Influence of Basal Medium and Organic Additives on In Vitro Germination and Plant Growth of Endangered Orchid Gastrochilus fuscopunctatus
Abstract
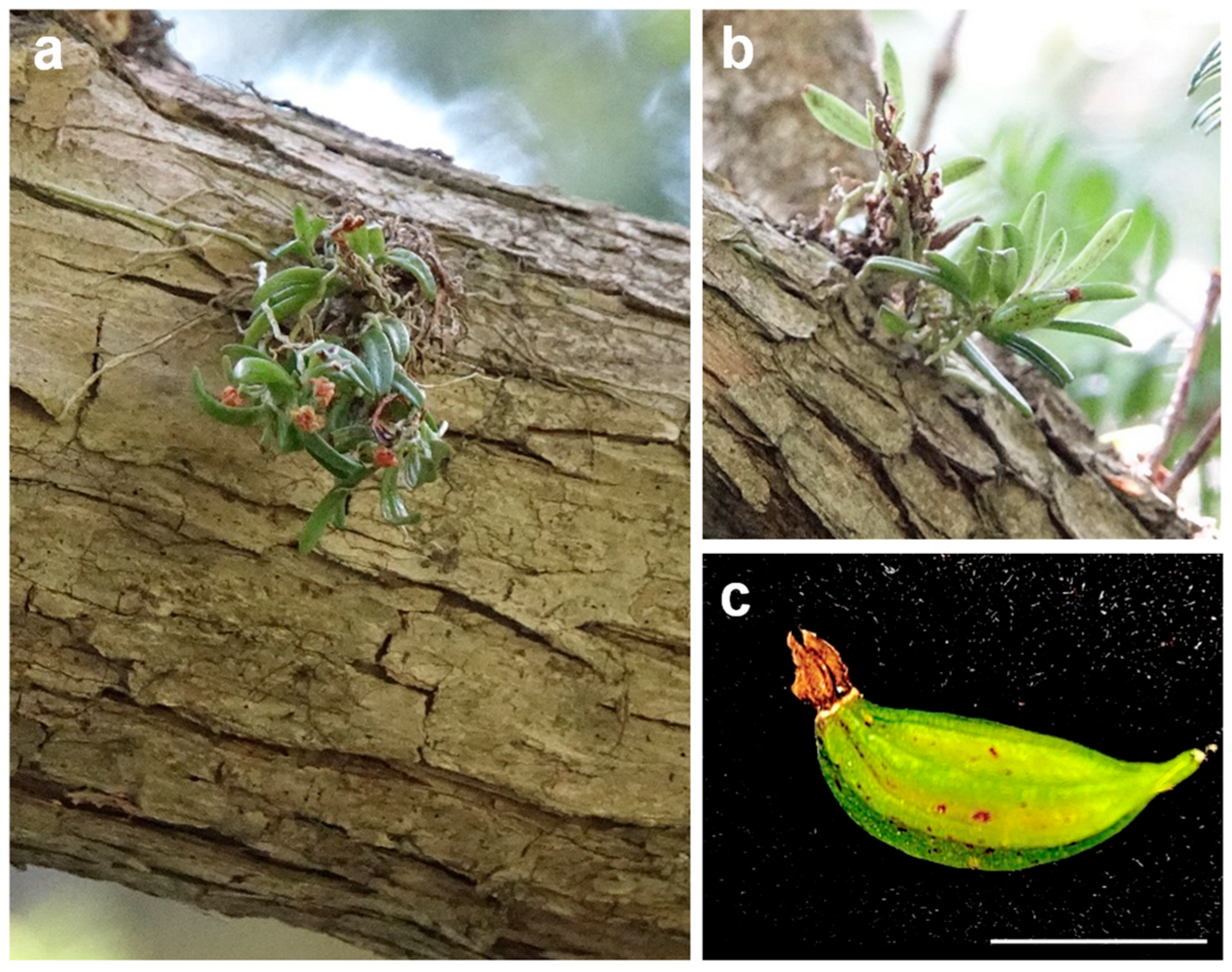
1. Introduction
2. Results and Discussion
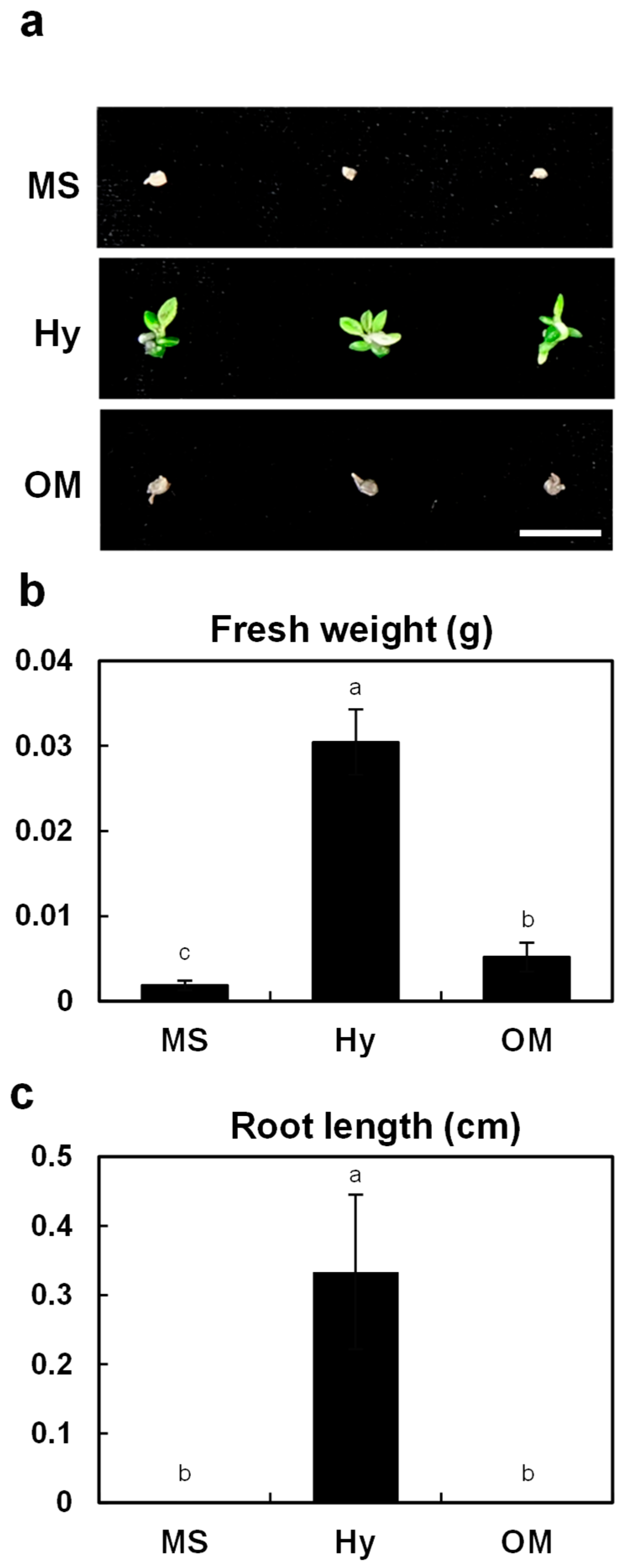
2.1. Effects of Basal Medium Composition on Asymbiotic Germination and Early Protocorm Development
2.2. Effect of Different Basal Media on Seedling Growth
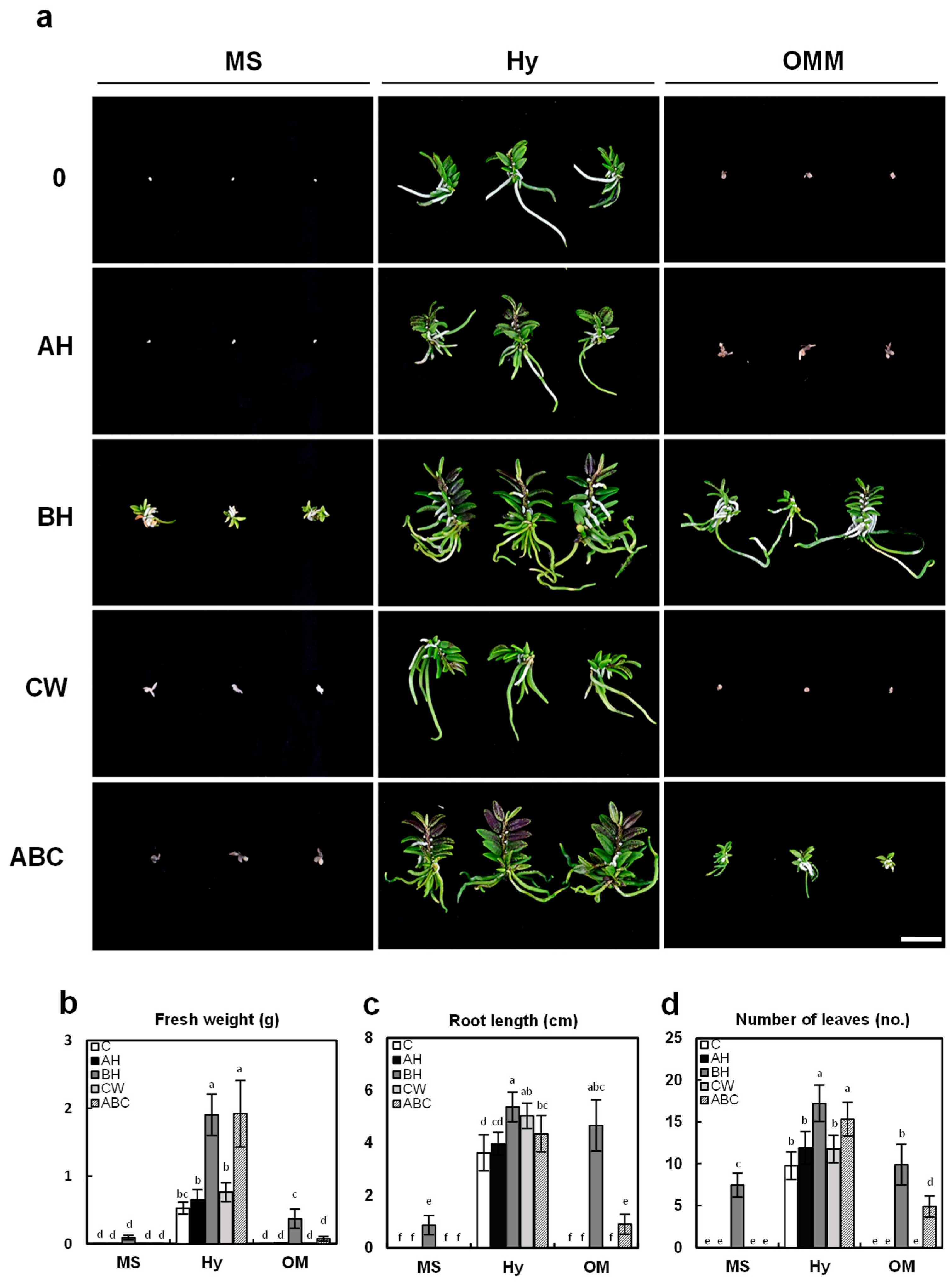
2.3. Effect of Organic Additives on Early Seedling Growth
2.4. Effect of Basal Media and Organic Additives on Plant Growth at Later Plant Development Stages
3. Materials and Methods
3.1. Plant Material and Surface Sterilization of Capsules
3.2. Germination of the Seeds and Protocorm Development
3.3. Culture Media for In Vitro Seedling Growth
3.4. Measurement of Plant Development and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chase, M.W.; Cameron, K.M.; Freudenstein, J.V.; Pridgeon, A.M.; Salazar, G.; Van den Berg, C.; Schuiteman, A. An Updated Classification of Orchidaceae. Bot. J. Linn. Soc. 2015, 177, 151–174. [Google Scholar] [CrossRef]
- Christenhusz, M.J.M.; Byng, J.W. The Number of Known Plant Species in the World and Its Annual Increase. Phytotaxa 2016, 261, 201–217. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2023-2. Available online: https://www.iucnredlist.org (accessed on 14 August 2025).
