Abstract
Edible bird nest (EBN) has a longstanding tradition in Chinese herbal medicine as a natural supplement for enhancing health and well-being. Current scientific research indicates that EBN possesses properties conducive to improving male reproductive function. These properties include promoting cell proliferation, containing essential reproductive hormones, and exhibiting antioxidant activity. Despite these promising characteristics, the potential of EBN’s specific effects on male reproductive health, particularly in addressing infertility, remains sparsely discussed. Hereby, this review aims to present the available scientific evidence, discuss potential benefits, and underscore the importance of future research in advancing our understanding of EBN’s role in male reproductive health. This work is imperative to expand our knowledge of the biological underpinnings of EBN so that it can be developed as a food supplement and alternative treatment for male infertility.
1. Introduction
Edible bird nest (EBN) has been valued in traditional Chinese medicine as a food supplement with diverse health-promoting effects. It is produced from the saliva of male swiftlets during the breeding season [1]. About 24 swiftlet species have been recognised globally and grouped into four genera. Among these, Hydrochous, Schoutedenapus, and Collocalia are non-echolocating swiftlets, while the genus Aerodramus is echolocating. The white nest produced by Aerodramus fuciphagus is the most common and commercially important type in Southeast Asia [2,3,4].
Research on EBN began more than two centuries ago. Early studies on EBN primarily focused on the nature of the nest, including the materials used in its construction [5], and the glandular source of the mucus that binds the nest [6,7]. Over time, research interest shifted towards its nutritional composition. Studies have established that EBN predominantly comprises protein followed by carbohydrates, fat, ash, and trace moisture [8,9,10]. It also contains essential minerals such as sodium and calcium, which are in higher concentrations than magnesium, potassium, phosphorus and iron, and amino acids necessary for physiological functions [10,11]. This distinctive nutritional profile has driven further research into its bioactive and therapeutic potential.
Traditionally, EBN has been credited with multiple health benefits, including improving digestion, easing asthma, dissolving phlegm, and promoting the speedy recovery of illnesses, surgery, and wounds [2,12]. It has also been associated with enhanced complexion, delayed ageing process, improved voice and attention, and increased libido [2,12].
Scientific studies have begun to validate these claims, and various reports showed that EBN possesses antimicrobial and antiviral properties [13,14], anti-inflammatory effects [15,16,17], antioxidant activity [17,18,19], and anti-ageing potential [20]. In addition, EBN has been reported to exert anti-osteoarthritis effects [21,22], enhance wound healing [23], and promote cell proliferation [15,24,25]. In vivo studies further support its therapeutic value, demonstrating improvements in osteoporosis [26], anti-diabetic activity [27], and enhancing spatial learning and memory [28,29].
Among the many reported benefits, EBN is increasingly recognised for its potential role in reproductive health. Traditional claims suggest that EBN enhances libido [2,12,30]. Libido is a term used to describe a sexual drive or desire that involves a complex interaction involving steroid hormones, motivating external environment, and cognitive function [31]. However, whether the libido in the traditional claim of EBN refers to one or both sexual partners is unclear. Nevertheless, the reproductive function requires more than a sexual urge. The significant component of reproductive function is to produce gametes so that fertilisation and subsequent pregnancy can occur.
Interestingly, specific properties of EBN are directly relevant to the nature of the reproductive system. Its cell-proliferative activity [15,24,25] parallels the cellular processes involved in gametogenesis. EBN also contains vital hormones [12] crucial to the reproductive system’s physiological function. Additionally, its antioxidant activity [17,18,19] protects gametes from oxidative stress, a key factor in gamete integrity.
Despite these promising features, their implications for male reproductive health have been poorly discussed. Therefore, this review comprehensively discussed characteristics of EBN in the male reproductive system. Additionally, this review aimed to compile all recent scientific information supporting EBN’s ability to improve various conditions related to the male reproductive system. This evaluation could serve as a platform for EBN’s potential as a food supplement to enhance the reproductive system, particularly as the world’s fertility crisis gains momentum.
2. Nutritional Composition of EBN
Edible bird’s nest (EBN) is valued not only for its cultural significance but also for its rich nutritional profile. Proteins comprise the most significant portion of their content, followed by carbohydrates, ash, fat, and a small amount of moisture [8,9,10,11]. Alongside these macronutrients, EBN provides a wide variety of amino acids, both essential, such as lysine, threonine, leucine, and valine, and non-essential, including serine, glutamic acid, proline, and tyrosine [9,32]. Some amino acids, like tyrosine and glutamic acid, have even been proposed as markers to distinguish nests from different sources [33]. These amino acids play vital roles in reproductive physiology, as glutamic acid contributes to spermatogonial cell energy metabolism [34]. Meanwhile, proline and lysine are essential substrates for collagen synthesis that contribute to the structural integrity of tissues, including the seminiferous tubules that support spermatogenesis [35].
EBN is also a source of complex carbohydrates, particularly in the form of glycoproteins. These include sialic acid and several oligosaccharides such as D-mannose, galactose, N-acetylgalactosamine (GalNAc), N-acetylglucosamine (GlcNAc), and N-acetylneuraminic acid (NANA) [32,36]. These glycans may support male reproductive function by facilitating cell–cell recognition, sperm–oocyte interaction, and protection of sperm membranes from oxidative damage [37].
In addition, EBN contains minerals, most notably sodium and calcium in higher amounts than magnesium, potassium, phosphorus, and iron [10,11]. Calcium, in particular, is essential for sperm motility and acrosome reaction [38], while magnesium and zinc are known cofactors in spermatogenesis and testosterone biosynthesis [39].
Interestingly, reproductive hormones, including testosterone, estradiol (E2), progesterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), and prolactin, have also been identified in EBN extracts [40]. Though present in small amounts, these hormonal components may contribute to its traditional reputation for improving vitality and reproductive health.
This diverse composition provides not only basic nutrition but also bioactive compounds that may synergistically support male reproductive physiology through energy provision, antioxidant protection, hormonal modulation, and spermatogenic maintenance. A detailed summary of its major constituents, including proteins, carbohydrates, amino acids, minerals, and vitamins, is presented in Table 1.

Table 1.
Composition of EBN and its potential benefit to the male reproductive system.
3. Characteristics of EBN Attributed to Male Reproductive Function
The beneficial effects of EBN on male reproductive health can be understood through three major characteristics that directly support reproductive function: (1) its proliferative effect on germ cells, (2) the presence of reproductive hormonal content, and (3) its potent antioxidant activity.
3.1. Proliferative Effect
EBN has been shown to exhibit biological activity similar to epidermal growth factor (EGF) [44], a single-chain polypeptide of 53 amino acids first described by Stanley Cohen in 1962 [45]. Since the discovery of EGF-like activity in EBN, multiple approaches have been employed to investigate its proliferative potential using different cell models.
Supplementation of processed and unprocessed EBN at five ppm promoted significant proliferation of human colonic adenocarcinoma (Caco-2) cells [24]. While the proliferative effect was initially attributed to EGF, further evidence suggested that sialic acid, GalNAc, and GlcNAc, the primary carbohydrate moieties in EBN, may also contribute to this characteristic. These glycans are known to mediate cell–cell and carbohydrate–protein interactions as well as downstream signalling [45,46], which are critical for cell proliferation.
EBN’s proliferative effects were observed through various in vitro studies. For instance, EBN supplementation accelerated the proliferation of spleen B-cells, while T-cells showed little response [47]. Furthermore, EBN was added as a serum replacement at varying concentrations to human adipose-derived stem cells (hADSCs), normal human fibroblasts (NHFs), and cancer cell lines (MCF-7 and Hep2B). At 2000 ppm, EBN increased proliferation of hADSCs and NHFs by 34% and 38%, respectively. In the meantime, no proliferative effect on cancer cell lines was recorded [25].
The differing results observed between Caco-2 [24] and other cancer cell lines [25] suggest that EBN’s proliferative effects may depend on factors such as cell type, EBN concentration, type and source of EBN, as well as the EBN doses applied under different experimental conditions. Although EBN tends to promote cell growth in normal cells, these findings should be interpreted with caution. Current evidence is not yet sufficient to confirm that EBN selectively supports normal cell proliferation or that it is inherently safe in relation to cancer risk. Therefore, further in vivo studies are essential to clarify its biological effects, including detailed dose–response evaluations, long-term exposure assessments, and comprehensive toxicological testing to ensure the safety of EBN consumption in humans.
Nevertheless, the proliferative effect of EBN appears to involve activation of the activator protein-1 pathway (AP-1) and nuclear factor kappa B (NF-κB), which in turn stimulate production of interleukin-6 (IL-6) and vascular endothelial growth factor (VEGF) through the p44/42 and p38 mitogen-activated protein kinase (MAPK) pathways [20,25].
