PET/CT Volumetric Parameters as Predictors of the Peritoneal Cancer Index in Advanced Ovarian Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Peritoneal Cancer Index Assessment by Surgery
2.3. 2-[18F]FDG PET/CT
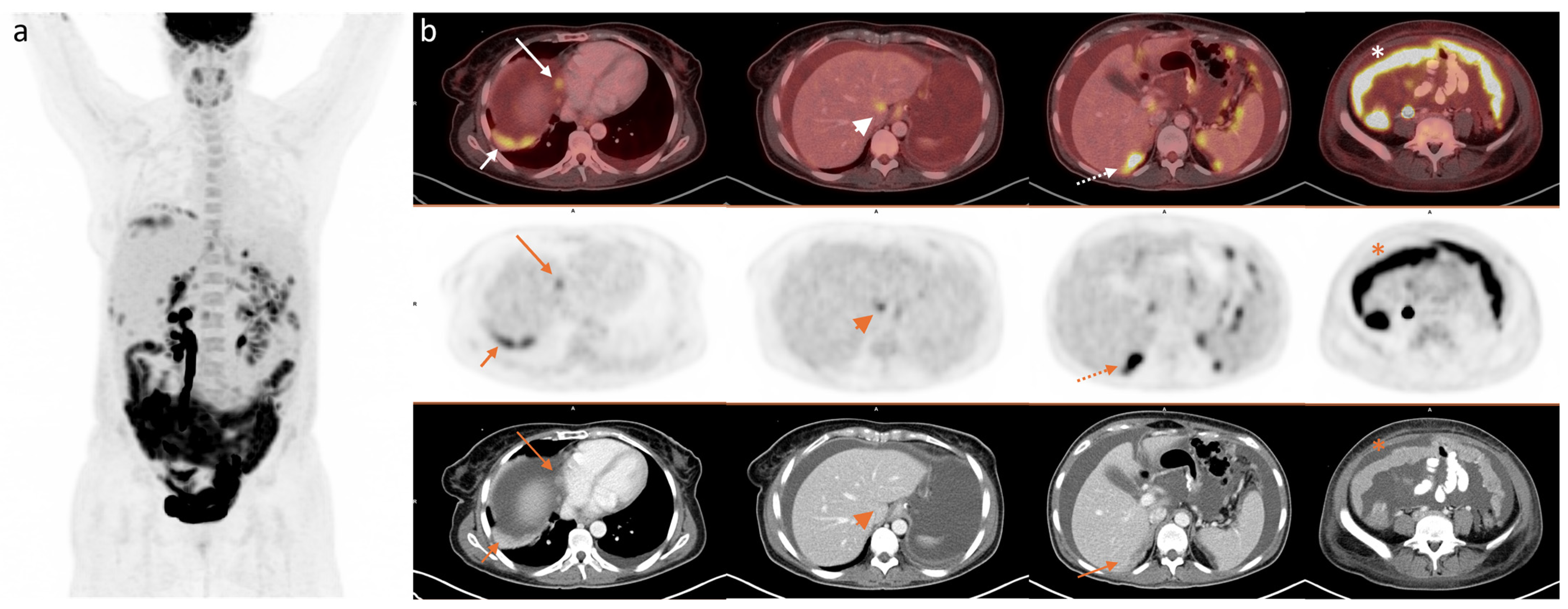
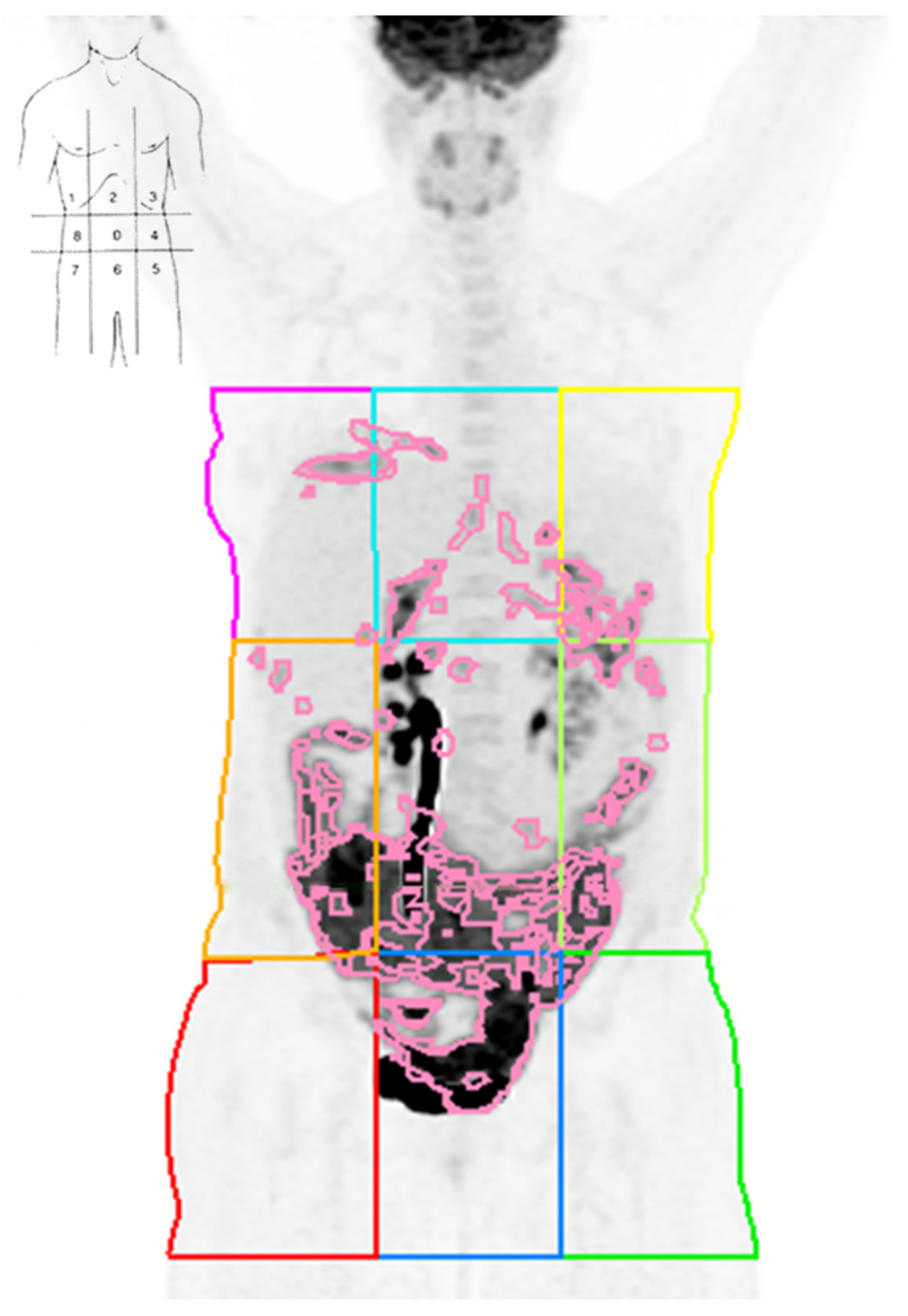
2.4. Image Analysis
2.5. Statistical Analysis
3. Results
3.1. Patient Selection and Baseline Characteristics
3.2. PET/CT Parameters, Global Tumour Burden, and Surgical PCI
3.3. Volumetric Parameters and PCI Stratification Thresholds
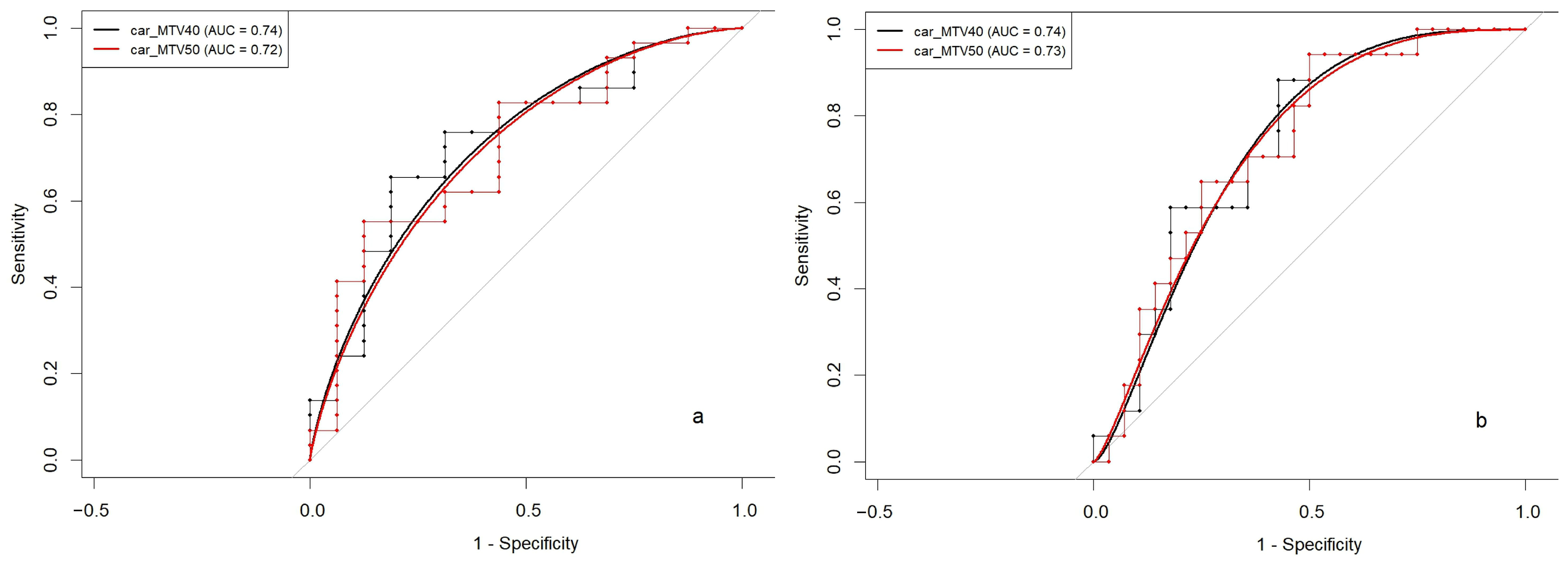
3.4. Quadrant-Level Volumetric Parameters and PCI: Correlation and Diagnostic Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOC | Advanced Ovarian Cancer |
| FIGO | International Federation of Gynaecology and Obstetrics |
| PDS | Primary Debulking Surgery |
| PCI | Peritoneal Cancer Index |
| FDG | Fluorodeoxyglucose |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| MTV | Metabolic Tumour Volume |
| TLG | Total Lesion Glycolysis |
| SUV | Standardised Uptake Value |
| SUVmax | Maximum Standardised Uptake Value |
| HGSOC | High-Grade Serous Ovarian Carcinoma |
| UOC | Undifferentiated Ovarian Carcinoma |
| NACT | Neoadjuvant Chemotherapy |
| VOI | Volume of Interest |
