Impact of Temperature Stresses on Wheat Quality: A Focus on Starch and Protein Composition
Abstract
1. Introduction
2. Materials and Methods
3. Effect of Temperature on Wheat Quality
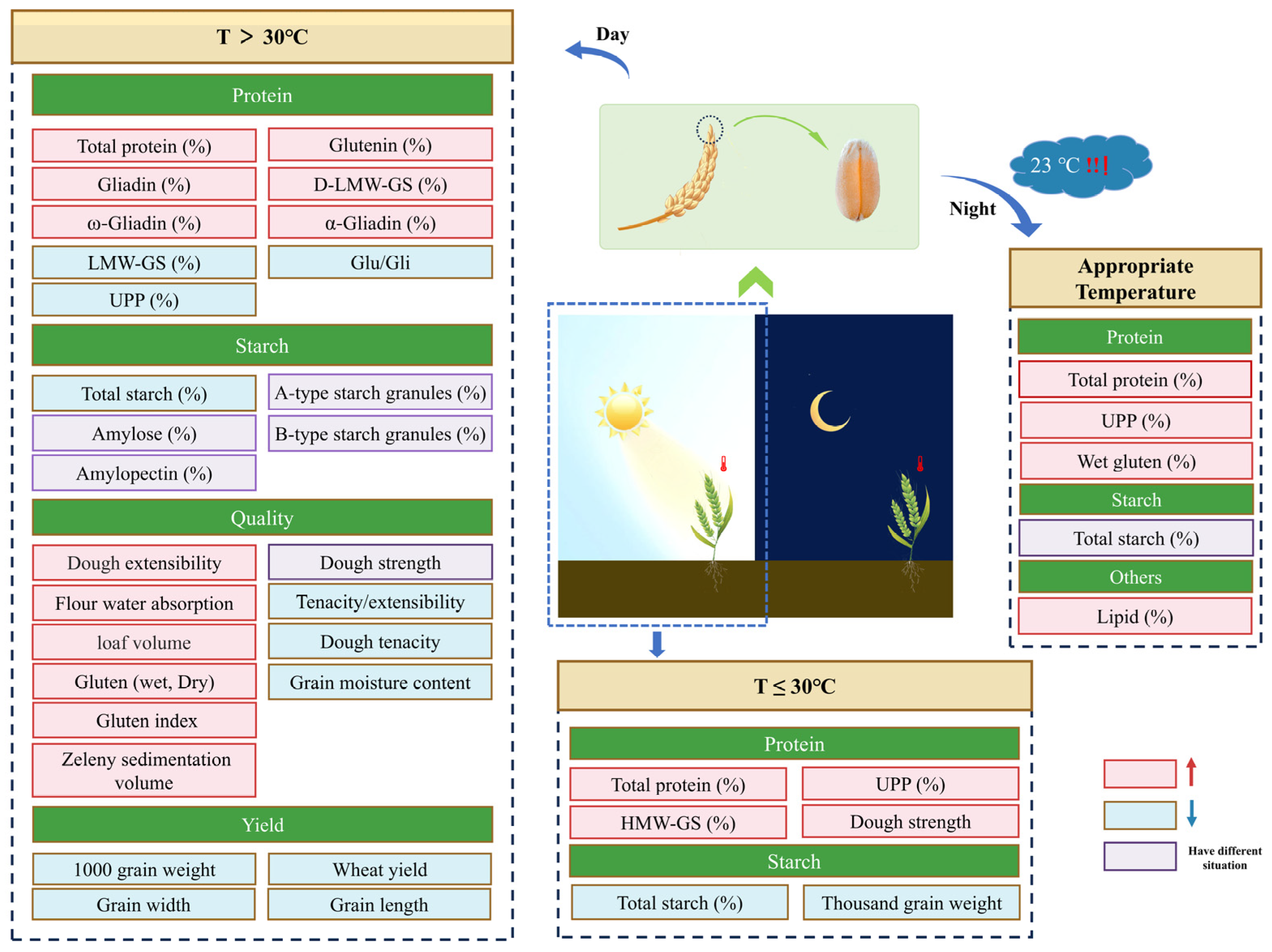
3.1. High Temperature
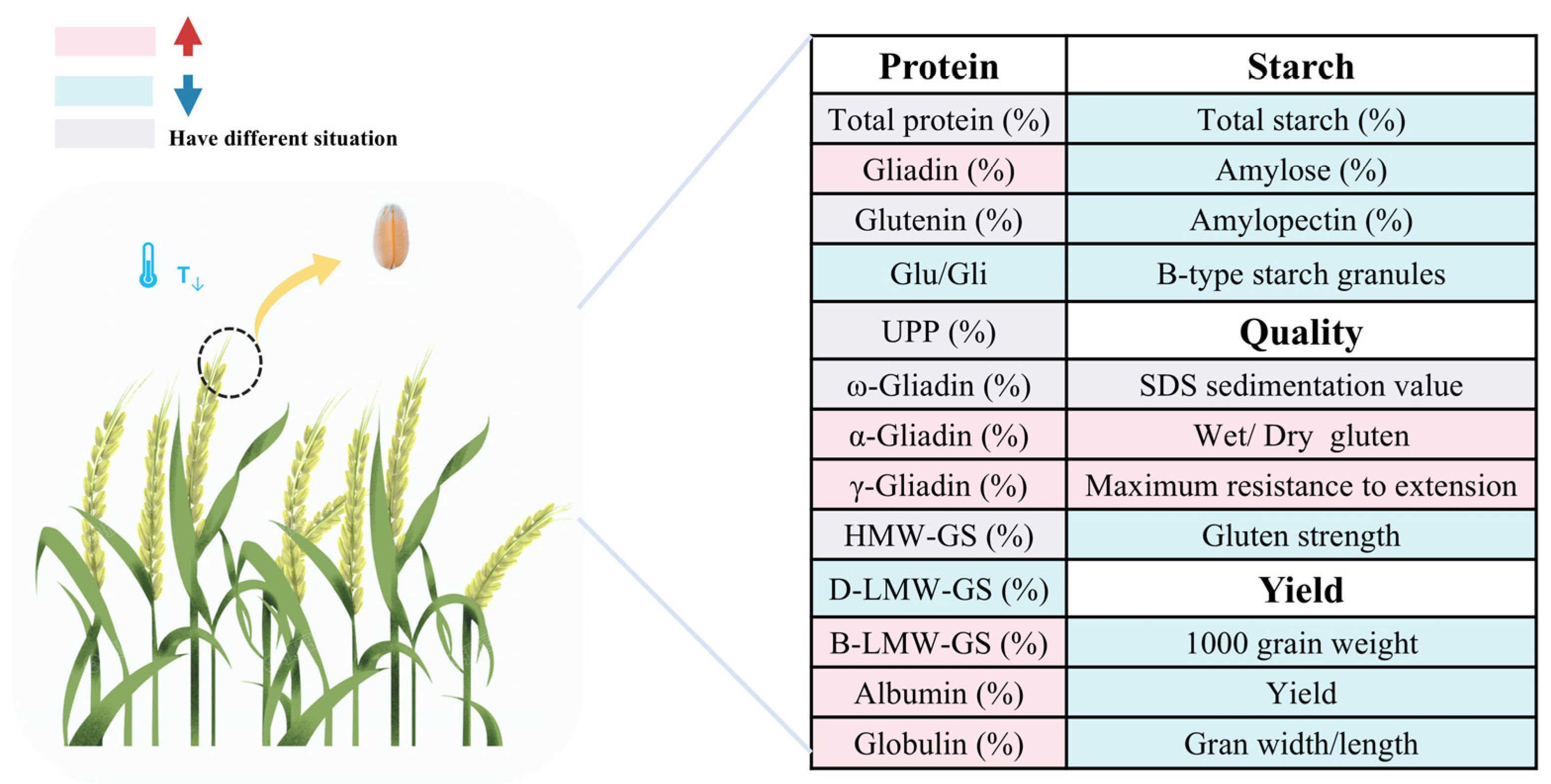
3.2. Low Temperature
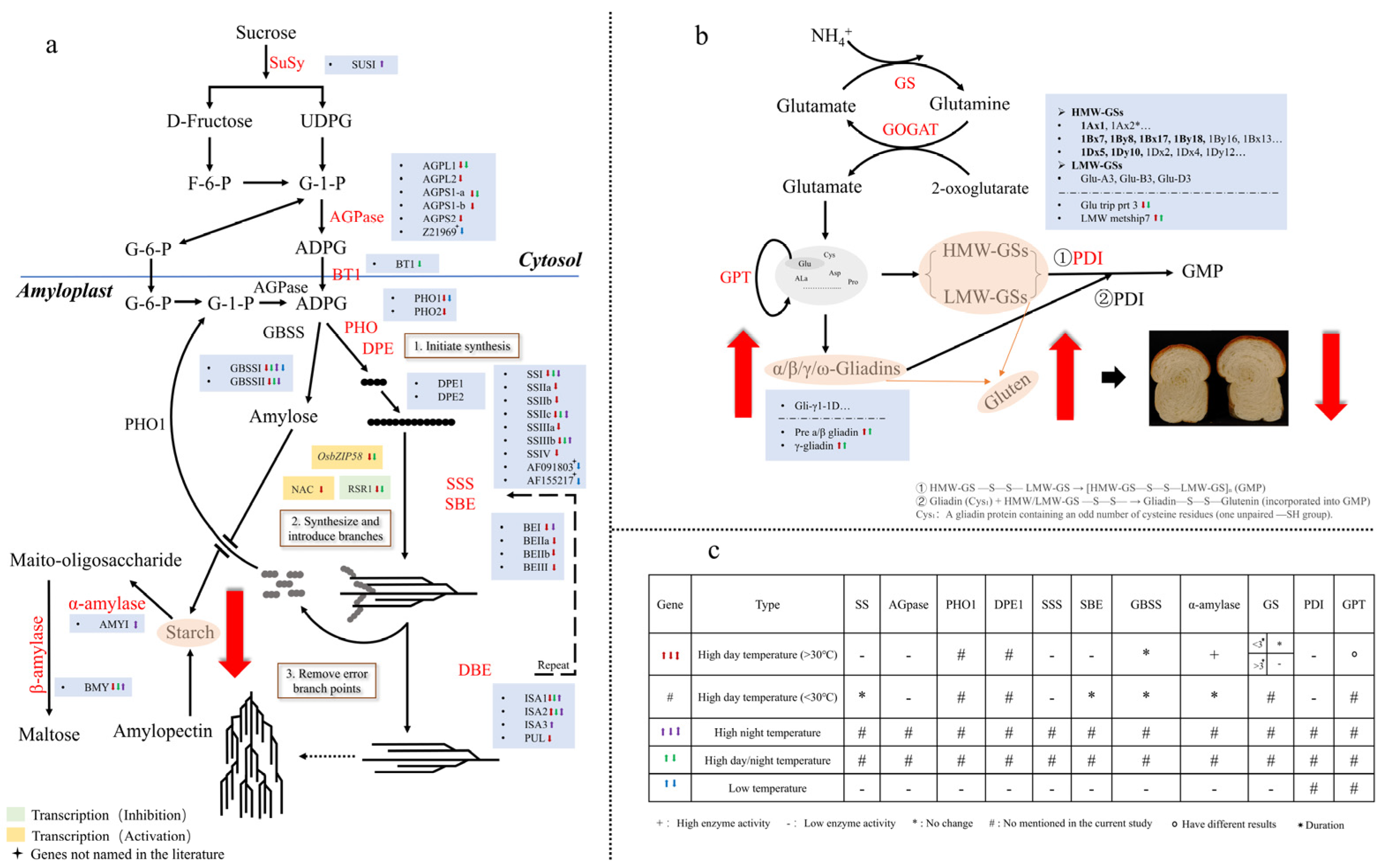
4. Mechanism Underlying Temperature-Driven Quality Changes in Wheat Grains
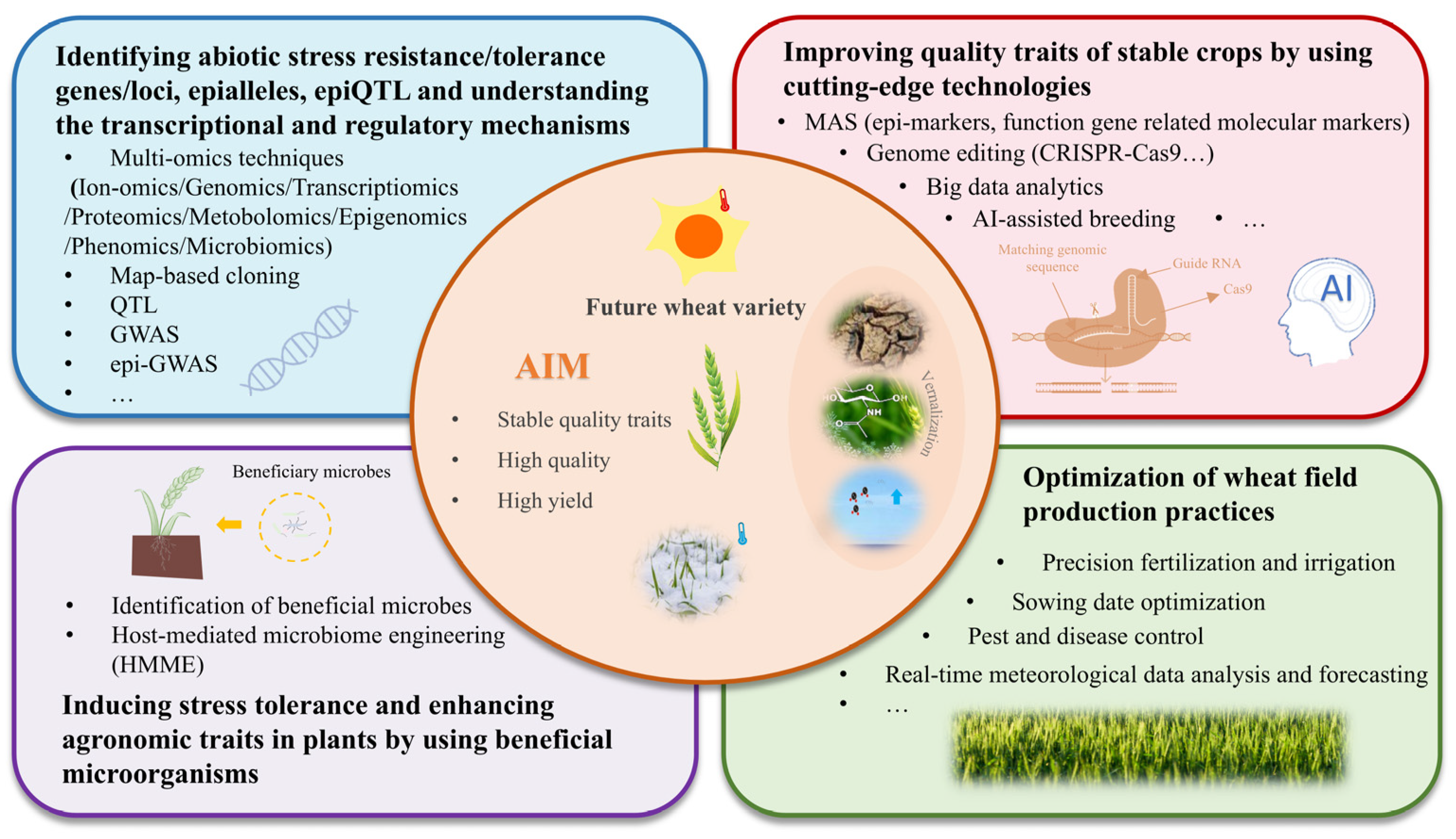
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, X.; Hou, R.; Yang, G. Effects of climactic warming on the starch and protein content of winter wheat grain under conservation tillage in the North China Plain. Soil Till. Res. 2024, 238, 105995. [Google Scholar] [CrossRef]
- Troccoli, A.; Borrelli, G.M.; De Vita, P.; Fares, C.; Di Fonzo, N. Mini Review: Durum Wheat Quality: A Multidisciplinary Concept. J. Cereal Sci. 2000, 32, 99–113. [Google Scholar] [CrossRef]
- Niu, L.; Wang, W.; Zhang, Y.; Zou, j.; Wang, Z.; Lu, L.; Wang, F.; Wang, W.; Yu, L. Wheat Quality and Yield Traits: Effects on Scores of Steamed Bread and Noodles. Chin. Agric. Sci. Bull. 2022, 38, 129–133. [Google Scholar] [CrossRef]
- Yin, B.; Jia, J.; Sun, X.; Hu, X.; Ao, M.; Liu, W.; Tian, Z.; Liu, H.; Li, D.; Tian, W.; et al. Dynamic metabolite QTL analyses provide novel biochemical insights into kernel development and nutritional quality improvement in common wheat. Plant Commun. 2024, 5, 100792. [Google Scholar] [CrossRef]
- Ibrahim, S.; Saleem, B.; Rehman, N.; Zafar, S.A.; Naeem, M.K.; Khan, M.R. CRISPR/Cas9 mediated disruption of Inositol Pentakisphosphate 2-Kinase 1 (TaIPK1) reduces phytic acid and improves iron and zinc accumulation in wheat grains. J. Adv. Res. 2022, 37, 33–41. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, G.; Li, Y.; Lv, H. Changes in quality characteristics and metabolites composition of wheat under different storage temperatures. J. Stored Prod. Res. 2024, 105, 102229. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, X.; He, H.; Kamruzzaman, M. Advancements, limitations and challenges in hyperspectral imaging for comprehensive assessment of wheat quality: An up-to-date review. Food Chem. X 2024, 21, 101235. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, R.; Li, S.; Ullah, R.; Yang, F.; Wang, Z.; Guo, W.; You, M.; Li, B.; Xie, C.; et al. TaMADS29 interacts with TaNF-YB1 to synergistically regulate early grain development in bread wheat. Sci. China Life Sci. 2023, 66, 1647–1664. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Hu, K.; He, Y. Enhancing wheat protein through low-water-fertility under climate change without yield penalty. Agric. Water Manag. 2024, 300, 108909. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, W.; Han, C.; Cao, H.; Xu, Y.; Zhang, W.; Yan, Y. Synthesis and Accumulation of Glutenin Subunits during Grain Development in Bread Wheat as Revealed by Reversed-phase Ultra-performance Liquid Chromatography (RP-UPLC). Cereal Res. Commun. 2016, 44, 461–471. [Google Scholar] [CrossRef][Green Version]
- Lin, J.F. Advances in Cereal Science and Technology. J. Assoc. Off. Anal. Chem. 1979, 62, 700b–701. [Google Scholar] [CrossRef]
- Yu, Z.; Han, C.; Wang, S.; Lv, D.; Chen, G.; Li, X.; Jiang, G.-L.; Yan, Y. Fast separation and characterization of water-soluble proteins in wheat grains by reversed-phase ultra performance liquid chromatography (RP-UPLC). J. Cereal Sci. 2013, 57, 288–294. [Google Scholar] [CrossRef]
- Zhen, S.; Deng, X.; Xu, X.; Liu, N.; Zhu, D.; Wang, Z.; Yan, Y. Effect of high-nitrogen fertilizer on gliadin and glutenin subproteomes during kernel development in wheat (Triticum aestivum L.). Crop. J. 2020, 8, 38–52. [Google Scholar] [CrossRef]
- Guo, J.; Guo, Z.; Wang, H.; Lian, X. Effects of three glutenins extracted in acidic, neutral and alkaline urea solutions on the retrogradation of wheat amylose and amylopectin. Int. J. Biol. Macromol. 2023, 233, 123576. [Google Scholar] [CrossRef] [PubMed]
- Emide, D.; Magni, C.; Saitta, F.; Cardone, G.; Botticella, E.; Fessas, D.; Iametti, S.; Lafiandra, D.; Sestili, F.; Marti, A.; et al. Molecular insights into the role of amylose/amylopectin ratio on gluten protein organization. Food Chem. 2023, 404, 134675. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhou, J.; Liu, Y.; Lu, X.; Han, C.; Zhang, W.; Xu, Y.; Yan, Y. Biosynthesis and Regulation of Wheat Amylose and Amylopectin from Proteomic and Phosphoproteomic Characterization of Granule-binding Proteins. Sci. Rep. 2016, 6, 33111. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Wen, J.; Geng, H.; Zhan, X.; Liu, Y. Physicochemical properties, structural properties and gels 3D printing properties of wheat starch. Int. J. Biol. Macromol. 2024, 261, 129885. [Google Scholar] [CrossRef]
- Watanabe, S.; Nishitsuji, Y.; Hayakawa, K.; Shi, Y.-C. Pasting properties of A- and B-type wheat starch granules and annealed starches in relation to swelling and solubility. Int. J. Biol. Macromol. 2024, 261, 129738. [Google Scholar] [CrossRef]
- Guo, L.; Wang, Q.; Chen, H.; Wu, D.; Dai, C.; Chen, Y.; Ma, Y.; Wang, Z.; Li, H.; Cao, X.; et al. Moderate addition of B-type starch granules improves the rheological properties of wheat dough. Food Res. Int. 2022, 160, 111748. [Google Scholar] [CrossRef]
- Williams, R.; O’brien, L.; Eagles, H.A.; Solah, V.A.; Jayasena, V. The influences of genotype, environment, and genotype×environment interaction on wheat quality. Crop Pasture Sci. 2008, 59, 95–111. [Google Scholar] [CrossRef]
- Labuschagne, M.T.; Elago, O.; Koen, E. The influence of temperature extremes on some quality and starch characteristics in bread, biscuit and durum wheat. J. Cereal Sci. 2009, 49, 184–189. [Google Scholar] [CrossRef]
- Zhu, G.; Yan, X.; Zhu, D.; Deng, X.; Wu, J.-S.; Xia, J.; Yan, Y. Lysine acetylproteome profiling under water deficit reveals key acetylated proteins involved in wheat grain development and starch biosynthesis. J. Proteom. 2018, 185, 8–24. [Google Scholar] [CrossRef]
- Fábián, A.; Jäger, K.; Rakszegi, M.; Barnabás, B. Embryo and endosperm development in wheat (Triticum aestivum L.) kernels subjected to drought stress. Plant Cell Rep. 2011, 30, 551–563. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, D.; Deng, X.; Zhen, S.; Wang, Z.; Yan, Y. Effects of water deficit on breadmaking quality and storage protein compositions in bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2018, 98, 4357–4368. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2024, 15, 1507628. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, Y.; Zhao, J.; Chen, Z.; Huang, X.; Fan, G. Regulating the composition and secondary structure of wheat protein through canopy shading to improve dough performance and nutritional index. Food Res. Int. 2023, 173, 113399. [Google Scholar] [CrossRef]