The relevance of these findings to male reproduction lies in the fact that spermatogenesis depends heavily on cell proliferation within the testis. Spermatozoa are produced in the seminiferous tubules, where spermatogonia stem cells (SSCs) undergo mitosis to maintain their pool and generate progenitors for spermatogenesis [48]. SSCs can divide symmetrically to produce identical daughter cells or asymmetrically to yield one SSC and one progenitor. These divisions give rise to Apaired and Aaligned spermatogonia, collectively referred to as undifferentiated spermatogonia [49]. Upon differentiation, these cells progress through successive mitotic divisions (A1–A4, intermediate, and B spermatogonia) before entering meiosis as spermatocytes [50] (Figure 1). This highly regulated process is controlled by Sertoli cells and influenced by growth factors, such as glial cell line-derived neurotrophic factor (GDNF), fibroblast growth factor (FGF), and EGF, which activate signalling cascades including Ras-ERK, PI3K/AKT, and MAPK pathways [51,52]. Given that EBN has been shown to contain EGF and to activate MAPK signalling [25], its supplementation may support spermatogonia proliferation and enhance spermatogenesis.
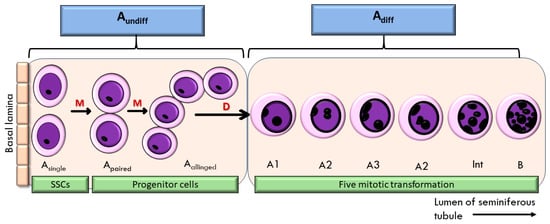
Figure 1.
Proliferation of spermatogonia stem cells (SSC) in the seminiferous tubule of the testis. M—mitosis; D—differentiation; Aundiff—undifferentiated spermatogonia; Adiff—differentiated spermatogonia.
Beyond growth factor-like activity and carbohydrate moieties, EBN also contains vitamin A [36], an essential factor for the transition of A-aligned spermatogonia into differentiating A1 spermatogonia. The active derivative of vitamin A, retinoic acid (RA), regulates this transition through receptor phosphorylation. In the absence of RA, spermatogonia differentiation and subsequent meiosis cannot proceed [53,54]. While no studies have yet examined whether RA derived from EBN directly influences germ cell proliferation in the testis, the presence of this nutrient provides an additional plausible mechanism through which EBN may support spermatogenesis.
Taken together, EBN supplementation may enhance SSC proliferation through multiple complementary mechanisms: (i) growth factor-like activity, particularly via EGF and MAPK signalling; (ii) carbohydrate-mediated cell signalling; and (iii) provision of vitamin A/RA to drive spermatogonia differentiation.
In summary, both in vitro and in vivo evidence demonstrate that EBN can stimulate proliferation while remaining safe for non-cancerous cells. Its ability to enhance proliferation and differentiation through MAPK and RA-related mechanisms, and its protective effects against environmental and chemical insults, highlight EBN’s potential role in supporting spermatogenesis and addressing oligospermia-related male infertility.
3.2. Reproductive Hormonal Content
The physiological function of the male reproductive system is tightly regulated by reproductive hormones, particularly FSH, LH, and testosterone. FSH acts on Sertoli cells to promote spermatogonia proliferation and initiate spermatogenesis [55], activating multiple signalling pathways, including cAMP/PKA, MAPK, calcium, phosphoinositide 3-kinase (PI3K), and phospholipase A2 [56].
It also regulates retinoic acid signalling to stimulate spermatogonia differentiation [57]. On the other hand, LH stimulates Leydig cells to produce testosterone, which is essential for spermatogonia development, meiosis, and spermiation [58,59].
In addition, progesterone receptors, although the hormone is often regarded as female-specific, are also present in the seminal vesicles and testes [60]. Similarly, estradiol receptors α (ERα) and β (ERβ) have been identified in germ cells and along the spermatic ducts, highlighting the importance of estrogen in testicular function [61]. Meanwhile, evidence suggests that both estradiol and prolactin have been shown to influence spermatogenesis through the hypothalamic–pituitary–gonadal (HPG) axis and to contribute to the regulation of libido and erectile function [62,63].
In line with these physiological roles, EBN has been shown to contain several reproductive hormones, including testosterone, E2, progesterone, LH, FSH, and prolactin [12]. Advanced profiling using Orbitrap LC–MS analysis further confirmed the presence of testosterone, E2, and progesterone in EBN extracts [64]. For this EBN hormonal content, it is essential to note that these hormones are present only in trace concentrations. In addition, its bioavailability following oral consumption remains uncertain. Digestion and absorption processes may further limit their capacity to influence systemic hormone levels in humans.
Nevertheless, functional studies support the presence of these hormonal contents in EBN extract. For instance, EBN administration increased serum testosterone and LH in castrated male Wistar rats [40]. Meanwhile, another study observed a non-significant dose-dependent increase in serum testosterone, FSH, and LH in male Sprague Dawley rats [64]. Furthermore, 8 weeks of EBN supplementation in female rats significantly elevated E2, progesterone, and prolactin, with the highest dose (120 mg/kg/day) producing the most pronounced effects [65]. All these studies administered EBN via oral or intragastric routes.
Collectively, these findings suggest that EBN consumption may influence systemic hormone levels. In the context of male reproductive physiology, alterations in circulating hormone concentrations could, in turn, affect spermatogenesis and the HPG feedback loop. For instance, if systemic testosterone levels rise to a physiologically active range following EBN supplementation, this may suppress local intratesticular testosterone synthesis through negative feedback on gonadotropin release [66]. Such suppression could potentially impair spermatogenesis, as locally produced testosterone within the testis is essential for Sertoli cell function and germ cell development [67].
Therefore, while the hormonal components of EBN may contribute to reproductive regulation, their effects must be carefully balanced to ensure they do not disrupt the physiological homeostasis required for optimal sperm production and function. However, since these observations are based solely on animal studies, further evaluation is needed to determine whether such effects are physiologically significant in humans.
Although the systemic relevance of these hormonal effects remains uncertain, the presence of these reproductive hormones in EBN may partly explain the longstanding traditional claims that EBN enhances libido. However, its influence on libido remains hypothetical, as no direct evidence from animal or human studies is currently available. Further experimental and clinical investigations are needed to determine whether EBN supplementation has any measurable effect on sexual desire or performance.
Beyond this cultural perspective, the hormonal content of EBN highlights its potential as a supportive intervention for male infertility associated with hormonal imbalances. By influencing key regulators such as testosterone, LH, and FSH, EBN supplementation could help restore hormonal homeostasis and improve reproductive function. Nevertheless, its use should be approached cautiously in healthy men without infertility, as unnecessary exposure to exogenous hormones may carry unintended risks.
3.3. Antioxidant Activity
Its antioxidative activity represents the third prominent characteristic of EBN relevant to male reproductive function. The antioxidative activity of EBN was first demonstrated when its supplementation protected HEPG2 cells against toxicity induced by hydrogen peroxide (H2O2). Moreover, it exhibited free radical-scavenging capacity in 2,2′-azino-bis [3-ethylbenzothiazoline-6-sulphonic acid] (ABTS) and oxygen radical absorbance capacity (ORAC) assay [18].
Consistently, EBN prevented SH-SY5Y cells exposed to neurotoxin 6-hydroxypamine (6-OHDA), a model to imitate the onset of Parkinson’s disease [19]. Furthermore, EBN supplementation showed an increase in the expression of hepatic antioxidant genes, including superoxide dismutase (SOD) 1 and 2, glutathione reductase (Gsr), and glutathione peroxidase (Gpx), in a dose-dependent manner in rats that were fed with a high-fat diet [17].
Several EBN constituents appear to mediate these antioxidant effects. A comparison of the amino acid profile between EBN and chicken eggs showed that EBN demonstrated stronger 1,1-diphenyl-2-picrylhydrazyl (DPPH) and ABTS, primarily attributed to its high content of histidine, proline, phenylalanine, and tryptophan [68]. In addition, two pentapeptides derived from EBN hydrolysates, including Pro-Phe-His-Pro-Tyr and Leu-Leu-Gly-Asp-Pro, also demonstrated high ORAC value and could significantly protect HEPG2 cells from H2O2-induced oxidative damage [69]. These findings support the notion that the protein-rich composition of EBN is a valuable natural source of antioxidants [70].
In addition, bioactive compounds such as ovotransferrin, lactoferrin [71], sialic acid [72], and several vitamins, including vitamins A, D, and C, may also contribute to EBN’s antioxidative activity [73]. Thus, current evidence suggests that EBN exerts its antioxidant effects mainly through non-enzymatic mechanisms, although whether it also engages enzymatic antioxidant pathways remains to be clarified.
