Abstract
Grain amaranths are recalcitrant to conventional in vitro plant regeneration by organogenesis de novo or through somatic embryogenesis. Consequently, floral organogenesis by these methods, representing the culminating developmental point in angiosperms, is rarely achieved. In the present study, the manipulation of in vitro flowering was explored as part of a strategy designed to overcome grain amaranth’s regeneration recalcitrance. It led to an efficient and reproducible in vitro protocol in which half-longitudinally dissected zygotic embryos generated fully developed Amaranthus hypochondriacus (Ah) plants. The use of high-irradiance illumination with LED lamps with a 3:1 red–blue irradiance ratio was a critical factor, leading to a 70% rate of early flowering events under flowering-inhibiting long-day photoperiod conditions. Contrariwise, no flowering was induced under LED white lights. All in vitro flowering Ah plants yielded viable seeds. To understand the basic molecular mechanisms of the phenomenon observed, gene expression patterns and principal component analysis of key flowering-related genes were analyzed after cultivation in vitro for 4, 8, and 12 weeks under both lighting regimes. These coded for photoreceptors, photomorphogenetic regulators, embryogenic modulators, and flowering activators/repressors. The results highlighted the upregulation of key flowering-regulatory genes, including CONSTANS, FLOWERING LOCUS T, and LEAFY, together with the downregulation of the floral repressor TERMINAL FLOWER1. Ribosome biogenesis- and seed-development-related genes were also differentially expressed, supporting a key role in this process for protein synthesis and embryogenesis. A model is proposed to explain how this light-regulated molecular framework enables in vitro flowering and seed production in Ah plants kept under long-day photoperiods.
1. Introduction
Grain amaranths (Amaranthus hypochondriacus [Ah], A. cruentus, and A. caudatus) are ancient, highly nutritional, and drought-tolerant crops [,]. They are native to the American continent and are greatly valued for their, protein-rich seeds that have added nutraceutical properties, although their leaves may also be used as a vitamin-rich vegetable source [,,,]. Despite all the advantages provided by grain amaranths, there is not sufficient integration of these plants into large-scale agricultural production. An important contributing factor is the difficulty of employing mechanized methods in the grain amaranth production chain. This limitation is caused by several agronomic characteristics, such as small seed size, irregular seed maturation, and plant architecture, which complicate conventional mechanized harvesting and processing. Additionally, the lack of suitable equipment adapted for grain amaranth cultivation further restricts mechanized production. To solve these agronomical limitations, the introduction of favorable changes via genetic transformation and genome editing appears to be an attractive alternative, similar to numerous other related strategies that have had a demonstrated positive impact on several other economically important crops. Unfortunately, grain amaranths are known to be notoriously recalcitrant to plant transformation, mostly because of the difficulty in achieving plant regeneration in vitro using conventional methods, usually based on modifications of the auxin/cytokinin ratios to promote organogenesis de novo and/or somatic embryogenesis (SE) [,,].
In vitro flowering is an alternative tool for plant micropropagation designed to release new cultivars into the market with greater celerity. It involves the change from the apical meristematic region into a floral meristem, where the flowers will be produced. Until now, more than 100 different plant species have been reported to produce flowers in vitro []. The most important factors involved in flowering are the photoperiod, light quality and intensity, temperature, and growth regulators. Grain amaranths are predominantly short-day flowering crops, except for A. cruentus, generally known to be photoperiod-insensitive. Therefore, days shorter than 12 h will accelerate flowering in most grain amaranth species, while in A. cruentus, long days usually promote robust vegetative growth [,,,,,,]. Contrary to their recalcitrance to shoot–root and SE plant regeneration, amaranth plants readily undergo floral development under in vitro conditions. Tisserat and Galletta [] found that five Amaranthus species, i.e., A. caudatus, A. gangeticus, Ah, A. retroflexus, and A. viridis exhibit prolific flowering in vitro (e.g., 80% in A gangeticus, 30% in Ah). The inflorescences could be maintained separately for several months, whereas the life cycle could be completed under tissue culture conditions within 24 to 32 weeks. Later, the use of controlled cultivation conditions based on gradual day length manipulation was able to reduce flowering and generation times to 4 and 8 weeks, respectively [].
Plants have developed a complex mechanism to sense light and use it, together with the circadian rhythm, to distinguish the proper moment to start the flowering process [,,,,,]. There are several signaling pathways that regulate a plant’s transition from vegetative growth to the reproductive phase. Among the most important is the photoperiod signaling pathway, which allows plants to respond to day length. Under long-day conditions, the CONSTANS (CO) protein accumulates in leaves and activates the expression of FLOWERING LOCUS T (FT), which acts as a mobile signal, or florigen, that is transported to the apical meristem to initiate flowering []. The light quality is perceived by the PHYTOCHROME B (PHYB; red), PHYTOCHROME A (PHYA; far red), and CRYPTOCHROME2 (CRY2; blue) photoreceptors. The CRY2 and PHYB photoreceptors are also known to be involved in the photoperiodic control of many of the plant development processes [,]. These further interact with proteins such as CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) and SUPPRESSOR OF PHYA1 (SPA1) to modulate CO stability and influence FT expression []. In addition, PHYTOCHROME-DEPENDENT LATE-FLOWERING (PHL) physically interacts with CO to suppress its degradation by PHYB [].
This work describes the induction of flowering in Ah plants grown in vitro using 3:1 red–blue LED luminaries (R-BLL) under a long-day photoperiod. The plants developed rapidly and successfully completed their reproductive cycle, generating flowers and viable seeds that germinated normally. Subsequently, a STRING database v12.0 network was queried to select the genes whose expression patterns could test the working hypothesis stating that enriched red light illumination accelerated flowering in Ah under restrictive long-day photoperiod conditions by altering the regulatory mechanisms of CONSTANS and FLOWERING LOCUS T. This information was integrated into a model in which the observed increase in CO and FT transcripts under red–blue LED conditions in a long-day photoperiod suggested a possible alternative or modified flowering-regulatory mechanism in Ah, distinct from the classical A. thaliana model. Still, the proposed mechanism remains hypothetical and will require further experimentation to be validated.
2. Results
2.1. Flowering Induction
In vitro flowering of Ah plantlets was induced from zygotic embryos dissected longitudinally and cultured in MS medium with 3% sucrose and 3 g/L gelrite under R-BLL. Non-inductive light conditions were implemented using WLL. Long-day (16 h light/8 h dark) photoperiods within a 18 °C (dark) to 28 °C (light) temperature range were applied in both illumination regimes. Under the R-BLL experimental conditions, which were replicated five times, 90% of the cultured embryos developed into mature plants, 71% of which successfully flowered and yielded viable seeds (an average of 139 seeds per plant), which had a mean 70% germination rate (Figure 1; Table 1). Seed setting started in flowering plants 12 weeks after the experiments were started.
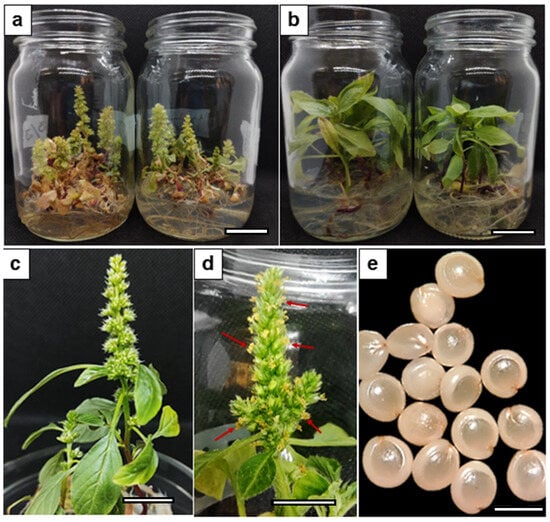
Figure 1.
Aspects of induced flowering in A. hypochondriacus (Ah) plants grown under long-day photoperiods. (a) In vitro flowering of Ah, grown in MS medium with 3% sucrose and 3 g/l gelrite under red–blue LED light (R-BLL) illumination; (b) non-flowering Ah plants grown under white LED light (WLL) conditions; (c) monoecious Ah flowers; (d) pollen-producing Ah inflorescences (indicated by red arrows), and (e) mature seeds produced from Ah plants grown in vitro under R-BLL illumination. Measuring bars represent 1.5 cm in (a,b), 1 cm in (c,d), and 1 mm in (e).

