Abstract
Osteoporosis is one of the most common extraintestinal complications among patients suffering from inflammatory bowel diseases. The role of vitamin D and calcium in the prevention of a decreased bone mineral density is well known, although other nutrients, including micronutrients, are also of extreme importance. Despite the fact that zinc, copper, selenium, iron, cadmium, silicon and fluorine have not been frequently discussed with regard to the prevention of osteoporosis, it is possible that a deficiency or excess of the abovementioned elements may affect bone mineralization. Additionally, the risk of malnutrition, which is common in patients with ulcerative colitis or Crohn’s disease, as well as the composition of gut microbiota, may be associated with micronutrients status.
1. Introduction
More and more people suffer from inflammatory bowel disease (IBD). In 2017, IBD affected 6.8 million people worldwide [1]. In Europe, in 2010, the incidence of ulcerative colitis (UC) and Crohn’s disease (CD) amounted to 9.8 and 6.3 per 100,000 inhabitants, respectively [2]. Moreover, due to their condition, IBD patients are particularly at a risk of developing malnutrition and extraintestinal manifestations [3].
According to the World Health Organization, malnutrition is a deficiency, imbalance or an excess intake of energy or/and nutrients, including micronutrients [4]. In fact, it constitutes one of the risk factors in the development of osteoporosis in patients with IBD [5]. Malnutrition in patients suffering from IBD is multifactorial and has been associated with malabsorption, a decreased calories consumption, pharmacological treatment, nutrient loss in the gastrointestinal tract and an increased energy expenditure [6]. Additionally, malnutrition has also been associated with a poorer quality of life [7].
Calcium and vitamin D are the most frequently discussed nutrients in the prevention of low bone mineral density (BMD) [8]. However, other nutrients, including micronutrients, should also be included in the discussion, since they play an essential role in proper bone mineralization [9,10]. A deficiency of micronutrients does not cause direct clinical symptoms; thus, its diagnosis is challenging in standard laboratory tests. Therefore, a decreased level of micronutrients is often overlooked in establishing the causes of the ailments. Furthermore, a deficiency of micronutrients may be associated with intestinal microbiota [11].
2. Nutrition in IBD
Malnutrition, including the micronutrients deficiency, may affect both UC (ulcerative colitis) and CD (Crohn’s disease) patients, although it is more common among patients with Crohn’s disease. Therefore, IBD patients should be regularly monitored with regard to malnutrition [12]. Calcium and vitamin D are the most commonly discussed micronutrients in terms of osteoporosis prevention [13,14,15,16]; nevertheless, vitamin B12, folic acid, vitamin K, vitamin C, phosphate, magnesium and sodium also play an important role in the prevention of osteoporosis [17]. According to Owczarek et al., the key nutrients for IBD patients comprise iron, calcium, vitamin D, vitamins B, vitamin A and zinc [18], but other components of the diet, such as other micronutrients, should be taken into consideration as well. Figure 1 presents the sources of micronutrients. Moreover, the European Society of Clinical Nutrition and Metabolism (ESPEN) indicate that patients suffering from IBD should be particularly monitored for micronutrient deficiency, since it can affect aspects such as normal growth and bone health [12].
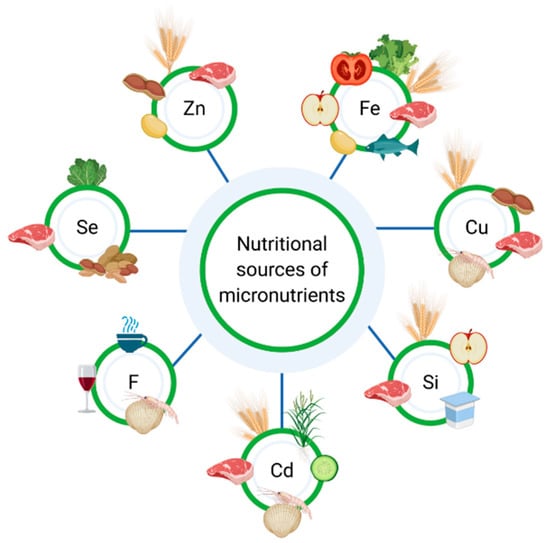
Figure 1.
Nutritional sources of micronutrients.
3. Osteoporosis in IBD
Musculoskeletal disorders, including osteoporosis, are the most common extraintestinal complications in IBD patients [19]. Osteoporosis constitutes a bone disorder which stems from an imbalance between bone resorption and bone formation [20], and leads to a reduction in bone strength and an increased risk of fractures [21]. It is vital to notice that bone disorders may influence both morbidity and mortality [22]. A gold standard regarding the diagnosis of osteoporosis is dual-energy X-ray absorptiometry (DXA), which is used for the evaluation of the lumbar spine and femoral neck BMD [23].
Among the newly diagnosed IBD patients, osteoporosis and sarcopenia affects 11% and 46% subjects, respectively [24]. Krela-Kaźmierczak et al. reported that lumbar spine osteoporosis occurred in 11.7% and 3.8% patients with CD and UC, respectively. Additionally, femoral neck osteoporosis was diagnosed in 5.8% of patients with CD and in 2.9% of patients suffering from UC. It is generally accepted that factors affecting BMD include age, gender and peak bone mass. Interestingly, BMD correlates also with the BMI (Body Mass Index) [5], but the main risk factors of osteoporosis in IBD is steroids use. Additionally, chronic inflammation and malabsorption, which lead to a decreased absorption of nutrients and are essential for a proper bone mineralization, also influence BMD [23,25]. Moreover, genetic factors of osteoporosis in IBD have also been discussed [26]. The most vital system responsible for the development of osteoporosis in IBD is probably RANK/RANKL/osteoprotegerin. It is worth bearing in mind that the expression of RANL and osteoprotegerin depends on the single nucleotide polymorphism. However, Krela-Kaźmierczak et al. demonstrated that the molecular background of osteoporosis in CD and UC is different [27] Additionally, the concentration of pro-inflammatory cytokines (e.g., TNF-α) are increased among patients suffering from IBD, which, in turn, might affect BMD [28]. Furthermore, the risk factor of the development of osteoporosis is physical inactivity [29], whereas patients suffering from IBD often avoid exercise due to the gastrointestinal symptoms. In addition, cigarette smoking also affects bones, as cigarette smoke contains more than 7000 chemicals, including cadmium, which may be detrimental to the bone tissue [30]. Fortunately, in the recent years, the prevalence of cigarette smoking among IBD patients has reduced in Western countries [31]. It has been well established that the steroids and biopharmaceuticals used in the IBD therapy also affect bone mineral density [17]. In fact, Sole reported that infliximab increased BMD of the femoral neck in patients suffering from rheumatoid arthritis [32]. Additionally, Bernstein et al. demonstrated that infliximab improved BMD in patients suffering from CD [33]. Figure 2 presents risk factors of osteoporosis.
