Fabrication of Bioactive, 3D-Printed, Porous, Yttria-Stabilized Zirconia via Mg/Zn-Incorporated Modified Simulated Body Fluid Pretreatment
Abstract
1. Introduction
2. Results
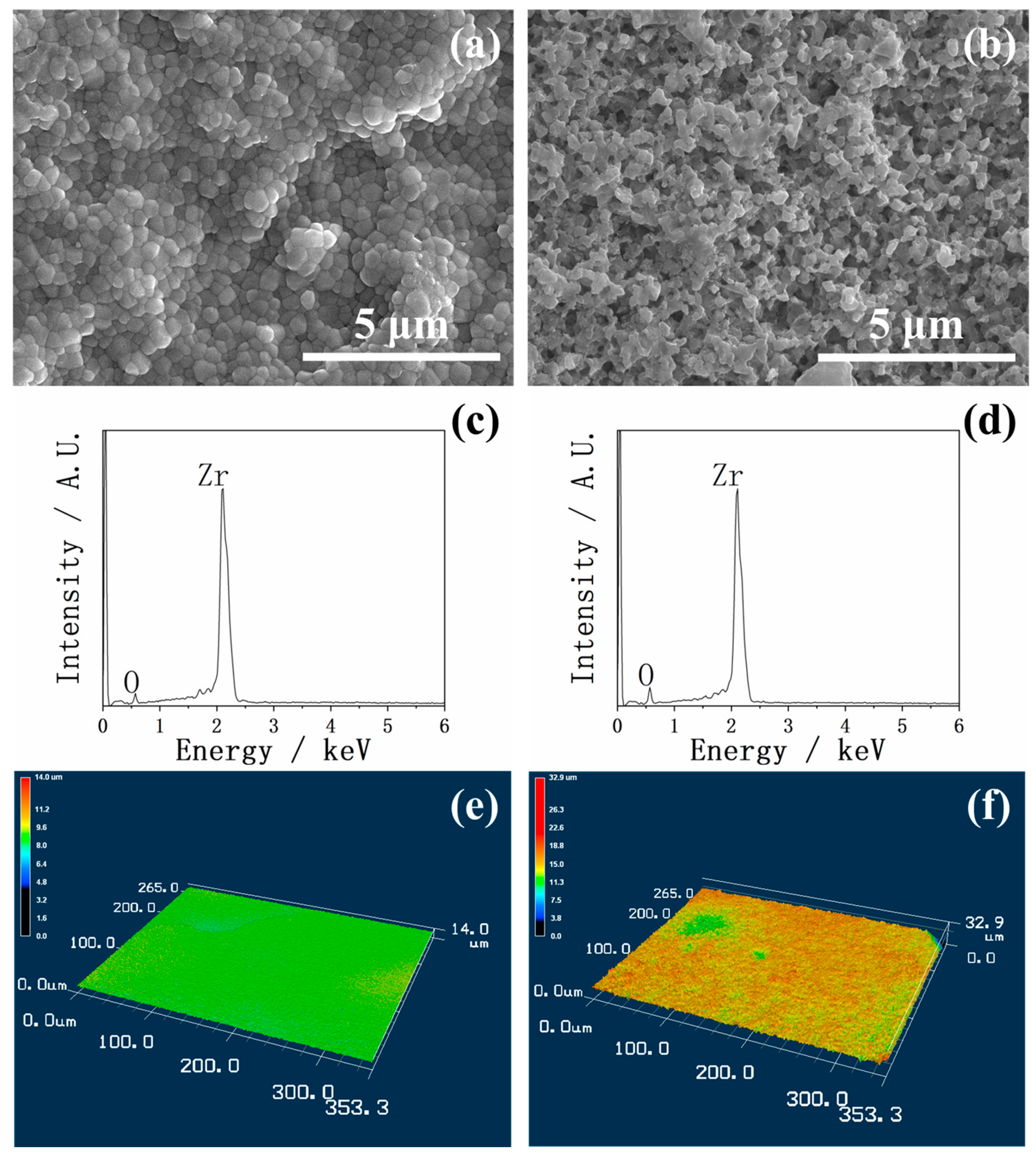
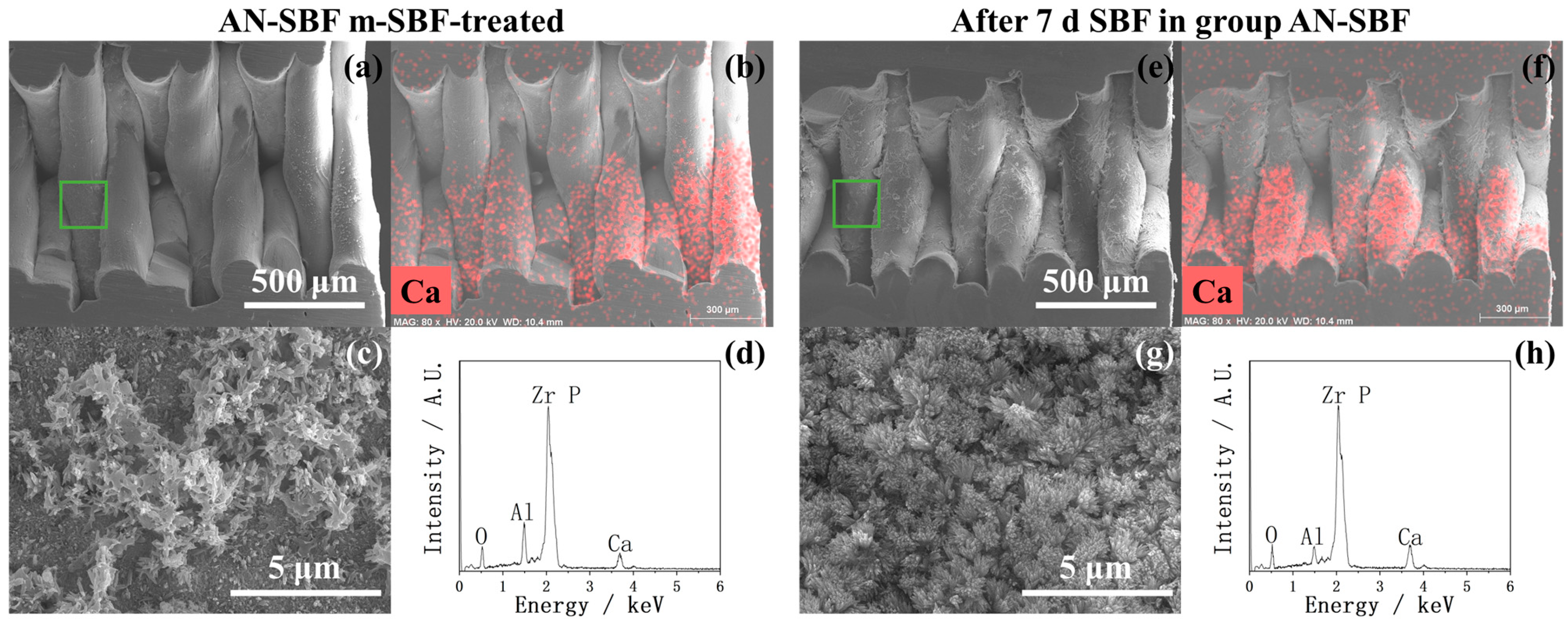
2.1. Surface Morphology Before and After HF Treatment
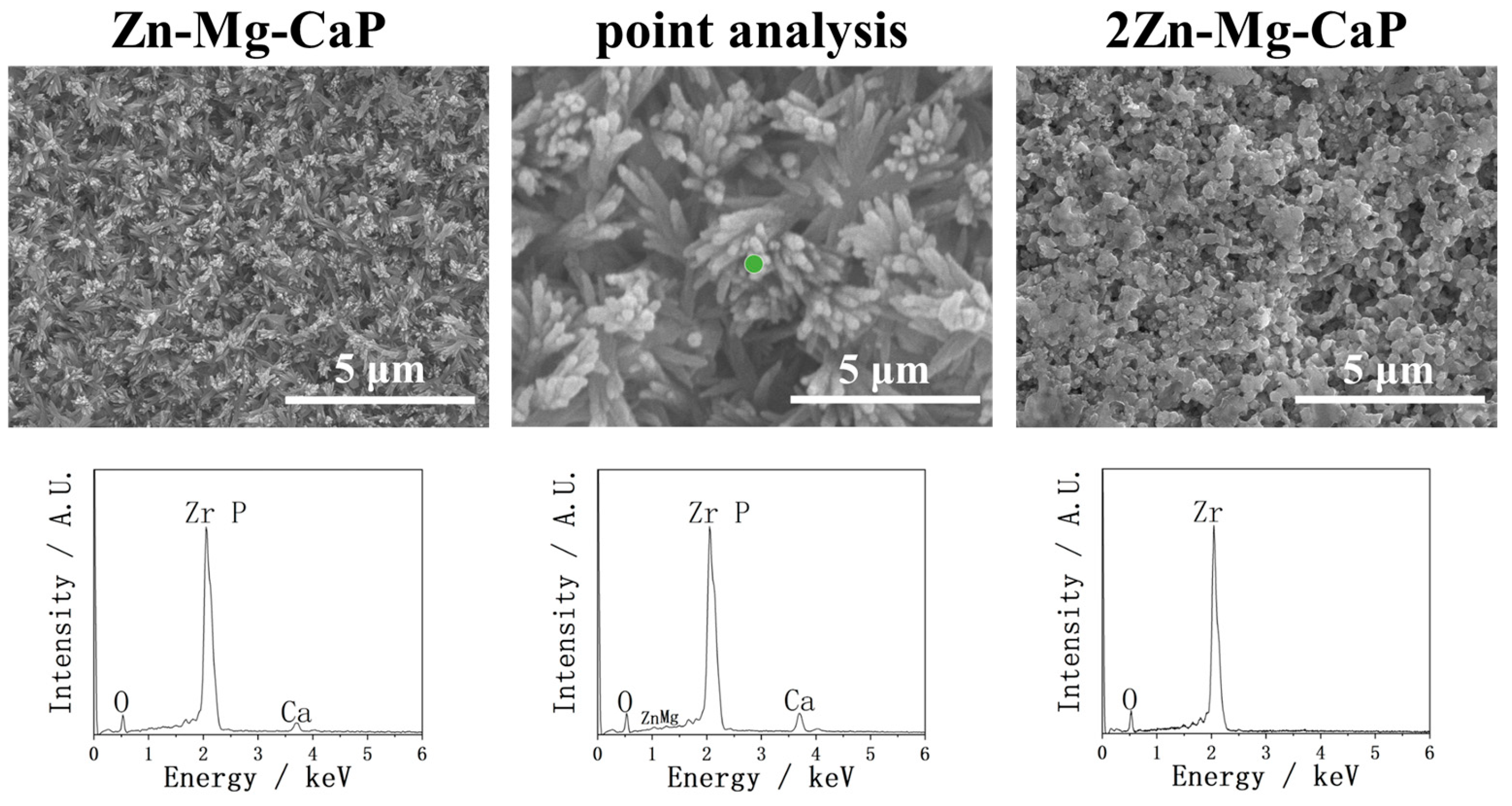
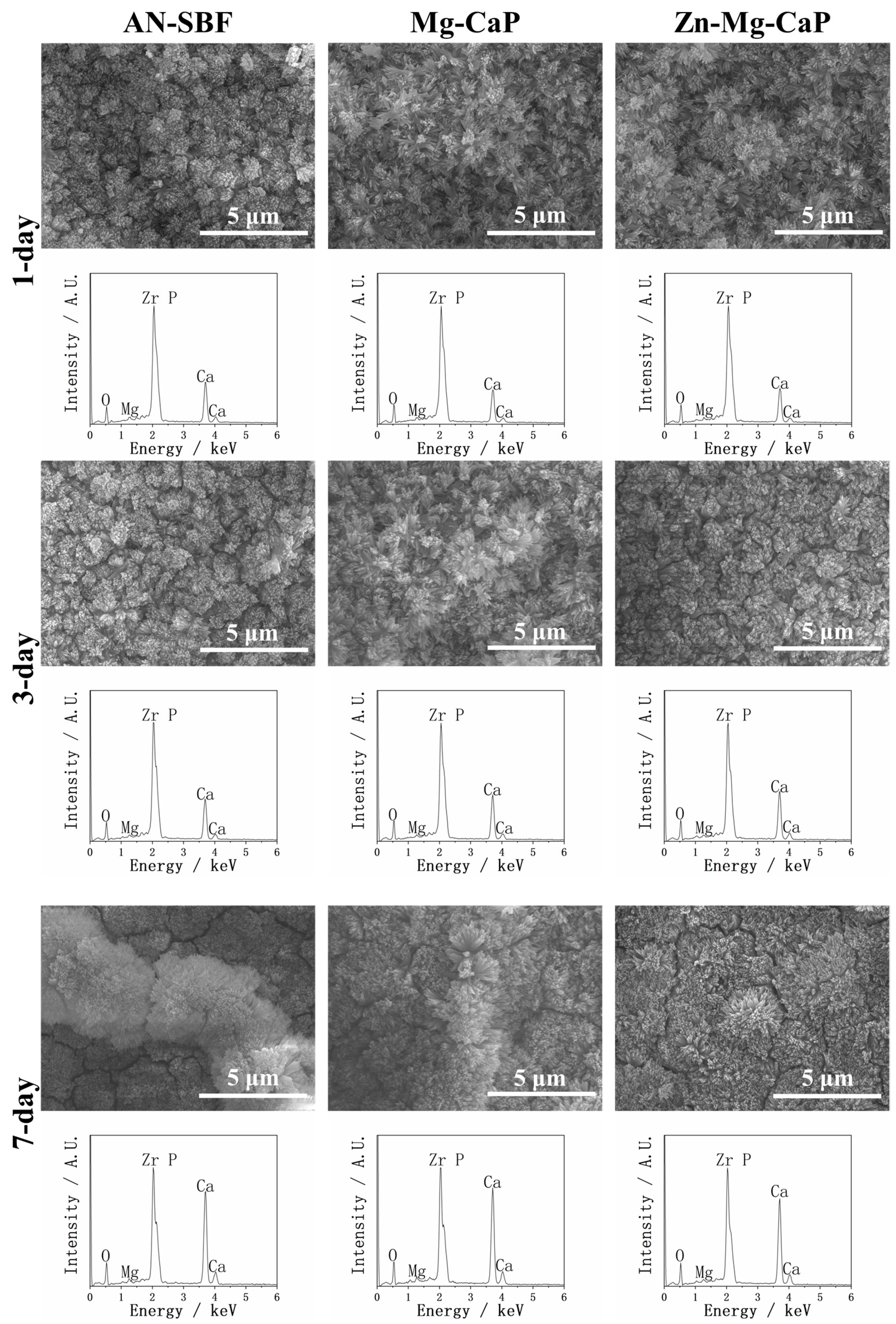
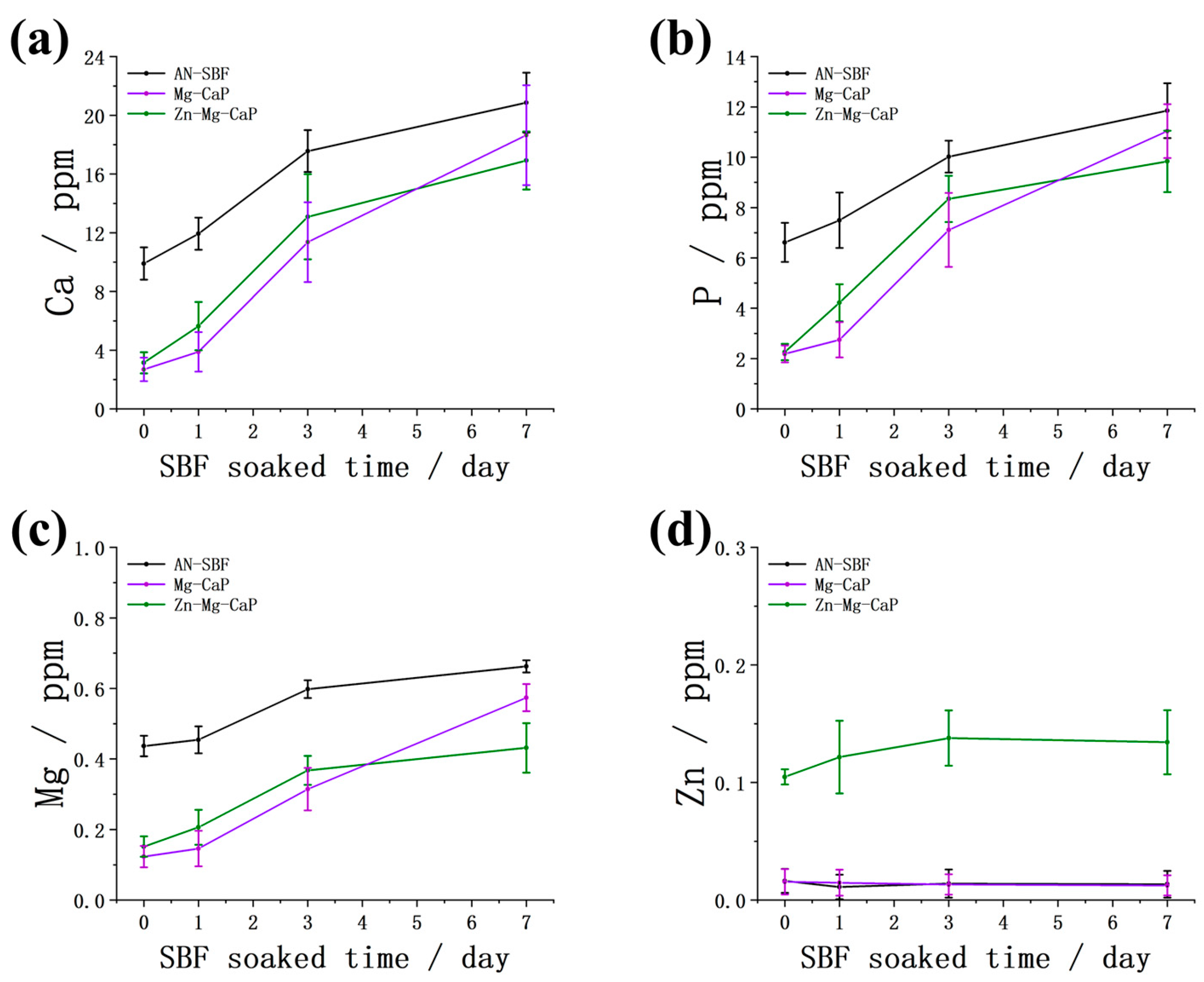
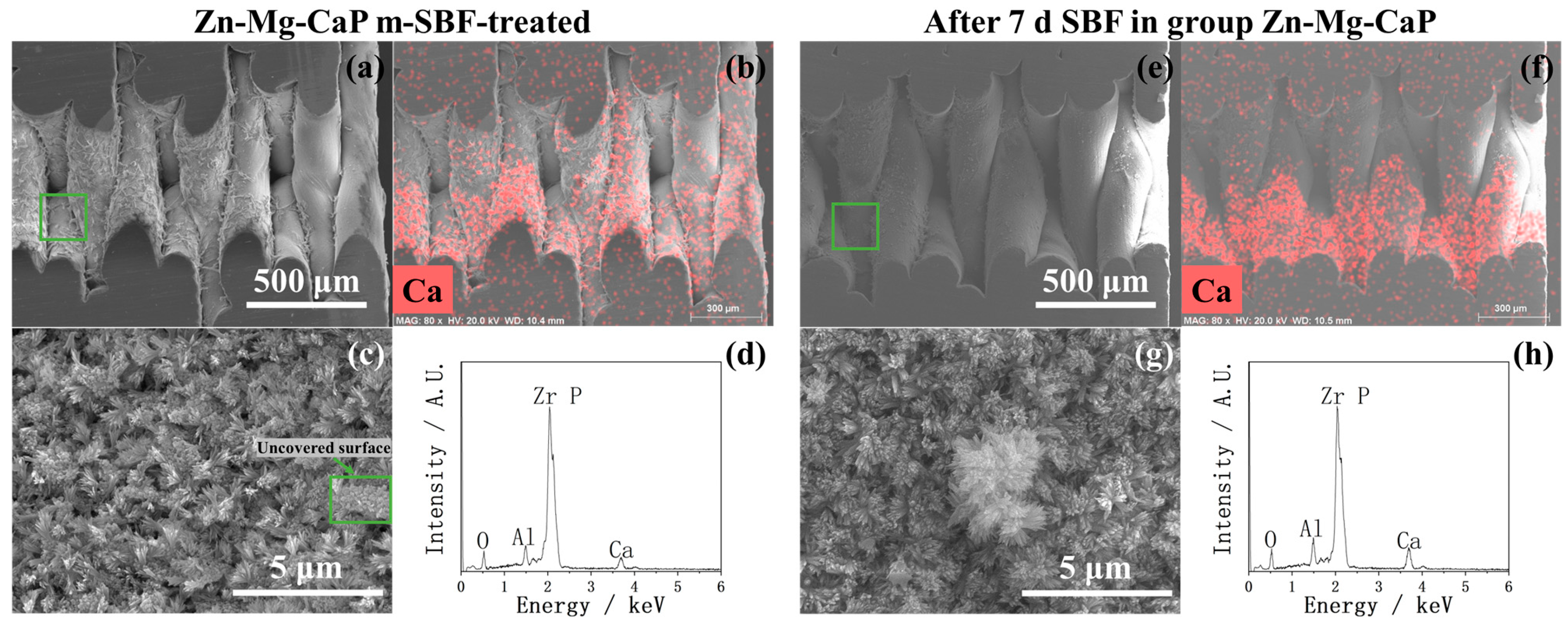
2.2. After m-SBF Treatment
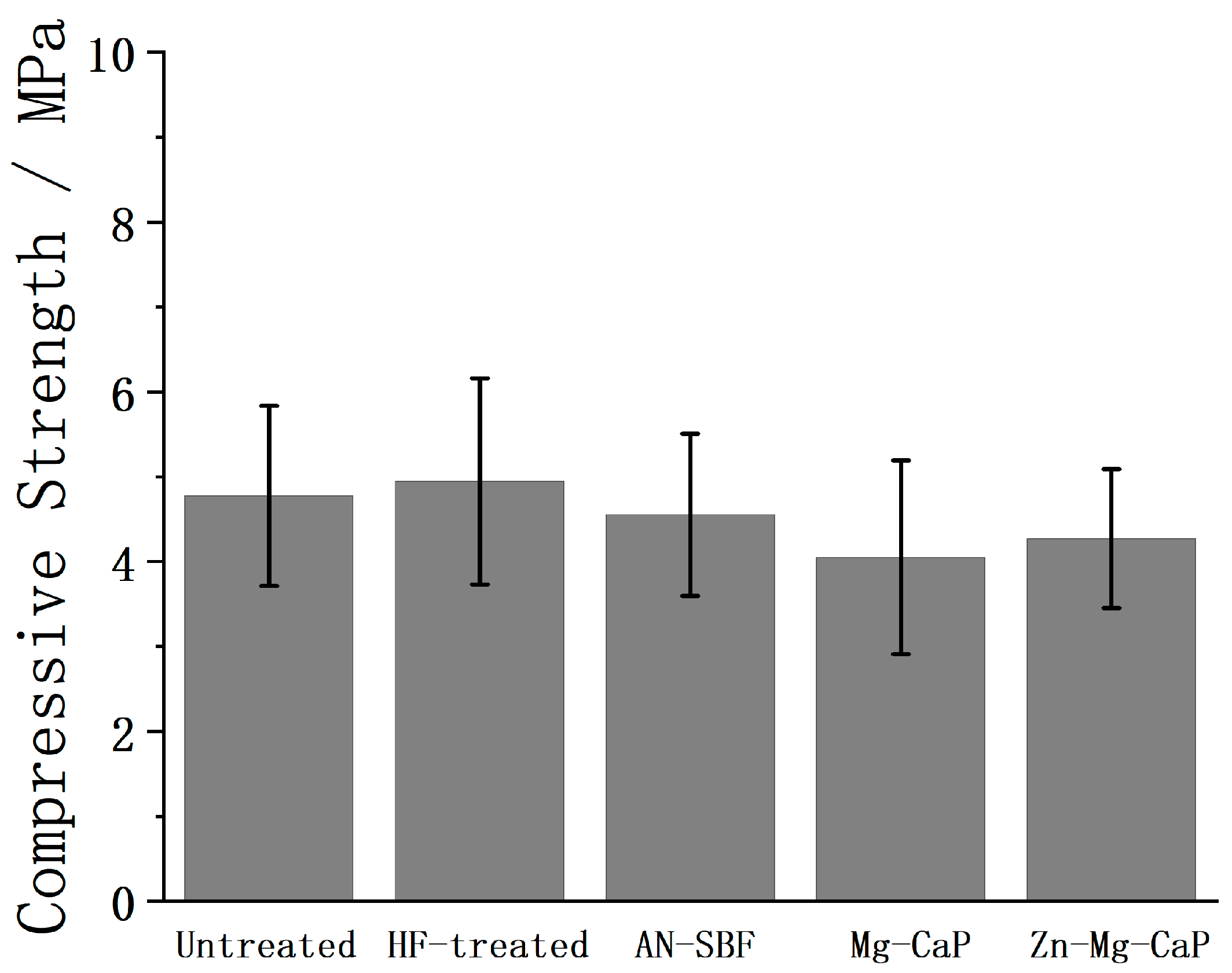
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Hydrofluoric Acid Treatment
4.3. Preparation of Standard SBF and m-SBFs
4.4. m-SBF Pretreatment
4.5. Evaluation of Bioactivity
4.6. Surface Characterization
4.7. Measurement of the Element Composition of Apatite by Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP)
