Abstract
Multiple Salmonella outbreaks linked to milk powders call for the need for effective pasteurization processes. Understanding the thermal inactivation kinetics of Salmonella in milk powder is crucial; however, the influence of macronutrient content (protein, fat, and carbohydrate) on these kinetics remains unclear. This study investigated the effects of common reconstitution temperature (45, 70, and 99 °C) on Salmonella survival in infant milk powder to raise public awareness about contamination risks. Seven milk powders with varying macronutrient compositions were used as model systems. After equilibrating to a uniform water activity (aw = 0.2), the thermal resistance of Salmonella was determined at 75, 80, and 85 °C. The goodness-of-fit of two primary models (log-linear and Weibull) was compared, and secondary response surface models were developed to predict the combined effects of temperature and macronutrient composition on Salmonella inactivation. Results showed that Salmonella could proliferate or be resuscitated when contaminated milk powder was reconstituted at conventional preparation temperatures (45 °C and 70 °C). Across the seven formulations, Salmonella thermal resistance (D-value) increased with protein content (10.44–90.18%) and decreased with carbohydrate content (0.35–63.24%). A significant protein–temperature interaction was observed, whereby the effect of protein content on the Salmonella D-value decreased as temperature increased from 75 to 85 °C. Fat content did not significantly affect thermal inactivation (p > 0.05). The log-linear model provided a better fit than the Weibull model in this study. Overall, this research quantifies how macronutrient composition impacts Salmonella thermal resistance and offers predictive models to improve pasteurization strategies for milk powder.
1. Introduction
In recent years, Salmonella contamination has posed a serious threat to the safety of low-moisture foods (LMFs), which are foods with water activity below 0.85 [1]. According to the Rapid Alert System for Food and Feed (RASFF), approximately 28.9% of reported foodborne Salmonella contamination cases between 2002 and 2025 were linked to LMFs [2]. Contaminated LMFs accounted for 21% of Salmonella outbreaks recorded by the U.S. Centers for Disease Control and Prevention (CDC) from 2007 to 2018 [3]. Milk powder, a typical LMF, is commonly used as an ingredient in many ready-to-eat foods (requiring no further cooking before consumption), such as confectionery [4], mixed beverages [5], seasonings [6], and nutrition bars [7]. However, milk powder is highly susceptible to Salmonella contamination, particularly due to Salmonella-harboring powder residues trapped in spray dryer wall cracks. Therefore, spray drying is usually not considered a microbial killing step. Besides, no processing step follows spray drying to pasteurize milk powders [8,9]. Poor hygiene, inadequate sanitation, and lack of process control also create opportunities for cross-contamination [10]. These pose a great threat to the microbiological safety of milk powder. In 2011, a Salmonella contamination incident in infant formula occurred in Spain, resulting in 285 infections. A similar outbreak affected at least 30 infants across 11 cities in France in 2018 [11]. Between 2020 and 2025, the RASFF reported six milk powder -related issues, with two cases being denied entry due to the detection of Salmonella [2].
Despite common assumptions, milk powder is not inherently safe from microbial hazards [12,13]. While its low water activity (aw) prevents bacterial proliferation, it simultaneously enhances the survival and thermal resistance of pathogens like Salmonella. This bacterium can persist for years in dry environments and becomes increasingly difficult to eliminate through heat as moisture levels decrease [14,15,16]. Recent Salmonella outbreaks linked to milk powder have exposed critical weaknesses in both industrial thermal processing and home preparation methods. Conventional hot-water reconstitution provides insufficient protection against these heat-adapted pathogens. Therefore, urgent research is needed to evaluate the impact of reconstitution conditions on the survival rate of Salmonella in milk powder and to develop reliable pasteurization techniques specifically designed for low-moisture dairy products.
The effectiveness of pasteurization in milk powder is influenced by multiple variables. Beyond aw, the thermal resistance of microorganisms in low-moisture foods is shaped by a combination of intrinsic and extrinsic factors. These include bacterial strain or serotype [17,18,19], inoculation methods [20,21], product geometry [22,23], product composition [9,24,25], and processing temperature [26]. Food composition—specifically the type and proportion of constituents—significantly affects thermal resistance parameters (D- and z-values). Zhang et al. [27] reported that the D-values of Salmonella Enteritidis on egg powder increased with increasing fat (0–56.7%, w.b.) and decreasing protein contents (83.59–31.81%, w.b.). Rachon et al. [12] found that at higher temperatures, the 5 log reduction time for Salmonella was slightly greater in confectionery than in chicken flour, indicating that sugars provide stronger heat protection. Certain compounds like lactose, mannitol, sucrose, and rhamnose are known to enhance Salmonella heat tolerance [9,28], whereas glucose may reduce it [28]. Previous studies have predominantly focused on examining the effects of one or two specific nutrients on Salmonella heat resistance in simplified low-moisture food matrices. However, the wide variety of milk powder products, such as whole milk powder, nonfat dry milk, whey protein powder, lactose-free infant milk, etc., which have different nutritional compositions may result in differences in bacterial heat resistance. This variability complicates the task for manufacturers who need to achieve consistent pathogen control while preserving nutritional quality across different product types. How macronutrients (proteins, fats, and total carbohydrates) in milk powder independently or synergistically influence Salmonella thermal resistance in combination with temperature remains an unresolved yet critical question for process design.
This research was designed to: (1) quantify the survival of Salmonella during the reconstitution of milk powder under various water temperatures; (2) define the role of macronutrients in shaping Salmonella inactivation kinetics; and (3) assess the synergistic effects of macronutrients and temperature on Salmonella thermal inactivation.
2. Materials and Methods
2.1. Milk Powder Products
Seven types of commercially sourced milk powder, including whole milk powder (WMP), nonfat dry milk (NFDM), whey protein powder (WPP), casein protein powder (CPP), infant milk powder (IMP), lactose-free infant milk (LFIM), and amino acid-based formula (AAF), were selected for this study. All samples, representing different production lots, were procured from Nestlé China Ltd. (Beijing, China). Upon arrival, each sample underwent proximate analysis and background microorganism testing according to the method of Wei et al. [14]. The composition of the seven powders is shown in Table 1. Additionally, the water activity of all samples was measured at 25 °C using a water activity meter ((HD-7, Wuxi Huake Instrument Co., Ltd., Wuxi, China).

Table 1.
Proximate composition (%) of whole milk powder (WMP), nonfat dry milk (NFDM), whey protein powder (WPP), casein protein powder (CPP), infant milk powder (IMP), lactose-free infant milk (LFIM), and amino acid-based formula (AAF).
2.2. Proximate Composition Analysis
Compositional analysis of the seven milk powder samples was performed using official methods [29]: ash (method 930.30), fat (method 932.06), protein (method 930.29), and moisture contents (method 927.05). The carbohydrate content was obtained by subtracting the sum of all other constituent weights from the total.
2.3. Bacterial Cultures and Inoculation Procedure
Procedures to prepare the Salmonella inoculum were similar to those of Wei et al. [14]. Salmonella enterica subsp. enterica Enteritidis PT30 was used in this study because of its relatively high tolerance to heat in LMFs [15,30]. The frozen bacterial stocks were maintained at −80 °C in trypticase soy broth (TSB; Hope Bio-technology Co., Ltd., Qingdao, China) with 0.6% (w/v) yeast extract (YE; Oxoid Limited, Basingstoke, UK) supplemented with 20% glycerol until use. After thawing a frozen Salmonella culture at 22 °C for 10 min, 1 mL was inoculated into 10 mL of TSBYE and incubated at 37 °C for 24 ± 2 h. A loopful of the overnight culture was streaked onto tryptic soy agar (TSA; Hope Bio-technology Co., Ltd., Qingdao, China) supplemented with 0.6% (w/v) yeast extract (YE). After incubation, a single isolated colony was transferred into 10 mL of TSBYE and incubated at 37 °C for 24 h for secondary enrichment. To prepare the inoculum, a 0.1 mL aliquot of the broth culture was subcultured onto TSAYE and incubated at 37 °C for 24 h. The resultant bacterial lawn was then suspended in 3 mL of 0.1% buffered peptone water (BPW; Hope Bio-technology Co., Ltd., Qingdao, China) with an L-shaped spreader. This procedure yielded a Salmonella inoculum with a final concentration of approximately 1010 CFU/mL. All sample inoculations were performed in a biosafety cabinet. Each of WMP, NFDM, WPP, CPP, IMP, LFIM, or AAF was weighed (100.0 ± 0.1 g) and placed uniformly on a sanitized aluminum tray. The seven milk powder samples were spray-inoculated with 10 mL of the prepared Salmonella inoculum, separately. Then, the milk powder was quickly stirred to promote uniform mixing with the suspension and prevent clumping of the milk powder.
