Identification of Potential Bioactive Peptides in Sheep Milk Kefir through Peptidomic Analysis at Different Fermentation Times
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Kefir Production
2.3. Measurement of Proteolytic Activity
2.4. Determination of Kefir Components
2.5. Peptide Extraction
2.6. microLC–timsTOF Pro-MS/MS Analysis
2.7. Protein and Peptide Identification by Bioinformatic Analysis
2.8. Data and Statistical Analysis
3. Results
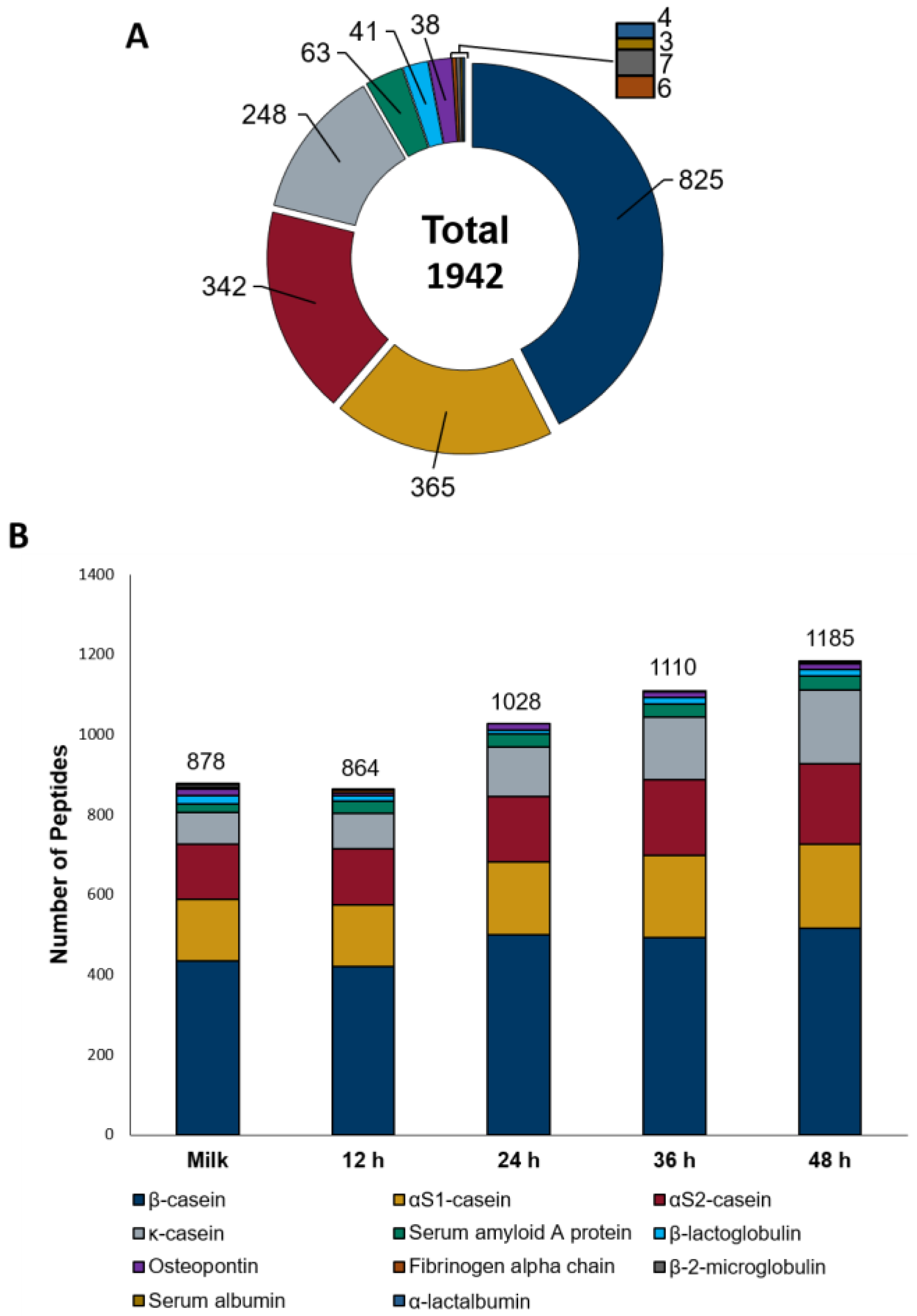
3.1. Changes in the Composition of Kefir during Fermentation
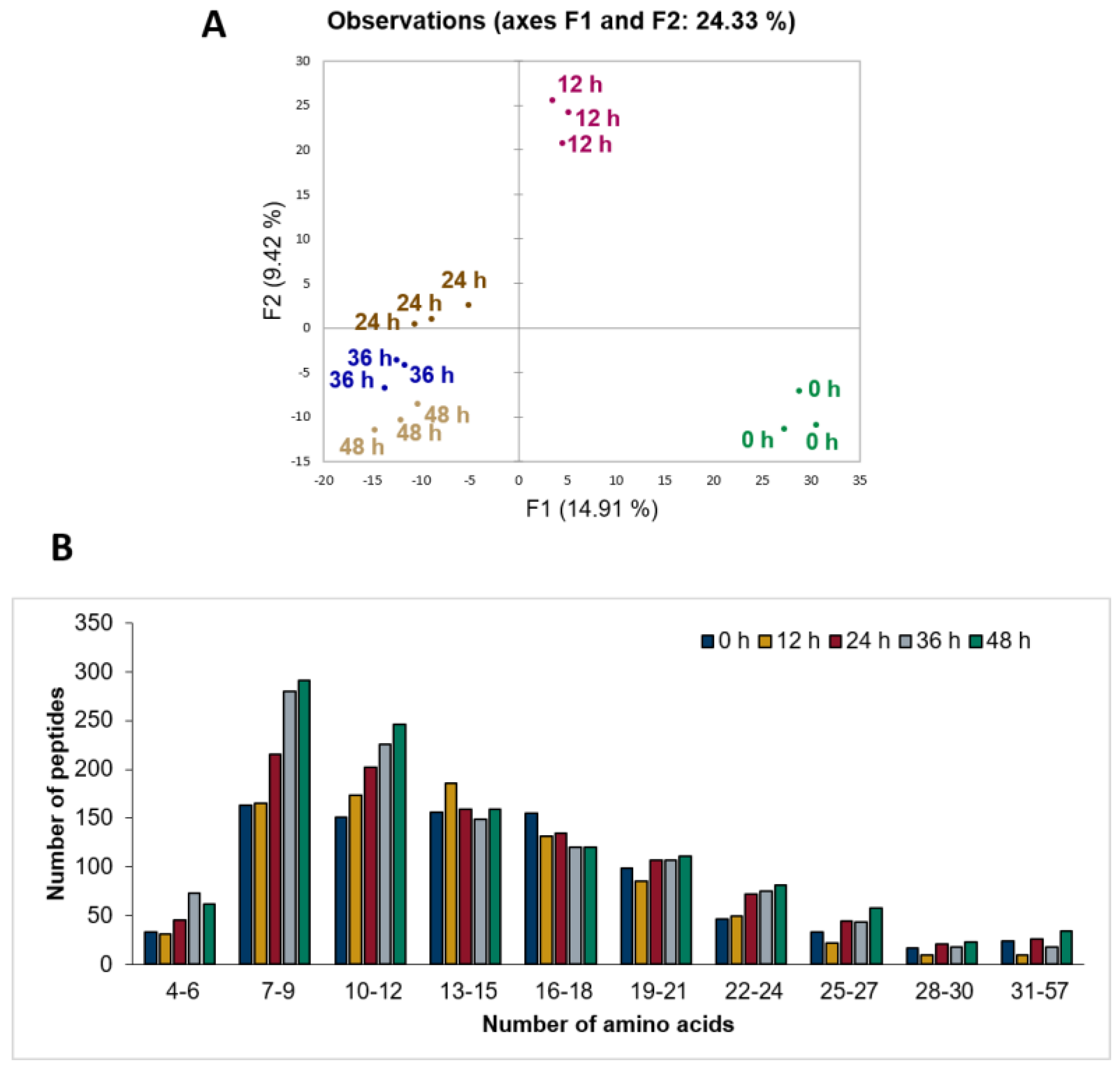
3.2. Untargeted Peptide Profiling of Sheep Milk Kefir throughout the Fermentation Process