- Ma, H.; Yang, Y.; Zhao, J.; Huang, X.; Yang, H.; Zheng, T.; Fan, G. Relationship between the baking quality of wheat (Triticum aestivum L.) and the protein composition and structure after shading. Food Chem. 2024, 441, 138392. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Zhuo, Q.; Zhang, B.; Wang, F.; Jiang, D. Starch Granule Size Distribution and Pasting Characteristic Response to Post-Anthesis Combined Stress of Waterlogging and Shading. Agriculture 2020, 10, 384. [Google Scholar] [CrossRef]
- Yang, H.; Zhao, J.; Ma, H.; Shi, Z.; Huang, X.; Fan, G. Shading affects the starch structure and digestibility of wheat by regulating the photosynthetic light response of flag leaves. Int. J. Biol. Macromol. 2023, 236, 123972. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Q.; Chang, X.; Wang, D.; Wang, Y.; Yang, Y.; Zhao, G.; Shi, S. Effects of Chemical Regulation on Wheat Yield and Quality under Different Soil Conditions. Crops 2021, 37, 96–100. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Ning, T.; Zhao, C.; Jiao, N.; Liu, Z.; Li, Z. Effect on Quality Traits and Grain Yield of the High-qualityWheat in Different Types of Soil. J. Shihezi Univ. (Nat. Sci.) 2006, 01, 75–78. [Google Scholar] [CrossRef]
- Smutná, P.; Tokatlidis, I.S. The influence of different soil types on rainfed wheat varieties for grain and protein yield. Cereal Res. Commun. 2020, 48, 391–398. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Wang, D.; Yang, Y.; Wang, Y.; Guo, D.; Liu, Z.; Chang, X.; Shi, S.; Zhao, G. Response of Yield and Quality of Strong Gluten Wheat to Different Soil Conditions and Nitrogen Levels. Crops 2022, 38, 167–173. [Google Scholar] [CrossRef]
- Dakora, F.D.; Li, H.; Zhao, J. Exploring the Impacts of Elevated CO2 on Food Security: Nutrient Assimilation, Plant Growth, and Crop Quality. Engineering 2025, 44, 234–244. [Google Scholar] [CrossRef]
- Gare, S.; Wagh, R.S.; Ingle, A.U. Evaluation of physiological, enzymatic, biochemical, nutritional parameters and stress tests in wheat genotypes. Ann. Phytomed. 2023, 12, 856–864. [Google Scholar] [CrossRef]
- Lobell, D.B.; Schlenker, W.; Costa-Roberts, J. Climate trends and global crop production since 1980. Science 2011, 333, 616–620. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Tao, Y.; Liu, M.; Yang, D.; Zhu, M.; Ding, J.; Zhu, X.; Guo, W.; Zhou, G.; Li, C. Does temporary heat stress or low temperature stress similarly affect yield, starch, and protein of winter wheat grain during grain filling? J. Cereal Sci. 2022, 103, 103408. [Google Scholar] [CrossRef]
- Lafiandra, D.; Shewry, P.R. Wheat Glutenin polymers 2. The role of wheat glutenin subunits in polymer formation and dough quality. J. Cereal Sci. 2022, 106, 103487. [Google Scholar] [CrossRef]
- Tanaka, H.; Gorafi, Y.S.A.; Fujita, M.; Sasaki, H.; Tahir, I.S.A.; Tsujimoto, H. Expression of seed storage proteins responsible for maintaining kernel traits and wheat flour quality in common wheat under heat stress conditions. Breed Sci. 2021, 71, 184–192. [Google Scholar] [CrossRef]
- Kong, X.; Hou, R.; Yang, G.; Ouyang, Z. Climate warming extends the effective growth period of winter wheat and increases grain protein content. Agric. Forest Meteorol. 2023, 336, 109477. [Google Scholar] [CrossRef]
- Payne, P.I.; Holt, L.M.; Krattiger, A.F.; Carrillo, J.M. Relationships between seed quality characteristics and HMW glutenin subunit composition determined using wheats grown in Spain. J. Cereal Sci. 1988, 7, 229–235. [Google Scholar] [CrossRef]
- Aono, S.; Nishitsuji, Y.; Iwaki, S.; Hayakawa, K. Effects of environmental temperature during maturation on protein characteristics in spring wheat (Triticum aestivum cv. Haruyokoi). J. Cereal Sci. 2024, 116, 103838. [Google Scholar] [CrossRef]
- Hernández-Espinosa, N.; Mondal, S.; Autrique, E.; Gonzalez-Santoyo, H.; Crossa, J.; Huerta-Espino, J.; Singh, R.P.; Guzmán, C. Milling, processing and end-use quality traits of CIMMYT spring bread wheat germplasm under drought and heat stress. Field Crops Res. 2018, 215, 104–112. [Google Scholar] [CrossRef]
- Guzmán, C.; Autrique, J.E.; Mondal, S.; Singh, R.P.; Govindan, V.; Morales-Dorantes, A.; Posadas-Romano, G.; Crossa, J.; Ammar, K.; Peña, R.J. Response to drought and heat stress on wheat quality, with special emphasis on bread-making quality, in durum wheat. Field Crops Res. 2016, 186, 157–165. [Google Scholar] [CrossRef]
- Randall, P.J.; Moss, H.J. Some effects of temperature regime during grain filling on wheat quality. Crop Pasture Sci. 1990, 41, 603–617. [Google Scholar] [CrossRef]
- Fleitas, M.C.; Mondal, S.; Gerard, G.S.; Hernández-Espinosa, N.; Singh, R.P.; Crossa, J.; Guzmán, C. Identification of CIMMYT spring bread wheat germplasm maintaining superior grain yield and quality under heat-stress. J. Cereal Sci. 2020, 93, 102981. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, L.; Hong, Y.; Li, Z.; Li, C.; Gu, Z. An extensive review: How starch and gluten impact dough machinability and resultant bread qualities. Crit. Rev. Food Sci. 2023, 63, 1930–1941. [Google Scholar] [CrossRef]
- Balla, K.; Rakszegi, M.; Li, Z.; Békés, F.; Bencze, S.; Veisz, O. Quality of winter wheat in relation to heat and drought shock after anthesis. Czech J. Food Sci. 2011, 29, 117–128. [Google Scholar] [CrossRef]
- Chunduri, V.; Kaur, A.; Kaur, S.; Kumar, A.; Sharma, S.; Sharma, N.; Singh, P.; Kapoor, P.; Kaur, S.; Kumari, A.; et al. Gene Expression and Proteomics Studies Suggest an Involvement of Multiple Pathways Under Day and Day-Night Combined Heat Stresses During Grain Filling in Wheat. Front. Plant Sci. 2021, 12, 660446. [Google Scholar] [CrossRef]
- Mahdavi, S.; Arzani, A.; Mirmohammady Maibody, S.A.M.; Kadivar, M. Grain and flour quality of wheat genotypes grown under heat stress. Saudi. J. Biol. Sci. 2022, 29, 103417. [Google Scholar] [CrossRef]
- Tahir, I.S.A.; Nakata, N.; Ali, A.M.; Mustafa, H.M.; Saad, A.S.I.; Takata, K.; Ishikawa, N.; Abdalla, O.S. Genotypic and temperature effects on wheat grain yield and quality in a hot irrigated environment. Plant Breed. 2006, 125, 323–330. [Google Scholar] [CrossRef]