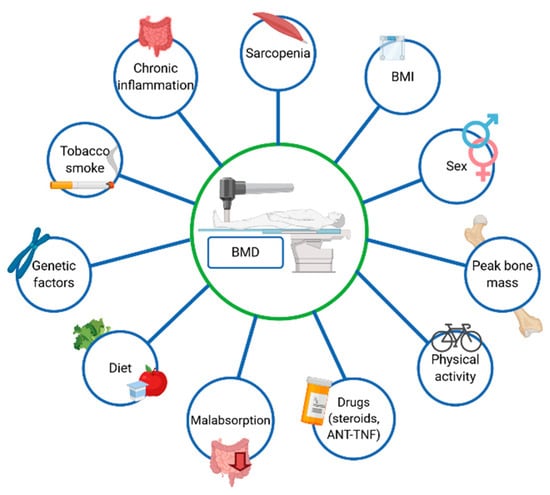
Figure 2.
Risk factors of osteoporosis in inflammatory bowel diseases.
4. Micronutrients in the Diet of IBD Patients
4.1. Zinc
Zinc (Zn) is a bone component and participates in bone turnover and metabolism [34]. It also affects the synthesis of collagen and the activity of alkaline phosphatase [35]. Both in vitro and in vivo studies show zinc is an anabolic factor for bone. Additionally, Zn stimulates bone formation and inhibits resorption of bone, leading to an increased bone mass [36]. Primary sources of zinc are meat, nut, bean and wholegrain products; however, the absorption of Zn from plant products is lower than from animal products [37].
Zinc deficiency occurred in 35% of children and adolescents at the time of the IBD diagnosis. Nevertheless, one-year supplementation of Zn did not improve Zn levels in all patients [38]. Furthermore, among adults suffering from IBD, the level of Zn was decreased in 68% of patients (normal range is estimated at 10–17 mmol/L) [39]. As a meta-analysis demonstrated, Zn concentration was significantly lower in patients with an autoimmune disease when compared to the control group [40]. In addition, many patients have to avoid nut, bean, and wholegrain products due to the occurrence of gastrointestinal symptoms after consumption.
Xiong et al. reported that an odds ratio of phalangeal osteoporosis was higher among individuals in the first zinc intake quartile than in the second, third and fourth. Moreover, Zn intake was negatively correlated with the risk of phalangeal osteoporosis in the entire population and men, but not in women [41]. Zinc intake and the serum concentration was decreased in men with lumbar spine and femoral neck osteoporosis when compared to the groups without osteoporosis. BMD of the femoral neck, lumbar spine and the distal wrist bones was significantly lower in the group in the lowest plasma zinc quartile [42]. Additionally, the zinc level was decreased among women with osteoporosis [43]. According to Mutly et al., Zn concentration in postmenopausal women who reported osteoporosis was significantly decreased in comparison with women suffering from osteopenia or with a normal bone mass [44]. Moreover, a six-month treatment with calcitonin increased Zn levels, which allowed to formulate the conclusion that the Zn level may be used for the evaluation of the osteoporosis therapy [43].
4.2. Copper
Copper (Cu) participates in various enzymatic process, nucleic acid synthesis, iron metabolism and immune system functions. Moreover, as a cofactor of antioxidant enzymes, Cu removes bone free radicals, leading to an increase of the osteoblasts activity [45]. It also affects bone formation and mineralization [35] and is responsible for lysine crosslink formation in elastin and collagen by means of the activation of lysyl oxidase [45]. Additionally, Cu is a cofactor of many enzymes in collagen synthesis [46]. Since it constitutes such a vital element, its deficiency may lead to disorders in bone and cholesterol metabolism [47], as well as contributing to the development of osteoporosis [48]. On the other hand, an excessive intake of Cu may induce oxidative stress, reduce cell proliferation and damage DNA [49]. Meat, seafood, nuts and grains are the primary sources of dietary copper [46].
Głąbska et al. demonstrated a lack of significant differences in Cu intake between men with UC and healthy men [50]. Moreover, the Cu concentration was not different among UC patients and healthy subjects [51]. On the other hand, serum level of copper among children with CD was significantly decreased in comparison with the healthy children [52].
Furthermore, a lack of differences in the Cu level was observed between postmenopausal women with osteoporosis, osteopenia and normal bone mass [44]. Another study revealed that copper concentration was significantly lower among postmenopausal women with osteoporosis than in women with normal BMD [43]. The intake of Cu in patients with tooth wear did not differ from the healthy individuals, although it decreased the lumbar spine BMD in the control group. In contrast, Cu content in enamel was significantly decreased in the study group than in the control group, but no difference in the Cu serum concentration level was found between the groups [53]. A decreased Cu serum level was associated with a significantly lower BMD of the femur and the femoral neck. Moreover, a high concentration of copper was connected with an increased prevalence of fractures, particularly among men [47].
However, research studies regarding the connection between copper and BMD are not well known and further research is necessary.
4.3. Selenium
Selenium (Se) is a component of over 25 selenoenzymes. Se deficiency can affect increased growth and bone metabolism. Additionally, Se is an antioxidant which reduces inflammation, and affects the proliferation and differentiation of bone cells. Selenium appears in food as selenomethionine, selenocysteine and Se-methylselenocysteine [54]. Content of Se in food is various and depends on the feed supplied to the animals (in animal products), including salmon, eggs, chicken, milk products, whereas in plant products, such as brazil nuts and garlic, Se content may be influenced by the substrate in which the plants are grown [55,56].
The serum level of selenium was significantly lower among patients with UC than in the healthy subjects [51]. The concentration of Se in pediatric patients suffering from CD or UC was significantly lower when compared to the healthy children [52]. Moreover, selenium deficiency occurred in 30% of IBD patients [57].
In terms of ageing men, Se level was positively correlated with BMD [58]. As Wang et al. showed, the elderly and ageing persons with a low selenium intake (≤ 29.2 μg/day) more frequently present with osteoporosis [59]. On the other hand, there was a lack of difference in Se concentration between the postmenopausal women with osteoporosis, osteopenia and normal BMD [60]. Odabasi et al. also reported that no difference in the serum level of Se was observed between subjects with and without osteoporosis [61]. Additionally, among women over 51 years of age, if calcium consumption was lower than 800 mg/day, a high intake of Se negatively affected bone mass [62].
The data concerning the impact of Se on bone are unclear and requires further research.
4.4. Iron
The most common consequence of iron (Fe) deficiency is anemia, although a decreased level of Fe may result in many disorders [63]. In fact, Fe affects collagen bone matrix synthesis and is a cofactor in the enzyme responsible for the metabolism of vitamin D [35]. Therefore, iron deficiency influences bone homeostasis. In contrast, iron overload may also lead to the development of osteoporosis, for instance by an increase in the reactive oxygen spices [64]. Products which are the sources of Fe can be divided into heme (meat, fish) and non-heme (grain, legumes, vegetables, fruits) [63].
Both the deficiency and the excess of iron can lead to the weakening of bones. Low concentration of Fe causes an increase in the expression of fibroblast growth factor 23 (FGF23) gene [65]. Moreover, iron is also a metal which may catalyze a formation of reactive oxygen spices [66], as well as affecting the differentiation and the activity of osteoblasts and osteoclasts [64].