Table 1.
Quantitative parameters obtained from the accelerated flowering of A. hypochondriacus plantlets cultured in vitro under 3:1 red–blue LED light luminaries in the context of a long-day photoperiod.
2.2. Selection of Relevant Ah Flowering-Related Genes for Analysis
A protein–protein interaction (PPI) network using STRING-based bioinformatic data with a 0.90 confidence level was constructed using information garnered from the A. thaliana genome (Figure 2). This exercise yielded a protein–protein interaction circuitry that was utilized to guide the selection of genes to be quantitatively analyzed during the early flowering process observed in in vitro Ah plants grown under R-BLL in long-day photoperiod conditions. This experimental strategy was implemented with the aim of identifying a possible signaling pathway able to explain the role of R-BLL light in the induction of the uncharacteristic long-day flowering trait observed in short-day flowering Ah plants.
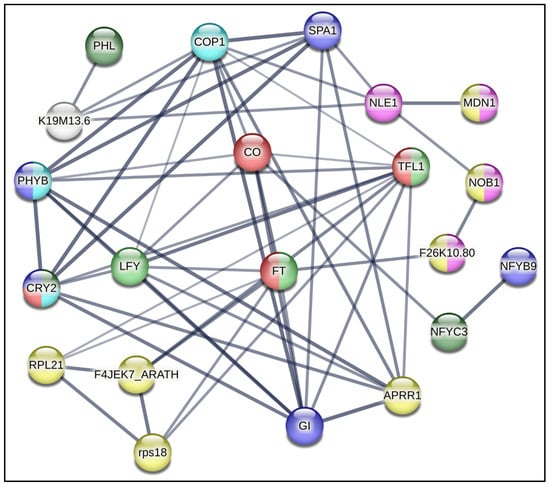
Figure 2.
Flowering-regulating protein–protein interaction network. A STRING-based bioinformatic protein–protein interaction network relevant during the flowering process, at a 0.900 confidence level, was constructed using information garnered from the A. thaliana genome. It constituted the basis for the selection of homologous Ah genes for quantitative expression analysis. The genes chosen for analysis were PHYB = PHYTOCHROME B and PHL = PHYTOCHROME-DEPENDENT LATE-FLOWERING, which sense changes in red/far red light and transduce light photoreceptor phytochrome B signaling, respectively; CRY2 = CRYPTOCHROME2, which regulates photomorphogenic events through the detection of UV-A/blue light; COP1 = CONSTITUTIVELY PHOTOMORPHOGENIC 1; SPA1 = SUPPRESSOR OF PHYA-1; CO = CONSTANS, coordinately regulated by red and blue photoreceptors to determine photoperiodic flowering regulation; FT = FLOWERING LOCUS T; TFL1 = TERMINAL FLOWER 1; LFY = LEAFY; GI = GIGANTEA; TIMING OF CHLOROPHYLL A/B BINDING PROTEIN/PSEUDO RESPONSE REGULATOR1 (TOC1/PRR1); RPS18 = RIBOSOMAL PROTEIN S18; RPL21C = RIBSOMAL PROTEIN L21 SUBUNIT C; NOB1 = NIN1 (ONE) BINDING PROTEIN 1; MDN1 = MIDASIN HOMOLOGUE 1; and LEC1 = LEAFY COTYLEDON1.
2.3. Gene Expression Analysis
The expression of homologous flowering-related genes from Ah plants, the selection of which was based on the exercise described above, was analyzed at 4 weeks after cultivation (WAC), when plants were in a robust vegetative stage, at 8 WAC, when the first apical flower buds appeared, and at 12 WAC, when diverse flower maturation stages were present due to the asynchronous reproductive maturation that is characteristic of Ah plants. The relative expression values of these genes, which were obtained under R-BLL conditions, were compared with those obtained from those Ah plants cultivated under WLL.
The R-BLL-to-WLL relative expression value ratios obtained during the different development periods are shown in Figure 3. In the first 4 weeks (Figure 3a), TOC/PRR1, TFL1, and RPS18 were among the genes most highly induced in the leaves and stem, whereas the former two were among the only genes expressed in the meristems. This showed a prevalence of factors that sustained the repression of flower development in the meristems at 4 WAC. Subsequently, in week 8 (Figure 3b), the FT gene, coding for the master regulator of flowering that promotes the transition from vegetative to reproductive development in plants [], increased to unexpectedly high expression levels under R-BLL, particularly in the meristems. Other genes that reverted to positive R-BLL to WLL relative expression ratios in the SAM/FM at 8 WAC were SPA1, LFY, GI, RSP18, NOB1, MDN1, LEC1, and PHL, while others such as RPL21C and, importantly, the TFL1 flowering-repression gene, showed reduced or negative expression ratios. This expression pattern was already indicative of a molecular organization positively orientated toward the induction of flowering and seed production, considering, for example, the roles of SPA1, PHL, NOB1, MDL1, and LEC1 in the control of photoperiodic flowering and the circadian clock, the promotion of flowering under red light, and seed, embryo, gametophyte, and pollen development, respectively [,,]. Lastly, at 12 WAC (Figure 3c), the most telling changes in gene expression observed in flowers were the induction of CO, the central controller of the photoperiod-sensing mechanism in plants that determines flowering by regulating FT, and of the PHYB and CRY2 photoreceptor genes. These could have possibly permitted the integration of upstream FT-activating signals transmitted by COP1 []. The R-BLL-to-WLL relative expression value ratios of LFY in flowers also markedly increased.
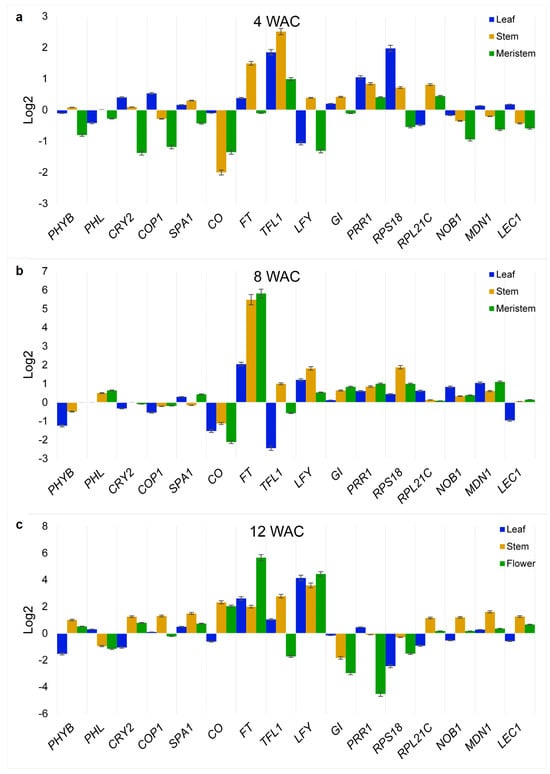
Figure 3.
Time-course quantitative PCR analysis of the selected A. hypochondriacus (Ah) flowering-related genes. Expression levels of genes selected from the protein-protein interaction network shown in Figure 2 were determined in leaves, stems, shoot apical meristems (SAMs) and/or flowering meristems (FMs), and flowers at three different developmental stages in Ah plantlets cultivated in vitro either under red–blue (R-BLL) or white LED light (WLL) illumination and in the context of a long photoperiod. Sampling was performed at (a) 4 weeks, (b) 8 weeks, and (c) 12 weeks after cultivation of the Ah half-embryo explants on MS media. The relative expression values in each experimental condition were calculated according to Livak and Schmittgen [] and normalized against the ELONGATION FACTOR 1α and β-TUBULIN Ah house-keeping genes. The results show the log2 of the ratio of relative expression levels detected under R-BLL and WLL, respectively. The genes analyzed were PHYB, PHL, CRY2, COP1, SPA1, CO, FT, TFL1, LFY, GI, TOC1/PRR1, RPS18, RPL21C, NOB1 = NIN1, MDN1, and LEC1 (for the meaning of the gene abbreviations, refer to Figure 2). The results represent at least three technical replicates of a biological experiment that was repeated at least thrice with similar results.