- Zhao, H.; Dai, T.; Jing, Q.; Jiang, D.; Cao, W.; Lu, W.; Tian, X. Effects of High Temperature during Grain Filling on Key Enzymes Involved in Starch Synthesis in Two Wheat Cultivars with Different Quality Types. Acta Agron. Sin. 2006, 32, 423–429. [Google Scholar]
- Kumari, A.; Kumar, R.R.; Singh, J.P.; Verma, P.; Singh, G.P.; Chinnusamy, V.; Praveen, S.; Goswami, S. Characterization of the starch synthase under terminal heat stress and its effect on grain quality of wheat. 3 Biotech 2020, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, J.; Tian, L.; Wang, S.; Tang, L.; Cao, W.; Zhu, Y. Effect of Postanthesis High Temperature on Grain Quality Formation for Wheat. Agron. J. 2017, 109, 1970–1980. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, D.; Dai, C.; Zhu, Y.; Zhu, M.; Ding, J.; Zhu, X.; Zhou, G.; Guo, W.; Li, C. Difference in Starch Structure and Physicochemical Properties between Waxy Wheat and Non-Waxy Wheat Subjected to Temporary Heat Stress during Grain Filling. Agronomy 2023, 13, 2067. [Google Scholar] [CrossRef]
- Liu, P.; Guo, W.; Jiang, Z.; Pu, H.; Feng, C.; Zhu, X.; Peng, Y.; Kuang, A.; Little, C.R. Effects of high temperature after anthesis on starch granules in grains of wheat (Triticum aestivum L.). J. Agric. Sci. 2011, 149, 159–169. [Google Scholar] [CrossRef]
- Lu, H.; Wang, C.; Guo, T.; Xie, Y.; Feng, W.; Li, S. Starch composition and its granules distribution in wheat grains in relation to post-anthesis high temperature and drought stress treatments. Starch Stärke 2014, 66, 419–428. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, R.Q.; Fu, K.Y.; Li, C.; Li, C. Effects of high temperature on starch morphology and the expression of genes related to starch biosynthesis and degradation. J. Cereal Sci. 2017, 73, 25–32. [Google Scholar] [CrossRef]
- Arenas-M, A.; Castillo, F.M.; Godoy, D.; Canales, J.; Calderini, D.F. Transcriptomic and Physiological Response of Durum Wheat Grain to Short-Term Heat Stress during Early Grain Filling. Plants 2022, 11, 59. [Google Scholar] [CrossRef]
- Shahid, M.R.; Wakeel, A.; Ullah, M.S.; Gaydon, D.S. Identifying changes to key APSIM-wheat constants to sensibly simulate high temperature crop response in Pakistan. Field Crops Res. 2024, 307, 109265. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Wang, X.; Wang, L.; Zhang, M.; Ren, M. Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop Pasture Sci. 2018, 69, 1208–1214. [Google Scholar] [CrossRef]
- Rangan, P.; Furtado, A.; Henry, R. Differential response of wheat genotypes to heat stress during grain filling. Exp. Agric. 2019, 55, 818–827. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Ding, M.; Min, D.; Wang, Z.; Gao, X. The influence of night warming treatment on the micro-structure of gluten in two wheat cultivars. Food Res. Int. 2019, 116, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Giménez, V.D.; Serrago, R.A.; García, G.A.; Miralles, D.J. How milling and breadmaking quality are modified by warmer nights in wheat? J. Cereal Sci. 2021, 102, 103343. [Google Scholar] [CrossRef]
- Impa, S.M.; Vennapusa, A.R.; Bheemanahalli, R.; Sabela, D.; Boyle, D.; Walia, H.; Jagadish, S.V.K. High night temperature induced changes in grain starch metabolism alters starch, protein, and lipid accumulation in winter wheat. Plant Cell Environ. 2020, 43, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P. Cereal Lipids. Nutr. Food Sci. 1984, 84, 15. [Google Scholar] [CrossRef]
- Impa, S.M.; Raju, B.; Hein, N.T.; Sandhu, J.; Prasad, P.V.V.; Walia, H.; Jagadish, S.V.K. High night temperature effects on wheat and rice: Current status and way forward. Plant Cell Environ. 2021, 44, 2049–2065. [Google Scholar] [CrossRef]
- Fan, Y.; Qin, B.; Yang, J.; Ma, L.; Cui, G.; He, W.; Tang, Y.; Zhang, W.; Ma, S.; Ma, C.; et al. Night warming increases wheat yield by improving pre-anthesis plant growth and post-anthesis grain starch biosynthesis. J. Integr. Agric. 2024, 23, 536–550. [Google Scholar] [CrossRef]
- Liu, L.; Song, H.; Shi, K.; Liu, B.; Zhang, Y.; Tang, L.; Cao, W.; Zhu, Y. Response of wheat grain quality to low temperature during jointing and booting stages—On the importance of considering canopy temperature. Agric. Forest Meteorol. 2019, 278, 107658. [Google Scholar] [CrossRef]
- Porter, J.R.; Gawith, M. Temperatures and the growth and development of wheat: A review. Eur. J. Agron. 1999, 10, 23–36. [Google Scholar] [CrossRef]
- Shi, K.; Yin, T.; Zhu, Y.; Liu, B.; Tang, L.; Cao, W.; Liu, L. Estimating the effect of low-temperature stress on the spatial distribution patterns of protein in wheat grains. J. Cereal Sci. 2022, 105, 103461. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, K.; Gu, D.; Zhang, S.; Wu, J. Quantifying the effect of low-temperature events on the grain quality formation of wheat. J. Cereal Sci. 2021, 100, 103257. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat Stress in Wheat during Reproductive and Grain-Filling Phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Graybosch, R.A.; Peterson, C.J.; Baenziger, P.S.; Shelton, D.R. Environmental modification of hard red winter wheat flour protein composition. J. Cereal Sci. 1995, 22, 45–51. [Google Scholar] [CrossRef]
- D’Ovidio, R.; Masci, S. The low-molecular-weight glutenin subunits of wheat gluten. J. Cereal Sci. 2004, 39, 321–339. [Google Scholar] [CrossRef]
- Koga, S.; Böcker, U.; Moldestad, A.; Tosi, P.; Shewry, P.R.; Mosleth, E.F.; Uhlen, A.K. Influence of temperature on the composition and polymerization of gluten proteins during grain filling in spring wheat (Triticum aestivum L.). J. Cereal Sci. 2015, 65, 1–8. [Google Scholar] [CrossRef]
- Koga, S.; Böcker, U.; Moldestad, A.; Tosi, P.; Shewry, P.R.; Mosleth, E.F.; Uhlen, A.K. Influence of temperature during grain filling on gluten viscoelastic properties and gluten protein composition. J. Sci. Food Agric. 2016, 96, 122–130. [Google Scholar] [CrossRef]
- Moldestad, A.; Fergestad, E.M.; Hoel, B.; Skjelvåg, A.O.; Uhlen, A.K. Effect of temperature variation during grain filling on wheat gluten resistance. J. Cereal Sci. 2011, 53, 347–354. [Google Scholar] [CrossRef]