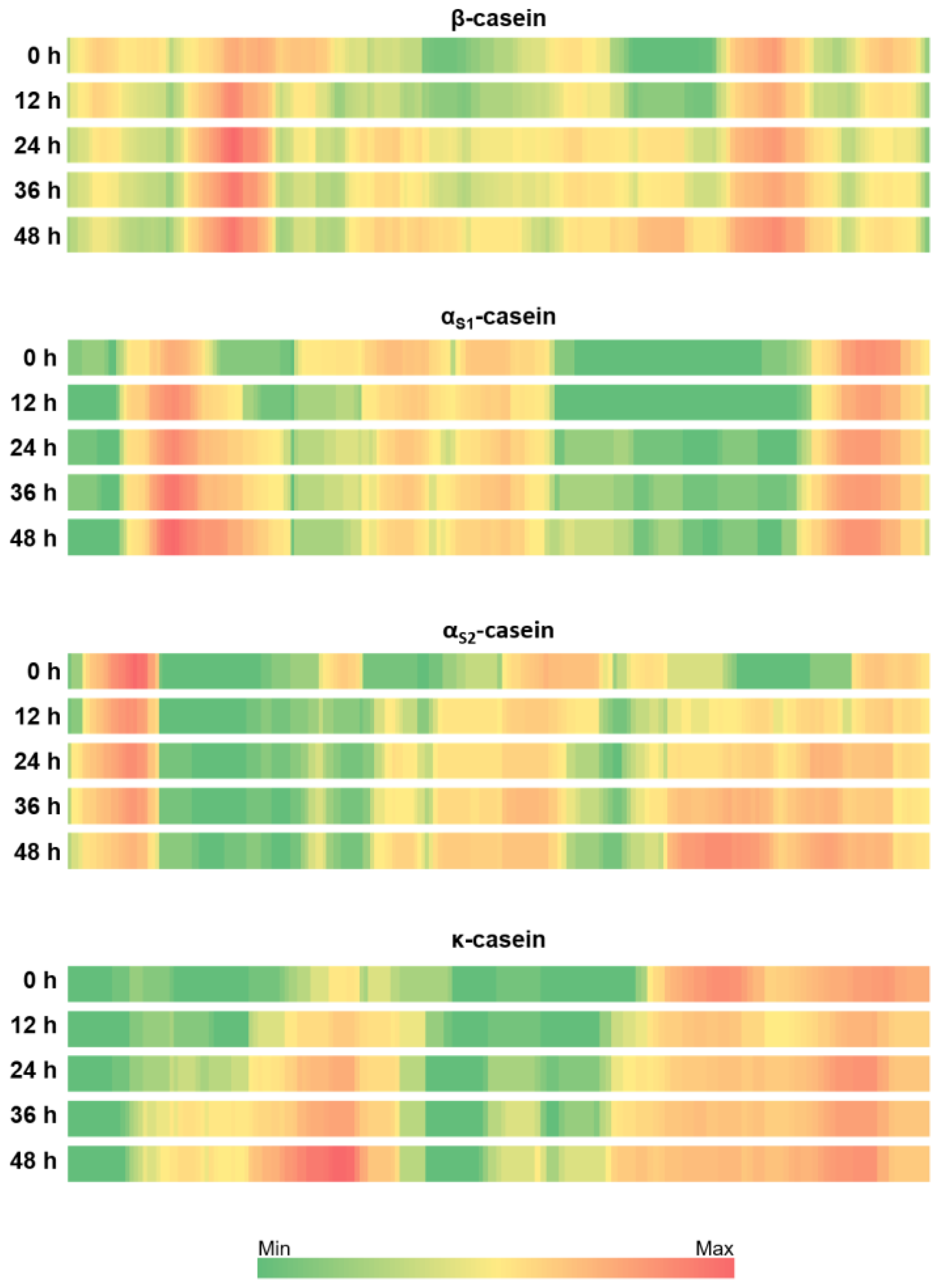
3.3. Patterns of Milk Protein Digestion by Microbial Proteases
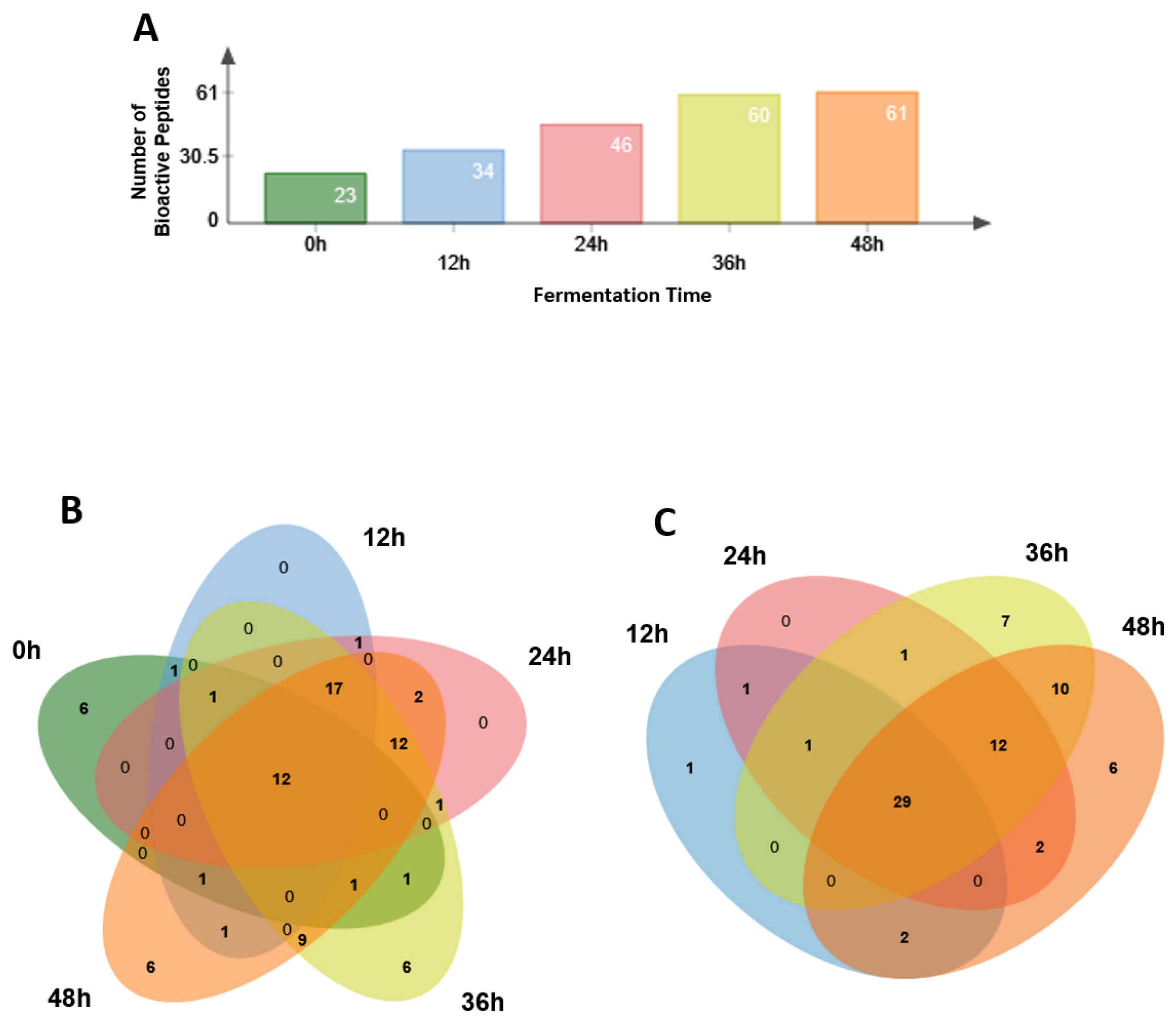
3.4. Identification of Potential Bioactive Peptides
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministerio de Agricultura. Pesca y Alimentación Caracterización del Sector Ovino y Caprino de Leche en España; Ministerio de Agricultura: Madrid, Spain, 2022. Available online: https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/caracterizacionovinoycaprinolechedatos2021_tcm30-562416.pdf (accessed on 20 January 2023).
- Ministerio de Agricultura. Pesca y Alimentación Indicadores Semestrales de Situación del Sector Ovino de Leche en España; Ministerio de Agricultura: Madrid, Spain, 2022. Available online: https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/dashboardovinoleche_diciembre_tcm30-553337.pdf (accessed on 20 January 2023).
- Ministerio de Agricultura. Pesca y Alimentación el Sector Ovino y Caprino de Leche en Cifras; Ministerio de Agricultura: Madrid, Spain, 2022. Available online: https://www.mapa.gob.es/es/ganaderia/temas/produccion-y-mercados-ganaderos/indicadoreseconomicosdelsectorovinoycaprinodeleche2022_act_tcm30-553590.pdf (accessed on 20 January 2023).
