoilage. However, this activity is often observed when bacteriocin is combined with surfactants, which contribute to cell wall destabilization and enhance bacteriocin effectiveness [91]. Furthermore, they serve as a natural alternative to chemical preservatives, addressing the growing consumer demand for healthier foods free from synthetic additives. Another important advantage of bacteriocins is that they do not alter the sensory characteristics of the products, such as taste, texture, or aroma, thereby preserving the sensory qualities of fermented foods [92].
Among all the studied bacteriocins, nisin, produced by Lac. Lactis exhibits strong antimicrobial activity against a broad spectrum of Gram-positive bacteria. Commercially, nisin is available under various trade names, including Nisaplin. Pediocin PA-1, a bacteriocin synthesized by P. acidilactici, demonstrates high efficacy against Listeria species and is commonly applied in meat. It is marketed under commercial names such as ALTA™ 2341 [93]. Bacteriocins like nisin have been shown to be highly effective in reducing pathogenic and spoilage microorganisms when applied in the food industry. However, bacteriocins are selected for specific purposes due to their limited effectiveness in the application. These processes by additives, chemical agents, and pH. These factors can alter physiological conditions and the integrity of the cell membrane, thereby affecting the interaction of bacteriocins with the target [94,95].
However, the application of bacteriocins also presents certain challenges. The spectrum of action of some bacteriocins can be limited; that is, they are effective against a restricted number of microorganisms, which may necessitate the use of supplementary preservation techniques. In addition, prolonged use of bacteriocins may promote the development of bacterial resistance, diminishing their effectiveness over time [96].
Another challenge relates to the instability of some bacteriocins under extreme processing conditions, such as high temperatures or pH variations, which can compromise their efficacy during certain production stages. The large-scale production of these substances can also be costly, potentially leading to increased food product prices. Finally, the use of bacteriocins encounters regulatory challenges because not all countries permit their use or require rigorous safety testing before approval, thereby limiting their application in specific markets [97].
Komora [85] evaluated a non-thermal multi-hurdle approach combining mild high hydrostatic pressure (HHP, 300 MPa), the bacteriophage Listex™ P100, and the pediocin PA-1-producing P. acidilactici HA 6111-2 to control L. monocytogenes in Alheira, a traditional fermented meat sausage from Northern Portugal. The combined treatment achieved the USDA-FSIS 5 log reduction, eliminating L. monocytogenes immediately after processing, whereas the dual combinations of HHP with Listex™ P100 or HHP with P. acidilactici were less effective. These findings highlight the potential of integrating bacteriophages, bacteriocinogenic LAB, and mild HHP as a novel strategy to enhance microbial safety in fermented sausages.
Barbosa [86] investigated the isolation of LAB with anti-Listeria activity from salami and characterized and semi-purified their bacteriocins to assess their effectiveness in controlling L. monocytogenes during salami production on a pilot scale. Two Ltb. curvatus strains were isolated from and identified by 16S rRNA sequencing. The bacteriocins MBSa2 and MBSa3 exhibited strong antimicrobial activity against L. monocytogenes and other Gram-positive bacteria. Purification analysis revealed that both strains produced two active peptides (4457.9 Da and 4360.1 Da) homologous to sakacin P and X. When the semi-purified bacteriocins from Ltb. curvatus MBSa2 were added to the experimentally contaminated salami batter (104–105 CFU/g L. monocytogenes), the pathogen counts were reduced by 2 log after 10 days and by 1.5 log after 20 days, demonstrating their potential to enhance salami safety during production.
The combined application of HHP (300 MPa, 5 min, 10 °C) and bacteriocin bacHA-6111-2, produced in situ or ex situ, was evaluated for its effectiveness in controlling L. innocua in Alheira, a traditional Portuguese fermented meat product. The impact of these treatments was assessed immediately after application and throughout 60 days of refrigerated storage (4 °C). Castro [88] demonstrated that a bacteriostatic effect was observed during early storage for higher initial L. innocua concentrations when pressure was applied alone or combined with ex situ bacteriocin production. Besides their technological relevance, certain LAB strains exhibit probiotic properties, thereby bridging the gap between food safety, product quality, and potential health benefits.
3.4. Probiotic LAB in Fermented Sausages
The growing interest in probiotic applications of LAB in fermented meats reflects a shift from purely technological functions toward health-oriented product innovation. There is a scientific consensus that LAB can provide benefits in biopreservative processes, and some strains can further contribute as probiotics (or postbiotics) in health-promoting benefits. This approach is rooted in microbial antagonism, employing nonpathogenic strains as protective cultures in food due to their ability to generate antimicrobial substances [98]. Moreover, when probiotic LAB is used in fermented sausages, it can modulate the microbiota of the consumers, thereby promoting additional health properties. The benefits of this treatment include improvement of gastrointestinal balance, stimulation of the immune system, and competition with some pathogens [99].
In general, the health benefits of probiotic LAB are based on the strains’ selection and viability, their adaptation to the food environment, and their resistance to the gastrointestinal tract, thereby generating health benefits for the consumer [100]. Fermented meat sausages do not require heat treatment, making them ideal for probiotic strain application. These meat products are hypothesized to effectively protect LAB under gastrointestinal conditions [101]. However, there is a potential negative impact on the cell viability of the probiotic strains when applied to meat products due to reactions, such as fermentation and drying, that occur in the product [102].
