Evaluation of Leguminous Plants as Phytoremediator Species in Soil with Pesticide and Vinasse Interactions
Abstract
1. Introduction
2. Results
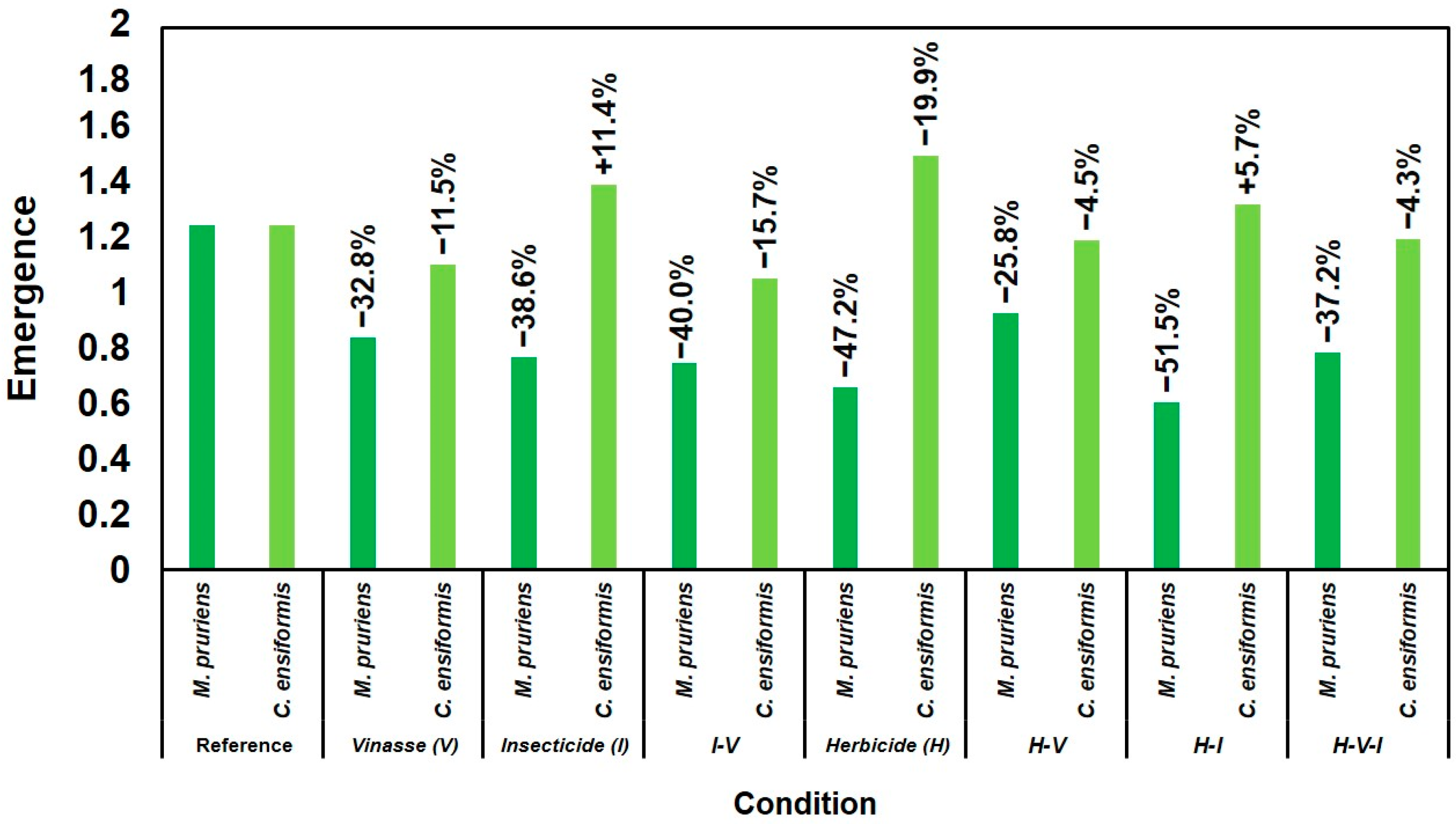
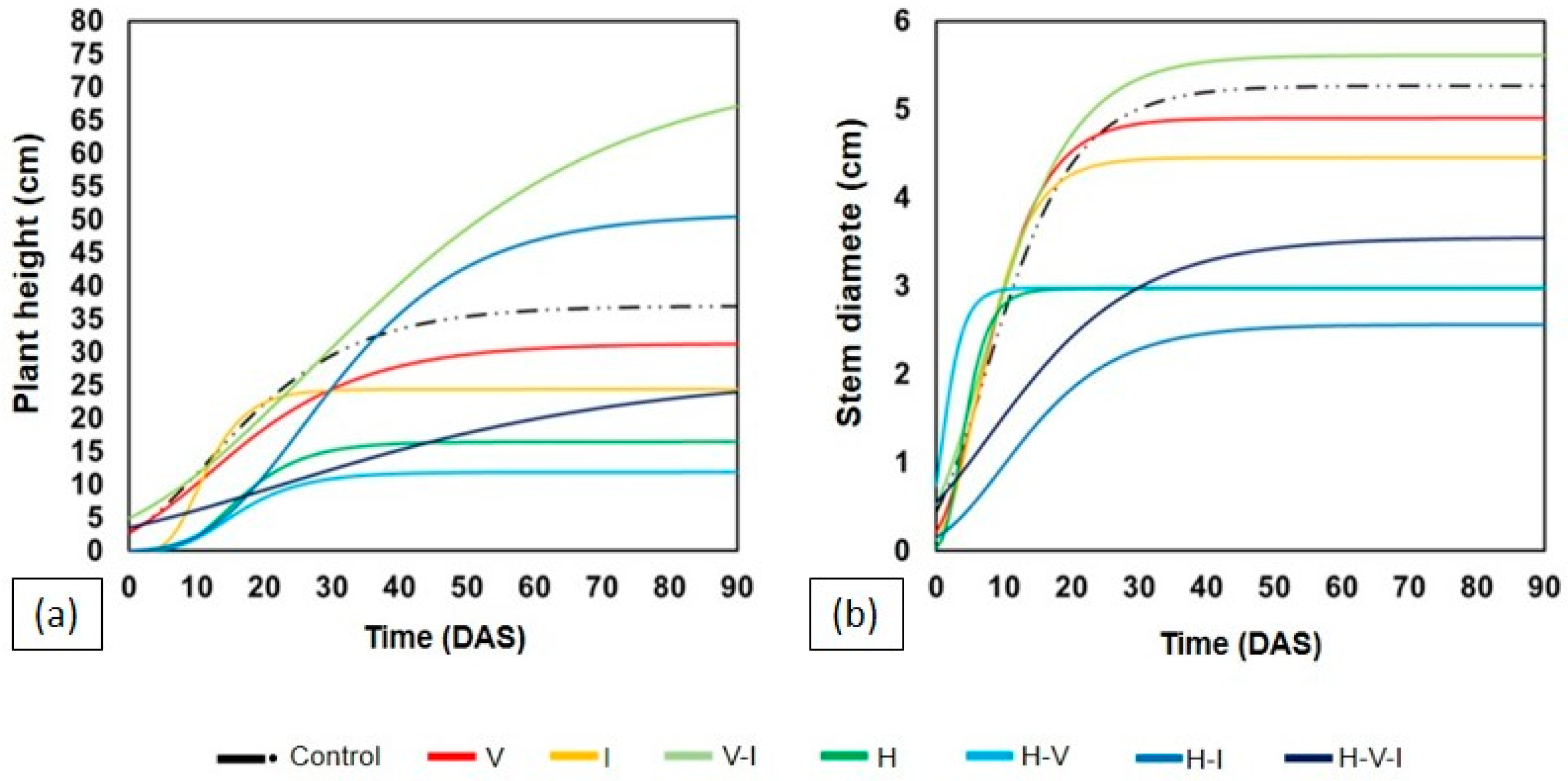
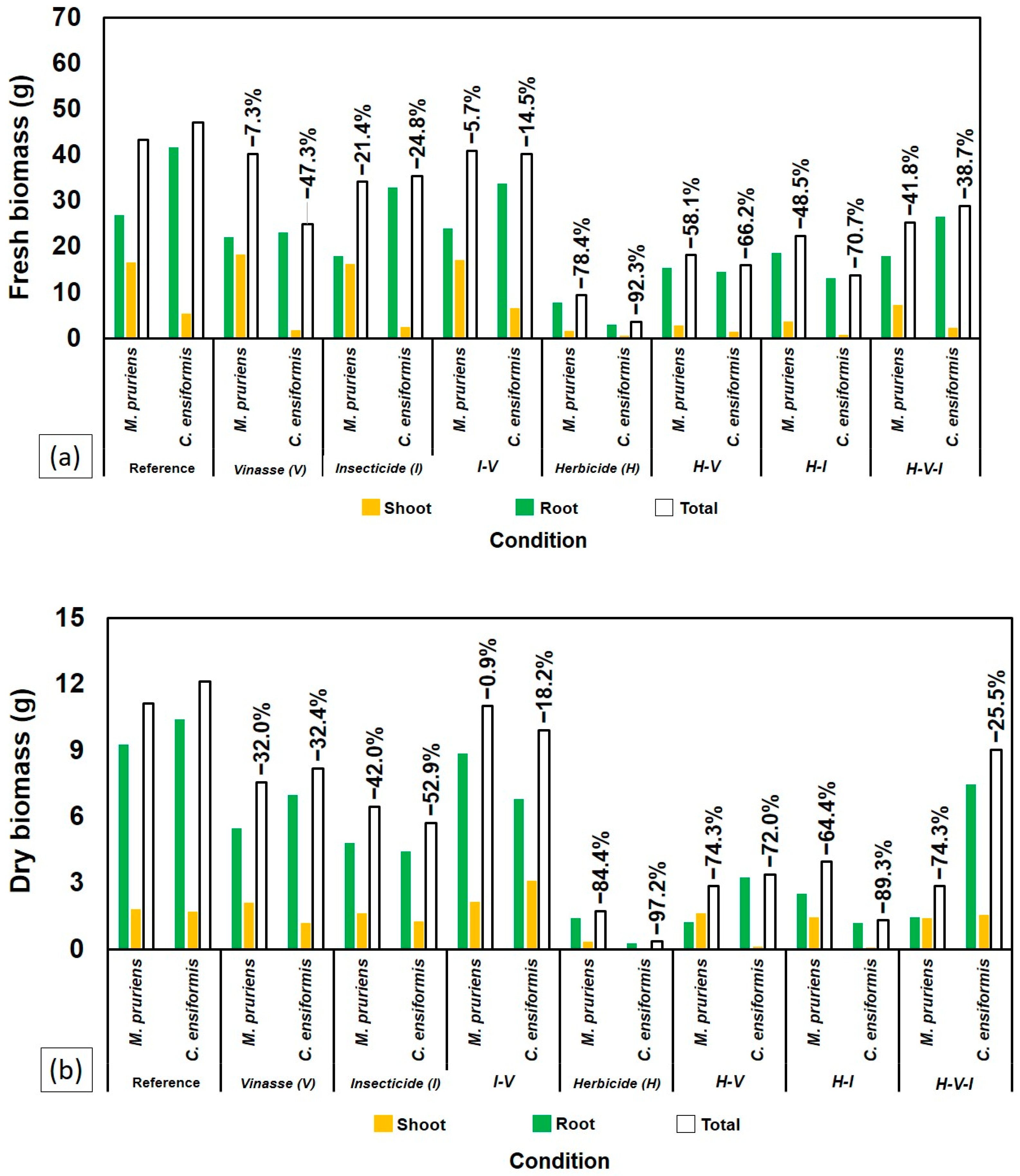
2.1. Growth and Biomass of Leguminous Species
2.2. Sentinel Species Response
2.3. Ecotoxicological Bioassays and Multivariate Analyses
3. Discussion
3.1. Phytotoxic Effects and Species Tolerance
3.2. Influence of Vinasse and Compound Interactions
3.3. Microbial and Environmental Implications of Phytoremediation
4. Materials and Methods
4.1. Location and Materials
4.2. Experimental Design and Analytical Methods
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, V.; Mol, H.G.J.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils—A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Huang, J.; Khan, M.T.; Perecin, D.; Coelho, S.T.; Zhang, M. Sugarcane for bioethanol production: Potential of bagasse in Chinese perspective. Renew. Sust. Energy Rev. 2020, 133, 110296. [Google Scholar] [CrossRef]
- Bassey, M.S.; Kolo, M.G.M.; Daniya, E.; Odofin, A.J. Trash mulch and weed management practice impact on some soil properties, weed dynamics and sugarcane (Saccharum officinarum L.) genotypes plant crop productivity. Sugar Tech 2021, 23, 395–406. [Google Scholar] [CrossRef]
- Brazil. Ministry of Agriculture, Livestock and Food Supply (Ministério da Agricultura, Pecuária e Abastecimento—MAPA). Phytosanitary Pesticides System—AGROFIT. Brasília, Brazil, 2025. Available online: https://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons (accessed on 15 August 2025).