Iron deficiency anemia affects over 19% and 21% of patients with CD and UC, respectively [67]. Nevertheless, iron deficiency was also diagnosed in 37% of IBD patients without anemia [68]. In fact, no difference in the intake of Fe was observed between men suffering from UC and the healthy controls [50]. However, the intake of total iron and heme iron was not associated with risk of UC and CD development [69].
Among postmenopausal women using hormone replacement therapy, the intake of iron was linked to positive changes in BMD of Ward’s triangle and trochanter [70]. Additionally, Fe intake was associated with a greater BMD of the lumbar spine (L2-L4), trochanter, femur neck, Ward’s triangle and the total body mass in non-smoking postmenopausal women [71]. Additionally, the prevalence of low BMD decreased according to the quartiles of hemoglobin in the healthy (without anemia) individuals over 60 years of age [72]. Ferritin concentration was positively correlated with BMD of the total lumbar spine, total femur and femur neck in men, but not in women [73]. On the other hand, Kim et al. reported that both the male and female patients in the first hemoglobin quartile presented a significantly faster loss of bone mass in the femur and the femoral neck [66].
4.5. Cadmium
Cadmium (Cd) may inhibit bone formation and mineralization, affect the collagen matrix, as well as increase urine calcium excretion [45]. Food products (such as seafood, meat, vegetables, grains and rice), cigarette smoke and the environment can constitute sources of cadmium. In fact, cadmium affects bone health, as it disrupts the metabolism of calcium and vitamin D in the intestines and kidneys [74,75]. Interestingly, a high concentration of cadmium occurs mainly in the industrial areas [45].
The risk of osteoporosis and fractures was 32% higher in individuals with a high Cd daily intake (≥13 μg/day, median) and 31% higher in subjects with a low intake (<13 μg/day) [76]. On the other hand, there was no association between BMD and cadmium intake (median of the intake was 25.29 μg/day) in postmenopausal women [77]. Moreover, BMD of the forearm was negatively correlated with cadmium excreted with urine, which suggests a dose–effect relationship between the Cd dose and BMD [78].
An excess intake of cadmium negatively affects the bone tissue. Therefore, in order to reduce cadmium absorption, patients should avoid products (especially vegetables) from industrial regions. Furthermore, an important element in the reduction of Cd supply is avoiding smoking.
4.6. Silicon
Silicon (Si) participates in a cross-link between collagen and proteoglycans formation, and may also participate in the process of electrochemical bone mineralization. Additionally, Si affects bone mineral density, although the exact mechanism has not been well understood [79].
Silicon (Si) is a non-metal which may be delivered to the human body with drugs, cosmetics, medical implants, water and food. The primary nutritional sources of Si are plant products (cereals, grains, some fruit and vegetables), dairy products and meat. As studies concerning tissues and osteoblasts have shown, Si increases dry bone mass, collagen and calcium content, and elevates the proliferation of trabecular cells [80].
Silicon intake correlated positively with BMD of four hip sites in men and premenopausal women, but not in postmenopausal women. Additionally, no association was observed between the lumbar spine BMD and Si intake [81]. A 12-week long supplementation with silicon-rich (86 mg/L) water did not alter the level of type 1 cross-linked N-telopeptide, procollagen type I intact, N-terminal propeptide, bone specific alkaline phosphatase and osteocalcin [82]. Nevertheless, an animal study has shown that Si supplementation affects BMD of the femur positively, although it does not change the concentration of alkaline phosphatase and osteocalcin [83].
4.7. Fluorine
Fluorine (F) may interact with the bone mineral matrix. Sodium fluoride has an anabolic effect, leading to an increase in bone mass; however, the mechanism of this action remains unknown. An in vitro study has shown that the impact of fluorine on osteoclasts depends on the concentration (15–30 mg/L) and leads to a decreased osteoclasts activity, whereas a concentration of 1 mg/L increases the activity of osteoclasts. Additionally, the narrow window of the therapeutic and toxic effect of fluorine makes it difficult to investigate the mechanism of fluorine impact on the bone tissue [84]. Additionally, the fluoride anion may change the crystalline structure in the bone tissue, since fluoride stimulates the formation of bone [85]. Products rich in fluoride comprise black and green tea, seafood and wine [86].
Research suggests that adding sodium fluoride to the supplementation of calcium and vitamin D did not increase osteocalcin level and did not decrease osteoprotegerin among patients suffering from CD [87]. A study by Abitbol et al. revealed that BMD of the lumbar spine increased in osteoporotic CD patients following the supplementation of Ca and vitamin D with and without the addition of fluorides. Moreover, no significant differences in BMD between groups were found [88].
The serum level of fluoride was not associated with BMD and the incidents of osteoporotic fractures in the course of four years of observations [89]. A meta-analysis showed that depending on the duration of the therapy, the treatment with fluoride elevated BMD of the spine and hip, although it did not affect the risk of hip and spine fractures. Additionally, a dose of ≤20 mg/day of fluoride equivalents was linked with a significantly decreased risk of fractures [85]. Phipps et al. investigated the impact of the consumption of fluorinated (continuous exposure) and non-fluorinated (no exposure) water on BMD. Continuous exposure resulted in a higher BMD of the lumbar spine, femoral neck and trochanter in the exposed women, whereas a smaller radius was observed in the same group as compared with the women who were not exposed. Moreover, women who consumed fluorinated water presented a lower risk of hip fractures and vertebral fractures by 31% and 27%, respectively, in comparison to women who consumed non-fluorinated water [90].
5. Gut Microbiota and Micronutrients
Studies have shown that many micronutrients participate in the bacterial colonization of the intestines. One of the elements affecting gut microbiota is selenium. A deficiency and excess of Se are linked to metabolic complications and increase the risk of developing certain neoplasms [11]. The impact of Se on gut microbiota depends on the dose. In fact, a daily supplementation 0.1–2.25 μg/kg increased the variety of gut microbiota [91], whereas a dose of 0.4 mg/kg increased the number of Akkermansia and Turicibacter, and a decreased the amount of Dorea and Mucispirillum [92].
The impact of zinc on the intestinal microbiota in patients suffering from IBD is crucial. Zn is indispensable for the integrity of the intestinal epithelium. On the other hand, an excess of Zn may negatively affect microbiota by increasing the amount of Clostridium and Enterococcus. The animal study demonstrated that the supplementation of Zn increased the number of Lactobacillus and decreased the amount of harmful bacteria, including Salmonella sp. [93,94]. Chronic zinc deficiency causes a decline in the number and diversity of Firmicutes, and leads to a decrease in the SCFA production [95].
