Abstract
Deodorants and antiperspirants available on the market are designed to reduce the discomfort associated with sweating. This study examined the types of active substances contained in deodorants and antiperspirants from international cosmetic brands available in Poland (part of the EU market) and the frequency of their use. Product compositions were analysed based on INCI (International Nomenclature of Cosmetic Ingredients) product labels. The investigation included the following 170 cosmetic products: 50 spray deodorants (from 50 different brands); 50 roll-on deodorants (from 50 brands); 20 stick deodorants (from 20 brands); 40 roll-on antiperspirants (from 40 brands); and 10 stick antiperspirants (from 10 brands). The most popular active components were Triethyl Citrate (51/120 formulations; 42.5%), followed by Alcohol (25.8%), Ethylhexylglycerin (25.0%), Caprylyl Glycol (12.5%), and Potassium Alum (10.0%). Antiperspirant products were dominated by aluminium-based compounds, with the most frequently used being the following aluminium-based salts: Aluminium Chlorohydrate (67.5%), Aluminium Sesquichlorohydrate (25.0%), and Aluminium Chloride (12.5%). In contrast, aluminium–zirconium complexes, such as Aluminum Zirconium Tri-, Penta-, and Octachlorohydrex Gly, were rarely used by cosmetic manufacturers. Additionally, composition complexity, i.e., the number of deodorizing and anti-sweating ingredients per single formulation, was examined for roll-on deodorants, stick deodorants, and roll-on antiperspirants. All tested antiperspirants and most deodorants contained fragrance-imparting ingredients; the most popular were Parfum/Fragrance, Limonene, Linalool, Citronellol, Citral, Benzyl Salicylate, Hexyl Cinnamal, and Geraniol.
Keywords:
antiperspirants; cosmetic ingredients; deodorants; frequency of use; market trends; odour; sweating 1. Introduction
Although sweating is a natural physiological thermoregulatory function, it has the undesirable effect of an unpleasant body odour that causes discomfort. Therefore, deodorants are used to counteract the odour caused by the bacterial decomposition of skin secretions in the armpits, feet, and other areas of the body and to increase the pleasantness of skin odour. Antiperspirants, on the other hand, prevent sweating by blocking the sweat glands [1,2].
Deodorants act on the effects of sweat secretion, but do not influence its composition or production. Depending on the active ingredients used, they work via different mechanisms. Deodorants work by inhibiting the growth of odour-causing bacteria, masking unpleasant odours, inhibiting the processes that lead to the formation of volatile compounds with an unpleasant odour, or by binding/absorbing such ingredients. Therefore, they act on the effects of sweat secretion, but do not influence its amount. Examples of deodorizing substances include Carylyl Glycol, Alcohol, C12-15 Alkyl Benzoate, and Zinc Ricinoleate [2,3,4]. The effect of deodorants can be enhanced by lowering the pH of products using weak acids, such as Lactic acid and Citric Acid, and antioxidants, such as BHT and Tocopheryl Acetate [5].
Antiperspirants reduce sweat secretion by creating a barrier on the skin surface. When sweat is not released from the sweat glands, its components do not undergo bacterial decay and, consequently, no unpleasant odour is produced. Most use aluminium-based compounds, such as Aluminium Chlorohydrate (ACH), Aluminium Sesquichlorohydrate, Aluminium Zirconium Tetrachlorohydrex Gly, and Potassium Alum, as active compounds. After an antiperspirant is applied to the underarm area, its component aluminium compounds polymerize under the influence of physiological pH, forming a gel which seals the openings of the sweat glands on the skin surface. This blockade is not permanent and disappears when the area is washed thoroughly with a cleanser, after very heavy sweating, or as a result of the keratinization process [2,3,4,6,7].
The market for deodorants and antiperspirants is very extensive, and products are sold in a variety of forms and applicators; most are roll-ons, sticks, or aerosols, with others being sold as creams, gels, powders, and pastes. Some contain additional moisturizing, soothing and anti-inflammatory compounds, such as pantenol, allantoin, hyaluronic acid, aloe barbadensis leaf extract, salvia hispanica, and seed oil, which provide more comprehensive care for the skin [4]. Almost all forms of deodorants and antiperspirants contain fragrance compositions of natural or synthetic origin to mask unpleasant odours. Fragrance components derived from essential oils, e.g., limonene, linalool, citronellol, geraniol, and cinnamyl alcohol, exhibit a range of other biological properties desirable for deodorants and antiperspirants, including antibacterial, antifungal, and anti-inflammatory effects [8,9].
In accordance with European Parliament and European Community (EC) Council Regulation No. 1223/2009, aluminium-zirconium chloride hydroxide complexes, AlxZr(OH)yClz, and aluminium zirconium chloride hydroxide glycine complexes may be used in “ready-to-use” antiperspirants at a maximum concentration of 20% as anhydrous aluminium zirconium chloride hydroxide or 5.4% as zirconium. Aluminium–zirconium compounds are also subject to the following restrictions: “The ratio of the number of aluminium atoms to that of zirconium atoms must be between 2 and 10. The ratio of the number of (Al + Zr) atoms to that of chlorine atoms must be between 0.9 and 2.1”; in addition, they should not be applied in aerosol dispensers or used on irritated or damaged skin [10].
There has been much controversy surrounding the safety of aluminium-based compounds in antiperspirants, which are alleged to cause breast cancer [11,12] and neurodegenerative diseases [13,14,15]. Although circumstantial evidence has linked aluminium to neurodegenerative diseases, no causal link has been proven. Similarly, there have been no published epidemiological studies that would support the claim that antiperspirants cause breast cancer [16,17]. The SCCS concludes that carcinogenicity is unlikely at the levels of aluminium exposure achieved through the use of cosmetics. Aluminium compounds’ ability to penetrate the epidermal layer is negligible, as it is only about 0.00052%. The SCCS considers aluminium compounds as safe when used at the following equivalent aluminium concentrations: up to 6.25% (in non-spray deodorants or non-spray antiperspirants) and up to 10.60% (in spray deodorants or spray antiperspirants) [18].
The first objective of this work is to take a snapshot of the current situation on the deodorant and antiperspirant market in terms of the use of active substances in these products. Studies such as this one repeated at certain time intervals can indicate trends in the development of the industry. There are widespread beliefs in the literature about the wide use of certain active substances, e.g., Triclosan, in products available on the market. These beliefs are not based on market reviews, but only on the intuition of authors.
Therefore, the second, more important goal of this survey is to check which of the legally permitted active substances are commonly used, which are sporadic, and which do not appear at all in preparations available on the market. This is to make it easier for researchers planning their research to choose the area on which to focus or the substances that will be points of reference for their research. The author also hopes that this work will influence the better selection of examples when teaching students of cosmetology.
2. Results
A total of 170 deodorants and antiperspirants were analysed to identify the type and frequency of the use of active ingredients, viz. odour-masking, odour-absorbing, antibacterial, and sweat-inhibiting compounds, and record their number per single product. In addition, the study examined the type and frequency of fragrances used for spray deodorants and stick antiperspirants.
2.1. Composition of Spray Deodorants (n = 50)
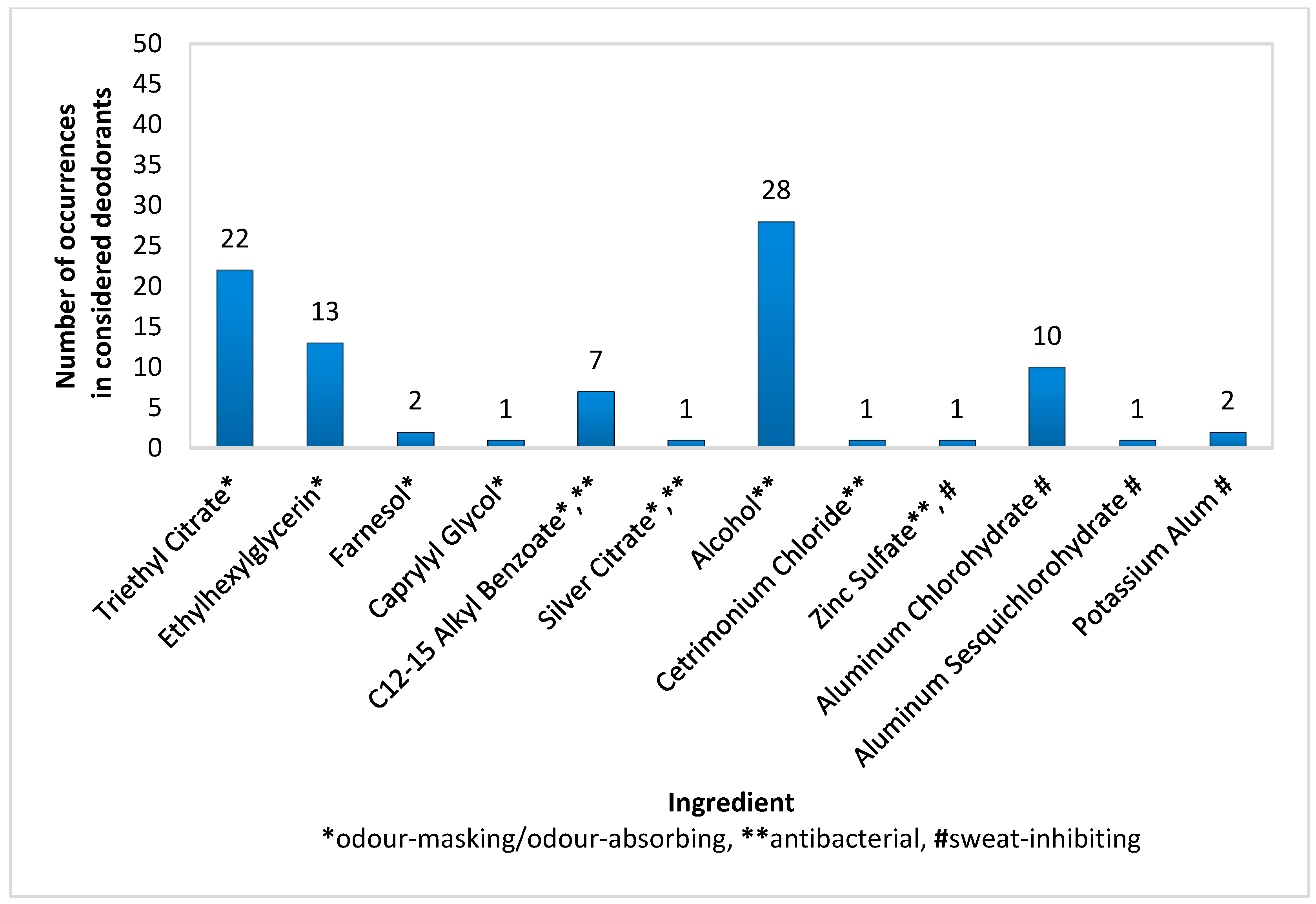
In spray deodorants (n = 50), the most common antibacterial ingredient, present in 56% of products, was Alcohol. The most common deodorant components used for masking or absorbing odour were Triethyl Citrate (44%) and Ethylhexylglycerin (26%), while Aluminium Chlorohydrate (20%) was the most commonly used for inhibiting sweat secretion (Figure 1). A relatively popular antibacterial and deodorizing component was C12-15 Alkyl Benzoate (14%). The remaining active substances, i.e., Farnesol, Caprylyl Glycol, Silver Citrate, Zinc Sulfate, Cetrimonium Chloride, Aluminium Sesquichlorohydrate, and Potassium Alum, were found in only one or two products (Figure 1). Interestingly, the examined spray deodorants also contained typical antiperspirant ingredients, such as Aluminium Chlorohydrate (20%), Aluminium Sesquichlorohydrate (2%), and Potassium Alum (4%); however, their concentrations were so low that their role was most likely to increase the bacteriostatic properties of the product.