Numerous reports have linked male infertility to an increase in reactive oxygen species (ROS) and a decrease in antioxidant levels, a condition known as oxidative stress [74,75]. Elevated ROS levels were reported to impair sperm motility through the increase in oxidative stress in the mitochondria of asthenozoospermic cases [76,77]. The decreased seminal antioxidant levels, on the other hand, were associated with increased single DNA breaks in teratozoospermic instances [78]. Additionally, specific diseases like varicocele [79], cryptorchidism [80], and the presence of bacteria and/or leukocytes in semen [81] are also connected to elevated ROS levels.
Given the growing recognition of oxidative stress in male infertility, numerous antioxidants have been investigated. This includes vitamin E [82], ascorbic acid [83], coenzyme Q10 [84], lycopene [85], and melatonin [86]. Various natural products were also evaluated, and this includes honey [87,88,89], curcumin [90], and broccoli [91]. Most of the studies employed oral supplementation [84,85,86,87,88,89] to assess the antioxidant properties of the abovementioned natural products and substances in clinical and animal studies. However, some studies used different approaches, such as adding to drinking water [92] or using a substrate in sperm preparation medium [90,93].
These approaches could be adapted to evaluate the antioxidative potential of EBN to mitigate oxidative stress in the male reproductive system. Oral administration remains the most practical method, consistent with its traditional consumption as soup, and is increasingly supported by modern formulations such as energy drinks [30]. Following supplementation, the level of oxidative stress in seminal plasma and its effect on the quality of sperm for clinical research subjects should be assessed. In animal research, a comprehensive assessment can be carried out by evaluating the oxidative status of blood serum and testicular homogenate, assessing testicular tissue sections by using proper antioxidant/oxidant probes, and analysing the gene expression levels of any relevant antioxidant system in the testis. As oxidative stress is a major contributor to male infertility, the presence of natural antioxidants in EBN further underscores its potential as a dietary supplement to promote male reproductive health, particularly in modern contexts where oxidative stress is prevalent.
The association between these three EBN characteristics and male reproductive function is illustrated in Figure 2. In addition, Table 2 summarizes the key molecules underlying each characteristic along with their probable effects on male reproductive outcomes.
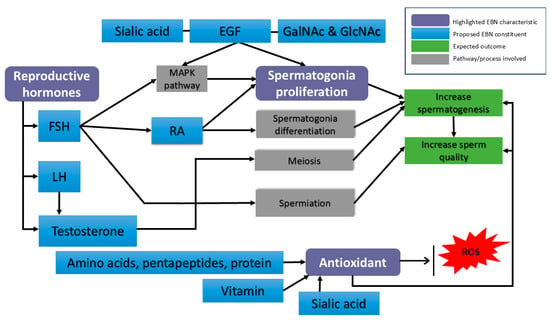
Figure 2.
Proposed molecular mechanisms of EBN action in male reproductive physiology. Purple boxes represent the EBN characteristic. Blue boxes represent potential constituents in EBN that might benefit spermatogenesis. EGF—Epidermal growth factor; FSH—follicle-stimulating hormone; LH—luteinizing hormone; RA—retinoic acid; ROS—reactive oxygen species; MAPK pathway—mitogen-activated protein kinase.

Table 2.
Summary of EBN characteristics, key molecules, and benefits for male reproductive function.
4. Scientific Evidence of EBN on the Male Reproductive System
Although reports on the effects of EBN on male reproductive health are relatively sparse, the existing evidence shows promising outcomes and underscores EBN’s potential as a male fertility supplement.
4.1. In Vivo Studies
The presence of reproductive hormones, including testosterone, E2, progesterone, LH, FSH, and prolactin in EBN extract was first identified by Ma and Liu [12]. The biological relevance of these hormones, particularly testosterone, was demonstrated when EBN was administered at 9 mg/kg/day to castrated male rats [40]. At this dose, EBN promoted penile, prostatic, and seminal vesicle development, enhanced serum testosterone and LH secretion, and increased endothelial nitric oxide synthase expression. These observations were comparable to those in rats injected intramuscularly with testosterone propionate, leading to the conclusion that the testosterone content of EBN is the key bioactive compound responsible for the observed effects. Such findings are consistent with the established role of testosterone in regulating erectile function through nitric oxide synthase pathways in penile erectile tissue [94,95,96,97].
Beyond erectile function, another vital dimension of male fertility is semen quality. Men worldwide are increasingly experiencing low sperm counts, a condition known as oligospermia. According to the World Health Organisation (WHO), oligospermia is defined as a sperm concentration below 2 × 106/mL [98]. A recent study by Jaffar et al. [64] reported that sperm concentration in adult Sprague Dawley rats increased gradually with higher doses of 250 mg/kg EBN supplementation. Furthermore, supplementation at 250 mg/kg significantly enhanced spermatogonia proliferation in rats exposed to radiofrequency electromagnetic field (RF-EMF) from a Wi-Fi router [99]. This effect was most likely mediated through hormonal regulation, particularly by FSH, rather than by modulating the c-KIT–SCF signalling pathway [99].
Complementary findings from another study showed that EBN supplementation of the same dose in Wi-Fi–exposed Sprague Dawley rat pups improved testicular oxidative stress status and sperm quality, further supporting its antioxidative role against RF-EMF–induced reproductive impairment [100]. On the same note, EBN supplementation also improved histomorphometry of the testis [101] and the hormonal profile of the male rats exposed to the RF-EMF [102].
Similarly, daily EBN supplementation at 250–1000 mg/kg for 28 days in a busulfan-induced oligospermia model significantly improved sperm concentration, seminiferous tubule diameter, germ cell height, and spermatogenesis index scores compared with busulfan-treated controls. Significantly, EBN also reduced germ cell apoptosis and upregulated p38 MAPK (MAPK14) expression, suggesting that its restorative effects are mediated through MAPK signalling [103]. Collectively, these results complement in vitro evidence and point to MAPK–PI3K–AKT pathways as central mediators of EBN’s proliferative action.
4.2. In Vitro Studies
A recent approach to exploring EBN’s fertility-enhancing potential has been its incorporation into commercial semen extenders. The incubation of stallion semen samples with EBN-supplemented E-Z mixin® (Animal Reproduction Systems, a division of Dupree, Inc., Chino, CA, USA) extender significantly increased total motility (TM) and progressive motility (PM). It also improved sperm kinematics, including average path velocity (VAP), straight-line velocity (VSL), and curvilinear velocity (VCL). However, other parameters of sperm kinematics, namely amplitude of lateral head displacement (ALH), linearity (LIN), straightness, and beat cross frequency, were not significantly affected [104]. By contrast, supplementation of EquiPlus® (Minitube, Tiefenbach, Germany) and I.N.R.A. 96® (IMV Technologies Group, L’Aigle, France) extenders with EBN did not produce significant improvements in sperm motility, progressive motility, or kinematic parameters. Similar negative findings were reported when stallion semen was incubated with EBN-supplemented INRA Freeze® (IMV Technologies Group, L’Aigle, France) and EquiPlus Freeze® Minitube, Tiefenbach, Germany) extenders [105].
All this scientific evidence was summarised in Table 3. Taken together, these findings highlight the need for further assessment of EBN’s effects on male fertility. While the evidence to date points to promising hormonal, proliferative, and antioxidative impacts, additional studies, including well-designed clinical trials, are required to validate EBN’s therapeutic potential in improving male reproductive outcomes.

Table 3.
Scientific evidence of EBN effects on the male reproductive system.
5. Concern and Safety of EBN on the Male Reproductive System
Even though EBN is an efficient cell proliferation promoter, the currently available scientific proof was generated in vitro. The primary concern with cell proliferation is the likelihood that EBN can encourage excessive cell growth, which would then initiate the development of malignant tissue. Nevertheless, Roh et al. [25] reported that no significant proliferation occurs in the human cancer cell lines of MCF-7 and Hep2B. Similarly, there is no significant growth of human breast adenocarcinoma cells (MCF-7), human alveolar adenocarcinoma cells (A549), human epithelial colorectal adenocarcinoma cells (Caco-2), and human colorectal carcinoma cells (HCT116) was reported [106]. In addition to in vitro testing on different cancer cell lines, in vitro SSC propagation can best validate the proliferative effect of EBN on spermatogenesis [107]. This cultivation method was successfully shown in rodent gonocytes and spermatogonia, but it is still difficult to apply to primate SSCs [108].
Besides an in vitro assessment of various cancer cell lines, EBN supplementation and its potential to cause abnormal proliferation of cells should be evaluated in an in vivo system. Intricate interactions occur between diverse niches in an in vivo system, which could lead to numerous outcomes. EBN has been linked to increased spermatogonia proliferation against Wi-Fi radiation [99] and considerable gonadotrophic cell proliferation in the pituitary gland to alleviate lead toxicity in female rats [109]. Both investigations examined in vivo cell proliferation and found no evidence of aberrant tissue growth. However, additional monitoring is necessary when alternative EBN sources, types, and doses are implemented.
