Nutritional and Metabolic Imbalance in Keratoconus
Abstract
:1. Introduction
2. Pathogenesis of Keratoconus
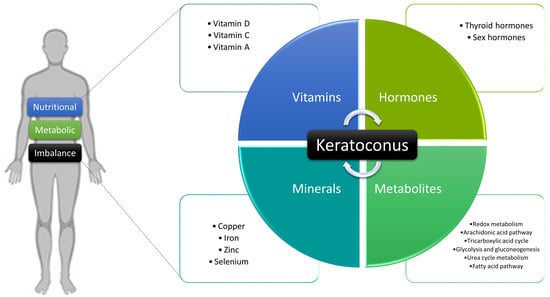
3. Nutritional and Metabolic Alterations in Keratoconus
3.1. Vitamins
3.2. Minerals
3.3. Hormones
3.3.1. Thyroid Hormones
3.3.2. Sex Hormones
3.4. Metabolites
3.4.1. Redox Metabolism
3.4.2. Arachidonic Acid Pathway
3.4.3. Tricarboxylic Acid Cycle
3.4.4. Glycolysis and Gluconeogenesis
3.4.5. Urea Cycle Metabolism
3.4.6. Fatty Acid Metabolism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tuft, S.J.; Moodaley, L.C.; Gregory, W.M.; Davison, C.R.; Buckley, R.J. Prognostic Factors for the Progression of Keratoconus. Ophthalmology 1994, 101, 439–447. [Google Scholar] [CrossRef]
- Ferrari, G.; Rama, P. The keratoconus enigma: A review with emphasis on pathogenesis. Ocul. Surf. 2020, 18, 363–373. [Google Scholar] [CrossRef]
- Hashemi, H.; Heydarian, S.; Hooshmand, E.; Saatchi, M.; Yekta, A.; Aghamirsalim, M.; Valadkhan, M.; Mortazavi, M.; Hashemi, A.; Khabazkhoob, M. The Prevalence and Risk Factors for Keratoconus: A Systematic Review and Meta-Analysis. Cornea 2020, 39, 263–270. [Google Scholar] [CrossRef]
- Anitha, V.; Vanathi, M.; Raghavan, A.; Rajaraman, R.; Ravindran, M.; Tandon, R. Pediatric keratoconus—Current perspectives and clinical challenges. Indian J. Ophthalmol. 2021, 69, 214–225. [Google Scholar] [CrossRef]
- Mukhtar, S.; Ambati, B.K. Pediatric keratoconus: A review of the literature. Int. Ophthalmol. 2018, 38, 2257. [Google Scholar] [CrossRef]
- Chan, E.; Chong, E.W.; Lingham, G.; Stevenson, L.J.; Sanfilippo, P.G.; Hewitt, A.W.; Mackey, D.A.; Yazar, S. Prevalence of Keratoconus Based on Scheimpflug Imaging: The Raine Study. Ophthalmology 2021, 128, 515–521. [Google Scholar] [CrossRef]
- Woodward, M.A.; Blachley, T.S.; Stein, J.D. The association between sociodemographic factors, common systemic diseases, and keratoconus an analysis of a nationwide heath care claims database. Ophthalmology 2016, 123, 457–465.e2. [Google Scholar] [CrossRef] [Green Version]
- Gordon-Shaag, A.; Millodot, M.; Shneor, E.; Liu, Y. The genetic and environmental factors for keratoconus. BioMed Res. Int. 2015, 2015, 795738. [Google Scholar] [CrossRef] [Green Version]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Lass, J.H.; Lembach, R.G.; Park, S.B.; Hom, D.L.; Fritz, M.E.; Svilar, G.M.; Nuamah, I.F.; Reinhart, W.J.; Stocker, E.G.; Keates, R.H.; et al. Clinical Management of Keratoconus: A Multicenter Analysis. Ophthalmology 1990, 97, 433–445. [Google Scholar] [CrossRef]
- Wang, Y.; Rabinowitz, Y.S.; Rotter, J.I.; Yang, H. Genetic epidemiological study of keratoconus: Evidence for major gene determination. Am. J. Med. Genet. 2000, 93, 403–409. [Google Scholar] [CrossRef]
- Hawkes, E.; Nanavaty, M.A. Eye Rubbing and Keratoconus: A Literature Review. Int. J. Keratoconus Ectatic Corneal Dis. 2014, 3, 118–121. [Google Scholar] [CrossRef]
- Bawazeer, A.M.; Hodge, W.G.; Lorimer, B. Atopy and keratoconus: A multivariate analysis. Br. J. Ophthalmol. 2000, 84, 834–836. [Google Scholar] [CrossRef] [Green Version]
- Pedrotti, E.; Demasi, C.L.; Fasolo, A.; Bonacci, E.; Brighenti, T.; Gennaro, N.; Ferrari, M.; Marchini, G. Obstructive Sleep Apnea Assessed by Overnight Polysomnography in Patients With Keratoconus. Cornea 2018, 37, 470–473. [Google Scholar] [CrossRef]
- Sharif, K.W.; Casey, T.A.; Coltart, J. Prevalence of mitral valve prolapse in keratoconus patients. J. R. Soc. Med. 1992, 85, 446–448. [Google Scholar] [CrossRef]