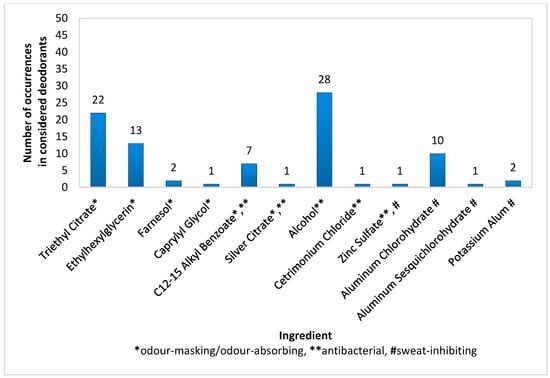
Figure 1.
Frequency of use of active ingredients in spray deodorants (n = 50).
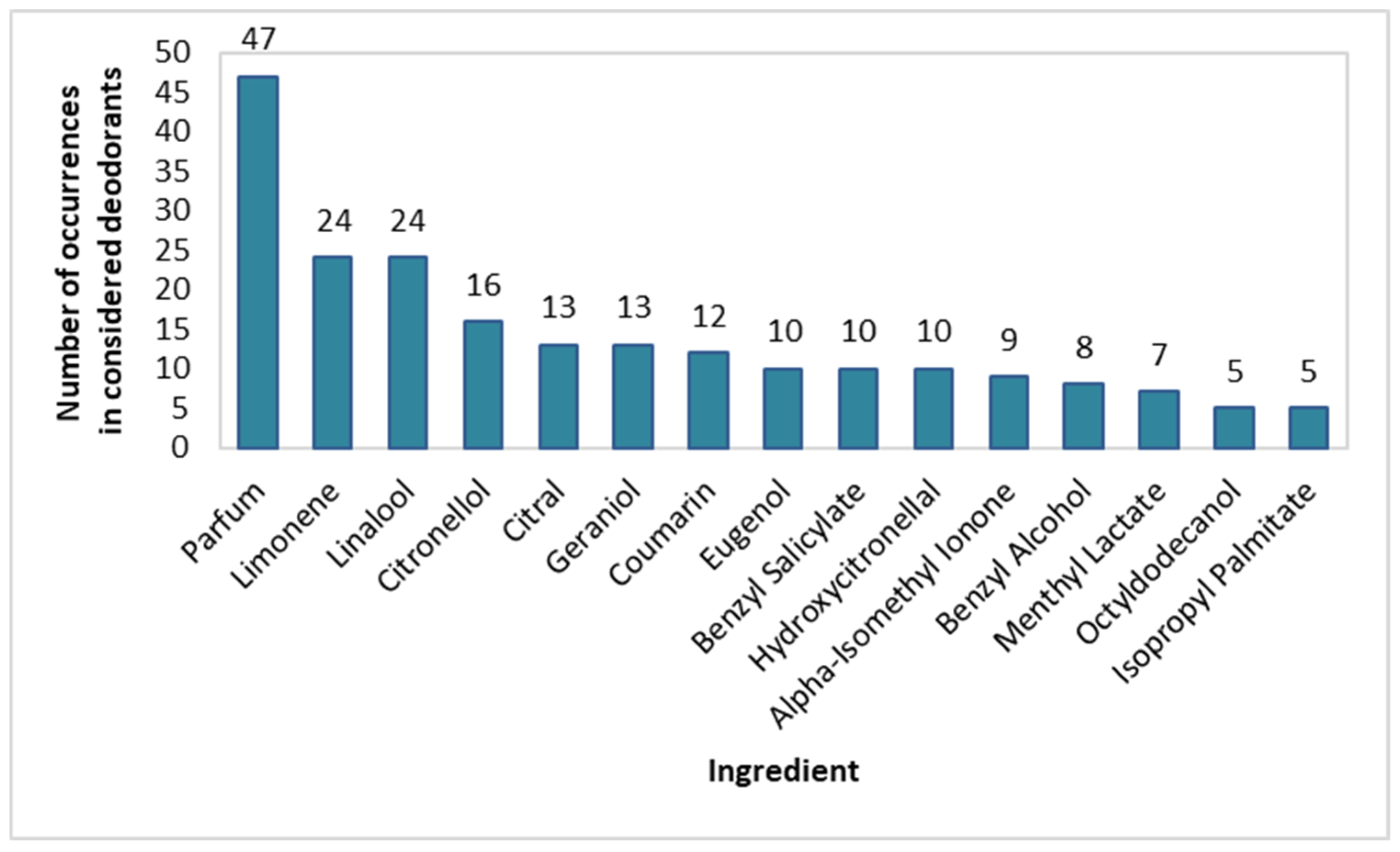
In spray deodorants, the type and frequency of use of fragrance-giving substances were also determined. All substances with a content greater than or equal to 10% are presented in Figure 2. The most popular fragrances, apart from Parfum (94% of products), were Limonene (48%), Linalool (48%), Citronellol (32%), Citral (26%), Geraniol (26%), and Coumarin (24%). Fragrances such as Eugenol, Benzyl Salicylate, Hydroxycitronellal, Alpha-Isomethyl Ionone, Benzyl Alcohol, Menthyl Lactate, Octyldodecanol, and Isopropyl Palmitate were present in 10–20% of the investigated spray deodorants.
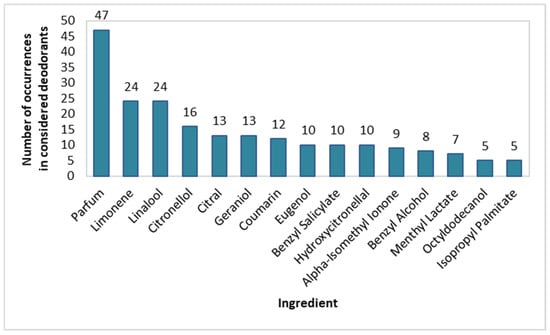
Figure 2.
Frequency of use of fragrance-impairing ingredients in spray deodorants (n = 50).
The following fragrance-giving ingredients were found in fewer than five products: Dimethyl Phenylbutanol, Dimethyl Phenylpropanol, Citronellyl Methylcrotonate, Cinnamyl Alcohol, Cocos Nucifera Oil/Coconut Oil, T-Butyl Alcohol, Levulinic Acid, Vanillyl Butyl Ether, Benzyl Benzoate, Ammonium Glycyrrhizate, and Isopropyl Myristate.
The following fragrant plant extracts and essential oils, which also have antibacterial and astringent effects on the skin, were found in the examined spray deodorants: Myristica Fragrans Kernel Oil, Helianthus Annuus Seed Oil, Calendula Officinalis Flower Extract, Rosa Damascena Extract, Salvia Officinalis Oil; Salvia Officinalis Leaf Extract, Elettaria Cardamomum Seed Extract, Rosmarinus Officinalis Leaf Extract, Rosa Moschate Leaf Extract and Equisetum Arvense Extract. These plant extracts and essential oils were found in approximately one-quarter of the analysed spray deodorants, and often several of them were found in the same single product.