- Guimarães, A.C.D.; Paula, D.F.; Mendes, K.F.; Sousa, R.N.; Araújo, G.R.; Inoue, M.H.; Tornisielo, V.L. Can soil type interfere in sorption-desorption, mobility, leaching, degradation, and microbial activity of the 14C-tebuthiuron herbicide? J. Hazard. Mater. Adv. 2022, 6, 100074. [Google Scholar] [CrossRef]
- Conciani, P.A.; Mendes, K.F.; de Sousa, R.N.; Ribeiro, A.P.; Pimpinato, R.F.; Tornisielo, V.L. Peanut and sorghum are excellent phytoremediators of 14C-tebuthiuron in herbicide-contaminated soil. Adv. Weed Sci. 2023, 41, e020220068. [Google Scholar] [CrossRef]
- Frias, Y.A.; Lima, E.W.; Aragão, M.B.; Nantes, L.S.; Moreira, B.R.A.; Cruz, V.H.; Tomaz, R.S.; Lopes, P.R.M. Mucuna pruriens cannot develop phytoremediation of tebuthiuron in agricultural soil with vinasse: A morphometrical and ecotoxicological analysis. Front. Bioeng. Biotechnol. 2023, 11, 1156751. [Google Scholar] [CrossRef]
- Cruz, V.H.; Moreira, B.R.A.; Valério, T.S.; Frias, Y.A.; Silva, V.L.; Morais, E.B.; Vasconcelos, L.G.; Tropaldi, L.; Prado, E.P.; Montagnolli, R.N.; et al. Leguminous Plants and Microbial Inoculation: An Approach for Biocatalytic Phytoremediation of Tebuthiuron in Agricultural Soil. Agronomy 2024, 14, 2805. [Google Scholar] [CrossRef]
- Pereira, J.M.; Fernandes, P.M.; Veloso, V.R.S. Physiological effect of the insecticide thiamethoxam on sugarcane. Arq. Inst. Biol. 2010, 77, 159–164. [Google Scholar] [CrossRef]
- Brazil. Brazilian Institute of Environment and Renewable Natural Resources (Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis—IBAMA). Environmental Profile: Thiamethoxam. Brasília, Brazil, 2019. Available online: http://www.ibama.gov.br/phocadownload/agrotoxicos/perfis-ambientais/2019/Perfil%20Ambiental%20-%20Tiametoxam%20-%2002_10_2019.pdf (accessed on 23 August 2025).
- Actara_750_SG_2023; A Registered on Ministry of Agriculture, Livestock and Supply, n. 05313. Syngenta. Syngenta Crop Protection Ltd.: Basel, Switzerland, 2023.