Besides the monitoring of any abnormal tissue growth, the potential of EBN to mitigate the decline in male reproductive status can be achieved by using a few well-established models of male fertility in rodents. These models include the busulfan-induced model [110,111], cimetidine-induced model [112], and nicotine-induced model [113]. All of these in vivo models were proven to decrease semen parameters such as sperm motility, sperm morphology, and sperm count. It also disturbs sexual hormones, increases testicular oxidative stress, and alters the histological structure of the seminiferous tubule [111,112,113].
The hormonal content of EBN may also need to be evaluated. The most noticeable hormonal properties of EBN are its estrogenic properties [114,115]. Estrogen is thought to play a more dominant role in females than in the male reproductive system. However, the primary form of estrogen, E2, also has a significant effect on male sexual function. For instance, the libido of a male depends on the balance between testosterone and E2 (T: E). An increase in E2 level leading to a low T: E ratio may negatively affect libido [63,116] and cause erectile dysfunction [117,118].
EBN also contains prolactin, which is another well-known female hormone that is crucial for milk production during lactation [119]. Although prolactin receptors are recognised in interstitial cells, Sertoli cells, and germ cells, their role in maintaining the male reproductive system is still debatable [62]. However, its excessive level is known to contribute to male infertility by impairing sperm production [120], causing erectile dysfunction and a decrease in libido [121].
Given that the reproductive hormone strictly regulates all the reproductive functions in males, any additional external source of hormones may alter the normal male physiological reproductive function. Therefore, monitoring the hormonal content of EBN, particularly its estrogenic action, is essential so that the beneficial effects of EBN on the male reproductive system can be highlighted rather than its adverse effects.
Toxicity evaluations of EBN supplementation are sparse. To date, Hou et al. [27] monitored the effect of 0.3, 0.6, and 1.2 g/kg on a menopausal animal model. Their study reported that all doses cause no changes in the levels of liver alanine transaminase (ALT), alkaline phosphatase (ALP), and gamma-glutamyl transferase (GGT). Urea and creatinine also show no significant changes in monitoring the kidney function upon receiving the doses. No reproductive toxicity testing of EBN has ever been performed. Given that EBN contains numerous bioactive compounds, high levels of nitrate and nitrite [122], heavy metals [123], and adulteration [124], this issue needs to be addressed immediately.
To address this concern, Malaysian regulations have established specific maximum limits for lead, arsenic, and mercury in raw cleaned edible bird’s nest (EBN). In contrast, other jurisdictions generally apply broader food-group limits. Nevertheless, studies have reported substantial variability in contaminant levels among EBN samples, with some exceeding these national thresholds [125]. These findings highlight the necessity for continuous surveillance, standardised processing practices, and stringent quality assurance measures to ensure the safety and regulatory compliance of EBN intended for human consumption. This concern has been listed in Table 4.

Table 4.
Toxicological issues, current regulatory limits, and research needs for EBN.
6. Future Perspective of EBN on the Male Reproductive System
According to the available evidence and safety concerns regarding EBN, particularly as it relates to the male reproductive system, there is currently a dearth of information regarding the effects of EBN on male infertility. To date, EBN’s effects are limited to its ability to treat erectile dysfunction, improve several sperm parameters, and preserve spermatogonia proliferation against Wi-Fi exposure. Nevertheless, the data of each study remains limited.
Extensive research still needs to be conducted, particularly to determine the potential of EBN in addressing issues with male infertility. Although a study by Jaffar et al. [64] indicated an improvement in sperm quality, the impact of EBN should be further assessed by using known infertile models. An induced animal model can be used to cause infertility problems such as oligozoospermia, asthenozoospermia, or teratozoospermia. Work involving such models will contribute to the knowledge of EBN’s potential to address male infertility issues.
Future research should assess the effectiveness of EBN in reducing oxidative stress in male infertility cases. The male reproductive system can experience oxidative stress in a variety of ways. One of them is to adopt elements of contemporary lifestyles like cigarette smoking, consuming a high-fat diet to induce obesity, or developing metabolic disorders such as diabetes (Figure 3). These elements contribute to male infertility.
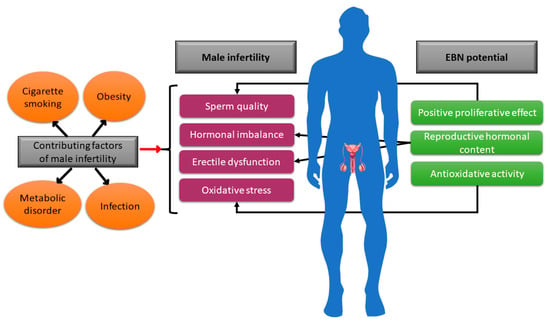
Figure 3.
Depiction of a few male infertility contributing factors and the possible action of EBN to augment the infertility status in males.
No study has yet evaluated how the traditional EBN claim affects male libido. Male libido may be increased by several key reproductive hormones found in EBN, thereby supporting the conventional superstition. This EBN characteristic might be helpful to attenuate male infertility linked to hormonal imbalance, which frequently occurs in obese males [128]. However, monitoring of all reproductive hormonal levels in EBN is necessary because of concerns regarding its effects on the male reproductive system. Furthermore, an evaluation of EBN toxicity, particularly about the reproductive system, is urgently needed.
Finally, a comparison of EBN and the present male infertility supplement regimen offered by fertility clinics is an interesting topic for future research. The current method of treating male infertility involves using antioxidant supplements, such as Profortil [129], coenzyme Q10 [84], and zinc [130]. Given that EBN has three or possibly more bioactive compounds in a single supplement that is essential for the reproductive system, it may become an effective food supplement for male fertility.
Author Contributions
Conceptualization, F.H.F.J. and S.F.I.; methodology, N.A.A. and S.M.M.; software, N.A.A. and S.M.M.; validation, F.H.F.J., S.F.I. and K.O.; formal analysis, K.O.; investigation, F.H.F.J., N.A.A. and S.M.M.; resources, F.H.F.J., S.F.I. and K.O.; data curation, K.O.; writing—original draft preparation, F.H.F.J.; writing—review and editing, F.H.F.J.; visualization, S.F.I. and K.O.; supervision, S.F.I. and K.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data sets were generated or analyzed during the present study.
Acknowledgments
All authors would like to acknowledge Department of Physiology, Faculty of Medicine, UKM, for the support to complete this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ABTS | 2,2′-azino-bis [3-ethylbenzothiazoline-6-sulphonic acid] |
| AP-1 | Activator protein-1 pathway |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
| EBN | Edible Bird Nest |
| E2 | Estradiol |
| EGF | Epidermal growth factor |
| ERα | Estradiol receptors α |
| ERβ | Estradiol receptors β |
| FGF | Fibroblast growth factor |
| FSH | Follicle-stimulating hormone |
| GalNAc | N-acetylgalactosamine |
| GlcNAc | N-acetylglucosamine |
| GDNF | Glial cell line-derived neurotrophic factor |
| Gpx | Glutathione peroxidase |
| Gsr | Glutathione reductase |
| H2O2 | Hydrogen peroxide |
| hADSCs | Human adipose-derived stem cells |