2.4. PCA of the Gene Expression Levels Produced Under R-BLL and a Long-Day Photoperiod at 12 WAC
Component 1 of the PCA in the 12 WAC flowering stage (x-axis, increasing average expression) indicated that 63.98% of the genes analyzed had significantly modified levels of expression. Here, the FT gene exhibited the higher contribution to the detected changes in gene expression (i.e., 90.41), making it, by far, the most relevant gene in flowering development under R-BLL conditions. It was followed by LFY (1.33), CO (0.86), CRY2 (0.73), SPA1 (0.70), LEC1 (0.70), MDN1 (0.68), PHYB (0.67), RPL21C (0.63), NOB1 (0.58), COP1 (0.50), PRR1 (0.49), PHL (0.49), GI (0.47), TFL1 (0.35), and RPS18 (0.34) (Figure 4a). Component 2 (PCA2) (y-axis, increasing positive trend) explained 33.61% of the variance. The most relevant gene recognized at PCA2 during flowering at 12 WAC was TFL1 (44.46). It was followed by RPL18 (17.00), LFY (9.8), PRR1 (7.97), GI (5.9), CRY2 (5.86), CO (4.13), LEC1 (2.27), PHYB (1.34), PHL (0.42), NOB1 (0.30), MDN1 (0.22), RPL21C (0.14), FT (0.12), SPA1 (0.016), and COP1 (0.001) (Figure 4a). Components 1 and 3 were constituted in ca. equal measures by genes predominantly expressed during the 8 and 12 WAC sampling time points. These corresponded to the initiation of flowering and active flower development stages, respectively, while component 2 was constituted by genes whose maximum expression levels were detected at 4 WAC, when vegetative growth was still prevalent, and flowering had not yet started (Figure 4b). The PCA results obtained from the gene expression assays in the stems and in leaves are shown as Supplementary Materials (Figures S2 and S3).
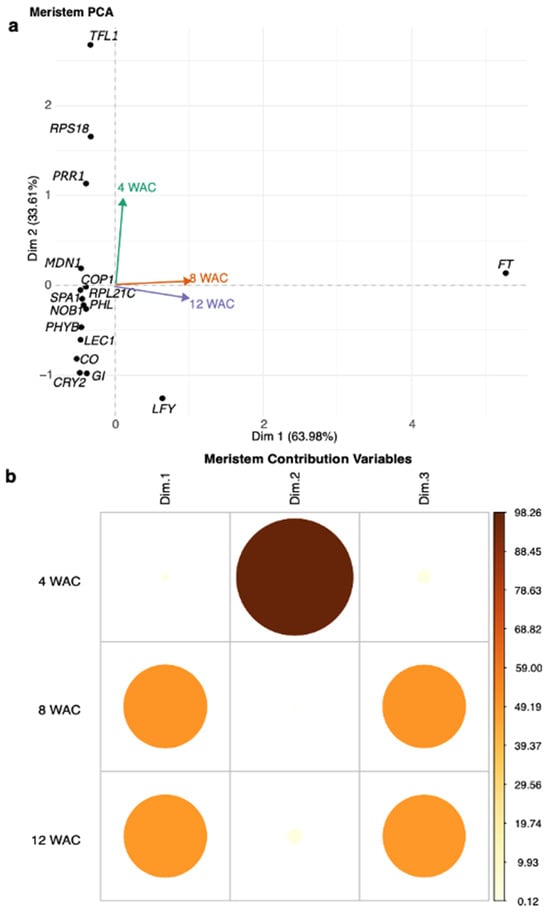
Figure 4.
Principal component analysis (PCA) of the quantitative gene expression assays detected in apical meristems and flowers. In (a) the PCA1 (x-axis, increasing average expression) indicates that 63.98% of the genes analyzed had significantly modified levels of expression. Here, the FT gene exhibited the higher gene contribution in gene expression (i.e., 90.41), making it, by far, the most relevant gene in flowering development under R-BLL conditions 12 WAC. PCA2 (y-axis, increasing positive trend) explained 33.61% of the variance. It indicated that the most relevant contribution to this component during flowering at 12 WAC was from TFL1 (44.46). It was followed by RPL18 (17.00), LFY (9.8), and PRR1 (7.97). (b) Components 1 and 3 were enriched in genes whose maximum levels were reached at 8 and 12 WAC, while component 2 grouped those genes whose expression was predominant at 4 WAC, representing the pre-flowering vegetative stage.
2.5. Analysis of the Cis-Acting Elements in the Ah FT Gene
PLANTCARE was used to analyze the 2000 pb upstream promoter sequence of the Ah FT gene and showed that the promoter region of the FT gene contained 21 light-responsive elements (LREs), implying that they may have contributed to the R-BLL activation of this gene under R-B enriched light and consequently to the induction of flowering under long-day photoperiod conditions (Figure 5).

Figure 5.
Light-responsive elements (LREs) present in the promoter region of the A. hypochondriacus FT gene. The figure shows 2000 nucleotides upstream of the 5’-UTR of the Ah FT gene. Nucleotide sequences representing potential LREs are highlighted in different colors, representing Box4 motifs (green boxes); GT1 motifs, (orange boxes); G-Box motifs, (blue boxes); TCT motifs, (red boxes); GATA motif (mustard-yellow box); and I-box motif (pink box). The “+” and “−” symbols indicate base pairs downstream and upstream of the transcription start site, respectively.
3. Discussion
The differentiation of the shoot apical meristem into a floral meristem promoted by FT is a unique feature of the evolution of Gymnosperms and Angiosperms []. It is highly relevant to agriculture because of its obvious implications for sexual reproduction and plant productivity. Although flowering development under in vitro conditions has been demonstrated in more than 100 plant species [], the molecular mechanisms responsible for the orchestration of such a significant event under these particular growing conditions have not been analyzed in depth, particularly under controlled light regimes and in underutilized crops like Ah [,].
The present study describes the induced flowering of Ah under in vitro conditions in which plants were continuously exposed to R-BLL under a non-flowering-promoting long-day photoperiod. In order to gain an insight into the possible molecular mechanisms involved in this remarkable process, a number of key flowering-transition-associated genes were analyzed at different development stages that included the vegetative-to-reproductive growth transition. The gene expression analysis was based on a PPI network derived from the STRING database based on the A. thaliana genome, the results of which should referred to as tentative, considering that these mechanistic inferences are based on homologous genes and prior knowledge from A. thaliana. Thus, their functional conservation in Ah remains to be validated. As expected, a significant increase in FT expression in meristems and flowers was detected in R-BLL-exposed plants during the initiation (8 WAC) and fulfillment (12 WAC) of flower development. Moreover, the PCA1 analysis clearly suggested the relevance of high FT gene expression levels in the meristems and in flowers and, therefore, in the R-BLL-induced flowering in in vitro-cultured Ah plants maintained under a long-day photoperiod. Following in importance were the LFY and CO genes, whereas the TFL1 repressor was strongly downregulated. The latter is in accordance with the notion that all flowering pathways necessarily converge at the florigen–anti-florigen hormone system that dictates, for example, that the balance between FT and TFL1 will define the plant growth stage as indeterminate or determinate by modulating the formation of vegetative or reproductive tissues in the apical and axillary meristems []. The observed reduction in TFL1 expression from 8 WAC onwards and in its varying relevance in the PCA were in accordance with its role as a key regulator of FT expression, and thereby of flower induction, which is defined through the 14-3-3 protein-mediated competition for the FD bZIP transcription factor (TF) []. The proposal that FT/TFL1 expression ratio may reflect a shift in floral developmental programming in Ah, based solely on transcriptional profiles, will require further experimentation, e.g., using protein-level or interaction assays, to be functionally validated.
