Abstract
The intensive use of chemical pesticides has significantly impacted the environment and human health, encouraging the search for more sustainable and environmentally safe pest management strategies. In this context, botanical pesticides emerge as a promising solution, distinguished by their natural origin and lower toxicity. Although botanical insecticides have demonstrated their potential, these solutions are still little explored in scientific and technological terms, representing an expanding field to develop safer agricultural biological pesticides. The Meliaceae family, especially the Trichilia genus, has been recognized for its richness in bioactive compounds with insecticidal potential. However, Trichilia claussenii remains little studied, despite its occurrence in Brazilian ecosystems. Therefore, this article aims to analyze the information on the botanical and phytochemical characteristics and bioinsecticidal activity of T. claussenii, aiming to highlight its potential as a natural resource for biological pest control. The leaf and fruit extracts of T. claussenii revealed the presence of bioactive metabolites. The group of terpenes is highlighted, notable for their role in the chemical defense of plants and for their recognized insecticidal activity. In the leaf extract, terpenes were the most abundant class, representing 46.3% of the total identified. In the fruit extract, terpenes were also prominent, although to a lesser extent (34.5%). Additionally, a phytochemical screening of the aqueous leaf extract indicated the presence of coumarins, flavonoids, and alkaloids, compounds commonly associated with insecticidal activity. By consolidating this knowledge, we aim to encourage new research and the development of a botanical bioinsecticide based on this species.
1. Introduction
The search for more sustainable alternatives applied to the management of agricultural pests is growing due to the environmental and social impacts caused by the intensive use of chemical pesticides [1]. In this scenario, botanical pesticides are distinguished as a promising product in biological control, as they come from plant extracts with natural bioactive compounds, being less aggressive to the environment and human health [2]. These products are made from different parts of plants and can have multiple modes of action [3]. The generally lower toxicity makes their waste biodegradable with agroecological practices and organic production systems [4].
In the context of searching for sustainable solutions, the potential of Brazilian biodiversity as a source of new bioactive compounds is highlighted. Brazil is recognized as the country with the greatest plant diversity in the world, where approximately 43% of the cataloged plant species are endemic, that is, exclusive to the national territory [5]. This wide variety of plant species represents a strategic genetic heritage for scientific research, with great potential to discover and develop innovative botanical insecticides. In recent years, the Brazilian biopesticide market, including botanical insecticides, has shown annual growth of 45% [6]. On the international scenario, the global market for biopesticides, including botanicals, is projected to reach around US$27.9 billion by 2028, boosted by safe environmental solutions and the reduction in chemical pesticides.
The history of using natural pesticides has deep roots. The use of plant extracts in biological control dates back to ancestral agricultural practices, where certain people used oils and powders from plants with insecticidal properties to protect crops [7]. These solutions were prepared by hand and applied manually by spraying or spreading directly on the plants. Although empirical, this knowledge formed the basis for future scientific research, fostering the development of commercial botanical pesticides and sustainable agricultural technologies.
Therefore, the bioactive compounds found in plants, such as alkaloids, terpenoids, flavonoids, and phenols, among others, play defensive roles in the natural environment [8]. In this context, the Meliaceae family is highlighted, comprising approximately 740 species distributed in tropical and subtropical regions and widely recognized for the production of limonoids with high insecticidal activity [9]. Among the species of the Meliaceae family, the Trichilia genus is a strategic source of active ingredients for bioinsecticide formulations.
Despite this favorable scenario, the use of botanical extracts remains little explored at the scientific and technological levels. Unlike other species of the genus Trichilia spp., Trichilia claussenii is little studied. Although there are records of its occurrence in Brazilian ecosystems, the species is notably underexplored in the scientific literature. In addition, T. claussenii has economic and ecological relevance due to its distribution across different regions of the South American continent, being found mainly in Brazil but also reported in other South American countries [10]. This broad distribution reinforces its potential as a strategic botanical resource, both for scientific exploration and for possible applications in sustainable agricultural management. This lack of information is even more evident regarding its bioinsecticidal potential, representing an important gap that justifies the need for a comprehensive review of the species. In addition, agronomic aspects such as cultivation requirements, seasonal availability of biomass, and climatic limitations must be considered, since factors such as water regime, light availability, and edaphoclimatic conditions directly influence plant growth and the synthesis of bioactive metabolites.
Considering this background, this study aims to fill this gap by overview of knowledge about T. claussenii and offering a scientific basis on its main botanical characteristics, chemical composition, and its potential as an alternative in biological control to guide future investigations and practical applications of its botanical extract in agriculture.
2. Brief Bibliometric Assessment
2.1. Data Collection
This study was conducted as a narrative review supported by bibliometric mapping. The bibliometric mapping approach is based on the creation of a map as a visual representation of the research topic, illustrating the relationship between key terms in the field. The bibliometric analysis was conducted using the VOSViewer® software, version 1.6.20 (Centre for Science and Technology Studies (CWTS), Leiden University, Leiden, The Netherlands), based on data provided by the Elsevier® library service, through the Scopus® scientific platform.
The keywords and logical connectors “Meliaceae” and “Trichilia” and “extract” or “botanical” were used, seeking results in titles, abstracts, and keywords for a broad scope on the topic. The bibliographic coupling resulted in 187 scientific documents, of which 177 are research articles and 10 are reviews. During the data collection on T. claussenii, the keywords and logical connectors “Trichilia” and “claussenii” were used, totaling 27 scientific documents, of which 26 are research articles and 1 review. A minimum occurrence of three citations was established for the keywords to improve the understanding and visualization of the generated bibliographic maps. Only those that appeared in at least 3 studies between 1985 and 2025 were considered.
2.1.1. Meliaceae and Trichilia spp.
The parameterization of scientific studies on the proposed theme involves visualizing the network based on the frequency of occurrence of keywords in the analyzed documents, as illustrated in Figure 1a,b. In total, 212 keywords were considered in this research. These items were organized into six clusters, the largest of which was composed of 43 terms. The main keywords identified were: “Meliaceae” (157 occurrences), “plant extract” (129 occurrences), “chemistry” (57 occurrences), “medicinal plant” (47 occurrences), “Trichilia catigua” (39 occurrences), and “limonoid” (33 occurrences).
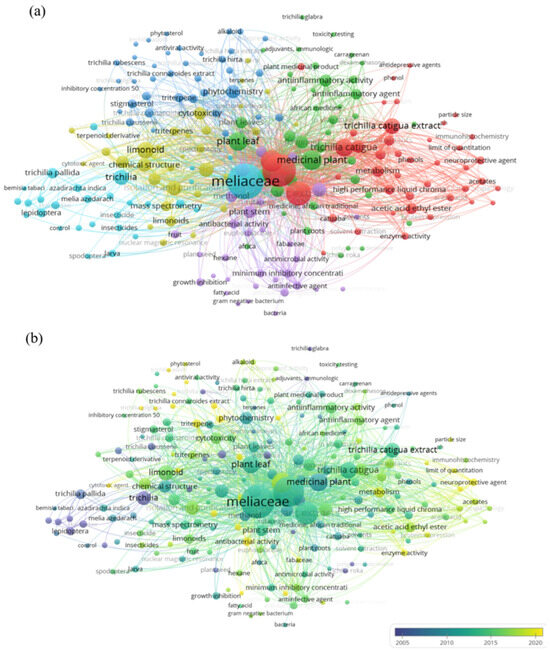
Figure 1.
Periodic (a) and temporal (b) surveys in VOSviewer® of bibliographic coupling (187 documents) of keywords with occurrences from 1985 to 2025, based on the scientific platform Scopus®, according to the keywords “Meliaceae” and “Trichilia” and “extract” or botanical”; a total of 212 keywords were indicated in groupings.