2.2. Composition of Roll-On Deodorants (n = 50)
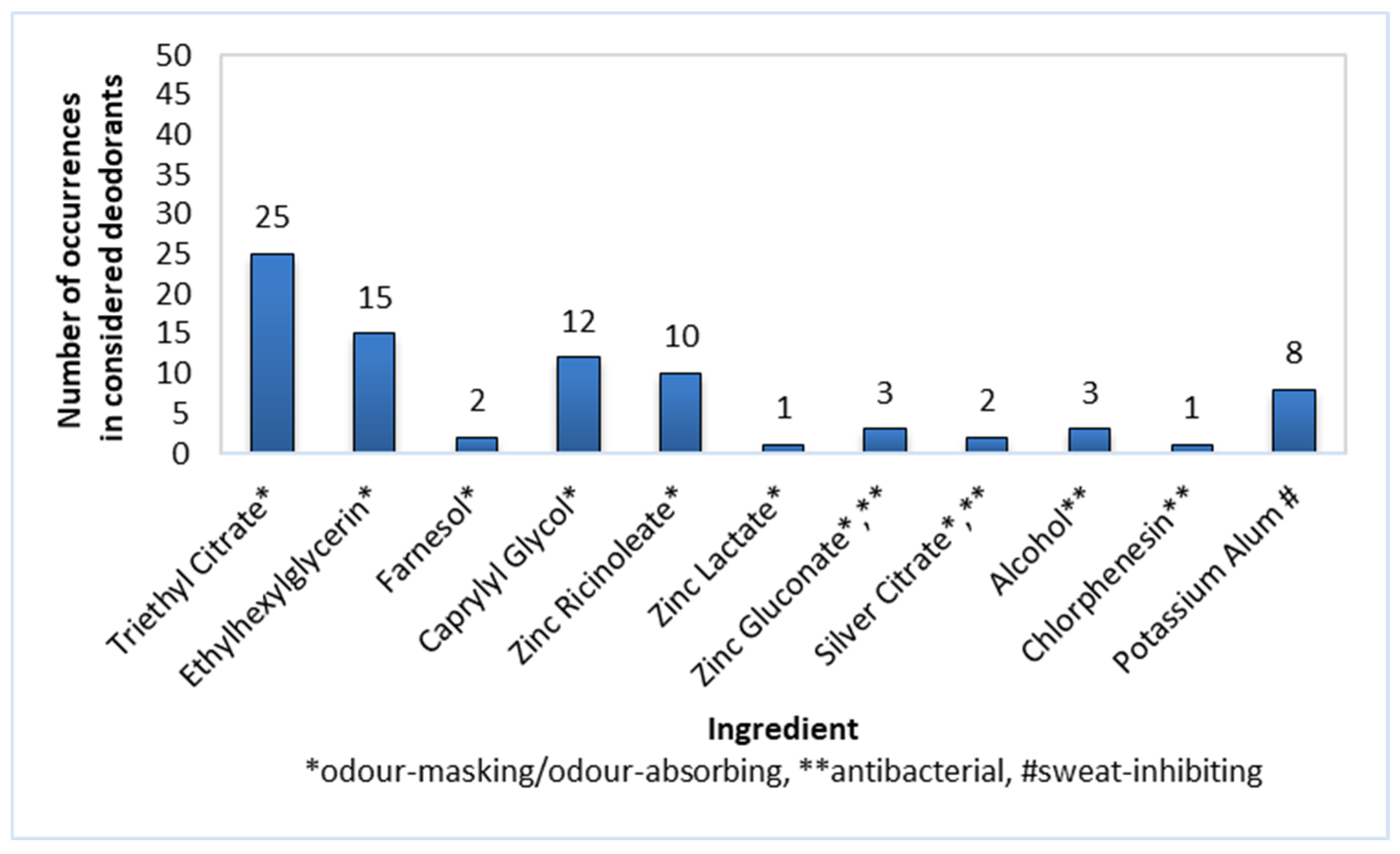
The masking and odour-absorbing substances found to be present in the composition of roll-on deodorants (n = 50) included Triethyl Citrate (50% of products), Ethylhexylglycerin (30%), Caprylyl Glycol (24%), and Zinc Ricinoleate (20%; Figure 3). Less popular compounds with the above-mentioned functions, namely Farnesol, Zinc Lactate, Zinc Gluconate, and Silver Citrate, were noted in 6% or less of the products.
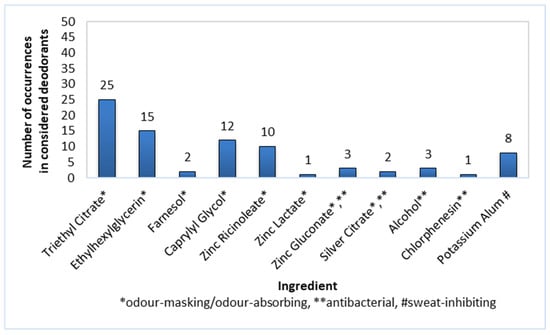
Figure 3.
Frequency of use of active ingredients in roll-on deodorants (n = 50).
Antibacterial ingredients such as Zinc Gluconate, Silver Citrate, Alcohol, and Chlophenesin were not commonly used in roll-on deodorants—their prevalence was 6% or less among the considered preparations.
Potassium Alum was used as a sweat-inhibiting component and was present in 16% of the roll-on deodorants.
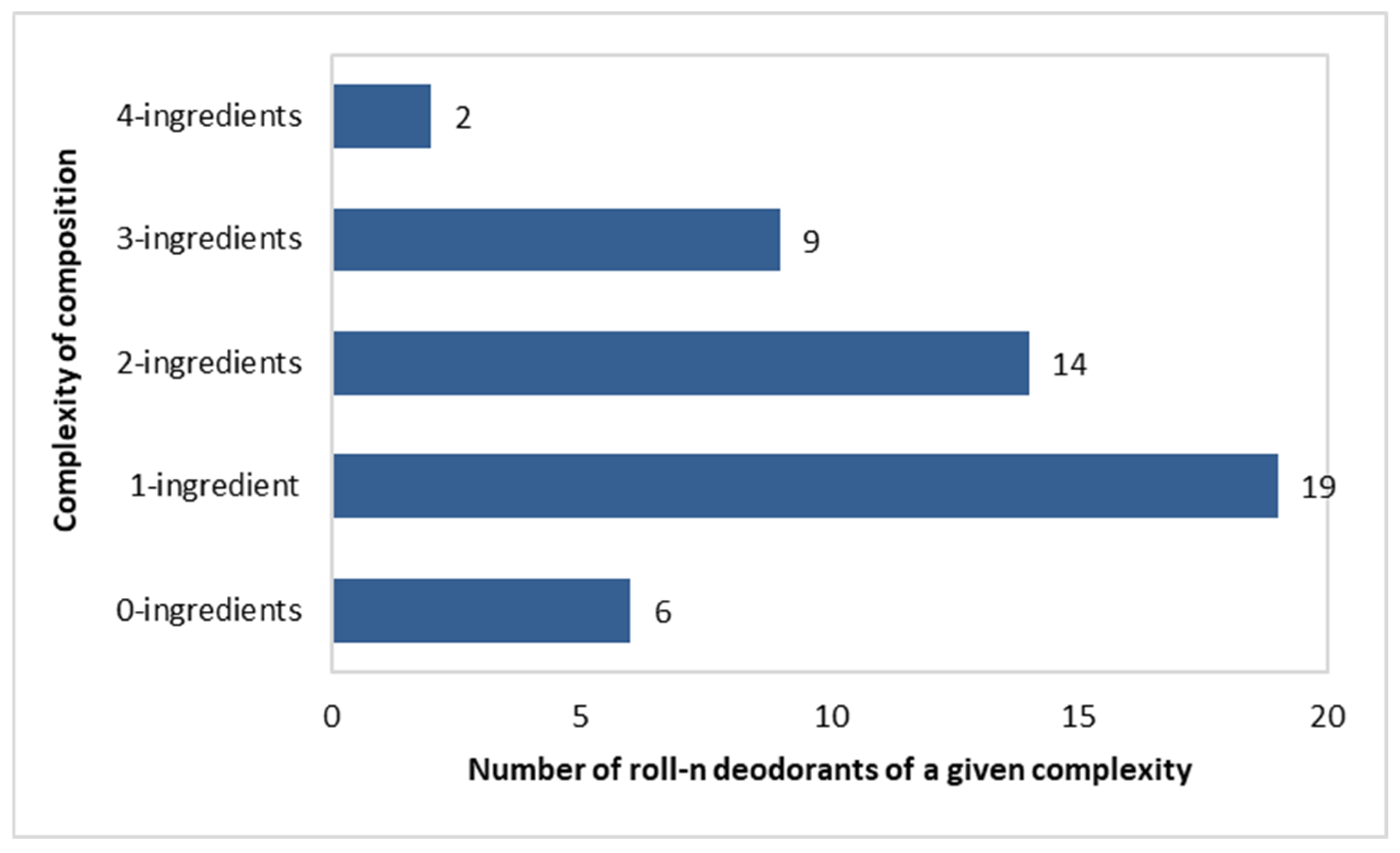
Complexity of Composition of the Investigated Roll-On Deodorants
Composition complexity was calculated as the number of deodorizing and anti-sweating ingredients present in a single formulation. The analysis only included components with these functions.
As many as 6 out of 50 roll-on deodorants did not contain any of the active ingredients listed in Figure 3. In addition, 19 out of 50 deodorants included a single component, as follows: Potassium Alum (in 4 deodorants); Zinc Ricinolate (in 1); Caprylyl Glycol (in 3); Ethylhexylglycerin (in 4); Zinc Gluconate (in 2); and Triethyl Citrate (in 5). Subsequently, fourteen preparations had two components, nine had three components, and two had four components. The following pairs of active ingredients were found: (Triethyl Citrate + Zinc Ricinolate)—in five products; (Triethyl Citrate + Ethylhexylglycerin)—in three products; (Ethylhexylglycerin + Caprylyl Glycol)—in three products; (Triethyl Citrate + Alcokol)—in one product; (Triethyl Citrate + Zinc Lactate)—in one product; and (Potassium Alum + Zinc Ricinolate)—in one product (Figure 4).