2.4. Sample Equilibration and Storage Stability
To achieve a target aw of 0.20 at 25 °C, the inoculated samples were placed in a custom-built controlled-humidity chamber where the relative humidity was maintained at 20%. Water activity is one of the key environmental factors affecting microbial growth. This operation provides an experimental model for studying the survival of Salmonella under low water activity conditions. At days 0, 1, 2, 3, 4, 5, 8, 10, 16, and 20, a 3 ± 0.1 g subsample was collected from each batch of inoculated powder and placed into a sterile homogenizing bag for enumeration. The enumeration method was based on the approach described by Wei et al. [31] with minor modifications. A 10-fold dilution was performed by adding 27 mL of 0.1% BPW to each sample, followed by 5 min of homogenization. Homogenized sample were serially diluted in 9 mL of 0.1% BPW.A 100 μL aliquot of the dilution was aspirated and inoculated in duplicate onto m-TSA (TSAYE supplemented with 0.05% ammonium iron citrate, and 0.03% sodium thiosulfate) and incubated for 24 h at 37 ± 2 °C. After incubation, colonies with a black center were enumerated as Salmonella.
2.5. Reconstitution Experiment of IMP
This study explores the impact of reconstitution with water at different temperatures (45 °C, 70 °C, and boiling water) on the survival of Salmonella inoculated on IMP. After 3 days of equilibration in the chamber, inoculated IMP was mixed with water at a fixed ratio of 14%, reflecting standard infant formula preparation. Temperature-controlled water was added directly to the powder in the beaker and immediately mixed using a magnetic stirrer for 30 s to ensure homogeneity. The reconstituted milk was placed at room temperature and microbiologically analyzed after 0, 2, 4, and 6 h to evaluate Salmonella contamination levels in IMP at different consumption time points.
2.6. Isothermal Treatment
Isothermal treatments were performed on inoculated milk powder equilibrated to a aw of 0.20 ± 0.02 for 3 days using a custom thermal lethality testing device developed by Chung et al. [32]. The selected treatment temperatures of 75, 80, and 85 °C were determined through preliminary experiments to optimize between sample quality preservation and processing efficiency, as higher temperatures cause discoloration while lower temperatures require impractical processing durations. Each thermal death curve was established through six sampling time points designed to achieve a minimum 3-log reduction of Salmonella populations. Immediately following each thermal treatment, samples underwent rapid cooling in an ice-water bath for one minute to terminate thermal inactivation, with subsequent microbial enumeration conducted according to the previously detailed methodology for sample equilibration and stability assessment.
2.7. Primary Model Fitting
This study employed two primary inactivation models to describe the survival kinetics in milk powders: the log-linear model (1) and the Weibull-type model (2), following the methodology of Peleg and Cole [33]:
The log-linear model parameters were defined as follows:
and
(CFU/g) represent the concentration of Salmonella at time
and time zero, respectively;
(min) is the treatment duration; and
(min) denotes the decimal reduction time at temperature T (°C);
is the scale parameter representing the overall steepness of the survival curve, where
approaching 1 indicates the sterilization process is approximately log-linear, while
deviating from 1 suggests a complex decontamination process. The shape parameter α determines the curve’s concavity: α > 1 produces downward concavity, α < 1 creates upward concavity, and α = 1 represents approaching a straight line, reverting to the first-order kinetic model.
Model performance was assessed with the root mean squared error (RMSE) and the corrected Akaike Information Criterion (AICc; [34]).
In this formulation,
and
denote the observed and model-predicted logarithmic microbial populations, respectively, while
represents the observation count.
is the sum of squares of the residuals, and
equals the number of estimated parameters plus one. Reduced RMSE and AICc values indicate enhanced model prediction accuracy [34,35].
2.8. Secondary Model Fitting
The combined effects of temperature and nutritional composition on the thermal inactivation kinetics of Salmonella in seven milk powder varieties were examined via Response Surface Methodology (RSM; Equation (5)). The model incorporated only factors demonstrating statistical significance (p < 0.05). All RSM parameters were estimated using analysis of variance and response surface functions implemented in the open-source statistical software R (software R (Version 4.5.0, https://www.R-project.org/, accessed on 10 June 2025; [36]).
where Mac represents macronutrients like proteins, carbohydrates, and lipids.
2.9. Statistical Analysis
All experiments were conducted in triplicate. Data are presented as mean ± standard deviation (SD). Microsoft® Excel 2010 was used for primary data processing. Additionally, the model analysis described in Section 2.7 and Section 2.8 was implemented using the Python® 3.10 (Python Software Foundation, Wilmington, DE, USA). Data visualization was performed using Origin® 2017 (Origin Lab Corp., Northampton, MA, USA). Statistical significance was evaluated by analysis of variance (ANOVA) in SPSS® 19 (IBM Co., Chicago, IL, USA), with a p-value of less than 0.05 considered significant.
3. Results
3.1. Proximate Composition Estimation
The composition of the seven powders is shown in Table 1. Different milk powders were selected to represent the macronutrient compositions in commercially available powder products. WMP (27.46% fat) and NFDM (<1% fat) were compared to evaluate the effect of fat on microbial thermal resistance WPP and CPP, with protein contents of 88.18% and 90.18% respectively, represent high-protein, low-fat and low-carbohydrate milk powders. Therefore, they can be used to assess the independent effect of protein on the thermal resistance of microorganisms Furthermore, WPP and CPP differ in protein type—the former contains whey protein while the latter consists of casein, enabling evaluation of how different protein sources affect microbial thermal resistance.
IMP, LFIM, and AAF have carbohydrate contents of 57.59%, 58.55%, and 63.24% respectively, representing high carbohydrate content milk powders while maintaining similar lipid and protein contents. Compared with other milk powders, the independent effect of carbohydrate on the thermal resistance of microorganisms can be assessed. Specifically, LFIM and AAF were strategically selected to isolate the effects of lactose and hydrolysis degree, respectively.
The differential composition of the selected milk powders enabled a comparative analysis of the effects exerted by fat, protein type, and carbohydrate source on the thermal resistance of Salmonella.