In contrast, fermented meat sausage contains high amounts of NaCl (2–4% total product weight), nitrite, and nitrate, which reduce the pH to 4.5 and water activity to 0.90 [103]. Therefore, the potential probiotic strains must be able to thrive in this harsh environment. The selection of strains with these capabilities is fundamental for their application in fermented sausages, due to the characteristics required for the fermented meat matrix process [104]. Some strains of BAL, such as Bifidobacterium longum KACC91563; E. faecium CECT410; Lbs. casei SJRP66; Lbs. paracasei DTA83; Lbs. rhamnosus LOCK900; Lpb. plantarum 299v; Lactobacillus acidophilus CRL1014; Ltb. sakei 23K; Limosilactobacillus fermentum R6; and Staphylococcus simulans NJ201, have potential applications in fermented sausages. The production of fermented meat sausage with probiotics shows their successful colonization of the meat matrix and their ability to maintain high counts throughout processing. This is attributed to their resistance to NaCl and nitrate/nitrite salts, with minimal effects on product quality, such as color, pH, oxidative status, texture, and sensory attributes. However, human studies using probiotic LAB strains in fermented sausages are still scarce [96,97].
When selecting LAB strains with probiotic potential for application in meat products, various factors must be assessed through both in vitro and in vivo analyses to ensure that the selected strains can effectively survive and function within both the meat matrix and the human digestive system [105]. Survival in the GIT is critical, with a focus on resistance to pH fluctuations, digestive enzymes, salts, and interaction with other members of the host microbiome. Furthermore, the strains must be able to adapt to the distinctive characteristics of the meat environment, which include high protein content, specific water activity (aw) levels, moisture content, and fats. LAB are particularly well-suited for meat fermentation because of their capacity to facilitate acidification, flavor and texture development, and color stabilization. Additionally, the probiotic efficacy of LAB in meat products must be demonstrated through their antimicrobial activity and low resistance to antibiotics, ensuring both product safety and health benefits for consumers [106].
Liu et al. [107] evaluated the antimicrobial and antioxidant activities of Ltb. sakei, Lpb. plantarum, and P. pentosaceus and their applicability as starter cultures in sausage fermentation. When applied individually or in combination, these strains exhibited significant inhibitory effects against E. coli and S. aureus, along with pronounced antioxidant capacity, as evidenced by DPPH, ABTS, and hydroxyl radical scavenging activities, reducing power, and elevated antioxidant enzyme activities. Moreover, the inoculation of probiotic LAB maintained the physicochemical and sensory attributes of naturally fermented sausages while improving color and texture. Among the tested strains, Lpb. plantarum notably yielded higher sensory values.
4. Conclusions
The reformulation of fermented sausages through the reduction of sodium chloride and other curing agents remains a significant technological and safety challenge, particularly in the context of increasing consumer demand for healthier, clean-label products. Maintaining product integrity, microbiological safety, and sensory quality is essential, and LAB represents a multifunctional approach to address these objectives. LAB can function as bioprotective cultures, inhibiting spoilage and pathogenic microorganisms, while certain strains also confer probiotic properties, providing additional health benefits. Future research should prioritize the systematic selection and characterization of LAB strains capable of delivering both technological and probiotic functions under reduced-sodium conditions. Evaluating strain performance across diverse meat matrices, investigating interactions with natural preservatives and flavor modulators, assessing long-term effects on sensory attributes and consumer acceptance, and optimizing starter culture combinations and inoculation levels to balance microbial safety, product quality, and functional properties. Advancing these aspects will generate actionable knowledge to guide the development of safer, nutritionally optimized, and consumer acceptable fermented sausages.