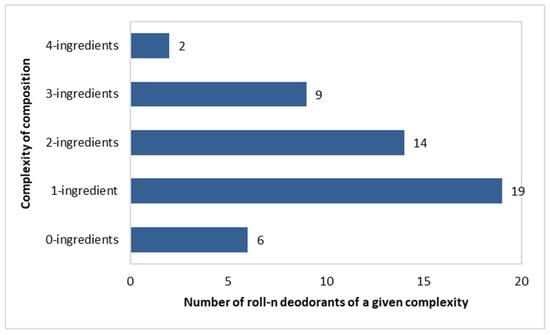
Figure 4.
Number of roll-on deodorants (n = 50) with specific numbers of active ingredients per single product.
2.3. Composition of Stick Deodorants (n = 20)
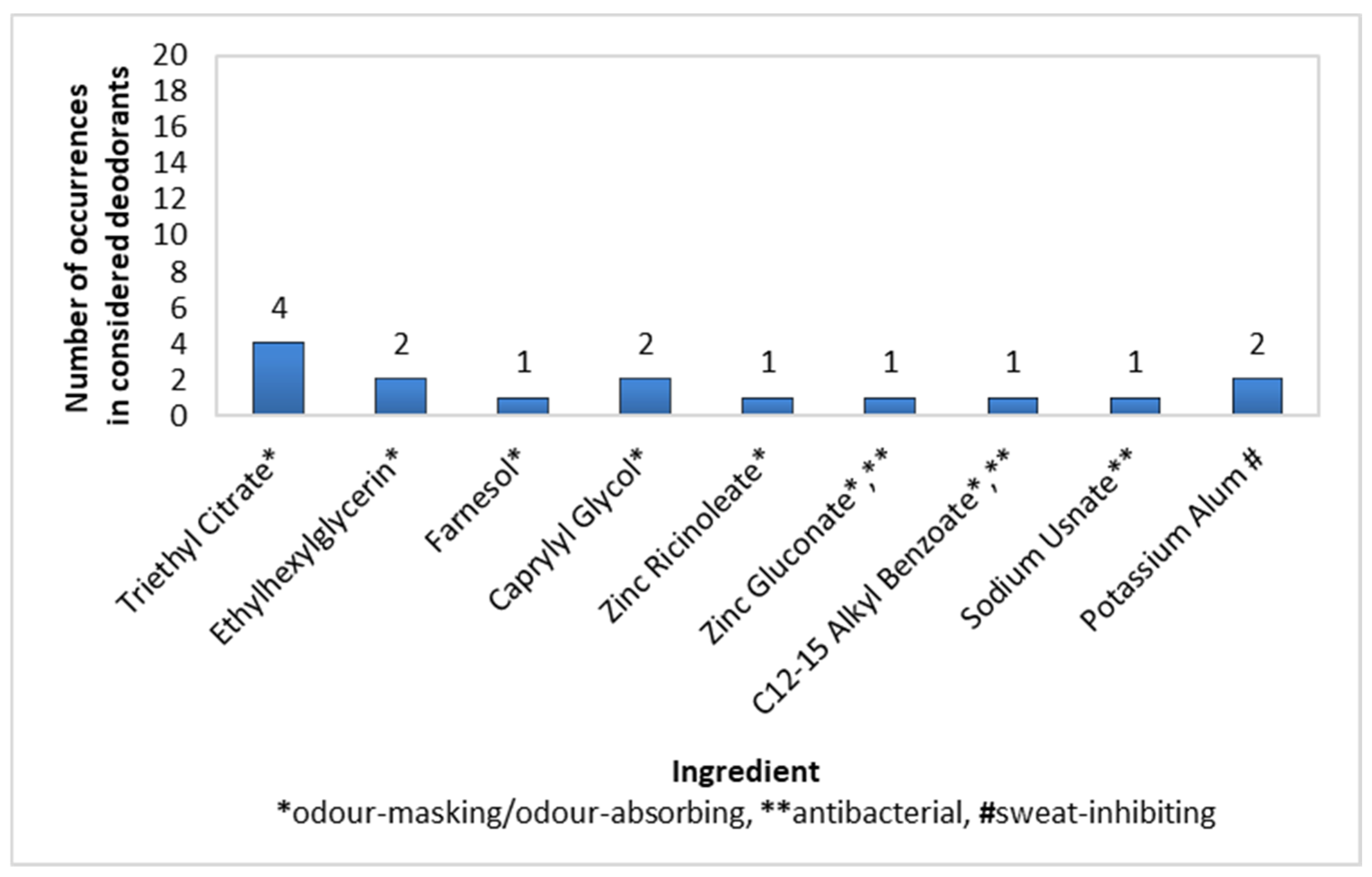
The prevalence of active ingredients in stick deodorants was rather poor. The largest share was demonstrated by Triethyl Citrate (20% of preparations), followed by Ehtylhexylglycerin, Caprylyl Glycol, and Potassium Alum, each present in 10% of deodorants, and then Farnesol, Zinc Ricinolate, Zinc Gluconate, C12-15 Alkyl Benzoate, and Sodium Usnate, each present in 5% of the considered products (Figure 5).
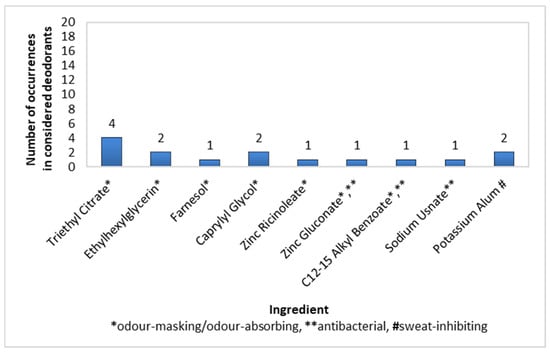
Figure 5.
Frequency of use of active ingredients in stick deodorants (n = 20).
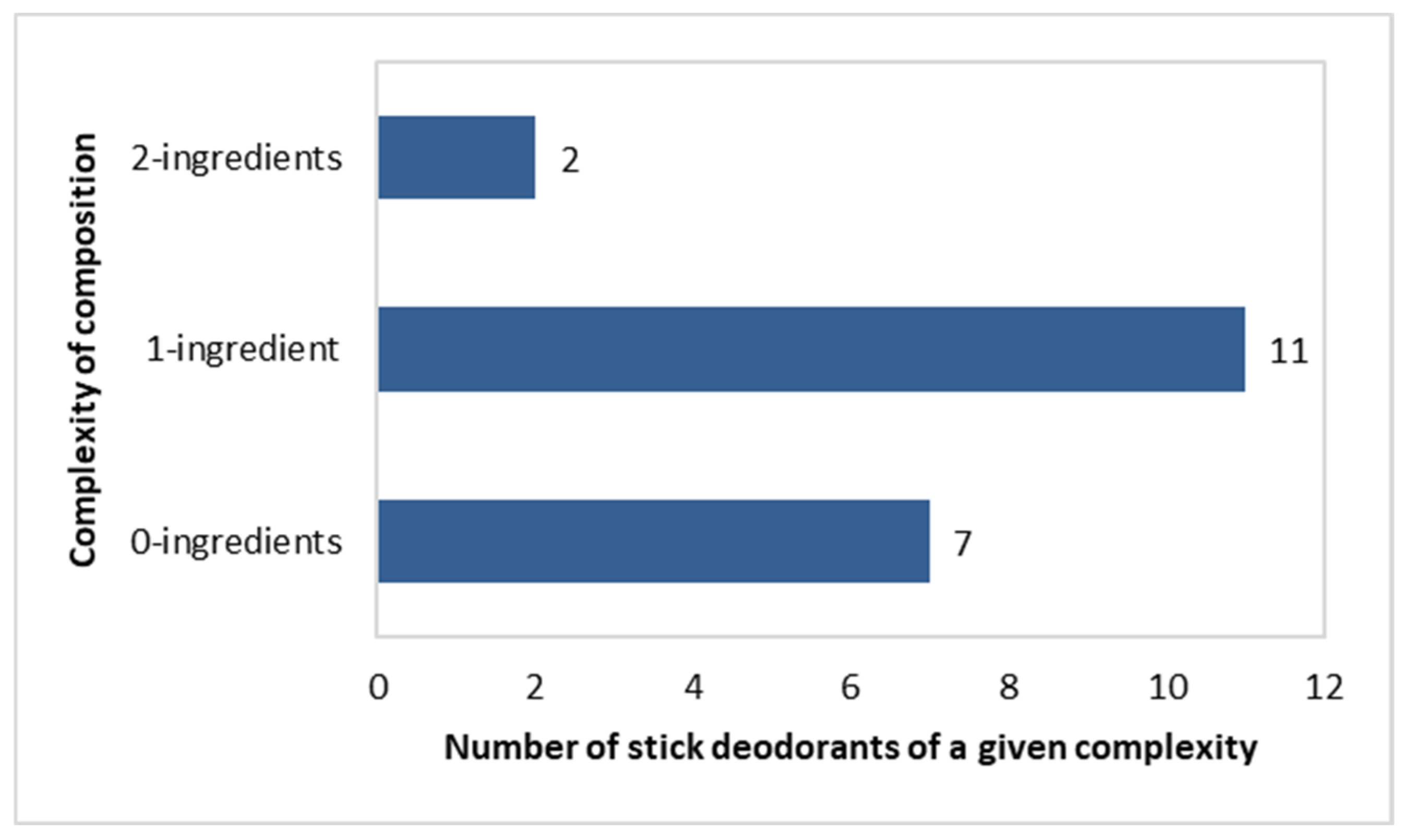
Complexity of Composition of the Stick Deodorants
Most preparations in the stick deodorants were of a “one-component” complexity (n = 11), as follows: Triethyl Citrate (three products), Potassium Alum (two products), Caprylyl Glycol (one product), Ethylhexylglycerin (one product), Zinc Gluconate (one product), Farnesol (one product), C12-15 Alkyl Benzoate (one product), and Sodium Usnate (one product). In total, 7 out of 20 deodorants did not contain any of the active ingredients shown in Figure 5. In turn, two of the “two-component” formulations contained the following pairs of components: (Triethyl Citrate + Zinc Ricinolate) and (Ethylhexylglycerin + Caprylyl Glycol) (Figure 6).