- Beardsley, T.L.; Foulks, G.N. An Association of Keratoconus and Mitral Valve Prolapse. Ophthalmology 1982, 89, 35–37. [Google Scholar] [CrossRef]
- Lee, R.; Hafezi, F.; Bradley Randleman, J. Bilateral keratoconus induced by secondary hypothyroidism after radioactive iodine therapy. J. Refract. Surg. 2018, 34, 351–353. [Google Scholar] [CrossRef]
- Marsack, J.D.; Benoit, J.S.; Kollbaum, P.S.; Anderson, H.A. Application of Topographical Keratoconus Detection Metrics to Eyes of Individuals with Down Syndrome. Optom. Vis. Sci. 2019, 96, 664–669. [Google Scholar] [CrossRef]
- Alio, J.L.; Vega-Estrada, A.; Sanz, P.; Osman, A.A.; Kamal, A.M.; Mamoon, A.; Soliman, H. Corneal morphologic characteristics in patients with down syndrome. JAMA Ophthalmol. 2018, 136, 971–978. [Google Scholar] [CrossRef] [Green Version]
- Ottas, A.; Fishman, D.; Okas, T.L.; Püssa, T.; Toomik, P.; Märtson, A.; Kingo, K.; Soomets, U. Blood serum metabolome of atopic dermatitis: Altered energy cycle and the markers of systemic inflammation. PLoS ONE 2017, 12, e0188580. [Google Scholar] [CrossRef] [Green Version]
- Shechter, A. Obstructive sleep apnea and energy balance regulation: A systematic review. Sleep Med. Rev. 2017, 34, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, D.; Wyka, J. Down syndrome—Genetic and nutritional aspects of accompanying disorders. Rocz. Państwowego Zakładu Hig. 2015, 66, 189–194. [Google Scholar]
- Penders, J.; Thijs, C.; Van Den Brandt, P.A.; Kummeling, I.; Snijders, B.; Stelma, F.; Adams, H.; Van Ree, R.; Stobberingh, E.E. Gut microbiota composition and development of atopic manifestations in infancy: The KOALA birth cohort study. Gut 2007, 56, 661–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burns, D.M.; Johnston, F.M.; Frazer, D.G.; Patterson, C.; Jackson, A.J. Keratoconus: An analysis of corneal asymmetry. Br. J. Ophthalmol. 2004, 88, 1252–1255. [Google Scholar] [CrossRef]
- Ambekar, R.; Toussaint, K.C.; Wagoner Johnson, A. The effect of keratoconus on the structural, mechanical, and optical properties of the cornea. J. Mech. Behav. Biomed. Mater. 2011, 4, 223–236. [Google Scholar] [CrossRef]
- Kenney, M.C.; Nesburn, A.B.; Burgeson, R.E.; Butkowski, R.J.; Ljubimov, A. V Abnormalities of the extracellular matrix in keratoconus corneas. Cornea 1997, 16, 345–351. [Google Scholar] [CrossRef]
- Kaldawy, R.M.; Wagner, J.; Ching, S.; Seigel, G.M. Evidence of apoptotic cell death in keratoconus. Cornea 2002, 21, 206–209. [Google Scholar] [CrossRef]
- Mannion, L.S.; Tromans, C.; O’Donnell, C. An evaluation of corneal nerve morphology and function in moderate keratoconus. Contact Lens Anterior Eye 2005, 28, 185–192. [Google Scholar] [CrossRef]
- Shetty, R.; D’Souza, S.; Khamar, P.; Ghosh, A.; Nuijts, R.M.M.A.; Sethu, S. Biochemical markers and alterations in keratoconus. Asia-Pac. J. Ophthalmol. 2020, 9, 533–540. [Google Scholar] [CrossRef]
- Pinnell, S.R. Regulation of collagen biosynthesis by ascorbic acid: A review. Yale J. Biol. Med. 1985, 58, 553–559. [Google Scholar]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D.P. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: Relevance in keratoconus. Clin. Exp. Optom. 2013, 96, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Senni, C.; Bernabei, F.; Cicero, A.F.G.; Vagge, A.; Maestri, A.; Scorcia, V.; Giannaccare, G. The role of nutrition and nutritional supplements in ocular surface diseases. Nutrients 2020, 12, 952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, D.A.; Rocha, E.M.; Aragona, P.; Clayton, J.A.; Ding, J.; Golebiowski, B.; Hampel, U.; Mcdermott, A.M.; Schaumberg, D.A.; Srinivasan, S.; et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul. Surf. 2017, 15, 284–333. [Google Scholar] [CrossRef]
