Transcriptomic Profiling and Histological Validation of Preputial Fibrosis in Hypospadias
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Enrollment
2.2. Sample Collection
2.3. Histological Staining
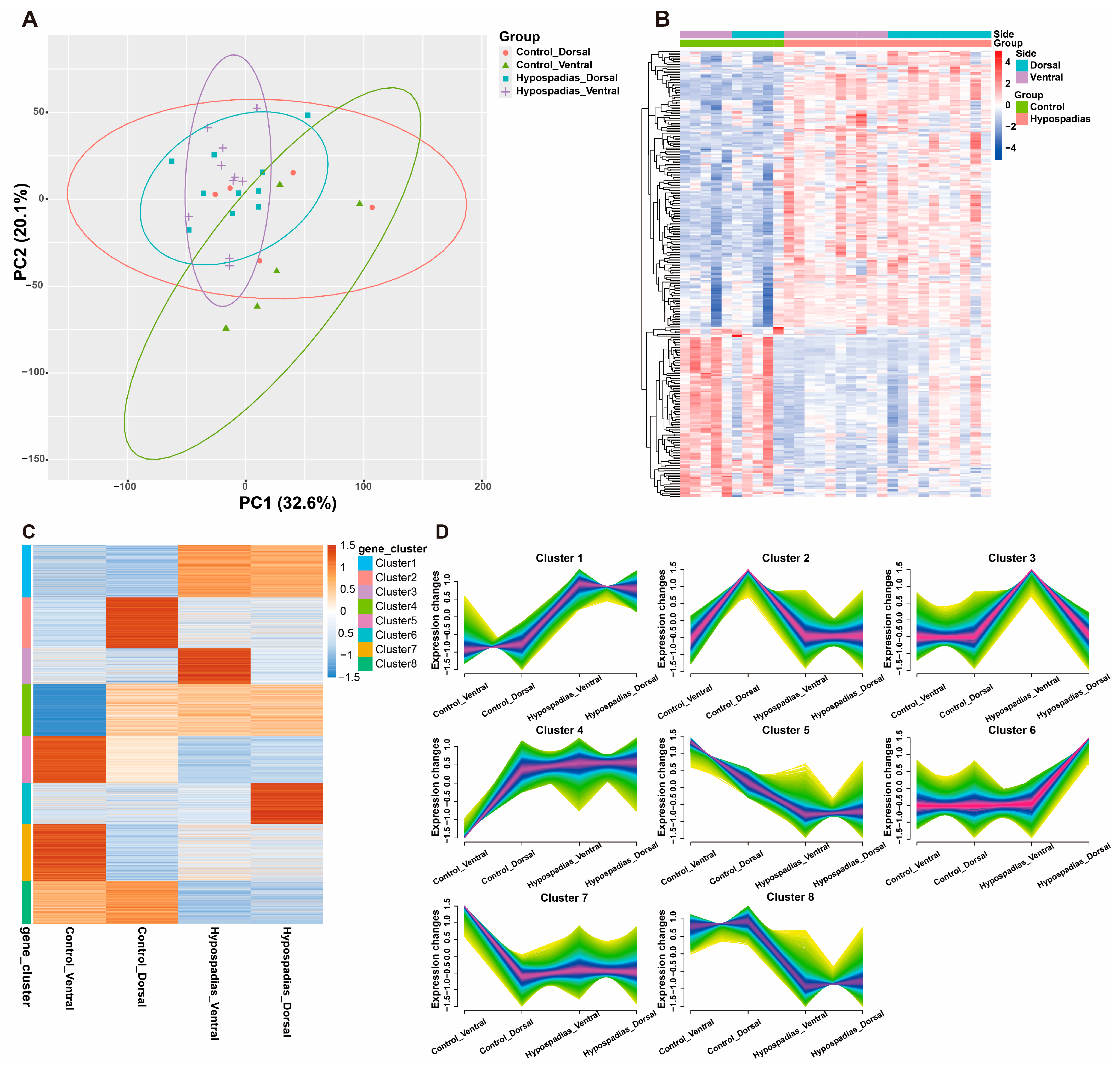
2.4. Transcriptomic Sequencing and Analysis
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Gene Expression Profiles
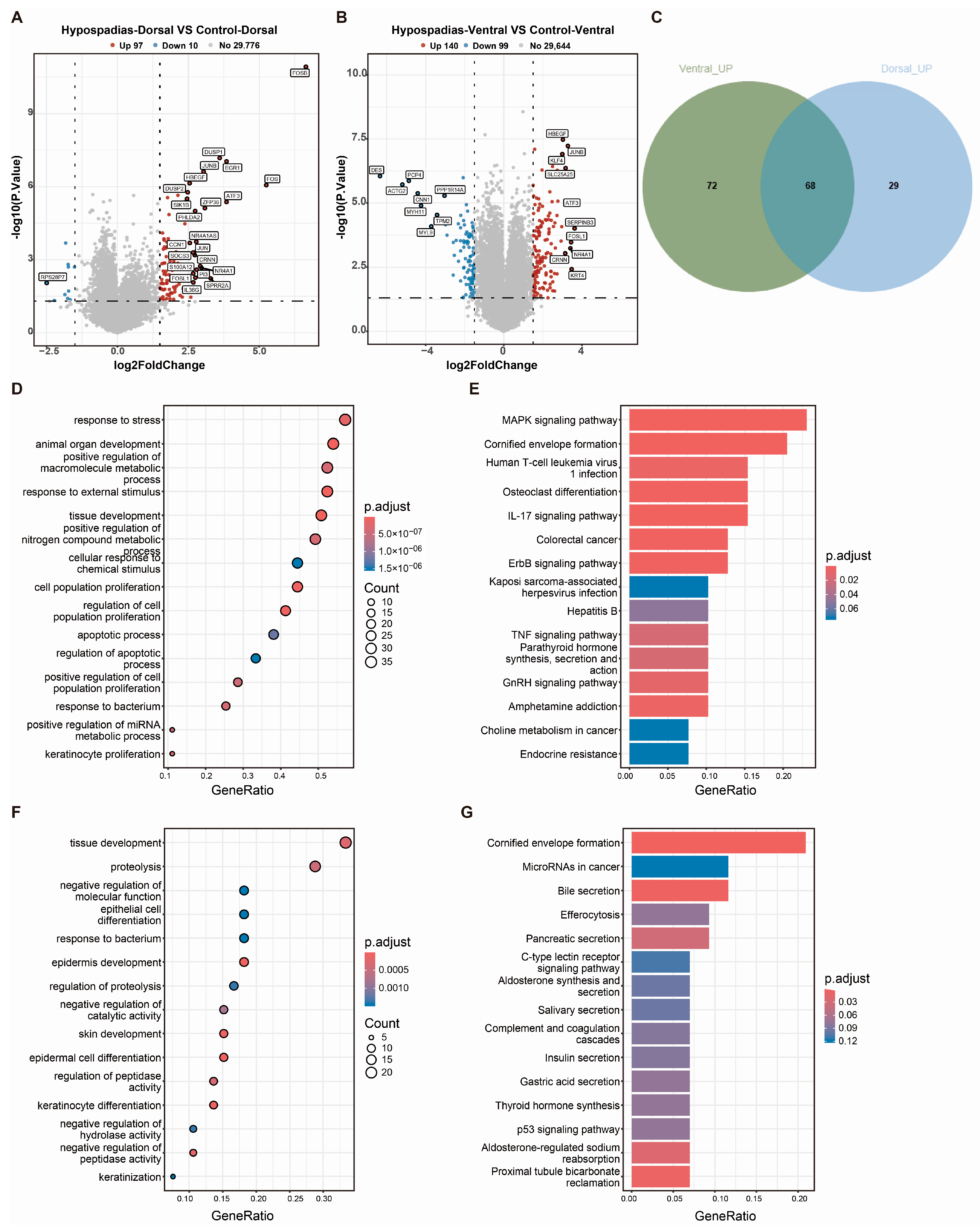
3.3. DEGs Between Hypospadias Children and Controls
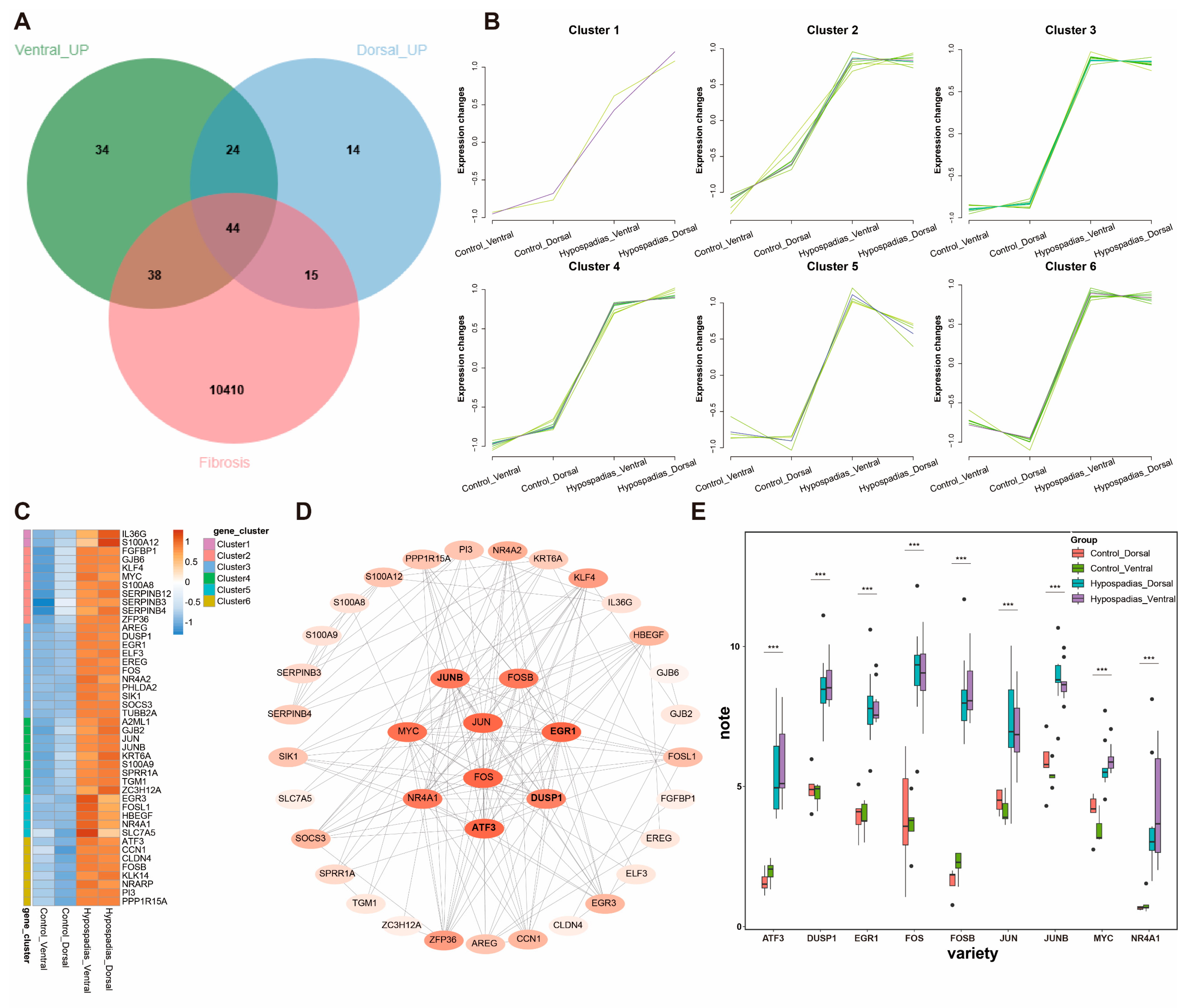
3.4. Fibrosis-Related DEGs in Hypospadias
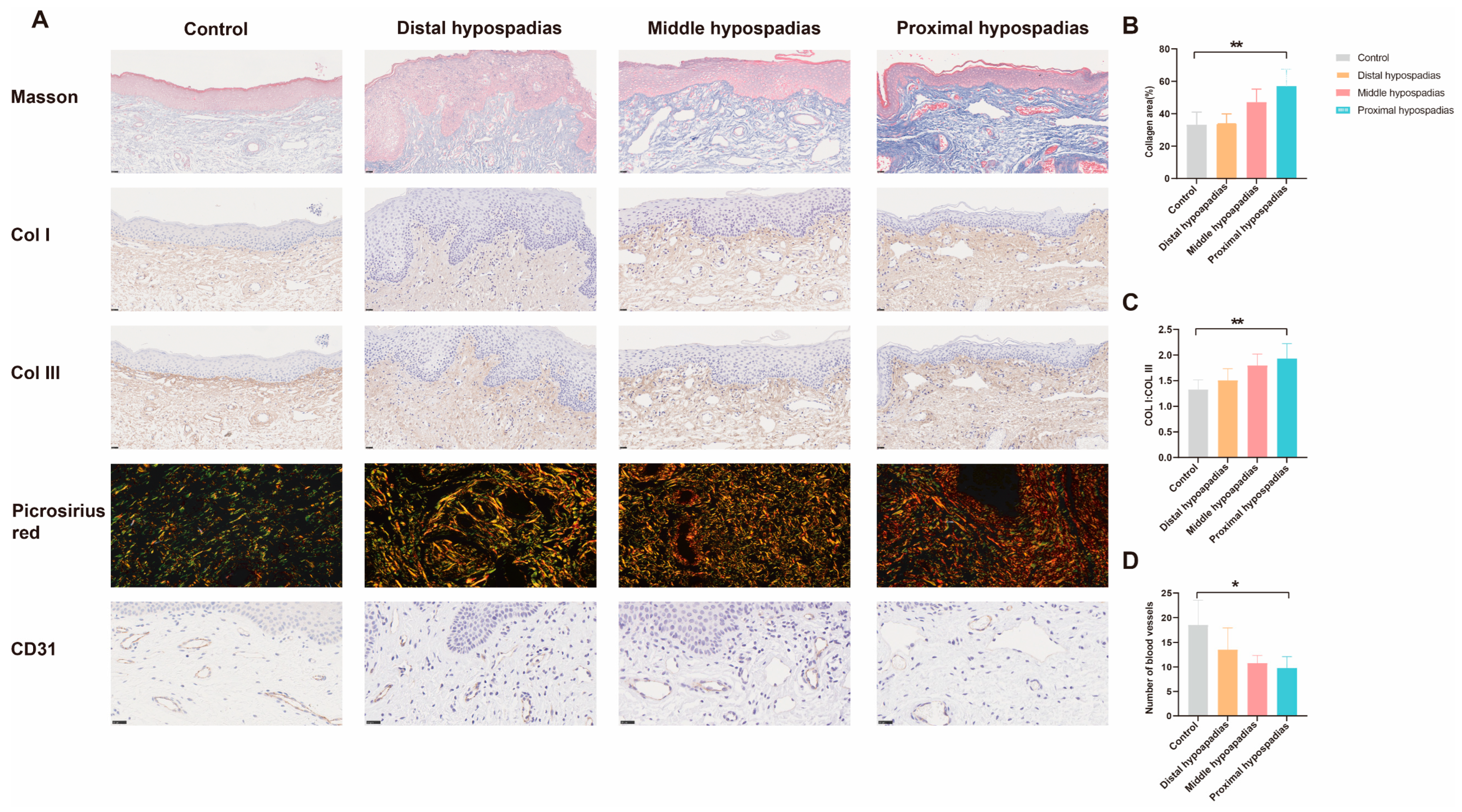
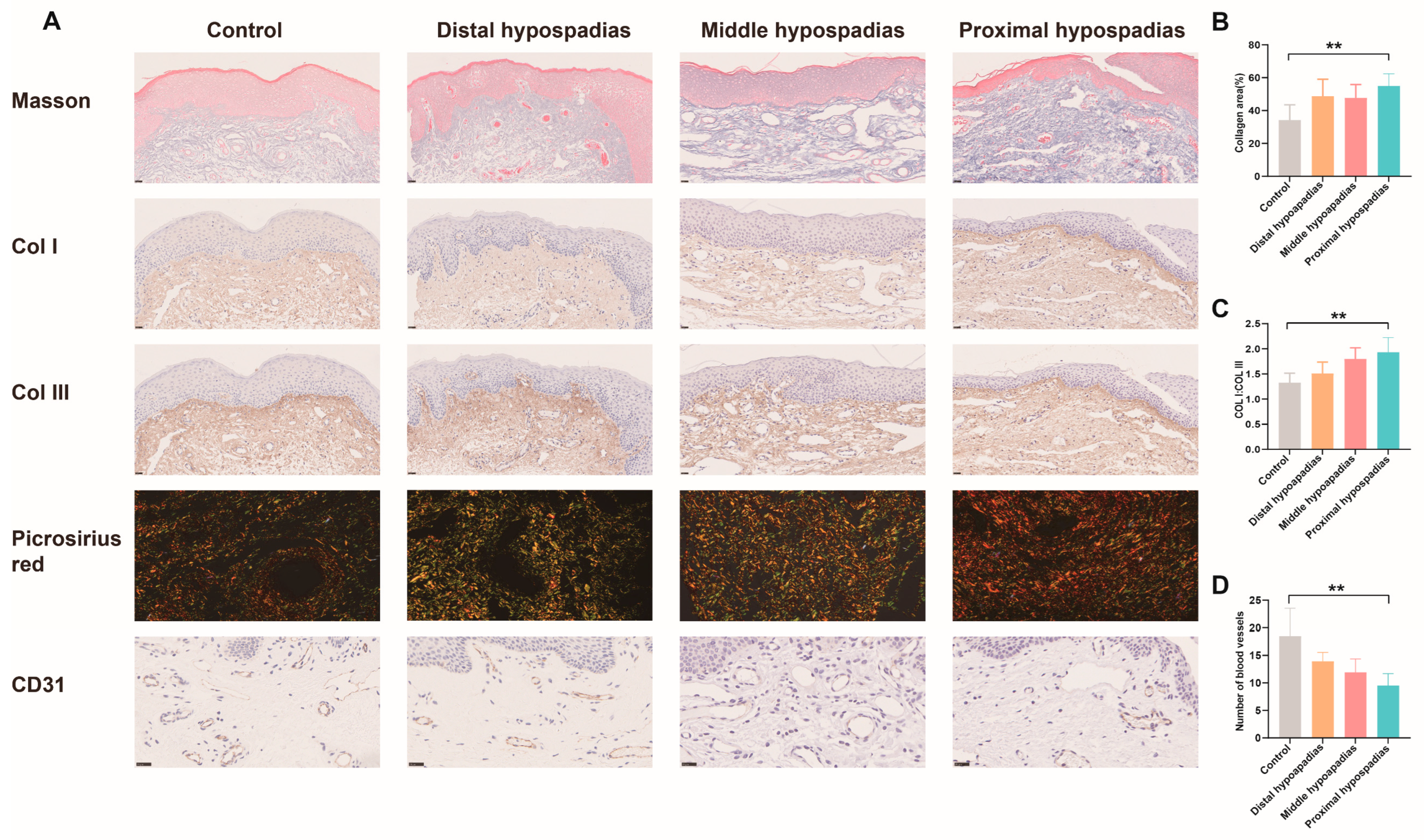
3.5. Histological Validation of Preputial Fibrosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anttila, A.; Lahdes-Vasama, T.; Pakkasjärvi, N.; Taskinen, S. Cumulative re-operation rates during follow-up after hypospadias repair. BJU Int. 2024, 134, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Hild, O.; Fotso Kamdem, A.; Boulard, N.; Auber, F.; Chaussy, Y. Primary hypospadias repair outcomes: Results from a retrospective cohort of 292 children. World J. Urol. 2024, 42, 137. [Google Scholar] [CrossRef] [PubMed]