Indirect support for the results of the present study can be drawn from a report describing that the overexpression of FT in Brachypodium distachyon led to early flowering and a determinate inflorescence structure in a day-length-independent manner []. Likewise, differences in the number and type of cis-acting elements in the promoter regions of PEBP genes such as FT have been proposed to define the plant’s perception of the photoperiod and its translation into long-day and short-day flowering habits []. Thus, contrary to short-day-flowering Ah plants, long-day-flowering A. thaliana lacks specific light-response elements, such as AE-Boxes and Gap-motifs []. In this context, the analysis of the cis-acting elements of the Ah FT gen promoter found twenty-one light-responsive cis-acting elements, including eleven box-4 (ATTAAT) motifs, four GT1-motifs, two TCT-motifs, two G-boxes, one I-box, and one GATA-motif. Among these, G-boxes [], I-boxes [], and GT1-motifs [] are known to be regulated by red light, whereas GATA and I box motifs most likely mediated blue light sensitivity, similarly to previous findings reported in A. thaliana [,]. Moreover, box-4 cis-acting elements present in Dof family genes, also identified in closely related Chenopodium quinoa plants, were shown to be enhanced by both red and blue light []. An admission should be made at this point that although the presence of light-responsive cis-elements suggests potential regulation by light quality, experimental validation, e.g., by means of promoter–reporter studies, will be required to confirm their functionality in Ah. Nonetheless, this analysis provides an interesting preliminary framework to hypothesize potential gene regulatory functions in Ah, thereby opening up avenues for future experimental investigation to better understand the molecular mechanisms underlying flowering induction in this species.
In addition to FT, the proposed relevance of the LFY gene in the R-BLL-induced early and photoperiod-insensitive flowering in Ah was congruent with its role as a unique plant-specific TF involved in meristem floral fate and flower initiation []. Thus, in the present study, the induction of LFY gradually increased to reach the highest expression levels in the flowering stage, which were between ca. 9- and 50-fold higher than those detected in earlier vegetative and vegetative-to-reproductive transition stages. The chromatin-modifying capacity of LFY allows the binding of several other TFs to modulate several developmental processes, including flowering. It achieves the latter by acting downstream of FT to regulate floral organ development []. In this sense, it is tempting to speculate that its role in R-BLL-induced early and photoperiod-independent flowering could have involved the recruitment of a whole gamut of bZIP TFs and others that are known to regulate flowering time via changes in the structure of SWI/SNF-type complexes [,,]. Also, the high levels of LFY expression detected in flowers of in vitro-R-BLL-treated Ah plants could have also contributed to promoting the accelerated flowering detected, similar to the early-flowering-related phenotypes observed in transgenic rice, tobacco, and hybrid aspen and citrus plants overexpressing the LFY gene [,,,]. Additionally, a transcriptome analysis of flower induction in ginger (Zingiber officinale) plants kept under red light indicated that, in coincidence with the present study, LFY, CO, and FT were highly upregulated and associated with flowering []. However, these comparisons should be taken with caution until more detailed evolutionary analyses and functional studies can clarify the conservation and divergence of these mechanisms, particularly in non-model species. In addition, similar gene expression patterns under red light in Z. officinale, an evolutionary divergent species, limit their direct applicability to Ah.
The additional importance allegedly assigned to CO in the early and photoperiod-independent flowering produced in in vitro Ah plantlets grown under R-BLL is supported by the role that this gene is known to play in the photoperiod-mediated control of flowering in other plant models. In A. thaliana, for example, CO is part of the regulatory hub in the photoperiodic flowering pathway that promotes long-day flowering by inducing FT expression in leaves []. Following the cautionary tone of this discussion, it must be acknowledged that although the evidence linking CO with the red–blue light induction of flowering under long-day conditions in Ah is compelling, its precise functional role in these plants and whether it operates similarly to short-day photoperiods remain to be determined.
Likewise, the PCA obtained during flowering revealed that the fourth most relevant gene coded for the PHL protein, which is known to suppress the degradation of CO by complexing with the PHYB sensor of red light []. Its projected importance in the accelerated flowering phenotype observed in Ah plants cultured under R-BLL and a long-day photoperiod is highlighted by the fact that A. thaliana phl mutants showed late-flowering phenotypes under long-day conditions, partly due to reduced CO levels []. In this sense, the importance of the CRY2 blue light sensor is derived from its positive regulation of CO. However, it remains to be determined how the known antagonistic action of cry2 and phyb mutants observed toward flowering development in response to different light wavelengths [] may have affected the accelerated flowering of Ah plants in response to R-BLL during long-day photoperiods. On the other hand, the reduced expression of the SPA1 gene observed in the flowers of R-BLL-treated Ah plants could have affected its capacity to regulate the photoperiodic regulation of flowering time [], thereby facilitating early flowering under long-day photoperiods in a plant with a short-day flowering habit. Also, despite the fact that PHYB suppresses the expression of flowering genes by promoting CO degradation, its positive expression, detected in the flowers of R-BLL-treated Ah plants, may have had a similar effect to that observed in A thaliana PHYB-overexpressing plants, which showed an early-flowering phenotype []. Additionally, the R-BLL exposure used to produce early-flowering Ah plants under long-day photoperiods led to induced levels of the CRY2 gene in the periods corresponding to the initialization and culmination of flowering. This suggested that in response to the blue light component of the R-BLLs, the photoexcited CRY2 could have interacted with SPA1 or COP1 to suppress COP1 activity and, consequently, avoid CO degradation, thereby initiating the floral transition that, in the experimental conditions employed, could have been photoperiod-insensitive []. Conversely, the high-red-light conditions used in the present study could have promoted the binding of PHYB with SPA1, as proposed based on previous findings in A. thaliana, thereby preventing the formation of the COP1–SPA1 protein complex to enable the accumulation of photomorphogenesis-promoting proteins []. It should be emphasized, once more, that further experimental validation in Ah is required to certify the possible scenarios mentioned above.
Further, the PCA indicated the relevance, at 12 WAC, of LEC1, coding for a nuclear TF Y subunit B-9, known to be a transcriptional activator of genes required for both embryo maturation and cellular differentiation. Its importance at this stage probably contributed to the successful development of mature viable grain amaranth seeds with a high germination rate []. Similarly, the induction of the NOB1 gene, coding for the NIN1 (ONE) BINDING PROTEIN1, in the 8 WAC pre-flowering stage in Ah grown under R-BLLs could be significant considering that this protein is essential for embryogenesis and pollen development processes required for adequate seed development []. In contrast, the downregulated expression of the ribosomal protein RPS18 gene detected at 12 WAC was suggestive of its minor influence on the flowering process in Ah, despite the fact that this protein is required for cell division [] and is specifically expressed in meristems and in tissues with high cell division activity [], whereas the RPL21C gene, coding for a large-subunit (60S) ribosomal protein, was induced predominantly in Ah’s stems, meristems, and flowers. The latter pattern could have been indicative of this gene’s key role in the maintenance of cellular activity in Ah plants, similarly to its participation in the regulation of chloroplast functions in A. thaliana. Additionally, it could have favored flowering in Ah due to its capacity to interact with epigenetic factors that regulate FLC, a major repressor of flowering []. Furthermore, it has been shown that it is essential for embryonic development []. On the other hand, the generally downregulated expression of the GIGANTEA gene observed in Ah plants suggested that its participation in the regulation of the flowering time via a CO-independent pathway that involves the involvement of miR172 and the further activation of photoperiodic flowering through the induction of FT was probably irrelevant in the context of the acceleration of flowering by R-BLL in Ah []. PRR1, known to play a critical role in the regulation of the circadian clock in close relation to PHYB activity, was highly ranked in component 2 of the PCA at 12 WAC, together with other genes expressed when flowering in Ah was repressed. Thereby, its strongly downregulated expression during active flowering could have favored early flowering considering that, under red light conditions, the expression of PRR1 was found to decline and release FT from repression [].
Another possible explanation for the accelerated flowering produced in Ah plants exposed to R-BLLs could be linked to the high irradiance emitted by the R-BLLs (i.e., 200 μmol m−2s−1), compared with WLLs, whose emissions reached only 94 μmol m−2s−1. This difference was consistent with findings indicating that increased irradiance and long-day conditions can accelerate flower induction in several commercially important ornamental plants []. Likewise, cis-elements like the G-box and GATA motif present in the Ah FT gene promoter could have represented a contributing flowering-habit-altering factor, considering that they were found to respond positively to continuous high-irradiance light in A. thaliana [].