The periodic survey (Figure 1a) reveals the marked presence of the term “Meliaceae” and highlights the focus of the research, while the high occurrence of the term “plant extract” reveals the scientific and academic interest in the compounds obtained from the species of this family, especially in the context of agricultural and pharmacological applications [11]. The word “chemistry” indicates that most of the studies are focused on exploring the phytochemical characterization of these extracts to identify the main bioactive compounds responsible for the biological activities [12]. The occurrence of “medicinal plant” reinforces the pharmacological value of the Meliaceae species, suggesting that species of this family are used for therapeutic purposes, motivating scientific investigations on this focus [13]. The keyword “Trichilia catigua”, the most explored species among those of the genus, points to the direction of the research within Trichilia spp. [14]. The term “limonoid”, a class of bioactive compounds of the Meliaceae family, highlights the interest in investigating secondary metabolites with potential insecticide and antimicrobial effects [15]. Although the biological and chemical potential of Meliaceae is recognized, this survey reveals that studies are still concentrated on a few species, which reinforces the expansion of species of the genus Trichilia spp.
Using the color scale (Figure 1b), which ranges from blue (older publications) to yellow (more recent), it was observed that the highest concentration of publications was between 2010 and 2015, represented by the most intense shades. This period marks a peak of scientific interest in the study of plant extracts and bioactive compounds from plants of the Meliaceae group. However, after 2018, there has been a noticeable decrease in the number of new studies, indicated by the small presence of relationships in light yellow shades, reflecting a decrease in recent research focused on the subject. This gap presents a strategic opportunity for the scientific community to invest in innovative studies on little-explored species of Meliaceae, seeking to contribute to the development of more effective and safer botanical insecticides.
2.1.2. Research Data: Trichilia claussenii
The frequency of keywords in the analyzed documents can be seen in Figure 2a,b. In total, 50 keywords were considered in this research, organized into 2 groups, the largest of which was composed of 21 terms. The main keywords identified were: Trichilia claussenii (17 occurrences), Meliaceae (8 occurrences), plant extract (6 occurrences), and Brazil (4 occurrences).
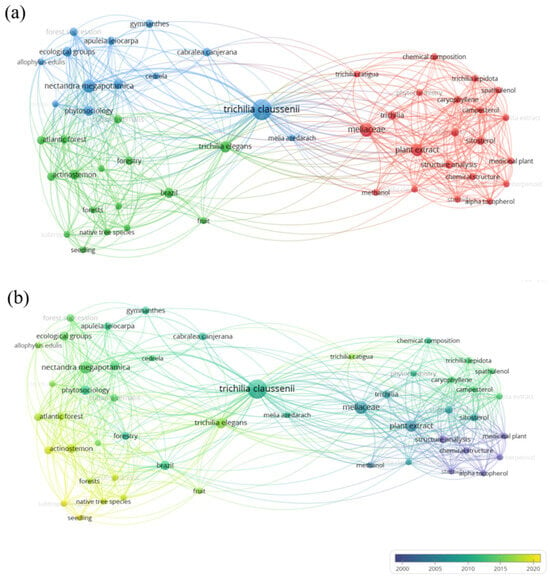
Figure 2.
Periodic (a) and temporal (b) surveys in VOSviewer® of bibliographic coupling (27 documents) of keywords with occurrences from 1985 to 2025, based on the scientific platform Scopus®, according to the keywords “Trichilia” and “claussenii”; a total of 50 keywords were indicated in 2 groupings.
The bibliometric analysis focused on the species T. claussenii revealed a scenario of significant scientific scarcity, evidenced by the identification of a few articles related to the species in the analyzed databases. The recurrence of the word “Meliaceae” reinforces its insertion within the taxonomic context of the family, known for bioactives such as limonoids and terpenoids [16]. The association with “plant extract” shows that the few studies are focused on the extraction and analysis of chemical compounds from the plant [17]. The word “Brazil”, although less frequent, is relevant to indicate the predominant geographic origin of the studies, which is in agreement with the natural distribution of the species in Brazilian ecosystems [18]. These data highlight the need for further scientific study of the species T. claussenii, since this species remains on the margins of contemporary scientific research.
The temporal analysis of the articles shows that most studies were conducted between 2005 and 2015, as indicated by the dominant shades. After 2015, there was a noticeable decline in publications related to rare occurrences, represented by yellow tones, which reflect more recent research. The first scientific study addressing the use of T. claussenii for controlling insect pests was in 2003 within a doctoral thesis [19]. This indicates that, although the species garnered some scientific interest in the past decade, research has not continued in recent years. Consequently, there is a new opportunity for studies that aim to establish the role of T. claussenii as a strategic bioactive resource in sustainable agriculture.
3. Trichilia claussenii
T. claussenii belongs to the Meliaceae family and is a tree species native to South America. Its full scientific name is Trichilia claussenii C.DC., with “C.DC.” being the abbreviation of the Swiss botanist Casimir De Candolle, responsible for the official description of the species [20]. Many species of the Trichilia genus are endemic to Brazil, a country that stands out worldwide for its high potential for botanical research, as it is home to approximately 20% of global biodiversity [21]. Figure 3 depicts the simplified phylogenetic classification of the Meliaceae family and the genetic position of the Trichilia genus.
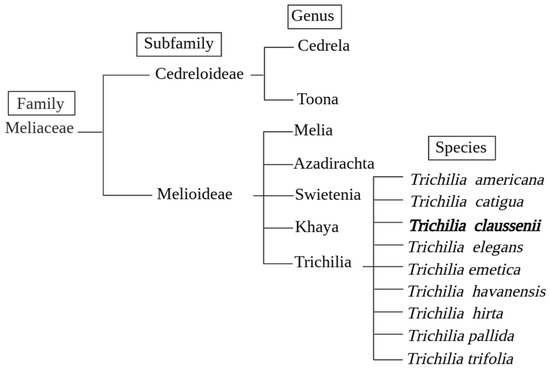
Figure 3.
Simplified phylogenetic classification of the Meliaceae family. The species Trichilia claussenii shown in bold is the focus of this study.
The taxonomic analysis of the species T. claussenii is presented in Table 1 to detail its scientific classification and facilitate the understanding of its systematic position in the plant kingdom.

Table 1.
Taxonomic classification of T. claussenii.
Popularly known in Brazil as “catiguá-vermelho”, the T. claussenii has been recorded in Brazil from the states of Minas Gerais and Mato Grosso do Sul to Rio Grande do Sul, being predominant in forest areas, especially in the rainforest zone of the Atlantic slope [22]. The main phytogeographic domains are the Cerrado and the Atlantic Forest, where it demonstrates the capacity to adapt to different plant formations, such as Riparian or Gallery Forest, Ombrophilous Forest, and Mixed Ombrophilous Forest. In the state of Rio Grande do Sul, records of the species were identified in the municipalities of Santa Maria, Nova Palma, Nova Prata, Pelotas, Nova Bassano, Derrubadas, and Porto Alegre, according to information from the “Flora Digital” [23].
3.1. Botanical Aspects
The main botanical characteristics of T. claussenii were described elsewhere [24]. The species is a medium-sized tree, which can reach between 6 and 12 m in height and 20 to 30 cm in diameter. The main botanical characteristics include alternate leaves, generally with dark green leaflets and a leathery texture. The flowers are small, whitish, or yellowish, and are arranged in panicle-type inflorescences. The fruits are woody capsules that, when ripe, open to expose seeds covered by a reddish aril, a structure that facilitates dispersal by birds. Fruiting occurs between January and May in Brazil, while flowering occurs from August to October. Ecologically, the species plays an important role, as its seeds, when consumed by birds, are dispersed effectively, contributing to its propagation. In addition, its relatively fast growth makes it useful in the recovery of degraded areas. The main botanical characteristics of T. claussenii can be seen in Figure 4.
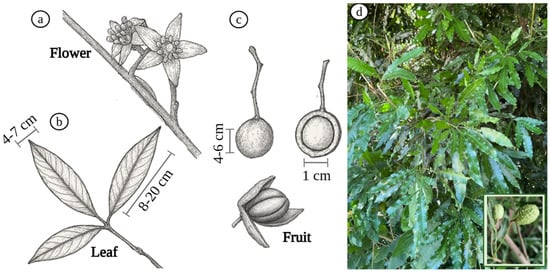
Figure 4.
Botanical characteristics of T. claussenii: (a) flower; (b) leaves; (c) fruit, and (d) tree and its fruit.