- Boyazoglu, J.; Morand-Fehr, P. Mediterranean Dairy Sheep and Goat Products and Their Quality. A Critical Review. Small Rumin. Res. J. Int. Goat Assoc. 2001, 40, 1–11. [Google Scholar] [CrossRef]
- Li, S.; Delger, M.; Dave, A.; Singh, H.; Ye, A. Seasonal Variations in the Composition and Physicochemical Characteristics of Sheep and Goat Milks. Foods 2022, 11, 1737. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.F.; Kumar, M.R.; Yeap, S.K.; Abdullah, J.O.; Khalid, M.; Omar, A.R.; Osman, M.A.; Mortadza, S.A.S.; Alitheen, N.B. Kefir and Its Biological Activities. Foods 2021, 10, 1210. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, X.; Sun, Y.; Sun, X.; Guo, M. Differences in Protein Profiles of Kefir Grains from Different Origins When Subcultured in Goat Milk. J. Agric. Food Chem. 2022, 70, 7515–7524. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shi, J.; Yang, X.; Nan, B.; Liu, Y.; Wang, Z. Chemical and Physical Characteristics and Antioxidant Activities of the Exopolysaccharide Produced by Tibetan Kefir Grains during Milk Fermentation. Int. Dairy J. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk Kefir: Nutritional, Microbiological and Health Benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Chourasia, R.; Chiring Phukon, L.; Abedin, M.M.; Padhi, S.; Singh, S.P.; Rai, A.K. Bioactive Peptides in Fermented Foods and Their Application: A Critical Review. Syst. Microbiol. Biomanuf. 2023, 3, 88–109. [Google Scholar] [CrossRef]
- Mudgil, P.; Gan, C.-Y.; Affan Baig, M.; Hamdi, M.; Mohteshamuddin, K.; Aguilar-Toalá, J.E.; Vidal-Limon, A.M.; Liceaga, A.M.; Maqsood, S. In-Depth Peptidomic Profile and Molecular Simulation Studies on ACE-Inhibitory Peptides Derived from Probiotic Fermented Milk of Different Farm Animals. Food Res. Int. 2023, 168, 112706. [Google Scholar] [CrossRef]
- Tomazou, M.; Oulas, A.; Anagnostopoulos, A.K.; Tsangaris, G.T.; Spyrou, G.M. In Silico Identification of Antimicrobial Peptides in the Proteomes of Goat and Sheep Milk and Feta Cheese. Proteomes 2019, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Amorim, F.G.; Coitinho, L.B.; Dias, A.T.; Friques, A.G.F.; Monteiro, B.L.; Rezende, L.C.D.D.; Pereira, T.D.M.C.; Campagnaro, B.P.; De Pauw, E.; Vasquez, E.C.; et al. Identification of New Bioactive Peptides from Kefir Milk through Proteopeptidomics: Bioprospection of Antihypertensive Molecules. Food Chem. 2019, 282, 109–119. [Google Scholar] [CrossRef]
- Dallas, D.C.; Citerne, F.; Tian, T.; Silva, V.L.M.; Kalanetra, K.M.; Frese, S.A.; Robinson, R.C.; Mills, D.A.; Barile, D. Peptidomic Analysis Reveals Proteolytic Activity of Kefir Microorganisms on Bovine Milk Proteins. Food Chem. 2016, 197, 273–284. [Google Scholar] [CrossRef]
- Ebner, J.; Aşçı Arslan, A.; Fedorova, M.; Hoffmann, R.; Küçükçetin, A.; Pischetsrieder, M. Peptide Profiling of Bovine Kefir Reveals 236 Unique Peptides Released from Caseins during Its Production by Starter Culture or Kefir Grains. J. Proteom. 2015, 117, 41–57. [Google Scholar] [CrossRef]
- Piovesana, S.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Samperi, R.; Zenezini Chiozzi, R.; Laganà, A. Peptidome Characterization and Bioactivity Analysis of Donkey Milk. J. Proteom. 2015, 119, 21–29. [Google Scholar] [CrossRef]
- Izquierdo-González, J.J.; Amil-Ruiz, F.; Zazzu, S.; Sánchez-Lucas, R.; Fuentes-Almagro, C.A.; Rodríguez-Ortega, M.J. Proteomic Analysis of Goat Milk Kefir: Profiling the Fermentation-Time Dependent Protein Digestion and Identification of Potential Peptides with Biological Activity. Food Chem. 2019, 295, 456–465. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Guo, H.; Chen, F.; Zhao, L.; He, L.; Ou, Y.; Huang, M.; Zhang, Y.; Guo, B.; Cao, Y.; et al. Antibacterial Effects of a Cell-Penetrating Peptide Isolated from Kefir. J. Agric. Food Chem. 2016, 64, 3234–3242. [Google Scholar] [CrossRef] [PubMed]
- de Lima, M.D.S.F.; da Silva, R.A.; da Silva, M.F.; da Silva, P.A.B.; Costa, R.M.P.B.; Teixeira, J.A.C.; Porto, A.L.F.; Cavalcanti, M.T.H. Brazilian Kefir-Fermented Sheep’s Milk, a Source of Antimicrobial and Antioxidant Peptides. Probiotics Antimicrob. Proteins 2018, 10, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.P.; Rosario, A.I.L.S.; Lelis, C.A.; Rekowsky, B.S.S.; Carvalho, A.P.A.; Rosário, D.K.A.; Elias, T.A.; Costa, M.P.; Foguel, D.; Conte-Junior, C.A. Bioactive Compounds from Kefir and Their Potential Benefits on Health: A Systematic Review and Meta-Analysis. Oxidative Med. Cell. Longev. 2021, 2021, 9081738. [Google Scholar] [CrossRef]
- Church, F.C.; Porter, D.H.; Catignani, G.L.; Swaisgood, H.E. An O-Phthalaldehyde Spectrophotometric Assay for Proteinases. Anal. Biochem. 1985, 146, 343–348. [Google Scholar] [CrossRef]
- Rodríguez-Ortega, M.J. “Shaving” Live Bacterial Cells with Proteases for Proteomic Analysis of Surface Proteins. Methods Mol. Biol. 2018, 1722, 21–29. [Google Scholar] [CrossRef]