Data regarding the impact of iron on the gut microbiota content are ambiguous, and the effect probably largely depends on the dose. The supplementation of Fe for the deficiency treatment decreased the number of the beneficial microorganisms, simultaneously increasing the amount of harmful intestinal microbes. Furthermore, dysbiosis elevated intestinal inflammation [96]. On the other hand, iron supplementation in smaller doses (50 mg/day in 4 days/week for 38 weeks) did not change the significant concentration of a dominant group of bacteria (both beneficial and harmful) in the intestine among children [97]. The intake of grains enriched in Fe decreased the number of Bifidobacteriacea (51% vs. 37%) and increased Bacteroidetes (5% vs. 14%) in the stool samples [98]. A decreased number of Bifidobacterium in the stool sample was more significant among children with a higher dose (6.4 mg/day) of Fe supplementation than the lower dose (1.2 mg/day). An excess of Fe has been associated with an increase in Defluviitaleaceae, Ruminococcaceae and Coprococcus and a decrease in Lachnospiraceae and Allobaculum [99]. A higher dose of iron was also associated with an elevated virulence of the pathogenic bacteria [100]. Interestingly, a dose of less than 60 mg/day did not change the composition of the stool microbiome among women with overweight and obesity in early pregnancy [101]. Contrary to the use of supplementation, a diet with a higher content of iron resulted in an increased amount of Bifidobacterium among Japanese women [102]. Nevertheless, the type of supplemented iron also constitutes an important factor. A study showed that non-heme iron increased the amount of Firmicutes, whereas heme iron decreased the amount of Firmicutes [103]. Additionally, the oral intake of iron resulted in a decreased number of Fecalibacterium prausnitzii, Ruminococcus bromii, Collinsella aerofaciens and Dorea, when compared with the intravenous administration. Moreover, the administration in drops (a standard dose) may decrease the relative abundance of lactobacilli, simultaneously increasing the susceptibility to bacterial infections. Table 1 summarizes the information concerning the role of microbiota in the composition of gut microbiota.

Table 1.
The impact of the selected micronutrients on gut microbiota.
6. Summary
The prevention of osteoporosis in patients suffering from IBD is an important element of medical care. A proper diet, preventing a deficiency of various nutrients, including micronutrients (Table 2), is one of the factors involved in the prevention of bone mineral density loss. Nutritional education needs to be focused on preventing both the gastrointestinal discomfort, as well as the consequences of IBD, including osteoporosis. Patients ought to be educated with regard to nutrition, taking into account the sources of calcium and vitamin D, but also of zinc, copper, selenium, iron, silicon and fluoride. Nevertheless, many patients have to eliminate certain products (such as grains or beans, which are sources of microelements) due to gastrointestinal symptoms, e.g., abdominal pain or diarrhea (Figure 3). Therefore, it is vital to emphasize the fact that an elimination diet increases the risk of microelements deficiency, leading to an elevated risk of osteoporosis, which often remains undiagnosed. Thus, patients may present nutritional deficiency, despite clinical remission and healing of the mucosa.

Table 2.
Summary of connection between micronutrient, their deficiency in IBD and association with osteoporosis.
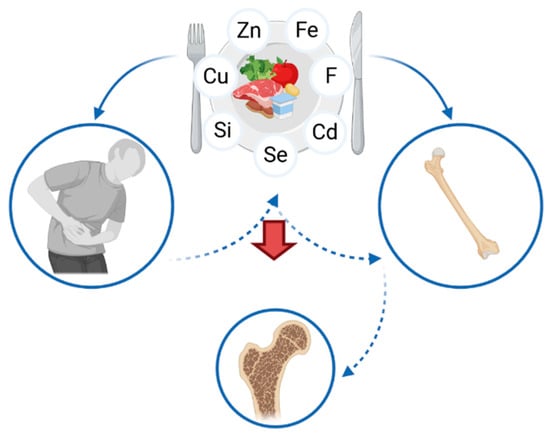
Figure 3.
Association between the intake of micronutrients, inflammatory bowel diseases and osteoporosis.
Therefore, patients with a chronic elimination diet should be subject to screening, including micronutrients status testing. Additionally, patients suffering from IBD ought to be educated with regard to nutrition, since a well-balanced personal diet is one of the most essential components in the prevention of both malnutrition and nutritional deficiency. Moreover, the cooperation between gastroenterologists, dieticians and other specialists is vital for comprehensive patient care and the prevention of complications, including osteoporosis.
Nevertheless, further studies concerning the role of micronutrients in the development of osteoporosis in patients suffering from IBD are necessary.
Author Contributions
Conceptualization, A.E.R. and I.K.-K.; writing—original draft preparation, A.E.R., A.M.R. and A.Z.; writing—review and editing, A.M.R.; supervision, A.D. and I.K.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Statement excluded.
Acknowledgments
Figures were created with Biorender.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alatab, S.; Sepanlou, S.G.; Ikuta, K.; Vahedi, H.; Bisignano, C.; Safiri, S.; Sadeghi, A.; Nixon, M.R.; Abdoli, A.; Abolhassani, H.; et al. The Global, Regional, and National Burden of Inflammatory Bowel Disease in 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [Google Scholar] [CrossRef]
- Burisch, J.; Munkholm, P. The Epidemiology of Inflammatory Bowel Disease. Scand. J. Gastroenterol. 2015, 50, 942–951. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Wu, T.-C.; Lo, Y.-C.; Wang, L.-S. Gastrointestinal Complications and Extraintestinal Manifestations of Inflammatory Bowel Disease in Taiwan: A Population-Based Study. J. Chin. Med. Assoc. 2017, 80, 56–62. [Google Scholar] [CrossRef]
- Fact Sheets—Malnutrition. Available online: https://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed on 15 January 2021).
