Association between the Erythrocyte Membrane Fatty Acid Profile and Cognitive Function in the Overweight and Obese Population Aged from 45 to 75 Years Old
Abstract
:1. Introduction
2. Materials and Methods
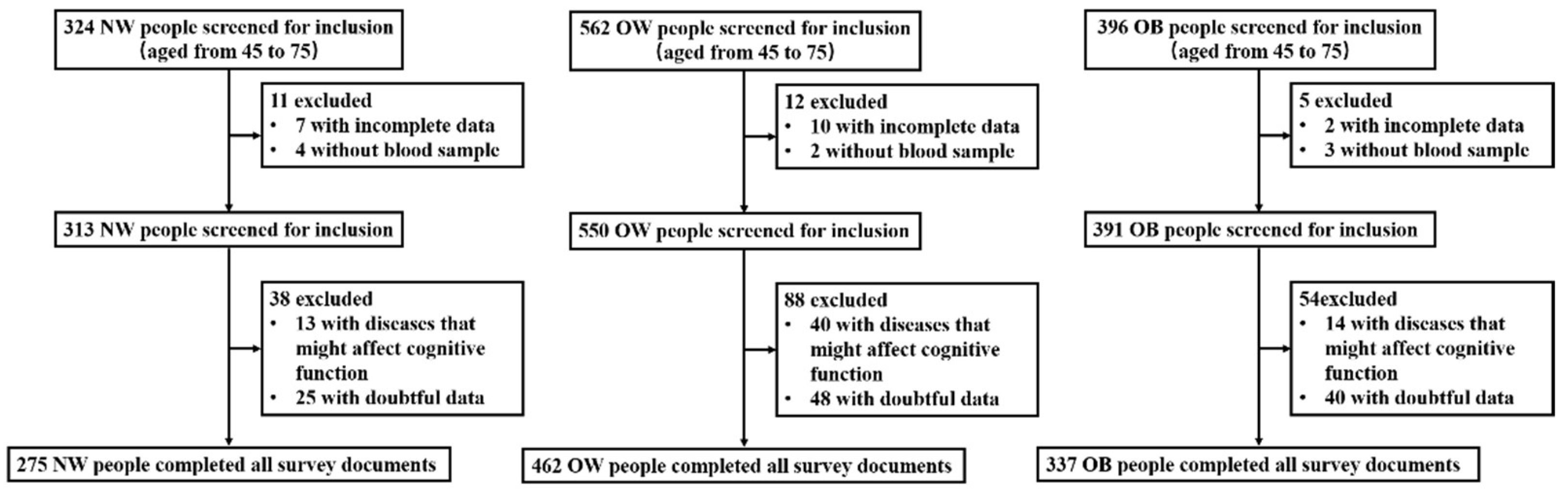
2.1. Participants and Group
2.2. Physical Examinations and Blood Sample Collection
2.3. Questionnaire Survey
2.3.1. Sociodemographic Characteristics
2.3.2. Assessment of Cognitive Function
2.3.3. Dietary Intake Survey
2.4. Blood Biochemistry Parameter Detection
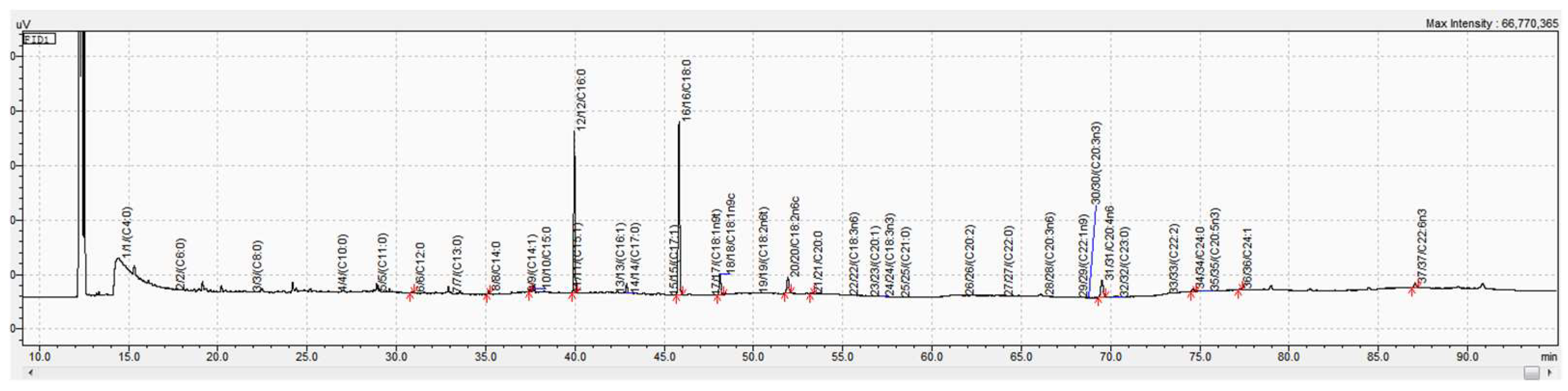
2.5. Fatty Acid Analysis
2.5.1. Collection of the Erythrocyte Membranes
2.5.2. Fatty Acid Analysis
2.6. Statistical Analysis
3. Results
3.1. Basic Information
3.2. Blood Biochemistry Parameters for Comparison among the Three Groups
3.3. Cognitive Scores for Comparison among the Three Groups
3.4. The Fatty Acid Composition of the Erythrocyte Membranes for Comparison among the Three Groups
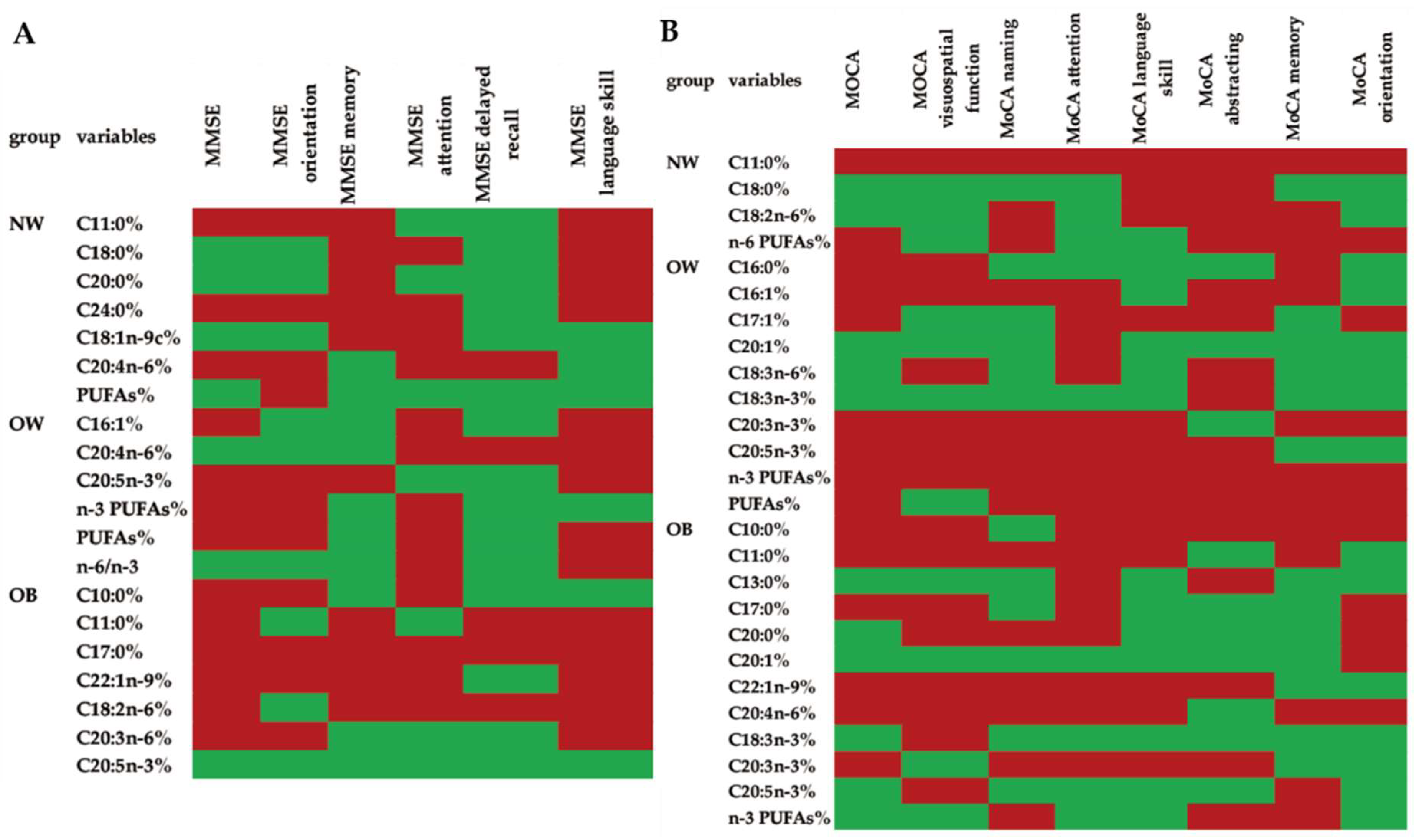
3.5. Associations between the Scores in All of the Cognitive Domains Measured by MMSE or MoCA and the Fatty Acid Composition of the Erythrocyte Membranes in Different Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Callaghan, B.C.; Reynolds, E.L.; Banerjee, M.; Chant, E.; Villegas-Umana, E.; Gardner, T.W.; Votruba, K.; Giordani, B.; Pop-Busui, R.; Pennathur, S.; et al. The Prevalence and Determinants of Cognitive Deficits and Traditional Diabetic Complications in the Severely Obese. Diabetes Care 2020, 43, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. Fact Sheets-Obesity-and-Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 24 January 2022).
- Chinese Nutrition and Chronic Disease Status Report in 2020. Available online: https://baike.so.com/doc/24597977-26899070.html (accessed on 24 January 2022).
- Qin, S.; Wang, A.; Gu, S.; Wang, W.; Gao, Z.; Tang, X.; Yan, L.; Wan, Q.; Luo, Z.; Qin, G.; et al. Association between obesity and urinary albumin-creatinine ratio in the middle-aged and elderly population of Southern and Northern China: A cross-sectional study. BMJ Open 2021, 11, e040214. [Google Scholar] [CrossRef]
- National Bureau of Statistics of China 2019 China Statistical Yearbook. Available online: http://www.stats.gov.cn/tjsj/ndsj/2019/indexeh.htm (accessed on 24 January 2022).
- Jia, L.; Du, Y.; Chu, L.; Zhang, Z.; Li, F.; Lyu, D.; Li, Y.; Zhu, M.; Jiao, H.; Song, Y.; et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: A cross-sectional study. Lancet Public Health 2020, 5, e661–e671. [Google Scholar] [CrossRef]
- Ding, B.; Xiao, R.; Ma, W.; Zhao, L.; Bi, Y.; Zhang, Y. The association between macronutrient intake and cognition in individuals aged under 65 in China: A cross-sectional study. BMJ Open 2018, 8, e018573. [Google Scholar] [CrossRef] [Green Version]
- Murphy, T.; Thuret, S. The systemic milieu as a mediator of dietary influence on stem cell function during ageing. Ageing Res. Rev. 2015, 19, 53–64. [Google Scholar] [CrossRef]
- Janssen, C.I.; Kiliaan, A.J. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: The influence of LCPUFA on neural development, aging, and neurodegeneration. Prog. Lipid Res. 2014, 53, 1–17. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Custodero, C.; Lozupone, M.; Imbimbo, B.P.; Valiani, V.; Agosti, P.; Schilardi, A.; D’Introno, A.; La Montagna, M.; Calvani, M.; et al. Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer’s Disease and Late-Life Cognitive Disorders: A Systematic Review. J. Alzheimer’s Dis. JAD 2017, 59, 815–849. [Google Scholar] [CrossRef] [Green Version]
- Alsumari, S.R.; AlNouri, D.M.; El-Sayed, M.M.A.; El-Din, M.F.S.; Arzoo, S. The sociodemographic characteristics and dietary and blood plasma fatty acid profiles of elderly Saudi women with Alzheimer disease. Lipids Health Dis. 2019, 18, 77. [Google Scholar] [CrossRef] [Green Version]
- Finucane, O.M.; Lyons, C.L.; Murphy, A.M.; Reynolds, C.M.; Klinger, R.; Healy, N.P.; Cooke, A.A.; Coll, R.C.; McAllan, L.; Nilaweera, K.N.; et al. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes 2015, 64, 2116–2128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abete, I.; Goyenechea, E.; Zulet, M.A.; Martínez, J.A. Obesity and metabolic syndrome: Potential benefit from specific nutritional components. Nutr. Metab. Cardiovasc. Dis. NMCD 2011, 21 (Suppl. S2), B1–B15. [Google Scholar] [CrossRef] [PubMed]