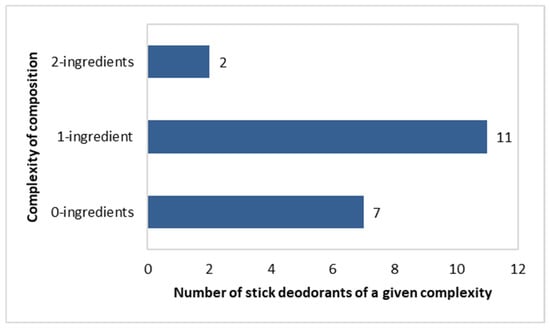
Figure 6.
Number of stick deodorants (n = 20) with specific numbers of active ingredients per single product.
The number of occurrences of active substances according to the form of the deodorant (viz. spray, roll-on, and stick) and their total quantities (incl. %) are summarized in Table 1. The most frequently used component was the scent-giving “parfum”, found in 81.7% of products. Many deodorants also contained natural fragrance-giving ingredients of the essential oils specified in their composition, e.g., Limonene, Linalool, Citronellol, Citral, and Geraniol.

Table 1.
Number of occurrences of active substances according to individual forms of deodorants.
The most popular substance was Triethyl Citrate, present in 51/120 (42.5%) formulations, followed by Alcohol, in 31 (25.8%), Ethylhexylglycerin in 30 (25.0%), Caprylyl Glycol in 15 (12.5%), and Potassium Alum in 12 (10.0%). The remaining ingredients were only present in 10% or less of products, e.g., Zinc Ricinoleate (9.2%), Aluminum Chlorohydrate (8.3%), and C12-15 Alkyl Benzoate (6.7%).
2.4. Composition of Stick Antiperspirants (n = 10)
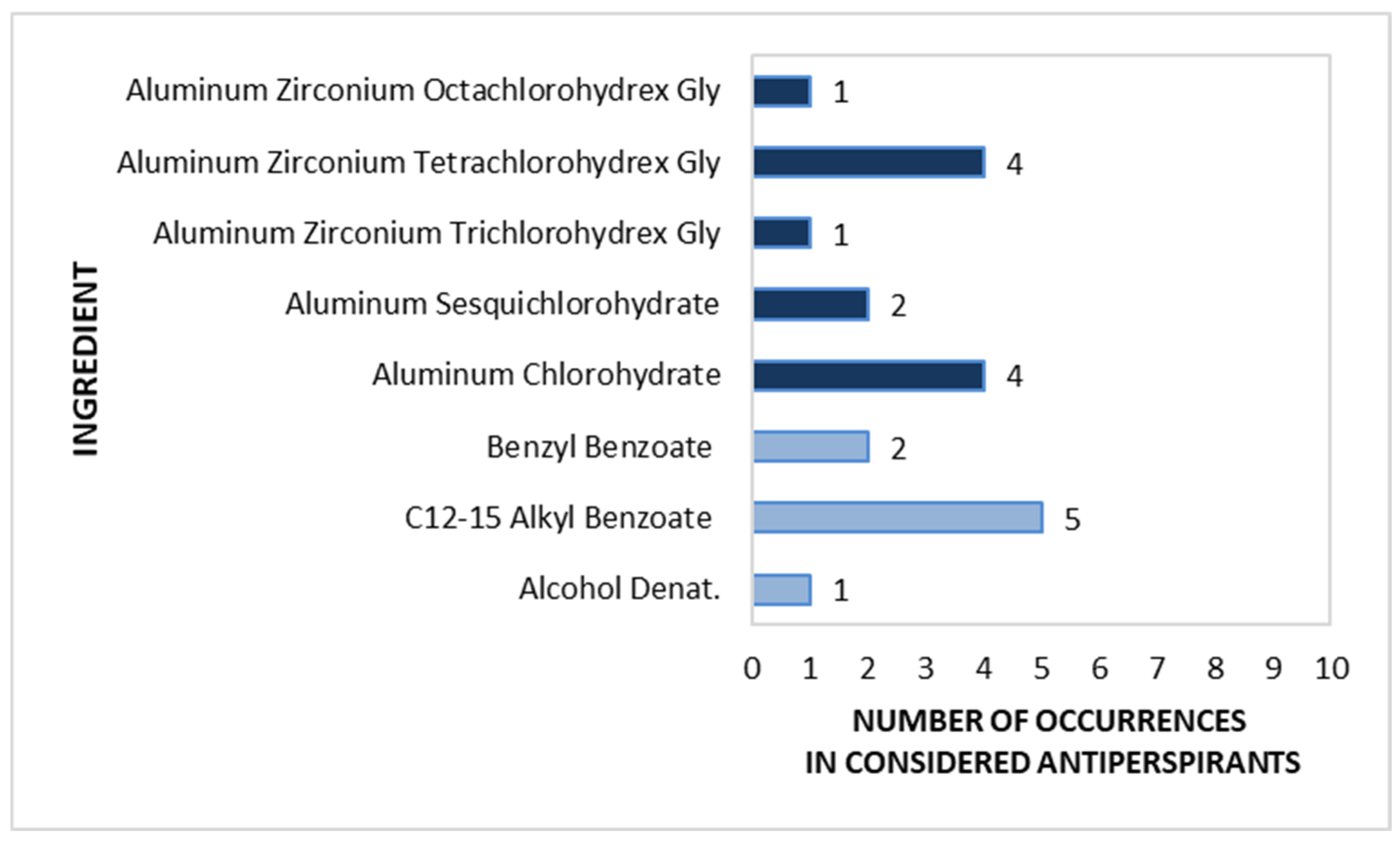
Stick antiperspirants are not as common on the market as other forms. Since the analysis assumed the testing of only one product from a given brand, for this cosmetic form from the antiperspirant category, only 10 products were collected from the entire analysed market. Most antiperspirant components that inhibit sweat secretion are aluminium-based compounds and aluminium zirconium complexes. They are marked in dark blue in Figure 7. The most frequently used examples were Aluminium Zirconium Tetrachlorohydrex Gly and Aluminium Chlorohydrate, with each substance noted in four products, followed by Aluminium Sesquichlorohydrate in two products and Aluminium Zirconium Trichlorohydrex Gly and Aluminium Zirconium Octachlorohydrex Gly in only one product each. The odour-masking and antibacterial ingredient C12-15 Alkyl Benzoate was found in 50% of the investigated antiperspirants. All substances with deodorizing functions found in the stick antiperspirants are shown in light blue in Figure 7.
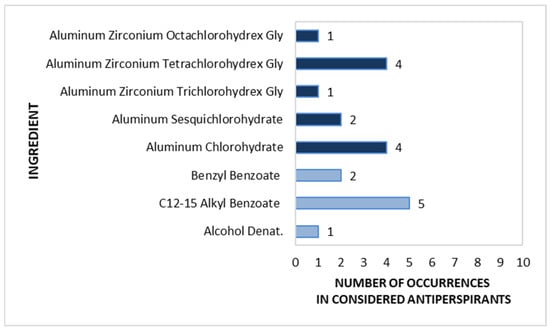
Figure 7.
Frequency of use of active ingredients in stick antiperspirants (n = 10).