- Waterloos, M.; Verla, W.; Spinoit, A.F.; Oosterlinck, W.; Van Laecke, E.; Hoebeke, P.; Lumen, N. Urethroplasty for urethral injuries and trauma-related strictures in children and adolescents: A single-institution experience. J. Pediatr. Urol. 2019, 15, 176.e1–176.e7. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Guo, H.L.; Shu, H.Q.; Gu, J.; Jin, C.R.; Chen, F.; Sa, Y.L. Surgical treatment of pelvic fracture urethral distraction defects in boys: Which approach is suitable? Asian J. Androl. 2020, 22, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Sreeranga, Y.L.; Joshi, P.M.; Bandini, M.; Kulkarni, S.B. Comprehensive analysis of paediatric pelvic fracture urethral injury: A reconstructive centre experience. BJU Int. 2022, 130, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhang, R.; Lu, C.; Chen, Y. Factors to Consider in Augmentation Urethroplasty with Oral Mucosa Graft or Penile Skin Flap for Anterior Urethral Stricture: A Systematic Review and Meta-analysis. Eur. Urol. Open Sci. 2023, 50, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Manasherova, D.; Kozyrev, G.; Nikolaev, V.; Abdullaev, F.; Abdulkarimov, G.; Kushnir, B.; Gazimiev, M. Bracka’s Method of Proximal Hypospadias Repair: Preputial Skin or Buccal Mucosa? Urology 2020, 138, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Cağrı Savaş, M.; Kapucuoğlu, N.; Gürsoy, K.; Başpınar, S. The microvessel density of the hypospadiac prepuce in children. J. Pediatr. Urol. 2011, 7, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Elbakry, A.; Matar, A.; Zalata, K.; Zakaria, A.; Al Atrash, G. Microvasculature and healing potential of the inner versus outer preputial skin: Preliminary immunohistochemical observations. Int. Urol. Nephrol. 2015, 47, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, R.; Turkyilmaz, Z.; Sonmez, K.; Kumas, G.; Ergun, S.; Ergun, M.; Basaklar, A. Twenty-Four Genes are Upregulated in Patients with Hypospadias. Balk. J. Med. Genet. 2013, 16, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Piñeyro-Ruiz, C.; Serrano, H.; Jorge, I.; Miranda-Valentin, E.; Pérez-Brayfield, M.R.; Camafeita, E.; Mesa, R.; Vázquez, J.; Jorge, J.C. A Proteomics Signature of Mild Hypospadias: A Pilot Study. Front. Pediatr. 