- Sletvold, N.; Dahlgren, J.P.; Øien, D.I.; Moen, A.; Ehrlén, J. Climate Warming Alters Effects of Management on Population Viability of Threatened Species: Results from a 30-Year Experimental Study on a Rare Orchid. Glob. Change Biol. 2013, 19, 2729–2738. [Google Scholar] [CrossRef]
- Fay, M.F. Orchid Conservation: How Can We Meet the Challenges in the Twenty-First Century? Bot. Stud. 2018, 59, 16. [Google Scholar] [CrossRef]
- Arditti, J.; Ghani, A.K.A. Tansley Review No. 110. Numerical and Physical Properties of Orchid Seeds and Their Biological Implications. New Phytol. 2000, 145, 367–421. [Google Scholar] [CrossRef]
- Batygina, T.B.; Bragina, E.A.; Vasilyeva, V.E. The Reproductive System and Germination in Orchids. Acta Biol. Crac. Ser. Bot. 2003, 45, 21–34. [Google Scholar]
- Mayo, M.J.; Wochok, Z.S.; Arditti, J. Tissue Culture of Orchids. In Micropropagation of Orchids; Arditti, J., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 41–85. [Google Scholar]
- Uslu, O.S.; Babur, E.; Alma, M.H.; Solaiman, Z.M. Walnut Shell Biochar Increases Seed Germination and Early Growth of Seedlings of Fodder Crops. Agriculture 2020, 10, 427. [Google Scholar] [CrossRef]
- Uslu, Ö.S.; Gedik, O.; Kaya, A.R.; Erol, A.; Babur, E.; Khan, H.; Seleiman, M.F.; Wasonga, D.O. Effects of Different Irrigation Water Sources Contaminated with Heavy Metals on Seed Germination and Seedling Growth of Different Field Crops. Water 2025, 17, 892. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, K.; da Silva, J.A.T.; Zhang, J.; Chen, Z.; Xia, N.; Duan, J. Asymbiotic Seed Germination, Seedling Development and Reintroduction of Paphiopedilum wardii Sumerh., an Endangered Terrestrial Orchid. Sci. Hortic. 2012, 138, 198–209. [Google Scholar] [CrossRef]
- Lee, Y.-I.; Yeung, E.C.; Chung, M.-C. Embryo Development of Orchids. In Orchid Biotechnology; Arditti, J., Ed.; World Scientific: Singapore, 2007; pp. 23–44. [Google Scholar]
- Kauth, P.J.; Vendrame, W.A.; Kane, M.E. In Vitro Seed Culture and Seedling Development of Calopogon tuberosus. Plant Cell Tissue Organ Cult. 2006, 85, 91–102. [Google Scholar] [CrossRef]
- Stewart, S.L.; Kane, M.E. Asymbiotic Seed Germination and In Vitro Seedling Development of Habenaria macroceratitis (Orchidaceae), a Rare Florida Terrestrial Orchid. Plant Cell Tissue Organ Cult. 2006, 86, 147–158. [Google Scholar] [CrossRef]
- Zhang, Y.; Lee, Y.I.; Deng, L.; Zhao, S. Asymbiotic Germination of Immature Seeds and the Seedling Development of Cypripedium macranthos Sw., an Endangered Lady’s Slipper Orchid. Sci. Hortic. 2013, 164, 130–136. [Google Scholar] [CrossRef]
- Utami, E.S.W.; Hariyanto, S. Organic Compounds: Contents and Their Role in Improving Seed Germination and Protocorm Development in Orchids. Int. J. Agron. 2020, 2020, 2795108. [Google Scholar] [CrossRef]
- Winarto, B.; da Silva, J.A.T. Use of Coconut Water and Fertilizer for in Vitro Proliferation and Plantlet Production of Dendrobium ‘Gradita 31′. Vitro Cell. Dev. Biol.-Plant 2015, 51, 303–314. [Google Scholar] [CrossRef]