4.8. Cross-Sectional Characterization
4.9. Compressive Strength
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- do Nascimento, C.; Pita, M.S.; Fernandes, F.H.N.C.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Bacterial Adhesion on the Titanium and Zirconia Abutment Surfaces. Clin. Oral Implant. Res. 2014, 25, 337–343. [Google Scholar] [CrossRef]
- Chevalier, J. What Future for Zirconia as a Biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Lughi, V.; Sergo, V. Low Temperature Degradation -Aging- of Zirconia: A Critical Review of the Relevant Aspects in Dentistry. Dent. Mater. 2010, 26, 807–820. [Google Scholar] [CrossRef]
- Tchinda, A.; Chézeau, L.; Pierson, G.; Kouitat-Njiwa, R.; Rihn, B.H.; Bravetti, P. Biocompatibility of ZrO2 vs. Y-TZP Alloys: Influence of Their Composition and Surface Topography. Materials 2022, 15, 4655. [Google Scholar] [CrossRef]
- Marques, A.; Miranda, G.; Silva, F.; Pinto, P.; Carvalho, Ó. Review on Current Limits and Potentialities of Technologies for Biomedical Ceramic Scaffolds Production. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2021, 109, 377–393. [Google Scholar] [CrossRef] [PubMed]
- Resende-Gonçalves, C.I.; Sampaio, N.; Moreira, J.; Carvalho, O.; Caramês, J.; Manzanares-Céspedes, M.C.; Silva, F.; Henriques, B.; Souza, J. Porous Zirconia Blocks for Bone Repair: An Integrative Review on Biological and Mechanical Outcomes. Ceramics 2022, 5, 161–172. [Google Scholar] [CrossRef]