Author Contributions
M.L.B.: conceptualization, investigation, writing—original draft, writing—review and editing, visualization, methodology, formal analysis, data curation. C.V.B.S.: investigation, writing—original draft, writing—review and editing. C.A.A.J.: writing—review and editing. S.D.T.: writing—review and editing. A.L.B.P.: conceptualization, writing—review and editing, supervision. A.C.d.S.B.: conceptualization, writing—review and editing, project administration, supervision, funding acquisition, and methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Brazilian National Council for Scientific and Technological Development (CNPq, Process No. 150738/2022-6) and by the Brazilian Federal Agency for Support and Evaluation of Graduate Education (CAPES, Process No. 88887.624270/2021-00).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, J.; Huang, X.-H.; Zhang, Y.-Y.; Li, S.; Dong, X.; Qin, L. Effect of sodium salt on meat products and reduction sodium strategies—A review. Meat Sci. 2023, 205, 109296. [Google Scholar] [CrossRef]
- Orsi, A.S.; Junior, W.J.L.; Alegbeleye, O.O.; Muniz, D.C.; Horita, C.N.; Sant’Ana, A.S. Sodium chloride reduction in meat processing: Microbial shifts, spoilage risks, and metagenomic insights. Meat Sci. 2025, 226, 109848. [Google Scholar] [CrossRef]
- Alves Junior, C.A.; Bellucci, E.R.B.; Santos, J.M.D.; Bertuci, M.L.; Barretto, A.C.D. L-lysine and dietary fiber improve the physicochemical properties of sausage without added phosphate and reduced salt levels. Sci. Agric. 2023, 80, e20220026. [Google Scholar] [CrossRef]
- Cittadini, A.; Domínguez, R.; Sarriés, M.V.; Pateiro, M.; Lorenzo, J.M. Study of Pansalt® or Laminaria Ochroleuca seaweed powder as potential NaCl replacers in dry-cured foal “cecina”. Meat Sci. 2023, 204, 109253. [Google Scholar] [CrossRef]
- Alves Junior, C.A.; Bellucci, E.R.B.; Bertuci, M.L.; dos Santos, J.M.; Barretto, A.C.D. Effect of collagen in Italian type salami with NaCl reduction on the physicochemical and technological properties. Int. J. Food Sci. Technol. 2024, 59, 7589–7597. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of lactic acid bacteria for the biopreservation of meat products: A systematic review. Meat Sci. 2022, 183, 108661. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Tang, Y.; Hu, Y.; Lu, Y.; Sun, Q.; Lv, Y.; Zhang, Q.; Wu, C.; Zhu, M.; He, Q.; et al. Sodium Reduction in Traditional Fermented Foods: Challenges, Strategies, and Perspectives. J. Agric. Food Chem. 2021, 69, 8065–8080. [Google Scholar] [CrossRef]
- Jia, S.; Shen, H.; Wang, D.; Liu, S.; Ding, Y.; Zhou, X. Novel NaCl reduction technologies for dry-cured meat products and their mechanisms: A comprehensive review. Food Chem. 2024, 431, 137142. [Google Scholar] [CrossRef]
- Rios-Mera, J.D.; Selani, M.M.; Patinho, I.; Saldaña, E.; Contreras-Castillo, C.J. Modification of NaCl structure as a sodium reduction strategy in meat products: An overview. Meat Sci. 2021, 174, 108417. [Google Scholar] [CrossRef]
- Bertuci, M.L.; Alves Junior, C.A.; Souza, C.V.B.; Penna, A.L.B.; da Silva Barretto, A.C. Bio preservation capacity of potentially probiotic Lacticaseibacillus strains in fermented sausage. Int. J. Food Sci. Technol. 2023, 58, 6253–6262. [Google Scholar] [CrossRef]
- Rendueles, C.; Duarte, A.C.; Escobedo, S.; Fernández, L.; Rodríguez, A.; García, P.; Martínez, B. Combined use of bacteriocins and bacteriophages as food biopreservatives. A review. Int. J. Food Microbiol. 2022, 368, 109611. [Google Scholar] [CrossRef]
- Karbowiak, M.; Szymański, P.; Zielińska, D. Synergistic Effect of Combination of Various Microbial Hurdles in the Biopreservation of Meat and Meat Products—Systematic Review. Foods 2023, 12, 1430. [Google Scholar] [CrossRef]
- Todorov, S.D.; Popov, I.; Weeks, R.; Chikindas, M.L. Use of Bacteriocins and Bacteriocinogenic Beneficial Organisms in Food Products: Benefits, Challenges, Concerns. Foods 2022, 11, 3145. [Google Scholar] [CrossRef]
- Afraei, M.; Soleimanian-Zad, S.; Fathi, M. Improvement the texture of nitrite-free fermented sausages using microencapsulation of fermenting bacteria. Food Biosci. 2022, 50, 102010. [Google Scholar] [CrossRef]
- Comi, G.; Muzzin, A.; Corazzin, M.; Iacumin, L. Lactic Acid Bacteria: Variability Due to Different Pork Breeds, Breeding Systems and Fermented Sausage Production Technology. Foods 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Pei, H.; Liu, R.; Chen, L.; Gao, X.; Gu, Y.; Hou, Q.; Yin, Y.; Yu, H.; Wu, M.; et al. Effects of Lactobacillus plantarum NJAU-01 from Jinhua ham on the quality of dry-cured fermented sausage. LWT 2019, 101, 513–518. [Google Scholar] [CrossRef]