| IL-6 | Interleukin-6 |
| LC-MS | Liquid chromatography mass spectrometry |
| LH | Luteinizing hormone |
| MAPK | Mitogen-activated protein kinase |
| NF-κB | Nuclear factor kappa B |
| NANA | N-acetylneuraminic acid |
| ORAC | Oxygen radical absorbance capacity |
| PI3K | Phosphoinositide 3-kinase |
| RA | Retinoic acid |
| RF-EMF | Radiofrequency electromagnetic field |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| SSCs | Spermatogonia stem cells |
| VEGF | Vascular endothelial growth factor |
References
- Looi, Q.H.; Omar, A.R. Swiftlets and edible bird’s nest industry in Asia. Pertanika J. Sch. Res. Rev. 2016, 2, 32–48. [Google Scholar]
- Babji, A.; Nurfatin, M.; Etty Syarmila, I.; Masitah, M. Secrets of edible bird nest. UTAR Agric. Sci. J. 2015, 1, 32–37. [Google Scholar]
- Chua, L.S.; Zukefli, S.N. A comprehensive review of edible bird nests and swiftlet farming. J. Integr. Med. 2016, 14, 415–428. [Google Scholar] [CrossRef]
- Kang, N.; Hails, C.; Sigurdsson, J. Nest construction and egg-laying in Edible-nest Swiftlets Aerodramus spp. and the implications for harvesting. IBIS 1991, 133, 170–177. [Google Scholar] [CrossRef]
- Green, J. The edible bird’s-nest, or nest of the Java swift (Collocalia nidifica). J. Physiol. 1885, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Home, E. Some account of the nests of the Java swallow, and of the glands that secrete the mucus of which they are composed. Proc. R. Soc. Lond. 1833, 2, 277–278. [Google Scholar]
- Marshall, A.; Folley, S. The origin of nest-cement in edible-nest swiftlets (Collocalia spp.). J. Zool. 1956, 126, 383–390. [Google Scholar] [CrossRef]
- Hamzah, Z.; Ibrahim, N.H.; Sarojini, J.; Hussin, K.; Hashim, O.; Lee, B.-B. Nutritional properties of edible bird nest. J. Asian Sci. Res. 2013, 3, 600. [Google Scholar]
- Marcone, M.F. Characterization of the edible bird’s nest the “Caviar of the East”. Food Res. Int. 2005, 38, 1125–1134. [Google Scholar] [CrossRef]
- Saengkrajang, W.; Matan, N.; Matan, N. Nutritional composition of the farmed edible bird’s nest (Collocalia fuciphaga) in Thailand. J. Food Compos. Anal. 2013, 31, 41–45. [Google Scholar] [CrossRef]
- Norhayati, M.; Azman, O.; Nazaimoon, W. Preliminary study of the nutritional content of Malaysian edible bird’s nest. Malays. J. Nutr. 2010, 16, 389–396. [Google Scholar]
- Ma, F.; Liu, D. Sketch of the edible bird’s nest and its important bioactivities. Food Res. Int. 2012, 48, 559–567. [Google Scholar] [CrossRef]
- Biddle, F.; Belyavin, G. The haemagglutination inhibitor in edible bird-nest: Its biological and physical properties. Microbiology 1963, 31, 31–44. [Google Scholar] [CrossRef]
- Saengkrajang, W.; Matan, N.; Matan, N. Antimicrobial activities of the edible bird’s nest extracts against food-borne pathogens. Thai J. Agric. Sci. 2011, 44, 326–330. [Google Scholar]
- Aswir, A.; Wan Nazaimoon, W. Effect of edible bird’s nest on cell proliferation and tumor necrosis factor-alpha (TNF-α) release in vitro. Int. Food Res. J. 2011, 18, 1123–1127. [Google Scholar]
- Vimala, B.; Hussain, H.; Nazaimoon, W.W. Effects of edible bird’s nest on tumour necrosis factor-alpha secretion, nitric oxide production and cell viability of lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Agric. Immunol. 2012, 23, 303–314. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Hou, Z.; Abdullah, M.A.; Ideris, A.; Ismail, N. Edible Bird’s Nest attenuates high fat diet-induced oxidative stress and inflammation via regulation of hepatic antioxidant and inflammatory genes. BMC Complement. Altern. Med. 2015, 15, 310. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M. In vitro bioaccessibility and antioxidant properties of edible bird’s nest following simulated human gastro-intestinal digestion. BMC Complement. Altern. Med. 2014, 14, 468. [Google Scholar] [CrossRef] [PubMed]
- Yew, M.Y.; Koh, R.Y.; Chye, S.M.; Othman, I.; Ng, K.Y. Edible bird’s nest ameliorates oxidative stress-induced apoptosis in SH-SY5Y human neuroblastoma cells. BMC Complement. Altern. Med. 2014, 14, 391. [Google Scholar] [CrossRef]
- Kim, K.C.; Kang, K.A.; Lim, C.M.; Park, J.H.; Jung, K.S.; Hyun, J.W. Water extract of edible bird’s nest attenuated the oxidative stress-induced matrix metalloproteinase-1 by regulating the mitogen-activated protein kinase and activator protein-1 pathway in human keratinocytes. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 347–354. [Google Scholar] [CrossRef]
- Nakagawa, H.; Hama, Y.; Sumi, T.; Li, S.C.; Maskos, K.; Kalayanamitra, K.; Mizumoto, S.; Sugahara, K.; Li, Y.-T. Occurrence of a nonsulfated chondroitin proteoglycan in the dried saliva of Collocalia swiftlets (edible bird’s-nest). Glycobiology 2007, 17, 157–164. [Google Scholar] [CrossRef][Green Version]
- Chua, K.-H.; Lee, T.-H.; Nagandran, K.; Md Yahaya, N.H.; Lee, C.-T.; Tjih, E.T.T.; Abdul Aziz, R. Edible Bird’s nest extract as a chondro-protective agent for human chondrocytes isolated from osteoarthritic knee: In vitro study. BMC Complement. Altern. Med. 2013, 13, 19. [Google Scholar] [CrossRef]
- Zainal Abidin, F.; Hui, C.K.; Luan, N.S.; Mohd Ramli, E.S.; Hun, L.T.; Abd Ghafar, N. Effects of edible bird’s nest (EBN) on cultured rabbit corneal keratocytes. BMC Complement. Altern. Med. 2011, 11, 94. [Google Scholar] [CrossRef] [PubMed]
- Aswir, A.R.; Nazaimoon, W.M.W. Effect of edible bird’s nest on Caco-2 cell proliferation. J. Food Technol. 2010, 8, 126–130. [Google Scholar] [CrossRef]
- Roh, K.-B.; Lee, J.; Kim, Y.-S.; Park, J.; Kim, J.-H.; Lee, J.; Park, D. Mechanisms of Edible Bird’s Nest Extract-Induced Proliferation of Human Adipose-Derived Stem Cells. eCAM 2012, 2012, 797520. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, N.; Matsumoto, M.; Bukawa, W.; Chiji, H.; Nakayama, K.; Hara, H.; Tsukahara, T. Improvement of bone strength and dermal thickness due to dietary edible bird’s nest extract in ovariectomized rats. Biosci. Biotechnol. Biochem. 2011, 75, 590–592. [Google Scholar] [CrossRef]
- Hou, Z.; Imam, M.U.; Ismail, M.; Ooi, D.J.; Ideris, A.; Mahmud, R. Nutrigenomic effects of edible bird’s nest on insulin signaling in ovariectomized rats. Drug Des. Devel Ther. 2015, 9, 4115–4125. [Google Scholar] [CrossRef]
- Hou, Z.; He, P.; Imam, M.U.; Qi, J.; Tang, S.; Song, C.; Ismail, M. Edible bird’s nest prevents menopause-related memory and cognitive decline in rats via increased hippocampal sirtuin-1 expression. Oxid. Med. Cell. Longev. 2017, 2017, 7205082. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Lin, X.; Zhang, D.; Yin, J.; Yuan, M.; Zhang, W.; Li, R.; Xu, B.; Wang, D.; et al. Maternal edible bird’s nest diet improves learning and memory function of offspring rats via the ERK-CREB-BDNF pathway. J. Funct. Foods 2023, 108, 105757. [Google Scholar] [CrossRef]
- Lee, T.H.; Wani, W.A.; Lee, C.H.; Cheng, K.K.; Shreaz, S.; Wong, S.; Hamdan, N.; Azmi, N.A. Edible bird’s nest: The functional values of the prized animal-based bioproduct from Southeast Asia–A review. Front. Pharmacol. 2021, 12, 626233. [Google Scholar] [CrossRef]
- Herkommer, K.; Meissner, V.H.; Dinkel, A.; Jahnen, M.; Schiele, S.; Kron, M.; Ankerst, D.P.; Gschwend, J.E. Prevalence, lifestyle, and risk factors of erectile dysfunction, premature ejaculation, and low libido in middle-aged men: First result of the Bavarian men’s health-study. Andrology 2024, 12, 801–808. [Google Scholar] [CrossRef]
- Kathan, R.H.; Weeks, D.I. Structure studies of collocalia mucoid: I. Carbohydrate and amino acid composition. Arch. Biochem. Biophys. 1969, 134, 572–576. [Google Scholar] [CrossRef]
- Seow, E.K.; Ibrahim, B.; Muhammad, S.A.; Lee, L.H.; Cheng, L.H. Differentiation between house and cave edible bird’s nests by chemometric analysis of amino acid composition data. LWT 2016, 65, 428–435. [Google Scholar] [CrossRef]