Based on the information discussed above, a hypothetical model based on homology with A. thaliana was constructed to propose the possible mechanisms by means of which flowering induction under a long-day photoperiod was accelerated in Ah plants kept under R-BLLs with a 2.2-fold-higher irradiance than WLL, in addition to much more intense red and blue peaks (Figure 6). This model predicts that under R-BLL conditions, the upregulation of FT, the master regulator of flowering, was greatly favored, as well as the induction of other light-sensitive flowering regulators such as LFY, CO, CRY2, SPA1, PHYB, LEC1, MDN1, NOB1, RPL21, and COP1. Moreover, successful flowering was accompanied by the production of mature and viable seeds. In contrast, the model proposes that under WLLs, flowering was repressed by the favoring of protein–protein interaction that inhibited CO accumulation and flower-promoting FT activity.
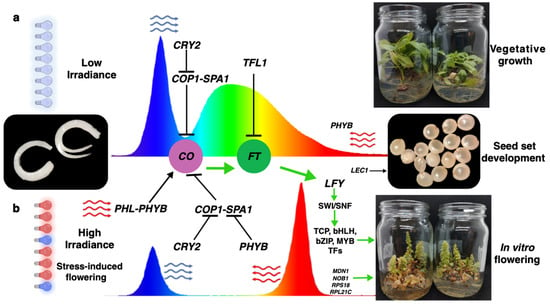
Figure 6.
Induced flowering in in vitro-cultured A. hypochondriacus plantlets maintained under red–blue LED lights in the context of a non-flowering-inducing long-day photoperiod: proposed model based on homology with A. thaliana. Two hypothetical scenarios are shown: (a) half-zygotic embryos cultivated during long-day photoperiods under white LED light (WLL) luminaries with low irradiance; the CRY2 blue light receptor becomes the dominant regulator. This triggers the day-time repression of CO that contributes to blocking the formation of the SPA1-COP1 complex. Furthermore, under these conditions, the TFL-mediated repression of flowering via FT is favored. (b) In vitro cultivation maintained under 3:1 red–blue (R-B) LED luminaries with high irradiance activates a different flower-inducing process that involves the formation of a PHL-PHYB complex that favors the activation of CO; this step is further promoted by the inhibited formation of the SPA1-COP1 complex by the PHYB red light receptor and CRY2. The sum of these events promotes the activation of FT, which leads to the downstream accumulation of LFY that allows the chromatin opening of the SWI/SNF complex and the binding of transcription factors (TFs) that stimulate flower development. At the same time, ribosomal biogenesis via RPS18 and other proteins aids in the formation of in vitro flowers capable of generating fertile seeds via LEC1.
As is frequently the case with explanatory models, it must be recognized that the one above is based on speculative associations or suggests putative functions rather than demonstrate direct causality. Therefore, future functional validation experiments, such as gene knockouts, overexpression studies, or protein interaction assays, will be needed to conclusively determine the causal roles played by these genes in flowering regulation in Ah.
4. Materials and Methods
4.1. Plant Materials
Seeds of Ah cv. Revancha were collected from mature plants grown under greenhouse conditions for 90 days in the spring–summer of 2023. During this period, the maximum and minimum temperatures recorded in the greenhouse were 32 °C and 17 °C, respectively. The collected seeds were sterilized by placing them for 20 min in a desiccator containing 30 mL of sodium hypochlorite and 30 mL of hydrochloric acid 6 N. For in vitro culture, the seeds were dissected using longitudinal excisions that permitted the extraction of two symmetrical embryo halves.
4.2. Flowering Induction Conditions
Half-zygotic embryos of Ah were cultured in MS medium [], complemented with either 3% or 5% sucrose and 3 or 5 g/L of Gelrite (GELZAN; Sigma-Aldrich, St. Louis, MO, USA), respectively. All media were set at a pH of 5.8. A total of fifty flasks were cultured with ten embryo halves per flask, half of which were placed under white LED lights (WLL; ECOFIT T8 LED lamps, Philips; Amsterdam, The Netherlands) with a photosynthetic photon flux (PPF) of 29.3 µmol/s (PPF-Blue [B]), 24.5 µmol/s (PPF-Red [R]) and a total irradiance of 94 µmol/m2 s. The rest were maintained under LED luminaries emitting light with a 3:1 R: B ratio (R-BLL; VIVIDGRO T8 lamps; Lighting Science Group Corporation, West Warwick, RI, USA). These emitted only two peaks in the visible spectrum: the largest, at the red light-end, had a PPF-R of 124.3 µmol/s, and the smaller, at the opposite, blue light end, had a PPF-B of 36.1 µmol/s. The lamps had 200 µmol/m2 s irradiance. All flasks were cultured inside a growth room maintained under long-day photoperiod conditions (16 h light/8 h darkness), with a light/dark temperature of 28/18 °C (for more information about the light quality, refer to Figure S1 and Table S1).
4.3. Interaction Analysis of Proteins Involved in Amaranth Flowering
A gene network analysis with 0.90 confidence was performed using STRING v11.5 [] based on A. thaliana and using homologous Ah genes deposited in the Phytozome network []. STRING was used as a preliminary tool to identify potential candidate genes with known roles in flowering regulation in order to generate hypothesis-generating data.
Only those genes coding for A. thaliana homologs with protein sequence identities greater than 38% were selected, to increase the likelihood of conservation of both functional domains and likely biological roles. These included the photoreceptors of red PHYB (AT2G18790) and blue CRY2 (AT1G04400) light; the photomorphogenesis regulators COP1 (AT2G32950) and SPA1 (AT2G46340); the flowering regulators CO (AT5G15840), FT (AT1G65480), TFL1 (AT5G03840), LFY (AT5G61850), and PHL (AT5G29000); the circadian rhythms sensors GI (AT1G22770) and TIMING OF CHLOROPHYLL A/B BINDING PROTEIN/PSEUDO RESPONSE REGULATOR1 (TOC1/PRR1) (AT5G61380); the ribosomal proteins RPS18 (ATCG00650) and RPL21C (AT1G35680); the ribosome biogenesis regulators NOB1 (AT5G41190) and MDN1 (AT1G67120); and the seed and embryogenesis development regulator LEC1 (AT1G72390). Homologous sequences in Ah with greater than 60% sequence similitude with A. thaliana proteins were considered for the design of primers used for the qPCR assays (see below).
4.4. Isolation of RNA and qPCR Analysis
The gene expression assay was performed with RNA isolates from plants cultured under at two light conditions (R-BLL and WLL) and sampled at three different development stages: 4, 8, and 12 weeks after the in vitro cultivation (WAC) of half-zygotic Ah embryos. Stems, leaves, dissected meristems, and flowers were collected, if applicable, at each sampling stage for subsequent RNA extraction. Total RNA from each different organ was isolated using Trizol (Invitrogen, Carlsbad, CA, USA); its purity and concentration were determined using a NanoDrop 2000 apparatus as instructed (ThermoFisher Scientific; Waltham, MA, USA), and its integrity was confirmed by 1% agarose gel electrophoresis.
Total RNA samples (1 μg) were reverse-transcribed to generate the first-strand cDNA as previously described []. The cDNA obtained was diluted 25-fold with deionized–distilled water prior to quantitative PCR (qPCR) analyses. These were performed using SYBR Green detection chemistry and a CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). The primers used are listed in Table S2. The primers were designed using IDT Primer3 software (version 2.2.3; Integrated DNA Technologies, Inc., Coralville, IA, USA). The expression of the genes EF1 α (elongation factor 1 α) and β-TUBULIN was used as a reference for calculating the relative expression of the target genes under WLL and R-BLL using the 2−∆∆CT method [,]. Log2-transformed gene expression values were subsequently employed to calculate the proportional changes in gene expression between WLL and R-BLL conditions.
4.5. Principal Component Analysis (PCA) of Gene Expression
All data derived from the quantitative gene expression assays under R-BLL and WLL conditions were subjected to a PCA after standardizing to unit variance. The resulting factor scores of PC1 and PC2 were tested in a two-way analysis of variance (ANOVA). Data analyses were carried out using Rstudio version 2024.09.0 + 375 [].