- Giello, M.; la Storia, A.; de Filippis, F.; Ercolini, D.; Villani, F. Impact of Lactobacillus curvatus 54M16 on microbiota composition and growth of Listeria monocytogenes in fermented sausages. Food Microbiol. 2018, 72, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.I.; Mizan, M.F.R.; Roy, P.K.; Nahar, S.; Toushik, S.H.; Ashrafudoulla, M.; Jahid, I.K.; Lee, J.; Ha, S.-D. Listeria monocytogenes biofilm inhibition on food contact surfaces by application of postbiotics from Lactobacillus curvatus B.67 and Lactobacillus plantarum M.2. Food Res. Int. 2021, 148, 110595. [Google Scholar] [CrossRef]
- Todorov, S.D.; Baretto Penna, A.L.; Venema, K.; Holzapfel, W.H.; Chikindas, M.L. Recommendations for the use of standardised abbreviations for the former Lactobacillus genera, reclassified in the year 2020. Benef. Microbes 2023, 15, 1–4. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Muthuvelu, K.S.; Ethiraj, B.; Pramnik, S.; Raj, N.K.; Venkataraman, S.; Rajendran, D.S.; Bharathi, P.; Palanisamy, E.; Narayanan, A.S.; Vaidyanathan, V.K.; et al. Biopreservative technologies of food: An alternative to chemical preservation and recent developments. Food Sci. Biotechnol. 2023, 32, 1337–1350. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- Kim, J.; Knowles, S.; Ahmad, R.; Day, L. Objective Measurements Associated with the Preferred Eating Qualities of Fermented Salamis. Foods 2021, 10, 2003. [Google Scholar] [CrossRef]
- Santos, J.M.D.; Ignácio, E.O.; Bis-Souza, C.V.; Silva-Barretto, A.C.D. Performance of reduced fat-reduced salt fermented sausage with added microcrystalline cellulose, resistant starch and oat fiber using the simplex design. Meat Sci. 2021, 175, 108433. [Google Scholar] [CrossRef]
- Zampouni, K.; Soniadis, A.; Dimakopoulou-Papazoglou, D.; Moschakis, T.; Biliaderis, C.G.; Katsanidis, E. Modified fermented sausages with olive oil oleogel and NaCl–KCl substitution for improved nutritional quality. LWT 2022, 158, 113172. [Google Scholar] [CrossRef]
- Dimakopoulou-Papazoglou, D.; Katsanidis, E. Osmotic processing of meat: Mathematical modeling and quality parameters. Food Eng. Rev. 2020, 12, 32–47. [Google Scholar] [CrossRef]
- Tapia, M.S.; Alzamora, S.M.; Chirife, J. Effects of water activity (aw) on microbial stability as a hurdle in food preservation. In Water Activity in Foods: Fundamentals and Applications; Wiley: Hoboken, NJ, USA, 2020; pp. 323–355. [Google Scholar] [CrossRef]
- Inguglia, E.S.; Zhang, Z.; Tiwari, B.K.; Kerry, J.P.; Burgess, C.M. Salt reduction strategies in processed meat products—A review. Trends Food Sci. Technol. 2017, 59, 70–78. [Google Scholar] [CrossRef]
- Padilla-Moseley, J.; Sivakumar, B.; Flexner, N.; Grajeda, R.; Gamble, B.; Blanco-Metzler, A.; Arcand, J. Factors Impacting the Uptake of Research into Dietary Sodium Reduction Policies in Five Latin American Countries: A Qualitative Study. Curr. Dev. Nutr. 2023, 7, 100073. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Yong, H.I.; Jung, S.; Kim, H.-W.; Choi, Y.-S. Effect of reducing sodium chloride based on the sensory properties of meat products and the improvement strategies employed: A review. J. Anim. Sci. Technol. 2021, 63, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.; Laranjo, M.; Potes, M.E.; Agulheiro-Santos, A.C.; Fernandes, M.J.; Garcia, R.; Fraqueza, M.J. Impact of a 25% Salt Reduction on the Microbial Load, Texture, and Sensory Attributes of a Traditional Dry-Cured Sausage. Foods 2020, 9, 554. [Google Scholar] [CrossRef] [PubMed]
- Corral, S.; Belloch, C.; López-Díez, J.J.; Salvador, A.; Flores, M. Yeast inoculation as a strategy to improve the physico-chemical and sensory properties of reduced salt fermented sausages produced with entire male fat. Meat Sci. 2017, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Hu, Y.; Wen, R.; Wang, Y.; Qin, L.; Kong, B. Characterisation of the flavour profile of dry fermented sausages with different NaCl substitutes using HS-SPME-GC-MS combined with electronic nose and electronic tongue. Meat Sci. 2021, 172, 108338. [Google Scholar] [CrossRef]
- Le, B.; Yu, B.; Amin, M.S.; Liu, R.; Zhang, N.; Soladoye, O.P.; Aluko, R.E.; Zhang, Y.; Fu, Y. Salt taste receptors and associated salty/salt taste-enhancing peptides: A comprehensive review of structure and function. Trends Food Sci. Technol. 2022, 129, 657–666. [Google Scholar] [CrossRef]
- Tabanelli, G.; Barbieri, F.; Soglia, F.; Magnani, R.; Gardini, G.; Petracci, M.; Gardini, F.; Montanari, C. Safety and technological issues of dry fermented sausages produced without nitrate and nitrite. Food Res. Int. 2022, 160, 111685. [Google Scholar] [CrossRef] [PubMed]