- Voigt, A.L.; Thiageswaran, S.; de Lima e Martins Lara, N.; Dobrinski, I. Metabolic Requirements for Spermatogonial Stem Cell Establishment and Maintenance In Vivo and In Vitro. Int. J. Mol. Sci. 2021, 22, 1998. [Google Scholar] [CrossRef]
- Albaugh, V.L.; Mukherjee, K.; Barbul, A. Proline precursors and collagen synthesis: Biochemical challenges of nutrient supplementation and wound healing. J. Nutr. 2017, 147, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Yu-Qin, Y.; Liang, X.; Hua, W.; Hui-Xing, Z.; Xin-Fang, Z.; Bu-Sen, L. Determination of edible bird’s nest and its products by gas chromatography. J. Chromatogr. Sci. 2000, 38, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; You, S.; Xu, Y.; Shi, W.; Zhu, B.; Shen, J.; Wu, J.; Li, C.; Chen, Z.; Su, Y.; et al. Precision glycoproteomics reveals distinctive N-glycosylation in human spermatozoa. Mol. Cell. Proteom. 2022, 21, 100214. [Google Scholar] [CrossRef]
- Torrezan-Nitao, E.; Brown, S.G.; Mata-Martínez, E.; Treviño, C.L.; Barratt, C.; Publicover, S. [Ca2+]i oscillations in human sperm are triggered in the flagellum by membrane potential-sensitive activity of CatSper. Hum. Reprod. 2021, 36, 293–304. [Google Scholar] [CrossRef]
- Maciejewski, R.; Radzikowska-Büchner, E.; Flieger, W.; Kulczycka, K.; Baj, J.; Forma, A.; Flieger, J. An Overview of essential microelements and common metallic nanoparticles and their effects on male fertility. Int. J. Environ. Res. Public Health 2022, 19, 11066. [Google Scholar] [CrossRef]
- Ma, F.; Liu, D.-C.; Dai, M.-X. The effects of the edible bird’s nest on sexual function of male castrated rats. Afr. J. Pharm. Pharmacol. 2012, 6, 2875–2879. [Google Scholar] [CrossRef]
- Noor, H.S.M.; Babji, A.; Lim, S.J. Nutritional composition of different grades of edible bird’s nest and its enzymatic hydrolysis. AIP Conf. Proc. 2018, 1940, 020088. [Google Scholar]
- Lu, Y.; Han, D.; Wang, J.; Wang, D.; He, R.; Han, L. Study on the main ingredients of the three species of edible swift’s nest of Yunnan Province. Zool. Res. 1995, 16, 385–391. [Google Scholar]
- Quek, M.C.; Chin, N.L.; Yusof, Y.A.; Law, C.L.; Tan, S.W. Characterization of edible bird’s nest of different production, species and geographical origins using nutritional composition, physicochemical properties and antioxidant activities. Food Res. Int. 2018, 109, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S. Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J. Biol. Chem. 1962, 237, 1555–1562. [Google Scholar] [CrossRef]
- Gupta, R.; Leon, F.; Rauth, S.; Batra, S.K.; Ponnusamy, M.P. A systematic review on the implications of O-linked glycan branching and truncating enzymes on cancer progression and metastasis. Cells 2020, 9, 446. [Google Scholar] [CrossRef]
- Ghosh, S. Sialic acid and biology of life: An introduction. In Sialic Acids and Sialoglycoconjugates in the Biology of Life, Health and Disease; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–61. [Google Scholar]
- Zhao, R.; Li, G.; Kong, X.-J.; Huang, X.-Y.; Li, W.; Zeng, Y.-Y.; Lai, X.-P. The improvement effects of edible bird’s nest on proliferation and activation of B lymphocyte and its antagonistic effects on immunosuppression induced by cyclophosphamide. Drug Des. Dev. Ther. 2016, 10, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Kubota, H. Heterogeneity of Spermatogonial Stem Cells. In Stem Cells Heterogeneity in Different Organs. Advances in Experimental Medicine and Biology; Birbrair, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 225–242. [Google Scholar]
- Mäkelä, J.-A.; Hobbs, R.M. Molecular regulation of spermatogonial stem cell renewal and differentiation. Reproduction 2019, 158, R169–R187. [Google Scholar] [CrossRef]
- Griswold, M.D. Spermatogenesis: The commitment to meiosis. Physiol. Rev. 2016, 96, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.H.; Hao, S.L.; Yang, W.X. Regulation of spermatogonial stem cell self-renewal and proliferation in mammals. Histol. Histopathol. 2022, 37, 825–838. [Google Scholar]
- Lee, R.; Park, H.-J.; Lee, W.-Y.; Han, M.-G.; Park, J.H.; Moon, J.; Kwon, D.A.; Song, H. Effect of epidermal growth factor on the colony-formation ability of porcine spermatogonial germ cells. Biotechnol. Bioprocess. Eng. 2021, 26, 677–687. [Google Scholar] [CrossRef]
- Li, X.; Long, X.-Y.; Xie, Y.-J.; Zeng, X.; Chen, X.; Mo, Z.-C. The roles of retinoic acid in the differentiation of spermatogonia and spermatogenic disorders. Clin. Chim. Acta 2019, 497, 54–60. [Google Scholar] [CrossRef]
- Griswold, M.D. Cellular and molecular basis for the action of retinoic acid in spermatogenesis. J. Mol. Endocrinol. 2022, 69, T51–T57. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-M.; Li, Z.-F.; Yang, W.-X.; Tan, F.-Q. Follicle-stimulating hormone signaling in Sertoli cells: A licence to the early stages of spermatogenesis. Reprod. Biol. Endocrinol. 2022, 20, 97. [Google Scholar] [CrossRef]
- Ruwanpura, S.M.; McLachlan, R.I.; Meachem, S.J. Hormonal regulation of male germ cell development. J. Endocrinol. 2010, 205, 117–131. [Google Scholar] [CrossRef]
- Khanehzad, M.; Abbaszadeh, R.; Holakuyee, M.; Modarressi, M.H.; Nourashrafeddin, S.M. FSH regulates RA signaling to commit spermatogonia into differentiation pathway and meiosis. Reprod. Biol. Endocrinol. 2021, 19, 4. [Google Scholar] [CrossRef]
- Zirkin, B.R.; Papadopoulos, V. Leydig cells: Formation, function, and regulation. Biol. Reprod. 2018, 99, 101–111. [Google Scholar] [CrossRef]
- O’Shaughnessy, P.J. Hormonal control of germ cell development and spermatogenesis. In Seminars in Cell & Developmental Biology; Academic Press: New York, NY, USA, 2014; pp. 55–65. [Google Scholar]
- Dmitrieva, A.D.; Morozov, I.A.; Karkhov, A.M.; Rubtsov, P.M.; Smirnova, O.V.; Shchelkunova, T.A. Distribution of progesterone receptors and the membrane component of the progesterone receptor in various organs and tissues of male and female rats. Biochem. Moscow Suppl. Ser. A 2024, 18 (Suppl. S1), S33–S47. [Google Scholar] [CrossRef]
- Rosati, L.; Falvo, S.; Chieffi Baccari, G.; Santillo, A.; Di Fiore, M.M. The aromatase–estrogen system in the testes of non-mammalian vertebrates. Animals 2021, 11, 1763. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Deshpande, S.; Balasinor, N.H. Unveiling the role of prolactin and its receptor in male reproduction. Horm. Metab. Res. 2019, 51, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440. [Google Scholar] [CrossRef]
- Jaffar, F.H.F.; Osman, K.; Hui, C.K.; Zulkefli, A.F.; Ibrahim, S.F. Edible bird’s nest supplementation improves male reproductive parameters of Sprague Dawley rat. Front. Pharmacol. 2021, 12, 631402. [Google Scholar] [CrossRef]
- Albishtue, A.A.; Yimer, N.; Zakaria, M.Z.A.; Abubakar, A.A.; Almhanawi, B.H. Effects of EBN on embryo implantation, plasma concentrations of reproductive hormones, and uterine expressions of genes of PCNA, steroids, growth factors and their receptors in rats. Theriogenology 2019, 126, 310–319. [Google Scholar] [CrossRef]
- Chung, J.Y.; Brown, S.; Chen, H.; Liu, J.; Papadopoulos, V.; Zirkin, B. Effects of pharmacologi-cally induced Leydig cell testosterone production on intratesticular testosterone and spermatogenesis. Biol. Reprod. 2019, 102, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Navanukraw, P.; Chotimanukul, S.; Kemthong, T.; Choowongkomon, K.; Chatdarong, K. Impaired testicular function without altering testosterone concentration using an anti-follicular-stimulating hormone receptor (Anti-FSHr) single-chain variable fragment (scFv) in long-tailed macaques (Macaca fascicularis). Animals 2023, 13, 2282. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.M.; Noor, H.S.M.; Chong, P.K.; Babji, A.S.; Lim, S.J. Comparison of amino acids profile and antioxidant activities between edible bird nest and chicken egg. Malays. Appl. Biol. 2019, 48, 63–69. [Google Scholar]