3.2. Chemical Composition: Case Study
For the chemical characterization of the leaves and fruits of the species T. claussenii (Meliaceae), the extraction of the bioactive compounds was performed through Ultrasound-Assisted Extraction (UAE). Extractions were performed using approximately 50 g of biomass (leaves or fruits) and 500 mL of solvent (distilled water) at a concentration of 10% (w/v). The ultrasound was adjusted with a pulse cycle (0.9) and an amplitude of 100%. The extraction time was set at 10 min. The species was collected in the municipality of Nova Palma, in the Quarta Colônia region, state of Rio Grande do Sul (Brazil), at the approximate geographic coordinates of 29°28′45″ S latitude and 53°29′03″ W longitude. The leaves and fruits were collected in the afternoon, around 5:00 p.m. Figure 5 represents the steps involved in the process of characterizing the botanical components of T. claussenii with insecticidal properties.
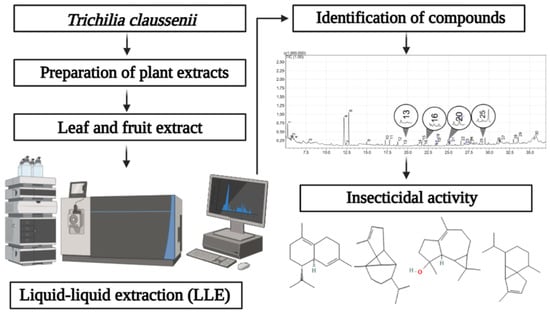
Figure 5.
General representation of the steps involved in the process of characterizing substances of botanical origin from T. claussenii with insecticidal properties.
Liquid–liquid extraction (LLE) was employed to isolate the analytes from the samples. The extraction process involved three different solvents: hexane, dichloromethane, and acetonitrile. Gas chromatography analyses were conducted using a GC-2010 Plus gas chromatograph (Shimadzu, Kyoto, Japan) coupled to a GCMS-QP2010 Ultra mass spectrometer (Shimadzu, Kyoto, Japan) equipped with an automatic AOC-20is series injector (Shimadzu, Kyoto, Japan). The chromatographic separation was achieved using an Rtx®-5 ms GC column (30 m × 0.25 mm, 0.25 μm film thickness) composed of 5% diphenyl and 95% dimethyl polysiloxane (Restek Corporation, Bellefonte, PA, USA). Helium was used as the carrier gas at a flow rate of 1.20 mL min−1. The injector temperature was maintained at 250 °C. Volatile compounds were injected in splitless mode. The initial oven temperature was set at 40 °C for 1 min, then increased to 90 °C at a rate of 1 °C min−1, followed by an increase to 140 °C at a rate of 2 °C min−1, and finally to 250 °C at a rate of 40 °C min−1, where it was held for 17.25 min. Both the interface and ion source temperatures were maintained at 250 °C. Mass spectra were acquired over the m/z range of 50–550 amu with a scan rate of 0.30 scans s−1. Identification of volatiles was performed using a single quadrupole mass spectrometer in electron impact (EI) mode at 70 eV in scan acquisition mode. Individual components were identified by comparing their calculated relative retention indices with those obtained from a C7–C30 homologous n-alkanes series (49452-U; Supelco, Bellefonte, PA, USA) and by matching their mass fragmentation patterns with those in the Wiley Registry of Mass Spectral Data (Palisade Corporation, Newfield, NY, USA). Only compounds with a mass spectral matching score greater than 80% were considered for further analysis.
A total of 41 and 29 compounds were extracted from leaves and fruits, respectively, predominantly classified as phenols, terpenes, sterols, esters, and carboxylic acids. The terpene class was found in larger quantities in the botanical parts (Figure 6).
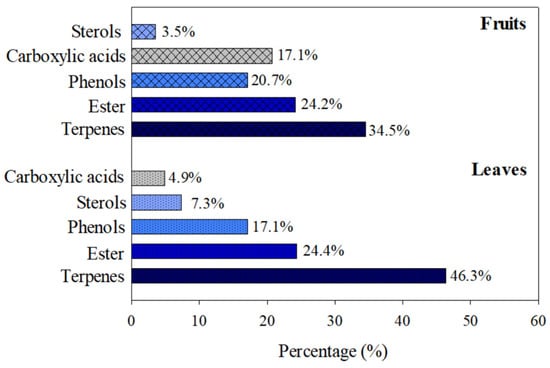
Figure 6.
Classes of compounds identified in T. claussenii extracts.
The verification of the extracts of leaves and fruits of T. claussenii demonstrated a varied composition of bioactive metabolites. In the extract obtained from leaves, the terpenes class was the most abundant, with 46.3% of the total. Esters (24.4%) and phenols (17.1%) were the other predominant classes, while sterols and carboxylic acids presented lower concentrations, with 7.3% and 4.9%, respectively. In the fruit extract, terpenes were also predominant, although in a smaller proportion (34.5%). The esters remained in a similar proportion to the leaves (24.2%), while phenols registered an increase, with 20.7%. The carboxylic acids presented (17.1%), and sterols presented a lower percentage, with 3.5%.
Furthermore, it was possible to identify in the analyzed groups the main compounds related to the antimicrobial, insecticidal and cytotoxic activities present in the extracts of Trichilia claussenii. Among them, the following stand out: squalene, 9,12-octadecadienoic acid, neophytadiene, delta-cadinene, α-copaene, isospathulenol, trans-caryophyllene, caryophyllene, trans-p-Mentha-2,8-dienol, α-cubebene, α-cadinol, 2,4-bis(1,1-dimethylethyl)-phenol, and nerolidol.
The compounds trans-caryophyllene, α-copaene, α-cubebene, and delta-cadinene, belonging to the terpene group, are notable for their role in chemical defense and insecticidal activity, and are considered essential for pest control [25]. Trans-caryophyllene has repellent and insecticidal properties, acting directly on the insect nervous system, mainly through acetylcholinesterase inhibition and interference in cholinergic transmission [26]. Alpha-copaene is recognized for its repellent effect against various pest species [27], while α-cubebene has direct toxic effects on certain insects [28]. Delta-cadinene interferes with the insect hormonal system by inhibiting the action of the molting hormone (ecdysone), thereby impairing the regulation of metamorphosis and development [29].
Among the other compounds, squalene (triterpene) stands out for its antioxidant properties and its contribution to plant resistance [30], while nerolidol has been demonstrated to be effective as an insect repellent [31]. 2,4-bis(1,1-dimethylethyl)-phenol also plays an important role, inhibiting the growth of pathogenic microorganisms by altering cell membrane integrity and inhibiting essential enzymes [32]. Therefore, the identification of these bioactive metabolites reinforces the potential of T. claussenii as a promising source of natural substances for the biological management of agricultural pests.
Table 2 presents different bioactive compounds of plant origin found in the species T. claussenii and their modes of action on insect pests. Understanding these modes of action is essential for the development of integrated pest management strategies.

Table 2.
Compounds present in T. claussenii extracts and their modes of action.
Phytochemical Screening
Table 3 presents the results of the qualitative phytochemical screening of T. claussenii leaves, highlighting the presence or absence of the main secondary metabolites detected in the plant extract. The identification of phytochemical components was performed according to the methodology described in the scientific literature [38].

Table 3.
Phytochemical screening of T. claussenii.
Qualitative screening of the aqueous extract of T. claussenii leaves indicated the presence of coumarin, flavonoids, and alkaloids. However, tannins, saponins, and quinone compounds are absent. Saponins showed negative results in both the foam formation test and thin layer chromatography (TLC). The coumarin analysis showed a slightly greenish coloration, indicating the presence of volatile coumarins. Flavonoids were detected and classified as belonging to the flavonol class.
The phytochemicals observed in T. claussenii demonstrate similarities with the results of other species of the genus Trichilia, indicating a promising phytochemical pattern within the group. However, it is important to emphasize that there are still no published data on a preliminary phytochemical characterization specific to T. claussenii, which makes direct comparisons with the same species difficult.
Studies with related species reinforce the presence of secondary metabolites with bioactive potential. For example, the methanolic extract of Trichilia monadelpha leaves [38,39] and Trichilia hirta [40] revealed the presence of alkaloids and flavonoids. Similarly, hydroalcoholic extracts of Trichilia silvatica leaves and stems presented coumarin and flavonoids [41].