- Zeng, Y.; Yu, J.; Cang, J.; Liu, L.; Mu, Y.; Wang, J.; Zhang, D. Detection of sugar accumulation and expression levels of correlative key enzymes in winter wheat (Triticum aestivum) at low temperatures. Biosci. Biotechnol. Biochem. 2011, 75, 681–687. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Li, L.; Xu, X.; Yang, L.; Luo, Z.; Wang, B.; Ma, S.; Fan, Y.; Huang, Z. The Effects of Short-Term Exposure to Low Temperatures During the Booting Stage on Starch Synthesis and Yields in Wheat Grain. Front. Plant Sci. 2021, 12, 684784. [Google Scholar] [CrossRef]
- Zhang, A.; Li, Z.; Zhou, Q.; Zhao, J.; Zhao, Y.; Zhao, M.; Ma, S.; Fan, Y.; Huang, Z.; Zhang, W. An integrated physiology and proteomics analysis reveals the response of wheat grain to low temperature stress during booting. J. Integr. Agric. 2023, 24, 114–131. [Google Scholar] [CrossRef]
- Du, X.; Gao, Z.; Sun, X.; Bian, D.; Ren, J.; Yan, P.; Cui, Y. Increasing temperature during early spring increases winter wheat grain yield by advancing phenology and mitigating leaf senescence. Sci. Total Environ. 2022, 812, 152557. [Google Scholar] [CrossRef]
- Liu, Z.; Guo, D.; Chang, X.; Wang, D.; Yang, Y.; Liu, X.; Wang, Y.; Shi, S.; Wang, Y.; Zhao, G. Effects of Nitrogen Dressing Time and Proportion on Wheat Grain Filling and Its Physiological Mechanism. Crops 2024, 40, 174–179. [Google Scholar] [CrossRef]
- Dias, A.S.; Lidon, F.C. Evaluation of Grain Filling Rate and Duration in Bread and Durum Wheat, under Heat Stress after Anthesis. J. Agron. Crop Sci. 2009, 195, 137–147. [Google Scholar] [CrossRef]
- Sofield, I.; Evans, L.T.; Cook, M.G.; Wardlaw, I.F. Factors Influencing the Rate and Duration of Grain Filling in Wheat. Funct. Plant Biol. 1977, 4, 785–797. [Google Scholar] [CrossRef]
- Xu, S.; Dong, Q.; Deng, M.; Lin, D.; Xiao, J.; Cheng, P.; Xing, L.; Niu, Y.; Gao, C.; Zhang, W.; et al. The vernalization-induced long non-coding RNA VAS functions with the transcription factor TaRF2b to promote TaVRN1 expression for flowering in hexaploid wheat. Mol. Plant 2021, 14, 1525–1538. [Google Scholar] [CrossRef]
- He, D.; Fang, S.; Liang, H.; Wang, E.; Wu, D. Contrasting yield responses of winter and spring wheat to temperature rise in China. Environ. Res. Lett. 2020, 15, 124038. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, B.; Yao, Y.; Guo, Z.; Jia, H.; Kong, L.; Zhang, A.; Ma, W.; Ni, Z.; Xu, S.; et al. Wheat genomic study for genetic improvement of traits in China. Sci. China Life Sci. 2022, 65, 1718–1775. [Google Scholar] [CrossRef]
- Wang, X.; Hou, L.; Lu, Y.; Wu, B.; Gong, X.; Liu, M.; Wang, J.; Sun, Q.; Vierling, E.; Xu, S. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J. Exp. Bot. 2018, 69, 5531–5545. [Google Scholar] [CrossRef]
- Bonfil, D.J.; Abbo, S.; Svoray, T. Sowing Date and Wheat Quality as Determined by Gluten Index. Crop Sci. 2015, 55, 2294–2306. [Google Scholar] [CrossRef]
- Slafer, G.A.; Savin, R.; Pinochet, D.; Calderini, D.F. Chapter 3—Wheat. In Crop Physiology Case Histories for Major Crops; Sadras, V.O., Calderini, D.F., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 98–163. [Google Scholar] [CrossRef]
- Su, H. Effects of Asymmetric Warming on Yield and Quality of Winter Wheat. Master’s Thesis, Nanjing University of Information Science & Technology, Nanjing, China, 2016. [Google Scholar]
- Lazauskas, S.; Povilaitis, V.; Antanaitis, S.; Miliauskienė, J.; Sakalauskienė, S.; Pšibišauskienė, G.; Auskalniene, O.; Raudonius, S.; Duchovskis, P. Winter wheat leaf area index under low and moderate input management and climate change. J. Food Agric. Environ. 2012, 10, 588–593. [Google Scholar]
- Wan, S.; Xia, J.; Liu, W.; Niu, S. Photosynthetic overcompensation under nocturnal warming enhances grassland carbon sequestration. Ecology 2009, 90, 2700–2710. [Google Scholar] [CrossRef]
- Hou, R.; Ouyang, Z.; Li, Y.; Wilson, G.V.; Li, H. Is the change of winter wheat yield under warming caused by shortened reproductive period? Ecol. Evol. 2012, 2, 2999–3008. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Xu, X.; Ouyang, Z. Effect of experimental warming on nitrogen uptake by winter wheat under conventional tillage versus no-till systems. Soil Till Res. 2018, 180, 116–125. [Google Scholar] [CrossRef]
- Uprety, D.C.; Sen, S.; Dwivedi, N. Rising atmospheric carbon dioxide on grain quality in crop plants. Physiol. Mol. Biol. Pla. 2010, 16, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Gebbing, T.; Schnyder, H.; KÜhbauch, W. The utilization of pre-anthesis reserves in grain filling of wheat. Assessment by steady-state 13CO2/12CO2 labelling. Plant Cell Environ. 1999, 22, 851–858. [Google Scholar] [CrossRef]
- He, J.; Goyal, R.; Laroche, A.; Zhao, M.; Lu, Z. Water stress during grain development affects starch synthesis, composition and physicochemical properties in triticale. J. Cereal Sci. 2012, 56, 552–560. [Google Scholar] [CrossRef]
- Fahy, B.; Siddiqui, H.; David, L.C.; Powers, S.J.; Borrill, P.; Uauy, C.; Smith, A.M. Final grain weight is not limited by the activity of key starch-synthesising enzymes during grain filling in wheat. J. Exp. Bot. 2018, 69, 5461–5475. [Google Scholar] [CrossRef]
- Clifton, M. The Effects of Elevated Temperature Stress on Grain-Filling in Wheat (Triticum aestivum L.). Ph.D. Thesis, The University of Sydney, Sydney, Australia, 2023. [Google Scholar]
- Impa, S.M.; Sunoj, V.S.J.; Krassovskaya, I.; Bheemanahalli, R.; Obata, T.; Jagadish, S.V.K. Carbon balance and source-sink metabolic changes in winter wheat exposed to high night-time temperature. Plant Cell Environ. 2019, 42, 1233–1246. [Google Scholar] [CrossRef]
- Posch, B.C.; Kariyawasam, B.C.; Bramley, H.; Coast, O.; Richards, R.A.; Reynolds, M.P.; Trethowan, R.; Atkin, O.K. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J. Exp. Bot. 2019, 70, 5051–5069. [Google Scholar] [CrossRef]
- Kaushik, M.; Mulani, E.; Kumar, A.; Chauhan, H.; Saini, M.R.; Bharti, A.; Gayatri; Iyyappan, Y.; Madhavan, J.; Sevanthi, A.M.; et al. Starch and storage protein dynamics in the developing and matured grains of durum wheat and diploid progenitor species. Int. J. Biol. Macromol. 2024, 267, 131177. [Google Scholar] [CrossRef] [PubMed]
- Fowler, D.B.; Brydon, J.; Darroch, B.A.; Entz, M.H.; Johnston, A.M. Environment and Genotype Influence on Grain Protein Concentration of Wheat and Rye. Agron. J. 1990, 82, 655–664. [Google Scholar] [CrossRef]
- Panozzo, J.F.; Eagles, H.A. Cultivar and environmental effects on quality characters in wheat. II. Protein. Crop Pasture Sci. 2000, 51, 629–636. [Google Scholar] [CrossRef]