- Krela-Kaźmierczak, I.; Michalak, M.; Szymczak-Tomczak, A.; Łykowska-Szuber, L.; Stawczyk-Eder, K.; Waszak, K.; Kucharski, M.A.; Dobrowolska, A.; Eder, P. Prevalence of Osteoporosis and Osteopenia in a Population of Patients with Inflammatory Bowel Diseases from the Wielkopolska Region. Pol. Arch. Intern. Med. 2018, 128, 447–454. [Google Scholar] [CrossRef]
- Scaldaferri, F.; Pizzoferrato, M.; Lopetuso, L.R.; Musca, T.; Ingravalle, F.; Sicignano, L.L.; Mentella, M.; Miggiano, G.; Mele, M.C.; Gaetani, E.; et al. Nutrition and IBD: Malnutrition and/or Sarcopenia? A Practical Guide. Gastroenterol. Res. Pract. 2017, 2017. [Google Scholar] [CrossRef]
- Pulley, J.; Todd, A.; Flatley, C.; Begun, J. Malnutrition and Quality of Life among Adult Inflammatory Bowel Disease Patients. JGH Open 2020, 4, 454–460. [Google Scholar] [CrossRef]
- Yamada, S.; Inaba, M. [Osteoporosis and mineral intake]. Clin. Calcium 2004, 14, 96–99. [Google Scholar]
- Karaaslan, F.; Mutlu, M.; Mermerkaya, M.U.; Karaoğlu, S.; Saçmaci, Ş.; Kartal, Ş. Comparison of Bone Tissue Trace-Element Concentrations and Mineral Density in Osteoporotic Femoral Neck Fractures and Osteoarthritis. Clin. Interv. Aging 2014, 9, 1375–1382. [Google Scholar] [CrossRef]
- Liu, S.-Z.; Yan, H.; Xu, P.; Li, J.-P.; Zhuang, G.-H.; Zhu, B.-F.; Lu, S.-M. Correlation Analysis between Bone Mineral Density and Serum Element Contents of Postmenopausal Women in Xi’an Urban Area. Biol. Trace Elem. Res. 2009, 131, 205–214. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium Intake, Status, and Health: A Complex Relationship. Horm. Athens Greece 2020, 19, 9–14. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Escher, J.; Hébuterne, X.; Kłęk, S.; Krznaric, Z.; Schneider, S.; Shamir, R.; Stardelova, K.; Wierdsma, N.; Wiskin, A.E.; et al. ESPEN Practical Guideline: Clinical Nutrition in Inflammatory Bowel Disease. Clin. Nutr. Edinb. Scotl. 2020, 39, 632–653. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alexander, D.D.; Boushey, C.J.; Dawson-Hughes, B.; Lappe, J.M.; LeBoff, M.S.; Liu, S.; Looker, A.C.; Wallace, T.C.; Wang, D.D. Calcium plus Vitamin D Supplementation and Risk of Fractures: An Updated Meta-Analysis from the National Osteoporosis Foundation. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2016, 27, 367–376. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Gamsjaeger, S.; Hassler, N.; Fahrleitner-Pammer, A.; Dobnig, H.; Stepan, J.J.; Pavo, I.; Eriksen, E.F.; Klaushofer, K. Vitamin D and Calcium Supplementation for Three Years in Postmenopausal Osteoporosis Significantly Alters Bone Mineral and Organic Matrix Quality. Bone 2017, 95, 41–46. [Google Scholar] [CrossRef]
- Reyes-Garcia, R.; Mendoza, N.; Palacios, S.; Salas, N.; Quesada-Charneco, M.; Garcia-Martin, A.; Fonolla, J.; Lara-Villoslada, F.; Muñoz-Torres, M. Effects of Daily Intake of Calcium and Vitamin D-Enriched Milk in Healthy Postmenopausal Women: A Randomized, Controlled, Double-Blind Nutritional Study. J. Womens Health 2002 2018, 27, 561–568. [Google Scholar] [CrossRef]
- Henriksen, K.; Byrjalsen, I.; Andersen, J.R.; Bihlet, A.R.; Russo, L.A.; Alexandersen, P.; Valter, I.; Qvist, P.; Lau, E.; Riis, B.J.; et al. A Randomized, Double-Blind, Multicenter, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Oral Salmon Calcitonin in the Treatment of Osteoporosis in Postmenopausal Women Taking Calcium and Vitamin D. Bone 2016, 91, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, A.E.; Rychter, A.M.; Zawada, A.; Dobrowolska, A.; Krela-Kaźmierczak, I. Nutrients in the Prevention of Osteoporosis in Patients with Inflammatory Bowel Diseases. Nutrients 2020, 12, 1702. [Google Scholar] [CrossRef] [PubMed]
- Owczarek, D.; Rodacki, T.; Domagała-Rodacka, R.; Cibor, D.; Mach, T. Diet and Nutritional Factors in Inflammatory Bowel Diseases. World J. Gastroenterol. 2016, 22, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, J. Musculoskeletal Manifestation in Inflammatory Bowel Disease. Korean J. Gastroenterol. 2019, 73, 276–284. [Google Scholar] [CrossRef]
- Föger-Samwald, U.; Dovjak, P.; Azizi-Semrad, U.; Kerschan-Schindl, K.; Pietschmann, P. Osteoporosis: Pathophysiology and Therapeutic Options. EXCLI J. 2020, 19, 1017–1037. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Hampson, G. The Pathogenesis, Diagnosis, Investigation and Management of Osteoporosis. J. Clin. Pathol. 2011, 64, 1042–1050. [Google Scholar] [CrossRef]
- Wang, N.; Xie, D.; Wu, J.; Wu, Z.; He, H.; Yang, Z.; Yang, T.; Wang, Y. Selenium and Bone Health: A Protocol for a Systematic Review and Meta-Analysis. BMJ Open 2020, 10, e036612. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.E. Epidemiology, Etiology, and Diagnosis of Osteoporosis. Am. J. Obstet. Gynecol. 2006, 194, S3–S11. [Google Scholar] [CrossRef] [PubMed]
- Adriani, A.; Pantaleoni, S.; Luchino, M.; Ribaldone, D.G.; Reggiani, S.; Sapone, N.; Sguazzini, C.; Isaia, G.; Pellicano, R.; Astegiano, M. Osteopenia and Osteoporosis in Patients with New Diagnosis of Inflammatory Bowel Disease. Panminerva Med. 2014, 56, 145–149. [Google Scholar]
- Pellicano, R.; Ribaldone, D.G. Osteoporosis, Osteopenia, and Inflammatory Bowel Disease: Lessons from a Real-world Study. Pol. Arch. Intern. Med. 2018, 128, 411–413. [Google Scholar] [CrossRef]
- Hoffmann, P.; Krisam, J.; Kasperk, C.; Gauss, A. Prevalence, Risk Factors and Course of Osteoporosis in Patients with Crohn’s Disease at a Tertiary Referral Center. J. Clin. Med. 2019, 8, 2178. [Google Scholar] [CrossRef]
- Krela-Kaźmierczak, I.; Kaczmarek-Ryś, M.; Szymczak, A.; Michalak, M.; Skrzypczak-Zielińska, M.; Drwęska-Matelska, N.; Marcinkowska, M.; Eder, P.; Łykowska-Szuber, L.; Wysocka, E.; et al. Bone Metabolism and the c.-223C > T Polymorphism in the 5′UTR Region of the Osteoprotegerin Gene in Patients with Inflammatory Bowel Disease. Calcif. Tissue Int. 2016, 99, 616–624. [Google Scholar] [CrossRef]
- Lima, C.A.; Lyra, A.C.; Rocha, R.; Santana, G.O. Risk Factors for Osteoporosis in Inflammatory Bowel Disease Patients. World J. Gastrointest. Pathophysiol. 2015, 6, 210–218. [Google Scholar] [CrossRef]