- Scorza Júnior, R.P.; Rigitano, R.L.O. Sorption, degradation and leaching of the insecticide thiamethoxam in two soils of Mato Grosso do Sul. Rev. Bras. Eng. Agríc. Ambient. 2012, 16, 564–572. [Google Scholar] [CrossRef]
- Soto, M.A.; Basso, J.B.; Kiang, C.H. Impact of sugarcane fertigation with vinasse on soil physical, chemical, and hydraulic properties. In Sugarcane and Its Impacts: An Academic Perspective; Canal 6: Bauru, Brazil, 2017; pp. 1–275. [Google Scholar]
- Pinto, L.E.V.; Araujo, F.F. Use of vinasse as biofertilizer: Effect on nodulation, growth, and nutrient accumulation in soybean cultivation (Uso de vinhaça como biofertilizante: Efeito na nodulação, crescimento e acúmulo de nutrientes no cultivo da soja). Colloq. Agrariae 2019, 15, 97–109. [Google Scholar] [CrossRef]
- Brito, L.F.P.; Espíndola, E.L.G.; Ogura, A.P. Tolerance of free-floating aquatic macrophytes to sugarcane vinasse and its implications for phytoremediation strategies. J. Water, Sanit. Hyg. Dev. 2024, 14, 325–331. [Google Scholar]
- Pinto, T.J.S.; Moreira, R.A.; Freitas, J.S.; Silva, L.C.M.; Yoshii, M.P.C.; Lopes, L.F.P.; Ogura, A.P.; Gabriel, G.V.M.; Rosa, L.M.T.; Schiesari, L.; et al. Responses of Chironomus sancticaroli to the simulation of environmental contamination by sugarcane management practices: Water and sediment toxicity. Sci. Total Environ. 2023, 857, 159643. [Google Scholar] [CrossRef]
- Morato, L.F.C.; Ruiz, G.C.M.; Lessa, C.J.A.; Olivier, D.S.; Amaral, M.S.; Gomes, O.P.; Pazin, W.M.; Batagin-Neto, A.; Oliveira JR, O.N.; Constantino, C.J.L. Combined impact of pesticides on mono- and bilayer lipid membranes. Chem. Phys. Lipids 2025, 268, 105474. [Google Scholar] [CrossRef]