In the context of agricultural pest control, the detected compounds gain relevance. In laboratory tests, plant extracts of Trichilia emetica containing alkaloids demonstrated efficacy in controlling Spodoptera frugiperda, suggesting a relevant insecticidal potential for this class of metabolites [42]. Alkaloids have been increasingly studied due to their widespread occurrence and strong bioactivity; it is estimated that more than 12,000 alkaloids have already been isolated from different plant species [43], many of which have demonstrated toxicity against insect pests [44].
Flavonoids are phenolic compounds widely distributed in plants and recognized for their negative effects on insect development. They can induce malformations, retard growth, and reduce pest fecundity, in addition to directly affecting the nervous system of insects through changes in neural channels [45]. A striking example is the flavonoid chrysoeriol, isolated from Melientha suavis, which showed significant toxicity against Spodoptera litura [46].
Coumarins, also blocked in species of the genus, arouse great interest in the scientific community due to their insecticidal activity [47]. These substances act as toxins and food inhibitors, interfering with the metabolic processes of insects. By reducing appetite, they directly affect growth, development, and reproduction, contributing to the control of agricultural guidelines [48].
4. Use of T. claussenii in Biological Control
Biological control using living organisms or natural substances from plants has become a viable alternative for reducing agricultural pest populations [49]. In this scenario, studies indicate that plants of the Trichilia genus have bioinsecticidal properties aimed at pests such as lepidopterans and coleoptera, which cause great damage to crops [42]. Additionally, its use is compatible with integrated pest management (IPM) practices, preserving natural enemies and reducing the risk of resistance. However, despite the potential benefits, the use of T. claussenii in biological control still faces challenges. To better understand its role in the agricultural context, Table 4 presents the main advantages and limitations of using T. claussenii in biological control.

Table 4.
Advantages and limitations of using T. claussenii in biological control.
From a toxicological point of view, botanical extracts have low acute toxicity to humans and other non-target organisms, such as bees and natural enemies of pests. This is an important difference to conventional pesticides, which cause poisoning, food contamination, and harm to human health [50]. The compounds present in the plant must undergo standardized tests to guarantee long-term safety. The mistaken belief that natural products do not negatively affect other organisms leads to the neglect of these assessments. Therefore, it is feasible to conduct studies on the physiological or ecological selectivity of botanical insecticides about toxicity to natural enemies and humans.
Concerning environmental impacts, the use of botanical insecticides is advantageous because they are biodegradable and have a shorter persistence in soil and water [51]. This reduces the risk of environmental contamination and imbalances in agricultural ecosystems. The indiscriminate extraction of plant raw materials for the production of bioinsecticides can pose a threat to biodiversity, especially if the species is exploited in an unsustainable manner. In addition, variations in chemical composition due to environmental and genetic factors can affect the efficacy and safety of plant extracts [52]. Therefore, it is essential to balance the responsible use of the plant with sustainable management practices. Table 5 shows the positive aspects and limitations of the use of T. claussenii from a toxicological and environmental perspective, contributing to a critical analysis of its viability as a biological control agent.

Table 5.
Toxicological and environmental aspects of the use of T. claussenii in the context of botanical insecticides.
5. Mechanisms of Action of T. claussenii
The mode of action of an insecticide is the way it interferes with the insect’s biological processes, mainly affecting the nervous system, development, or energy production [60]. Determining this mechanism in plant compounds is a challenge, as it involves multiple actions and biological targets. However, this knowledge is essential to apply products strategically, aiming to ensure effectiveness in control and avoid insect resistance. The insecticidal action of T. claussenii extracts, as well as botanical insecticides, occurs through different mechanisms of action, depending on the type of compound and the formulation used, as shown in Figure 7. The effects can be behavioral, such as repellency, feeding inhibition, direct toxicity, and growth interruption.
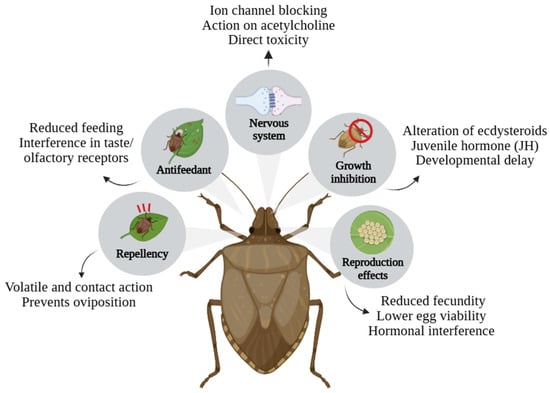
Figure 7.
Main modes of action of T. claussenii on insects.
The antifeedant action is caused mainly by phagodeterrents, which are natural substances from plants that inhibit the appetite of insects, causing interruption of feeding, weight loss, and death by starvation [61]. This mode of action occurs by interfering with the insect’s sensory systems, such as taste and smell, preventing it from recognizing the plant as suitable. This process can occur upon contact with the plant treated with the botanical extract, in which the insect lands or comes into contact with the plant surface that contains antifeedant substances, and its stimuli in the taste sensors, located in the mouthparts, palps, antennae, and tarsi, interact with the receptor proteins in the chemoreceptors and cause repulsion, causing rejection of the food after a brief tasting or even without starting to consume it [62]. The result of this interruption is reduced intake, lower weight gain, and incomplete development that can result in reduced fertility or even death.
Growth inhibition is an efficient mode of action, as it acts sublethally, interfering with the development, ecdysis (molting), cuticle formation, and metamorphosis of insects, resulting in interruptions in the life cycle of the insect pest [63]. This process can occur through hormonal interference, blocking of chitin formation, and changes in the endocrine and enzymatic systems. During insect growth, there are two main hormones at this stage: ecdysteroids, responsible for stimulating the cuticle, and juvenile hormone (JH), which maintains the larval state and prevents premature changes [64].
Some plant compounds can inhibit or block these hormones. Analogous to JH, they can keep the insect in a juvenile state, preventing the transition to the adult stage and resulting in deformed or sterile adults [65]. However, when botanical bioactives interfere as ecdysteroid blockers, they prevent molting inhibition and the larva enters a lethargic state and dies [66]. Growth interruption can also occur through inhibition of chitin synthesis, which is a component of the insect cuticle. Some plant components inhibit the enzyme chitin synthase, blocking the formation of a new cuticle after the ecdysis phase [67]. At the same time, changes in metabolism and body growth are also observed in the action that some extracts reduce the activity of digestive enzymes or affect the metabolism of proteins and lipids, leading to decreased body mass gain and delayed growth.
Direct action on insect pests can cause direct toxicity, affecting the nervous system and energy metabolism, causing paralysis, lethargy, and death of the insects [68]. Insects have a central and peripheral nervous system that functions as a basis for the transmission of electrical and chemical impulses between neurons, with GABA (gamma-aminobutyric acid), acetylcholine, and glutamic acid being the main neurotransmitters involved in this stage [69]. Acetylcholinesterase (AchE) is an enzyme that degrades the neurotransmitter acetylcholine; compounds such as alkaloids, terpenoids, and flavonoids can inhibit AChE, leading to the accumulation of acetylcholine in synapses, which are the junctions that allow the transmission of nerve impulses [70]. As a consequence, insect pests may exhibit tremors, neuronal hyperstimulation, muscle paralysis, and death due to neural exhaustion. The action on neuronal ion channels targets sodium channels (Na+), which are essential for the conduction of nerve impulses, and chloride channels mediated by GABA, which is a neurotransmitter that regulates the inhibition of insect neurons [71]. Terpenoids and alkaloids from plant extracts block or excessively open these channels, which deregulate action potentials and prevent communication between neurons, leading to inhibition of motor coordination, spasms, and even death.