- Zheng, B.; Chenu, K.; Fernanda Dreccer, M.; Chapman, S.C. Breeding for the future: What are the potential impacts of future frost and heat events on sowing and flowering time requirements for Australian bread wheat (Triticum aestivium) varieties? Glob. Change Biol. 2012, 18, 2899–2914. [Google Scholar] [CrossRef] [PubMed]
- Cromey, M.G.; Wright, D.S.C.; Boddington, H.J. Effects of frost during grain filling on wheat yield and grain structure. New Zeal. J. Crop Hort. 1998, 26, 279–290. [Google Scholar] [CrossRef]
- Li, X.; Pu, H.; Liu, F.; Zhou, Q.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Winter Wheat Photosynthesis and Grain Yield Responses to Spring Freeze. Agron. J. 2015, 107, 1002–1010. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, S.; Malik, J.A.; Berger, J.D.; Nayyar, H. Cold stress effects on reproductive development in grain crops: An overview. Environ. Exp. Bot. 2010, 67, 429–443. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Wang, P.; Wang, H.; Huang, W.; Wu, Y.; Yin, Y. Freeze resistance analysis of different wheat cultivars based on the relationships between physiological indices and grain yield. Ying Yong Sheng Tai Xue Bao 2011, 22, 1477–1484. [Google Scholar]
- Bhattacharya, A. Effect of Low-Temperature Stress on Germination, Growth, and Phenology of Plants: A Review. In Physiological Processes in Plants Under Low Temperature Stress; Bhattacharya, A., Ed.; Springer: Singapore, 2022; pp. 1–106. [Google Scholar] [CrossRef]
- Tecson, M.C.B.; Geluz, C.; Cruz, Y.; Greene, E.R. Glutamine Synthetase: Diverse Regulation and Functions of an Ancient Enzyme. Biochemistry 2025, 64, 547–554. [Google Scholar] [CrossRef]
- Ma, W.; Appels, R.; Bekes, F.; Larroque, O.; Morell, M.K.; Gale, K.R. Genetic characterisation of dough rheological properties in a wheat doubled haploid population: Additive genetic effects and epistatic interactions. Theor. Appl. Genet. 2005, 111, 410–422. [Google Scholar] [CrossRef]
- Lu, H.; Wang, C.; Guo, T.; Yin, Y. Effects of high-temperature and drought stress on protein concentration and key enzyme activities in relation to nitrogen metabolism in wheat grains during the early stage of grain filling. Acta Ecol. Sin. 2014, 34, 3612–3619. [Google Scholar] [CrossRef]
- Zhang, S.; Ghatak, A.; Bazargani, M.M.; Bajaj, P.; Varshney, R.K.; Chaturvedi, P.; Jiang, D.; Weckwerth, W. Spatial distribution of proteins and metabolites in developing wheat grain and their differential regulatory response during the grain filling process. Plant J. 2021, 107, 669–687. [Google Scholar] [CrossRef]
- Hu, J.; Yu, M.; Chang, Y.; Tang, H.; Wang, W.; Du, L.; Wang, K.; Yan, Y.; Ye, X. Functional analysis of TaPDI genes on storage protein accumulation by CRISPR/Cas9 edited wheat mutants. Int. J. Biol. Macromol. 2022, 196, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Zhen, J.; Song, T. Individual and combined effects of heat and drought and subsequent recovery on winter wheat (Triticum aestivum L.) photosynthesis, nitrogen metabolism, cell osmoregulation, and yield formation. Plant Physiol. Bioch. 2023, 196, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dai, T.; Jiang, D.; Cao, W. Effects of High Temperature on Key Enzymes Involved in Starch and Protein Formation in Grains of Two Wheat Cultivars. J. Agron. Crop Sci. 2008, 194, 47–54. [Google Scholar] [CrossRef]
- Majláth, I.; Darko, E.; Palla, B.; Nagy, Z.; Janda, T.; Szalai, G. Reduced light and moderate water deficiency sustain nitrogen assimilation and sucrose degradation at low temperature in durum wheat. J. Plant Physiol. 2016, 191, 149–158. [Google Scholar] [CrossRef]
- Ning, P.; Yang, L.; Li, C.; Fritschi, F.B. Post-silking carbon partitioning under nitrogen deficiency revealed sink limitation of grain yield in maize. J. Exp. Bot. 2018, 69, 1707–1719. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, L.; Li, H.; Yang, C.; Wang, D.; Lin, X.; Lin, Y.; Zhang, H.; Li, X.; Zhao, P.; et al. TaNF-Y-PRC2 orchestrates temporal control of starch and protein synthesis in wheat. bioRxiv 2023. bioRxiv:2023.2012.2026.573020. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Wang, C.; Liu, W.; Ma, G.; Han, Q.; Ma, D. Effects of High Temperature and Drought Stress on the Expression of Gene Encoding Enzymes and the Activity of Key Enzymes Involved in Starch Biosynthesis in Wheat Grains. Front. Plant Sci. 2019, 10, 1414. [Google Scholar] [CrossRef]
- Harris, P.J.; Burrell, M.M.; Emes, M.J.; Tetlow, I.J. Effects of Post-Anthesis High-Temperature Stress on Carbon Partitioning and Starch Biosynthesis in a Spring Wheat (Triticum aestivum L.) Adapted to Moderate Growth Temperatures. Plant Cell Physiol. 2023, 64, 729–745. [Google Scholar] [CrossRef]
- Singletary, G.W. Starch synthesis and grain filling in wheat. In Developments in Crop Science; Gupta, A.K., Kaur, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 26, pp. 79–105. [Google Scholar] [CrossRef]
- Satoh, H.; Shibahara, K.; Tokunaga, T.; Nishi, A.; Tasaki, M.; Hwang, S.K.; Okita, T.W.; Kaneko, N.; Fujita, N.; Yoshida, M.; et al. Mutation of the plastidial alpha-glucan phosphorylase gene in rice affects the synthesis and structure of starch in the endosperm. Plant Cell 2008, 20, 1833–1849. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Seijo, J.A.; Ruzanski, C.; Krucewicz, K.; Meier, S.; Hägglund, P.; Svensson, B.; Palcic, M.M. Functional and structural characterization of plastidic starch phosphorylase during barley endosperm development. PLoS ONE 2017, 12, e0175488. [Google Scholar] [CrossRef] [PubMed]
- Van der Maarel, M.J.E.C.; Leemhuis, H. Starch modification with microbial alpha-glucanotransferase enzymes. Carbohyd. Polym. 2013, 93, 116–121. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, A.; Zhou, Q.; Fang, R.; Zhao, Y.; Li, Z.; Zhao, J.; Zhao, M.; Ma, S.; Fan, Y.; et al. Low-temperature at booting reduces starch content and yield of wheat by affecting dry matter transportation and starch synthesis. Front. Plant Sci. 2023, 14, 1207518. [Google Scholar] [CrossRef]
- Liu, B.; Martre, P.; Ewert, F.; Porter, J.R.; Challinor, A.J.; Müller, C.; Ruane, A.C.; Waha, K.; Thorburn, P.J.; Aggarwal, P.K.; et al. Global wheat production with 1.5 and 2.0 °C above pre-industrial warming. Glob. Change Biol. 2019, 25, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhao, M.; Yin, W.; Li, Y.; Guo, R.; Jiao, Z.; Zhang, Y. Physiological mechanism and gene discovery for high temperature tolerance of wheat: A review. J. South. Agric. 2022, 53, 2885–2893. [Google Scholar]