- Nantes, L.S.; Aragão, M.B.; Moreira, B.R.A.; Frias, Y.A.; Valerio, T.S.; Lima, E.W.; Viana, R.S.; Lopes, P.R.M. Synergism and antagonism in environmental behavior of tebuthiuron and thiamethoxam in soil with vinasse by natural attenuation. Int. J. Environ. Sci. Technol. 2022, 20, 4883–4892. [Google Scholar] [CrossRef]
- Barroso, G.M.; dos Santos, E.A.; Pires, F.R.; Galon, L.; Cabral, C.M.; dos Santos, J.B. Phytoremediation: A green and low-cost technology to remediate herbicides in the environment. Chemosphere 2023, 334, 138943. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, K.E.; Huang, X.-D.; Glick, B.R.; Greenberg, B.M. Phytoremediation and rhizoremediation of organic soil contaminants: Potential and challenges. Plant Sci. 2009, 176, 20–30. [Google Scholar] [CrossRef]
- Mendes, K.F.; Maset, B.A.; Mielke, K.C.; Sousa, R.N.; Martins, B.A.B.; Tornisielo, V.L. Phytoremediation of quinclorac and tebuthiuron-polluted soil by green manure plants. Int. J. Phytoremediat. 2020, 23, 474–481. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Moreira, B.R.A.; Montagnolli, R.N.; Prado, E.P.; Viana, R.S.; Tomaz, R.S.; Cruz, J.M.; Bidoia, E.D.; Frias, Y.A.; Lopes, P.R.M. Green manure species for phytoremediation of soil with tebuthiuron and vinasse. Front. Bioeng. Biotechnol. 2021, 8, 613642. [Google Scholar] [CrossRef]
- Frias, Y.A.; Moreira, B.R.A.; Prado, E.P.; Valério, T.S.; Vasconcelos, L.G.; Lopes, P.R.M. Pennisetum glaucum in reducing ecotoxicity in soil with tebuthiuron, thiamethoxam and vinasse. J. Agric. Food Res. 2024, 18, 101470. [Google Scholar] [CrossRef]
- Diogo, S.M.; Nunes, J.S.; Matos, C.D.C. Growth of legumes in coexistence with weed. Recital—Rev. Educ., Ciência e Tecnol. Almenara/MG 2023, 5, 117–130. [Google Scholar]