Repellent action is also a mode of action in which the effect prevents the insect from coming into contact with a certain area or surface. This process can occur via olfactory (volatile) or gustatory (direct contact) pathways [72]. Insects use a combination of olfactory chemoreceptors on the antennae and palps to detect volatile odors, and gustatory receptors present on the mouthparts, tarsi, and antennae, to detect substances upon landing. These receptors are linked to sensory neurons, which transmit signals to the insect’s brain, guiding its behavior. Although the number of plant substances with known insecticidal action has increased, many compounds need to be further studied, especially regarding their mode of action. Thus, the valorization of compounds already identified and the exploration of their modes of action and bioactivity are essential strategies for advancing this area.
6. Use of T. claussenii in Integrated Pest Management
IPM is a sustainable approach that guides control decisions based on economic, social, and environmental criteria, promoting the combination of different management strategies [73]. One of its fundamental pillars is biological control, which uses beneficial organisms to keep pest populations below damage levels. T. claussenii is a promising plant for this purpose, as its application in IPM programs can reduce the frequency and quantity of chemical insecticide use, decreasing the risk of resistance development in pests when used in rotation with other methods [74]. In addition, T. claussenii extract is compatible with biological control agents, such as parasitoids and natural predators, which allows its integration with other sustainable strategies.
The use must be associated with a systematic monitoring program of pest populations, allowing for specific and targeted applications of the extracts only when infestation levels reach economic control levels. Furthermore, its application can be combined with biological control, through the release or conservation of natural enemies, since the extract is compatible with many of these organisms [75]. It can also be included in rotation or alternation schemes with other types of insecticides, whether botanical, microbiological, or synthetic, to avoid the selection of resistant populations. In agroecological or organic-based systems, the extract can also be used with cultural practices, such as soil management, intercropping, and crop rotation, creating a management system that reduces conditions favorable to the development of pests.
Another important point in IPM is the planning of applications, which must consider crop phenology, pest biology, and environmental conditions that affect both the persistence of extracts and the population dynamics of insects [76]. Thus, the use of T. claussenii should not be isolated, but rather articulated with a set of integrated actions, guided by technical diagnosis, strategic planning, and constant evaluation of effectiveness. The function of IPM, therefore, is not to completely replace other methods, but rather to complement management to expand control possibilities and reinforce the sustainability of the agricultural system. Figure 8 presents a simplified flowchart with the insertion of T. claussenii in IPM.
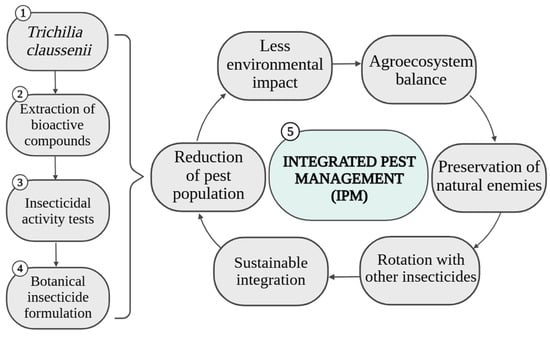
Figure 8.
Simplified flowchart representing the use of T. claussenii in IPM.
Synergy of T. claussenii and Microbial Agents
The use of T. claussenii with microbial agents in the control of agricultural pests represents a promising strategy in IPM. This approach combines the insecticidal effects of the bioactive compounds present in the plant extract with the specific action of microorganisms (fungi, bacteria, viruses, nematodes, protozoa) [53,77]. Table 6 presents the benefits of the combination of T. claussenii with entomopathogenic microorganisms.

Table 6.
Benefits of the synergy of T. claussenii and microbial agents.
T. claussenii extract, when combined with microorganisms, can enhance the toxic, infectious, or physiological effects of these agents on insect pests. These integrated modes of action explore different routes of infection and intoxication, increasing control. Table 7 shows the types of microbial agents and their modes of action integrated with T. claussenii extract.

Table 7.
Combined modes of action of T. claussenii (plant extract) and microbial agents.
There are still no records of specific studies investigating the association of T. claussenii extracts with microbial agents. This gap represents a promising research opportunity, especially based on the growing trend of integrating botanical extracts with microorganisms in biological control strategies in IPM.
7. Limitations and Challenges in the Development of Botanical Insecticides
Although several groups of plants have already been explored for their insecticidal effects, the number of species studied is still small compared to the vast botanical diversity available, especially in Brazil. Despite the advances achieved, challenges remain to improve the effectiveness of research with botanical insecticides. In this context, despite the recognized insecticidal potential of T. claussenii, there are still several gaps that limit its practical application in the biological control of agricultural pests. The main issues related to the subject are highlighted below, based on Ribeiro et al. [24].
One of the main obstacles is related to standardization and regulation. Progress in this field depends not only on the correct identification of plant species but also on the improvement of extraction techniques, formulation processes, and quality control methods. This requires a multidisciplinary approach to ensure effectiveness, safety, and commercial viability. Furthermore, the evaluation of bioactivity must go beyond insect mortality, encompassing effects on insect behavior and development, as well as the adoption of reliable bioassay methods that allow for more accurate comparisons across studies.
Another critical issue refers to the standardization of extracts and the effectiveness of formulations. Extraction methods directly influence the chemical composition and, consequently, the insecticidal activity of the compounds, reinforcing the need for optimized techniques and more stable formulations. In this regard, inconsistency in extract concentrations represents an additional difficulty, since variations in preparation methods may compromise the reproducibility of results and hinder the consolidation of scientific protocols.
Another relevant point refers to the limitations of the extraction process. The choice of solvent, the technique employed (such as maceration, Soxhlet, ultrasound-assisted extraction, or supercritical fluid extraction), and the experimental conditions (time, temperature, and plant/solvent ratio) can significantly alter the final chemical composition of the extract, directly influencing its bioactivity. These methodological variations also make it difficult to compare different studies and extrapolate results to practical conditions, reinforcing the importance of advances in the optimization and validation of extraction techniques.
In addition, the lack of continuity in long-term research programs limits consistent progress in this area. Many studies remain restricted to laboratory assays, without transition to field conditions or product development, reducing the representativeness and applicability of the findings. The scarcity of scientific publications, particularly on T. claussenii, also limits access to reliable information, hindering the establishment of a solid knowledge base.
Finally, the absence of specific regulations for botanical insecticides poses another significant obstacle. At present, many products must still comply with requirements designed for synthetic pesticides, which makes their registration and commercialization more difficult or slower. Overcoming these challenges requires integrated efforts among researchers, funding agencies, and regulatory bodies to strengthen scientific advances and ensure the practical feasibility of botanical insecticides in sustainable agriculture.
8. Conclusions
Although botanical insecticides represent a sustainable and environmentally friendly alternative for pest control, they are still underexplored in the context of biological control, particularly when it comes to lesser-known species. In this regard, the review on Trichilia claussenii revealed important gaps, such as the absence of patent records directly associated with the species, with its scientific and technological potential still unexplored. Future perspectives include the necessity for further phytochemical studies for the identification of bioactive compounds, genetic and biotechnological analyses to optimize metabolite production, and the development of stable and effective formulations that enable large-scale agricultural use. Field trials, ecotoxicological studies, and the evaluation of combined uses with microbial agents are also promising pathways. Thus, T. claussenii may become a strategic resource for the development of commercial bioinsecticides, contributing to more sustainable agricultural practices and reducing dependence on synthetic insecticides.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by L.P.O., A.d.B.F.F., E.d.M. and G.U. The first draft of the manuscript was written by L.P.O. and G.L.Z. and all authors commented on previous versions of the manuscript. The final revision of the manuscript was done by M.A.M., M.V.T. and G.L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Coordination for the Improvement of Higher Education Personnel (CAPES), the National Council of Technological and Scientific Development (CNPq: 404308/2023-6), and the Research Support Foundation of the State of Rio Grande do Sul (FAPERGS: 24/2551-0001977-4). G. L. Zabot (308067/2021-5), M. A. Mazutti, and M. V. Tres thank CNPq for the productivity grants.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ngegba, P.M.; Cui, G.; Khalid, M.Z.; Zhong, G. Use of botanical pesticides in agriculture as an alternative to synthetic Pesticides. Agriculture 2022, 12, 600. [Google Scholar] [CrossRef]
- Aremu, A.O.; Omogbene, T.O.; Fadiji, T.; Lawal, I.O.; Opara, U.L.; Fawole, O.A. Plants as an alternative to the use of chemicals for crop protection against biotic threats: Trends and future perspectives. Eur. J. Plant Pathol. 2024, 170, 711–766. [Google Scholar] [CrossRef]
- Divekar, P. Botanical pesticides: An eco-friendly approach for management of insect pests. Acta Sci. Agric. 2023, 7, 75–81. [Google Scholar] [CrossRef]
- Sarmah, K.; Anbalagan, T.; Marimuthu, M.; Mariappan, P.; Angappan, S.; Vaithiyanathan, S. Innovative formulation strategies for botanical- and essential oil-based insecticides. J. Pest Sci. 2024, 98, 1–30. [Google Scholar] [CrossRef]
- Abdala, V. Mais de 25 mil Espécies da Flora só Existem no Brasil, Mostra Estudo. Available online: https://agenciabrasil.ebc.com.br (accessed on 20 June 2021).