- McMillan, J. Spectrum of Darkness, Agent of Light: Myopia, Keratoconus, Ocular Surface Disease, and Evidence for a Profoundly Vitamin D-dependent Eye. Cureus 2018, 10, e2744. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Watsky, M.A. Influence of Vitamin D on corneal epithelial cell desmosomes and hemidesmosomes. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4074–4083. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Pintea, V.; Lin, Y.; Hammock, B.D.; Watsky, M.A. Vitamin D enhances corneal epithelial barrier function. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7359–7364. [Google Scholar] [CrossRef] [Green Version]
- Cankaya, C.; Cumurcu, T.; Gunduz, A. Corneal endothelial changes in patients with Vitamin D deficiency. Indian J. Ophthalmol. 2018, 66, 1256–1261. [Google Scholar] [CrossRef]
- Reins, R.Y.; Baidouri, H.; McDermott, A.M. Vitamin D Activation and Function in Human Corneal Epithelial Cells During TLR-Induced Inflammation. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7715. [Google Scholar] [CrossRef] [Green Version]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.T.; Cerquinho, R.G.; Perez, M.M.; da Alves, B.C.A.; Pereira, E.C.; Azzalis, L.A.; Junqueira, V.B.C.; Soares, L.R.; Fonseca, F.L.A. Determination of vitamin D in tears of healthy individuals by the electrochemiluminescence method. J. Clin. Lab. Anal. 2019, 33, 22830. [Google Scholar] [CrossRef]
- Lee, H.K.; Jung, E.H.; Cho, B.J. Epidemiological Association between Systemic Diseases and Keratoconus in a Korean Population: A 10-Year Nationwide Cohort Study. Cornea 2020, 39, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Blackberg, S.N.; Knapp, A.A. Ocular Changes Accompanying Disturbances of Calcium-Phosphorus Metabolism. Arch. Ophthalmol. 1934, 11, 665. [Google Scholar] [CrossRef]
- Knapp, A.A. Results of vitamin-D-complex treatment of keratoconus. Preliminary study. Am. J. Ophthalmol. 1939, 22, 289–292. [Google Scholar] [CrossRef]
- Akkaya, S.; Ulusoy, D.M. Serum Vitamin D Levels in Patients with Keratoconus. Ocul. Immunol. Inflamm. 2020, 28, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Aslan, M.G.; Fındık, H.; Okutucu, M.; Aydın, E.; Oruç, Y.; Arpa, M.; Uzun, F. Serum 25-Hydroxy Vitamin D, Vitamin B12, and Folic Acid Levels in Progressive and Nonprogressive Keratoconus. Cornea 2021, 40, 334–341. [Google Scholar] [CrossRef]
- Zarei-Ghanavati, S.; Yahaghi, B.; Hassanzadeh, S.; Mobarhan, M.G.; Hakimi, H.R.; Eghbali, P. Serum 25-hydroxyvitamin D, selenium, zinc and copper in patients with keratoconus. J. Curr. Ophthalmol. 2020, 32, 26–31. [Google Scholar] [CrossRef]
- López-López, M.; Regueiro, U.; Bravo, S.B.; del Chantada-Vázquez, M.P.; Varela-Fernández, R.; Ávila-Gómez, P.; Hervella, P.; Lema, I. Tear proteomics in keratoconus: A quantitative SWATH-MS analysis. Investig. Ophthalmol. Vis. Sci. 2021, 62, 30. [Google Scholar] [CrossRef]
- Saika, S.; Uenoyama, K.; Hiroi, K.; Tanioka, H.; Takase, K.; Hikita, M. Ascorbic acid phosphate ester and wound healing in rabbit corneal alkali burns: Epithelial basement membrane and stroma. Graefe’s Arch. Clin. Exp. Ophthalmol. 1993, 231, 221–227. [Google Scholar] [CrossRef]
- Guo, X.; Hutcheon, A.E.K.; Melotti, S.A.; Zieske, J.D.; Trinkaus-Randall, V.; Ruberti, J.W. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2007, 48, 4050–4060. [Google Scholar] [CrossRef]