- Hidese, S.; Ota, M.; Matsuo, J.; Ishida, I.; Hiraishi, M.; Yoshida, S.; Noda, T.; Sato, N.; Teraishi, T.; Hattori, K.; et al. Association of obesity with cognitive function and brain structure in patients with major depressive disorder. J. Affect. Disord. 2018, 225, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Zhao, L.; Ding, B.J.; Xiao, R.; Ma, W.W. The association of blood non-esterified fatty acid, saturated fatty acids, and polyunsaturated fatty acids levels with mild cognitive impairment in Chinese population aged 35–64 years: A cross-sectional study. Nutr. Neurosci. 2021, 24, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Djoussé, L.; Biggs, M.L.; Lemaitre, R.N.; King, I.B.; Song, X.; Ix, J.H.; Mukamal, K.J.; Siscovick, D.S.; Mozaffarian, D. Plasma omega-3 fatty acids and incident diabetes in older adults. Am. J. Clin. Nutr. 2011, 94, 527–533. [Google Scholar] [CrossRef] [Green Version]
- Schoeniger, A.; Adolph, S.; Fuhrmann, H.; Schumann, J. The impact of membrane lipid composition on macrophage activation in the immune defense against Rhodococcus equi and Pseudomonas aeruginosa. Int. J. Mol. Sci. 2011, 12, 7510–7528. [Google Scholar] [CrossRef]
- Tram, L.; Krogh Venø, S.; Dahm, C.C.; Thomsen, B.H.; Berg Johansen, M.; Overvad, K.; Berg Schmidt, E. Adipose Tissue Lipophilic Index and Risk of Ischemic Stroke—A Danish Case-Cohort Study. Nutrients 2018, 10, 1570. [Google Scholar] [CrossRef] [Green Version]
- Weston, A.D.; Hood, L. Systems biology, proteomics, and the future of health care: Toward predictive, preventative, and personalized medicine. J. Proteome Res. 2004, 3, 179–196. [Google Scholar] [CrossRef]
- Huang, N.J.; Pishesha, N.; Mukherjee, J.; Zhang, S.; Deshycka, R.; Sudaryo, V.; Dong, M.; Shoemaker, C.B.; Lodish, H.F. Genetically engineered red cells expressing single domain camelid antibodies confer long-term protection against botulinum neurotoxin. Nat. Commun. 2017, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Yuan, L.; Zhen, J.; Ma, W.; Cai, C.; Huang, X.; Xiao, R. The Erythrocyte Fatty Acid Profile and Cognitive Function in Old Chinese Adults. Nutrients 2016, 8, 385. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Lu, F.C. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed. Environ. Sci. BES 2004, 17, 1–36. [Google Scholar] [PubMed]
- Diabetes Society of Chinese Medical Association. Guidelines for the Prevention and Treatment of Type 2 Diabetes in China (2017 Edition); Peking University Medical Publisher: Beijing, China, 2018; pp. 292–344. [Google Scholar]
- Yuan, G.; Al-Shali, K.Z.; Hegele, R.A. Hypertriglyceridemia: Its etiology, effects and treatment. Can. Med. Assoc. J. 2007, 176, 1113–1120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Yean Lee, W.; Hilal, S.; Saini, M.; Wong, T.Y.; Chen, C.L.; Venketasubramanian, N.; Ikram, M.K. Comparison of the Montreal Cognitive Assessment and the Mini-Mental State Examination in detecting multi-domain mild cognitive impairment in a Chinese sub-sample drawn from a population-based study. Int. Psychogeriatr. 2013, 25, 1831–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Li, Q.; Shi, L.; Lu, K.; Shang, Q.; Yao, L.; Ye, G. Investigation of dietary intake of cadmium in certain polluted area of south in China. J. Hyg. Res. 2009, 38, 552–554, 557. [Google Scholar]
- Amirkalali, B.; Sohrabi, M.R.; Esrafily, A.; Jalali, M.; Gholami, A.; Hosseinzadeh, P.; Keyvani, H.; Shidfar, F.; Zamani, F. Erythrocyte membrane fatty acid profile & serum cytokine levels in patients with non-alcoholic fatty liver disease. Indian J. Med. Res. 2018, 147, 352–360. [Google Scholar]
- Choi, H.N.; Yim, J.E. Effects of Erythrocyte Membrane Polyunsaturated Fatty Acids in Overweight, Obese, and Morbidly Obese Korean Women. J. Cancer Prev. 2017, 22, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Moussavi Javardi, M.S.; Madani, Z.; Movahedi, A.; Karandish, M.; Abbasi, B. The correlation between dietary fat quality indices and lipid profile with Atherogenic index of plasma in obese and non-obese volunteers: A cross-sectional descriptive-analytic case-control study. Lipids Health Dis. 2020, 19, 213. [Google Scholar] [CrossRef]