3.2. Survival of Salmonella After Reconstitution
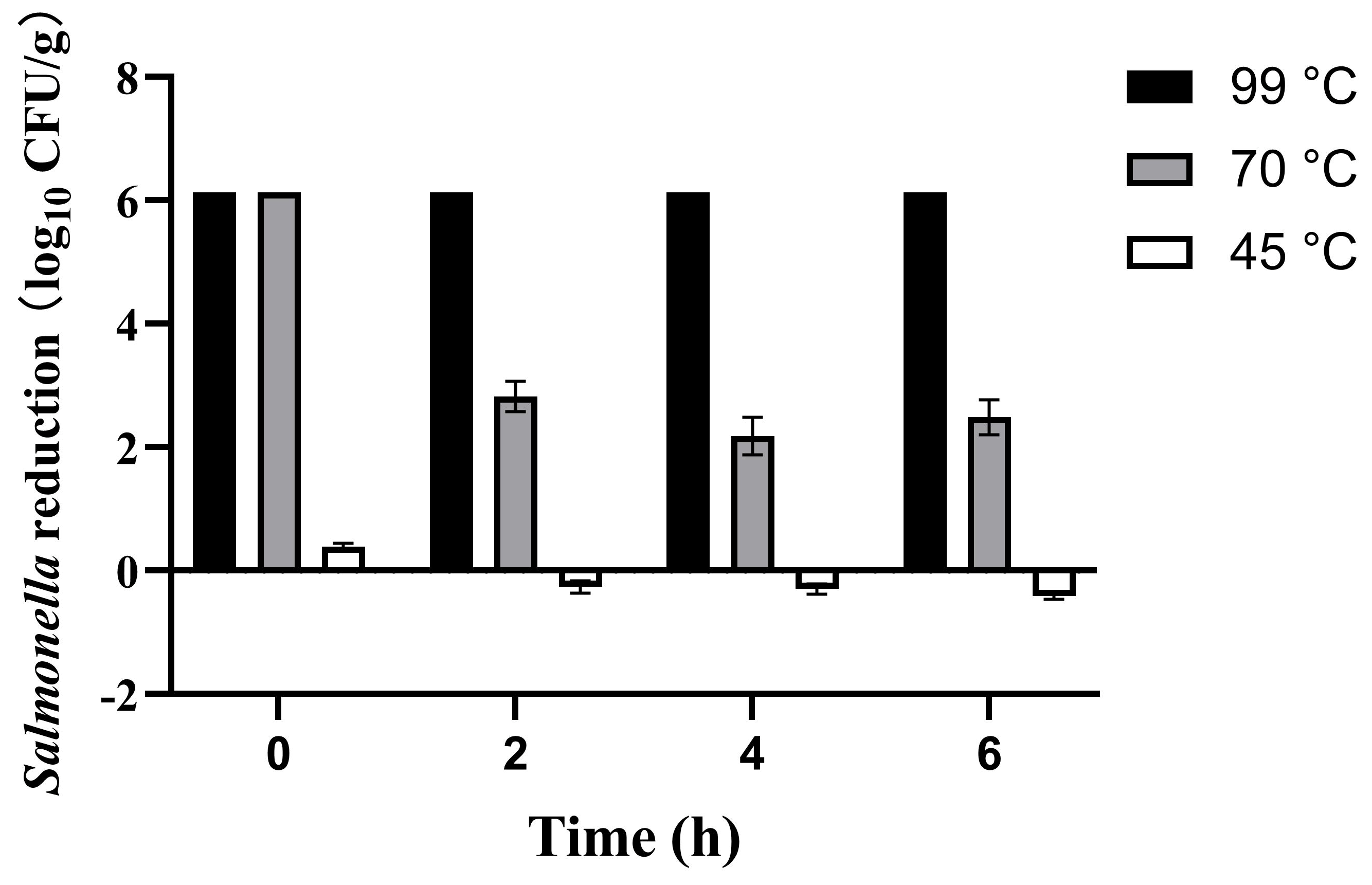
IMP inoculated with Salmonella was reconstituted at three distinct temperatures (45, 70, and 99 °C), corresponding to the manufacturer’s recommended preparation temperature, WHO’s advised infant formula temperature [37], and boiling water temperature, respectively. Salmonella reduction was investigated at 0, 2, 4, and 6 h. This is because prepared formula is not consumed immediately in practice; it typically undergoes a period of shaking for homogenization and subsequent cooling to a suitable feeding temperature. Furthermore, in outdoor scenarios such as traveling with an infant, the time between preparation and actual consumption might be prolonged. Therefore, the 0–6-h time selection help simulate potential time-temperature abuse scenarios that may occur during actual infant formula preparation and feeding practices. The results presented in Figure 1.
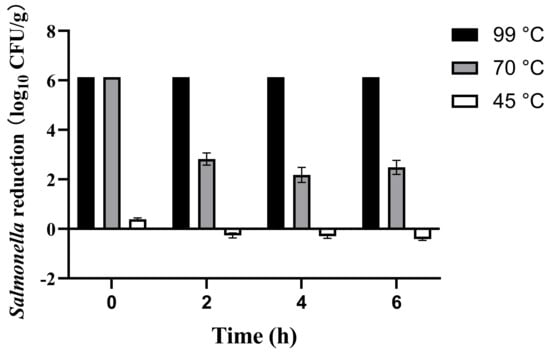
Figure 1.
Survival of Salmonella in infant milk powder (IMP) after reconstituting at 45, 70, and 99 °C.
The experimental results demonstrated significant temperature-dependent effects on Salmonella reduction in IMP reconstitution. Boiling water achieved complete microbial inactivation (>6 log reduction) immediately after preparation, maintaining this effect throughout the 6-h observation. When reconstituting with 70 °C waters, the Salmonella on IMP was initially reduced below detection limits (>6 log). However, the Salmonella population was found to recover by more than 3 log at 2 h. This indicated that the Salmonella on IMP gradually recovered over time after this mild heat treatment. Notably, only 0.39 ± 0.06 log initial reduction was observed when reconstituting with water at a temperature of 45 °C, and then the number of Salmonella gradually returned to the original level over 6 h. This indicated that the manufacturer’s recommended preparation methods not only failed to inactivate Salmonella, but also promoted bacterial proliferation. These results suggest that Salmonella in IMP exhibited notable heat resistance and might resuscitate and proliferate under favorable temperature conditions.
Shi et al. [38] reported a similar finding that Salmonella counts in black and green tea showed no significant reduction after brewing at 25 or 55 °C for 10 min (p > 0.05). Significant counts reductions (p < 0.05) occurred at 75 °C (>4 log) and 100 °C (>8 log). However, 75 °C treatment induced viable but non-culturable (VBNC) Salmonella, which was generally avirulent but could resuscitate and regain virulence under favorable conditions [39]. However, high-temperature reconstitution may damage milk powder nutrients and reduce probiotic viability in infant formula [40]. They reported that probiotic viability in infant formula was reduced by 93.4% when reconstituting at 70 °C compared to 40 °C. Similar findings were observed in other foods: green beans lost 17% vitamin A after boiling at 97 °C for 60 s [41], while leeks showed 29% vitamin C loss after boiling at 94–96 °C for 90 s [42].
Contaminated IMP poses a potential risk to infants’ health under regular infant formula preparation procedures. Therefore, it is necessary to maintain its food microbial safety. However, the formula of IMP designed for different stages of infants differ in fat, protein and carbohydrate contents and types. The difference in macronutrients in different milk powder products could affect the thermal resistance of Salmonella, thus requiring different pasteurization conditions. Therefore, investigating the effect of micronutrition on heat resistance of Salmonella could build a universal model to assist in the selection of optimal treatment conditions for the pasteurization of different milk powder products.
3.3. Storage Stability of Powder Inoculation
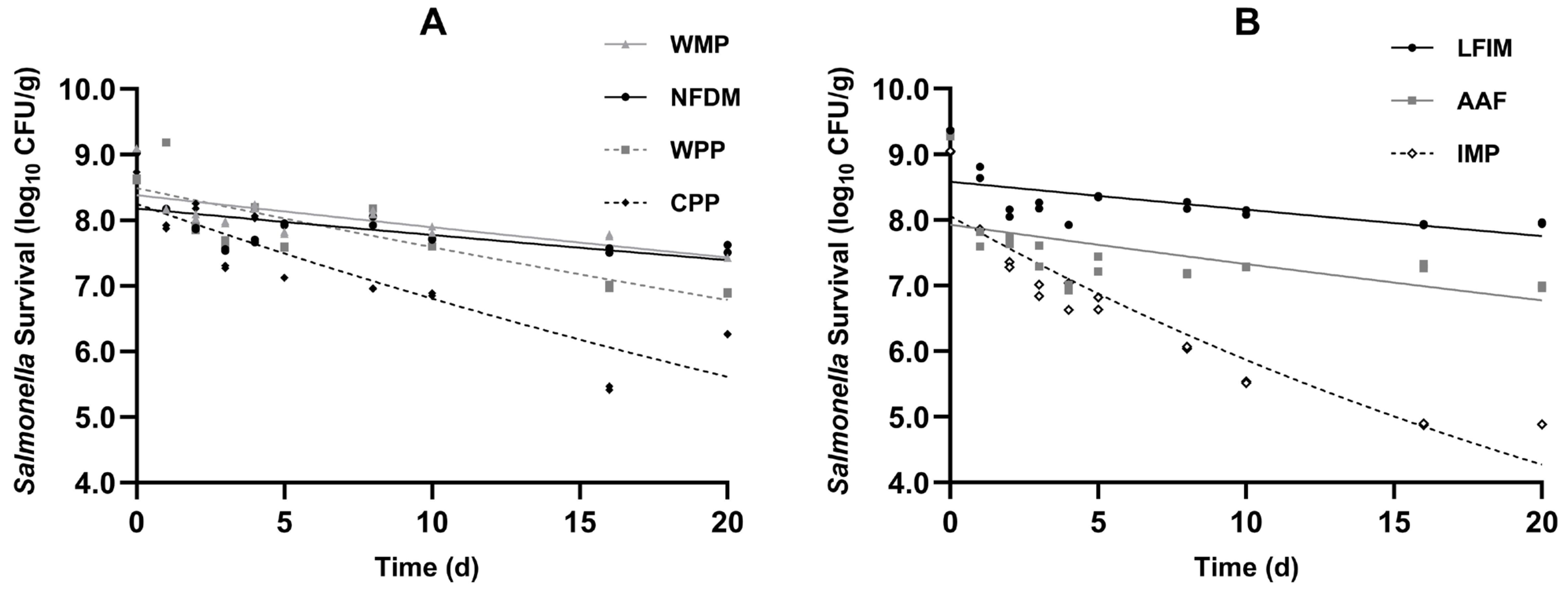
The survival data of Salmonella in four types of milk powder products and three infant formulas are presented in Figure 2. All inoculated samples showed good homogeneity and exhibited nonlinear survival curves with declining trends during 20 days.