- Fu, J.; Ristic, Z. Analysis of transgenic wheat (Triticum aestivum L.) harboring a maize (Zea mays L.) gene for plastid EF-Tu: Segregation pattern, expression and effects of the transgene. Plant Mol. Biol. 2010, 73, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Song, N.; Zheng, M.; Liu, X.; Liu, Z.; Xing, J.; Ma, J.; Guo, W.; Yao, Y.; Peng, H.; et al. Histone acetyltransferase GCN5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis. Plant J. 2015, 84, 1178–1191. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, J.; Zhang, N.; Xin, M.; Peng, H.; Hu, Z.; Ni, Z.; Du, J. The wheat NAC transcription factor TaNAC2L is regulated at the transcriptional and post-translational levels and promotes heat stress tolerance in transgenic Arabidopsis. PLoS ONE 2015, 10, e0135667. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, X.; Wang, F.; Zhang, L.; Xin, M.; Hu, Z.; Yao, Y.; Ni, Z.; Sun, Q.; Peng, H. Characterization of wheat MYB genes responsive to high temperatures. BMC Plant Biol. 2015, 17, 1–14. [Google Scholar] [CrossRef]
- Geng, X.; Zang, X.; Li, H.; Liu, Z.; Zhao, A.; Liu, J.; Peng, H.; Yao, Y.; Hu, Z.; Ni, Z.; et al. Unconventional splicing of wheat TabZIP60 confers heat tolerance in transgenic Arabidopsis. Plant Sci. 2018, 274, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Weichert, H.; Högy, P.; Mora-Ramirez, I.; Fuchs, J.; Eggert, K.; Koehler, P.; Weschke, W.; Fangmeier, A.; Weber, H. Grain yield and quality responses of wheat expressing a barley sucrose transporter to combined climate change factors. J. Exp. Bot. 2017, 68, 5511–5525. [Google Scholar] [CrossRef]
- Zang, X.; Geng, X.; Wang, F.; Liu, Z.; Zhang, L.; Zhao, Y.; Tian, X.; Ni, Z.; Yao, Y.; Xin, M.; et al. Overexpression of wheat ferritin gene TaFER-5B enhances tolerance to heat stress and other abiotic stresses associated with the ROS scavenging. BMC Plant Biol. 2017, 17, 14. [Google Scholar] [CrossRef]
- Zang, X.; Geng, X.; Liu, K.; Wang, F.; Liu, Z.; Zhang, L.; Zhao, Y.; Tian, X.; Hu, Z.; Yao, Y.; et al. Ectopic expression of TaOEP16-2-5B, a wheat plastid outer envelope protein gene, enhances heat and drought stress tolerance in transgenic Arabidopsis plants. Plant Sci. 2017, 258, 1–11. [Google Scholar] [CrossRef]
- Zang, X.; Geng, X.; He, K.; Wang, F.; Tian, X.; Xin, M.; Yao, Y.; Hu, Z.; Ni, Z.; Sun, Q.; et al. Overexpression of the Wheat (Triticum aestivum L.) TaPEPKR2 Gene Enhances Heat and Dehydration Tolerance in Both Wheat and Arabidopsis. Front. Plant Sci. 2018, 9, 1710. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, X.; Zhang, H.; Zhou, C.; Zhao, A.; Wang, F.; Zhao, Y.; Tian, X.; Hu, Z.; Xin, M. Isolation and characterization of heat -responsive gene TaGASR1 from wheat (Triticum aestivum L.). J. Plant Biol. 2017, 60, 57–65. [Google Scholar] [CrossRef]
- Hu, X.; Chen, D.; Lynne Mclntyre, C.; Fernanda Dreccer, M.; Zhang, Z.B.; Drenth, J.; Kalaipandian, S.; Chang, H.; Xue, G. Heat shock factor C2a serves as a proactive mechanism for heat protection in developing grains in wheat via an ABA-mediated regulatory pathway. Plant Cell Environ. 2018, 41, 79–98. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Z.; Duan, S.; Zhang, Y.; Li, G.; Guo, X. Cloning and Characterization of Heat Shock Transcription Factor Gene TaHsfB2d and Its Regulating Role in Thermotolerance. Acta Agron. Sin. 2018, 44, 53–62. [Google Scholar] [CrossRef]
- Liu, Z.; Li, G.; Zhang, H.; Zhang, Y.; Zhang, Y.; Duan, S.; Sheteiwy, M.S.A.; Zhang, H.; Shao, H.; Guo, X. TaHsfA2-1, a new gene for thermotolerance in wheat seedlings: Characterization and functional roles. J. Plant Physiol. 2020, 246–247, 153135. [Google Scholar] [CrossRef]
- Li, G.; Liu, Z.; Zhang, H.; Zhao, B.; Zhang, Y.; Ma, Z.; Duan, S.; Meng, X.; Guo, X. Molecular characterization of a novel heat shock transcription factor gene TaHsfA2-11 and its overexpression improves thermotolerance in wheat. Environ. Exp. Bot. 2024, 218, 105609. [Google Scholar] [CrossRef]
- Xue, G.; Drenth, J.; McIntyre, C. TaHsfA6f is a transcriptional activator that regulates a suite of heat stress protection genes in wheat (Triticum aestivum L.) including previously unknown Hsf targets. J. Exp. Bot. 2015, 66, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Feng, M.; Jiang, Y.; Du, D.; Dong, C.; Zhang, Z.; Wang, W.; Liu, J.; Liu, X.; Li, S.; et al. Thermosensitive SUMOylation of TaHsfA1 defines a dynamic ON/OFF molecular switch for the heat stress response in wheat. Plant Cell 2023, 35, 3889–3910. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, W.; Wang, W.; Zhang, G.; Liu, Y.; Wang, Y.; Wang, W. Wheat F-Box Protein Gene TaFBA1 Is Involved in Plant Tolerance to Heat Stress. Front. Plant. Sci. 2018, 9, 521. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Dong, J.; Tian, X.; Wang, J.; Palta, J.A.; Xu, S.; Fang, Y.; Wang, Z. Over-Expression of the Heat-Responsive Wheat Gene TaHSP23.9 in Transgenic Arabidopsis Conferred Tolerance to Heat and Salt Stress. Front. Plant Sci. 2020, 11, 243. [Google Scholar] [CrossRef]
- Tian, X.; Wang, F.; Zhao, Y.; Lan, T.; Yu, K.; Zhang, L.; Qin, Z.; Hu, Z.; Yao, Y.; Ni, Z.; et al. Heat shock transcription factor A1b regulates heat tolerance in wheat and Arabidopsis through OPR3 and jasmonate signalling pathway. Plant Biotechnol. J. 2020, 18, 1109–1111. [Google Scholar] [CrossRef]
- Thomelin, P.; Bonneau, J.; Brien, C.; Suchecki, R.; Baumann, U.; Kalambettu, P.; Langridge, P.; Tricker, P.; Fleury, D. The wheat Seven in absentia gene is associated with increases in biomass and yield in hot climates. J. Exp. Bot. 2021, 72, 3774–3791. [Google Scholar] [CrossRef]
- Tian, X.; Qin, Z.; Zhao, Y.; Wen, J.; Lan, T.; Zhang, L.; Wang, F.; Qin, D.; Yu, K.; Zhao, A.; et al. Stress granule-associated TaMBF1c confers thermotolerance through regulating specific mRNA translation in wheat (Triticum aestivum). New Phytol. 2022, 233, 1719–1731. [Google Scholar] [CrossRef]
- Lin, J.; Song, N.; Liu, D.; Liu, X.; Chu, W.; Li, J.; Chang, S.; Liu, Z.; Chen, Y.; Yang, Q.; et al. Histone acetyltransferase TaHAG1 interacts with TaNACL to promote heat stress tolerance in wheat. Plant Biotechnol. J. 2022, 20, 1645–1647. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Yu, T.; Peng, C.; Fu, Y.; Fang, Y.; Li, Y.; Xu, Z.; Chen, J.; Dong, H.; Ma, Y.; et al. Somatic embryogenetic receptor kinase TaSERL2 regulates heat stress tolerance in wheat by influencing TaBZR2 protein stability and transcriptional activity. Plant Biotechnol. J. 2025, 1–17. [Google Scholar] [CrossRef]