- Santos, T.; Fonseca, L.C.; Tedrus, G.; Delbue, J.L. Alzheimer’s disease: Nutritional status and cognitive aspects associated with disease severity. Nutr. Hosp. 2018, 35, 1298–1304. [Google Scholar] [CrossRef]
- Ferguson, A.C.; Tank, R.; Lyall, L.M.; Ward, J.; Welsh, P.; Celis-Morales, C.; McQueenie, R.; Strawbridge, R.J.; Mackay, D.F.; Pell, J.P.; et al. Association of SBP and BMI with cognitive and structural brain phenotypes in UK Biobank. J. Hypertens. 2020, 38, 2482–2489. [Google Scholar] [CrossRef]
- Houle, B.; Gaziano, T.; Farrell, M.; Gómez-Olivé, F.X.; Kobayashi, L.C.; Crowther, N.J.; Wade, A.N.; Montana, L.; Wagner, R.G.; Berkman, L.; et al. Cognitive function and cardiometabolic disease risk factors in rural South Africa: Baseline evidence from the HAALSI study. BMC Public Health 2019, 19, 1579. [Google Scholar] [CrossRef]
- Hou, Q.; Guan, Y.; Yu, W.; Liu, X.; Wu, L.; Xiao, M.; Lü, Y. Associations between obesity and cognitive impairment in the Chinese elderly: An observational study. Clin. Interv. Aging 2019, 14, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.; Choi, S.; Lyu, J. Body mass index and trajectories of cognitive decline among older Korean adults. Aging Ment. Health 2020, 24, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Palta, P.; Carlson, M.C.; Crum, R.M.; Colantuoni, E.; Sharrett, A.R.; Yasar, S.; Nahin, R.L.; DeKosky, S.T.; Snitz, B.; Lopez, O.; et al. Diabetes and Cognitive Decline in Older Adults: The Ginkgo Evaluation of Memory Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 73, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerber, Y.; VanWagner, L.B.; Yaffe, K.; Terry, J.G.; Rana, J.S.; Reis, J.P.; Sidney, S. Non-alcoholic fatty liver disease and cognitive function in middle-aged adults: The CARDIA study. BMC Gastroenterol. 2021, 21, 96. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, T.; Lee, D.; Yang, L.; Li, S. Body mass index across adult life and cognitive function in the American elderly. Aging 2020, 12, 9344–9353. [Google Scholar] [CrossRef]
- Gardener, H.; Caunca, M.; Dong, C.; Cheung, Y.K.; Rundek, T.; Elkind, M.S.V.; Wright, C.B.; Sacco, R.L. Obesity Measures in Relation to Cognition in the Northern Manhattan Study. J. Alzheimer’s Dis. JAD 2020, 78, 1653–1660. [Google Scholar] [CrossRef]
- Lipnicki, D.M.; Makkar, S.R.; Crawford, J.D.; Thalamuthu, A.; Kochan, N.A.; Lima-Costa, M.F.; Castro-Costa, E.; Ferri, C.P.; Brayne, C.; Stephan, B.; et al. Determinants of cognitive performance and decline in 20 diverse ethno-regional groups: A COSMIC collaboration cohort study. PLoS Med. 2019, 16, e1002853. [Google Scholar] [CrossRef]
- Takkunen, M.J.; de Mello, V.D.; Schwab, U.S.; Ågren, J.J.; Kuusisto, J.; Uusitupa, M.I. Associations of erythrocyte membrane fatty acids with the concentrations of C-reactive protein, interleukin 1 receptor antagonist and adiponectin in 1373 men. Prostaglandins Leukot. Essent. Fat. Acids 2014, 91, 169–174. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Zong, G.; Zhu, J.; Sun, L.; Ye, X.; Lu, L.; Jin, Q.; Zheng, H.; Yu, Z.; Zhu, Z.; Li, H.; et al. Associations of erythrocyte fatty acids in the de novo lipogenesis pathway with risk of metabolic syndrome in a cohort study of middle-aged and older Chinese. Am. J. Clin. Nutr. 2013, 98, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Bi, Y.; Ma, W.; He, L.; Yuan, L.; Feng, J.; Xiao, R. Long-term effects of high lipid and high energy diet on serum lipid, brain fatty acid composition, and memory and learning ability in mice. Int. J. Dev. Neurosci. 2010, 28, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.T.; Hsu, Y.J.; Chien, Y.W.; Huang, C.C.; Huang, W.C.; Chiu, W.C. Tea Seed Oil Prevents Obesity, Reduces Physical Fatigue, and Improves Exercise Performance in High-Fat-Diet-Induced Obese Ovariectomized Mice. Molecules 2019, 24, 980. [Google Scholar] [CrossRef] [Green Version]
- Perfilyev, A.; Dahlman, I.; Gillberg, L.; Rosqvist, F.; Iggman, D.; Volkov, P.; Nilsson, E.; Risérus, U.; Ling, C. Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 991–1000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okereke, O.I.; Rosner, B.A.; Kim, D.H.; Kang, J.H.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Willett, W.C.; Grodstein, F. Dietary fat types and 4-year cognitive change in community-dwelling older women. Ann. Neurol. 2012, 72, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Albrahim, T. The potential role of nutritional components in improving brain function among patients with Alzheimers disease: A meta-analysis of RCT studies. Neurosci. J. 2020, 25, 4–17. [Google Scholar]