- Kirchengast, S. Bone Loss and Physical Activity—A Bio Anthropological Perspective. J. Osteoporos. Phys. Act 2016. [Google Scholar] [CrossRef]
- Li, H.; Wallin, M.; Barregard, L.; Sallsten, G.; Lundh, T.; Ohlsson, C.; Mellström, D.; Andersson, E.M. Smoking-Induced Risk of Osteoporosis Is Partly Mediated by Cadmium From Tobacco Smoke: The MrOS Sweden Study. J. Bone Miner. Res. 2020, 35, 1424–1429. [Google Scholar] [CrossRef]
- Thomas, T.; Chandan, J.S.; Li, V.S.W.; Lai, C.Y.; Tang, W.; Bhala, N.; Kaplan, G.G.; Ng, S.C.; Ghosh, S. Global Smoking Trends in Inflammatory Bowel Disease: A Systematic Review of Inception Cohorts. PLoS ONE 2019, 14, e0221961. [Google Scholar] [CrossRef]
- Sole, K. Infliximab Increases Bone Mineral Density in Patients with Rheumatoid Arthritis. Nat. Clin. Pract. Rheumatol. 2006, 2, 120. [Google Scholar] [CrossRef]
- Bernstein, M.; Irwin, S.; Greenberg, G.R. Maintenance Infliximab Treatment Is Associated with Improved Bone Mineral Density in Crohn’s Disease. Am. J. Gastroenterol. 2005, 100, 2031–2035. [Google Scholar] [CrossRef]
- Bekheirnia, M.R.; Shamshirsaz, A.A.; Kamgar, M.; Bouzari, N.; Erfanzadeh, G.; Pourzahedgilani, N.; Tabatabaie, S.M.; Abdollah Shamshirsaz, A.; Kimiagar, M.; Ezzati, F.; et al. Serum Zinc and Its Relation to Bone Mineral Density in Beta-Thalassemic Adolescents. Biol. Trace Elem. Res. 2004, 97, 215–224. [Google Scholar] [CrossRef]
- Palacios, C. The Role of Nutrients in Bone Health, from A to Z. Crit. Rev. Food Sci. Nutr. 2006, 46, 621–628. [Google Scholar] [CrossRef]
- Yamaguchi, M. Role of Nutritional Zinc in the Prevention of Osteoporosis. Mol. Cell. Biochem. 2010, 338, 241–254. [Google Scholar] [CrossRef]
- Tucker, K.L. Vegetarian Diets and Bone Status. Am. J. Clin. Nutr. 2014, 100 (Suppl. 1), 329S–335S. [Google Scholar] [CrossRef]
- Santucci, N.R.; Alkhouri, R.H.; Baker, R.D.; Baker, S.S. Vitamin and Zinc Status Pretreatment and Posttreatment in Patients with Inflammatory Bowel Disease. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 455–457. [Google Scholar] [CrossRef]
- Naber, T.H.; Van den Hamer, C.J.; Baadenhuysen, H.; Jansen, J.B. The Value of Methods to Determine Zinc Deficiency in Patients with Crohn’s Disease. Scand. J. Gastroenterol. 1998, 33, 514–523. [Google Scholar] [CrossRef]
- Sanna, A.; Firinu, D.; Zavattari, P.; Valera, P. Zinc Status and Autoimmunity: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 68. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, Y.; Wu, J.; He, H.; Wang, N.; Lei, G. [Cross-sectional association between dietary zinc intake and phalangeal osteoporosis]. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 784–789. [Google Scholar] [CrossRef]
- Hyun, T.H.; Barrett-Connor, E.; Milne, D.B. Zinc Intakes and Plasma Concentrations in Men with Osteoporosis: The Rancho Bernardo Study. Am. J. Clin. Nutr. 2004, 80, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Gür, A.; Colpan, L.; Nas, K.; Cevik, R.; Saraç, J.; Erdoğan, F.; Düz, M.Z. The Role of Trace Minerals in the Pathogenesis of Postmenopausal Osteoporosis and a New Effect of Calcitonin. J. Bone Miner. Metab. 2002, 20, 39–43. [Google Scholar] [CrossRef]
- Mutlu, M.; Argun, M.; Kilic, E.; Saraymen, R.; Yazar, S. Magnesium, Zinc and Copper Status in Osteoporotic, Osteopenic and Normal Post-Menopausal Women. J. Int. Med. Res. 2007, 35, 692–695. [Google Scholar] [CrossRef]
- Zofková, I.; Nemcikova, P.; Matucha, P. Trace Elements and Bone Health. Clin. Chem. Lab. Med. CCLM 2013, 51, 1555–1561. [Google Scholar] [CrossRef]
- Pepa, G.D.; Brandi, M.L. Microelements for Bone Boost: The Last but Not the Least. Clin. Cases Miner. Bone Metab. 2016, 13, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; He, Z.; Qiao, H.; Zhai, Z.; Mao, Z.; Yu, Z.; Dai, K. Serum Copper Levels Are Associated with Bone Mineral Density and Total Fracture. J. Orthop. Transl. 2018, 14, 34–44. [Google Scholar] [CrossRef]
- Mir, E.; Hossein-nezhad, A.; Bahrami, A.; Bekheirnia, M.R.; Javadi, E.; Naderi, A.A.; Larijani, B. Adequate Serum Copper Concentration Could Improve Bone Density, Postpone Bone Loss and Protect Osteoporosis in Women. Iran. J. Public Health 2007, 36, 24–29. [Google Scholar]
- Royer, A.; Sharman, T. Copper Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Głąbska, D.; Guzek, D.; Lech, G. Analysis of the Nutrients and Food Products Intake of Polish Males with Ulcerative Colitis in Remission. Nutrients 2019, 11, 2333. [Google Scholar] [CrossRef]
- Poursadegh, F.; Ahadi, M.; Vosoughinia, H.; Salehi, M.; Beheshti Namdar, A.; Farzanehfar, M.R.; Memar, B.; Ziaolhagh, R. A STROBE Compliant Observational Study on Trace Elements in Patients with Ulcerative Colitis and Their Relationship with Disease Activity. Medicine (Baltimore) 2018, 97, e13523. [Google Scholar] [CrossRef] [PubMed]
- Ojuawo, A.; Keith, L. The Serum Concentrations of Zinc, Copper and Selenium in Children with Inflammatory Bowel Disease. Cent. Afr. J. Med. 2002, 48, 116–119. [Google Scholar]
- Sierpinska, T.; Konstantynowicz, J.; Orywal, K.; Golebiewska, M.; Szmitkowski, M. Copper Deficit as a Potential Pathogenic Factor of Reduced Bone Mineral Density and Severe Tooth Wear. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2014, 25, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Cao, J.J.; Combs, G.F. Selenium in Bone Health: Roles in Antioxidant Protection and Cell Proliferation. Nutrients 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in Food and the Human Body: A Review. Sci. Total Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef]
- Han, Y.M.; Yoon, H.; Lim, S.; Sung, M.-K.; Shin, C.M.; Park, Y.S.; Kim, N.; Lee, D.H.; Kim, J.S. Risk Factors for Vitamin D, Zinc, and Selenium Deficiencies in Korean Patients with Inflammatory Bowel Disease. Gut Liver 2017, 11, 363–369. [Google Scholar] [CrossRef]