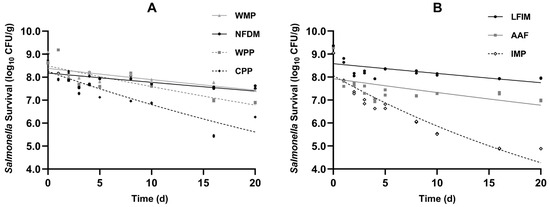
Figure 2.
Viability of Salmonella population in seven types of milk powders (A) whole milk powder (WMP), nonfat dry milk (NFDM), whey protein powder (WPP), casein protein powder (CPP), (B) infant milk powder (IMP), lactose-free infant milk (LFIM), and amino acid-based formula (AAF) during storage period (25 °C). Error bars indicate 1 SD of 3 subsamples.
Consistent with published reports [14,15,43,44], Salmonella populations in the various milk powders exhibited two distinct phases during storage, characterized by an initial rapid decline followed by a slower death rate. This pattern is commonly observed in low-water-activity foods, such as ground black pepper [45], egg white powder [46], dried basil leaves [47,48], and chia seeds [49]. This was because the desiccated environment (aw < 0.70; [50]) created stress for the pathogenic bacteria, which resulted in an initial rapid decline. Through adaptation to desiccation stress, Salmonella developed progressively enhanced environmental resistance [51].
The initial population of Salmonella on inoculated WMP and NFDM was 9.09 and 9.03 log10 CFU/g, respectively. During 20 days of storage, the reductions of Salmonella in WMP and NFDM were observed to be 1.66 and 1.40 log. Similar findings were reported by Wei et al. [14]. They reported that after WMP and NFDM were adjusted to an aw of 0.20, about 1.1 and 0.5 log reductions of Salmonella were observed, respectively. Both independent experiments demonstrated greater Salmonella reduction in WMP than NFDM, with no significant further decline observed during 20 days of storage. The initial bacterial counts of Salmonella in CPP and WPP were at a level of 8.62 and 8.68 log10 CFU/g, respectively, and dropped by 1.73 and 2.41 log, respectively, after 20 days of storage.
The initial population of Salmonella on inoculated LFIM and AAF was 9.37 and 9.31 log10 CFU/g, respectively. During 20 days of storage, the reductions of Salmonella were observed to be 1.41 and 2.32 log. The reduction in Salmonella in AAF was greater than that in LFIM. This phenomenon may be attributed to the release of anti-Salmonella active peptides in AAF, which could disrupt the bacterial membrane or virulence factors of Salmonella. The initial bacterial count of Salmonella in IMP was 9.05 log10 CFU/g and dropped by 4.83 log. Day et al. [52] studied Salmonella and Shigella survival in IMP, reporting 2.9 and 0.81 log reductions after 12-week storage, respectively. The greater reduction observed in our study might reflect differences in inoculation methods. The 20-day stability tests demonstrated that Salmonella remains viable in milk powders for extended periods, emphasizing the necessity of a kill step to guarantee product safety.
3.4. Thermal Resistance of Salmonella in Seven Types of Milk Powder
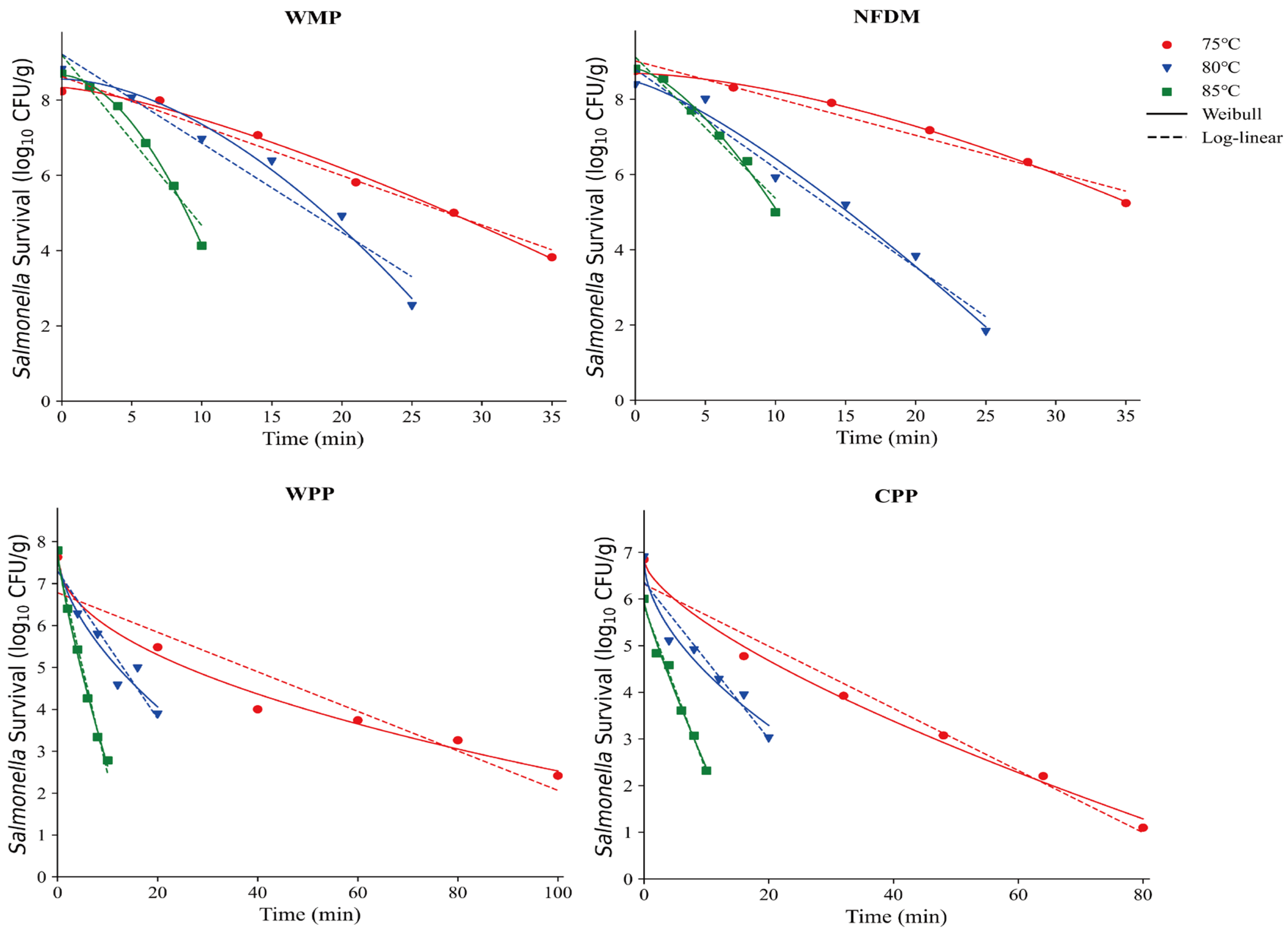
This research investigated how macronutrient composition affects Salmonella thermal inactivation kinetics in seven dairy matrices: WMP, NFDM, WPP, CPP, IMP, LFIM, and AAF. The isothermal inactivation curves and thermal inactivation kinetics of Salmonella in WMP, NFDM, WPP and CPP are shown in Figure 3 and Table 2, respectively. The survival curve was steeper at higher treatment temperature, indicating a higher inactivation rate. For instance, the -values of NFDM and WPP decreased significantly from 10.12 to 2.66 min and 21.21 to 1.98 min, respectively, as the treatment temperatures were increased from 75 to 85 °C.