| MIP | Maximum Intensity Projection |
| AUC | Area Under the Curve |
| IQR | Interquartile Range |
| SD | Standard Deviation |
| sCr | Serum Creatinine |
| MTV40 | Metabolic Tumour Volume Calculated with 40% SUVmax Threshold |
| MTV50 | Metabolic Tumour Volume Calculated with 50% SUVmax Threshold |
| TLG40 | Total Lesion Glycolysis Calculated Using MTV40 |
| TLG50 | Total Lesion Glycolysis Calculated Using MTV50 |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Prat, J. Staging Classification for Cancer of the Ovary, Fallopian Tube, and Peritoneum. Obstet. Gynecol. 2015, 126, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Matias-Guiu, X.; Amant, F.; Concin, N.; Davidson, B.; Fotopoulou, C.; González-Martin, A.; Gourley, C.; Leary, A.; Lorusso, D.; et al. ESGO–ESMO–ESP Consensus Conference Recommendations on Ovarian Cancer: Pathology and Molecular Biology and Early, Advanced and Recurrent Disease. Ann. Oncol. 2024, 35, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Chéreau, E.; Ballester, M.; Selle, F.; Cortez, A.; Daraï, E.; Rouzier, R. Comparison of Peritoneal Carcinomatosis Scoring Methods in Predicting Resectability and Prognosis in Advanced Ovarian Cancer. Am. J. Obstet. Gynecol. 2010, 202, 178.e1–178.e10. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical Research Methodologies in Diagnosis and Staging of Patients with Peritoneal Carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [PubMed]
- Gasimli, K.; Braicu, E.I.; Richter, R.; Chekerov, R.; Sehouli, J. Prognostic and Predictive Value of the Peritoneal Cancer Index in Primary Advanced Epithelial Ovarian Cancer Patients After Complete Cytoreductive Surgery: Study of Tumor Bank Ovarian Cancer. Ann. Surg. Oncol. 2015, 22, 2729–2737. [Google Scholar] [CrossRef] [PubMed]
- Lampe, B.; Kroll, N.; Piso, P.; Forner, D.M.; Mallmann, P. Prognostic Significance of Sugarbaker’s Peritoneal Cancer Index for the Operability of Ovarian Carcinoma. Int. J. Gynecol. Cancer 2015, 25, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Gouy, S.; Belghiti, J.; Uzan, C.; Canlorbe, G.; Gauthier, T.; Morice, P. Accuracy and Reproducibility of the Peritoneal Cancer Index in Advanced Ovarian Cancer during Laparoscopy and Laparotomy. Int. J. Gynecol. Cancer 2013, 23, 1699–1703. [Google Scholar] [CrossRef] [PubMed]
- Tentes, A.-A.K.; Tripsiannis, G.; Markakidis, S.K.; Karanikiotis, C.N.; Tzegas, G.; Georgiadis, G.; Avgidou, K. Peritoneal Cancer Index: A Prognostic Indicator of Survival in Advanced Ovarian Cancer. Eur. J. Surg. Oncol. 2003, 29, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Escrig, J.; Serra-Rubert, A.; Gomez-Quiles, L.; Rivadulla, I.; Játiva-Porcar, R.; Moreno-Clarí, E.; Montañés-Pauls, B.; Granel-Villach, L.; Villegas-Cánovas, C.; et al. Prognostic Value of Peritoneal Cancer Index in Primary Advanced Ovarian Cancer. Eur. J. Surg. Oncol. 2018, 44, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Elzarkaa, A.A.; Shaalan, W.; Elemam, D.; Mansour, H.; Melis, M.; Malik, E.; Soliman, A.A. Peritoneal Cancer Index as a Predictor of Survival in Advanced Stage Serous Epithelial Ovarian Cancer: A Prospective Study. J. Gynecol. Oncol. 2018, 29, e47. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Serra, A.; Rivadulla, I.; Gomez, L.; Escrig, J. Prediction of Suboptimal Cytoreductive Surgery in Patients with Advanced Ovarian Cancer Based on Preoperative and Intraoperative Determination of the Peritoneal Carcinomatosis Index. World J. Surg. Oncol. 2018, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Guidelines: Ovarian Cancer/Fallopian Tube Cancer/Primary Peritoneal Cancer. Version 2. 2023. Available online: https://www.nccn.org/Guidelines/Guidelines-Detail?Category=1&id=1453 (accessed on 27 August 2023).