2020, 8, 586287. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Fu, W.; Hu, J.; Tang, X.; Cui, Y.; Jia, W. Quantitative proteomics reveals specific protein regulation of severe hypospadias. Transl. Androl. Urol. 2022, 11, 495–508. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.A.; de Marins, R.L.; Rondon, A.; Damião, R. Age-related structural changes of the urethral plate in hypospadias. J. Pediatr. Urol. 2013, 9 Pt B, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Mizuno, K.; Kojima, Y.; Moritoki, Y.; Nishio, H.; Kato, T.; Kurokawa, S.; Kamisawa, H.; Kohri, K. Characterization of the urethral plate and the underlying tissue defined by expression of collagen subtypes and microarchitecture in hypospadias. Int. J. Urol. 2011, 18, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Huang, Y.; Lyu, Y.; Xi, Z.; Xie, H.; Fu, Q.; Song, L.; Chen, F. A Histomorphological Study of the Divergent Corpus Spongiosum Surrounding the Urethral Plate in Hypospadias. Urology 2020, 144, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sun, W.; Ye, Z.; Jia, W. HNF-1α promotes urethral fibrosis by up-regulating STAT3 transcriptional activity in mice hypospadias. Biochim. Biophys. Acta Mol. Basis Dis. 2025, 1871, 167893. [Google Scholar] [CrossRef] [PubMed]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Wang, S.; Kong, X.; Yu, Y.; Shen, L.; Long, C.; Liu, X.; Wei, G.H. c-Fos is upregulated in the genital tubercle of DEHP-induced hypospadiac rats and the prepuce of patients with hypospadias. Syst. Biol. Reprod. Med. 2021, 67, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Perske, C.; Sennert, M.; Fawzy, M.; Wirmer, J.; Hadidi, A.T. Hormone receptor expression in hypospadias. J. Pediatr. Urol. 2023, 19, 697.e1–697.e8. [Google Scholar] [CrossRef] [PubMed]
| No. | Age/m (Range) | Rrethral Orifice | |
|---|---|---|---|
| Control | 21 | 38.52 (5,72) | Normal |
| Hypospadias | 31 | 34.55 (9,79) | |
| Distal | 11 | 43.81 (13–79) | Coronal groove, subglanular |
| Middle | 9 | 25.78 (12–66) | Penile shaft |