- Hooper, C.; De Souto Barreto, P.; Payoux, P.; Salabert, A.S.; Guyonnet, S.; Andrieu, S.; Sourdet, S.; Delrieu, J.; Vellas, B. Association of Cortical β-Amyloid with Erythrocyte Membrane Monounsaturated and Saturated Fatty Acids in Older Adults at Risk of Dementia. J. Nutr. Health Aging 2017, 21, 1170–1175. [Google Scholar] [CrossRef]
- Heude, B.; Ducimetière, P.; Berr, C. Cognitive decline and fatty acid composition of erythrocyte membranes—The EVA Study. Am. J. Clin. Nutr. 2003, 77, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Mumme, K.; Stonehouse, W. Effects of medium-chain triglycerides on weight loss and body composition: A meta-analysis of randomized controlled trials. J. Acad. Nutr. Diet. 2015, 115, 249–263. [Google Scholar] [CrossRef]
- Assunção, M.L.; Ferreira, H.S.; dos Santos, A.F.; Cabral, C.R., Jr.; Florêncio, T.M. Effects of dietary coconut oil on the biochemical and anthropometric profiles of women presenting abdominal obesity. Lipids 2009, 44, 593–601. [Google Scholar] [CrossRef]
- Nishimura, Y.; Moriyama, M.; Kawabe, K.; Satoh, H.; Takano, K.; Azuma, Y.T.; Nakamura, Y. Lauric Acid Alleviates Neuroinflammatory Responses by Activated Microglia: Involvement of the GPR40-Dependent Pathway. Neurochem. Res. 2018, 43, 1723–1735. [Google Scholar] [CrossRef]
- Karazurna, N.A.; Porter, C.M.; Aytur, S.; Scott, T.; Mattei, J.; Noel, S.E.; Gonzalez, H.M.; Mossavar-Rahmani, Y.; Sotres-Alvarez, D.; Gallo, L.C.; et al. Associations between dietary fatty acid patterns and cognitive function in the Hispanic Community Health Study/Study of Latinos. Br. J. Nutr. 2021, 1–35. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Ghareghani, M.; Zibara, K.; Tajali Ardakani, M.; Jand, Y.; Azari, H.; Nikbakht, J.; Ghanbari, A. Alyssum homolocarpum seed oil (AHSO), containing natural alpha linolenic acid, stearic acid, myristic acid and β-sitosterol, increases proliferation and differentiation of neural stem cells in vitro. BMC Complement. Altern. Med. 2019, 19, 113. [Google Scholar] [CrossRef]
- Wang, J.T.; Yu, Z.Y.; Tao, Y.H.; Liu, Y.C.; Wang, Y.M.; Guo, Q.L.; Xue, J.Z.; Wen, X.H.; Zhang, Q.; Xu, X.D.; et al. A novel palmitic acid hydroxy stearic acid (5-PAHSA) plays a neuroprotective role by inhibiting phosphorylation of the m-TOR-ULK1 pathway and regulating autophagy. CNS Neurosci. Ther. 2021, 27, 484–496. [Google Scholar] [CrossRef]
- Dohrmann, D.D.; Putnik, P.; Kovačević, D.B.; Simal-Gandara, J.; Lorenzo, J.M.; Barba, F.J. Japanese, Mediterranean and Argentinean diets and their potential roles in neurodegenerative diseases. Food Res. Int. 2019, 120, 464–477. [Google Scholar] [CrossRef]
- Kim, S.W.; Schäfer, M.R.; Klier, C.M.; Berk, M.; Rice, S.; Allott, K.; Bartholomeusz, C.F.; Whittle, S.L.; Pilioussis, E.; Pantelis, C.; et al. Relationship between membrane fatty acids and cognitive symptoms and information processing in individuals at ultra-high risk for psychosis. Schizophr. Res. 2014, 158, 39–44. [Google Scholar] [CrossRef]
- Wang, J.; Wei, R.; Xie, G.; Arnold, M.; Kueider-Paisley, A.; Louie, G.; Mahmoudian Dehkordi, S.; Blach, C.; Baillie, R.; Han, X.; et al. Peripheral serum metabolomic profiles inform central cognitive impairment. Sci. Rep. 2020, 10, 14059. [Google Scholar] [CrossRef]
- Rostami, H.; Samadi, M.; Yuzbashian, E.; Zarkesh, M.; Asghari, G.; Hedayati, M.; Daneshafrooz, A.; Mirmiran, P.; Khalaj, A. Habitual dietary intake of fatty acids are associated with leptin gene expression in subcutaneous and visceral adipose tissue of patients without diabetes. Prostaglandins Leukot. Essent. Fat. Acids 2017, 126, 49–54. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, J.; Qiu, J.; Li, Y.; Wang, J.; Jiao, J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016, 103, 330–340. [Google Scholar] [CrossRef]