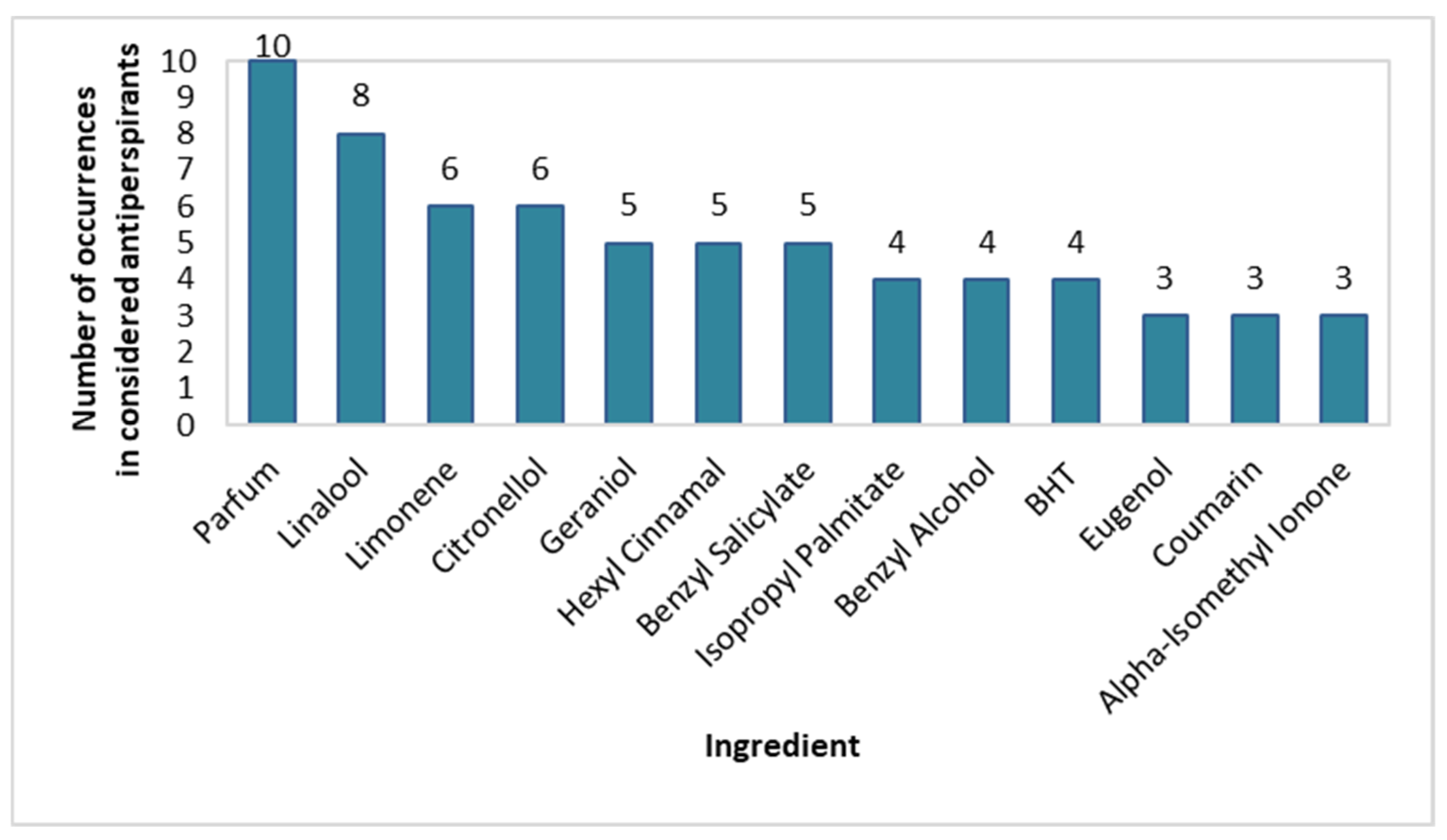
Similarly to spray deodorants, the prevalence of individual fragrance compounds was also checked in the stick antiperspirants (Figure 8). Similarly, the most frequently used fragrance substances were also Parfum (100% of products), Linalool (80%), Limonene (60%), and Citronellol (60%), however, they were more prevalent in stick than in spray deodorants. Benzyl Salicylate, Hexyl Cinnamal, and Geraniol were used in 50% of the considered antiperspirants, BHT, Benzyl Alcohol, and Isopropyl Palmitate were used in 40%, and Alpha-Isomethyl Ionone, Coumarin, and Eugenol were used in 30%. Hydroxycitronellal, Cetyl Palmitate, Citral, and Stearyl Alcohol were used less frequently.
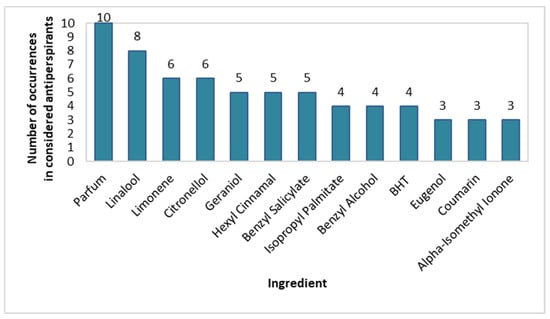
Figure 8.
Prevalence of fragrance-giving ingredients in stick antiperspirants (n = 10).
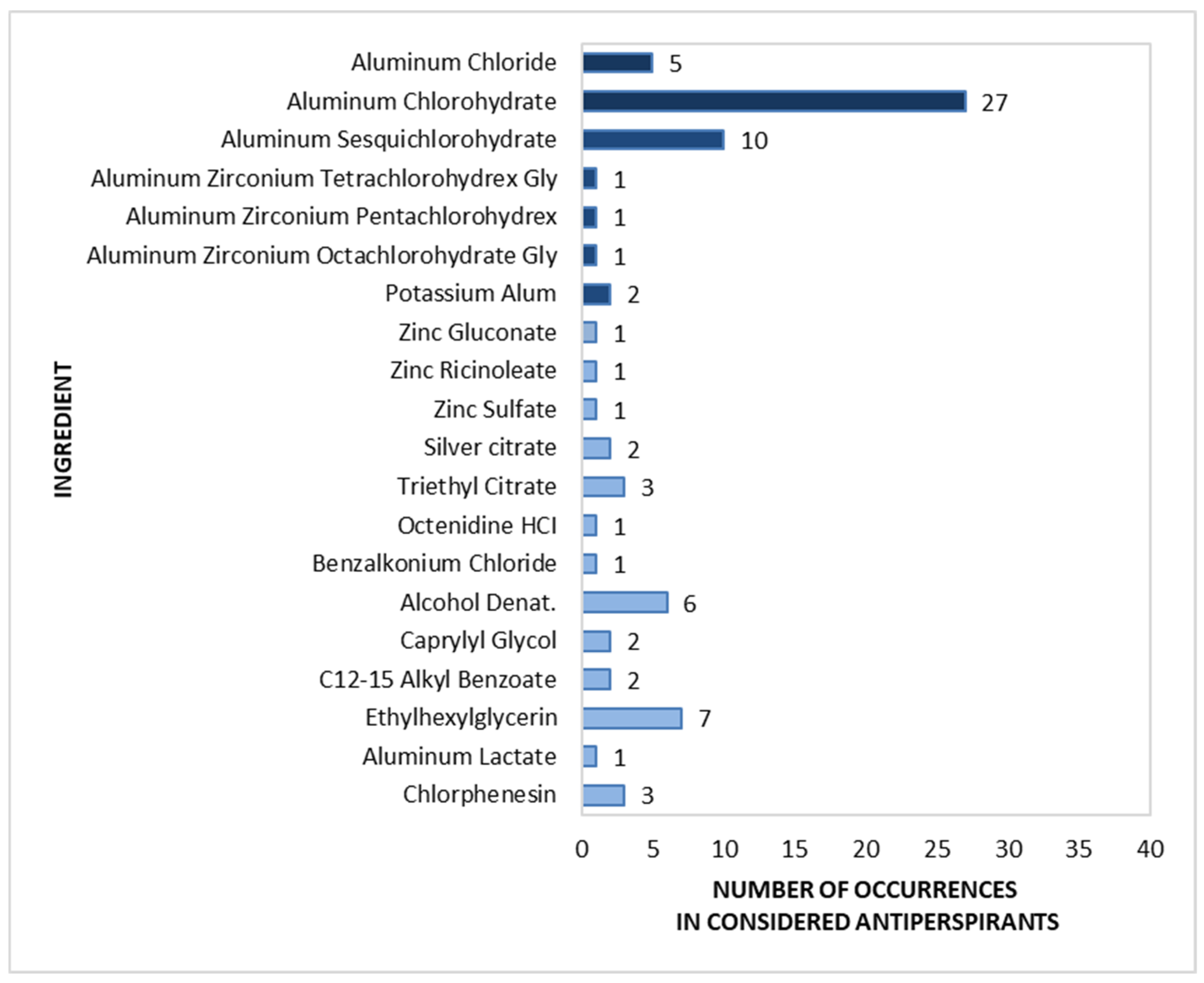
2.5. Composition of Roll-On Antiperspirants (n = 40)
A detailed analysis of the active components found in roll-on antiperspirants (n = 40) is shown in Figure 9. The most common group of substances (marked in dark blue in Figure 9) were aluminium salts, viz. Aluminium Chlorohydrate, found in 67.5% of the analysed preparations, and Aluminium Sesquichlorohydrate, found in 25.0%. These were followed by Aluminium Chloride (12.5%), and Potassium Alum (5.0%). Aluminium–zirconium compounds, such as Aluminium Zirconium Tetra-, Penta-, and Octachlorohydrex Gly, rarely appeared in roll-on antiperspirants, with each being identified in only one product.
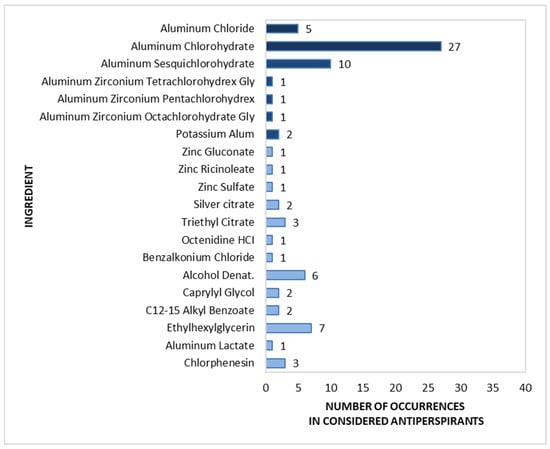
Figure 9.
Prevalence of active ingredients in roll-on antiperspirants (n = 40).
Among the non-antiperspirant components (marked in light blue in Figure 9), the most popular were antibacterial substances, including Alcohol (15.0%) and Chlorphenesin (7.5%), and the odour-masking compounds Ethylhexylglycerin (17.5%) and Triethyl Citrate (7.5%). Other deodorizing substances were observed in a maximum of 5.0% of products, as follows: C12-15 Alkyl Benzoate, Silver Citrate, Carpylyl Glycol, Zinc Gluconate, Zinc Ricinoleate, Zinc Sulfate, Benzalkonium Chloride, and Octenidine HCl.