- Cao, J.; Qin, Z.; Cui, G.; Chen, Z.; Cheng, X.; Peng, H.; Yao, Y.; Hu, Z.; Guo, W.; Ni, Z.; et al. Natural variation of STKc_GSK3 kinase TaSG-D1 contributes to heat stress tolerance in Indian dwarf wheat. Nat. Commun. 2024, 15, 2097. [Google Scholar] [CrossRef]
- Diao, D.; Li, Y.; Meng, X.; Ji, S.; Sun, Y.; Ma, X.; Li, J.; Feng, Y.; Li, C.; Wu, J.; et al. Cloning and Heat Tolerance Function of Wheat TaGRAS34-5A Gene. Sci. Agric. Sin. 2025, 58, 617–634. [Google Scholar] [CrossRef]
- Kovalchuk, N.; Chew, W.; Sornaraj, P.; Borisjuk, N.; Yang, N.; Singh, R.; Bazanova, N.; Shavrukov, Y.; Guendel, A.; Munz, E.; et al. The homeodomain transcription factor TaHDZipI-2 from wheat regulates frost tolerance, flowering time and spike development in transgenic barley. New Phytol. 2016, 211, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Snape, J.W.; Semikhodskii, A.; Fish, L.; Sarma, R.; Quarrie, S.A.; Galiba, G.; Sutka, J. Mapping frost tolerance loci in wheat and comparative mapping with other cereals. Acta Agron. Hung. 1997, 45, 265–270. [Google Scholar]
- Zhang, H.; Xue, X.; Guo, J.; Huang, Y.; Dai, X.; Li, T.; Hu, J.; Qu, Y.; Yu, L.; Mai, C.; et al. Association of the Recessive Allele vrn-D1 With Winter Frost Tolerance in Bread Wheat. Front. Plant Sci. 2022, 13, 879768. [Google Scholar] [CrossRef]
- Pan, X.; Nie, X.; Gao, W.; Yan, S.; Feng, H.; Cao, J.; Lu, J.; Shao, H.; Ma, C.; Chang, C.; et al. Identification of genetic loci and candidate genes underlying freezing tolerance in wheat seedlings. Theor. Appl. Genet. 2024, 137, 57. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Yu, T.; Chen, J.; Chen, M.; Zhou, Y.; Ma, Y.; Xu, Z.; Min, D. Response of Wheat Zinc-Finger Transcription Factor TaDi19A to Cold and Its Screening of Interacting Proteins. Sci. Agric. Sin. 2017, 50, 2411–2422. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, N.; Wang, S.; Tian, H.; Liu, L.; Pei, D.; Yu, X.; Zhao, L.; Chen, F. A TaSnRK1α Modulates TaPAP6L-Mediated Wheat Cold Tolerance through Regulating Endogenous Jasmonic Acid. Adv. Sci. 2023, 10, e2303478. [Google Scholar] [CrossRef]
- Ekele, J.U.; Webster, R.; Perez de Heredia, F.; Lane, K.E.; Fadel, A.; Symonds, R.C. Current impacts of elevated CO2 on crop nutritional quality: A review using wheat as a case study. Stress Biol. 2025, 5, 34. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Wahid, A.; Al Masruri, M.H.; Ullah, A.; Siddique, K.H.M.; Farooq, M. Impact of climate change on wheat grain composition and quality. J. Sci. Food Agric. 2023, 103, 2745–2751. [Google Scholar] [CrossRef]
- Mao, H.; Jiang, C.; Tang, C.; Nie, X.; Du, L.; Liu, Y.; Cheng, P.; Wu, Y.; Liu, H.; Kang, Z.; et al. Wheat adaptation to environmental stresses under climate change: Molecular basis and genetic improvement. Mol. Plant 2023, 16, 1564–1589. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Zhang, Z.; Lin, X.; Xiao, J. Epigenetic perspectives on wheat speciation, adaptation, and development. Trends in Genet. 2025, S0168-9525(25)00083-6. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Wang, R.; Yang, X.; Zhang, A.; Liu, D. Molecular Markers and Their Applications in Marker-Assisted Selection (MAS) in Bread Wheat (Triticum aestivum L.). Agriculture 2023, 13, 642. [Google Scholar] [CrossRef]
- Zhou, X.; Zhao, Y.; Ni, P.; Ni, Z.; Sun, Q.; Zong, Y. CRISPR-mediated acceleration of wheat improvement: Advances and perspectives. J. Genet. Genomics 2023, 50, 815–834. [Google Scholar] [CrossRef]
- Gaillochet, C.; Develtere, W.; Jacobs, T.B. CRISPR screens in plants: Approaches, guidelines, and future prospects. Plant Cell 2021, 33, 794–813. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Li, W.; Zhang, H.; Li, L. Big data and artificial intelligence-aided crop breeding: Progress and prospects. J. Integr. Plant Biol. 2025, 67, 722–739. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sharifi, R.; Khan, M.S.S.; Islam, F.; Bhat, J.A.; Kui, L.; Majeed, A. Wheat Microbiome: Structure, Dynamics, and Role in Improving Performance Under Stress Environments. Front. Microbiol. 2021, 12, 821546. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, R.; Gaikwad, K.B.; Babu, P.; Kumar Bainsla, N.; Dharmateja, P.; Ahmad Chaudhary, A.; Ansari, R. Deciphering the environmental impact on spike architectural traits for grain yield consolidation in bread wheat (T. aestivum L.). Saudi J. Biol. Sci. 2022, 29, 2800–2810. [Google Scholar] [CrossRef]
| Treatment Stage | Temperature | Variety | Change 1 | Reference |
|---|---|---|---|---|
| Controlled Phytotron Glasshouse | ||||
| Starch—Total starch content (%) | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [69] |
| Jointing period, Booting period | Three temperature levels: day/night, 6/16 °C (CK), 0/10 °C, −6/4 °C Two treatment durations: 3/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [72] |
| Booting period | Four temperature levels: −2, 0 or 2 °C from 19:00 to 07:00, and 5 °C from 07:00 to 19:00, without cold stress (CK) | Semi-winter (Yannong 19), spring wheat (Yangmai 18) | Decrease | [80] |
| Filling period | 18/8 °C (day/night), 3 d | Winter wheat (Ningmai 13, Zhenmai 12) | Decrease | [37] |
| Filling period | −5.5 °C, 3 h | Spring wheat (Kariega) | Decrease | [21] |
| Starch—Amylose (%) | ||||
| Jointing period, Booting period | Three temperature levels: day/night, 6/16 °C (CK), 0/10 °C, −6/4 °C Two treatment durations: 3/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [72] |
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [69] |
| Booting period | Four temperature levels: day/night, 12/12 h, 2/5 °C, 0/5 °C, −2/5 °C, without Low temperature treatment (CK) | Winter wheat (Wanmai 52), semi-winter (Yannong 19) | Decrease | [81] |
| Starch—Amylopectin (%) | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [69] |
| Jointing period, Booting period | Three temperature levels: day/night, 6/16 °C (CK), 0/10 °C, −6/4 °C Two treatment durations: 3/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [72] |
| Booting period | Four temperature levels: day/night, 12/12 h, 2/5 °C, 0/5 °C, −2/5 °C, without Low temperature treatment (CK) | Winter wheat (Wanmai 52), semi-winter (Yannong 19) | Decrease | [81] |
| Filling period | 18/8 °C (day/night), 3 d | Winter wheat (Ningmai 13, Zhenmai 12) | Decrease | [37] |