- Glickman, A.; Paredes, P.; Carreras-Diéguez, N.; Niñerola-Baizán, A.; Gaba, L.; Pahisa, J.; Fusté, P.; del Pino, M.; Díaz-Feijóo, B.; González-Bosquet, E.; et al. Evaluation of Patients with Advanced Epithelial Ovarian Cancer before Primary Treatment: Correlation between Tumour Burden Assessed by [18F]FDG PET/CT Volumetric Parameters and Tumour Markers HE4 and CA125. Eur. Radiol. 2022, 32, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Gallicchio, R.; Nardelli, A.; Venetucci, A.; Capacchione, D.; Pelagalli, A.; Sirignano, C.; Mainenti, P.; Pedicini, P.; Guglielmi, G.; Storto, G. F-18 FDG PET/CT Metabolic Tumor Volume Predicts Overall Survival in Patients with Disseminated Epithelial Ovarian Cancer. Eur. J. Radiol. 2017, 93, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hernández, G.; Delgado-Bolton, R.C.; Fernández-Pérez, C.; Lapeña-Gutiérrez, L.; Carreras-Delgado, J.L. Meta-Analysis of the Diagnostic Efficacy of FDG-PET in Patients with Suspected Ovarian Cancer Recurrence. Rev. Esp. Med. Nucl. 2005, 24, 161–173. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Lee, E.Y.P.; Chiu, K.; Chang, C. The Emerging Roles of Functional Imaging in Ovarian Cancer with Peritoneal Carcinomatosis. Clin. Radiol. 2018, 73, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Mallet, E.; Angeles, M.A.; Cabarrou, B.; Chardin, D.; Viau, P.; Frigenza, M.; Navarro, A.S.; Ducassou, A.; Betrian, S.; Martínez-Gómez, C.; et al. Performance of Multiparametric Functional Imaging to Assess Peritoneal Tumor Burden in Ovarian Cancer. Clin. Nucl. Med. 2021, 46, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Suh, C.H.; Kim, Y.J.; Woo, S.; Kim, H. Prognostic Value of Volume-Based Metabolic Parameters of 18F-FDG PET/CT in Ovarian Cancer: A Systematic Review and Meta-Analysis. Ann. Nucl. Med. 2018, 32, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Liu, S.; Zhou, S.; Xiang, L.; Wu, X.; Yang, H. The Role of 18F-FDG PET/CT-Based Quantitative Metabolic Parameters in Patients with Ovarian Clear Cell Carcinoma. Cancer Biomark. 2019, 27, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Palma, M.D.; Gregianin, M.; Nardin, M.; Roma, A.; Nicoletto, M.O.; Nardelli, G.B.; Zagonel, V. Diagnostic and Prognostic Evaluation of Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography and Its Correlation with Serum Cancer Antigen-125 (CA125) in a Large Cohort of Ovarian Cancer Patients. J. Turk. Ger. Gynecol. Assoc. 2015, 16, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, M.; Harter, P.; Bjørn, S.F.; Høgdall, C. Specific Regions, Rather than the Entire Peritoneal Carcinosis Index, Are Predictive of Complete Resection and Survival in Advanced Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2018, 28, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Delgado Bolton, R.C.; Calapaquí Terán, A.K.; Pellet, O.; Ferrero, A.; Giammarile, F. The Search for New 2-18F-FDG PET/CT Imaging Biomarkers in Advanced Ovarian Cancer Patients. Clin. Nucl. Med. 2021, 46, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.; Park, S.-Y.; Lim, M.C. Peritoneal Cancer Index in Ovarian Cancer. J. Gynecol. Oncol. 2019, 30, 2018–2019. [Google Scholar] [CrossRef] [PubMed]