- Pang, Z.; Tayyab, M.; Kong, C.; Liu, Q.; Liu, Y.; Hu, C.; Huang, J.; Weng, P.; Islam, W.; Lin, W.; et al. Continuous sugarcane planting negatively impacts soil microbial community structure, soil fertility, and sugarcane agronomic parameters. Microorganisms 2021, 9, 2008. [Google Scholar] [CrossRef]
- Ding, M.; Dai, H.; He, Y.; Liang, T.; Zhai, Z.; Zhang, S.; Hu, B.; Cai, H.; Dai, B.; Xu, Y.; et al. Continuous cropping system altered soil microbial communities and nutrient cycles. Front. Microbiol. 2024, 15, 1374550. [Google Scholar] [CrossRef]
- Lima, E.W.; Brunaldi, B.P.; Frias, Y.A.; Moreira, B.R.A.; Alves, L.S.; Lopes, P.R.M. A synergistic bacterial pool decomposes tebuthiuron in soil. Sci. Rep. 2022, 12, 9225. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, J.; Kah, M.; Brown, C.D. Adsorption and degradation of four acidic herbicides in soils from southern Spain. Pest Manag. Sci. 2008, 64, 703–710. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.A.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araujo Filho, J.C.; Lima, H.N.; Marques, F.A.; et al. Brazilian Soil Classification System; Embrapa: Brasília, Brazil, 2025. [Google Scholar]
- Pires, F.R.; Procópio, S.O.; Santos, J.B.; Souza, C.M.; Dias, R.R. Evaluation of tebuthiuron phytoremediation using Crotalaria juncea as an indicator plant. Rev. Ciên. Agron. 2008, 39, 245–250. [Google Scholar]
- CETESB—Environmental Company of the State of São Paulo (Companhia Ambiental do Estado de São Paulo). Standard Technique P4.231: Vinasse—Criterions and Procedures for Application to Agricultural Soil (Vinhaça—Critérios e Procedimentos para Aplicação no solo Agrícola), 3rd ed.; CETESB: São Paulo, Brazil, 2015; 15p. [Google Scholar]
- ABNT. Brazilian Association of Norms Techniques. Procedure for Obtaining Solubilized Extract from Solid Waste; ABNT: Rio de Janeiro, Brazil, 2004; NBR 10006. [Google Scholar]