- Baque, M.A.; Shin, Y.K.; Elshmari, T.; Lee, E.J.; Paek, K.Y. Effect of Light Quality, Sucrose and Coconut Water Concentration on the Microporpagation of Calanthe Hybrids (‘Bukduseong’ × ‘Hyesung’ and ‘Chunkwang’ × ‘Hyesung’). Aust. J. Crop Sci. 2011, 5, 1247–1254. [Google Scholar]
- National Institute of Biological Resources. Rare and Endangered Plants of Korea; National Institute of Biological Resources: Incheon, Republic of Korea, 2018. [Google Scholar]
- Ministry of Environment. The Revised List of Endangered Wildlife in Korea; Ministry of Environment: Sejong, Republic of Korea, 2017. [Google Scholar]
- Kang, H.; Kang, K.W.; Kim, D.H.; Sivanesan, I. In Vitro Propagation of Gastrochilus matsuran (Makino) Schltr., an Endangered Epiphytic Orchid. Plants 2020, 9, 524. [Google Scholar] [CrossRef]
- Park, H.B.; An, J.; Bae, K.H.; Hong, S.H.; Park, H.J.; Kim, S.; Lee, C.W.; Lee, B.D.; Baek, J.H.; Kim, N.Y.; et al. Asymbiotic Seed Germination and In Vitro Seedling Development of the Endangered Orchid Species Cypripedium guttatum. Plants 2023, 12, 3788. [Google Scholar] [CrossRef]
- Hwang, J.E.; Park, H.B.; Jeon, D.Y.; Park, H.J.; Kim, S.; Lee, C.W.; Kim, Y.J.; Yoon, Y.J. Effect of Different Basal Media and Organic Supplements on In Vitro Seedling Development of the Endangered Orchid Species Dendrobium moniliforme (L.) Swartz. Plants 2024, 13, 2721. [Google Scholar] [CrossRef]
- An, J.; Kim, P.B.; Park, H.B.; Kim, S.; Park, H.J.; Lee, C.W.; Lee, B.D.; Kim, N.Y.; Hwang, J.E. Effects of Different Growth Media on In Vitro Seedling Development of an Endangered Orchid Species Sedirea japonica. Plants 2021, 10, 1193. [Google Scholar] [CrossRef]
- Yeung, E.C.; Li, Y.Y.; Lee, Y.I. Understanding Seed and Protocorm Development in Orchids. In Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols; Springer: New York, NY, USA, 2018; pp. 3–26. [Google Scholar]
- Colli, S.; Kerbauy, G.B. Direct Root Tip Conversion of Catasetum into Protocorm-Like Bodies. Effects of Auxin and Cytokinin. Plant Cell Tissue Organ Cult. 1993, 33, 39–44. [Google Scholar] [CrossRef]
- Knudson, L. A New Nutrient Solution for the Germination of Orchid Seeds. Am. Orchid Soc. Bull. 1946, 15, 214–217. [Google Scholar]
- Vacin, E.F.; Went, F.W. Some pH Changes in Nutrient Solutions. Bot. Gaz. 1949, 110, 605–613. [Google Scholar] [CrossRef]
- Kano, K. Studies on the Media for Orchid Seed Germination. Mem. Fac. Agric. Kagawa Univ. 1965, 20, 68. [Google Scholar]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bae, K.H.; Kim, N.Y.; Song, J.M.; Song, G. In Vitro Propagation and Protocorm-Like Body Formation of Endangered Species, Dendrobium moniliforme. J. For. Environ. Sci. 2014, 30, 126–132. [Google Scholar] [CrossRef]
- Alam, M.K.; Rashid, M.H.; Hossain, M.S.; Salam, M.A.; Rouf, M.A. In Vitro Seed Propagation of Dendrobium (Dendrobium transparens) Orchid as Influenced by Different Media. Int. J. Biotechnol. 2002, 1, 2–4. [Google Scholar] [CrossRef]
- Safitri, Y.; Yalapuspita, D.C.; Handini, E.; Aprilianti, P.; Isnaini, Y.; Semiarti, E. Improvement of Growth Rate in In Vitro Culture of Paphiopedilum primulinum MW Wood & P. Taylor and Paphiopedilum glaucophyllum JJ Smith Using Banana Enrichment Media. Trop Life Sci. Res. 2024, 35, 109–120. [Google Scholar]