- Delgado Bolton, R.C.; Aide, N.; Colletti, P.M.; Ferrero, A.; Paez, D.; Skanjeti, A.; Giammarile, F. EANM Guideline on the Role of 2-[18F]FDG PET/CT in Diagnosis, Staging, Prognostic Value, Therapy Assessment and Restaging of Ovarian Cancer, Endorsed by the American College of Nuclear Medicine (ACNM), the Society of Nuclear Medicine and Molecular Imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3286–3302. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.G.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging: Version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jaume, S.; Skaltsa, K.; Pallarès, N.; Carrasco, J.L. ThresholdROC: Optimum Threshold Estimation Tools for Continuous Diagnostic Tests in R. J. Stat. Softw. 2017, 82, 1–21. [Google Scholar] [CrossRef]
- Boria, F.; Chiva, L.; Carbonell, M.; Gutierrez, M.; Sancho, L.; Alcazar, A.; Coronado, M.; Hernández Gutiérrez, A.; Zapardiel, I. 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography (18F-FDG PET/CT) Predictive Score for Complete Resection in Primary Cytoreductive Surgery. Int. J. Gynecol. Cancer 2022, 32, 1427–1432. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Cho, A.; Lee, J.-H.; Yun, M.; Lee, J.D.; Kim, Y.T.; Kang, W.J. The Role of Metabolic Tumor Volume and Total Lesion Glycolysis on 18F-FDG PET/CT in the Prognosis of Epithelial Ovarian Cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Mayoral, M.; Fernandez-Martinez, A.; Vidal, L.; Fuster, D.; Aya, F.; Pavia, J.; Pons, F.; Lomeña, F.; Paredes, P. Prognostic Value of 18F-FDG PET/CT Volumetric Parameters in Recurrent Epithelial Ovarian Cancer. Rev. Esp. Med. Nucl. Imagen Mol. 2016, 35, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Woo, S.; Suh, C.H.; Lee, J.J. Performance of Pre-Treatment 18 F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Detecting Metastasis in Ovarian Cancer: A Systematic Review and Meta-Analysis. J. Gynecol. Oncol. 2018, 29, e98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Sang-Woo, L. Diagnostic Accuracy of 18 F-FDG PET/CT for Detection of Peritoneal Carcinomatosis; a systematic review and meta-analysis. Br. Inst. Radiol. 2017, 90, 1081. [Google Scholar]
- De Iaco, P.; Musto, A.; Orazi, L.; Zamagni, C.; Rosati, M.; Allegri, V.; Cacciari, N.; Al-Nahhas, A.; Rubello, D.; Venturoli, S.; et al. FDG-PET/CT in Advanced Ovarian Cancer Staging: Value and Pitfalls in Detecting Lesions in Different Abdominal and Pelvic Quadrants Compared with Laparoscopy. Eur. J. Radiol. 2011, 80, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Berthelot, C.; Morel, O.; Girault, S.; Verrièle, V.; Poirier, A.L.; Moroch, J.; Boucher, Y.; Le Jeune, J.J.; Lorimier, G. Use of FDG-PET/CT for Peritoneal Carcinomatosis before Hyperthermic Intraperitoneal Chemotherapy. Nucl. Med. Commun. 2011, 32, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Chong, G.O.; Jeong, S.Y.; Lee, Y.H.; Lee, H.J.; Lee, S.W.; Han, H.S.; Hong, D.G.; Lee, Y.S. The Ability of Whole-Body SUVmax in F-18 FDG PET/CT to Predict Suboptimal Cytoreduction during Primary Debulking Surgery for Advanced Ovarian Cancer. J. Ovarian Res. 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Meuli, R.A.; Achtari, C.; Prior, J.O. Peritoneal Carcinomatosis in Primary Ovarian Cancer Staging. Clin. Nucl. Med. 2015, 40, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Elekonawo, F.M.K.; Starremans, B.; Laurens, S.T.; Bremers, A.J.A.; de Wilt, J.H.W.; Heijmen, L.; de Geus-Oei, L.F. Can [18F]F-FDG PET/CT Be Used to Assess the Pre-Operative Extent of Peritoneal Carcinomatosis in Patients with Colorectal Cancer? Abdom. Radiol. 2020, 45, 301–306. [Google Scholar] [CrossRef] [PubMed]