- Bigornia, S.J.; Scott, T.M.; Harris, W.S.; Tucker, K.L. Prospective Associations of Erythrocyte Composition and Dietary Intake of n-3 and n-6 PUFA with Measures of Cognitive Function. Nutrients 2018, 10, 1235. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Xu, M.; Kalueff, A.V.; Song, C. Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1β administration. Eur. J. Nutr. 2018, 57, 1781–1791. [Google Scholar] [CrossRef]
- García-Cerro, S.; Rueda, N.; Vidal, V.; Puente, A.; Campa, V.; Lantigua, S.; Narcís, O.; Velasco, A.; Bartesaghi, R.; Martínez-Cué, C. Prenatal Administration of Oleic Acid or Linolenic Acid Reduces Neuromorphological and Cognitive Alterations in Ts65dn Down Syndrome Mice. J. Nutr. 2020, 150, 1631–1643. [Google Scholar] [CrossRef]
- Wysoczański, T.; Sokoła-Wysoczańska, E.; Pękala, J.; Lochyński, S.; Czyż, K.; Bodkowski, R.; Herbinger, G.; Patkowska-Sokoła, B.; Librowski, T. Omega-3 Fatty Acids and their Role in Central Nervous System—A Review. Curr. Med. Chem. 2016, 23, 816–831. [Google Scholar] [CrossRef]
- Haution-Bitker, M.; Gilbert, T.; Vignoles, A.; Lecardonnel, C.; Watelet, S.; Blond, E.; Drai, J.; Bonnefoy, M. Associations between Plasmatic Polyunsaturated Fatty Acids Concentrations and Cognitive Status and Decline in Neurocognitive Disorders. J. Nutr. Health Aging 2018, 22, 718–725. [Google Scholar] [CrossRef]
- Urso, C.J.; Zhou, H. Palmitic Acid Lipotoxicity in Microglia Cells Is Ameliorated by Unsaturated Fatty Acids. Int. J. Mol. Sci. 2021, 22, 9093. [Google Scholar] [CrossRef]
- MacDonald-Wicks, L.; McEvoy, M.; Magennis, E.; Schofield, P.W.; Patterson, A.J.; Zacharia, K. Dietary Long-Chain Fatty Acids and Cognitive Performance in Older Australian Adults. Nutrients 2019, 11, 711. [Google Scholar] [CrossRef] [Green Version]
- González, S.; Huerta, J.M.; Fernández, S.; Patterson, A.M.; Lasheras, C. The relationship between dietary lipids and cognitive performance in an elderly population. Int. J. Food Sci. Nutr. 2010, 61, 217–225. [Google Scholar] [CrossRef]
| NW (n = 275) | OW (n = 462) | OB (n = 337) | p | ||
|---|---|---|---|---|---|
| Continuous variable | M ± SD | M ± SD | M ± SD | ||
| Age | 61.476 ± 7.493 | 60.738 ± 6.937 | 59.362 ± 6.885 ab | 0.001 ** | |
| BMI (kg/m2) | 22.142 ± 1.365 | 25.935 ± 1.131 a | 30.819 ± 2.430 ab | <0.001 ** | |
| Waist-hip ratio | 0.898 ± 0.066 | 0.910 ± 0.057 | 0.923 ± 0.066 a | 0.001 ** | |
| Energy intake (kcal) | 1789.213 ± 1041.815 | 1747.080 ± 940.751 | 1884.528 ± 922.536 | 0.126 | |
| Categorical variable | n (%) | n (%) | n (%) | ||
| Gender | male | 94 (34.18%) | 158 (34.20%) | 103 (30.56%) | 0.503 |
| female | 181 (65.82%) | 304 (65.80%) | 234 (69.44%) | ||
| Culture | illiterate | 16 (5.86%) | 30 (6.52%) | 27 (8.06%) | 0.961 |
| primary school | 64 (23.44%) | 108 (23.48%) | 71 (21.19%) | ||
| junior high school | 142 (52.01%) | 239 (51.96%) | 175 (52.24%) | ||
| senior middle school | 45 (16.48%) | 72 (15.65%) | 50 (14.93%) | ||
| technical secondary school | 3 (1.10%) | 5 (1.09%) | 6 (1.79%) | ||
| junior college | 1 (0.37%) | 3 (0.65%) | 5 (1.49%) | ||
| undergraduate or above | 2 (0.73%) | 3 (0.65%) | 1 (0.30%) | ||
| Smoking | never | 194 (72.66%) | 346 (76.38%) | 269 (80.54%) | 0.208 |
| continuous smoking for at least 6 months | 67 (25.09%) | 97 (21.41%) | 57 (17.07%) | ||
| smoking for at least 6 months but not continuous | 6 (2.25%) | 10 (2.21%) | 8 (2.40%) | ||
| Drinking | never | 197 (73.23%) | 334 (73.25%) | 235 (71.00%) | 0.836 |
| former | 10 (3.72%) | 15 (3.29%) | 16 (4.83%) | ||
| current | 62 (23.05%) | 107 (23.46%) | 80 (24.17%) | ||
| Exercise | no | 36 (13.14%) | 57 (12.47%) | 44 (13.29%) | 0.935 |
| yes | 238 (86.86%) | 400 (87.53%) | 287 (86.71%) | ||
| History of hypertension | no | 180 (65.45%) | 238 (51.52%) a | 154 (45.70%) a | <0.001 ** |
| yes | 91 (33.09%) | 223 (48.27%) a | 181 (53.71%) a | ||
| unknow | 4 (1.45%) | 1 (0.22%) a | 2 (0.59%) | ||
| Diabetes mellitus | no | 228 (82.91%) | 390 (84.42%) | 264 (78.34%) | 0.080 |
| yes | 47 (17.09%) | 72 (15.58%) | 73 (21.66%) | ||
| Hypertriglyceridemia | no | 210 (76.36%) | 290 (62.77%) a | 192 (56.97%) a | <0.001 ** |
| yes | 65 (23.64) | 172 (37.23%) a | 145 (43.03%) a |
| Variables | NW (n = 275) | OW (n = 462) | OB (n = 337) | p |
|---|---|---|---|---|
| TC (mmol/L) | 5.311 ± 1.030 | 5.210 ± 1.052 | 5.190 ± 1.066 | 0.295 |
| TG (mmol/L) | 1.379 ± 1.004 | 1.831 ± 1.654 a | 1.895 ± 1.181 ab | <0.001 ** |