- Acemi, A. Growth Enhancing Effects of Banana Homogenate on a Glucomannan-Rich Orchid Species: Serapias vomeracea (Burm. f.) Briq. J. Fur Kult. 2020, 72, 167–175. [Google Scholar]
- Arum, I.; Semiarti, E. Effects of Banana Homogenate and Light Quality on Growth and Development of Phalaenopsis amabilis Orchid Plantlets. J. Trop. Life Sci. 2022, 12, 89–98. [Google Scholar]
- Momtaj, S.; Kaur, S. Role of Organic Growth Supplement in In Vitro Multiplication of Orchid Species—A Review. J. Trop. Life Sci. 2021, 11, 145–160. [Google Scholar] [CrossRef]
- Utami, E.S.W.; Hariyanto, S. In Vitro Seed Germination and Seedling Development of a Rare Indonesian Native Orchid Phalaenopsis amboinensis JJ Sm. Scientifica 2019, 2019, 8105138. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zeng, S.; Lin, D.; Teixeira da Silva, J.A.; Bu, Z.; Zhang, J.; Duan, J. In Vitro Propagation and Reintroduction of the Endangered Renanthera imschootiana Rolfe. PLoS ONE 2014, 9, e110033. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef]
- Wongsa, T.; Piapukiew, J.; Kuenkaew, K.; Somsanook, C.; Sapatee, O.; Linjikao, J.; Kongbangkerd, A. Asymbiotic Seed Germination and In Vitro Propagation of the Thai Rare Orchid Species Eulophia bicallosa (D. Don) PF Hunt & Summerh. Plants 2025, 14, 2212. [Google Scholar]
- Ragu, V.S.; Ombokou, R.; Repin, R.; Molidin, D.; Miadin, R.; Aziz, Z.A. In Vitro Seed Germination of Paphiopedilum lowii, an Endangered Slipper Orchid in North Borneo. Biodiversitas 2022, 23, 11. [Google Scholar] [CrossRef]
- Baltazar-Bernal, O.; Mora-González, E.G. Reproductive Phenology and Asymbiotic Germination for Conservation of Endangered Miniature Orchid Specklinia digitale. Horticulturae 2025, 11, 311. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.E.; Park, H.B.; Tho, J.-H.; Kim, M.; Park, H.J.; Kim, S.; Lee, C.W.; Kim, Y.-J. Influence of Basal Medium and Organic Additives on In Vitro Germination and Plant Growth of Endangered Orchid Gastrochilus fuscopunctatus. Plants 2025, 14, 3133. https://doi.org/10.3390/plants14203133
Hwang JE, Park HB, Tho J-H, Kim M, Park HJ, Kim S, Lee CW, Kim Y-J. Influence of Basal Medium and Organic Additives on In Vitro Germination and Plant Growth of Endangered Orchid Gastrochilus fuscopunctatus. Plants. 2025; 14(20):3133. https://doi.org/10.3390/plants14203133
Chicago/Turabian StyleHwang, Jung Eun, Hyeong Bin Park, Jae-Hwa Tho, Myojin Kim, Hwan Joon Park, Seongjun Kim, Chang Woo Lee, and Young-Joong Kim. 2025. "Influence of Basal Medium and Organic Additives on In Vitro Germination and Plant Growth of Endangered Orchid Gastrochilus fuscopunctatus" Plants 14, no. 20: 3133. https://doi.org/10.3390/plants14203133
APA StyleHwang, J. E., Park, H. B., Tho, J.-H., Kim, M., Park, H. J., Kim, S., Lee, C. W., & Kim, Y.-J. (2025). Influence of Basal Medium and Organic Additives on In Vitro Germination and Plant Growth of Endangered Orchid Gastrochilus fuscopunctatus. Plants, 14(20), 3133. https://doi.org/10.3390/plants14203133