- Beukhof, C.M.; Medici, M.; Van den Beld, A.W.; Hollenbach, B.; Hoeg, A.; Visser, W.E.; De Herder, W.W.; Visser, T.J.; Schomburg, L.; Peeters, R.P. Selenium Status Is Positively Associated with Bone Mineral Density in Healthy Aging European Men. PLoS ONE 2016, 11, e0152748. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, D.; Li, J.; Long, H.; Wu, J.; Wu, Z.; He, H.; Wang, H.; Yang, T.; Wang, Y. Association between Dietary Selenium Intake and the Prevalence of Osteoporosis: A Cross-Sectional Study. BMC Musculoskelet. Disord. 2019, 20, 585. [Google Scholar] [CrossRef] [PubMed]
- Arikan, D.C.; Coskun, A.; Ozer, A.; Kilinc, M.; Atalay, F.; Arikan, T. Plasma Selenium, Zinc, Copper and Lipid Levels in Postmenopausal Turkish Women and Their Relation with Osteoporosis. Biol. Trace Elem. Res. 2011, 144, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, E.; Turan, M.; Aydin, A.; Akay, C.; Kutlu, M. Magnesium, Zinc, Copper, Manganese, and Selenium Levels in Postmenopausal Women with Osteoporosis. Can Magnesium Play a Key Role in Osteoporosis? Ann. Acad. Med. Singapore 2008, 37, 564–567. [Google Scholar]
- Pedrera-Zamorano, J.D.; Calderon-García, J.F.; Roncero-Martin, R.; Mañas-Nuñez, P.; Moran, J.M.; Lavado-Garcia, J.M. The Protective Effect of Calcium on Bone Mass in Postmenopausal Women with High Selenium Intake. J. Nutr. Health Aging 2012, 16, 743–748. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Balogh, E.; Paragh, G.; Jeney, V. Influence of Iron on Bone Homeostasis. Pharmaceuticals 2018, 11, 107. [Google Scholar] [CrossRef]
- Imel, E.A.; Liu, Z.; McQueen, A.K.; Acton, D.; Acton, A.; Padgett, L.R.; Peacock, M.; Econs, M.J. Serum Fibroblast Growth Factor 23, Serum Iron and Bone Mineral Density in Premenopausal Women. Bone 2016, 86, 98–105. [Google Scholar] [CrossRef]
- Kim, B.-J.; Ahn, S.H.; Bae, S.J.; Kim, E.H.; Lee, S.-H.; Kim, H.-K.; Choe, J.W.; Koh, J.-M.; Kim, G.S. Iron Overload Accelerates Bone Loss in Healthy Postmenopausal Women and Middle-Aged Men: A 3-Year Retrospective Longitudinal Study. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2012, 27, 2279–2290. [Google Scholar] [CrossRef]
- Madanchi, M.; Fagagnini, S.; Fournier, N.; Biedermann, L.; Zeitz, J.; Battegay, E.; Zimmerli, L.; Vavricka, S.R.; Rogler, G.; Scharl, M.; et al. The Relevance of Vitamin and Iron Deficiency in Patients with Inflammatory Bowel Diseases in Patients of the Swiss IBD Cohort. Inflamm. Bowel Dis. 2018, 24, 1768–1779. [Google Scholar] [CrossRef] [PubMed]
- González Alayón, C.; Pedrajas Crespo, C.; Marín Pedrosa, S.; Benítez, J.M.; Iglesias Flores, E.; Salgueiro Rodríguez, I.; Medina Medina, R.; García-Sánchez, V. Prevalence of Iron Deficiency without Anaemia in Inflammatory Bowel Disease and Impact on Health-Related Quality of Life. Gastroenterol. Hepatol. 2018, 41, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Khalili, H.; De Silva, P.S.; Ananthakrishnan, A.N.; Lochhead, P.; Joshi, A.; Garber, J.J.; Richter, J.R.; Sauk, J.; Chan, A.T. Dietary Iron and Heme Iron Consumption, Genetic Susceptibility, and Risk of Crohn’s Disease and Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1088–1095. [Google Scholar] [CrossRef]
- Maurer, J.; Harris, M.M.; Stanford, V.A.; Lohman, T.G.; Cussler, E.; Going, S.B.; Houtkooper, L.B. Dietary Iron Positively Influences Bone Mineral Density in Postmenopausal Women on Hormone Replacement Therapy. J. Nutr. 2005, 135, 863–869. [Google Scholar] [CrossRef]
- Harris, M.M.; Houtkooper, L.B.; Stanford, V.A.; Parkhill, C.; Weber, J.L.; Flint-Wagner, H.; Weiss, L.; Going, S.B.; Lohman, T.G. Dietary Iron Is Associated with Bone Mineral Density in Healthy Postmenopausal Women. J. Nutr. 2003, 133, 3598–3602. [Google Scholar] [CrossRef]
- Kim, H.-S.; Park, H.-M.; Lee, H.S.; Lee, Y.-J. Hemoglobin Levels and Low Bone Mineral Density in Non-Anemic Older Adults: Secondary Analysis of the Korean National Health and Nutrition Examination Survey. Exp. Gerontol. 2019, 126, 110706. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Jang, J.S.; Lee, D.R.; Kim, Y.H.; Nam, G.E.; Han, B.-D.; Do Han, K.; Cho, K.H.; Kim, S.M.; Choi, Y.S.; et al. Serum Ferritin Levels Are Positively Associated with Bone Mineral Density in Elderly Korean Men: The 2008-2010 Korea National Health and Nutrition Examination Surveys. J. Bone Miner. Metab. 2014, 32, 683–690. [Google Scholar] [CrossRef]
- Ohta, H.; Ichikawa, M.; Seki, Y. Effects of Cadmium Intake on Bone Metabolism of Mothers during Pregnancy and Lactation. Tohoku J. Exp. Med. 2002, 196, 33–42. [Google Scholar] [CrossRef]
- Chunhabundit, R. Cadmium Exposure and Potential Health Risk from Foods in Contaminated Area, Thailand. Toxicol. Res. 2016, 32, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Engström, A.; Michaëlsson, K.; Vahter, M.; Julin, B.; Wolk, A.; Åkesson, A. Associations between Dietary Cadmium Exposure and Bone Mineral Density and Risk of Osteoporosis and Fractures among Women. Bone 2012, 50, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Lavado-García, J.M.; Puerto-Parejo, L.M.; Roncero-Martín, R.; Moran, J.M.; Pedrera-Zamorano, J.D.; Aliaga, I.J.; Leal-Hernández, O.; Canal-Macias, M.L. Dietary Intake of Cadmium, Lead and Mercury and Its Association with Bone Health in Healthy Premenopausal Women. Int. J. Environ. Res. Public. Health 2017, 14, 1437. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, H.; Shi, Y.; Weng, S.; Jin, T.; Kong, Q.; Nordberg, G.F. Environmental Cadmium Exposure and Forearm Bone Density. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2004, 17, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Price, C.T.; Koval, K.J.; Langford, J.R. Silicon: A Review of Its Potential Role in the Prevention and Treatment of Postmenopausal Osteoporosis. Int. J. Endocrinol. 2013, 2013, 316783. [Google Scholar] [CrossRef] [PubMed]
- Jugdaohsingh, R. Silicon and Bone Health. J. Nutr. Health Aging 2007, 11, 99–110. [Google Scholar]