- Fraqueza, M.J.; Laranjo, M.; Elias, M.; Patarata, L. Microbiological hazards associated with salt and nitrite reduction in cured meat products: Control strategies based on antimicrobial effect of natural ingredients and protective microbiota. Curr. Opin. Food Sci. 2021, 38, 32–39. [Google Scholar] [CrossRef]
- Pavli, F.G.; Argyri, A.A.; Chorianopoulos, N.G.; Nychas, G.-J.E.; Tassou, C.C. Effect of Lactobacillus plantarum L125 strain with probiotic potential on physicochemical, microbiological and sensorial characteristics of dry-fermented sausages. LWT 2020, 118, 108810. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Jia, J.; Peng, H.; Qian, Q.; Pan, Z.; Liu, D. Nitrite and nitrate in meat processing: Functions and alternatives. Curr. Res. Food Sci. 2023, 6, 100470. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Q.; Xia, C.; Yang, F.; Xu, N.; Wu, Q.; Hu, Y.; Xia, L.; Wang, C.; Zhou, M. Effect of selenium supplements on the antioxidant activity and nitrite degradation of lactic acid bacteria. World J. Microbiol. Biotechnol. 2019, 35, 61. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, L.; Yang, Q. Partial replacement of nitrite with a novel probiotic Lactobacillus plantarum on nitrate, color, biogenic amines and gel properties of Chinese fermented sausages. Food Res. Int. 2020, 137, 109351. [Google Scholar] [CrossRef]
- Morales, P.; Aguirre, J.; Troncoso, M.; Figueroa, G. Comparison of in vitro and in situ antagonism assays as tools for the selection of bio-preservative lactic acid bacteria (LAB) in poultry meat. LWT 2020, 118, 108846. [Google Scholar] [CrossRef]
- Graça, C.; Lima, A.; Raymundo, A.; Sousa, I. Sourdough Fermentation as a Tool to Improve the Nutritional and Health-Promoting Properties of Its Derived-Products. Fermentation 2021, 7, 246. [Google Scholar] [CrossRef]
- Kong, L.; Deng, J.; Cai, K.; Wu, Y.; Ge, J.; Xu, B. Evaluating the colour formation and oxidation effect of Leuconostoc mesenteroides subsp. IMAU:80679 combining with ascorbic acid in fermented sausages. Food Biosci. 2023, 52, 102478. [Google Scholar] [CrossRef]
- Stasiak-Różańska, L.; Berthold-Pluta, A.; Pluta, A.S.; Dasiewicz, K.; Garbowska, M. Effect of Simulated Gastrointestinal Tract Conditions on Survivability of Probiotic Bacteria Present in Commercial Preparations. Int. J. Environ. Res. Public Health 2021, 18, 1108. [Google Scholar] [CrossRef]
- Qin, S.; Zeng, X.M.; Jiang, M.; Rui, X.; Li, W.; Dong, M.S.; Chen, X.H.; Zhang, Q.Q. Genomic and biogenic amine-reducing characterization of Lactiplantibacillus plantarum JB1 isolated from fermented dry sausage. Food Control 2023, 154, 109971. [Google Scholar] [CrossRef]
- Martínez-Miranda, J.G.; Chairez, I.; Durán-Páramo, E. Mannitol Production by Heterofermentative Lactic Acid Bacteria: A Review. Appl. Biochem. Biotechnol. 2022, 194, 2762–2795. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; de Melo Franco, B.D.G.; Tagg, J.R. Bacteriocins of Gram-positive bacteria having activity spectra extending beyond closely-related species. Benef. Microbes 2019, 10, 315–328. [Google Scholar] [CrossRef]
- de Azevedo, P.O.S.; Converti, A.; Gierus, M.; Oliveira, R.P.S. Antimicrobial activity of bacteriocin-like inhibitory substance produced by Pediococcus pentosaceus: From shake flasks to bioreactor. Mol. Biol. Rep. 2019, 46, 461–469. [Google Scholar] [CrossRef]
- Habiba, M.U.; Augustin, M.A.; Varela, C.; Morris, H.; Rahman, M.M.; Bozkurt, H. Probiotic Dairy Innovations: Exploring Buffalo Milk Potential for Food Product Development. Compr. Rev. Food Sci. Food Saf. 2025, 24, 4. [Google Scholar] [CrossRef] [PubMed]
- Wanderley Porto, M.C.; de Souza de Azevedo, P.O.; Lourenço, F.R.; Converti, A.; Vitolo, M.; Oliveira, R.P.S. Effect of Polydextrose on the Growth of Pediococcus pentosaceus as Well as Lactic Acid and Bacteriocin-like Inhibitory Substances (BLIS) Production. Microorganisms 2022, 10, 1898. [Google Scholar] [CrossRef]
- Jawan, R.; Abbasiliasi, S.; Mustafa, S.; Kapri, M.R.; Halim, M.; Ariff, A.B. In Vitro Evaluation of Potential Probiotic Strain Lactococcus lactis Gh1 and Its Bacteriocin-Like Inhibitory Substances for Potential Use in the Food Industry. Probiotics Antimicrob. Proteins 2021, 13, 422–440. [Google Scholar] [CrossRef]
- Piazentin, A.C.M.; Mendonça, C.M.N.; Vallejo, M.; Mussatto, S.I.; de Souza Oliveira, R.P. Bacteriocin-like inhibitory substances production by Enterococcus faecium 135 in co-culture with Ligilactobacillus salivarius and Limosilactobacillus reuteri. Braz. J. Microbiol. 2022, 53, 131–141. [Google Scholar] [CrossRef]