- Meier, F.; Beck, S.; Grassl, N.; Lubeck, M.; Park, M.A.; Raether, O.; Mann, M. Parallel Accumulation-Serial Fragmentation (PASEF): Multiplying Sequencing Speed and Sensitivity by Synchronized Scans in a Trapped Ion Mobility Device. J. Proteome Res. 2015, 14, 5378–5387. [Google Scholar] [CrossRef]
- The UniProt Consortium; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; et al. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk Kefir: Composition, Microbial Cultures, Biological Activities, and Related Products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Chaudhary, K.; Sharma, M.; Nagpal, G.; Chauhan, J.S.; Singh, S.; Gautam, A.; Raghava, G.P.S. AHTPDB: A Comprehensive Platform for Analysis and Presentation of Antihypertensive Peptides. Nucleic Acids Res. 2015, 43, D956–D962. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, G. APD: The Antimicrobial Peptide Database. Nucleic Acids Res. 2004, 32, D590–D592. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, C.; Chen, H.; Xue, J.; Guo, X.; Liang, M.; Chen, M. BioPepDB: An Integrated Data Platform for Food-Derived Bioactive Peptides. Int. J. Food Sci. Nutr. 2018, 69, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Waghu, F.H.; Barai, R.S.; Gurung, P.; Idicula-Thomas, S. CAMPR3: A Database on Sequences, Structures and Signatures of Antimicrobial Peptides. Nucleic Acids Res. 2016, 44, D1094–D1097. [Google Scholar] [CrossRef]
- Pirtskhalava, M.; Amstrong, A.A.; Grigolava, M.; Chubinidze, M.; Alimbarashvili, E.; Vishnepolsky, B.; Gabrielian, A.; Rosenthal, A.; Hurt, D.E.; Tartakovsky, M. DBAASP v3: Database of Antimicrobial/Cytotoxic Activity and Structure of Peptides as a Resource for Development of New Therapeutics. Nucleic Acids Res. 2021, 49, D288–D297. [Google Scholar] [CrossRef] [PubMed]
- Zamyatnin, A.A.; Borchikov, A.S.; Vladimirov, M.G.; Voronina, O.L. The EROP-Moscow Oligopeptide Database. Nucleic Acids Res. 2006, 34, D261–D266. [Google Scholar] [CrossRef]
- Panyayai, T.; Ngamphiw, C.; Tongsima, S.; Mhuantong, W.; Limsripraphan, W.; Choowongkomon, K.; Sawatdichaikul, O. FeptideDB: A Web Application for New Bioactive Peptides from Food Protein. Heliyon 2019, 5, e02076. [Google Scholar] [CrossRef]
- Nielsen, S.D.; Beverly, R.L.; Qu, Y.; Dallas, D.C. Milk Bioactive Peptide Database: A Comprehensive Database of Milk Protein-Derived Bioactive Peptides and Novel Visualization. Food Chem. 2017, 232, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Shtatland, T.; Guettler, D.; Kossodo, M.; Pivovarov, M.; Weissleder, R. PepBank—A Database of Peptides Based on Sequence Text Mining and Public Peptide Data Sources. BMC Bioinform. 2007, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- Bardou, P.; Mariette, J.; Escudié, F.; Djemiel, C.; Klopp, C. Jvenn: An Interactive Venn Diagram Viewer. BMC Bioinform. 2014, 15, 293. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pischetsrieder, M. Identification and Relative Quantification of Bioactive Peptides Sequentially Released during Simulated Gastrointestinal Digestion of Commercial Kefir. J. Agric. Food Chem. 2017, 65, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Du, A.; Fan, Z.; Shi, L. Novel Insight into the Transformation of Peptides and Potential Benefits in Brown Fermented Goat Milk by Mesoporous Magnetic Dispersive Solid Phase Extraction-Based Peptidomics. Food Chem. 2022, 389, 133110. [Google Scholar] [CrossRef]
- Boudonck, K.J.; Mitchell, M.W.; Wulff, J.; Ryals, J.A. Characterization of the Biochemical Variability of Bovine Milk Using Metabolomics. Metabolomics 2009, 5, 375–386. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A. Biochemical Characteristics of Ewe and Goat Milk: Effect on the Quality of Dairy Products. Small Rumin. Res. 2011, 101, 33–40. [Google Scholar] [CrossRef]
- Quirós, A.; Hernández-Ledesma, B.; Ramos, M.; Amigo, L.; Recio, I. Angiotensin-Converting Enzyme Inhibitory Activity of Peptides Derived from Caprine Kefir. J. Dairy Sci. 2005, 88, 3480–3487. [Google Scholar] [CrossRef]
- Yilmaz-Ersan, L.; Ozcan, T.; Akpinar-Bayizit, A.; Sahin, S. Comparison of Antioxidant Capacity of Cow and Ewe Milk Kefirs. J. Dairy Sci. 2018, 101, 3788–3798. [Google Scholar] [CrossRef]
- Czyżak-Runowska, G.; Wójtowski, J.A.; Łęska, B.; Bielińska-Nowak, S.; Pytlewski, J.; Antkowiak, I.; Stanisławski, D. Lactose Content and Selected Quality Parameters of Sheep Milk Fermented Beverages during Storage. Animals 2022, 12, 3105. [Google Scholar] [CrossRef]
- Silva, T.; Pires, A.; Gomes, D.; Viegas, J.; Pereira-Dias, S.; Pintado, M.E.; Henriques, M.; Pereira, C.D. Sheep’s Butter and Correspondent Buttermilk Produced with Sweet Cream and Cream Fermented by Aromatic Starter, Kefir and Probiotic Culture. Foods 2023, 12, 331. [Google Scholar] [CrossRef] [PubMed]