- Sobrero, M.C.; Ronco, A. Acute toxicity test with lettuce seeds (Lactuca sativa L.). In Toxicological Tests and Water Quality Assessment Methods: Standardization; Intercalibration; Results and Applications (Ensayos Toxicológicos y Métodos de Evaluación de Calidad de aguas: Standerización; Intercalibración; Resultados y Aplicaciones); Morales, G.C., Ed.; IMTA: Guadalajara, Mexico, 2004; pp. 71–77. [Google Scholar]
- Pezzini, R.V.; Cargnelutti Filho, A.; De Bem, C.M.; Souza, J.M.; Chaves, G.G.; Neu, I.M.M.; Procedi, A. Gompertz and Logistic models for morphological traits of sudangrass cultivars during sowing seasons. Semina: Ciênc. Agrár. 2019, 40, 3399–3418. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Gu, Z. Complex heatmap visualization. Imeta 2022, 1, e43. [Google Scholar] [CrossRef]
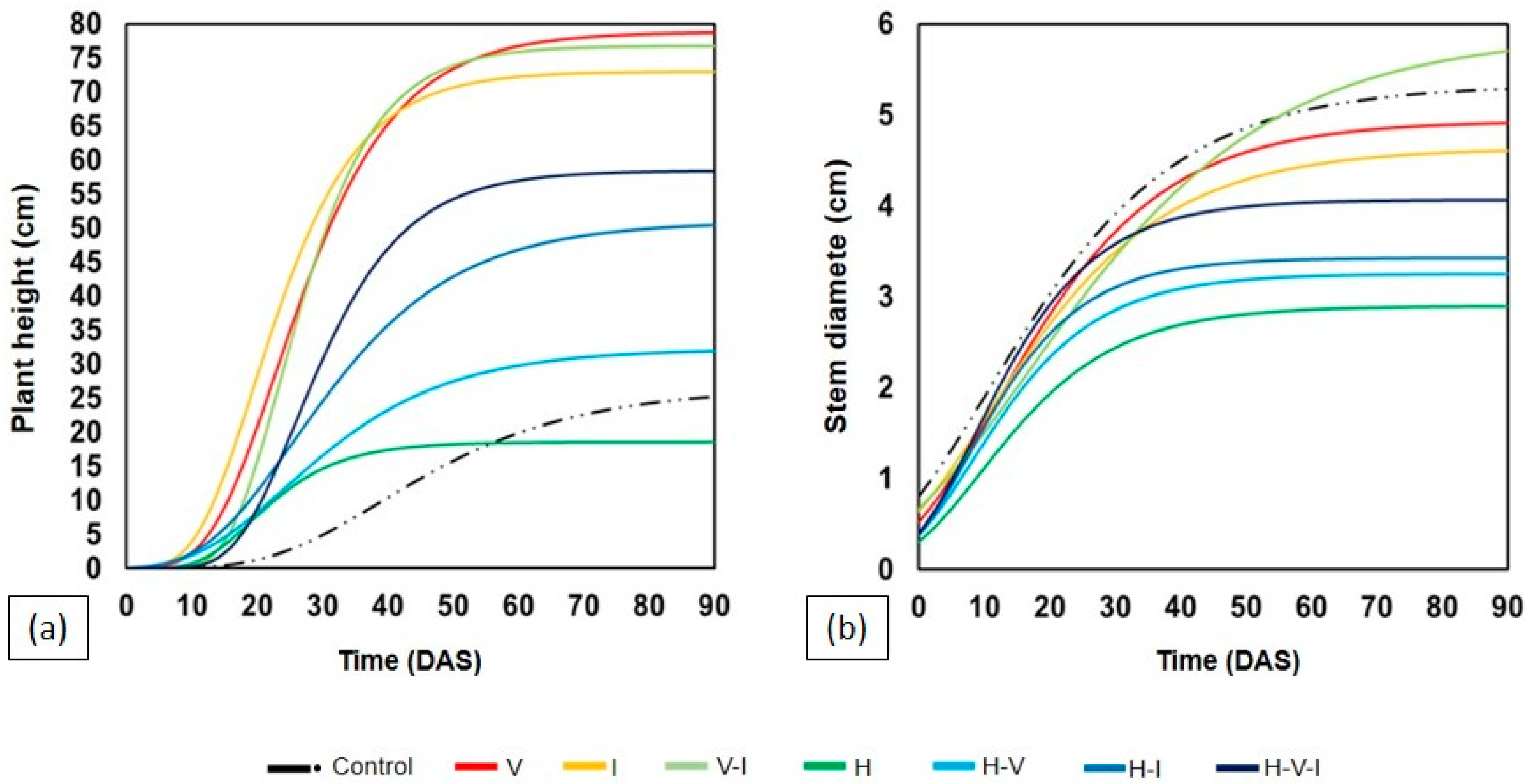
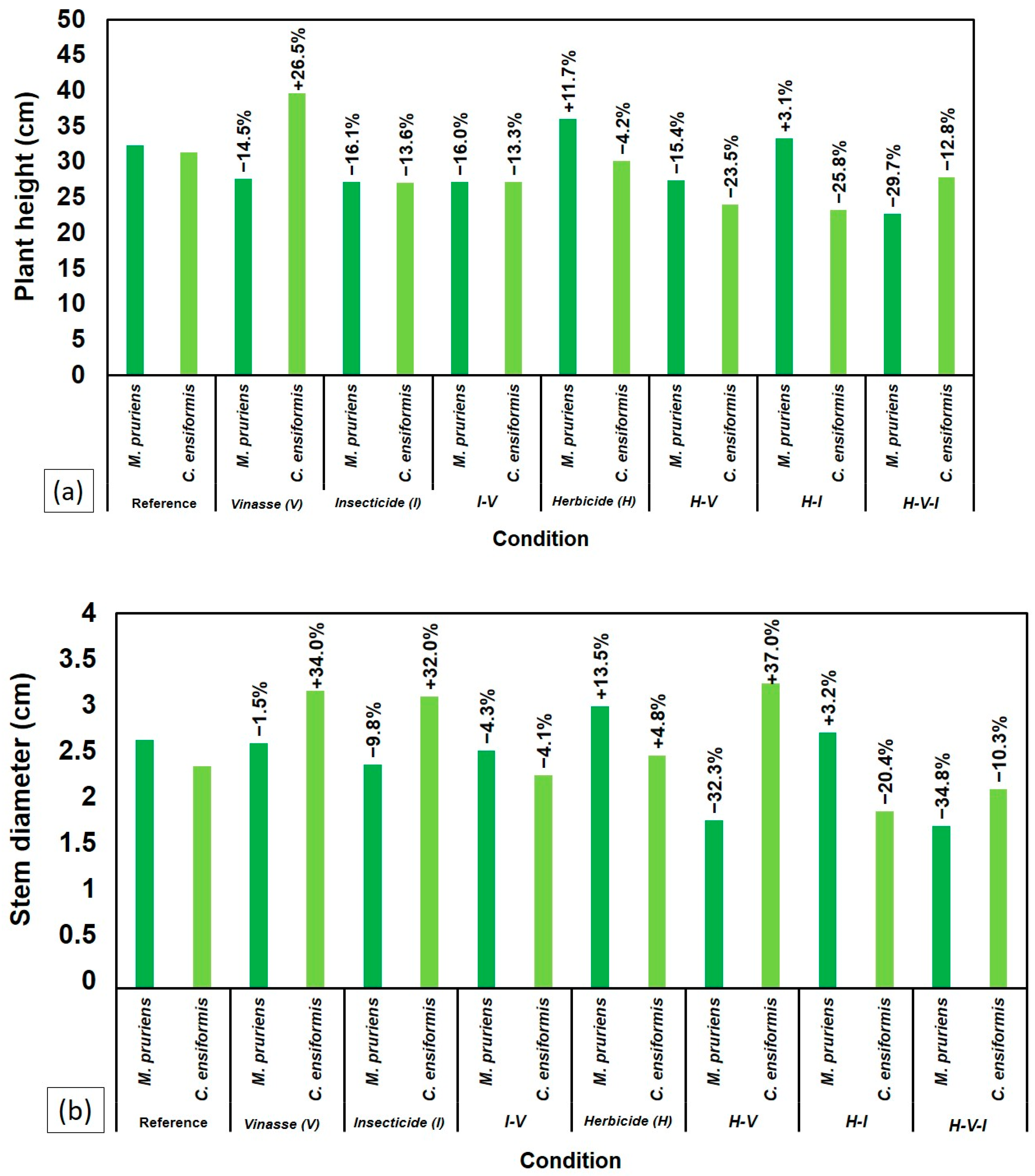
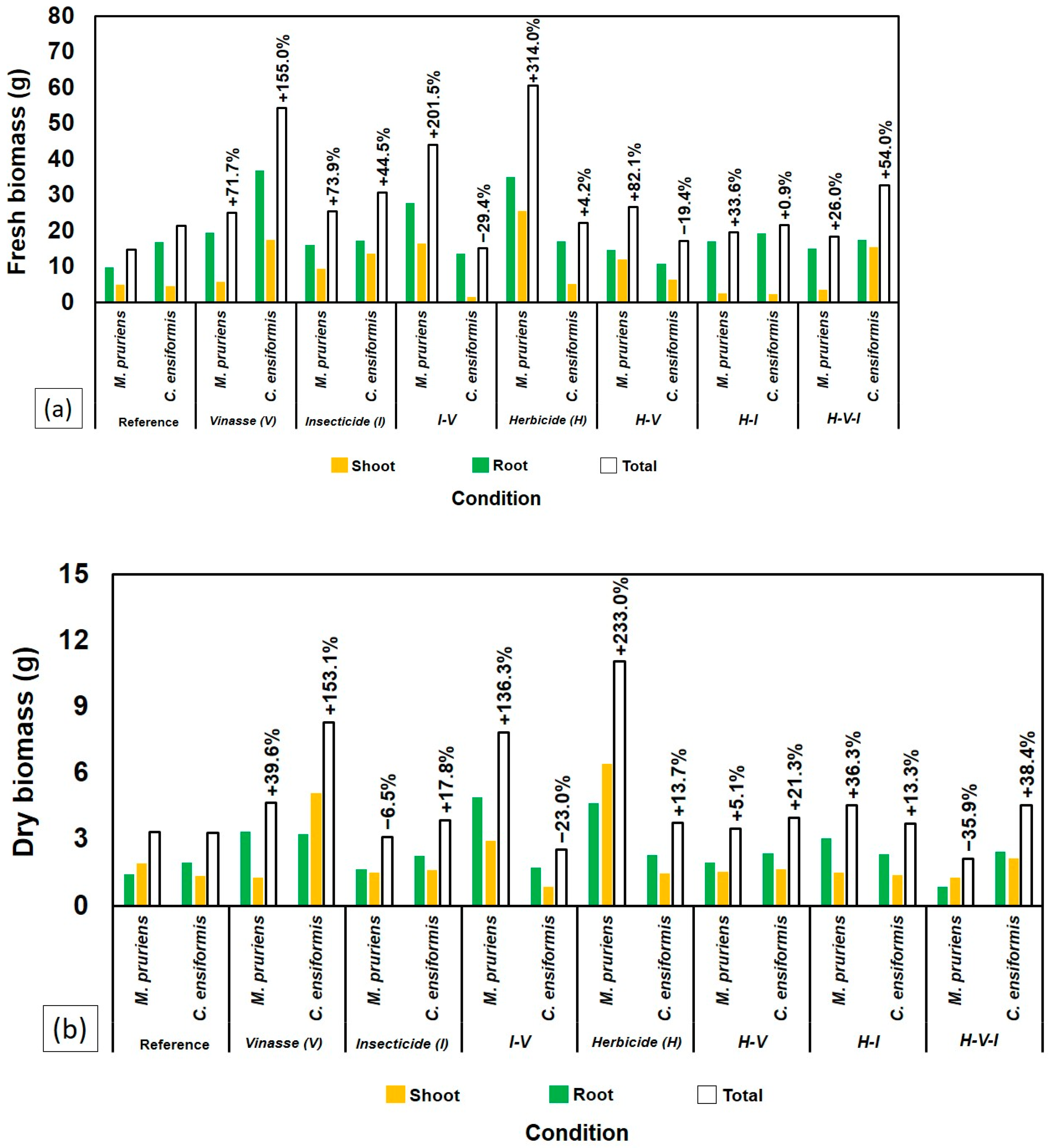
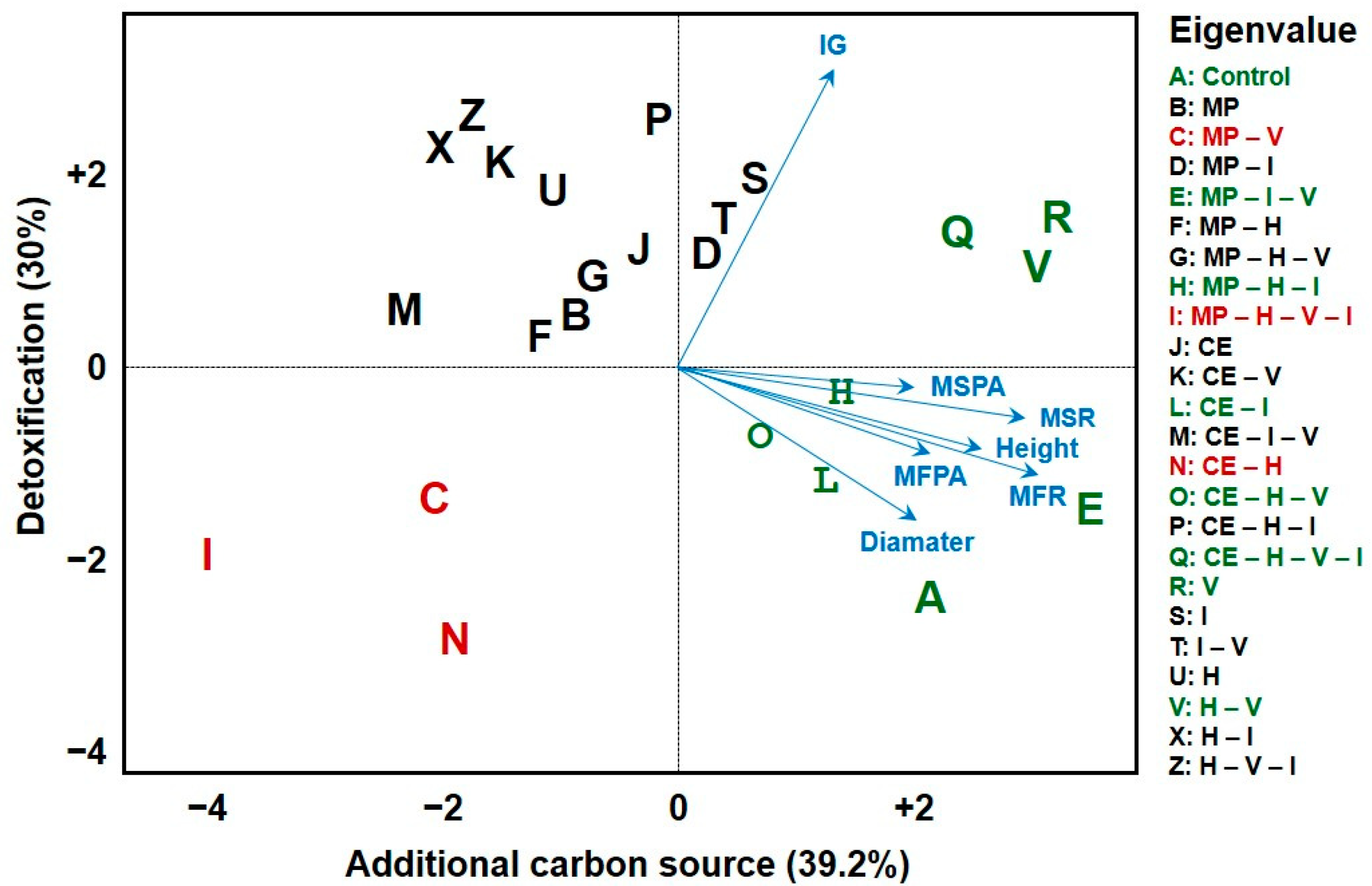
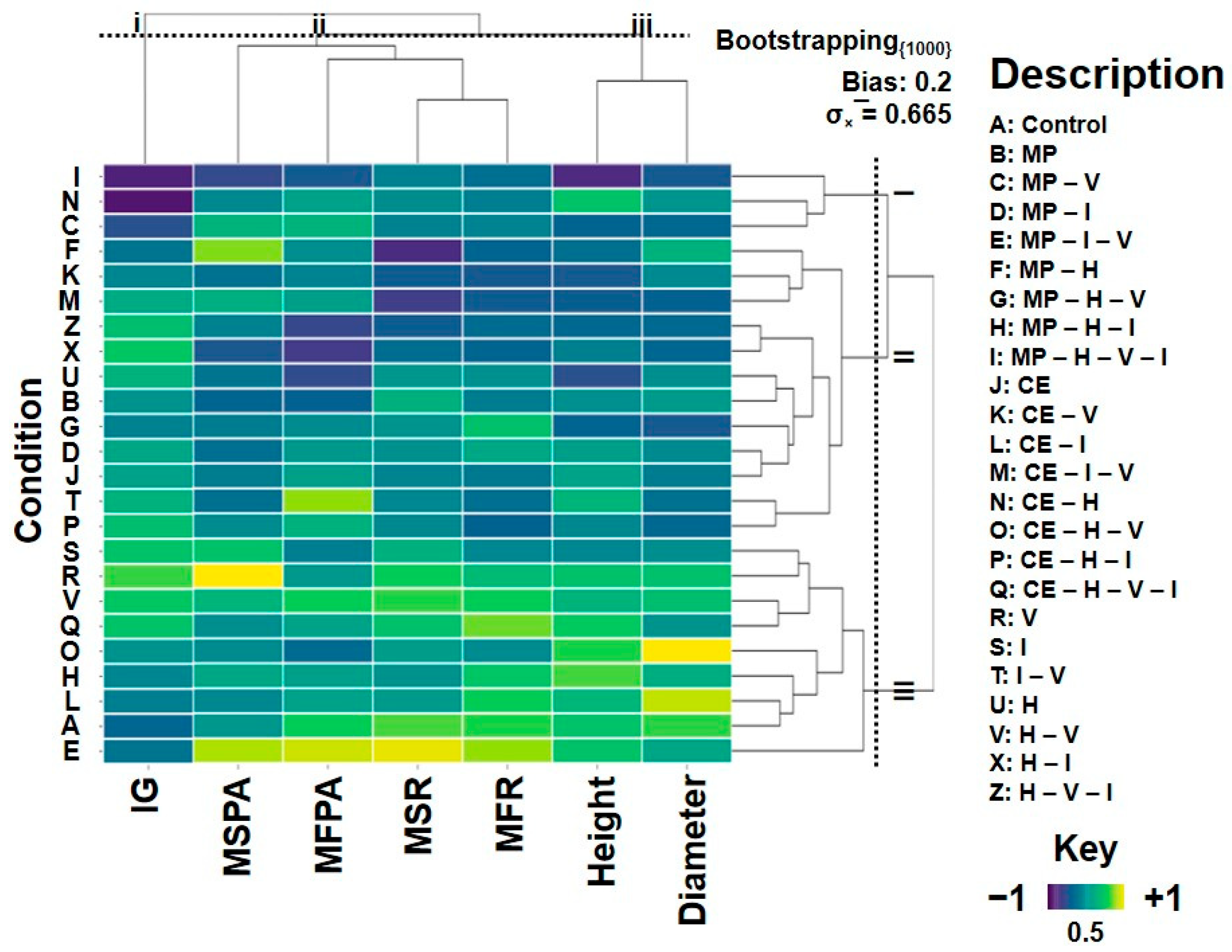
| Treatments | Mucuna pruriens | |||||||
|---|---|---|---|---|---|---|---|---|
| Plant Height (cm) | Stem Diameter (mm) | Fresh Biomass (g) | Dry Biomass (g) | |||||
| Control | 70.75 | a | 5.63 | a | 38.75 | a | 10.88 | a |
| V | 67.60 | a | 4.90 | ab | 38.23 | a | 7.34 | abc |
| I | 67.00 | a | 4.73 | ab | 31.13 | a | 6.44 | abc |
| V–I | 76.25 | a | 5.46 | ab | 43.73 | a | 9.68 | ab |
| H | 23.75 | b | 4.49 | ab | 7.77 | b | 1.75 | c |
| H–V | 40.50 | ab | 3.83 | b | 7.45 | b | 5.53 | abc |
| H–I | 68.33 | a | 4.39 | ab | 22.86 | ab | 5.02 | abc |
| H–V–I | 63.50 | ab | 5.46 | ab | 23.97 | ab | 3.70 | bc |
| Treatments | Canavalia ensiformis | |||||||
|---|---|---|---|---|---|---|---|---|
| Plant Height (cm) | Stem Diameter (mm) | Fresh Biomass (g) | Dry Biomass (g) | |||||