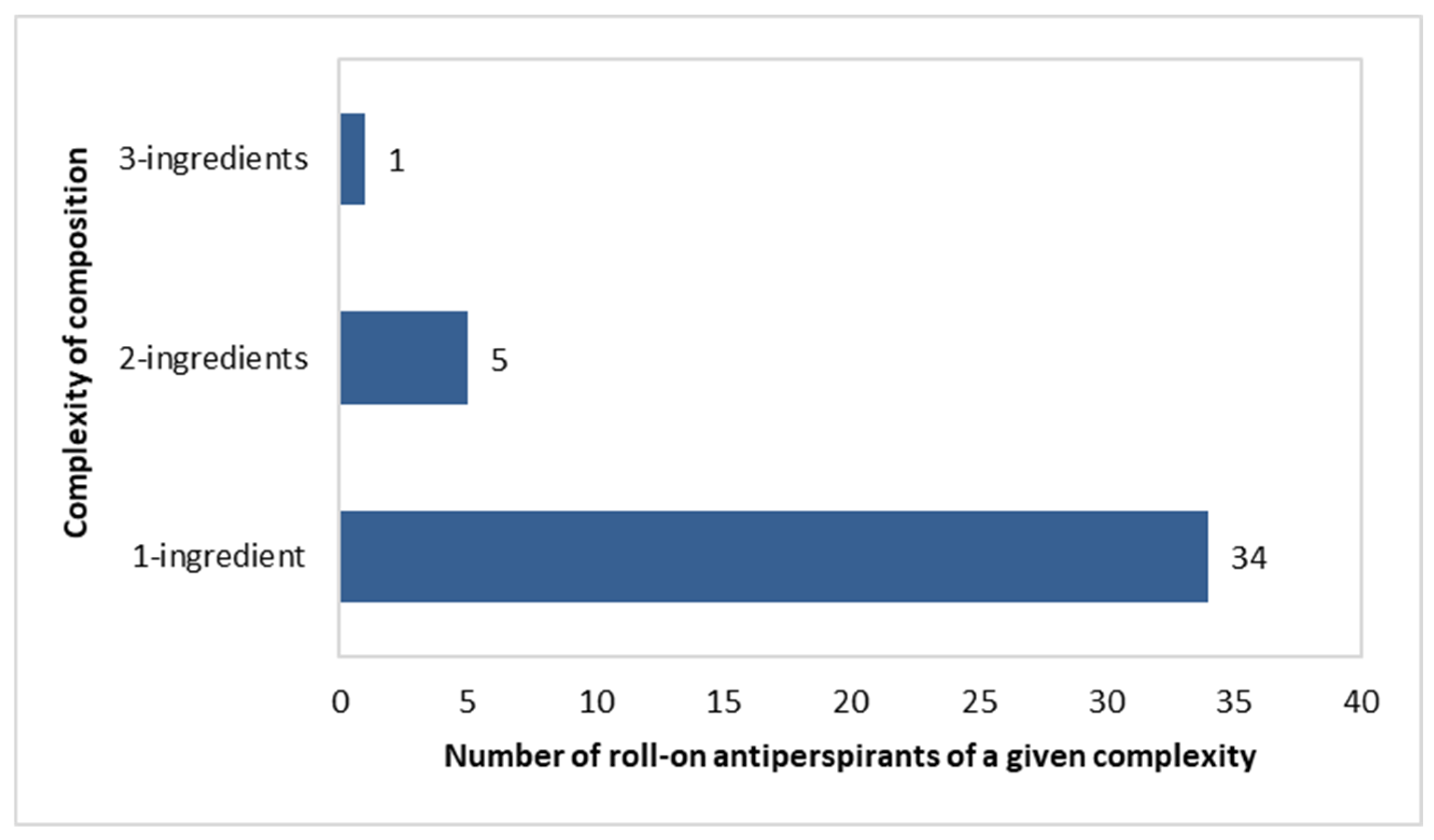
Complexity of Composition of the Investigated Roll-On Antiperspirants
Composition complexity is defined here as the number of aluminium-based ingredients in a single formulation.
Roll-on antiperspirants were dominated by “single-ingredient” products, with this being noted in 34 out of 40 products. Five formulations included a pair of antiperspirant ingredients, as follows: (Aluminium Chlorohydrate and Aluminium Sesquichlorohydrate) in three products, (Aluminium Chlorohydrate and Potassium Alum) in one product, and (Aluminium Chloride and Aluminium Sesquichlorhydrate) in one product. Only one “three-component” formulation was noted, containing Aluminium Zirconium Tetrachlorohydrex Gly, Aluminium Sesquichlorohydrate, and Aluminium Chlorohydrate (Figure 10).
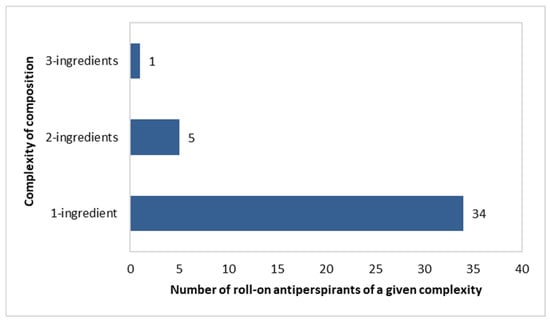
Figure 10.
Number of roll-on antiperspirants (n = 40) with specific numbers of aluminium-based compounds per single product.
Composition complexity was firstly examined for selected product forms from both categories, deodorants (roll-on and stick) and antiperspirants (roll-on). Since the results of these studies did not show any surprising trend, it was decided not to conduct this type of analysis for the remaining product forms.
The total numbers of active substance occurrences in antiperspirants according to roll-on or stick form are given in Table 2. In both forms, the dominant ingredients were Parfum (86.0%), Aluminium Chlorohydrate (62.0%), and Aluminium Sesquichlorohydrate (24.0%). Aluminium Chloride (10.%) was present mainly in roll-on antiperspirants, and Aluminium Zirconium Tetrachlorohydrex Gly (10.0%) was present mainly in stick antiperspirants. The remaining aluminium zirconium compounds were used very rarely in antiperspirants (from 2% to 4% of products). In addition to substances that inhibit sweat secretion, these preparations also included antibacterial or odour-masking substances, such as C12-15 Alkyl Benzoate, Alcohol, and Ethylhexylglycerin (each 14.0%), as well as Trietyl Cirate and Chlorphenesin (each 6.0%).

Table 2.
Number of occurrences of active substances according to the form of antiperspirant.
Regardless of their form, the deodorants and antiperspirants included absorbents such as Magnesium Aluminium Silicate, Perlite, Talc, Magnesium Trisilicate, Magnesium Oxide, Sodium Starch Octenylsuccinate, Maltodextrin, and Aluminium Starch Octenylsuccinate. They act by absorbing moisture, which diminishes unpleasant odours by eliminating the skin flora that causes unpleasant odours.
3. Materials and Methods
The INCI compositions of 170 deodorants and antiperspirants from international cosmetic brands available online in Poland were surveyed.
The following product types were included in the analysis:
- -
- Spray deodorants n = 50 (50 products from 50 different brands);
- -
- Roll-on deodorants n = 50 (50 products from 50 different brands);
- -
- Stick deodorants n = 20 (20 products from 20 different brands);
- -
- Roll-on antiperspirants n = 40 (40 products from 40 different brands);
- -
- Stick antiperspirants n = 10 (10 products from 10 different brands).
The analysis assumed the testing of only one product from an individual brand. Thus, a given tested brand was never repeated within the same cosmetic form (spray, stick, or roll-on) from a given product category. The same brand could be tested, but did not have to be tested for different cosmetic forms within a given product category (deodorants and antiperspirants). This means that a given brand could be tested for a maximum of five products (three deodorant forms: spray, roll-on, and stick, and two antiperspirant forms: roll-on and stick).
4. Discussion
Underarm deodorants and antiperspirants are widely used throughout the world. As such, an extensive range of products are currently available on the market. Nevertheless, while every effort was made to ensure that this analysis was as complete as possible, some online product labels lacked INCI data and, hence, could not be included in the study; despite this, 170 products from five categories were analysed.
This is the first such detailed review of the deodorants and antiperspirants available on the market in the European Union. Therefore, it is not possible to determine whether there has been a clear downward or upward trend in the use of the tested active ingredients.
Despite this, many scientific papers on deodorants mention the widespread use of antibacterial substances like Triclosan, Benzalkonium Chloride, and Chlorhexidine in deodorant compositions [1,2,19]. However, the current survey of this group of cosmetic products shows a different tendency. None of the 120 examples tested contained Triclosan or Chlorhexidine, despite being considered safe and effective. Only one of the investigated antiperspirants was found to have Benzalkonium Chloride in its INCI composition. The most popular antibacterial agents in the deodorants were found to be Alcohol, C12-15 Alkyl Benzoate, and Zinc Gluconate.
Aluminium-based compounds have long been used to inhibit perspiration. The first such compound, Aluminium Chloride, was introduced as an antiperspirant in 1916 by Stillians. The compound proved effective in inhibiting sweat secretion, but its use was associated with skin irritation and clothing damage due to its high acidity. Less acidic aluminium salts such as Aluminium Chlorohydrate (ACH) were discovered in the 1940s, and zirconium was added to increase the effectiveness of these salts 30 years later.