- Bohara, S.; Suthakorn, J. Surface Coating of Orthopedic Implant to Enhance the Osseointegration and Reduction of Bacterial Colonization: A Review. Biomater. Res. 2022, 26, 1–17. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- Yao, T.; Hibino, M.; Yamaguchi, S.; Okada, H. Method for Stabilizing Calcium Phosphate Fine Particles, Process for Production of Calcium Phosphate Fine Particles by Utilizing the Method, and Use Thereof. U.S. Patent 8178066; Japanese Patent 5261712, 15 May 2012. [Google Scholar]
- Kim, H.-M.; Himeno, T.; Kawashita, M.; Kokubo, T.; Nakamura, T. The Mechanism of Biomineralization of Bone-like Apatite on Synthetic Hydroxyapatite: An in Vitro Assessment. J. R. Soc. Interface 2004, 1, 17–22. [Google Scholar] [CrossRef]
- Wu, Y.; Takai, S.; Yabutsuka, T. Enhancement of Bioactivity of Zr-50Ti Alloys through Sulfuric Acid Treatment Followed by Modified Simulated Body Fluid Treatment. J. Jpn. Soc. Powder Powder Metall. 2025, 72, S971–S976. [Google Scholar] [CrossRef]
- Ding, H.; Pan, H.; Xu, X.; Tang, R. Toward a Detailed Understanding of Magnesium Ions on Hydroxyapatite Crystallization Inhibition. Cryst. Growth Des. 2014, 14, 763–769. [Google Scholar] [CrossRef]
- He, L.Y.; Zhang, X.M.; Liu, B.; Tian, Y.; Ma, W.H. Effect of Magnesium Ion on Human Osteoblast Activity. Braz. J. Med. Biol. Res. 2016, 49, 1–6. [Google Scholar] [CrossRef]
- Wu, Y.; Takai, S.; Yabutsuka, T. Development of Rapid Bioactivity-Expressed Zr-50Ti Alloys by Surface Treatment with Modified Simulated Body Fluid. Int. J. Mol. Sci. 2024, 25, 6587. [Google Scholar] [CrossRef]
- Ren, F.; Xin, R.; Ge, X.; Leng, Y. Characterization and Structural Analysis of Zinc-Substituted Hydroxyapatites. Acta Biomater. 2009, 5, 3141–3149. [Google Scholar] [CrossRef]
- Ortiz, I.Y.; Raybolt dos Santos, A.; Costa, A.M.; Mavropoulos, E.; Tanaka, M.N.; Prado da Silva, M.H.; de Souza Camargo, S. In Vitro Assessment of Zinc Apatite Coatings on Titanium Surfaces. Ceram. Int. 2016, 42, 15502–15510. [Google Scholar] [CrossRef]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Motelica-Heino, M.; Guegan, R.; Buton, N. Evaluation of Antibacterial Activity of Zinc-Doped Hydroxyapatite Colloids and Dispersion Stability Using Ultrasounds. Nanomaterials 2019, 9, 515. [Google Scholar] [CrossRef]
- ISO 23317:2025; Implants for Surgery—Materials—Simulated Body Fluid (SBF) Preparation Procedure and Test Method to Detect Apatite Formation in SBF for Initial Screening of Bone-Contacting Implant Materials. ISO: Geneva, Switzerland, 2025.