| HDL-C (mmol/L) | 1.515 ± 0.326 | 1.391 ± 0.309 a | 1.343 ± 0.295 a | <0.001 ** |
| LDL-C (mmol/L) | 3.168 ± 0.807 | 3.166 ± 0.791 | 3.214 ± 0.838 | 0.633 |
| FBG (mmol/L) | 6.066 ± 2.916 | 5.932 ± 2.423 | 6.335 ± 2.388 ab | <0.001 ** |
| Apo E (mg/L) | 51.778 ± 14.882 | 54.906 ± 18.927 | 55.996 ± 17.280 a | 0.019 ** |
| Variables | NW (n = 275) | OW (n = 462) | OB (n = 337) | p |
|---|---|---|---|---|
| MMSE | 26.920 ± 2.843 | 26.409 ± 2.996 | 26.751 ± 2.826 | 0.049 * |
| MMSE orientation | 9.447 ± 0.996 | 9.429 ± 0.916 | 9.504 ± 0.890 | 0.224 |
| MMSE memory | 2.865 ± 0.419 | 2.846 ± 0.422 | 2.905 ± 0.349 | 0.054 |
| MMSE attention | 4.047 ± 1.346 | 3.745 ± 1.447 a | 3.878 ± 1.367 | 0.014 * |
| MMSE delayed recall | 2.284 ± 0.858 | 2.232 ± 0.899 | 2.303 ± 0.858 | 0.532 |
| MMSE language skills | 8.262 ± 1.048 | 8.180 ± 1.016 | 8.157 ± 1.016 | 0.136 |
| MOCA | 21.567 ± 4.235 | 20.781 ± 4.142 a | 21.478 ± 4.075 | 0.011 * |
| MOCA visuospatial function | 2.967 ± 1.265 | 2.879 ± 1.224 | 2.997 ± 1.252 | 0.271 |
| MoCA naming | 2.738 ± 0.570 | 2.721 ± 0.568 | 2.721 ± 0.587 | 0.843 |
| MoCA attention | 5.102 ± 1.103 | 4.970 ± 1.140 | 5.169 ± 0.990 | 0.044 * |
| MoCA language skills | 1.982 ± 0.949 | 1.907 ± 0.914 | 1.958 ± 0.941 | 0.477 |
| MoCA abstracting | 1.007 ± 0.863 | 0.937 ± 0.860 | 0.935 ± 0.857 | 0.498 |
| MoCA memory | 2.193 ± 1.671 | 1.829 ± 1.608 a | 2.119 ± 1.582 b | 0.005 ** |
| MoCA orientation | 5.578 ± 0.786 | 5.539 ± 0.741 | 5.579 ± 0.752 | 0.311 |
| Variables | NW (n = 275) | OW (n = 462) | OB (n = 337) | p |
|---|---|---|---|---|
| C4:0% | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.000 |
| C6:0% | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.000 |
| C8:0% | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.000 |
| C10:0% | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.003 ± 0.059 | 0.335 |
| C11:0% | 0.139 ± 2.299 | 0.139 ± 2.245 | 0.235 ± 2.379 | 0.138 |
| C12:0% | 0.238 ± 0.703 | 0.217 ± 0.659 | 0.370 ± 0.898 | 0.130 |
| C13:0% | 0.069 ± 0.340 | 0.079 ± 0.461 | 0.164 ± 0.715 | 0.109 |
| C14:0% | 0.239 ± 0.452 | 0.214 ± 0.434 | 0.334 ± 0.673 | 0.251 |
| C15:0% | 0.859 ± 1.251 | 1.010 ± 1.921 | 1.088 ± 2.172 | 0.289 |
| C16:0% | 27.651 ± 5.262 | 28.636 ± 5.032 a | 29.333 ± 5.852 ab | <0.001 ** |
| C17:0% | 0.132 ± 0.272 | 0.168 ± 0.591 | 0.098 ± 0.273 ab | 0.001 ** |
| C18:0% | 17.880 ± 4.959 | 17.667 ± 5.058 | 18.836 ± 5.363 b | 0.015 * |
| C20:0% | 0.017 ± 0.099 | 0.007 ± 0.070 | 0.008 ± 0.064 | 0.176 |
| C21:0% | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.001 ± 0.020 | 0.335 |
| C22:0% | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.000 |
| C23:0% | 0.010 ± 0.102 | 0.000 ± 0.000 | 0.007 ± 0.069 | 0.071 |
| C24:0% | 0.038 ± 0.156 | 0.042 ± 0.176 | 0.038 ± 0.191 | 0.448 |
| SFAs% | 47.273 ± 8.336 | 48.179 ± 9.219 | 50.516 ± 9.989 ab | 0.001 ** |
| C14:1% | 0.000 ± 0.000 | 0.011 ± 0.110 | 0.017 ± 0.165 | 0.121 |
| C15:1% | 0.018 ± 0.158 | 0.022 ± 0.182 | 0.039 ± 0.243 | 0.414 |
| C16:1% | 0.099 ± 0.148 | 0.077 ± 0.125 | 0.073 ± 0.143 a | 0.024 * |
| C17:1% | 0.016 ± 0.058 | 0.021 ± 0.109 | 0.008 ± 0.038 b | 0.009 ** |
| C18:1n-9% | 11.172 ± 1.775 | 10.983 ± 2.149 | 10.547 ± 2.371 ab | <0.001 ** |
| C20:1% | 0.021 ± 0.175 | 0.026 ± 0.117 | 0.057 ± 0.228 | 0.375 |
| C22:1n-9% | 0.000 ± 0.000 | 0.001 ± 0.011 | 0.001 ± 0.020 | 0.683 |
| C24:1% | 0.942 ± 1.797 | 0.832 ± 1.593 | 1.089 ± 2.205 | 0.615 |
| MUFAs% | 12.268 ± 2.301 | 11.971 ± 2.616 | 11.831 ± 2.961 | 0.051 |
| C18:2n-6% | 13.703 ± 2.746 | 13.417 ± 3.068 | 12.788 ± 3.210 ab | 0.002 ** |
| C18:3n-6% | 0.001 ± 0.011 | 0.004 ± 0.043 | 0.010 ± 0.083 | 0.322 |
| C20:3n-6% | 1.283 ± 0.607 | 1.249 ± 0.677 | 1.128 ± 0.681 b | 0.028 * |
| C20:4n-6% | 20.488 ± 4.356 | 20.113 ± 4.612 | 18.579 ± 5.384 ab | <0.001 ** |
| n-6 PUFAs% | 35.474 ± 6.398 | 34.784 ± 7.093 | 32.504 ± 8.040 ab | <0.001 ** |
| C18:3n-3% | 0.032 ± 0.153 | 0.036 ± 0.190 | 0.057 ± 0.299 | 0.918 |
| C20:3n-3% | 0.091 ± 1.502 | 0.107 ± 1.623 | 0.454 ± 3.162 | 0.031 * |
| C20:5n-3% | 0.006 ± 0.074 | 0.003 ± 0.041 | 0.002 ± 0.030 | 0.979 |
| C22:6n-3% | 4.816 ± 1.379 | 4.877 ± 1.458 | 4.605 ± 1.558 ab | 0.005 ** |
| n-3 PUFAs% | 4.944 ± 2.059 | 5.023 ± 2.037 | 5.118 ± 3.743 b | 0.015 * |
| C20:2% | 0.041 ± 0.113 | 0.042 ± 0.116 | 0.032 ± 0.101 | 0.385 |