- Smaoui, S.; Echegaray, N.; Kumar, M.; Chaari, M.; D’Amore, T.; Shariati, M.A.; Rebezov, M.; Lorenzo, J.M. Beyond conventional meat preservation: Saddling the control of bacteriocin and lactic acid bacteria for clean label and functional meat products. Appl. Biochem. Biotechnol. 2024, 196, 3604–3635. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Nanda, P.K.; Pateiro, M.; Lorenzo, J.M.; Dhar, P.; Das, A.K. Lactic Acid Bacteria and Bacteriocins: Novel Biotechnological Approach for Biopreservation of Meat and Meat Products. Microorganisms 2022, 10, 2058. [Google Scholar] [CrossRef]
- Sadeghi, A.; Katouzian, I.; Ebrahimi, M.; Assadpour, E.; Tan, C.; Jafari, S.M. Bacteriocin-like inhibitory substances as green bio-preservatives; nanoliposomal encapsulation and evaluation of their in vitro/in situ anti-Listerial activity. Food Control 2023, 150, 109725. [Google Scholar] [CrossRef]
- de Marco, I.; Fusieger, A.; Nero, L.A.; Kempka, A.P.; Moroni, L.S. Bacteriocin-like inhibitory substances (BLIS) synthesized by Lactococcus lactis LLH20: Antilisterial activity and application for biopreservation of minimally processed lettuce (Lactuca sativa L.). Biocatal. Agric. Biotechnol. 2022, 42, 102355. [Google Scholar] [CrossRef]
- Haryani, Y.; Halid, N.A.; Guat, G.S.; Nor-Khaizura, M.A.R.; Hatta, A.; Sabri, S.; Radu, S.; Hasan, H. Characterization, molecular identification, and antimicrobial activity of lactic acid bacteria isolated from selected fermented foods and beverages in Malaysia. FEMS Microbiol. Lett. 2023, 370, fnad023. [Google Scholar] [CrossRef] [PubMed]
- Thuy, T.T.D.; Lu, H.-F.; Bregente, C.J.B.; Huang, F.-C.A.; Tu, P.-C.; Kao, C.-Y. Characterization of the broad-spectrum antibacterial activity of bacteriocin-like inhibitory substance-producing probiotics isolated from fermented foods. BMC Microbiol. 2024, 24, 85. [Google Scholar] [CrossRef]
- Jiang, S.; Cai, L.; Lv, L.; Li, L. Pediococcus pentosaceus, a future additive or probiotic candidate. Microb. Cell Fact. 2021, 20, 45. [Google Scholar] [CrossRef]
- Giaouris, E. Application of lactic acid bacteria and their metabolites against foodborne pathogenic bacterial biofilms. In Recent Trends in Biofilm Science and Technology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 205–232. [Google Scholar] [CrossRef]
- Milani, G.; Tabanelli, G.; Barbieri, F.; Montanari, C.; Gardini, F.; Belloso Daza, M.V.; Castellone, V.; Bozzetti, M.; Cocconcelli, P.S.; Bassi, D. Technological traits and mitigation activity of autochthonous lactic acid bacteria from Mediterranean fermented meat-products. LWT 2024, 196, 115861. [Google Scholar] [CrossRef]
- Siddi, G.; Piras, F.; Spanu, V.; Meloni, M.P.; Sanna, R.; Carta, N.; Errico, M.; Cuccu, M.; de Santis, E.P.L.; Scarano, C. Selection of commercial protective cultures to be added in Sardinian fermented sausage to control Listeria monocytogenes. Ital. J. Food Saf. 2022, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Parada Fabián, J.C.; Álvarez Contreras, A.K.; Natividad Bonifacio, I.; Hernández Robles, M.F.; Vázquez Quiñones, C.R.; Quiñones Ramírez, E.I.; Vázquez Salinas, C. Toward safer and sustainable food preservation: A comprehensive review of bacteriocins in the food industry. Biosci. Rep. 2025, 45, 277–302. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Mohammadi, M.; Mortazavian, A.M.; Yousefi, M. Antibacterial Activity of Pediocin and Pediocin-Producing Bacteria Against Listeria monocytogenes in Meat Products. Front. Microbiol. 2021, 12, 709959. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, X.; Shan, C.; Xia, X.; Wang, Y.; Dong, M.; Zhou, J. Novel bacteriocin produced by Lactobacillus alimentarius FM-MM 4 from a traditional Chinese fermented meat Nanx Wudl: Purification, identification and antimicrobial characteristics. Food Control 2017, 77, 290–297. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Georgescu, C.; Turcuş, V.; Olah, N.K.; Mathe, E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018, 143, 922–935. [Google Scholar] [CrossRef]
- De Prisco, A.; Mauriello, G. Probiotication of foods: A focus on microencapsulation tool. Trends Food Sci. Technol. 2016, 48, 27–39. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of lactic acid bacteria and their metabolites on the techno-functional properties and health benefits of fermented dairy products. Crit. Rev. Food Sci. Nutr. 2023, 63, 4819–4841. [Google Scholar] [CrossRef] [PubMed]
- Chittora, D.; Meena, B.; Jain, T.; Sharma, K. Biopreservation: Bacteriocins and lactic acid bacteria. J. Postharvest Technol. 2022, 10, 1–15. Available online: https://journals.acspublisher.com/index.php/jpht/article/view/15040 (accessed on 25 April 2025).