- Tzusuki, A.; Embrapa. Pesticidas Biológicos Cresceram 45% Nos Últimos Cinco Anos. 2024. Available online: https://www.embrapa.br/ (accessed on 25 June 2024).
- Pila, D.; Maqueda, R.H. Traditional knowledge and use of plants as agricultural insecticides from a gender perspective in three rural communities of the Ecuadorian Andes. Ethnobot. Res. Appl. 2023, 26, 1–12. [Google Scholar] [CrossRef]
- Bilal, T.; Mushtaq, T.; Ahmad, P.I.; Gangoo, S.A.; Behar, B.; Ayoob, B.; Farooq, S.; Mushtaq, I. Botanicals their use as antimicrobial, antifungal and anti insecticides. Pharma. Innov. J. 2022, 11, 1521–1528. [Google Scholar]
- Lin, M.; Liu, X.; Chen, J.; Huang, J.; Zhou, L. Insecticidal triterpenes in Meliaceae III: Plant species, molecules, and activities in Munronia–Xylocarpus. Int. J. Mol. Sci. 2024, 25, 7818. [Google Scholar] [CrossRef] [PubMed]
- Robaina, R.R.; Braz-Filho, R.; Vieira, I.J. An updated review of the structural chemistry and biological activity of compounds from Trichilia species. Phytochem. Rev. 2025, 8, 1–37. [Google Scholar] [CrossRef]
- Ogunlakin, A.D.; Sonibare, M.A. Phytochemistry and biological activities of Tetracera species. Trends Phytochem. Res. 2022, 6, 339–352. [Google Scholar] [CrossRef]
- Grau, L.; Díaz, R.; Guerrero, D.; Viera, J.; Acosta, A. Compuestos bioactivos y actividad antioxidante in vitro del extracto etanólico de hojas de Melia azedarach Linn (Meliaceae). Steviana 2024, 16, 33–41. [Google Scholar] [CrossRef]
- Kandeda, A.K.; Lewale, S.; Djeuzong, E.; Kouamouo, J.; Dimo, T. An aqueous extract of Khaya senegalensis (Desv.) A. Juss. (Meliaceae) prevents seizures and reduces anxiety in kainate-treated rats: Modulation of GABA neurotransmission, oxidative stress, and neuronal loss in the hippocampus. Heliyon 2022, 8, e09549. [Google Scholar] [CrossRef]
- Bernardo, J.; Santos, A.C.; Videira, R.A.; Valentão, P.; Veiga, F.; Andrade, P.B. Trichilia catigua and Turnera diffusa phyto-phospholipid nanostructures: Physicochemical characterization and bioactivity in cellular models of induced neuroinflammation and neurotoxicity. Int. J. Pharm. 2022, 620, 121774. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Wang, Z.; Yang, L. Limonoids from the genus Melia (Meliaceae): Phytochemistry, synthesis, bioactivities, pharmacokinetics, and toxicology. Front. Pharmacol. 2022, 12, 795565. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.D.; Lopes, A.V.; Marques, V.P.; Bellonzi, T.K.; Cerdan, I.P.; Magalhães, R.; Gasparino, E.C. Pollen morphology of Meliaceae from Brazilian forest fragments of Cerrado, Brazil. Palynology 2025, 49, 1925769. [Google Scholar] [CrossRef]
- Passos, M.S.; Nogueira, T.R.; Azevedo, O.A.; Vieira, M.C.; Terra, W.S.; Braz-Filho, R.; Vieira, I.J. Limonoids from the genus Trichilia and biological activities: Review. Phytoc. Rev. 2021, 20, 1055–1086. [Google Scholar] [CrossRef]
- Barros, M.E.; Sousa, L.M.; Santos, F.D.; Loiola, M.I. Flora of Ceará, Brazil: Meliaceae. Orig. Papers. 2024, 75, e01762023. [Google Scholar] [CrossRef]
- Bogorni, P.C.; Vendramim, J.D. Efeito subletal de extratos aquosos de Trichilia spp. sobre o desenvolvimento de Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) em Milho. Neotrop. Entomol. 2003, 34, 311–317. [Google Scholar] [CrossRef]
- Castro, T.M.; Moraes, G.J. Diversity of phytoseiid mites (Acari: Mesostigmata: Phytoseiidae) in the Atlantic Forest of São Paulo. Syst. Biodivers. 2010, 8, 301–307. [Google Scholar] [CrossRef]
- Abranches, S. Biological megadiversity as a tool of soft power and development for Brazil. Braz. Polit. Sci. Rev. 2020, 14, e0006. [Google Scholar] [CrossRef]
- Maffra, C.R. Características físicas e germinativas de sementes de Trichilia claussenii C.DC. para a região do Médio Alto Uruguai-RS. Braz. J. Biosyst. Eng. 2019, 13, 65–71. [Google Scholar] [CrossRef]
- Flora. Flora Digital—Trichilia claussenii. Available online: https://floradigital.ufsc.br/busca.php (accessed on 10 June 2021).
- Ribeiro, L.P.; Vendramim, J.D.; Baldin, E.L. Inseticidas Botânicos No Brasil: Aplicações, Potencialidades e Perspectivas; FEALQ: Piracicaba, Brazil, 2023; p. 652. Available online: https://www.google.com.br/books (accessed on 10 June 2024).