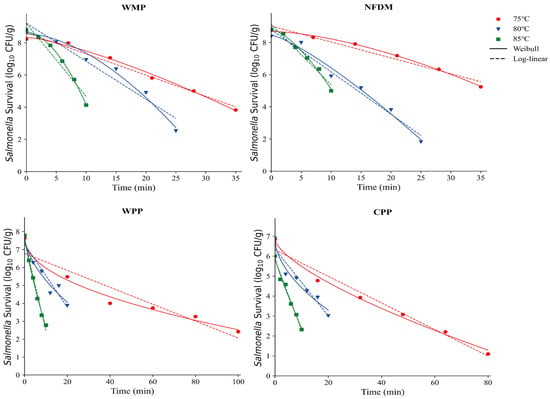
Figure 3.
Thermal inactivation kinetics of Salmonella in whole milk powder (WMP), nonfat dry milk (NFDM), whey protein powder (WPP), and casein protein powder (CPP) at various temperatures.

Table 2.
Parameter estimates for log-linear and Weibull models for inactivation of Salmonella in whole milk powder (WMP), nonfat dry milk (NFDM), whey protein powder (WPP), casein protein powder (CPP), infant milk powder (IMP), lactose-free infant milk (LFIM), and amino acid-based formula (AAF).
The -values of WMP and NFDM were greater than 1 (Table 2), indicating that their thermal inactivation curves exhibited upward convexity and the lethality rate increased over time. In contrast, the α values of WPP and CPP were less than 1, resulting in concave thermal inactivation curves, which implies a decreasing lethality rate with time. The -values of WMP (, , ) were significantly lower than those of WPP and CPP (p < 0.05, Table 2). Compositional analysis revealed that the protein content of WPP and CPP was higher than that of WMP (Table 1). Proteins are a key structural component of microbial cells, the proportion of protein in milk powders would influence microbial heat resistance. In this study, the higher protein contents provided additional protection to microorganisms, reducing heat-induced damage and resulting in greater heat resistance. In contrast, the lower protein content in WMP and NFDM likely made Salmonella more heat-sensitive, thereby affecting the shape of the thermal inactivation curves and its heat resistance. A similar finding has been observed by Jin et al. [25] that the D-values in high-protein matrices were larger than the D-values in high-fat matrices under high temperature conditions. Rachon et al. [12] demonstrated that Salmonella exhibits greater heat resistance than Listeria monocytogenes in high-protein matrices, while their heat resistance was comparable in high-salt seasoning. However, Zhang et al. [27] found that the -values of S. enteritidis increased exponentially with decreasing protein contents (from 83.59 % to 31.81 %) in egg powders. This difference might be due to variations in samples, type of proteins and the different ranges of protein contents (from 10.44 % to 90.18 %) in this study.
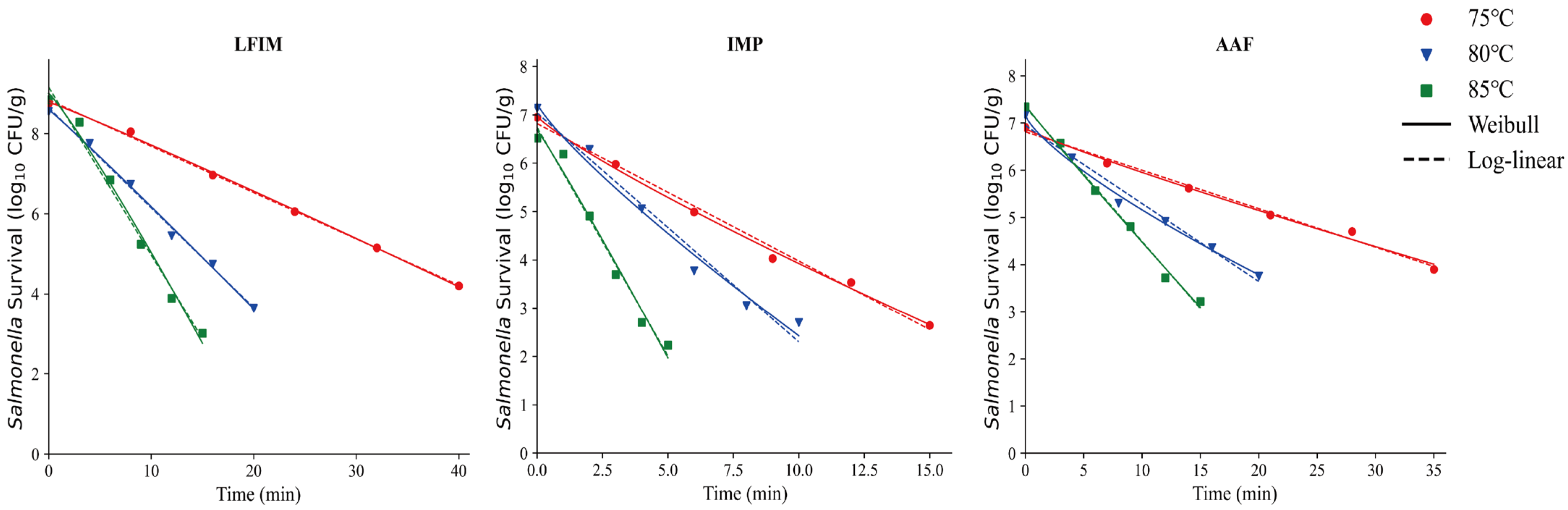
The isothermal inactivation curves of Salmonella in LFIM, AAF and IMP are shown in Figure 4. These three types of infant formula exhibit notably high carbohydrate content (Table 1). Although the carbohydrate content could provide an additional energy source for microorganisms, it did not enhance their heat resistance in this study. A similar conclusion was reached by Barnes [53], the author found that under low water activity conditions, the addition of sugar did not significantly alter the survival rate of Salmonella. But this finding differs from some previously published studies [9,12], which suggested that carbohydrates enhance microbial heat resistance. Moats et al. [23] reported that the results with carbohydrates were variable, with mannitol, sucrose, and rhamnose providing substantial protection while glucose decreased heat resistance. These differences may be due to differences in the types of carbohydrates examined. Other research has emphasized specific types of sugars while this study focused on total carbohydrates. Moreover, the physical properties of the samples (e.g., melting behavior) further contributed to these discrepancies. Alshammari et al. [54] suggested that easily meltable samples or sugars might cover the bacteria cells, limiting migration of moisture from the headspace into the bacterial cells. In contrast, the total carbohydrates in our milk powder samples did not melt easily, likely accounting for the observed differences.
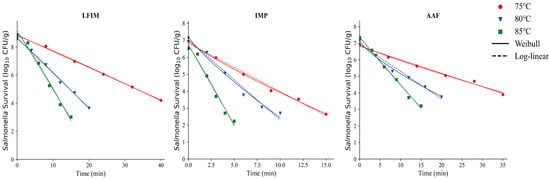
Figure 4.
Thermal inactivation kinetics of Salmonella in infant milk powder (IMP), lactose-free infant milk (LFIM), and amino acid-based formula (AAF) at various temperatures.
This study employed the log-linear model and the Weibull model to stimulate bacterial thermal inactivation kinetics (Figure 3 and Figure 4), with specific parameter values detailed in Table 2. Although the Weibull model provided a good fit in some cases, the log-linear model was more straightforward for linear relationships and directly yielded the D-values of Salmonella in different milk powders, which makes it the preferred fitting model for this study.