- Grobe, G.M.; Reichl, S. Characterization of Vitamin C-induced cell sheets formed from primary and immortalized human corneal stromal cells for tissue engineering applications. Cells Tissues Organs 2013, 197, 283–297. [Google Scholar] [CrossRef]
- Aghaei, N.; Ramin, S.; Aghaei, A. In Vitro Effects of Ascorbic Acid on Corneal Collagen Cross-Linking in Keratoconus. World Fam. Med. J./Middle East J. Fam. Med. 2017, 15, 133–140. [Google Scholar] [CrossRef]
- Snytnikova, O.A.; Yanshole, L.V.; Iskakov, I.A.; Yanshole, V.V.; Chernykh, V.V.; Stepakov, D.A.; Novoselov, V.P.; Tsentalovich, Y.P. Quantitative metabolomic analysis of the human cornea and aqueous humor. Metabolomics 2017, 13, 152. [Google Scholar] [CrossRef]
- Sharif, R.; Sejersen, H.; Frank, G.; Hjortdal, J.; Karamichos, D. Effects of collagen cross-linking on the keratoconus metabolic network. Eye 2018, 32, 1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saijyothi, A.V.; Fowjana, J.; Madhumathi, S.; Rajeshwari, M.; Thennarasu, M.; Prema, P.; Angayarkanni, N. Tear fluid small molecular antioxidants profiling shows lowered glutathione in keratoconus. Exp. Eye Res. 2012, 103, 41–46. [Google Scholar] [CrossRef]
- Mutch, J.R.; Richards, M.B. Keratoconus Experimentally Produced in The Rat by Vitamin A Deficiency. Br. J. Ophthalmol. 1939, 23, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, J.; Wang, L.; Huang, Y. Topical retinoic acid induces corneal strengthening by upregulating transglutaminase 2 in murine cornea. Exp. Eye Res. 2022, 214, 108850. [Google Scholar] [CrossRef]
- Yilmaz, M.; Arikan, S.; Türkön, H. Plasma homocysteine levels in patients with keratoconus. Clin. Exp. Optom. 2020, 103, 804–807. [Google Scholar] [CrossRef]
- Dudakova, L.; Liskova, P.; Jirsova, K. Is copper imbalance an environmental factor influencing keratoconus development? Med. Hypotheses 2015, 84, 518–524. [Google Scholar] [CrossRef]
- Bamdad, S.; Owji, N.; Bolkheir, A. Association between advanced keratoconus and serum levels of zinc, calcium, magnesium, iron, copper, and selenium. Cornea 2018, 37, 1306–1310. [Google Scholar] [CrossRef]
- Ortak, H.; Söǧüt, E.; Taş, U.; Mesci, C.; Mendil, D. The relation between keratoconus and plasma levels of MMP-2, zinc, and SOD. Cornea 2012, 31, 1048–1051. [Google Scholar] [CrossRef]
- Kiliç, R.; Bayraktar, A.C.; Bayraktar, S.; Kurt, A.; Kavutçu, M. Evaluation of serum superoxide dismutase activity, malondialdehyde, and zinc and copper levels in patients with keratoconus. Cornea 2016, 35, 1512–1515. [Google Scholar] [CrossRef] [PubMed]
- Avetisov, S.E.; Mamikonian, V.R.; Novikov, I.A. The role of tear acidity and Cu-cofactor of lysyl oxidase activity in the pathogenesis of keratoconus. Vestn. Oftalmol. 2011, 127, 3–8. [Google Scholar] [PubMed]
- Avetisov, S.E.; Mamikonyan, V.R.; Novikov, I.A.; Pateyuk, L.S.; Osipyan, G.A.; Kiryushchenkova, N.P. Abnormal distribution of trace elements in keratoconic corneas. Ann. Ophthalmol. Vestn. Oftal’Mologii 2015, 131, 34. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Lin, L.; Wu, Z.; Jin, X.; Ni, H. Kayser-Fleischer ring with keratoconus: A coincidence? A case report. BMC Ophthalmol. 2020, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Kagan, H.M.; Trackman, P.C. Properties and function of lysyl oxidase. Am. J. Respir. Cell Mol. Biol. 1991, 5, 206–210. [Google Scholar] [CrossRef]
- Cantemir, A.; Alexa, A.I.; Ciobica, A.; Balmus, I.M.; Antioch, I.; Stoica, B.; Chiselita, D.; Costin, D. Evaluation of antioxidant enzymes in keratoconus. Rev. Chim. 2016, 67, 1538–1541. [Google Scholar]