- Yu, N.; Chen, Z.; Yang, J.; Li, R.; Zou, W. Integrated transcriptomic and metabolomic analyses reveal regulation of terpene biosynthesis in the stems of Sindora glabra. Tree Physiol. 2021, 41, 1087–1102. [Google Scholar] [CrossRef]
- Ikawati, S.; Himawan, T.; Abadi, A.; Sarno, H.; Fajarudin, A. In silico study of eugenol and trans-caryophyllene also clove oil fumigant toxicity on Tribolium castaneum. J. Trop. Life Sci. 2022, 12, 339–349. [Google Scholar] [CrossRef]
- Magnani, R.F.; Volpe, H.X.; Luvizotto, R.A.; Mulinari, T.A.; Agostini, T.T.; Bastos, J.K.; Ribeiro, V.P.; Carmo-Sousa, M.; Wulff, N.A.; Pena, L.; et al. α-Copaene is a potent repellent against the Asian Citrus Psyllid Diaphorina citri. Sci. Rep. 2025, 15, 3564. [Google Scholar] [CrossRef]
- Rohimatun, M.D.N.; Puspasari, L.T.; Rusmin, D. Toxicity and chemical compounds of Piper aduncum fruit extract against storage pest Sitophilus oryzae and Callosobruchus maculatus. IOP Conf. Ser. Earth Environ. Sci. 2023, 1253, 012001. [Google Scholar] [CrossRef]
- Natchiappan, S.; Ramasamy, S.; Ganguly, S. Effect of Lantana camara L. Essential oil: A natural solution for managing stored grain insects. Int. J. Zool. Appl. Biosci. 2024, 9, 34–40. [Google Scholar] [CrossRef]
- Joshi, S.S.; Sumathi, E.; Murugan, M.; Haran, R.; Priya, S.S.; Shandeep, G.; Mohankumar, S.; Uma, D.; Nelson, A. Correction: Exploration of bioactive molecules from Sesbania grandiflora L.: Identification of squalene as an effective compound against the two-spotted spider mite, Tetranychus urticae Koch, through molecular docking. Exp. Appl. Acarol. 2025, 94, 27. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, K.; Hamadah, K.H.; Selim, S.H.; Waheeb, H. Biopesticidal potential of nerolidol, a sesquiterpene compound, and its drastic impact on growth and metamorphosis of the cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae). Sch. Acad. J. Biosci. 2021, 9, 36–57. [Google Scholar] [CrossRef]
- Devi, C.J.; Saikia, K.; Mazumdar, R.; Das, R.; Bharadwaj, P.; Thakur, D. Identification, biocontrol and plant growth promotion potential of endophytic Streptomyces sp. a13. Curr. Microbiol. 2025, 82, 64. [Google Scholar] [CrossRef]
- Liu, Z.; Li, Q.X.; Song, B. Pesticidal activity and mode of action of monoterpenes. J. Agric. Food Chem. 2022, 70, 4556–4571. [Google Scholar] [CrossRef]
- Hernandez-Trejo, A.; Rodríguez-Herrera, R.; Sáemz-Galindo, A.; López-Badillo, C.M.; Flores-Gallegos, A.C.; Ascacio-Valdez, J.A.; Estrada-Drouaillet, B.; Osorio-Hernández, E.O. Insecticidal capacity of polyphenolic seed compounds from neem (Azadirachta indica) on Spodoptera frugiperda (J. E. Smith) larva. J. Environ. Sci. Health 2021, 56, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Mwamba, S.; Kihika-Opanda, R.; Murungi, L.; Losenge, T.; Beck, J.J.; Torto, B. Identification of repellents from four non-host Asteraceae plants for the root knot nematode, Meloidogyne incognita. J. Agric. Food Chem. 2021, 69, 15145–15456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Guleria, N.; Deeksha, M.; Kumari, N.; Kumar, R.; Jha, A.K.; Parmar, N.; Ganguly, P.; Andrade, E.H.; Ferreira, O.O.; et al. From an invasive weed to an insecticidal agent: Exploring the potential of Lantana camara in insect management strategies—A review. Int. J. Mol. Sci. 2024, 25, 12788. [Google Scholar] [CrossRef]
- El-Ashmouny, R.S.; Rady, M.H.; Merdan, B.A.; El-Sheikh, T.A.; Hassan, R.E.; Gohary, E. Larvicidal and pathological effects of green synthesized silver nanoparticles from Artemisia herba-alba against Spodoptera littoralis through feeding and contact application. Egypt. J. Basic Appl. Sci. 2022, 9, 239–253. [Google Scholar] [CrossRef]
- Simões, C.M.; Schenkel, E.P.; Gosmann, G.; Mello, J.C. Farmacognosia: Da Planta ao Medicamento, 6th ed.; UFRGS: Porto Alegre, Brazi, 2017; p. 1104. Available online: https://bibliotecadebiomedicina.blogspot.com/2019/01/livro-farmacognosia-da-planta-ao.html (accessed on 20 April 2024).
- Clark, P.D.; Omo-Udoyo, E. A comparative assessment on antioxidant and phytochemical of Trichilia monadelpha (Thonn) J.J. De Wilde (Meliaceae) plant extracts. Chem. Sci. Int. J. 2021, 30, 24–33. [Google Scholar] [CrossRef]
- Sierra, J.D.; Contreras, O.I.; Angulo, A.A. Phytochemical screening and antifungal activity in vitro of Trichilia hirta L. fruit extracts against clinical isolates of Candida spp. Indian J. Nat. Prod. Resour. 2022, 13, 170–175. [Google Scholar] [CrossRef]
- Silveira, L.L.; Dias, M.M.; Pelinsari, S.M.; Paula, R.A.; Castro, A.S.; Almeida, V.L.; Gonçalves, R.V. Trichilia silvatica extracts modulate the oxinflammatory response: An in vitro analysis. J. Ethnopharmacol. 2025, 349, 119973. [Google Scholar] [CrossRef]
- Monjane, J.; Tambo, T.R. Evaluation of the insecticidal activity of the extracts of Trichilia emetica, Trichilia capitata, and Azadirachta indica against the Spodoptera frugiperda (Fall Armyworm) on maize crop. Alger. J. Nat. Prod. 2021, 9, 830–837. [Google Scholar] [CrossRef]
- Zandavar, H.; Babazad, M. Secondary metabolites: Alkaloids and flavonoids in medicinal plants. In Herbs Spices—New Advances; IntechOpen: London, UK, 2023; pp. 1–28. [Google Scholar] [CrossRef]
- Bhambhani, S.; Kondhare, K.R.; Giri, A.P. Diversity in chemical structures and biological properties of plant alkaloids. Molecules 2021, 26, 3374. [Google Scholar] [CrossRef]
- Pereira, V.; Figueira, O.; Castilho, P.C. Flavonoids as insecticides in crop protection—A review of current research and future prospects. Plants 2024, 13, 776. [Google Scholar] [CrossRef]
- Ruttanaphan, T.; Thitathan, W.; Piyasaengthong, N.; Nobsathian, S.; Bullangpoti, V. Chrysoeriol isolated from Melientha suavis Pierre with activity against the agricultural pest Spodoptera litura. Chem. Biol. Technol. Agric. 2022, 9, 21. [Google Scholar] [CrossRef]
- Xia, T.; Liu, Y.; Lu, Z.; Yu, H. Natural coumarin shows toxicity to Spodoptera litura by inhibiting detoxification enzymes and glycometabolism. Int. J. Mol. Sci. 2023, 24, 13177. [Google Scholar] [CrossRef]
- Zaynab, M.; Khan, J.; Al-Yahyai, R.; Sadder, M.; Li, S. Toxicity of coumarins in plant defense against pathogens. Toxicon 2024, 250, 108118. [Google Scholar] [CrossRef] [PubMed]
- Ivase, T.J.; Nyakuma, B.B.; Otitolaiye, V.O.; Utume, L.N.; Ayoosu, M.I.; Jagun, Z.T.; Oladokun, O.; Dodo, Y.A. Standardization, quality control, and bio- enhancement of botanical insecticides: A review. DRC Sustain. Futur. J. Environ. Agric. Energy 2021, 2, 104–111. [Google Scholar] [CrossRef]
- Iqbal, T.; Ahmed, N.; Shahjeer, K.; Ahmed, S.; Al-Mutairi, K.A.; Khater, H.F.; Ali, R.F. Botanical insecticides and their potential as anti-insect/pests: Are they successful against insects and pests? In Global Decline of Insects; IntechOpen: London, UK, 2021; Volume 1, pp. 1–14. [Google Scholar] [CrossRef]
- Haritha, D.; Ahmed, M.F.; Bala, S.; Choudhury, D. Eco-friendly plant based on botanical pesticides. Plant Arch. 2021, 21, 2197–2204. [Google Scholar] [CrossRef]
- Heinrich, M.; Jalil, B.; Abdel-Tawab, M.; Echeverria, J.; Kulic, Z.; McGaw, L.; Pezzuto, J.M.; Potterat, O.; Wang, J. Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research—The ConPhyMP—Guidelines. Front. Pharmacol. 2022, 13, 953205. [Google Scholar] [CrossRef]