- Jugdaohsingh, R.; Tucker, K.L.; Qiao, N.; Cupples, L.A.; Kiel, D.P.; Powell, J.J. Dietary Silicon Intake Is Positively Associated with Bone Mineral Density in Men and Premenopausal Women of the Framingham Offspring Cohort. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Karp, H.; Zerlin, A.; Lee, T.Y.A.; Carpenter, C.; Heber, D. Absorption of Silicon from Artesian Aquifer Water and Its Impact on Bone Health in Postmenopausal Women: A 12 Week Pilot Study. Nutr. J. 2010, 9, 44. [Google Scholar] [CrossRef]
- Bae, Y.-J.; Kim, J.-Y.; Choi, M.-K.; Chung, Y.-S.; Kim, M.-H. Short-Term Administration of Water-Soluble Silicon Improves Mineral Density of the Femur and Tibia in Ovariectomized Rats. Biol. Trace Elem. Res. 2008, 124, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.T. Fluoride’s Effects on the Formation of Teeth and Bones, and the Influence of Genetics. J. Dent. Res. 2011, 90, 552–560. [Google Scholar] [CrossRef]
- Vestergaard, P.; Jorgensen, N.R.; Schwarz, P.; Mosekilde, L. Effects of Treatment with Fluoride on Bone Mineral Density and Fracture Risk--a Meta-Analysis. Osteoporos. Int. J. Establ. Result Coop. Eur. Found. Osteoporos. Natl. Osteoporos. Found. USA 2008, 19, 257–268. [Google Scholar] [CrossRef]
- Kanduti, D.; Sterbenk, P.; Artnik, B. Fluoride: A review of use and effects on health. Mater. Socio-Medica 2016, 28, 133–137. [Google Scholar] [CrossRef]
- Von Tirpitz, C.; Klaus, J.; Steinkamp, M.; Hofbauer, L.C.; Kratzer, W.; Mason, R.; Boehm, B.O.; Adler, G.; Reinshagen, M. Therapy of Osteoporosis in Patients with Crohn’s Disease: A Randomized Study Comparing Sodium Fluoride and Ibandronate. Aliment. Pharmacol. Ther. 2003, 17, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, V.; Mary, J.Y.; Roux, C.; Soulé, J.C.; Belaiche, J.; Dupas, J.-L.; Gendre, J.P.; Lerebours, E.; Chaussade, S. Groupe D’etudes Thérapeutiques des Affections Inflammatoires Digestives (GETAID) Osteoporosis in Inflammatory Bowel Disease: Effect of Calcium and Vitamin D with or without Fluoride. Aliment. Pharmacol. Ther. 2002, 16, 919–927. [Google Scholar] [CrossRef]
- Sowers, M.; Whitford, G.M.; Clark, M.K.; Jannausch, M.L. Elevated Serum Fluoride Concentrations in Women Are Not Related to Fractures and Bone Mineral Density. J. Nutr. 2005, 135, 2247–2252. [Google Scholar] [CrossRef]
- Phipps, K.R.; Orwoll, E.S.; Mason, J.D.; Cauley, J.A. Community Water Fluoridation, Bone Mineral Density, and Fractures: Prospective Study of Effects in Older Women. BMJ 2000, 321, 860–864. [Google Scholar] [CrossRef]
- Kasaikina, M.V.; Kravtsova, M.A.; Lee, B.C.; Seravalli, J.; Peterson, D.A.; Walter, J.; Legge, R.; Benson, A.K.; Hatfield, D.L.; Gladyshev, V.N. Dietary Selenium Affects Host Selenoproteome Expression by Influencing the Gut Microbiota. FASEB J. 2011, 25, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Cen, S.; Li, P.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Effects of Dietary Selenium Supplementation on Intestinal Barrier and Immune Responses Associated with Its Modulation of Gut Microbiota. Environ. Sci. Technol. Lett. 2018, 5, 724–730. [Google Scholar] [CrossRef]
- Starke, I.C.; Pieper, R.; Neumann, K.; Zentek, J.; Vahjen, W. The Impact of High Dietary Zinc Oxide on the Development of the Intestinal Microbiota in Weaned Piglets. FEMS Microbiol. Ecol. 2014, 87, 416–427. [Google Scholar] [CrossRef]
- Shao, Y.; Lei, Z.; Yuan, J.; Yang, Y.; Guo, Y.; Zhang, B. Effect of Zinc on Growth Performance, Gut Morphometry, and Cecal Microbial Community in Broilers Challenged with Salmonella Enterica Serovar Typhimurium. J. Microbiol. 2014, 52, 1002–1011. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.; Koren, O.; Tako, E. Chronic Zinc Deficiency Alters Chick Gut Microbiota Composition and Function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Frank, D.; Hendricks, A.; Ir, D.; Esamai, F.; Liechty, E.; Hambidge, K.; Krebs, N. Iron in Micronutrient Powder Promotes an Unfavorable Gut Microbiota in Kenyan Infants. Nutrients 2017, 9, 776. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran, E.K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The Effects of Iron Fortification on the Gut Microbiota in African Children: A Randomized Controlled Trial in Côte d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef] [PubMed]
- Qasem, W.; Azad, M.B.; Hossain, Z.; Azad, E.; Jorgensen, S.; Castillo San Juan, S.; Cai, C.; Khafipour, E.; Beta, T.; Roberts, L.J.; et al. Assessment of Complementary Feeding of Canadian Infants: Effects on Microbiome & Oxidative Stress, a Randomized Controlled Trial. BMC Pediatr. 2017, 17, 54. [Google Scholar] [CrossRef]
- Fang, S.; Zhuo, Z.; Yu, X.; Wang, H.; Feng, J. Oral Administration of Liquid Iron Preparation Containing Excess Iron Induces Intestine and Liver Injury, Impairs Intestinal Barrier Function and Alters the Gut Microbiota in Rats. J. Trace Elem. Med. Biol. 2018, 47, 12–20. [Google Scholar] [CrossRef]
- Kortman, G.A.M.; Dutilh, B.E.; Maathuis, A.J.H.; Engelke, U.F.; Boekhorst, J.; Keegan, K.P.; Nielsen, F.G.G.; Betley, J.; Weir, J.C.; Kingsbury, Z.; et al. Microbial Metabolism Shifts Towards an Adverse Profile with Supplementary Iron in the TIM-2 In Vitro Model of the Human Colon. Front. Microbiol. 2016, 6. [Google Scholar] [CrossRef]
- Dekker Nitert, M.; Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Anderson, G.J.; Frazer, D.M.; Callaway, L.K. Iron Supplementation Has Minor Effects on Gut Microbiota Composition in Overweight and Obese Women in Early Pregnancy. Br. J. Nutr. 2018, 120, 283–289. [Google Scholar] [CrossRef]
- Seura, T.; Yoshino, Y.; Fukuwatari, T. The Relationship between Habitual Dietary Intake and Gut Microbiota in Young Japanese Women. J. Nutr. Sci. Vitaminol. 2017, 63, 396–404. [Google Scholar] [CrossRef]
- Martin, O.C.B.; Olier, M.; Ellero-Simatos, S.; Naud, N.; Dupuy, J.; Huc, L.; Taché, S.; Graillot, V.; Levêque, M.; Bézirard, V.; et al. Haem Iron Reshapes Colonic Luminal Environment: Impact on Mucosal Homeostasis and Microbiome through Aldehyde Formation. Microbiome 2019, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. (Eds.) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academy of Sciences: Washington, DC, USA, 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).