Although aluminium-based compounds were introduced to cosmetics over 100 years ago, they remain the basic active substances for inhibiting sweat secretion [2,3]. Our findings indicate that the most commonly used compounds were Aluminium Chlorohydrate (62% of considered products), Aluminium Sesquichlorohydrate (24%), Aluminium Chloride (10%), and Aluminium Zirconium Tetrachlorohydrex Gly (10%). Surprisingly, the following aluminium–zirconium complexes were rarely used by cosmetic manufacturers: Aluminium Zirconium Octachlorohydrate Gly was used in only 4% of the included products, Aluminium Zirconium Pentachlorohydrex in 2%, Aluminium Zirconium Trichlorohydrex Gly in 2%, and Potassium Alum in 4%.
Zinc-based compounds, i.e., zinc salts of organic or inorganic acids, are also used as odour-absorbing agents, with Zinc Gluconate, Zinc Ricinoleate, Zinc Lactate, Zinc Phenolsulphate, Zinc Pyrrolidonecarboxylate (Zn PCA), and Zinc Sulphate being employed in deodorants [4,20]. Our data reveal that zinc-based compounds were mainly involved in the formulations of roll-on deodorants, as follows: Zinc Ricinoleate was present in 10 of 50 such products, Zinc Gluconate in 3, and Zinc Lactate and Zinc Sulphate in 1 product each. Only 3 of the 50 considered antiperspirants contained zinc salts, Zinc Gluconate and Zinc Sulphate.
Alternative active ingredients in deodorants and antiperspirants are constantly being sought, as well as new testing methods to evaluate their efficacy [19]. Potential candidates include bacterial extracts such as acetic acid bacterial (AAB) extract, including alcohol dehydrogenase and aldehyde dehydrogenase enzymes [21], plant extracts [e.g., the supercritical hop extract from the hop plant (Humulus lupulus L. and Cannabaceae) and Terminalia spp. plant extract], essential oils, and new synthetic compounds [19]. Another promising candidate is polyquaternium-16 (PQ-16), a copolymer of 1-vinyl-2-pyrrolidone and 1-vinyl-3-methylimidazolium chloride, which was found to possess antibacterial efficacy; a new roll-on formulation containing PQ-16 performed better than the commercially available roll-on and demonstrated a comparable efficacy to a formulation containing ACH [22]. Xylityl sesquicaprylate at 0.35% (w/w) in glycerin was found to possess deodorant activity in vitro; it also exhibited in vivo efficacy, as confirmed by human “sniffers” [23].
5. Conclusions
The antiperspirant market is dominated by aluminium-based compounds. The most common in roll-on antiperspirants are Aluminium Chlorohydrate (prevalence 67.5%), Aluminium Sesquichlorohydrate (25.0%), and Aluminium Chloride (12.5%). Aluminium–zirconium complexes, such as Aluminium Zirconium Tetra-, Penta-, and Octachlorohydrex Gly, are very rarely used in roll-on deodorants, with each being noted in only single products. However, such complexes (Aluminium Zirconium Tri-, Tetra-, and Octachlorohydrex Gly) are much more common in stick antiperspirants (60% of products), as are aluminium-based compounds such as Aluminium Chlorohydrate and Aluminium Sesquichlorohydrate (60% of products).
Regardless of the cosmetic form of a deodorant (i.e., spray, stick, or roll-on), the most commonly used ingredients are Triethyl Citrate, present in 51 of 120 (42.5%) formulations, followed by Alcohol in 31 (25.8%), Ethylhexylglycerin in 30 (25.0%), Caprylyl Glycol in 15 (12.5%), Potassium Alum in 12 (10.0%), and Zinc Ricinoleate in 11 (9.2%). Most of these are odour-masking and odour-absorbing agents. Antibacterial ingredients, with the exception of Alcohol in spray deodorants (56%), are rarely used by deodorant manufacturers (in less than 6% of the studied products). These included Zinc Gluconate, Zinc Sulfate, Sodium Usnate, Cetrimonium Chloride, Chlorphenesin, Silver Citrate, and C12-15 Alkyl Benzoate; the latter also has an odour-masking function.
In addition, 81.7% of deodorants and 100% of antiperspirants contain Parfum. However, other popular fragrance agents include Limonene, Linalool, Citronellol, Citral, Geraniol, and Coumarin.
It is likely that future trends in deodorants, in line with current trends in the entire cosmetics market, will go towards natural products, i.e., essential oils and substances isolated from them, as well as plant extracts. In addition to their ability to inhibit the growth of bacteria, they can impart a pleasant smell, which predisposes them to be effective deodorants. Probably, the deodorant market will completely reject synthetic antibacterial substances in favour of those of natural origin.
It is time for an alternative to aluminium compounds to finally appear on the antiperspirant market. The first aluminium antiperspirant, aluminium chloride, was introduced to the market over 100 years ago, and subsequent modifications also based on aluminium compounds.
Funding
This research was supported by the Medical University of Lodz, grant No. 503/3-066-02/503-31-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Callewaert, C.; Hutapea, P.; Van de Wiele, T. Deodorants and antiperspirants affect the axillary bacterial community. Arch. Dermatol. Res. 2014, 306, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.C.V.; Salvador, D.S.; Holsback, V.; Shultz, J.D.; Michniak-Kohn, B.B.; Leonardi, G.R. Deodorants and antiperspirants: Identification of new strategies and perspectives to prevent and control malodor and sweat of the body. Int. J. Dermatol. 2021, 60, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Benohanian, A. Antiperspirants and deodorants. Clin. Dermatol. 2001, 19, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Body malodours and their topical treatment agents. Int. J. Cosmet. Sci. 2011, 33, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Kemper, M.; Bielfeldt, S.; Knie, U.; Wilhelm, K.-P.; Abels, C. Significant Reduction of Body Odor in Older People with a pH 4.0 Emulsion. Cosmetics 2015, 2, 136–145. [Google Scholar] [CrossRef]
- Quatrale, R.P.; Coble, D.W.; Stoner, K.L.; Felger, C.B. The mechanism of antiperspirant action by aluminum salts. II Histological observations of human eccrine sweat glands inhibited by aluminum chlorhydrate. J. Soc. Cosmet. Chem. 1981, 32, 195–221. [Google Scholar]
- Martini, M.C. Déodorants et antitranspirants. Ann. Dermatol. Venereol. 2020, 147, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, E.; Lucia, A. Essential Oils and Their Individual Components in Cosmetic Products. Cosmetics 2021, 8, 114. [Google Scholar] [CrossRef]
- Lee, J.W.; Cong, T.T. Towards renewable flavors, fragrances and beyond. Curr. Opin. Biotechnol. 2020, 61, 168–180. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; Official Journal of the European Union L 342/59; Publications Office of the European Union: Luxembourg, 2009.
- Darbre, P.D. Aluminium and the human breast. Morphologie 2016, 100, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Gorgogietas, V.A.; Tsialtas, I.; Sotiriou, N.; Laschou, V.C.; Karra, A.G.; Leonidas, D.D.; Chrousos, G.P.; Protopapa, E.; Psarra, A.G. Potential interference of aluminum chlorohydrate with estrogen receptor signaling in breast cancer cells. J. Mol. Biochem. 2018, 7, 1–13. [Google Scholar] [PubMed]
- Exley, C. The toxicity of aluminium in humans. Morphologie 2016, 100, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Percy, M.E.; Kruck, T.P.; Pogue, A.I.; Lukiw, W.J. Towards the prevention of potential aluminium toxic effects and an effective treatment for Alzheimer’s disease. J. Inorg. Biochem. 2011, 105, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C. The neurotoxicity of environmental aluminium is still an issue. Neurotoxicology 2010, 31, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Mirick, D.K.; Davis, S.; Thomas, D.B. Antiperspirant use and the risk of breast cancer. J. Natl. Cancer Inst. 2002, 94, 1578–1580. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.F. Breast cancer and deodorants/antiperspirants: A systematic review. Cent. Eur. J. Public Health 2016, 24, 245–247. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Opinion on the Safety of Aluminium in Cosmetic Products, Preliminary Version of 30–31 October 2019; Final Version of 3–4 March 2020, SCCS/1613/19; SCCS (Scientific Committee on Consumer Safety): Luxembourg, 2020. [Google Scholar]
- Teerasumran, P.; Velliou, E.; Bai, S.; Cai, Q. Deodorants and antiperspirants: New trends in their active agents and testing methods. Int. J. Cosmet. Sci. 2023, 45, 426–443. [Google Scholar] [CrossRef] [PubMed]
- Ascione, J.M.; Forestier, S.; Rollat-Corvol, I. Deodorant Composition Comprising a Water-Soluble Zinc Salt as Odor-Absorbing Agent. U.S. Patent 6632421B2, 14 October 2003. [Google Scholar]
- Yoshioka, N.; Kurata, K.; Takahashi, T.; Ariizumi, M.; Mori, T.; Fujisawa, H.; Kameyama, N.; Okuyama, Y. Body odour aldehyde reduction by acetic acid bacterial extract including enzymes: Alcohol dehydrogenase and aldehyde dehydrogenase. Int. J. Cosmet. Sci. 2018, 40, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Traupe, B.; Fölster, H.; Max, H.; Schulz, J. Effective axillary malodour reduction by polyquaternium16-containing deodorants. Int. J. Cosmet. Sci. 2017, 39, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Mussi, L.; Baby, A.R.; Nogueira, C.; de Camargo Junior, F.B.; Magalhães, W.V. Deodorant Efficacy of Xylityl Sesquicaprylate Vehiculated into Roll-on and Stick Prototype Formulations. Cosmetics 2024, 11, 88. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).