- Basaid, K.; Furze, J. Botanical-microbial synergy—Fundaments of untapped potential of sustainable agriculture. J. Crop Health 2024, 76, 1263–1280. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, H.; Gao, J.; Zhang, J.; Li, X.; Zhou, J.; Liang, P.; Gao, X.; Gu, S. Plant volatile compound methyl benzoate is highly effective against Spodoptera frugiperda and safe to non-target organisms as an eco-friendly botanical-insecticide. Ecotoxicol. Environ. Saf. 2022, 245, 114101. [Google Scholar] [CrossRef]
- Catania, R.; Lima, M.A.; Potrich, M.; Sgolastra, F.; Zappalà, L.; Mazzeo, G. Are botanical biopesticides safe for bees (Hymenoptera, Apoidea)? Insects 2023, 14, 247. [Google Scholar] [CrossRef]
- Silva, R.S.; Tomaz, A.C.; Lopes, M.C.; Martins, J.C.; Xavier, V.M.; Picanço, M.C. Toxicity of botanical insecticides on Diaphania hyalinata, their selectivity for the predatory ant Paratrechina sp., and their potential phytotoxicity on pumpkin. Int. J. Pest Manag. 2016, 62, 95–104. [Google Scholar] [CrossRef]
- Peshin, R.; Dhawan, A.K. (Eds.) Integrated Pest Management: Concept, Opportunities and Challenges. In Integrated Pest Management: Innovation-Development Process; Springer: Dordrecht, The Netherlands, 2009; p. 667. [Google Scholar] [CrossRef]
- Santos, M.M.; Batista, A.C.; Silva, A.D.; Ganassoli, E.; Barradas, A.; Giongo, M. Characterization and dynamics of surface fuel of Cerrado grassland in Jalapão Region-Tocantins, Brazil. Floresta 2021, 51, 127–136. [Google Scholar] [CrossRef]
- Acevedo-Quintero, J.F.; Zamora-Abrego, J.G.; Chica-Vargas, J.P.; Mancera-Rodríguez, N.J. Functional traits of fruits of particular importance for seed dispersers in the tropical dry forest. Rev. Biol. Trop. 2023, 71, e52288. [Google Scholar]
- Nwonuma, C.B.; Omoniwa, B.P.; Elleke, T.E.; Aladele, P.; Ogundipe, O.E. The modes of action of biopesticidal compounds in insect control. Int. J. Trop. Insect Sci. 2025, 45, 513–523. [Google Scholar] [CrossRef]
- Souza, S.A.; Padial, I.M.; Souza, T.S.; Domingues, A.; Ferreira, E.A.; Mauad, M.; Cardoso, C.A.; Malaquias, J.B.; Oliveira, L.V.; Formagio, A.S.; et al. Evaluation of bioinseticide in the control of Plutella xylostella (Linnaeus, 1758): A laboratory study for large-scale implementation. Sustainability 2025, 17, 1626. [Google Scholar] [CrossRef]
- Jeon, H.; Tak, J. Gustatory habituation to essential oil induces reduced feeding deterrence and neuronal desensitization in Spodoptera litura. J. Pest Sci. 2024, 98, 321–336. [Google Scholar] [CrossRef]
- Saini, P.; Gupta, K.K. Bioefficacy of botanicals with special emphasis on Cassia fistula and nano-formulations on survival, growth, and development of insects: A sustainable approach of integrated pest management. Indian J. Nat. Prod. Resour. 2024, 15, 260–273. [Google Scholar] [CrossRef]
- Dai, H.; Liu, B.; Yang, L.; Yao, Y.; Liu, M.; Xiao, W.; Li, S.; Ji, R.; Sun, Y. Investigating the regulatory mechanism of the sesquiterpenol nerolidol from a plant on juvenile hormone-related genes in the insect Spodoptera exigua. Int. J. Mol. Sci. 2023, 24, 13330. [Google Scholar] [CrossRef]
- Hu, H.; Yin, X.; Pang, S.; Jiang, Y.; Weng, Q.; Hu, Q.; Wang, J. Mechanism of destruxin a inhibits juvenile hormone binding protein transporting juvenile hormone to affect insect growth. Pestic. Biochem. Physiol. 2023, 197, 105654. [Google Scholar] [CrossRef]
- Kilani-Morakchi, S.; Morakchi-Goudjil, H.; Sifi, K. Azadirachtin-based insecticide: Overview, risk assessments, and future directions. Front. Agron. 2021, 3, 676208. [Google Scholar] [CrossRef]
- Sun, X.; Li, W.; Yang, S.; Ni, X.; Han, S.; Wang, M.; Zhen, C.; Huang, X. Insecticidal activity and underlying molecular mechanisms of a phytochemical plumbagin against Spodoptera frugiperda. Front. Physiol. 2024, 15, 1427385. [Google Scholar] [CrossRef]
- Hoesain, M.; Suharto; Prastowo, S.; Pradana, A.P.; Alfarisy, F.K.; Adiwena, M. Investigating the plant metabolite potential as botanical insecticides against Spodoptera litura with different application methods. Cogent Food Agric. 2023, 9, 2229580. [Google Scholar] [CrossRef]
- Raisch, T.; Raunser, S. The modes of action of ion-channel-targeting neurotoxic insecticides: Lessons from structural biology. Nat. Struct. Mol. Biol. 2023, 30, 1411–1427. [Google Scholar] [CrossRef]
- Subba, B.; Thapa, S.; Gurung, B.; Pokhrel, P. Plant-based organic insecticides. In Recent Trend in Entomology; Cape Comorin Publisher: Tamil Nadu, India, 2023; Volume 9, pp. 154–164. Available online: https://www.researchgate.net/publication/373396771 (accessed on 15 February 2024).
- Coquerel, Q.R.; Démares, F.; Geldenhuys, W.; Ray, A.; Bréard, D.; Richomme, P.; Legros, C.; Norris, E.; Bloomquist, J. Toxicity and mode of action of the aporphine plant alkaloid liriodenine on the insect GABA receptor. Toxicon 2021, 201, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Cortese, D.; Da Silva, M.M.; Oliveira, G.S.; Mussury, R.M.; Fernandes, M.G. Repellency and reduction of offspring emergence potential of some botanical extracts against Sitophilus zeamais (Coleoptera: Curculionidae) in stored maize. Insects 2022, 13, 842. [Google Scholar] [CrossRef]
- Zhou, W.; Arcot, Y.; Medina, R.F.; Bernal, J.; Cisneros-Zevallos, L.; Akbulut, M. Integrated pest management: An update on the sustainability approach to crop protection. ACS Omega 2024, 9, 41130–41147. [Google Scholar] [CrossRef]
- Deguine, J.; Aubertot, J.; Flor, R.; Lescourret, F.; Wyckhuys, K.; Ratnadass, A. Integrated pest management: Good intentions, hard realities. A review. Agron. Sustain. Dev. 2021, 41, 38. [Google Scholar] [CrossRef]
- Ochieng, L.O.; Ogendo, J.O.; Bett, P.K.; Nyaanga, J.G.; Cheruiyot, E.K.; Mulwa, R.M.; Arnold, S.E.; Belmain, S.R.; Stevenson, P.C. Field margins and botanical insecticides enhance Lablab purpureus yield by reducing aphid pests and supporting natural enemies. J. Appl. Entomol. 2022, 146, 838–849. [Google Scholar] [CrossRef]
- Wiratno; Sutanto, K.D.; Nurawan, A.; Taufik, I.; Surdianto, Y.; Sutrisna, N.; Rizal, M.; Karmawati, E.; Siswanto; Soetopo, D.; et al. Eco-friendly botanical insecticides to control brown leafhoppers and their effects on the predators and aquatic environment. Glob. J. Environ. Sci. Manag. 2025, 11, 113–128. [Google Scholar] [CrossRef]
- Santos, W.P.; Lopes, L.M.; Silva, G.N.; Carvalho, M.S.; de Sousa, A.H. Piper aduncum Essential Oil: Toxicity to Sitophilus zeamais and Effects on the Quality of Corn Grains. Processes 2025, 13, 1363. [Google Scholar] [CrossRef]
- Ebani, V.V.; Mancianti, F. Entomopathogenic fungi and bacteria in a veterinary perspective. Biology 2021, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Chalivendra, S. Microbial toxins in insect and nematode pest biocontrol. Int. J. Mol. Sci. 2021, 22, 7657. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Wennmann, J.; Goergen, G.; Bryon, A.; Ros, V. Viruses of the fall armyworm spodoptera frugiperda: A review with prospects for biological control. Viruses 2021, 13, 2220. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Meléndez, V.; Ibarra-Rendón, J.; Contreras-Garduño, J. The evolution of entomopathogeny in nematodes. Ecol. Evol. 2024, 14, e10966. [Google Scholar] [CrossRef] [PubMed]
- Saddam, B.; Idrees, M.A.; Kumar, P.; Mahamood, M. Biopesticides: Uses and importance in insect pest control: A review. Int. J. Trop. Insect Sci. 2024, 44, 1013–1020. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).