- Tekin, S.; Seven, E. Assessment of serum catalase, reduced glutathione, and superoxide dismutase activities and malondialdehyde levels in keratoconus patients. Eye 2021. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D.P. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp. Eye Res. 2012, 96, 132–137. [Google Scholar] [CrossRef]
- Chaerkady, R.; Shao, H.; Scott, S.G.; Pandey, A.; Jun, A.S.; Chakravarti, S. The keratoconus corneal proteome: Loss of epithelial integrity and stromal degeneration. J. Proteom. 2013, 87, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Wójcik, K.A.; Synowiec, E.; Jiménez-García, M.P.; Kaminska, A.; Polakowski, P.; Blasiak, J.; Szaflik, J.; Szaflik, J.P. Polymorphism of the transferrin gene in eye diseases: Keratoconus and Fuchs endothelial corneal dystrophy. BioMed Res. Int. 2013, 2013, 247438. [Google Scholar] [CrossRef] [Green Version]
- Rouault, T.A. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nat. Chem. Biol. 2006, 2, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Thanos, S.; Oellers, P.; Meyer Zu Hörste, M.; Prokosch, V.; Schlatt, S.; Seitz, B.; Gatzioufas, Z. Role of thyroxine in the development of keratoconus. Cornea 2016, 35, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- El-Massry, A.; Doheim, M.F.; Iqbal, M.; Fawzy, O.; Said, O.M.; Yousif, M.O.; Badawi, A.E.; Tawfik, A.; Abousamra, A. Association between keratoconus and thyroid gland dysfunction: A cross-sectional case-control study. J. Refract. Surg. 2020, 36, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.J. Thyroid Gland Dysfunction and Keratoconus. Cornea 2018, 37, e3–e4. [Google Scholar] [CrossRef]
- AlHawari, H.H.; Khader, Y.S.; AlHawari, H.H.; Alomari, A.F.; Abbasi, H.N.; El-Faouri, M.S.; Al Bdour, M.D. Autoimmune Thyroid Disease and Keratoconus: Is There an Association? Int. J. Endocrinol. 2018, 2018, 7907512. [Google Scholar] [CrossRef] [PubMed]
- Flaskó, Z.; Zemova, E.; Eppig, T.; Módis, L.; Langenbucher, A.; Wagenpfeil, S.; Seitz, B.; Szentmáry, N. Hypothyroidism is Not Associated with Keratoconus Disease: Analysis of 626 Subjects. J. Ophthalmol. 2019, 2019, 3268595. [Google Scholar] [CrossRef]
- Kahán, I.L.; Varsányi-Nagy, M.; Tóth, M.; Nádrai, A. The possible role of tear fluid thyroxine in keratoconus development. Exp. Eye Res. 1990, 50, 339–343. [Google Scholar] [CrossRef]
- Stachon, T.; Stachon, A.; Hartmann, U.; Seitz, B.; Langenbucher, A.; Szentmáry, N. Urea, Uric Acid, Prolactin and fT4 Concentrations in Aqueous Humor of Keratoconus Patients. Curr. Eye Res. 2017, 42, 842–846. [Google Scholar] [CrossRef]
- Karamichos, D.; Escandon, P.; Vasini, B.; Nicholas, S.E.; Van, L.; Dang, D.H.; Cunningham, R.L.; Riaz, K.M. Anterior pituitary, sex hormones, and keratoconus: Beyond traditional targets. Prog. Retin. Eye Res. 2021, 101016. [Google Scholar] [CrossRef]
- Fink, B.A.; Sinnott, L.T.; Wagner, H.; Friedman, C.; Zadnik, K. The influence of gender and hormone status on the severity and progression of keratoconus. Cornea 2010, 29, 65–72. [Google Scholar] [CrossRef]
- Kennedy, R.H.; Bourne, W.M.; Dyer, J.A. A 48-year clinical and epidemiologic study of keratoconus. Am. J. Ophthalmol. 1986, 101, 267–273. [Google Scholar] [CrossRef]
- Stock, R.A.; Thumé, T.; Bonamigo, E.L. Acute corneal hydrops during pregnancy with spontaneous resolution after corneal cross-linking for keratoconus: A case report. J. Med. Case Rep. 2017, 11, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgihan, K.; Hondur, A.; Sul, S.; Ozturk, S. Pregnancy-induced progression of keratoconus. Cornea 2011, 30, 991–994. [Google Scholar] [CrossRef]