- Ghassem, M.; Arihara, K.; Mohammadi, S.; Sani, N.A.; Babji, A.S. Identification of two novel antioxidant peptides from edible bird’s nest (Aerodramus fuciphagus) protein hydrolysates. Food Funct. 2017, 8, 2046–2052. [Google Scholar] [CrossRef]
- Nurul Nadia, M.; Babji, A.; Ayub, M.; Nur’Aliah, D. Effect of enzymatic hydrolysis on antioxidant capacity of cave edible bird’s nests hydrolysate. Int. J. Chemtech Res. 2017, 10, 1100–1107. [Google Scholar]
- Hou, Z.; Imam, M.U.; Ismail, M.; Azmi, N.H.; Ismail, N.; Ideris, A.; Mahmud, R. Lactoferrin and ovotransferrin contribute toward antioxidative effects of Edible Bird’s Nest against hydrogen peroxide-induced oxidative stress in human SH-SY5Y cells. Biosci. Biotechnol. Biochem. 2015, 79, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Cheng, L.-J.; Shen, B.; Yuan, Z.-L.; Feng, Y.-Q.; Lu, S.-H. Antihypertensive and antioxidant properties of sialic acid, the major component of edible bird’s nests. Curr. Top. Nutraceutical Res. 2019, 17, 376–380. [Google Scholar]
- Sinbad, O.O.; Folorunsho, A.A.; Olabisi, O.L.; Ayoola, O.A.; Temitope, E.J. Vitamins as antioxidants. J. Food Sci. Nutr. Res. 2019, 2, 214–235. [Google Scholar] [CrossRef]
- Broussard, A.L.; Leader, B.; Russell, H.; Beydoun, H.; Colver, R.; Reuter, L.; Bopp, B.; Will, M.; Will, E.A.; Adaniya, G. Lifestyle factors and laboratory sperm processing techniques are correlated with sperm DNA fragmentation index, oxidative stress adducts, and high DNA stainability. Andrology 2023, 12, 1000292. [Google Scholar]
- Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From past to present: The link between reactive oxygen species in sperm and male infertility. Antioxidants 2019, 8, 616. [Google Scholar] [CrossRef]
- Lewis, S.E.; Simon, L. Clinical implications of sperm DNA damage. Hum. Fertil. 2010, 13, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Nowicka-Bauer, K.; Lepczynski, A.; Ozgo, M.; Kamieniczna, M.; Fraczek, M.; Stanski, L.; Olszewska, M.; Malcher, A.; Skrzypczak, W.; Kurpisz, M. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J. Physiol. Pharmacol. 2018, 69, 403–417. [Google Scholar]
- Ammar, O.; Mehdi, M.; Muratori, M. Teratozoospermia: Its association with sperm DNA defects, apoptotic alterations, and oxidative stress. Andrology 2020, 8, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Steger, K.; Yang, H.; Wang, H.; Hu, K.; Zhang, T.; Chen, B. A comprehensive investigation of sperm DNA damage and oxidative stress injury in infertile patients with subclinical, normozoospermic, and astheno/oligozoospermic clinical varicocoele. Andrology 2016, 4, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.; Philip, S.; Shittu, S.-T. Antioxidant activity of Moringa oleifera leaf extract on testicular oxidative stress during experimental cryptorchidism was optimal at low dose. Afr. J. Biomed. Res. 2021, 24, 109–114. [Google Scholar]
- Theam, O.C.; Dutta, S.; Sengupta, P. Role of leucocytes in reproductive tract infections and male infertility. Chem. Biol. Lett. 2020, 7, 124–130. [Google Scholar]
- Zubair, M. Effects of dietary vitamin E on male reproductive system. Asian Pac. J. Reprod. 2017, 6, 145–150. [Google Scholar] [CrossRef]
- Ahmad, G.; Agarwal, A.; Esteves, S.; Sharma, R.; Almasry, M.; Al-Gonaim, A.; AlHayaza, G.; Singh, N.; Al Kattan, L.; Sannaa, W. Ascorbic acid reduces redox potential in human spermatozoa subjected to heat-induced oxidative stress. Andrologia 2017, 49, e12773. [Google Scholar] [CrossRef]
- Alahmar, A.T.; Calogero, A.E.; Sengupta, P.; Dutta, S. Coenzyme Q10 improves sperm parameters, oxidative stress markers and sperm DNA fragmentation in infertile patients with idiopathic oligoasthenozoospermia. World J. Mens. Health 2021, 39, 346. [Google Scholar] [CrossRef]
- Nouri, M.; Amani, R.; Nasr-Esfahani, M.; Tarrahi, M.J. The effects of lycopene supplement on the spermatogram and seminal oxidative stress in infertile men: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 3203–3211. [Google Scholar] [CrossRef]
- Riviere, E.; Rossi, S.P.; Tavalieri, Y.E.; de Toro, M.M.M.; Ponzio, R.; Puigdomenech, E.; Levalle, O.; Martinez, G.; Terradas, C.; Calandra, R.S.; et al. Melatonin daily oral supplementation attenuates inflammation and oxidative stress in testes of men with altered spermatogenesis of unknown aetiology. Mol. Cell Endocrinol. 2020, 515, 110889. [Google Scholar] [CrossRef]
- Budin, S.B.; Jubaidi, F.F.; Azam, S.N.F.M.N.; Yusof, N.L.M.; Taib, I.S.; Mohamed, J. Kelulut honey supplementation prevents sperm and testicular oxidative damage in streptozotocin-induced diabetic rats. J. Teknol. 2017, 79, 89–95. [Google Scholar] [CrossRef]
- Fabunmi, O.A.; Ajibare, A.J.; Akintoye, O.O.; Olofinbiyi, B.A.; Olayaki, L.A. Honey ameliorates imbalance between reactive oxygen species and antioxidant enzymes in the testis of sleep deprived rats. Niger. Stethosc. 2021, 3, 40–48. [Google Scholar]
- Mohamed, M.; Sulaiman, S.A.; Jaafar, H.; Sirajudeen, K.N. Antioxidant protective effect of honey in cigarette smoke-induced testicular damage in rats. Int. J. Mol. Sci. 2011, 12, 5508–5521. [Google Scholar] [CrossRef]
- Tvrda, E.; Tušimová, E.; Kováčik, A.; Paál, D.; Greifova, H.; Abdramanov, A.; Lukáč, N. Curcumin has protective and antioxidant properties on bull spermatozoa subjected to induced oxidative stress. Anim. Reprod. Sci. 2016, 172, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh, M.; Akbari, A. The effects of broccoli and caraway extracts on serum oxidative markers, testicular structure and function, and sperm quality before and after sperm cryopreservation. Cryobiology 2021, 99, 11–19. [Google Scholar] [CrossRef]
- Sönmez, M.; Türk, G.; Yüce, A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology 2005, 63, 2063–2072. [Google Scholar] [CrossRef]
- Chi, H.; Kim, J.; Ryu, C.; Lee, J.; Park, J.; Chung, D.; Choi, S.; Kim, M.; Chun, E.; Roh, S. Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum. Reprod. 2008, 23, 1023–1028. [Google Scholar] [CrossRef]
- Zvara, P.; Sioufi, R.; Schipper, H.M.; Begin, L.; Brock, G. Nitric oxide mediated erectile activity is a testosterone dependent event: A rat erection model. Int. J. Impot. Res. 1995, 7, 209–219. [Google Scholar]
- Liao, M.; Huang, X.; Gao, Y.; Tan, A.; Lu, Z.; Wu, C.; Zhang, Y.; Yang, X.; Zhang, H.; Qin, X. Testosterone is associated with erectile dysfunction: A cross-sectional study in Chinese men. PLoS ONE 2012, 7, e39234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yassin, A.A.; Saad, F. Testosterone and erectile dysfunction. J. Androl. 2008, 29, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Isidori, A.M.; Buvat, J.; Corona, G.; Goldstein, I.; Jannini, E.A.; Lenzi, A.; Porst, H.; Salonia, A.; Traish, A.M.; Maggi, M. A critical analysis of the role of testosterone in erectile function: From pathophysiology to treatment—A systematic review. Eur. Urol. 2014, 65, 99–112. [Google Scholar] [CrossRef]
- Henkel, R. The sixth edition of the WHO manual for human semen analysis: A critical review and SWOT analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef]
- Jaffar, F.H.F.; Osman, K.; Hui, C.K.; Zulkefli, A.F.; Ibrahim, S.F. Long-term Wi-Fi exposure from pre-pubertal to adult age on the spermatogonia proliferation and protective effects of edible bird’s nest supplementation. Front. Physiol. 2022, 13, 828578. [Google Scholar] [CrossRef]