4.6. Analysis of Cis-Acting Regulatory Elements in the Ah FT Gene
The Plant CARE database [] was utilized to identify potential cis-acting regulatory elements within the promoter region of the Ah FT gene. The analysis spanned 2000 base pairs upstream of its transcription start site.
5. Conclusions
The results of this study present evidence suggesting that the coordinated action of CO, the integrator of the light signal into flowering, FT, the master regulator of flowering, and LFY, the controller of meristem fate and flower morphology, coupled with the much-reduced influence of the flowering repressor, TFL1, in meristems and in developing flowers, could have led to early flowering in Ah plants cultivated in vitro under R-BLLs and under a long-day photoperiod not conductive to reproductive development in these plants.
They also support the proposal that CRY2 and PHYB photoreceptors were probably important contributors via their possible inhibition of the formation of the COP1-SPA1 CO-repressing complex and subsequent blockage of FT induction. A high irradiance condition under R-BLLs compared with WLLs is also proposed as a contributing factor to the induction of early flowering in Ah plants cultivated in vitro and under a long-day photoperiod. Finally, increased protein synthesis mediated by the ribosome biogenesis factors NOB1 and MDN1 and the ribosomal protein RPL21 may have also contributed, not only to induce early flowering but also to ensure seed production in conjunction with LEC1.
The current protocol is being applied successfully in stable genetic transformation and genome-editing procedures of grain amaranths by particle bombardment and/or Agrobacterium tumefaciens mediation.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14203134/s1, Figure S1: Light spectra of the white and red/blue LED lamps employed in this study; Figure S2: Principal component analysis (PCA) of the quantitative gene expression assays detected in the leaves; Figure S3: Principal component analysis (PCA) of the quantitative gene expression assays detected in the stems; Table S1: Quality of light emitted by the red/blue and white LED lamps employed in this study; Table S2: Primer design of the A. hypochondriacus flowering-related genes that were analyzed in the present study.
Author Contributions
Conceptualization, A.R.B.-V. and J.P.D.-F.; methodology, A.R.B.-V., N.A.M.-G. and E.V.-L.; software, E.V.-L.; validation, A.R.B.-V. and J.P.D.-F.; formal analysis, A.R.B.-V., N.A.M.-G., E.V.-L. and J.P.D.-F.; investigation, A.R.B.-V. and J.P.D.-F.; data curation, A.R.B.-V., N.A.M.-G. and J.P.D.-F.; writing—original draft preparation, A.R.B.-V. and J.P.D.-F.; writing—review and editing, J.P.D.-F.; visualization, A.R.B.-V. and J.P.D.-F.; supervision, J.P.D.-F. All authors have read and agreed to the published version of the manuscript.
Funding
A.R.B.-V. was supported by postgraduate scholarships granted by The National Council for Humanities, Science and Technology (Conahcyt, México), code No. 1147108.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations were used in this manuscript:
| Ah | Amaranthus hypochondriacus |
| CO | CONSTANS |
| COP1 | CONSTITUTIVE PHOTOMORPHOGENESIS PROTEIN 1 |
| CRY2 | CRYPTOCHROME2 |
| FT | FLOWERING LOCUS T |
| GI | GIGANTEA |
| LEC1 | LEAFY COTYLEDON1 |
| LFY | LEAFY |
| NOB1 | NIN1 (0NE) BINDING PROTEIN1 |
| PCA | Principal component analysis |
| PHL | PHYTOCHROME-DEPENDENT LATE FLOWERING |
| PHYB | PHYTOCHROME B |
| PPI | Protein–protein interaction |
| R-BLL | Red–blue LED light |
| RPL21C | RIBOSOMAL PROTEIN L21 SUBUNIT C |
| RPS18 | RIBOSOMAL PROTEIN S18 |
| SPA1 | SUPPRESOR OF PHYA1 |
| TF | Transcription factor |
| TFL1 | TERMINAL FLOWER 1 |
| TOC1/PRR1 | TIMING OF CHLOROPHYLL A/B BINDING PROTEIN/PSEUDO RESPONSE REGULATOR1 |
| WLL | White LED light |
References
- Sauer, J.D. The Grain Amaranths: A Survey of Their History and Classification. Ann. Mo. Bot. Gard. 1950, 37, 561–632. [Google Scholar] [CrossRef]
- Brenner, D.M.; Baltensperger, D.D.; Kulakow, P.A.; Lehmann, J.W.; Myers, R.L.; Slabbert, M.M.; Sleugh, B.B. Genetic Resources and Breeding of Amaranthus. In Plant Breeding Reviews; Janick, J., Ed.; Wiley: Hoboken, NJ, USA, 2000; pp. 227–285. ISBN 978-0-471-38787-9. [Google Scholar]
- Rastogi, A.; Shukla, S. Amaranth: A New Millennium Crop of Nutraceutical Values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef]
- Venskutonis, P.R.; Kraujalis, P. Nutritional Components of Amaranth Seeds and Vegetables: A Review on Composition, Properties, and Uses. Comp. Rev. Food Sci. Food Safe 2013, 12, 381–412. [Google Scholar] [CrossRef]
- De Ron, A.M.; Sparvoli, F.; Pueyo, J.J.; Bazile, D. Editorial: Protein Crops: Food and Feed for the Future. Front. Plant Sci. 2017, 8, 105. [Google Scholar] [CrossRef]
- Hoidal, N.; Díaz Gallardo, M.; Jacobsen, S.-E.; Alandia, G. Amaranth as a Dual-Use Crop for Leafy Greens and Seeds: Stable Responses to Leaf Harvest Across Genotypes and Environments. Front. Plant Sci. 2019, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Flores, H.E.; Thier, A.; Galston, A.W. In Vitro Culture of Grain and Vegetable Amaranths (Amaranthus spp.). Am. J. Bot. 1982, 69, 1049–1054. [Google Scholar] [CrossRef]
- Bagga, S.; Venkateswarlu, K.; Sopory, S.K. In Vitro Regeneration of Plants from Hypocotyl Segments of Amaranthus paniculatus. Plant Cell Rep. 1987, 6, 183–184. [Google Scholar] [CrossRef] [PubMed]
- Bennici, A.; Schiff, S.; Bovelli, R. In Vitro Culture of Species and Varieties of Four Amaranthus L. Species. Euphytica 1992, 62, 181–186. [Google Scholar] [CrossRef]
- Murthy, K.S.R.; Kondamudi, R.; Rao, P.C.; Pullaiah, T. In Vitro Flowering-a Review. J. Agric. Technol. 2012, 8, 1517–1536. [Google Scholar]
- Zabka, G.G. Photoperiodism in Amaranthus caudatus. I. A Re-examination of the Photoperiodic Response. Am. J. Bot. 1961, 48, 21–28. [Google Scholar] [CrossRef]
- Kulakow, P.A.; Jain, S.K. The Inheritance of Flowering Time in Amaranthus Species. J. Genet. 1985, 64, 85–100. [Google Scholar] [CrossRef]
- Kauffman, C.S.; Weber, L.E. Grain Amaranth. In Advances in New Crops; Timber Press: Portland, OR, USA, 1990; pp. 127–139. [Google Scholar]