3.5. Influence of Temperature and Macronutrients on the Thermal Resistance of Salmonella
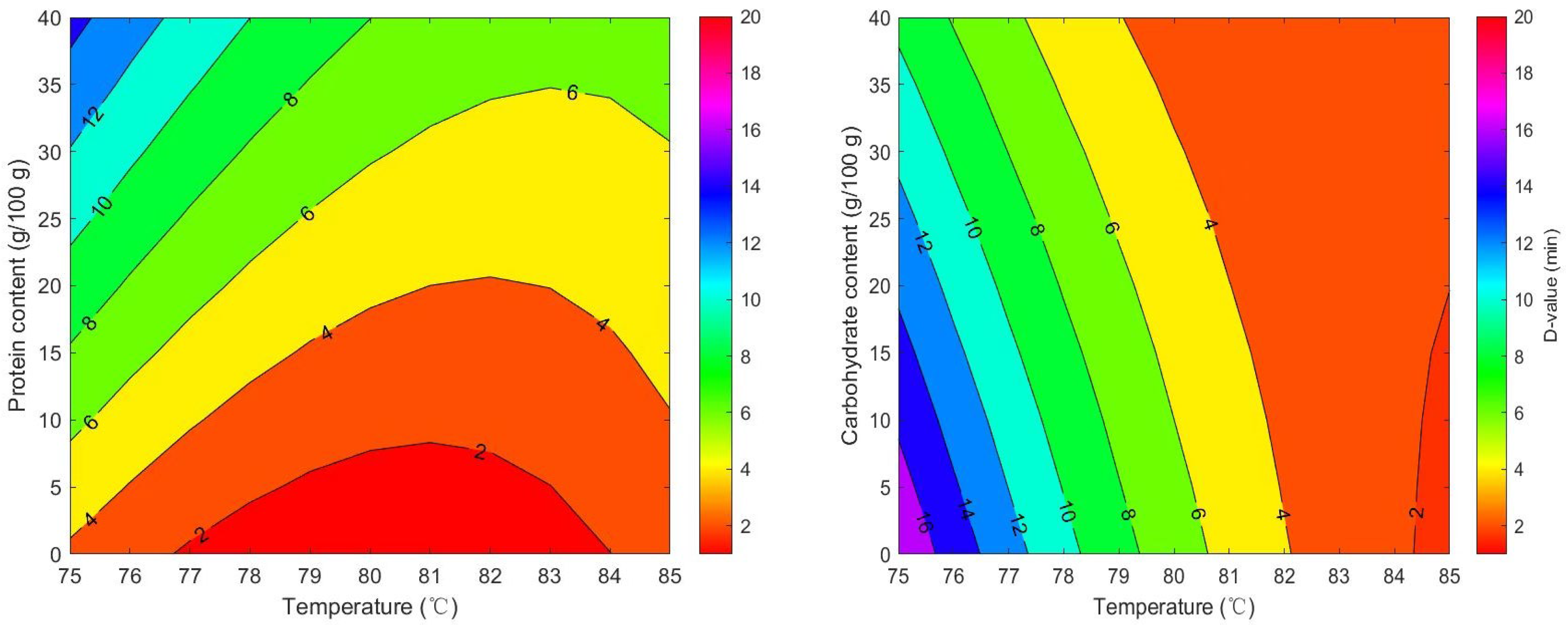
The RSM (Figure 5) were developed to estimate D-values of milk powders based on their macronutrient content and temperature. The following models were derived for D-values:
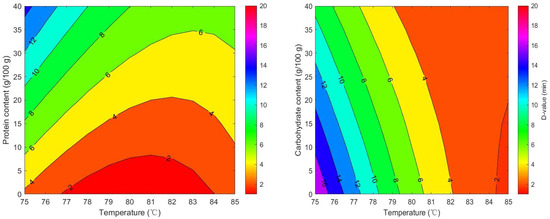
Figure 5.
Predicted D-values of Salmonella in seven milk powder varieties based on response surface modeling of temperature and macronutrient effects.
For protein:
D = 708.44 − 17.669 × T + 1.5793 × Wprotein − 0.017361 × T × Wprotein + 0.10994 × T2 − 0.0010818770 × Wprotein2
RMSE = 1.28; AICc = 163.86.
For carbohydrate:
where D = D-values of seven types of milk powder, T = isothermal treatment temperature (°C), and Wprotein/carbohydrate = protein or carbohydrate contents of the sample. The effect of fat content on D-values was found to be non-significant. Therefore, this variable was not incorporated into the final RSM. Although some studies have demonstrated that fat exerts a protective effect on microbial thermal inactivation [27,55], no such effect was detected in milk powders. Similar findings were observed by Wei et al. [14]. Most previous studies employed pure oil phases as test media, where the continuous oil matrix can readily encapsulate bacterial cells and form a physical moisture barrier [55]. In contrast, the fat in the milk powder studied here exists in the form of dispersed droplets or embedded particles rather than a continuous oil phase, resulting in limited localized protective effects. Moreover, the synergy of complex ingredients in mike powder may weaken the protective effect of fat [12].
D = 839.87 − 19.207 × T − 1.9082 × Wcarbohydrate + 0.022597 × T × Wcarbohydrate + 0.10994 × T2 − 0.00010876 × Wcarbohydrate2
RMSE = 1.62; AICc = 185.03.
As the temperature increased from 60 °C to 80 °C, the D-value of Salmonella significantly decreased (p < 0.05), indicating that its thermal inactivation rate accelerated significantly with rising temperature. An increase in protein content (10–20%) in milk powder significantly extended the D-value by 1.5–2 times. This indicated that, within the test range of this study, milk powder with higher protein content showed protective effect on Salmonella during thermal treatment. This observation was also reported by Manas et al. [56], Rachon et al. [12], Jin et al. [25]. Various peptide mixtures, some amino acids, protein components and divalent cations contribute to the protection of the Salmonella cell envelope against thermal damage [28]. Milk powder proteins might bind to bacterial heat-sensitive proteins, forming complexes that stabilize critical intracellular proteins and prevent thermal denaturation [28]. Additionally, there was a statistically significant interaction between temperature and protein content. This demonstrates that the thermal inactivation process of Salmonella is interactively influenced by both protein content and temperature. In the low-temperature range (75–79 °C), the D-value curves corresponding to different protein concentrations are relatively clustered, indicating that protein content has a comparatively greater influence on D-values within this temperature range. One possible reason is that temperatures increase the expression of heat shock genes like rpoH. These genes might help repair damaged proteins, maintain proper protein folding and protect bacterial cells from damage [57]. In the high-temperature range (80–85 °C), the protective effect of proteins diminishes as temperature increases. The influence of protein content on D-values becomes relatively minor, while temperature becomes the dominant factor.
It has been shown that when the carbohydrate content in milk powder exceeds 50%, the D-value of Salmonella decreases by 10–15%. This indicates that higher carbohydrate content reduced the heat resistance of Salmonella in this study. There was no significant interaction between carbohydrate content and temperature (p > 0.05). This suggests carbohydrates primarily affect Salmonella thermal inactivation, which did not depend on temperature. The mechanisms involve osmotic pressure regulation and the formation of maillard reaction products. High carbohydrate levels increase extracellular osmotic pressure, causing cellular dehydration and disrupting metabolic activity. Additionally, the Maillard reaction is a series of complex reactions between carbohydrates and amino acids under heating conditions, and its products possess antibacterial activity, inhibiting microbial growth and reproduction [58]. Therefore, carbohydrates can accelerate the inactivation of pathogens during food thermal processing.
In summary, in the case of protein-enriched milk formulations, enhanced thermal processing parameters are required to ensure microbiological safety standards. Conversely, for carbohydrate-dominant infant nutrition products, a strategic reduction in heat treatment severity could be employed to preserve nutritional integrity while maintaining adequate Salmonella control measures. The developed models provide industry practitioners with critical tools for optimizing pasteurization processes, enabling data-driven selection of temperature-time parameters that ensure microbial safety while accounting for compositional variations in milk powders.
4. Conclusions
Salmonella-contaminated milk powder remains a consumption risk even when reconstituted at conventional preparation temperatures. This investigation revealed that nutritional composition exerted a significant impact on Salmonella thermal resistance. Across the seven milk powder formulations investigated in this study, the heat resistance of Salmonella was positively correlated with protein content (10.44% to 90.18%) and negatively correlated with carbohydrate content (0.35% to 63.24%). Protein conferred protection against thermal inactivation and showed a significant interaction with temperature: its effect on Salmonella D-values decreased progressively as temperature increased from 75 to 85 °C. Assessment of Salmonella thermal inactivation in across various milk powder formulations provides important data for optimizing pasteurization processes to ensure powdered milk safety. These universal models provide the food industry with a practical tool to predict Salmonella D-values under specific combinations of macronutrient composition and processing temperature.