| Control | 43.00 | 5.61 | ab | 46.08 | a | 11.68 | ab | |
| V | 27.33 | 6.14 | ab | 11.54 | b | 2.66 | b | |
| I | 39.00 | 5.34 | b | 32.89 | ab | 8.76 | ab | |
| V–I | 41.00 | 7.59 | a | 50.37 | a | 14.75 | a | |
| H | ND | ND | ND | ND | ||||
| H–V | ND | ND | ND | ND | ||||
| H–I | ND | ND | ND | ND | ||||
| H–V–I | 27.50 | 5.80 | ab | 35.55 | ab | 7.03 | ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragão, M.B.; Ribeiro, E.R.; Frias, Y.A.; Cruz, V.H.; Valério, T.S.; Batista, A.R.; Ferreira, P.H.F.; Simionatto, H.H.; Lopes, P.R.M. Evaluation of Leguminous Plants as Phytoremediator Species in Soil with Pesticide and Vinasse Interactions. Plants 2025, 14, 3137. https://doi.org/10.3390/plants14203137
Aragão MB, Ribeiro ER, Frias YA, Cruz VH, Valério TS, Batista AR, Ferreira PHF, Simionatto HH, Lopes PRM. Evaluation of Leguminous Plants as Phytoremediator Species in Soil with Pesticide and Vinasse Interactions. Plants. 2025; 14(20):3137. https://doi.org/10.3390/plants14203137
Chicago/Turabian StyleAragão, Munick Beato, Emanuella Roberto Ribeiro, Yanca Araujo Frias, Victor Hugo Cruz, Thalia Silva Valério, Alexandre Ribeiro Batista, Paulo Henrique Frata Ferreira, Henzo Henrique Simionatto, and Paulo Renato Matos Lopes. 2025. "Evaluation of Leguminous Plants as Phytoremediator Species in Soil with Pesticide and Vinasse Interactions" Plants 14, no. 20: 3137. https://doi.org/10.3390/plants14203137
APA StyleAragão, M. B., Ribeiro, E. R., Frias, Y. A., Cruz, V. H., Valério, T. S., Batista, A. R., Ferreira, P. H. F., Simionatto, H. H., & Lopes, P. R. M. (2025). Evaluation of Leguminous Plants as Phytoremediator Species in Soil with Pesticide and Vinasse Interactions. Plants, 14(20), 3137. https://doi.org/10.3390/plants14203137