- Kauffman, C.S. The Status of Grain Amaranth for the 1990s. Food Rev. Int. 1992, 8, 165–185. [Google Scholar] [CrossRef]
- Lehmann, J.W.; Clark, R.L.; Frey, K.J. Biomass Heterosis and Combining Ability in Interspecific and Intraspecific Matings of Grain Amaranths. Crop Sci. 1991, 31, 1111–1116. [Google Scholar] [CrossRef]
- Brenner, D.M.; Widriechner, M. Amaranthus Seed Regeneration in Plastic Tents in Greenhouses. In Plant Genetic Resources Newsletter; FAO: Rome, Italy, 1998; pp. 1–4. [Google Scholar]
- Tisserat, B.; Galletta, P.D. In Vitro Flowering in Amaranthus. HortScience 1988, 23, 210–212. [Google Scholar] [CrossRef]
- Stetter, M.G.; Zeitler, L.; Steinhaus, A.; Kroener, K.; Biljecki, M.; Schmid, K.J. Crossing Methods and Cultivation Conditions for Rapid Production of Segregating Populations in Three Grain Amaranth Species. Front. Plant Sci. 2016, 7, 816. [Google Scholar] [CrossRef] [PubMed]
- Lazaro, A.; Valverde, F.; Piñeiro, M.; Jarillo, J.A. The Arabidopsis E3 Ubiquitin Ligase HOS1 Negatively Regulates CONSTANS Abundance in the Photoperiodic Control of Flowering. Plant Cell 2012, 24, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod Control of Plant Growth: Flowering Time Genes Beyond Flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2022, 41, 742–780. [Google Scholar] [CrossRef]
- Li, X.; Liang, T.; Liu, H. How plants coordinate their development in response to light and temperature signals. Plant Cell 2022, 34, 955–966. [Google Scholar] [CrossRef]
- Hughes, J.; Winkler, A. New Insight Into Phytochromes: Connecting Structure to Function. Annu. Rev. Plant Biol. 2024, 75, 153–183. [Google Scholar] [CrossRef]
- Wang, F.; Han, T.; Jeffrey Chen, Z. Circadian and Photoperiodic Regulation of the Vegetative to Reproductive Transition in Plants. Commun. Biol. 2024, 7, 579. [Google Scholar] [CrossRef]
- Turck, F.; Fornara, F.; Coupland, G. Regulation and Identity of Florigen: FLOWERING LOCUS T Moves Center Stage. Annu. Rev. Plant Biol. 2008, 59, 573–594. [Google Scholar] [CrossRef]
- Griffin, J.H.C.; Prado, K.; Sutton, P.; Toledo-Ortiz, G. Coordinating Light Responses between the Nucleus and the Chloroplast, a Role for Plant Cryptochromes and Phytochromes. Physiol. Plant 2020, 169, 515–528. [Google Scholar] [CrossRef]
- Romanowski, A.; Furniss, J.J.; Hussain, E.; Halliday, K.J. Phytochrome Regulates Cellular Response Plasticity and the Basic Molecular Machinery of Leaf Development. Plant Physiol. 2021, 186, 1220–1239. [Google Scholar] [CrossRef]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor Regulation of CONSTANS Protein in Photoperiodic Flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef]
- Endo, M.; Tanigawa, Y.; Murakami, T.; Araki, T.; Nagatani, A. PHYTOCHROME-DEPENDENT LATE-FLOWERING Accelerates Flowering through Physical Interactions with Phytochrome B and CONSTANS. Proc. Natl. Acad. Sci. USA 2013, 110, 18017–18022. [Google Scholar] [CrossRef]
- Zhu, Y.; Klasfeld, S.; Wagner, D. Molecular Regulation of Plant Developmental Transitions and Plant Architecture via PEPB Family Proteins: An Update on Mechanism of Action. J. Exp. Bot. 2021, 72, 2301–2311. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Gusmaroli, G.; Terzaghi, W.; Lau, O.S.; Yanagawa, Y.; Zhang, Y.; Li, J.; Lee, J.-H.; Zhu, D.; et al. Arabidopsis CULLIN4-Damaged DNA Binding Protein 1 Interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA Complexes to Regulate Photomorphogenesis and Flowering Time. Plant Cell 2010, 22, 108–123. [Google Scholar] [CrossRef]
- Missbach, S.; Weis, B.L.; Martin, R.; Simm, S.; Bohnsack, M.T.; Schleiff, E. 40S Ribosome Biogenesis Co-Factors Are Essential for Gametophyte and Embryo Development. PLoS ONE 2013, 8, e54084. [Google Scholar] [CrossRef]
- Li, K.; Wang, P.; Ding, T.; Hou, L.; Li, G.; Zhao, C.; Zhao, S.; Wang, X.; Li, P. Mutation of an Essential 60S Ribosome Assembly Factor MIDASIN 1 Induces Early Flowering in Arabidopsis. Int. J. Mol. Sci. 2022, 23, 6509. [Google Scholar] [CrossRef]
- Romero, J.M.; Serrano-Bueno, G.; Camacho-Fernández, C.; Vicente, M.H.; Ruiz, M.T.; Pérez-Castiñeira, J.R.; Pérez-Hormaeche, J.; Nogueira, F.T.S.; Valverde, F. CONSTANS, a HUB for All Seasons: How Photoperiod Pervades Plant Physiology Regulatory Circuits. Plant Cell 2024, 36, 2086–2102. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pin, P.A.; Nilsson, O. The Multifaceted Roles of FLOWERING LOCUS T in Plant Development. Plant Cell Environ. 2012, 35, 1742–1755. [Google Scholar] [CrossRef]
- Anuradha, K.M.; Zinta, G.; Chauhan, R.; Kumar, A.; Singh, S.; Singh, S. Genetic Resources and Breeding Approaches for Improvement of Amaranth (Amaranthus spp.) and Quinoa (Chenopodium quinoa). Front. Nutr. 2020, 10, 1129723. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, A.; Dhakte, P.; Raturi, G.; Vishwakarma, G.; Barbadikar, K.M.; Das, B.K.; Shivaraj, S.M.; Humira Sonah, H.; Deshmukh, R. Speed Breeding Opportunities and Challenges for Crop Improvement. J. Plant Growth Regul. 2023, 42, 46–59. [Google Scholar] [CrossRef]
- Moraes, T.S.; Dornelas, M.C.; Martinelli, A.P. FT/TFL1: Calibrating Plant Architecture. Front. Plant Sci. 2019, 10, 97. [Google Scholar] [CrossRef]
- Zhu, Y.; Klasfeld, S.; Jeong, C.W.; Jin, R.; Goto, K.; Yamaguchi, N.; Wagner, D. TERMINAL FLOWER 1-FD Complex Target Genes and Competition with FLOWERING LOCUS T. Nat. Commun. 2020, 11, 5118. [Google Scholar] [CrossRef]
- Liu, B.; Woods, D.P.; Li, W.; Amasino, R.M. INDETERMINATE1-Mediated Expression of FT Family Genes Is Required for Proper Timing of Flowering in Brachypodium Distachyon. Proc. Natl. Acad. Sci. USA 2023, 120, e2312052120. [Google Scholar] [CrossRef]
- Xu, H.; Guo, X.; Hao, Y.; Lu, G.; Li, D.; Lu, J.; Zhang, T. Genome-Wide Characterization of PEBP Gene Family in Perilla Frutescens and PfFT1 Promotes Flowering Time in Arabidopsis Thaliana. Front. Plant Sci. 2022, 13, 1026696. [Google Scholar] [CrossRef]
- Saidi, A.; Hajibarat, Z. In Silico Analysis of Floral MADS-BOX Gene in Brachypodium Distachyon. Bionature 2018, 38, 366–375. Available online: https://globalpresshub.com/index.php/BN/article/view/720 (accessed on 10 March 2025).