- Jaffar, F.H.F.; Osman, K.; Hui, C.K.; Zulkefli, A.F.; Ibrahim, F.H.F. Effect of Wi-Fi exposure and edible bird nest supplementation on the testicular oxidative stress status and sperm quality in male Sprague-Dawley rat pups. IJRR 2024, 22, 329–338. [Google Scholar] [CrossRef]
- Ibrahim, S.F.; Abu Bakar, N.F.; Paranthaman, N.S.; Gan, T.S.; Faith Childs, J.; Azhar, N.A.; Zulkefli, A.F.; Mat Ros, M.F.; Osman, K.; Jaffar, F.H.F. Testicular morphological alterations in 2.45 GHz Wi-Fi exposed rat pups and the mitigating effects of edible bird nest. Med. Health 2025, 20, 764–775. [Google Scholar]
- Maluin, S.M.; Jaffar, F.H.F.; Osman, K.; Zulkefli, A.F.; Mat Ros, M.F.; Ibrahim, S.F. Exploring edible bird nest’s potential in mitigating Wi-Fi’s impact on male reproductive health. Reprod. Med. Biol. 2024, 23, e12606. [Google Scholar] [CrossRef]
- Azhar, N.A.; Osman, K.; Jaffar, F.H.F.; Hui, C.K.; Mat Ros, M.F.; Zulkefli, A.F.; Ibrahim, S.F. Effect of edible bird nest supplementation against busulfan-induced oligospermia in adult rats. Sains Malays. 2024, 53, 1219–1234. [Google Scholar] [CrossRef]
- Al-Khaldi, K.; Yimer, N.; Al-Bulushi, S.; Haron, A.W.; Hiew, M.; Babji, A.S. A preliminary study on the effects of EZ Mixin® and EquiPlus® extenders supplemented with edible bird’s nest on the quality of chilled Arabian stallion semen. Anim. Reprod. 2021, 18, e20200027. [Google Scholar] [CrossRef] [PubMed]
- Al-Khaldi, K.; Yimer, N.; Sadiq, M.B.; Abdullah, F.F.J.B.; Babji, A.S.; Al-Bulushi, S. Edible bird’s nest supplementation in chilled and cryopreserved Arabian stallion semen. Saudi J. Biol. Sci. 2022, 29, 1577–1584. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.N.; Sani, D.; Lim, C.W.; Ideris, A.; Stanslas, J.; Lim, C.T.S. Proximate analysis and safety profile of farmed edible bird’s nest in Malaysia and its effect on cancer cells. eCAM 2020, 2020, 8068797. [Google Scholar] [CrossRef] [PubMed]
- Ibtisham, F.; Zhao, Y.; Wu, J.; Nawab, A.; Mei, X.; Li, G.; An, L. The optimized condition for the isolation and in vitro propagation of mouse spermatogonial stem cells. Biol. Futura 2019, 70, 79–87. [Google Scholar] [CrossRef]
- Ibtisham, F.; Honaramooz, A. Spermatogonial stem cells for in vitro spermatogenesis and in vivo restoration of fertility. Cells 2020, 9, 745. [Google Scholar] [CrossRef]
- Albishtue, A.A.; Yimer, N.; Zakaria, M.Z.A.; Haron, A.W.; Yusoff, R.; Almhanawi, B.H. Ameliorating effect of edible bird’s nest against lead acetate toxicity on the rat hypothalamic–pituitary–ovarian axis and expressions of epidermal growth factor and vascular endothelial growth factor in ovaries. Comp. Clin. Pathol. 2018, 27, 1257–1267. [Google Scholar] [CrossRef]
- Levi, M.; Stemmer, S.M.; Stein, J.; Shalgi, R.; Ben-Aharon, I. Treosulfan induces distinctive gonadal toxicity compared with busulfan. Oncotarget 2018, 9, 19317. [Google Scholar] [CrossRef]
- Mobarak, H.; Rahbarghazi, R.; Nouri, M.; Heidarpour, M.; Mahdipour, M. Intratesticular versus intraperitoneal injection of Busulfan for the induction of azoospermia in a rat model. BMC Pharmacol. Toxicol. 2022, 23, 50. [Google Scholar] [CrossRef]
- Al-Janabi, A.P.; Khalid, A.; Hadr, A.P. Effect of Cimetidine on semen parameters, FSH and LH in male rats. Int. J. Pharm. Bio-Med. Sci. 2023, 3, 240–244. [Google Scholar] [CrossRef]
- Efe, A.E.; Igho, O.E. Histomorphological effects of oral nicotine administration on the testes of adult wistar rats. J. Chem. Health Risks 2023, 13, 291. [Google Scholar]
- Ismail, M.; Hou, Z.P.; Stanslas, J.; Imam, M.U.; Zhang, Y.D.; Ideris, A.; Mahmud, R. Edible bird’s nest pretreatment prevents ovariectomy induced cognitive aging in Morris water maze. In Proceedings of the Edible Bird Nest Industry Conference, Putrajaya, Malaysia, 25–26 November 2014. [Google Scholar]
- Zhiping, H.; Imam, M.U.; Ismail, M.; Ismail, N.; Yida, Z.; Ideris, A.; Sarega, N.; Mahmud, R. Effects of edible bird’s nest on hippocampal and cortical neurodegeneration in ovariectomized rats. Food Funct. 2015, 6, 1701–1711. [Google Scholar] [CrossRef]
- Neto, R.P.; Nunes, R.d.S.S.; Nascimento, B.C.; Neto, C.M.B.; de Bessa Junior, J.; Srougi, M.; Nahas, W.C. Correlation between libido and testosterone: Estradiol ratio in men with sexual dysfunction. J. Sex. Med. 2022, 19, S232. [Google Scholar] [CrossRef]
- Chen, H.-R.; Tian, R.-H.; Li, P.; Chen, H.-X.; Xia, S.-J.; Li, Z. Estradiol is an independent risk factor for organic erectile dysfunction in eugonadal young men. Asian J. Androl. 2020, 22, 636–641. [Google Scholar] [CrossRef]
- Ponce, M.D.R.; Garolla, A.; Caretta, N.; De Toni, L.; Avogaro, A.; Foresta, C. Estradiol correlates with erectile dysfunction and its severity in type 2 diabetic patients. J. Diabetes Complicat. 2020, 34, 107728. [Google Scholar] [CrossRef]
- Ladyman, S.R.; Hackwell, E.C.; Brown, R.S. The role of prolactin in co-ordinating fertility and metabolic adaptations during reproduction. Neuropharmacology 2020, 167, 107911. [Google Scholar] [CrossRef]
- Dabbous, Z.; Atkin, S.L. Hyperprolactinaemia in male infertility: Clinical case scenarios. Arab. J. Urol. 2018, 16, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Salvio, G.; Martino, M.; Giancola, G.; Arnaldi, G.; Balercia, G. Hypothalamic–pituitary diseases and erectile dysfunction. J. Clin. Med. 2021, 10, 2551. [Google Scholar] [CrossRef]
- Quek, M.C.; Chin, N.L.; Yusof, Y.A.; Tan, S.W.; Law, C.L. Preliminary nitrite, nitrate and colour analysis of Malaysian edible bird’s nest. Inf. Process Agric. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Chen, J.; Lim, P.; Wong, S.; Mak, J. Determination of the presence and levels of heavy metals and other elements in raw and commercial edible bird nests. Malays. J. Nutr. 2014, 20, 377–391. [Google Scholar]
- Huang, X.; Li, Z.; Zou, X.; Shi, J.; Tahir, H.E.; Xu, Y.; Zhai, X.; Hu, X. A low cost smart system to analyze different types of edible Bird’s nest adulteration based on colorimetric sensor array. J. Food Drug Anal. 2019, 27, 876–886. [Google Scholar] [CrossRef] [PubMed]
- Yeo, B.H.; Tang, T.K.; Wong, S.F.; Tan, C.P.; Wang, Y.; Cheong, L.Z.; Lai, O.M. Potential residual contaminants in edible bird’s nest. Front. Pharmacol. 2021, 12, 631136. [Google Scholar] [CrossRef] [PubMed]
- Federation of Malaysian Manufacturers. Food Act 1983 Food (Amendment) (No. 3) Regulations 2014. Available online: https://www.fmm.org.my/images/articles/Draf-pindaan-PPM1985-Bil3-2014_draft%20food%20(amendment)%20reg%20(No%203)%202014.pdf (accessed on 18 September 2025).
- Jabatan Perkhidmatan Veterinar Malaysia, Standar Operating Procedures for Registration & Traceability System for Raw Unclean Edible-Birdnest Export to China. Available online: https://www.dvs.gov.my/dvs/resources/user_1/DVS%20pdf/SQIE/2018/SOPRUCEBN-2_Raw_unclean_primary_processing_establishment.pdf (accessed on 18 September 2025).
- Basar, M.M.; Avci, A.E. Obesity and male infertility: Energy imbalance to inflammation. Chem. Biol. Lett. 2021, 8, 162–170. [Google Scholar]
- Sun, C.G.; Da, A.L.; Sen, O.B.; Yusof, Z.; Chan, H.-K.; Balanathan, K.; Zain, M.M. Effects of 6-week micronutrient supplementation on sperm parameters and pregnancy outcomes in males with idiopathic infertility undergoing fertility interventions: A pilot cohort study. J. Pharm. Pract. Community Med. 2017, 3, 220–224. [Google Scholar] [CrossRef]
- Schisterman, E.F.; Clemons, T.; Peterson, C.M.; Johnstone, E.; Hammoud, A.O.; Lamb, D.; Carrell, D.T.; Perkins, N.J.; Sjaarda, L.A.; Van Voorhis, B.J. A randomized trial to evaluate the effects of folic acid and zinc supplementation on male fertility and livebirth: Design and baseline characteristics. Am. J. Epidemiol. 2020, 189, 8–26. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).