| C22:2% | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.000 |
| PUFAs% | 40.459 ± 7.128 | 39.848 ± 7.938 | 37.653 ± 8.622 ab | <0.001 ** |
| C18:1n-9 t % | 0.000 ± 0.000 | 0.001 ± 0.026 | 0.000 ± 0.000 | 0.512 |
| C18:2n-6t% | 0.000 ± 0.000 | 0.000 ± 0.000 | 0.000 ± 0.000 | 1.000 |
| TFAs% | 0.000 ± 0.000 | 0.001 ± 0.026 | 0.000 ± 0.000 | 0.512 |
| n-6/n-3 | 7.708 ± 2.155 | 7.321 ± 1.755 | 7.220 ± 2.000 | 0.061 |
| Variables | MMSE | MMSE Orientation | MMSE Memory | MMSE Attention | MMSE Delayed Recall | MMSE Language Skills | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | p | B | p | B | p | B | p | B | p | B | p | ||
| NW | |||||||||||||
| C11:0% | −0.011 | 0.846 | −0.094 | 0.114 | −0.026 | 0.015 * | 0.030 | 0.589 | 0.050 | 0.393 | −0.044 | 0.443 | |
| C18:0% | 0.064 | 0.048 * | 0.043 | 0.656 | −0.041 | 0.506 | −0.018 | 0.758 | 0.004 | 0.957 | −0.018 | 0.765 | |
| C20:0% | 0.089 | 0.125 | 0.051 | 0.412 | −0.098 | 0.103 | 1.618 | 0.037 * | 0.028 | 0.650 | −0.016 | 0.781 | |
| C24:0% | −0.078 | 0.166 | −0.070 | 0.237 | −0.033 | 0.570 | −1.117 | 0.021 * | 0.058 | 0.319 | −0.039 | 0.496 | |
| C18:1n-9c% | 0.042 | 0.458 | 0.062 | 0.344 | −0.055 | <0.001 ** | −0.009 | 0.873 | 0.049 | 0.416 | 0.076 | 0.188 | |
| C20:4n-6% | −0.040 | 0.600 | −0.044 | 0.695 | 0.022 | 0.723 | −0.008 | 0.892 | −0.031 | 0.007 ** | 0.091 | 0.117 | |
| PUFAs% | 0.013 | 0.882 | −0.021 | 0.011 * | 0.029 | 0.661 | 0.032 | 0.599 | 0.093 | 0.402 | 0.073 | 0.207 | |
| OW | |||||||||||||
| C16:1% | −0.050 | 0.287 | 0.031 | 0.541 | 0.020 | 0.674 | −1.198 | 0.028 * | 0.026 | 0.594 | −0.028 | 0.527 | |
| C20:4n-6% | 0.044 | 0.599 | 0.049 | 0.584 | 0.018 | 0.703 | −0.029 | 0.540 | −0.030 | 0.001 ** | −0.037 | 0.403 | |
| C20:5n-3% | −0.024 | 0.586 | −0.012 | 0.804 | −0.009 | 0.839 | 0.032 | 0.486 | 0.007 | 0.884 | −2.235 | 0.033 * | |
| n-3 PUFAs% | −0.061 | 0.218 | −0.069 | 0.194 | 0.040 | 0.390 | −0.081 | 0.017 * | 0.050 | 0.411 | 0.002 | 0.956 | |
| PUFAs% | −0.061 | <0.001 ** | −0.021 | <0.001 ** | 0.050 | 0.285 | −0.059 | 0.285 | 0.056 | 0.538 | −0.036 | 0.403 | |
| n-6/n-3 | 0.068 | 0.119 | 0.035 | 0.443 | 0.003 | 0.942 | −0.035 | 0.547 | 0.065 | 0.007 ** | −0.005 | 0.908 | |
| OB | |||||||||||||
| C10:0% | −0.066 | 0.182 | −0.087 | 0.097 | 0.011 | 0.827 | −3.169 | 0.010 * | 0.045 | 0.402 | 0.039 | 0.434 | |
| C11:0% | −0.064 | 0.190 | 0.037 | 0.486 | −0.031 | <0.001 ** | 0.019 | 0.724 | −0.061 | 0.001 ** | −0.023 | 0.646 | |
| C17:0% | −0.070 | 0.155 | −0.434 | 0.011 * | −0.017 | 0.744 | −0.012 | 0.821 | −0.037 | 0.500 | −0.034 | 0.503 | |
| C22:1n-9% | −0.036 | 0.471 | −0.025 | 0.631 | −2.658 | 0.004 ** | −0.028 | 0.603 | 0.037 | 0.495 | −0.041 | 0.413 | |
| C18:2n-6% | −0.084 | 0.088 | 0.049 | 0.421 | −0.021 | 0.682 | −0.064 | 0.238 | −0.033 | 0.024 * | −0.046 | 0.359 | |
| C20:3n-6% | −0.042 | 0.400 | −0.142 | 0.048 * | 0.031 | 0.545 | 0.039 | 0.475 | 0.020 | 0.748 | −0.002 | 0.966 | |
| C20:5n-3% | 9.965 | 0.030 * | 0.074 | 0.163 | 0.071 | 0.171 | 0.074 | 0.173 | 0.098 | 0.073 | 0.023 | 0.643 | |
| Variables | MoCA | MoCA Visuospatial Function | MoCA Naming | MoCA Attention | MoCA Language Skills | MoCA Abstracting | MoCA Memory | MoCA Orientation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | p | B | p | B | p | B | p | B | p | B | p | B | p | B | p | ||
| NW | |||||||||||||||||
| C11:0% | −0.095 | 0.065 | −0.028 | 0.621 | −0.040 | 0.006 ** | −0.039 | 0.490 | −0.052 | 0.022 * | −0.006 | 0.913 | −0.058 | 0.286 | −0.050 | 0.014 * | |
| C18:0% | 0.109 | 0.015 * | 0.033 | 0.567 | 0.035 | 0.562 | 0.053 | 0.354 | −0.089 | 0.200 | −0.086 | 0.335 | 0.088 | 0.108 | 0.028 | 0.767 | |
| C18:2n-6% | 0.050 | 0.425 | 0.108 | 0.060 | −0.035 | 0.548 | 0.094 | 0.095 | −0.068 | <0.001 ** | −0.030 | 0.766 | −0.060 | 0.276 | 0.068 | 0.499 | |
| n-6 PUFAs% | −0.003 | 0.971 | 0.043 | 0.459 | −0.066 | 0.268 | 0.003 | 0.965 | 0.101 | 0.278 | −0.019 | 0.020 * | −0.066 | 0.228 | −0.023 | 0.002 ** | |
| OW | |||||||||||||||||
| C16:0% | −0.058 | 0.385 | −0.016 | 0.725 | 0.062 | 0.184 | 0.024 | 0.605 | 0.049 | 0.323 | 0.055 | 0.230 | −0.055 | 0.018* | 0.042 | 0.576 | |
| C16:1% | −0.072 | 0.112 | −1.273 | 0.004 ** | −0.068 | 0.140 | −0.930 | 0.023 * | 0.041 | 0.388 | −0.012 | 0.789 | −0.045 | 0.351 | 0.045 | 0.370 | |