- Yin, H.; Luo, C.; Tian, Y.; Deng, Y. Altered expression of sex hormone receptors in keratoconus corneas. Biomed. Res. 2017, 28, 5089–5092. [Google Scholar]
- Ayan, B.; Yuksel, N.; Carhan, A.; Gumuşkaya Ocal, B.; Akcay, E.; Cagil, N.; Asik, M.D. Evaluation estrogen, progesteron and androgen receptor expressions in corneal epithelium in keratoconus. Contact Lens Anterior Eye 2019, 42, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Sharif, R.; Bak-Nielsen, S.; Sejersen, H.; Ding, K.; Hjortdal, J.; Karamichos, D. Prolactin-Induced Protein is a novel biomarker for Keratoconus. Exp. Eye Res. 2019, 179, 55–63. [Google Scholar] [CrossRef]
- McKay, T.B.; Hjortdal, J.; Sejersen, H.; Asara, J.M.; Wu, J.; Karamichos, D. Endocrine and Metabolic Pathways Linked to Keratoconus: Implications for the Role of Hormones in the Stromal Microenvironment. Sci. Rep. 2016, 6, 25534. [Google Scholar] [CrossRef] [Green Version]
- Karamichos, D.; Barrientez, B.; Nicholas, S.; Ma, S.; Van, L.; Bak-Nielsen, S.; Hjortdal, J. Gonadotropins in Keratoconus: The Unexpected Suspects. Cells 2019, 8, 1494. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D. Oxidative Stress. In Encyclopedia of Stress; Academic Press: Cambridge, MA, USA, 2007; pp. 45–48. ISBN 9780123739476. [Google Scholar]
- Wakamatsu, T.H.; Dogru, M.; Ayako, I.; Takano, Y.; Matsumoto, Y.; Ibrahim, O.M.A.; Okada, N.; Satake, Y.; Fukagawa, K.; Shimazaki, J.; et al. Evaluation of lipid oxidative stress status and inflammation in atopic ocular surface disease. Mol. Vis. 2010, 16, 2465–2475. [Google Scholar]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, M.P.; Chihuailaf, R.H. Antioxidants and the integrity of ocular tissues. Vet. Med. Int. 2011, 2011, 905153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cejka, C.; Cejkova, J. Oxidative stress to the cornea, changes in corneal optical properties, and advances in treatment of corneal oxidative injuries. Oxid. Med. Cell. Longev. 2015, 2015, 591530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gondhowiardjo, T.D.; Van Haeringen, N.J.; Volker-Dieben, H.J.; Beekhuis, H.W.; Kok, J.H.C.; Van Rij, G.; Pels, L.; Kijlstra, A. Analysis of corneal aldehyde dehydrogenase patterns in pathologic corneas. Cornea 1993, 12, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Buddi, R.; Lin, B.; Atilano, S.R.; Zorapapel, N.C.; Kenney, M.C.; Brown, D.J. Evidence of oxidative stress in human corneal diseases. J. Histochem. Cytochem. 2002, 50, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Chwa, M.; Atilano, S.R.; Reddy, V.; Jordan, N.; Kim, D.W.; Kenney, M.C. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Investig. Ophthalmol. Vis. Sci. 2006, 47, 1902–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnal, E.; Peris-Martínez, C.; Menezo, J.L.; Johnsen-Soriano, S.; Romero, F.J. Oxidative stress in keratoconus? Investig. Ophthalmol. Vis. Sci. 2011, 52, 8592–8597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atilano, S.; Lee, D.; Fukuhara, P.; Chwa, M.; Nesburn, A.; Udar, N.; Kenney, C. Corneal Oxidative Damage in Keratoconus Cells due to Decreased Oxidant Elimination from Modified Expression Levels of SOD Enzymes, PRDX6, SCARA3, CPSF3, and FOXM1. J. Ophthalmic Vis. Res. 2019, 14, 62. [Google Scholar] [CrossRef]