Author Contributions
Conceptualization, X.W. and S.L.; methodology, X.W. and Y.L.; software, X.W.; validation, Y.L., X.W. and S.L.; formal analysis, Y.L.; investigation, X.W. and Y.L.; resources, X.W.; data curation, X.W. and Y.L.; writing—original draft preparation, X.W. and Y.L.; writing—review and editing, S.L.; visualization, X.W.; supervision, X.W.and S.L.; project administration, X.W.; funding acquisition, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by Fujian Natural Science Foundation (No. 2023J05102) and National Natural Science Foundation of China (No. 32402262).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Codex Alimentarius Commission. Code of Hygienic Practice for Low-Moisture Foods; CAC/RCP 75-2015; Codex Alimentarius: Rome, Italy, 2015. [Google Scholar]
- RASFF RASFF Portal. 2025. Available online: https://webgate.ec.europa.eu/rasff-window/screen/search (accessed on 13 August 2025).
- Jayeola, V.; Farber, J.M.; Kathariou, S. Induction of the Viable-but-Nonculturable State in Salmonella Contaminating Dried Fruit. Appl. Environ. Microbiol. 2022, 88, e01733-21. [Google Scholar] [CrossRef]
- Millqvistfureby, A.; Smith, P. In-Situ Lecithination of Dairy Powders in Spray-Drying for Confectionery Applications. Food Hydrocoll. 2007, 21, 920–927. [Google Scholar] [CrossRef]
- Chuchird, P.; Pattarathitiwat, P.; Pongprajak, A. Formulation and Evaluation of Physical, Chemical and Sensory Properties Ofinstant Functional Beverage Powder Containing Pathum Thani Fragrance Rice, Soy Protein and Milk Powder. Food Res. 2024, 8, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Serna-Saldívar, S.O. (Ed.) Snack Foods: Processing, Innovation, and Nutritional Aspects, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; Volume 8–22, ISBN 978-1-003-12906-6. [Google Scholar]
- Banach, J.C.; Clark, S.; Lamsal, B.P. Particle Size of Milk Protein Concentrate Powder Affects the Texture of High-protein Nutrition Bars during Storage. J. Food Sci. 2017, 82, 913–921. [Google Scholar] [CrossRef]
- LiCari, J.J.; Potter, N.N. Salmonella Survival during Spray Drying and Subsequent Handling of Skimmilk Powder. II. Effects of Drying Conditions. J. Dairy Sci. 1970, 53, 871–876. [Google Scholar] [CrossRef]
- Ahmad, N.H.; Marks, B.P.; Ryser, E.T. Effect of Lactose and Milk Protein on Thermal Resistance of Enterococcus Faecium NRRL B-2354 and Salmonella in Dairy Powders. J. Food Prot. 2022, 85, 1865–1874. [Google Scholar] [CrossRef]
- Hebishy, E.; Yerlikaya, O.; Mahony, J.; Akpinar, A.; Saygili, D. Microbiological Aspects and Challenges of Whey Powders—I Thermoduric, Thermophilic and Spore-forming Bacteria. Int. J. Dairy Technol. 2023, 76, 779–800. [Google Scholar] [CrossRef]
- EFSA Multi-Country Outbreak of Salmonella Poona Infections Linked to Consumption of Infant Formula. EFSA Supporting Publications; John Wiley & Sons: Hoboken, NJ, USA, 2019; Volume 16. [Google Scholar] [CrossRef]
- Rachon, G.; Peñaloza, W.; Gibbs, P.A. Inactivation of Salmonella, Listeria Monocytogenes and Enterococcus Faecium NRRL B-2354 in a Selection of Low Moisture Foods. Int. J. Food Microbiol. 2016, 231, 16–25. [Google Scholar] [CrossRef]
- Deng, L.-Z.; Tao, Y.; Mujumdar, A.S.; Pan, Z.; Chen, C.; Yang, X.-H.; Liu, Z.-L.; Wang, H.; Xiao, H.-W. Recent Advances in Non-Thermal Decontamination Technologies for Microorganisms and Mycotoxins in Low-Moisture Foods. Trends Food Sci. Technol. 2020, 106, 104–112. [Google Scholar] [CrossRef]
- Wei, X.; Lau, S.K.; Chaves, B.D.; Danao, M.-G.C.; Agarwal, S.; Subbiah, J. Effect of Water Activity on the Thermal Inactivation Kinetics of Salmonella in Milk Powders. J. Dairy Sci. 2020, 103, 6904–6917. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, A.S.; Singh, A.; Unger, P.; Babb, M.; Yang, Y.; Michael, M. Survival and Thermal Resistance of Salmonella in Dry and Hydrated Nonfat Dry Milk and Whole Milk Powder during Extended Storage. Int. J. Food Microbiol. 2021, 337, 108950. [Google Scholar] [CrossRef]
- Wei, X. Microbial Challenge Studies of Radio Frequency Heating for Dairy Powders and Gaseous Technologies for Spices. Ph.D. Thesis, The University of Nebraska-Lincoln, Lincoln, NE, USA, 2021. [Google Scholar]
- Ma, L.; Zhang, G.; Gerner-Smidt, P.; Mantripragada, V.; Ezeoke, I.; Doyle, M.P. Thermal Inactivation of Salmonella in Peanut Butter. J. Food Prot. 2009, 72, 1596–1601. [Google Scholar] [CrossRef]
- Ng, H.; Bayne, H.G.; Garibaldi, J.A. Heat Resistance of Salmonella: The Uniqueness of Salmonella senftenberg 775W. Appl. Microbiol. 1969, 17, 78–82. [Google Scholar] [CrossRef]
- Quintavalla, S.; Larini, S.; Mutti, P.; Barbuti, S. Evaluation of the Thermal Resistance of Different Salmonella Serotypes in Pork Meat Containing Curing Additives. Int. J. Food Microbiol. 2001, 67, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.S.; Waterman, K.M.; Williams, R.C.; Ponder, M.A. Inoculation Preparation Affects Survival of Salmonella Enterica on Whole Black Peppercorns and Cumin Seeds Stored at Low Water Activity. J. Food Prot. 2015, 78, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, J.; Xie, L.; Zhu, M.-J.; Tang, J. Dry Inoculation Methods for Nonfat Milk Powder. J. Dairy Sci. 2019, 102, 77–86. [Google Scholar] [CrossRef]
- Ban, C.; Lee, D.H.; Jo, Y.; Bae, H.; Seong, H.; Kim, S.O.; Lim, S.; Choi, Y.J. Use of Superheated Steam to Inactivate Salmonella Enterica Serovars Typhimurium and Enteritidis Contamination on Black Peppercorns, Pecans, and Almonds. J. Food Eng. 2018, 222, 284–291. [Google Scholar] [CrossRef]
- Bedane, T.F.; Erdogdu, F.; Lyng, J.G.; Marra, F. Effects of Geometry and Orientation of Food Products on Heating Uniformity during Radio Frequency Heating. Food Bioprod. Process. 2021, 125, 149–160. [Google Scholar] [CrossRef]
- Verma, T.; Wei, X.; Lau, S.K.; Bianchini, A.; Eskridge, K.M.; Subbiah, J. Evaluation of Enterococcus faecium NRRL B-2354 as a Surrogate for Salmonella during Extrusion of Low-moisture Food. J. Food Sci. 2018, 83, 1063–1072. [Google Scholar] [CrossRef]
- Jin, Y.; Pickens, S.R.; Hildebrandt, I.M.; Burbick, S.J.; Grasso-Kelley, E.M.; Keller, S.E.; Anderson, N.M. Thermal Inactivation of Salmonella Agona in Low–Water Activity Foods: Predictive Models for the Combined Effect of Temperature, Water Activity, and Food Component. J. Food Prot. 2018, 81, 1411–1417. [Google Scholar] [CrossRef] [PubMed]
- Mattick, K.L.; Jørgensen, F.; Wang, P.; Pound, J.; Vandeven, M.H.; Ward, L.R.; Legan, J.D.; Lappin-Scott, H.M.; Humphrey, T.J. Effect of Challenge Temperature and Solute Type on Heat Tolerance of Salmonella Serovars at Low Water Activity. Appl. Environ. Microbiol. 2001, 67, 4128–4136. [Google Scholar] [CrossRef]
- Zhang, Y.; Pérez-Reyes, M.E.; Qin, W.; Hu, B.; Wu, Q.; Liu, S. Modeling the Effect of Protein and Fat on the Thermal Resistance of Salmonella Enterica Enteritidis PT 30 in Egg Powders. Food Res. Int. 2022, 155, 111098. [Google Scholar] [CrossRef]
- Moats, W.A.; Dabbah, R.; Edwards, V.M. Survival of Salmonella anatum Heated in Various Media. Appl. Microbiol. 1971, 21, 476–481. [Google Scholar] [CrossRef] [PubMed]
- AOAC International. Official Methods of Analysis, 17th ed.; Association of Official Agricultural Chemists: Washington, DC, USA, 2023; ISBN 978-0-19-761014-5. [Google Scholar]
- Xu, J.; Tang, J.; Jin, Y.; Song, J.; Yang, R.; Sablani, S.S.; Zhu, M.-J. High Temperature Water Activity as a Key Factor Influencing Survival of Salmonella Enteritidis PT30 in Thermal Processing. Food Control 2019, 98, 520–528. [Google Scholar] [CrossRef]
- Wei, X.; Agarwal, S.; Subbiah, J. Heating of Milk Powders at Low Water Activity to 95 °C for 15 Minutes Using Hot Air-Assisted Radio Frequency Processing Achieved Pasteurization. J. Dairy Sci. 2021, 104, 9607–9616. [Google Scholar] [CrossRef]
- Chung, H.-J.; Birla, S.L.; Tang, J. Performance Evaluation of Aluminum Test Cell Designed for Determining the Heat Resistance of Bacterial Spores in Foods. LWT-Food Sci. Technol. 2008, 41, 1351–1359. [Google Scholar] [CrossRef]
- Peleg, M.; Cole, M.B. Reinterpretation of Microbial Survival Curves. Crit. Rev. Food Sci. Nutr. 1998, 38, 353–380. [Google Scholar] [CrossRef]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: New York, NY, USA, 2004; ISBN 978-0-19-517179-2. [Google Scholar]
- Dolan, K.D.; Valdramidis, V.P.; Mishra, D.K. Parameter Estimation for Dynamic Microbial Inactivation: Which Model, Which Precision? Food Control 2013, 29, 401–408. [Google Scholar] [CrossRef]
- Lenth, R.V. Response-Surface Methods in R, Usingrsm. J. Stat. Softw. 2009, 32, 1–17. [Google Scholar] [CrossRef]
- WHO. Safe Preparation, Storage and Handling of Powdered Infant Formula: Guidelines; WHO: Geneva, Switzerland, 2007; p. 26.