- Cais-Sokolińska, D.; Wójtowski, J.; Pikul, J.; Danków, R.; Majcher, M.; Teichert, J.; Bagnicka, E. Formation of Volatile Compounds in Kefir Made of Goat and Sheep Milk with High Polyunsaturated Fatty Acid Content. J. Dairy Sci. 2015, 98, 6692–6705. [Google Scholar] [CrossRef]
- Leite, A.M.O.; Leite, D.C.A.; Del Aguila, E.M.; Alvares, T.S.; Peixoto, R.S.; Miguel, M.A.L.; Silva, J.T.; Paschoalin, V.M.F. Microbiological and Chemical Characteristics of Brazilian Kefir during Fermentation and Storage Processes. J. Dairy Sci. 2013, 96, 4149–4159. [Google Scholar] [CrossRef]
- Ohlsson, J.A.; Johansson, M.; Hansson, H.; Abrahamson, A.; Byberg, L.; Smedman, A.; Lindmark-Månsson, H.; Lundh, Å. Lactose, Glucose and Galactose Content in Milk, Fermented Milk and Lactose-Free Milk Products. Int. Dairy J. 2017, 73, 151–154. [Google Scholar] [CrossRef]
- Farag, M.A.; Jomaa, S.A.; El-Wahed, A.A.; El-Seedi, A.H.R. The Many Faces of Kefir Fermented Dairy Products: Quality Characteristics, Flavour Chemistry, Nutritional Value, Health Benefits, and Safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef]
- Basilicata, M.G.; Pepe, G.; Sommella, E.; Ostacolo, C.; Manfra, M.; Sosto, G.; Pagano, G.; Novellino, E.; Campiglia, P. Peptidome Profiles and Bioactivity Elucidation of Buffalo-Milk Dairy Products after Gastrointestinal Digestion. Food Res. Int. 2018, 105, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yu, Y.; Qi, Y.; Wang, F.; Yan, J.; Zou, H. Peptide Profiling and the Bioactivity Character of Yogurt in the Simulated Gastrointestinal Digestion. J. Proteom. 2016, 141, 24–46. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Peptidomics Profile, Bioactive Peptides Identification and Biological Activities of Six Different Cheese Varieties. Biology 2023, 12, 78. [Google Scholar] [CrossRef]
- Solieri, L.; Baldaccini, A.; Martini, S.; Bianchi, A.; Pizzamiglio, V.; Tagliazucchi, D. Peptide Profiling and Biological Activities of 12-Month Ripened Parmigiano Reggiano Cheese. Biology 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Pourjoula, M.; Picariello, G.; Garro, G.; D’Auria, G.; Nitride, C.; Rheza Ghaisari, A.; Ferranti, P. The Protein and Peptide Fractions of Kashk, a Traditional Middle East Fermented Dairy Product. Food Res. Int. 2020, 132, 109107. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, X.; Liu, N.; Cheng, J.; Wang, C.; Guo, M. Comparative Analysis of Caseins in Saanen Goat Milk from 3 Different Regions of China Using Quantitative Proteomics. J. Dairy Sci. 2022, 105, 5587–5599. [Google Scholar] [CrossRef] [PubMed]
- McGrath, B.A.; Kinsella, M.; Huppertz, T.; McSweeney, P.L.H.; Kelly, A.L. Proteomic Characterisation of Heat-Induced Hydrolysis of Sodium Caseinate. Int. Dairy J. 2016, 53, 51–59. [Google Scholar] [CrossRef]
- Guerrero, A.; Dallas, D.C.; Contreras, S.; Chee, S.; Parker, E.A.; Sun, X.; Dimapasoc, L.; Barile, D.; German, J.B.; Lebrilla, C.B. Mechanistic Peptidomics: Factors That Dictate Specificity in the Formation of Endogenous Peptides in Human Milk. Mol. Cell. Proteom. 2014, 13, 3343–3351. [Google Scholar] [CrossRef] [PubMed]
- Dallas, D.C.; Guerrero, A.; Parker, E.A.; Garay, L.A.; Bhandari, A.; Lebrilla, C.B.; Barile, D.; German, J.B. Peptidomic Profile of Milk of Holstein Cows at Peak Lactation. J. Agric. Food Chem. 2014, 62, 58–65. [Google Scholar] [CrossRef]
- Malta, S.M.; Batista, L.L.; Silva, H.C.G.; Franco, R.R.; Silva, M.H.; Rodrigues, T.S.; Correia, L.I.V.; Martins, M.M.; Venturini, G.; Espindola, F.S.; et al. Identification of Bioactive Peptides from a Brazilian Kefir Sample, and Their Anti-Alzheimer Potential in Drosophila Melanogaster. Sci. Rep. 2022, 12, 11065. [Google Scholar] [CrossRef] [PubMed]
- Zenezini Chiozzi, R.; Capriotti, A.L.; Cavaliere, C.; La Barbera, G.; Piovesana, S.; Samperi, R.; Laganà, A. Purification and Identification of Endogenous Antioxidant and ACE-Inhibitory Peptides from Donkey Milk by Multidimensional Liquid Chromatography and NanoHPLC-High Resolution Mass Spectrometry. Anal. Bioanal. Chem. 2016, 408, 5657–5666. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Bandeira, N.; Perez-Riverol, Y.; Sharma, V.; Carver, J.J.; Mendoza, L.; Kundu, D.J.; Wang, S.; Bandla, C.; Kamatchinathan, S.; et al. The ProteomeXchange Consortium at 10 Years: 2023 Update. Nucleic Acids Res. 2023, 51, D1539–D1548. [Google Scholar] [CrossRef]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE Database Resources in 2022: A Hub for Mass Spectrometry-Based Proteomics Evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
| Fermentation Time (h) | pH | Lactic Acid (g/L Kefir) | Ethanol (g/L Kefir) | Lactose (g/L Kefir) | D-Galactose (g/L Kefir) | Proteolytic Activity (A340) |
|---|---|---|---|---|---|---|
| 0 | 6.67 ± 0.03 | 0.056 ± 0.027 | 0.118 ± 0.107 | 31.893 ± 0.355 | 0.0475 ± 0.021 | 0.050 ± 0.004 |
| 12 | 5.20 ± 0.07 * | 0.728 ± 0.098 *** | 0.979 ± 0.261 ** | 31.509 ± 1.156 | 0.575 ± 0.096 ** | 0.101 ± 0.002 ** |