- Karamichos, D.; Zieske, J.D.; Sejersen, H.; Sarker-Nag, A.; Asara, J.M.; Hjortdal, J. Tear metabolite changes in keratoconus. Exp. Eye Res. 2015, 132, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Navel, V.; Malecaze, J.; Pereira, B.; Baker, J.S.; Malecaze, F.; Sapin, V.; Chiambaretta, F.; Dutheil, F. Oxidative and antioxidative stress markers in keratoconus: A systematic review and meta-analysis. Acta Ophthalmol. 2021, 99, e777–e794. [Google Scholar] [CrossRef]
- Behndig, A.; Karlsson, K.; Johansson, B.O.; Brännström, T.; Marklund, S.L. Superoxide dismutase isoenzymes in the normal and diseased human cornea. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2293–2296. [Google Scholar]
- Karamichos, D.; Hutcheon, A.E.K.; Rich, C.B.; Trinkaus-Randall, V.; Asara, J.M.; Zieske, J.D. In vitro model suggests oxidative stress involved in keratoconus disease. Sci. Rep. 2014, 4, 4608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toprak, I.; Kucukatay, V.; Yildirim, C.; Kilic-Toprak, E.; Kilic-Erkek, O. Increased systemic oxidative stress in patients with keratoconus. Eye 2014, 28, 285–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenney, M.C.; Brown, D.J. The cascade hypothesis of keratoconus. Contact Lens Anterior Eye 2003, 26, 139–146. [Google Scholar] [CrossRef]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Yarla, N.S.; Bishayee, A.; Sethi, G.; Reddanna, P.; Kalle, A.M.; Dhananjaya, B.L.; Dowluru, K.S.V.G.K.; Chintala, R.; Duddukuri, G.R. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin. Cancer Biol. 2016, 40–41, 48–81. [Google Scholar] [CrossRef]
- Radi, Z.A.; Render, J.A. The pathophysiologic role of cyclo-oxygenases in the eye. J. Ocul. Pharmacol. Ther. 2008, 24, 141–151. [Google Scholar] [CrossRef]
- Pouliquen, Y.; Bureau, J.; Mirshahi, M.; Mirshahi, S.S.; Assouline, M.; Lorens, G. Keratoconus and inflammatory processes. Bull. Soc. Belge Ophtalmol. 1996, 262, 25–28. [Google Scholar]
- Bureau, J.; Fabre, E.J.; Hecquet, C.; Pouliquen, Y.; Lorans, G. Modification of prostaglandin E2 and collagen synthesis in keratoconus fibroblasts, associated with an increase of interleukin 1α receptor number. Comptes Rendus l’Academie des Sci.-Ser. III 1993, 316, 425–430. [Google Scholar] [CrossRef]
- Bureau, J. Synthesis of t-PA, PAI, TGF-β1 in keratoconus cells. Vis. Res. 1995, 35, S178. [Google Scholar] [CrossRef]
- Daphne Teh, A.L.; Jayapalan, J.J.; Loke, M.F.; Wan Abdul Kadir, A.J.; Subrayan, V. Identification of potential serum metabolic biomarkers for patient with keratoconus using untargeted metabolomics approach. Exp. Eye Res. 2021, 211, 108734. [Google Scholar] [CrossRef]
- Galvis, V.; Sherwin, T.; Tello, A.; Merayo, J.; Barrera, R.; Acera, A. Keratoconus: An inflammatory disorder? Eye 2015, 29, 843–859. [Google Scholar] [CrossRef] [Green Version]
- Engelking, L.R. Leaks in the Tricarboxylic Acid (TCA) Cycle. In Textbook of Veterinary Physiological Chemistry; Academic Press: Cambridge, MA, USA, 2015; pp. 214–218. ISBN 978-0-12-391909-0. [Google Scholar]
- Rowan, S.; Jiang, S.; Chang, M.L.; Szymanski, J.; Korem, T.; Segal, E.; Cassalman, C.; McGuire, C.; Baleja, J.D.; Clish, C.B.; et al. Interaction of metabolome and microbiome contributes to dietary glycemia-induced age-related macular degeneration in aged C57BL/6J mice. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5002. [Google Scholar]
- Hartong, D.T.; Dange, M.; McGee, T.L.; Berson, E.L.; Dryja, T.P.; Colman, R.F. Insights from retinitis pigmentosa into the roles of isocitrate dehydrogenases in the Krebs cycle. Nat. Genet. 2008, 40, 1230–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: A Review. Ann. Bot. 2003, 91, 179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, M. Mini-review on Glycolysis and Cancer. J. Cancer Educ. 2013, 28, 454–457. [Google Scholar] [CrossRef]