- Shi, A.; Li, S.; Ma, H.; Du, X.-J.; Wang, S.; Lu, X. Survival of Salmonella in Tea under Different Storage Conditions and Brewing Methods. Front. Microbiol. 2022, 13, 816667. [Google Scholar] [CrossRef]
- Highmore, C.J.; Warner, J.C.; Rothwell, S.D.; Wilks, S.A.; Keevil, C.W. Viable-but-Nonculturable Listeria Monocytogenes and Salmonella Enterica Serovar Thompson Induced by Chlorine Stress Remain Infectious. Mbio 2018, 9, e00540-18. [Google Scholar] [CrossRef]
- Hongxin, J.; Liang, J.; Ni, L. Effect of Reconstitution Temperature on Survival Rate of Probiotics in Infant Formula. J. Dairy Sci. Technol. 2019, 42, 21–24. [Google Scholar] [CrossRef]
- Howard, L.A.; Wong, A.D.; Perry, A.K.; Klein, B.P. Β-carotene and Ascorbic Acid Retention in Fresh and Processed Vegetables. J. Food Sci. 1999, 64, 929–936. [Google Scholar] [CrossRef]
- Kmiecik, W.; Lisiewska, Z. Effect of Pretreatment and Conditions and Period of Storage on Some Quality Indices of Frozen Chive (Allium schoenoprasum L.). Food Chem. 1999, 67, 61–66. [Google Scholar] [CrossRef]
- Lian, F.; Zhao, W.; Yang, R.; Tang, Y.; Katiyo, W. Survival of Salmonella Enteric in Skim Milk Powder with Different Water Activity and Water Mobility. Food Control 2015, 47, 1–6. [Google Scholar] [CrossRef]
- Wei, X.; Agarwal, S.; Subbiah, J. Evaluation of Enterococcus Faecium NRRL B-2354 as a Surrogate for Salmonella Enterica in Milk Powders at Different Storage Times and Temperatures. J. Dairy Sci. 2021, 104, 198–210. [Google Scholar] [CrossRef]
- Wei, X.; Lau, S.K.; Stratton, J.; Irmak, S.; Subbiah, J. Radiofrequency Pasteurization Process for Inactivation of Salmonella spp. and Enterococcus faecium NRRL B-2354 on Ground Black Pepper. Food Microbiol. 2019, 82, 388–397. [Google Scholar] [CrossRef]
- Wei, X.; Lau, S.K.; Reddy, B.S.; Subbiah, J. A Microbial Challenge Study for Validating Continuous Radio-Frequency Assisted Thermal Processing Pasteurization of Egg White Powder. Food Microbiol. 2020, 85, 103306. [Google Scholar] [CrossRef] [PubMed]
- Verma, T.; Chaves, B.D.; Howell, T.; Subbiah, J. Thermal Inactivation Kinetics of Salmonella and Enterococcus Faecium NRRL B-2354 on Dried Basil Leaves. Food Microbiol. 2021, 96, 103710. [Google Scholar] [CrossRef]
- Verma, T.; Chaves, B.D.; Irmak, S.; Subbiah, J. Pasteurization of Dried Basil Leaves Using Radio Frequency Heating: A Microbial Challenge Study and Quality Analysis. Food Control 2021, 124, 107932. [Google Scholar] [CrossRef]
- Wason, S.; Subbiah, J. Gaseous Chlorine Dioxide for Inactivating Salmonella enterica and Enterococcus faecium NRRL B-2354 on Chia Seeds. Food Control 2023, 150, 109736. [Google Scholar] [CrossRef]
- Blessington, T.; Theofel, C.G.; Harris, L.J. A Dry-Inoculation Method for Nut Kernels. Food Microbiol. 2013, 33, 292–297. [Google Scholar] [CrossRef]
- Gruzdev, N.; Pinto, R.; Sela, S. Effect of Desiccation on Tolerance of Salmonella Enterica to Multiple Stresses. Appl. Environ. Microbiol. 2011, 77, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Day, J.B.; Sharma, D.; Siddique, N.; Hao, Y.D.; Strain, E.A.; Blodgett, R.J.; Al-Khaldi, S.F. Survival of Salmonella Typhi and Shigella dysenteriae in Dehydrated Infant Formula. J. Food Sci. 2011, 76, M324–M328. [Google Scholar] [CrossRef]
- Barnes, S.R. Influence of Sugar on the Survival of Salmonella in a Low—Water Activity Whey Protein-Based Model Food System. Master’s Thesis, University of Georgia, Athens, GA, USA, 2015. [Google Scholar]
- Alshammari, J.; Xu, J.; Tang, J.; Sablani, S.; Zhu, M.-J. Thermal Resistance of Salmonella in Low-Moisture High-Sugar Products. Food Control 2020, 114, 107255. [Google Scholar] [CrossRef]
- Yang, R.; Xie, Y.; Lombardo, S.P.; Tang, J. Oil Protects Bacteria from Humid Heat in Thermal Processing. Food Control 2021, 123, 107690. [Google Scholar] [CrossRef]
- Manas, P.; Pagan, R.; Sala, F.J.; Condon, S. Low Molecular Weight Milk Whey Components Protect Salmonella Senftenberg 775W against Heat by a Mechanism Involving Divalent Cations. J. Appl. Microbiol. 2001, 91, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Khoo, W.J.; Zheng, Q.; Chung, H.-J.; Yuk, H.-G. Growth Temperature Alters Salmonella Enteritidis Heat/Acid Resistance, Membrane Lipid Composition and Stress/Virulence Related Gene Expression. Int. J. Food Microbiol. 2014, 172, 102–109. [Google Scholar] [CrossRef]
- Lee, J.; Roux, S.; Le Roux, E.; Keller, S.; Rega, B.; Bonazzi, C. Unravelling Caramelization and Maillard Reactions in Glucose and Glucose + Leucine Model Cakes: Formation and Degradation Kinetics of Precursors, α-Dicarbonyl Intermediates and Furanic Compounds during Baking. Food Chem. 2022, 376, 131917. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).