- Fernández, M.; Hospital, X.F.; Caballero, N.; Jiménez, B.; Sánchez-Martín, V.; Morales, P.; Haza, A.I.; Hierro, E. Potential of selected bacteriocinogenic lactic acid bacteria to control Listeria monocytogenes in nitrite-reduced fermented sausages. Food Control 2023, 150, 109724. [Google Scholar] [CrossRef]
- Liu, X.; Qu, H.; Gou, M.; Guo, H.; Wang, L.; Yan, X. Application of Weissella cibaria X31 or Weissella confusa L2 as a starter in low nitrite dry-fermented sausages. Int. J. Food Eng. 2020, 16, 8. [Google Scholar] [CrossRef]
- Van der Veken, D.; Poortmans, M.; Dewulf, L.; Fraeye, I.; Michiels, C.; Leroy, F. Challenge tests reveal limited outgrowth of proteolytic Clostridium botulinum during the production of nitrate- and nitrite-free fermented sausages. Meat Sci. 2023, 200, 109158. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhou, C.; Ning, J.; Wang, S.; Nie, Q.; Wang, W.; Zhang, J.; Zhao, Z. Nitrite-reducing performance of two Lactobacillus strains isolated from traditional Sichuan fermented sausage in different production processes. Food Bioeng. 2022, 1, 307–318. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Zhu, J.; Kong, B.; Liu, Q.; Chen, Q. Improving the taste profile of reduced-salt dry sausage by inoculating different lactic acid bacteria. Food Res. Int. 2021, 145, 110391. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sui, Y.; Lu, J.; Ren, J.; Kong, B.; Li, Y.; Chen, Q.; Yang, W. Compensative role of autochthonous lactic acid bacteria in physical properties and taste profiles of dry sausage with partial substitution of NaCl by KCl. LWT 2024, 199, 116115. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Wang, Y.; Huang, Y.; Li, P.; Li, P. Lactobacillus plantarum LPL-1, a bacteriocin-producing strain, changed the bacterial community composition and improved the safety of low-salt fermented sausages. LWT 2020, 128, 109385. [Google Scholar] [CrossRef]
- de Almeida, M.A.; Saldaña, E.; da Silva Pinto, J.S.; Palacios, J.; Contreras-Castillo, C.J.; Sentandreu, M.A.; Fadda, S.G. A peptidomic approach of meat protein degradation in a low-sodium fermented sausage model using autochthonous starter cultures. Food Res. Int. 2018, 109, 368–379. [Google Scholar] [CrossRef]
- Oliveira Gomes, B.; Mesquita Oliveira, C.; Marins, A.R.; Gomes, R.G.; Feihrmann, A.C. Application of microencapsulated probiotic Bifidobacterium animalis ssp. lactis BB-12 in Italian salami. J. Food Process. Preserv. 2021, 45, 10. [Google Scholar] [CrossRef]
- Ștefan, G.; Predescu, C.N. Using the bioprotection culture for dry fermented salami-the control measure of Listeria monocytogenes growth. Sci. Pap. Ser. D Anim. Sci. 2024, 67, 1. Available online: https://animalsciencejournal.usamv.ro/pdf/2024/issue_1/Art72.pdf (accessed on 18 January 2025).
- García-López, J.D.; Barbieri, F.; Baños, A.; Madero, J.M.G.; Gardini, F.; Montanari, C.; Tabanelli, G. Use of two autochthonous bacteriocinogenic strains as starter cultures in the production of salchichónes, a type of Spanish fermented sausages. Curr. Res. Food Sci. 2023, 7, 100615. [Google Scholar] [CrossRef]
- Cosansu, S.; Geornaras, I.; Ayhan, K.; Sofos, J.N. Control of Listeria monocytogenes by bacteriocin-producing Pediococcus acidilactici 13 and its antimicrobial substance in a dry fermented sausage sucuk and in turkey breast. J. Food Nutr. Res. 2010, 49, 206–214. [Google Scholar]
- Casaburi, A.; di Martino, V.; Ferranti, P.; Picariello, L.; Villani, F. Technological properties and bacteriocins production by Lactobacillus curvatus 54M16 and its use as starter culture for fermented sausage manufacture. Food Control 2016, 59, 31–45. [Google Scholar] [CrossRef]
- de Souza Barbosa, M.; Todorov, S.D.; Ivanova, I.; Chobert, J.-M.; Haertlé, T.; de Melo Franco, B.D.G. Improving safety of salami by application of bacteriocins produced by an autochthonous Lactobacillus curvatus isolate. Food Microbiol. 2015, 46, 254–262. [Google Scholar] [CrossRef]
- Komora, N.; Maciel, C.; Amaral, R.A.; Fernandes, R.; Castro, S.M.; Saraiva, J.A.; Teixeira, P. Innovative hurdle system towards Listeria monocytogenes inactivation in a fermented meat sausage model—High pressure processing assisted by bacteriophage P100 and bacteriocinogenic Pediococcus acidilactici. Food Res. Int. 2021, 148, 110628. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Todorov, S.D.; Jurkiewicz, C.H.; Franco, B.D.G.M. Bacteriocin production by Lactobacillus curvatus MBSa2 entrapped in calcium alginate during ripening of salami for control of Listeria monocytogenes. Food Control 2015, 47, 147–153. [Google Scholar] [CrossRef]
- Todorov, S.D.; Vaz-Velho, M.; de Melo Franco, B.D.G.; Holzapfel, W.H. Partial characterization of bacteriocins produced by three strains of Lactobacillus sakei, isolated from salpicao, a fermented meat product from North-West of Portugal. Food Control 2013, 30, 111–121. [Google Scholar] [CrossRef]
- Castro, S.M.; Silva, J.; Casquete, R.; Queirós, R.; Saraiva, J.A.; Teixeira, P. Combined effect of pediocin bacHA-6111-2 and high hydrostatic pressure to control Listeria innocua in fermented meat sausage. Int. Food Res. J. 2018, 25, 553–560. Available online: http://www.ifrj.upm.edu.my/25%20(02)%202018/(14).pdf (accessed on 12 July 2025).