| Starch—B-type starch granules (%) | ||||
| Booting period | Four temperature levels: day/night, 12/12 h, 2/5 °C, 0/5 °C, −2/5 °C, without Low temperature treatment (CK) | Winter wheat (Wanmai 52), semi-winter (Yannong 19) | Decrease | [81] |
| Grain traits—Grain length | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [69] |
| Grain traits—Grain width | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [69] |
| Filling period | 18/8 °C (day/night), 3 d | Winter wheat (Ningmai 13, Zhenmai 12) | Decrease | [37] |
| Grain traits—Length–width ratio | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [69] |
| Grain traits—1000 grain weight | ||||
| Booting period | Four temperature levels: day/night, 12/12 h, 2/5 °C, 0/5 °C, −2/5 °C, without Low temperature treatment (CK) | Winter wheat (Wanmai 52), semi-winter (Yannong 19) | Decrease | [81] |
| Yield | ||||
| Booting period | Four temperature levels: day/night, 12/12 h, 2/5 °C, 0/5 °C, −2/5 °C, without Low temperature treatment (CK) | Winter wheat (Wanmai 52), semi-winter (Yannong 19) | Decrease | [81] |
| Protein—Total protein content (%) | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [69] |
| Jointing period, Booting period | Three temperature levels: day/night, 6/16 °C (CK), 0/10 °C, −6/4 °C Two treatment durations: 3/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [72] |
| Filling period | 18/8 °C (day/night), 3 d | Winter wheat (Ningmai 13, Zhenmai 12) | Increase | [37] |
| Filling period | −5.5 °C, 3 h | Spring bread wheat (Kariega, SST86), Durum wheat (Oranje), spring soft biscuit wheat (Snack) | Increase | [21] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Avle, Berserk, Bjarne, Zebra) | Decrease | [77] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Bjarne, Cadenza) | Decrease | [76] |
| Protein—Glu/Gli | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [69] |
| Protein—UPP (%) | ||||
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Cadenza) | Decrease | [76] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Bjarne) | Increase | [76] |
| Protein—Gliadin (%) | ||||
| Jointing period, Booting period | Three temperature levels: day/night, 6/16 °C (CK), 0/10 °C, −6/4 °C Two treatment durations: 3/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [72] |
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [69] |
| Protein—ω-gliadin (%) | ||||
| Filling period | 18/8 °C (day/night), 3d | Winter wheat (Ningmai 13, Zhenmai 12) | Increase | [37] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Bjarne, Cadenza) | Decrease | [76] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Avle, Berserk, Bjarne, Zebra) | Decrease | [77] |
| Protein—α-gliadin (%) | ||||
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Avle, Berserk, Bjarne, Zebra) | Increase | [77] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Bjarne, Cadenza) | Increase | [76] |
| Protein—γ-gliadin (%) | ||||
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Avle, Berserk, Bjarne, Zebra) | Increase | [77] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Bjarne, Cadenza) | Increase | [76] |
| Protein—Glutenin (%) | ||||
| Jointing period, Booting period | Three temperature levels: day/night, 6/16 °C (CK), 0/10 °C, −6/4 °C Two treatment durations: 3/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [72] |
| Jointing period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [69] |
| Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Decrease | [69] |
| Protein—HMW-GS (%) | ||||
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Avle, Berserk, Bjarne, Zebra) | unchanged | [77] |
| Filling period | 18/8 °C (day/night), 3 d | Winter wheat (Ningmai 13, Zhenmai 12) | Increase | [37] |
| Protein—D-type LMW-GS (%) | ||||
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Avle, Berserk, Bjarne, Zebra) | Decrease | [77] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Bjarne, Cadenza) | Decrease | [76] |
| Protein—B-type LMW-GS (%) | ||||
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Avle, Berserk, Bjarne, Zebra) | Increase | [77] |
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Bjarne, Cadenza) | Increase | [76] |
| Protein—Albumin (%) | ||||
| Jointing period, Booting period | Three temperature levels: day/night, 6/16 °C (CK), 0/10 °C, −6/4 °C Two treatment durations: 3/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [72] |
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [69] |
| Protein—Globulin (%) | ||||
| Jointing period, Booting period | Three temperature levels: day/night, 6/16 °C (CK), 0/10 °C, −6/4 °C Two treatment durations: 3/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [72] |
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [69] |
| Quality traits—Maximum resistance to extension (Rmax) | ||||
| Filling period | Three temperature levels: day/night, 13/10 °C, 18/15 °C, 23/20 °C | Spring wheat (Avle, Berserk, Bjarne, Zebra) | Increase | [77] |
| Quality traits—SDS sedimentation value | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [69] |
| Filling period | −5.5 °C, 3 h | Spring bread wheat (Kariega, SST86), Durum wheat (Oranje), spring Soft biscuit wheat (Snack) | Decrease | [21] |
| Quality traits—Wet gluten, Dry gluten | ||||
| Jointing period, Booting period | Four temperature levels: Tmin/Tavg/Tmax, 6/11/16 °C (CK), 2/3/8 °C, −4 /1/6 °C, −6/−1/4 °C Three treatment durations: 2/4/6 days | Winter wheat (Yangmai16, Xumai30) | Increase | [69] |
| Quality traits—Gluten strength | ||||
| Filling period | 17 to 18 °C | Spring wheat (Avle, Bastian, Bjarne, Zebra) | Decrease | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, P.; Wang, Y.; Sun, H. Impact of Temperature Stresses on Wheat Quality: A Focus on Starch and Protein Composition. Foods 2025, 14, 2178. https://doi.org/10.3390/foods14132178
Han P, Wang Y, Sun H. Impact of Temperature Stresses on Wheat Quality: A Focus on Starch and Protein Composition. Foods. 2025; 14(13):2178. https://doi.org/10.3390/foods14132178
Chicago/Turabian StyleHan, Pei, Yaping Wang, and Hui Sun. 2025. "Impact of Temperature Stresses on Wheat Quality: A Focus on Starch and Protein Composition" Foods 14, no. 13: 2178. https://doi.org/10.3390/foods14132178
APA StyleHan, P., Wang, Y., & Sun, H. (2025). Impact of Temperature Stresses on Wheat Quality: A Focus on Starch and Protein Composition. Foods, 14(13), 2178. https://doi.org/10.3390/foods14132178