- Toraya, H.; Yoshimura, M.; Somiya, S. Calibration Curve for Quantitative Analysis of the Monoclinic-Tetragonal ZrO2 System by X-Ray Diffraction. J. Am. Ceram. Soc. 1984, 67, C-119–C-121. [Google Scholar] [CrossRef]
- Flamant, Q.; Anglada, M. Hydrofluoric Acid Etching of Dental Zirconia. Part 2: Effect on Flexural Strength and Ageing Behavior. J. Eur. Ceram. Soc. 2016, 36, 135–145, Correction in J. Eur. Ceram. Soc. 2016, 36, 3547. [Google Scholar] [CrossRef]
- Xie, S.; Iglesia, E.; Bell, A.T. Water-Assisted Tetragonal-to-Monoclinic Phase Transformation of ZrO2 at Low Temperatures. Chem. Mater. 2000, 12, 2442–2447. [Google Scholar] [CrossRef]
- Sato, T.; Shimada, M. Transformation of Yttria-Doped Tetragonal ZrO2 Polycrystals by Annealing in Water. J. Am. Ceram. Soc. 1985, 68, 356. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Nawa, M.; Asano, T.; Tanaka, K.; Nakamura, T. Apatite-Forming Ability of a Zirconia/Alumina Nano-Composite Induced by Chemical Treatment. J. Biomed. Mater. Res. 2002, 60, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Barrere, F.; Van Blitterswijk, C.A.; De Groot, K.; Layrolle, P. Influence of Ionic Strength and Carbonate on the Ca-P Coating Formation from SBF×5 Solution. Biomaterials 2002, 23, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Urquia Edreira, E.R.; Wolke, J.G.C.; Birgani, Z.T.; Habibovic, P.; Jansen, J.A.; Tampieri, A.; Marcacci, M.; Leeuwenburgh, S.C.G.; Van Den Beucken, J.J.J.P. Substrate Geometry Directs the in Vitro Mineralization of Calcium Phosphate Ceramics. Acta Biomater. 2014, 10, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.A.; Müller, L.; Caillard, D.; Conforto, E. Preferred Growth Orientation of Biomimetic Apatite Crystals. J. Cryst. Growth 2007, 304, 464–471. [Google Scholar] [CrossRef]
- Pang, S.; He, Y.; He, P.; Luo, X.; Guo, Z.; Li, H. Fabrication of Two Distinct Hydroxyapatite Coatings and Their Effects on MC3T3-E1 Cell Behavior. Colloids Surf. B Biointerfaces 2018, 171, 40–48. [Google Scholar] [CrossRef]





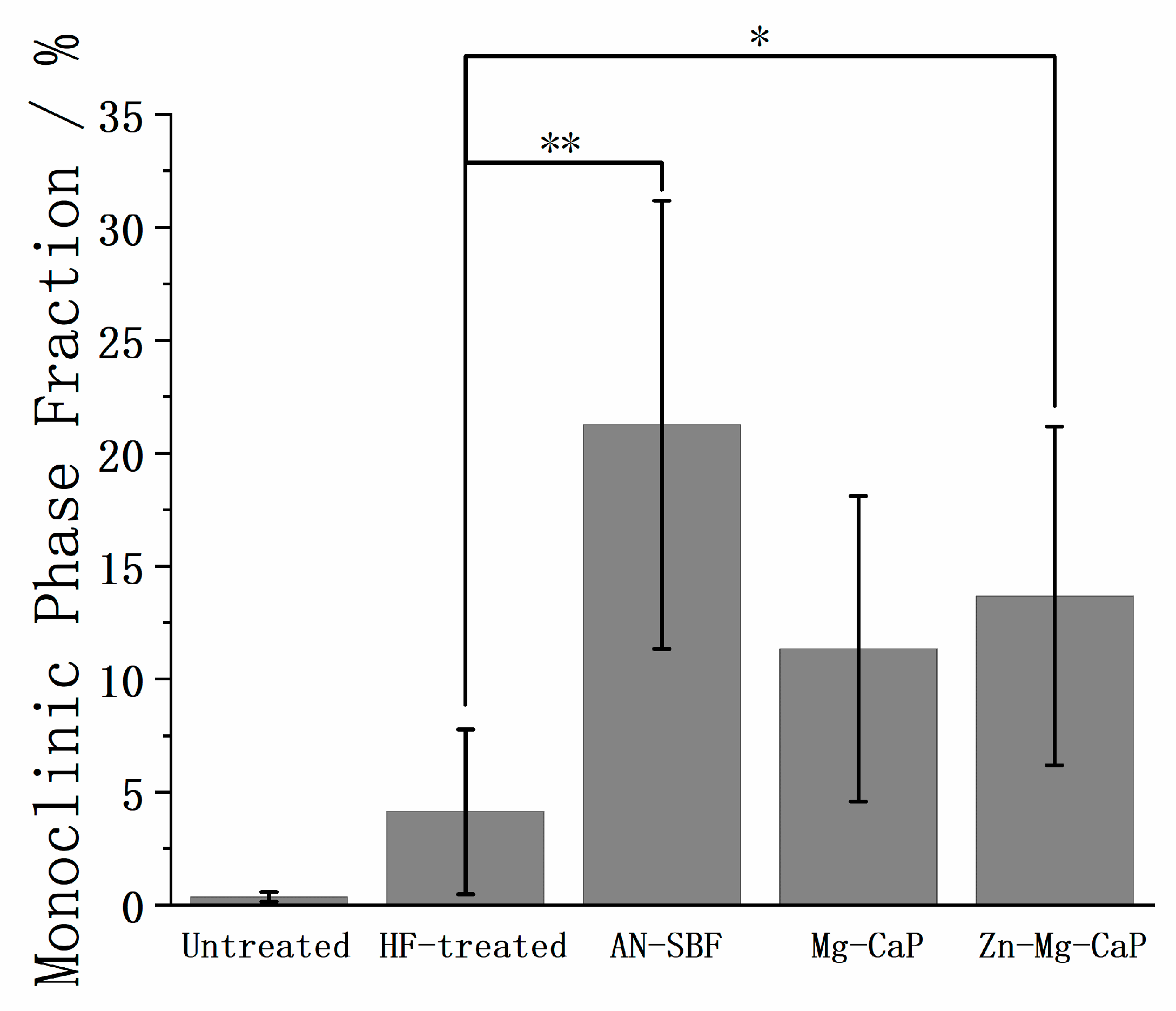



| AN-SBF | Mg-CaP | Zn-Mg-CaP | |