- Hatting, M.; Tavares, C.D.J.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2018, 1411, 21–35. [Google Scholar] [CrossRef]
- McKay, T.B.; Lyon, D.; Sarker-Nag, A.; Priyadarsini, S.; Asara, J.M.; Karamichos, D. Quercetin Attenuates Lactate Production and Extracellular Matrix Secretion in Keratoconus. Sci. Rep. 2015, 5, 9003. [Google Scholar] [CrossRef]
- Mckay, T.B.; Sarker-Nag, A.; Lyon, D.; Asara, J.M.; Karamichos, D. Quercetin modulates keratoconus metabolism in vitro. Cell Biochem. Funct. 2015, 33, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Whelchel, A.E.; McKay, T.B.; Priyadarsini, S.; Rowsey, T.; Karamichos, D. Association between Diabetes and Keratoconus: A Retrospective Analysis. Sci. Rep. 2019, 9, 13808. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Liu, Z.; Pan, S.; Jiang, Z.; Lu, H.; Amit, O.; Bradbury, E.M.; Hu, C.A.A.; Chen, X. Global investigation of p53-induced apoptosis through quantitative proteomic profiling using comparative amino acid-coded tagging. Mol. Cell. Proteom. 2004, 3, 998–1008. [Google Scholar] [CrossRef] [Green Version]
- McKay, T.B.; Hjortdal, J.; Sejersen, H.; Karamichos, D. Differential Effects of Hormones on Cellular Metabolism in Keratoconus in Vitro. Sci. Rep. 2017, 7, 42896. [Google Scholar] [CrossRef] [PubMed]
- McKay, T.B.; Priyadarsini, S.; Karamichos, D. Mechanisms of Collagen Crosslinking in Diabetes and Keratoconus. Cells 2019, 8, 1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, S.; Häberle, J.; Kido, J.; Mitsubuchi, H.; Endo, F.; Nakamura, K. Urea cycle disorders—Update. J. Hum. Genet. 2019, 64, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Kenchegowda, S.; Bazan, H.E.P. Significance of lipid mediators in corneal injury and repair. J. Lipid Res. 2010, 51, 879–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschetter, R.T. Lipid Analysis of the Human Cornea with and without Arcus Senilis. Arch. Ophthalmol. 1966, 76, 403–405. [Google Scholar] [CrossRef]
- Wojakowska, A.; Pietrowska, M.; Widlak, P.; Dobrowolski, D.; Wylegała, E.; Tarnawska, D. Metabolomic signature discriminates normal human cornea from Keratoconus—A pilot GC/MS study. Molecules 2020, 25, 2933. [Google Scholar] [CrossRef]
- McKay, T.B.; Hjortdal, J.; Priyadarsini, S.; Karamichos, D. Acute hypoxia influences collagen and matrix metalloproteinase expression by human keratoconus cells in vitro. PLoS ONE 2017, 12, e0176017. [Google Scholar] [CrossRef] [Green Version]
- Fadó, R.; Rodríguez-Rodríguez, R.; Casals, N. The return of malonyl-CoA to the brain: Cognition and other stories. Prog. Lipid Res. 2021, 81, 101071. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasagni Vitar, R.M.; Bonelli, F.; Rama, P.; Ferrari, G. Nutritional and Metabolic Imbalance in Keratoconus. Nutrients 2022, 14, 913. https://doi.org/10.3390/nu14040913
Lasagni Vitar RM, Bonelli F, Rama P, Ferrari G. Nutritional and Metabolic Imbalance in Keratoconus. Nutrients. 2022; 14(4):913. https://doi.org/10.3390/nu14040913
Chicago/Turabian StyleLasagni Vitar, Romina Mayra, Filippo Bonelli, Paolo Rama, and Giulio Ferrari. 2022. "Nutritional and Metabolic Imbalance in Keratoconus" Nutrients 14, no. 4: 913. https://doi.org/10.3390/nu14040913
APA StyleLasagni Vitar, R. M., Bonelli, F., Rama, P., & Ferrari, G. (2022). Nutritional and Metabolic Imbalance in Keratoconus. Nutrients, 14(4), 913. https://doi.org/10.3390/nu14040913