| 24 | 4.44 ± 0.14 ** | 1.434 ± 0.038 *** | 1.742 ± 0.600 ** | 31.882 ± 0.374 | 0.806 ± 0.073 *** | 0.127 ± 0.002 ** |
| 36 | 4.12 ± 0.08 *** | 1.500 ± 0.145 *** | 2.320 ± 0.111 *** | 30.832 ± 1.960 | 0.927 ± 0.132 *** | 0.128 ± 0.007 *** |
| 48 | 3.80 ± 0.13 *** | 1.689 ± 0.016 *** | 2.815 ± 0.216 *** | 29.049 ± 1.104 | 0.864 ± 0.141 ** | 0.178 ± 0.007 *** |
| # | Peptide Sequences | Mass | 0 | 12 | 24 | 36 | 48 | Protein | Start | End | Bioactivity | Cow | Goat |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LNVVGETVE | 958.50 | • | • | • | • | • | β-casein | 6 | 14 | ACE-inhibitory | ||
| 2 | FQSEEQQQTEDELQDK | 1980.85 | • | • | • | • | • | β-casein | 33 | 48 | Antithrombotic | ||
| 3 | DKIHPF | 755.40 | - | • | • | • | • | β-casein | 47 | 52 | ACE-inhibitory, Protein transport inhibitor | [7] | |
| 4 | LVYPFTGPIPN | 1216.65 | • | • | • | • | • | β-casein | 58 | 68 | ACE-inhibitory | ||
| 5 | TGPIPN | 597.31 | - | • | • | • | • | β-casein | 63 | 68 | ACE-inhibitory | ||
| 6 | TGPIPNSLPQ | 1022.54 | • | • | • | • | • | β-casein | 63 | 72 | ACE-inhibitory | ||
| 7 | LTQTPVVVPPF | 1196.68 | - | - | - | • | • | β-casein | 77 | 87 | ACE-inhibitory | [7,17] | |
| 8 | TQTPVVVPPFLQPE | 1550.83 | - | • | • | • | • | β-casein | 78 | 91 | Antioxidant | ||
| 9 | GVPKVKETMVPK | 1311.76 | - | • | • | • | • | β-casein | 94 | 105 | ACE-inhibitory | ||
| 10 | GVPKVKETMVPKH | 1448.82 | - | - | • | - | • | β-casein | 94 | 106 | ACE-inhibitory | ||
| 11 | HKEMPFPKYPVEPF | 1744.86 | - | • | • | • | • | β-casein | 106 | 119 | ACE-inhibitory | [15] | |
| 12 | HKEMPFPKYPVEPFTESQ | 2190.05 | • | • | • | • | • | β-casein | 106 | 123 | Antioxidant | ||
| 13 | MPFPKYPVEP | 1203.60 | - | - | • | • | • | β-casein | 109 | 118 | ACE-inhibitory, Neuropeptide | ||
| 14 | FPKYPVEPF | 1122.58 | - | - | • | • | • | β-casein | 111 | 119 | Antioxidant | ||
| 15 | YPVEPF | 750.36 | • | - | - | - | - | β-casein | 114 | 119 | ACE-inhibitory Antimicrobial, Neuropeptide, Opiate, Antioxidant, Opioid, Increase MUC4 expression | [36] | |
| 16 | PFTESQS | 794.34 | • | • | • | • | • | β-casein | 118 | 124 | ACE-inhibitory | ||
| 17 | TESQSLT | 764.36 | - | - | - | • | - | β-casein | 120 | 126 | ACE inhibitor | ||
| 18 | LTLTDVE | 789.41 | - | • | • | • | • | β-casein | 125 | 131 | ACE-inhibitory | [17] | |
| 19 | LHLPLP | 688.43 | • | - | - | • | • | β-casein | 133 | 138 | ACE-inhibitory, Neuropeptide | ||
| 20 | LHLPLPL | 801.51 | - | - | - | • | • | β-casein | 133 | 139 | ACE-inhibitory, Neuropeptide | ||
| 21 | HLPLPL | 688.43 | - | - | - | - | • | β-casein | 134 | 139 | ACE-inhibitory, Antiamnestic | ||
| 22 | FPPQSVL | 786.43 | - | • | • | • | • | β-casein | 157 | 163 | ACE-inhibitory | ||
| 23 | VLPVPQ | 651.40 | • | • | • | • | • | β-casein | 170 | 175 | ACE-inhibitory, Inhibition of cholesterol solubility | ||
| 24 | VLPVPQK | 779.49 | • | - | - | - | - | β-casein | 170 | 176 | ACE-inhibitory, Antioxidant, Antimicrobial, Inhibits enzymatic and nonenzymatic lipid peroxidation, Wound healing, Osteoanabolic, Anti-apoptotic effect | [37] | |
| 25 | RDMPIQAF | 976.48 | - | • | • | • | • | β-casein | 181 | 188 | ACE-inhibitory | [15,36] | |
| 26 | LYQEPVLGPVR | 1269.71 | • | - | - | - | - | β-casein | 190 | 200 | ACE-inhibitory, Anti-inflammatory | [37] | |
| 27 | YQEPVL | 747.38 | - | • | • | • | • | β-casein | 191 | 196 | ACE-inhibitory | ||
| 28 | YQEPVLGPVR | 1156.62 | - | - | - | - | • | β-casein | 191 | 200 | ACE-inhibitory, Immuno- and cyto-modulatory, Anticoagulant, Antioxidant, Anti-inflammatory, Antithrombotic, Immunomodulatory | [37] | |
| 29 | YQEPVLGPVRGPF | 1457.77 | - | • | - | - | • | β-casein | 191 | 203 | ACE-inhibitory | ||
| 30 | YQEPVLGPVRGPFPI | 1667.90 | • | • | • | • | • | β-casein | 191 | 205 | ACE-inhibitory, Antimicrobial | [36] | |
| 31 | QEPVL | 584.32 | - | - | • | • | • | β-casein | 192 | 196 | Immunomodulatory | ||
| 32 | EPVLGPVRGPFP | 1263.70 | • | • | - | - | • | β-casein | 193 | 204 | ACE-inhibitory, Neuropeptide | ||
| 33 | VLGPVRGPFP | 1037.60 | • | - | - | - | - | β-casein | 195 | 204 | ACE-inhibitory, Neuropeptide | ||
| 34 | LGPVRGPFPI | 1051.62 | - | - | • | • | • | β-casein | 196 | 205 | ACE-inhibitory | ||
| 35 | GPFPILV | 741.44 | • | • | • | • | - | β-casein | 201 | 207 | ACE-inhibitory | ||
| 36 | RPKHPIKH | 1011.61 | - | - | • | • | - | αS1-casein | 1 | 8 | ACE-inhibitory, Apoptosis inhibitory | ||
| 37 | RPKHPIKHQ | 1139.67 | - | - | - | • | - | αS1-casein | 1 | 9 | ACE-inhibitory, Neuropeptide | ||
| 38 | EVLNENLLRF | 1245.67 | • | • | - | - | - | αS1-casein | 14 | 23 | ACE-inhibitory | ||
| 39 | VLNENL | 700.38 | - | - | - | • | - | αS1-casein | 15 | 20 | ACE-inhibitory | ||
| 40 | VLNENLLR | 969.56 | - | • | • | • | • | αS1-casein | 15 | 22 | ACE-inhibitory, Antimicrobial | [15] | [7,17] |
| 41 | NENLLRF | 904.48 | • | • | • | • | • | αS1-casein | 17 | 23 | ACE-inhibitory | ||