| C17:1% | −0.050 | 0.230 | 0.033 | 0.459 | 0.025 | 0.595 | −1.424 | 0.002 ** | −0.049 | 0.279 | −0.035 | 0.457 | 0.002 | 0.955 | −0.020 | 0.672 | |
| C20:1% | 0.073 | 0.077 | 0.041 | 0.350 | 0.063 | 0.171 | −0.043 | 0.326 | 0.771 | 0.027 * | 0.020 | 0.664 | 0.073 | 0.106 | 0.022 | 0.639 | |
| C18:3n-6% | 0.005 | 0.901 | −0.067 | 0.138 | 0.035 | 0.456 | −0.020 | 0.648 | 2.071 | 0.030 * | −0.012 | 0.791 | 0.008 | 0.849 | 0.026 | 0.576 | |
| C18:3n-3% | 0.066 | 0.112 | 0.036 | 0.413 | 0.041 | 0.379 | 0.013 | 0.777 | 0.064 | 0.168 | −0.037 | 0.423 | 0.735 | 0.046 * | 0.015 | 0.748 | |
| C20:3n-3% | −0.260 | 0.012 * | −0.015 | 0.738 | −0.031 | 0.504 | −0.080 | 0.010 * | −0.004 | 0.945 | 0.002 | 0.961 | −0.045 | 0.308 | −0.051 | 0.270 | |
| C20:5n-3% | −0.061 | 0.139 | −0.066 | 0.135 | −0.046 | 0.326 | −0.013 | 0.766 | −2.636 | 0.008 ** | −0.008 | 0.863 | 0.029 | 0.515 | 0.022 | 0.633 | |
| n-3 PUFAs% | −0.031 | 0.700 | −0.035 | 0.449 | −0.043 | 0.355 | −0.010 | 0.881 | −0.066 | 0.001 ** | −0.038 | 0.408 | −0.031 | 0.536 | −0.052 | 0.327 | |
| PUFAs% | −0.091 | <0.001** | 0.029 | 0.547 | −0.088 | 0.056 | −0.030 | 0.535 | −0.032 | 0.542 | −0.038 | 0.415 | −0.060 | <0.001 ** | −0.019 | <0.001 ** | |
| OB | |||||||||||||||||
| C10:0% | −0.094 | 0.061 | −0.038 | 0.482 | 0.018 | 0.742 | −2.022 | 0.018 * | −0.061 | 0.267 | −0.012 | 0.825 | −0.030 | 0.583 | −0.104 | 0.053 | |
| C11:0% | −0.072 | 0.141 | −0.038 | 0.472 | −0.074 | 0.174 | −0.033 | 0.530 | −0.065 | 0.232 | 0.027 | 0.615 | −0.070 | 0.045 * | 0.047 | 0.372 | |
| C13:0% | 0.065 | 0.189 | 0.065 | 0.219 | 0.047 | 0.396 | −0.013 | 0.797 | 0.008 | 0.881 | −0.013 | 0.811 | 0.356 | 0.018 * | 0.036 | 0.503 | |
| C17:0% | −0.033 | 0.542 | −0.025 | 0.663 | 0.022 | 0.695 | −0.012 | 0.819 | 0.037 | 0.494 | 0.046 | 0.398 | 0.041 | 0.457 | −0.357 | 0.012 * | |
| C20:0% | 0.004 | 0.930 | −0.030 | 0.576 | −1.246 | 0.014 * | −0.015 | 0.771 | 1.670 | 0.034 * | 0.043 | 0.426 | 0.017 | 0.755 | −0.028 | 0.605 | |
| C20:1% | 1.733 | 0.043 * | 0.643 | 0.026 * | 0.050 | 0.358 | 0.070 | 0.189 | 0.095 | 0.082 | 0.035 | 0.518 | 0.096 | 0.077 | −0.023 | 0.692 | |
| C22:1n-9% | −0.067 | 0.177 | −0.032 | 0.554 | −4.441 | 0.006 ** | −5.091 | 0.048 * | −5.553 | 0.028 * | −0.034 | 0.526 | 0.000 | 0.993 | 0.021 | 0.688 | |
| C20:4n-6% | −0.007 | 0.882 | −0.017 | 0.750 | −0.027 | 0.629 | −0.061 | 0.401 | −0.035 | 0.527 | 0.027 | 0.625 | −0.101 | 0.069 | −0.016 | 0.038 * | |
| C18:3n-3% | 1.370 | 0.035 * | −0.031 | 0.560 | 0.222 | 0.040 * | 0.052 | 0.318 | 0.075 | 0.173 | 0.096 | 0.072 | 0.082 | 0.135 | 0.052 | 0.328 | |
| C20:3n-3% | −0.018 | 0.712 | 0.004 | 0.938 | −0.009 | 0.875 | −0.111 | 0.008 ** | −0.020 | 0.719 | −0.020 | 0.706 | 0.008 | 0.878 | 0.007 | 0.903 | |
| C20:5n-3% | 0.031 | 0.536 | −0.057 | 0.284 | 0.041 | 0.448 | 3.881 | 0.023 * | 0.094 | 0.090 | 0.010 | 0.860 | −0.062 | 0.259 | 0.064 | 0.232 | |
| n-3 PUFAs% | 0.005 | 0.916 | 0.034 | 0.522 | −0.006 | 0.906 | 0.072 | 0.042 * | 0.001 | 0.989 | −0.003 | 0.960 | −0.012 | 0.827 | 0.003 | 0.953 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Li, J.; Hua, Y.; Ding, B.; Zhou, C.; Yu, H.; Xiao, R.; Ma, W. Association between the Erythrocyte Membrane Fatty Acid Profile and Cognitive Function in the Overweight and Obese Population Aged from 45 to 75 Years Old. Nutrients 2022, 14, 914. https://doi.org/10.3390/nu14040914
Shen J, Li J, Hua Y, Ding B, Zhou C, Yu H, Xiao R, Ma W. Association between the Erythrocyte Membrane Fatty Acid Profile and Cognitive Function in the Overweight and Obese Population Aged from 45 to 75 Years Old. Nutrients. 2022; 14(4):914. https://doi.org/10.3390/nu14040914
Chicago/Turabian StyleShen, Jingyi, Jinchen Li, Yinan Hua, Bingjie Ding, Cui Zhou, Huiyan Yu, Rong Xiao, and Weiwei Ma. 2022. "Association between the Erythrocyte Membrane Fatty Acid Profile and Cognitive Function in the Overweight and Obese Population Aged from 45 to 75 Years Old" Nutrients 14, no. 4: 914. https://doi.org/10.3390/nu14040914
APA StyleShen, J., Li, J., Hua, Y., Ding, B., Zhou, C., Yu, H., Xiao, R., & Ma, W. (2022). Association between the Erythrocyte Membrane Fatty Acid Profile and Cognitive Function in the Overweight and Obese Population Aged from 45 to 75 Years Old. Nutrients, 14(4), 914. https://doi.org/10.3390/nu14040914