- Aalto-Araneda, M.; Pöntinen, A.; Pesonen, M.; Corander, J.; Markkula, A.; Tasara, T.; Stephan, R.; Korkeala, H. Strain variability of Listeria monocytogenes under NaCl stress elucidated by a high-throughput microbial growth data assembly and analysis protocol. Appl. Environ. Microbiol. 2020, 86, 6. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Li, X.; Zhang, H.; Chen, Q.; Kong, B. Application of lactic acid bacteria for improving the quality of reduced-salt dry fermented sausage: Texture, color, and flavor profiles. LWT 2022, 154, 112723. [Google Scholar] [CrossRef]
- NicAogáin, K.; O’Byrne, C.P. The role of stress and stress adaptations in determining the fate of the bacterial pathogen Listeria monocytogenes in the food chain. Front. Microbiol. 2016, 7, 1865. [Google Scholar] [CrossRef]
- Liang, Q.; Liu, Z.; Liang, Z.; Fu, X.; Li, D.; Zhu, C.; Kong, Q.; Mou, H. Current challenges and development strategies of bacteriocins produced by lactic acid bacteria applied in the food industry. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70038. [Google Scholar] [CrossRef] [PubMed]
- Field, D.; Fernandez de Ullivarri, M.; Ross, R.P.; Hill, C. After a century of nisin research—Where are we now? FEMS Microbiol. Rev. 2023, 47, fuad023. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic acid bacteria as antimicrobial agents: Food safety and microbial food spoilage prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef]
- Zimina, M.; Babich, O.; Prosekov, A.; Sukhikh, S.; Ivanova, S.; Shevchenko, M.; Noskova, S. Overview of global trends in classification, methods of preparation and application of bacteriocins. Antibiotics 2020, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Grujović, M.Ž.; Mladenović, K.G.; Semedo-Lemsaddek, T.; Laranjo, M.; Stefanović, O.D.; Kocić-Tanackov, S.D. Advantages and disadvantages of non-starter lactic acid bacteria from traditional fermented foods: Potential use as starters or probiotics. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1537–1567. [Google Scholar] [CrossRef]
- Fuochi, V.; Emma, R.; Furneri, P.M. Bacteriocins, a natural weapon against bacterial contamination for greater safety and preservation of food: A review. Curr. Pharm. Biotechnol. 2021, 22, 216–231. [Google Scholar] [CrossRef]
- Bis-Souza, C.V.; Penna, A.L.B.; da Silva Barretto, A.C. Applicability of potentially probiotic Lactobacillus casei in low-fat Italian type salami with added fructooligosaccharides: In vitro screening and technological evaluation. Meat Sci. 2020, 168, 108186. [Google Scholar] [CrossRef] [PubMed]
- Yim, D.-G.; Ali, M.; Nam, K.-C. Comparison of meat quality traits in salami added by nitrate-free salts or nitrate pickling salt during ripening. Food Sci. Anim. Resour. 2020, 40, 11–20. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Zhang, W.; Domínguez, R.; Xing, L.; Fierro, E.M.; Lorenzo, J.M. Autochthonous probiotics in meat products: Selection, identification, and their use as starter culture. Microorganisms 2020, 8, 1833. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; da Silva Barretto, A.C.; Santos, E.M.; Lorenzo, J.M. Functional fermented meat products with probiotics—A review. J. Appl. Microbiol. 2022, 133, 91–103. [Google Scholar] [CrossRef]
- Kaveh, S.; Hashemi, S.M.B.; Abedi, E.; Amiri, M.J.; Conte, F.L. Bio-preservation of meat and fermented meat products by lactic acid bacteria strains and their antibacterial metabolites. Sustainability 2023, 15, 10154. [Google Scholar] [CrossRef]
- Sirini, N.; Frizzo, L.S.; Aleu, G.; Soto, L.P.; Rosmini, M.R. Use of probiotic microorganisms in the formulation of healthy meat products. Curr. Opin. Food Sci. 2021, 38, 141–146. [Google Scholar] [CrossRef]
- Rachwał, K.; Gustaw, K. Lactic acid bacteria in sustainable food production. Sustainability 2024, 16, 3362. [Google Scholar] [CrossRef]
- Acedo, J.Z.; Chiorean, S.; Vederas, J.C.; van Belkum, M.J. The expanding structural variety among bacteriocins from Gram-positive bacteria. FEMS Microbiol. Rev. 2018, 42, 805–828. [Google Scholar] [CrossRef]
- Figueroa, R.H.H.; López-Malo, A.; Mani-López, E. Antimicrobial activity and applications of fermentates from lactic acid bacteria–a review. Sustain. Food Technol. 2024, 2, 292–306. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, S.; Cui, Y.; Wang, L.; Duan, J.; Yang, X.; Liu, X.; Zhang, S.; Sun, B.; Yu, H.; et al. Characteristics of lactic acid bacteria as potential probiotic starters and their effects on the quality of fermented sausages. Foods 2024, 13, 198. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).