| n = 45 | Mean/Median | (SD)/[IQR]/% |
|---|---|---|
| Age (years) | 61.9 | (SD = 10.9) |
| Histology | ||
| HGSOC | 40 ˙ | (88.9%) |
| UOC | 5 ˙ | (11.1%) |
| CA125 (U/mL) | 469 * | [1724] |
| HE4 (pmol/L) | 456 * | [1212] |
| sCr (mg/dL) | 0.73 * | [0.22] |
| FIGO stage | ||
| III | 23 ˙ | (51.1%) |
| IV | 22 ˙ | (48.9%) |
| PCI | 17 * | [9] |
| Primary treatment | ||
| PDS | 15 ˙ | (33.3%) |
| NACT | 30 ˙ | (66.7%) |
| n = 45 | Mean | SD |
|---|---|---|
| SUVmax | 18.4 | 8 |
| SUVmean | 7.84 | 4.01 |
| wb_MTV40 | 239 | 137 |
| wb_MTV50 | 147 | 88.4 |
| wb_TLG40 | 1757 | 1057 |
| wb_TLG50 | 1154 | 678 |
| car_MTV40 | 229 | 141 |
| car_MTV50 | 140 | 90.6 |
| car_TLG40 | 1687 | 1097 |
| car_TLG50 | 1101 | 704 |
| PCI Groups | <14 (n = 16) | ≥14 (n = 29) | p | <20 (n = 28) | ≥20 (n = 17) | p |
|---|---|---|---|---|---|---|
| car_MTV40 | 161 (118) | 266 (140) | 0.011 | 191(139) | 291 (124) | 0.016 |
| car_MTV50 | 99 (74.4) | 162 (92.0) | 0.016 | 117 (89.7) | 178 (80.7) | 0.023 |
| car_TLG40 | 1322 (936) | 1888 (1142) | 0.081 | 1506 (1097) | 1985 (1063) | 0.156 |
| car_TLG50 | 864 (592) | 1231 (737) | 0.077 | 966 (655) | 1322 (746) | 0.115 |
| Parameters | PCI | |
|---|---|---|
| r * | p | |
| All patients (n = 45) | ||
| MTV40_1 | 0.582 | <0.001 |
| TGL40_1 | 0.596 | <0.001 |
| MTV40_2 | 0.420 | 0.004 |
| TGL40_2 | 0.459 | 0.002 |
| MTV40_3 | 0.418 | 0.004 |
| TGL40_3 | 0.464 | 0.001 |
| MTV40_123 | 0.511 | <0.001 |
| TGL40_123 | 0.549 | <0.001 |
| PDS patients (n = 15) | ||
| MTV40_1 | 0.633 | 0.011 |
| TGL40_1 | 0.678 | 0.005 |
| MTV40_2 | 0.595 | 0.019 |
| TGL40_2 | 0.535 | 0.040 |
| MTV40_3 | 0.603 | 0.017 |
| TGL40_3 | 0.671 | 0.006 |
| MTV40_123 | 0.766 | 0.001 |
| TGL40_123 | 0.791 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glickman, A.; Gil-Ibáñez, B.; Niñerola-Baizán, A.; Tormo, M.; Carreras-Dieguez, N.; Fusté, P.; Del Pino, M.; González-Bosquet, E.; Romero-Zayas, I.; Celada-Castro, C.; et al. PET/CT Volumetric Parameters as Predictors of the Peritoneal Cancer Index in Advanced Ovarian Cancer Patients. Diagnostics 2025, 15, 1818. https://doi.org/10.3390/diagnostics15141818
Glickman A, Gil-Ibáñez B, Niñerola-Baizán A, Tormo M, Carreras-Dieguez N, Fusté P, Del Pino M, González-Bosquet E, Romero-Zayas I, Celada-Castro C, et al. PET/CT Volumetric Parameters as Predictors of the Peritoneal Cancer Index in Advanced Ovarian Cancer Patients. Diagnostics. 2025; 15(14):1818. https://doi.org/10.3390/diagnostics15141818
Chicago/Turabian StyleGlickman, Ariel, Blanca Gil-Ibáñez, Aida Niñerola-Baizán, Marta Tormo, Núria Carreras-Dieguez, Pere Fusté, Marta Del Pino, Eduardo González-Bosquet, Inmaculada Romero-Zayas, Cristina Celada-Castro, and et al. 2025. "PET/CT Volumetric Parameters as Predictors of the Peritoneal Cancer Index in Advanced Ovarian Cancer Patients" Diagnostics 15, no. 14: 1818. https://doi.org/10.3390/diagnostics15141818
APA StyleGlickman, A., Gil-Ibáñez, B., Niñerola-Baizán, A., Tormo, M., Carreras-Dieguez, N., Fusté, P., Del Pino, M., González-Bosquet, E., Romero-Zayas, I., Celada-Castro, C., Marina, T., Gaba, L., Hernández, A. R., Saco, A., Buñesch, L., Carrasco, J. L., Quintero, K., Fuster, D., Díaz-Feijóo, B., ... Paredes, P. (2025). PET/CT Volumetric Parameters as Predictors of the Peritoneal Cancer Index in Advanced Ovarian Cancer Patients. Diagnostics, 15(14), 1818. https://doi.org/10.3390/diagnostics15141818