| 42 | ENLLRF | 790.43 | • | • | • | • | • | αS1-casein | 18 | 23 | ACE-inhibitory | ||
| 43 | VVAPFPEVF | 1003.54 | - | - | - | - | • | αS1-casein | 24 | 32 | ACE-inhibitory | ||
| 44 | VAPFPE | 658.33 | - | - | - | • | • | αS1-casein | 25 | 30 | Inhibition of cholesterol solubility | ||
| 45 | VAPFPEV | 757.40 | - | - | • | - | • | αS1-casein | 25 | 31 | ACE-inhibitory | ||
| 46 | VAPFPEVF | 904.47 | - | - | • | • | • | αS1-casein | 25 | 32 | ACE-inhibitory | ||
| 47 | IQKEDVPSER | 1199.61 | • | - | - | - | - | αS1-casein | 81 | 90 | ACE-inhibitory | ||
| 48 | DVPSERYLG | 1034.50 | - | - | • | • | • | αS1-casein | 85 | 93 | ACE-inhibitory | ||
| 49 | YLGYLE | 756.37 | - | - | - | • | - | αS1-casein | 91 | 96 | ACE-inhibitory, Antioxidant, Opioid | ||
| 50 | YLGYLEQ | 884.43 | • | - | - | • | - | αS1-casein | 91 | 97 | Anxiolytic | ||
| 51 | GYLEQLLR | 990.55 | - | • | • | • | • | αS1-casein | 93 | 100 | ACE-inhibitory | ||
| 52 | YLEQLLR | 933.53 | - | • | • | - | - | αS1-casein | 94 | 100 | Antimicrobial | ||
| 53 | LEIVPK | 697.44 | - | • | • | • | • | αS1-casein | 109 | 114 | ACE-inhibitory | ||
| 54 | DAYPSGAW | 865.36 | - | - | • | • | • | αS1-casein | 157 | 164 | ACE-inhibitory, ACE-inhibitory | ||
| 55 | YTDAPSF | 799.34 | - | - | - | • | • | αS1-casein | 173 | 179 | ACE-inhibitory | ||
| 56 | IPNPIGSE | 825.42 | - | • | • | • | • | αS1-casein | 182 | 189 | ACE-inhibitory | ||
| 57 | ALNEINQFYQK | 1366.69 | - | • | • | • | • | αS2-casein | 82 | 92 | ACE-inhibitory | [7] | |
| 58 | YQKFPQY | 972.47 | - | - | - | - | • | αS2-casein | 90 | 96 | ACE-inhibitory, Antioxidant | [15,36] | [7,17] |
| 59 | YQKFPQYLQY | 1376.68 | - | • | • | • | • | αS2-casein | 90 | 99 | ACE-inhibitory | ||
| 60 | FPQYLQY | 957.46 | - | - | - | • | • | αS2-casein | 93 | 99 | ACE-inhibitory | ||
| 61 | NAGPFTPTVNREQLSTS | 1817.89 | - | - | - | • | - | αS2-casein | 116 | 132 | ACE-inhibitory | ||
| 62 | TVDQHQ | 726.33 | - | - | - | • | - | αS2-casein | 183 | 188 | ACE-inhibitory | ||
| 63 | PYVRYL | 809.44 | • | • | • | • | • | αS2-casein | 203 | 208 | ACE-inhibitory, Antioxidative, Antimicrobial | ||
| 64 | KYIPIQ | 760.45 | - | - | • | • | • | κ-casein | 24 | 29 | ACE-inhibitory | ||
| 65 | KYIPIQYVLS | 1222.70 | - | • | • | • | • | κ-casein | 24 | 33 | Antioxidant | ||
| 66 | YIPIQY | 795.42 | - | - | - | - | • | κ-casein | 25 | 30 | ACE-inhibitory, Antioxidative | ||
| 67 | YIPIQYVLSR | 1250.70 | - | - | • | • | • | κ-casein | 25 | 34 | ACE-inhibitory, Opioid (opioid antagonist), Neuropeptide, Immunomodulating, Ileum contracting, C3a Receptors agonist | ||
| 68 | IPIQYVL | 844.51 | - | - | - | • | • | κ-casein | 26 | 32 | Antioxidative | ||
| 69 | SRYPSY | 771.36 | - | - | - | • | • | κ-casein | 33 | 38 | Opioid | ||
| 70 | YPSYGLN | 812.37 | - | - | • | • | • | κ-casein | 35 | 41 | Opioid | ||
| 71 | FLPYPY | 798.40 | - | - | - | • | • | κ-casein | 55 | 60 | Opioid | ||
| 72 | YAKPVA | 647.36 | - | - | - | - | • | κ-casein | 61 | 66 | ACE-inhibitory | ||
| 73 | ARHPHPHLSF | 1197.62 | - | • | • | • | • | κ-casein | 96 | 105 | ACE-inhibitory | ||
| 74 | ARHPHPHLSFM | 1328.66 | - | - | • | • | • | κ-casein | 96 | 106 | Antioxidant | [15] | [17] |
| 75 | HPHPHLSF | 970.48 | • | - | - | - | - | κ-casein | 98 | 105 | ACE-inhibitory, Neuropeptide, Antioxidant | [37] | |
| 76 | PHPHLSF | 833.42 | - | - | - | • | • | κ-casein | 99 | 105 | Digestion inhibitor (chymosin digestion) | ||
| 77 | TAQVTSTEV | 934.46 | • | • | • | • | • | κ-casein | 163 | 171 | ACE-inhibitory | [17] | |
| 78 | QVTSTEV | 762.38 | - | - | • | • | • | κ-casein | 165 | 171 | ACE-inhibitory | ||
| Total | 23 | 34 | 46 | 60 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalabasmaz, S.; de la Torre, E.P.; Gensberger-Reigl, S.; Pischetsrieder, M.; Rodríguez-Ortega, M.J. Identification of Potential Bioactive Peptides in Sheep Milk Kefir through Peptidomic Analysis at Different Fermentation Times. Foods 2023, 12, 2974. https://doi.org/10.3390/foods12152974
Dalabasmaz S, de la Torre EP, Gensberger-Reigl S, Pischetsrieder M, Rodríguez-Ortega MJ. Identification of Potential Bioactive Peptides in Sheep Milk Kefir through Peptidomic Analysis at Different Fermentation Times. Foods. 2023; 12(15):2974. https://doi.org/10.3390/foods12152974
Chicago/Turabian StyleDalabasmaz, Sevim, Esther Prados de la Torre, Sabrina Gensberger-Reigl, Monika Pischetsrieder, and Manuel J. Rodríguez-Ortega. 2023. "Identification of Potential Bioactive Peptides in Sheep Milk Kefir through Peptidomic Analysis at Different Fermentation Times" Foods 12, no. 15: 2974. https://doi.org/10.3390/foods12152974
APA StyleDalabasmaz, S., de la Torre, E. P., Gensberger-Reigl, S., Pischetsrieder, M., & Rodríguez-Ortega, M. J. (2023). Identification of Potential Bioactive Peptides in Sheep Milk Kefir through Peptidomic Analysis at Different Fermentation Times. Foods, 12(15), 2974. https://doi.org/10.3390/foods12152974