| Proximal | 11 | 27.18 (9–56) | Scrotum, and perineum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Peng, Z.; Ding, Y.; Zhou, L.; Huang, J.; Wu, M.; Lv, Y.; Huang, Y.; Yu, M.; Chen, F. Transcriptomic Profiling and Histological Validation of Preputial Fibrosis in Hypospadias. Biomedicines 2025, 13, 2786. https://doi.org/10.3390/biomedicines13112786
Wang Y, Peng Z, Ding Y, Zhou L, Huang J, Wu M, Lv Y, Huang Y, Yu M, Chen F. Transcriptomic Profiling and Histological Validation of Preputial Fibrosis in Hypospadias. Biomedicines. 2025; 13(11):2786. https://doi.org/10.3390/biomedicines13112786
Chicago/Turabian StyleWang, Yaping, Zhiwei Peng, Yu Ding, Lijun Zhou, Jiacheng Huang, Min Wu, Yiqing Lv, Yichen Huang, Mingming Yu, and Fang Chen. 2025. "Transcriptomic Profiling and Histological Validation of Preputial Fibrosis in Hypospadias" Biomedicines 13, no. 11: 2786. https://doi.org/10.3390/biomedicines13112786
APA StyleWang, Y., Peng, Z., Ding, Y., Zhou, L., Huang, J., Wu, M., Lv, Y., Huang, Y., Yu, M., & Chen, F. (2025). Transcriptomic Profiling and Histological Validation of Preputial Fibrosis in Hypospadias. Biomedicines, 13(11), 2786. https://doi.org/10.3390/biomedicines13112786