|---|---|---|---|
| Zn/Ca | - | - | 3.33 |
| Mg/Ca | 4.41 | 4.59 | 4.82 |
| Ion Concentration (mM) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Blood Plasma | SBF | AN-SBF | Zn-SBF | CaP | Mg-CaP | Zn-Mg-CaP | 2Zn-Mg-CaP | C-Mg-CaP | |
| Na+ | 142.0 | 142.0 | 142.0 | 142.0 | - | - | - | - | 4.2 |
| K+ | 5.0 | 5.0 | 5.0 | 5.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Mg2+ | 1.5 | 1.5 | 1.5 | 1.5 | - | 1.5 | 1.5 | 1.5 | 1.5 |
| Ca2+ | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Cl− | 103.0 | 147.8 | 147.8 | 147.8 | 5.0 | 8.0 | 8.0 | 8.0 | 8.0 |
| HCO3− | 27.0 | 4.2 | 4.2 | 4.2 | - | - | - | - | 12.5 |
| HPO42− | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| SO42− | 0.5 | 0.5 | 0.5 | 0.5 | - | - | - | - | - |
| Zn2+ | 0.012–0.018 | - | - | 0.1 | - | - | 0.1 | 0.2 | - |
| pH (36.5 °C) | 7.2–7.4 | 7.4 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 | 8.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Takai, S.; Yabutsuka, T. Fabrication of Bioactive, 3D-Printed, Porous, Yttria-Stabilized Zirconia via Mg/Zn-Incorporated Modified Simulated Body Fluid Pretreatment. Int. J. Mol. Sci. 2025, 26, 10950. https://doi.org/10.3390/ijms262210950
Wu Y, Takai S, Yabutsuka T. Fabrication of Bioactive, 3D-Printed, Porous, Yttria-Stabilized Zirconia via Mg/Zn-Incorporated Modified Simulated Body Fluid Pretreatment. International Journal of Molecular Sciences. 2025; 26(22):10950. https://doi.org/10.3390/ijms262210950
Chicago/Turabian StyleWu, Yuwei, Shigeomi Takai, and Takeshi Yabutsuka. 2025. "Fabrication of Bioactive, 3D-Printed, Porous, Yttria-Stabilized Zirconia via Mg/Zn-Incorporated Modified Simulated Body Fluid Pretreatment" International Journal of Molecular Sciences 26, no. 22: 10950. https://doi.org/10.3390/ijms262210950
APA StyleWu, Y., Takai, S., & Yabutsuka, T. (2025). Fabrication of Bioactive, 3D-Printed, Porous, Yttria-Stabilized Zirconia via Mg/Zn-Incorporated Modified Simulated Body Fluid Pretreatment. International Journal of Molecular Sciences, 26(22), 10950. https://doi.org/10.3390/ijms262210950