- Argüello-Astorga, G.; Herrera-Estrella, L. Evolution of Light-Regulated Plant Promoters. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 525–555. [Google Scholar] [CrossRef]
- Dehesh, K.; Bruce, W.B.; Quail, P.H. A Trans-Acting Factor That Binds to a GT-Motif in a Phytochrome Gene Promoter. Science 1990, 250, 1397–1399. [Google Scholar] [CrossRef]
- Anderson, S.L.; Teakle, G.R.; Martino-Catt, S.J.; Kay, S.A. Circadian Clock- and Phytochrome-regulated Transcription Is Conferred by a 78 Bp Cis-acting Domain of the Arabidopsis CAB2 Promoter. Plant J. 1994, 6, 457–470. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Puente, P.; Deng, X.; Wei, N. Combinatorial Interaction of Light-responsive Elements Plays a Critical Role in Determining the Response Characteristics of Light-regulated Promoters in Arabidopsis. Plant J. 1998, 15, 69–77. [Google Scholar] [CrossRef]
- Qian, G.; Yang, J.; Wang, M.; Li, L. Identification of the Dof Gene Family in Quinoa and Its Potential Role in Regulating Flavonoid Synthesis Under Different Stress Conditions. Biology 2025, 14, 446. [Google Scholar] [CrossRef]
- Moyroud, E.; Kusters, E.; Monniaux, M.; Koes, R.; Parcy, F. LEAFY Blossoms. Trends Plant Sci. 2010, 15, 346–352. [Google Scholar] [CrossRef]
- Li, H.; Burgie, E.S.; Gannam, Z.T.K.; Li, H.; Vierstra, R.D. Plant Phytochrome B Is an Asymmetric Dimer with Unique Signalling Potential. Nature 2022, 604, 127–133. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Shi, H.; Guo, M.; Chai, M.; He, Q.; Yan, M.; Cao, D.; Zhao, L.; Cai, H.; et al. Evolutionary and Expression Analyses of Soybean Basic Leucine Zipper Transcription Factor Family. BMC Genom. 2018, 19, 159. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, Y.; Li, X.; Wang, G.; Zhou, Q.; Chen, Q.; Li, J.; Wang, X.; Tang, H. An Evolutionary Analysis of B-Box Transcription Factors in Strawberry Reveals the Role of FaBBx28c1 in the Regulation of Flowering Time. Int. J. Mol. Sci. 2021, 22, 11766. [Google Scholar] [CrossRef]
- Maassen, A.; Steciuk, J.; Wilga, M.; Szurmak, J.; Garbicz, D.; Sarnowska, E.; Sarnowski, T.J. SWI/SNF-Type Complexes–Transcription Factor Interplay: A Key Regulatory Interaction. Cell. Mol. Biol. Lett. 2025, 30, 30. [Google Scholar] [CrossRef]
- Weigel, D.; Nilsson, O. A Developmental Switch Sufficient for Flower Initiation in Diverse Plants. Nature 1995, 377, 495–500. [Google Scholar] [CrossRef]
- Nilsson, O.; Weigel, D. Modulating the Timing of Flowering. Curr. Opin. Biotechnol. 1997, 8, 195–199. [Google Scholar] [CrossRef]
- He, Z.; Zhu, Q.; Dabi, T.; Li, D.; Weigel, D.; Lamb, C. Transformation of Rice with the Arabidopsis Floral Regulator LEAFY Causes Early Heading. Transgenic Res. 2000, 9, 223–227. [Google Scholar] [CrossRef]
- Peña, L.; Martín-Trillo, M.; Juárez, J.; Pina, J.A.; Navarro, L.; Martínez-Zapater, J.M. Constitutive Expression of Arabidopsis LEAFY or APETALA1 Genes in Citrus Reduces Their Generation Time. Nat. Biotechnol. 2001, 19, 263–267. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, Y.; Liu, K.; Zheng, G.; Gao, L.; Li, Z.; Huang, M.; Jiang, Y. Transcriptome Profiles Reveal Gene Regulation of Ginger Flowering Induced by Photoperiod and Light Quality. Bot. Stud. 2023, 64, 12. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The Genetic Basis of Flowering Responses to Seasonal Cues. Nat. Rev. Genet. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Mockler, T.C.; Lin, C. Regulation of Flowering Time by Arabidopsis Photoreceptors. Science 1998, 279, 1360–1363. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kiba, T.; Chua, N. The Arabidopsis SPA1 Gene Is Required for Circadian Clock Function and Photoperiodic Flowering. Plant J. 2006, 46, 736–746. [Google Scholar] [CrossRef]
- Hajdu, A.; Ádám, É.; Sheerin, D.J.; Dobos, O.; Bernula, P.; Hiltbrunner, A.; Kozma-Bognár, L.; Nagy, F. High-level Expression and Phosphorylation of Phytochrome B Modulates Flowering Time in Arabidopsis. Plant J. 2015, 83, 794–805. [Google Scholar] [CrossRef]
- Lu, X.-D.; Zhou, C.-M.; Xu, P.-B.; Luo, Q.; Lian, H.-L.; Yang, H.-Q. Red-Light-Dependent Interaction of phyB with SPA1 Promotes COP1–SPA1 Dissociation and Photomorphogenic Development in Arabidopsis. Mol. Plant 2015, 8, 467–478. [Google Scholar] [CrossRef]
- Sheerin, D.J.; Menon, C.; Zur Oven-Krockhaus, S.; Enderle, B.; Zhu, L.; Johnen, P.; Schleifenbaum, F.; Stierhof, Y.-D.; Huq, E.; Hiltbrunner, A. Light-Activated Phytochrome A and B Interact with Members of the SPA Family to Promote Photomorphogenesis in Arabidopsis by Reorganizing the COP1/SPA Complex. Plant Cell 2015, 27, 189–201. [Google Scholar] [CrossRef]
- Santos-Mendoza, M.; Dubreucq, B.; Baud, S.; Parcy, F.; Caboche, M.; Lepiniec, L. Deciphering Gene Regulatory Networks That Control Seed Development and Maturation in Arabidopsis. Plant J. 2008, 54, 608–620. [Google Scholar] [CrossRef]
- Rogalski, M.; Ruf, S.; Bock, R. Tobacco Plastid Ribosomal Protein S18 Is Essential for Cell Survival. Nucleic Acids Res. 2006, 34, 4537–4545. [Google Scholar] [CrossRef]
- Van Lijsebettens, M.; Vanderhaeghen, R.; De Block, M.; Bauw, G.; Villarroel, R.; Van Montagu, M. An S18 Ribosomal Protein Gene Copy at the Arabidopsis PFL Locus Affects Plant Development by Its Specific Expression in Meristems. EMBO J. 1994, 13, 3378–3388. [Google Scholar] [CrossRef]
- Moin, M.; Bakshi, A.; Saha, A.; Dutta, M.; Madhav, S.M.; Kirti, P.B. Rice Ribosomal Protein Large Subunit Genes and Their Spatio-Temporal and Stress Regulation. Front. Plant Sci. 2016, 7, 1284. [Google Scholar] [CrossRef]
- Yin, T.; Pan, G.; Liu, H.; Wu, J.; Li, Y.; Zhao, Z.; Fu, T.; Zhou, Y. The Chloroplast Ribosomal Protein L21 Gene Is Essential for Plastid Development and Embryogenesis in Arabidopsis. Planta 2012, 235, 907–921. [Google Scholar] [CrossRef]
- Jung, J.-H.; Seo, Y.-H.; Seo, P.J.; Reyes, J.L.; Yun, J.; Chua, N.-H.; Park, C.-M. The GIGANTEA-Regulated MicroRNA172 Mediates Photoperiodic Flowering Independent of CONSTANS in Arabidopsis. Plant Cell 2007, 19, 2736–2748. [Google Scholar] [CrossRef]
- Mattson, N.S.; Erwin, J.E. The Impact of Photoperiod and Irradiance on Flowering of Several Herbaceous Ornamentals. Sci. Hortic. 2005, 104, 275–292. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING V11: Protein–Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-Wide Experimental Datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A Comparative Platform for Green Plant Genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Palmeros-Suárez, P.A.; Massange-Sánchez, J.A.; Martínez-Gallardo, N.A.; Montero-Vargas, J.M.; Gómez-Leyva, J.F.; Délano-Frier, J.P. The Overexpression of an Amaranthus Hypochondriacus NF-YC Gene Modifies Growth and Confers Water Deficit Stress Resistance in Arabidopsis. Plant Sci. 2015, 240, 25–40. [Google Scholar] [CrossRef]
- Bustin, S.A.; Beaulieu, J.-F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE Précis: Practical Implementation of Minimum Standard Guidelines for Fluorescence-Based Quantitative Real-Time PCR Experiments. BMC Mol. Biol. 2010, 11, 74. [Google Scholar] [CrossRef]
- Horton, N.J.; Kleinman, K. Using R and RStudio for Data Management, Statistical Analysis, and Graphics; Chapman and Hall/CRC: Boca Raton, FL, USA, 2015; ISBN 978-0-429-16156-8. [Google Scholar]
- Lescot, M. PlantCARE, a Database of Plant Cis-Acting Regulatory Elements and a Portal to Tools for in Silico Analysis of Promoter Sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).