Antisense Versus Antigene in the Computer-Aided Design of Triplex-Forming Oligonucleotides (TFO): Insights from a Dual-Method Review, Combining Bibliometric and Systematic Review
Abstract
1. Introduction
1.1. Motivation and Background
1.2. Related Works
| On Drug | On Class | Approval Date | Indication | Target Gene (AR) | Developing Company | Ref |
|---|---|---|---|---|---|---|
| Tofersen (Qalsody™) | siRNA | 2023 * | Amyotrophic lateral sclerosis (ALS) | SOD1 CNS (IT) | Ionis Pharma/ Biogen | [21] |
| Volanesorsen (Waylivra®) | siRNA | 2019 ** | Familial chylomicronemia syndrome | ApoC-III Liver (SC) | Ionis Pharma/ Akcea Tx | [33] |
| Inotersen (Tegsedi®) | Gapmer PS-ASO | 2018 * 2018 ** | Hereditary transthyretin amyloidosis | hTERT Liver (SC) | Ionis Pharma/ Akcea Tx | [34] |
| Mipomersen (Kynamro®) | Gamper PS-ASO | 2013 * | Homozygous familial hypercholesterolemia (HoFH) | ApoB-100 Liver (SC) | Ionis Pharma/Genzyme/Kastle Tx | [35] |
| Lumasiran (Oxlumo®) | siRNA/ GalNAc conjugate | 2020 * | Primary hyperoxaluria type 1 | HAO1 Liver (SC) | Alnylam Pharma | [36] |
| Inclisiran (Leqvio®) | siRNA/ GalNAc conjugate | 2020 ** | Hypercholesterolemia or mixed dyslipidemia | PCSK9 Liver (SC) | Alnylam Pharma /Medicines/ Novartis | [37] |
| Givosiran (Givlaari®) | siRNA/ GalNAc conjugate | 2019 * | Acute hepatic porphyria | ALAS1 Liver (SC) | Alnylam Pharma | [38] |
| Patisiran (Onpattro®) | LNP-siRNA | 2018 * | Transthyretin amyloidosis | hATTR Liver (IV) | Alnylam Pharma | [39] |
| Defibrotide (Defitelio®) | ssDNA/aptamer | 2016 * | VOD in HSCT | VOD/SOS Liver (IV) | Jazz Pharma | [40] |
| Vutrisiran (AmvuttraTM) | siRNA | 2022 * | Transthyretin amyloidosis | wtATTR Heart (SC) | Alnylam Pharma | [41] |
| Casimersen (Amondys 45®) | PMO ASO | 2021 * | Duchenne muscular dystrophy (DMD) | Dystrophin exon 45 Muscle (IV) | Sarepta Tx | [42] |
| Viltolarsen (Viltepso 53®) | PMO ASO | 2020 ** 2020 *** | Duchenne muscular dystrophy | Dystrophin exon 53 Muscle (IV) | Nippon Shinyaku Pharma | [43] |
| Golodirsen (Vyondys 53®) | PMO ASO | 2019 * | Duchenne muscular dystrophy | Dystrophin exon 53 Muscle (IV) | Sarepta Tx | [44] |
| Milasen (N-of-1 ASO) | Splice- switch ASO | 2018 * | Mila Makovec CLN7 gene associated with Batten disease | MFSD8 CNS (IT) | Boston Children’s Hospital | [45] |
| Nusinersen (Spinraza®) | Splice- switch ASO | 2016 * 2017 ** | Spinal muscular atrophy | SMN2 intron 7 CNS (IT) | Ionis Pharma/ Biogen | [46] |
| Eteplirsen (Exondys 51®) | PMO ASO | 2016 * | Duchenne muscular dystrophy | Dystrophin exon 51 Muscle (IV) | SareptanTx | [47] |
| Pegaptanib (Macugen®) | Aptamer | 2004 * | Macular degeneration | VEGFA Eye (ITV) | NeXstar Pharma/Eyetech Pharma | [48] |
| Fomivirsen (Vitravene®) | ASO | 1998 * 1999 ** | Cytomegalovirus retinitis | CMV IE2 Eye (ITV) | Ionis Pharma/Novartis | [49] |
1.3. Contributions
- Highlighting a clear imbalance: while antisense ONs have achieved significant clinical maturity, antigene approaches remain underexplored, revealing a persistent technological gap.
- To address this gap, we suggest incorporating advanced computational methods to overcome current challenges in TFO design and improve their therapeutic potential.
1.4. Manuscript Research Questions and Organization
- Bibliometric question: What are the publication trends, thematic clusters, and intellectual networks related to the design and application of TFO in antisense and antigene contexts?
- Systematic review question (PRISMA): What experimental criteria and design features have been reported for TFO in antigene strategies, and how do these compare to those used in antisense applications?
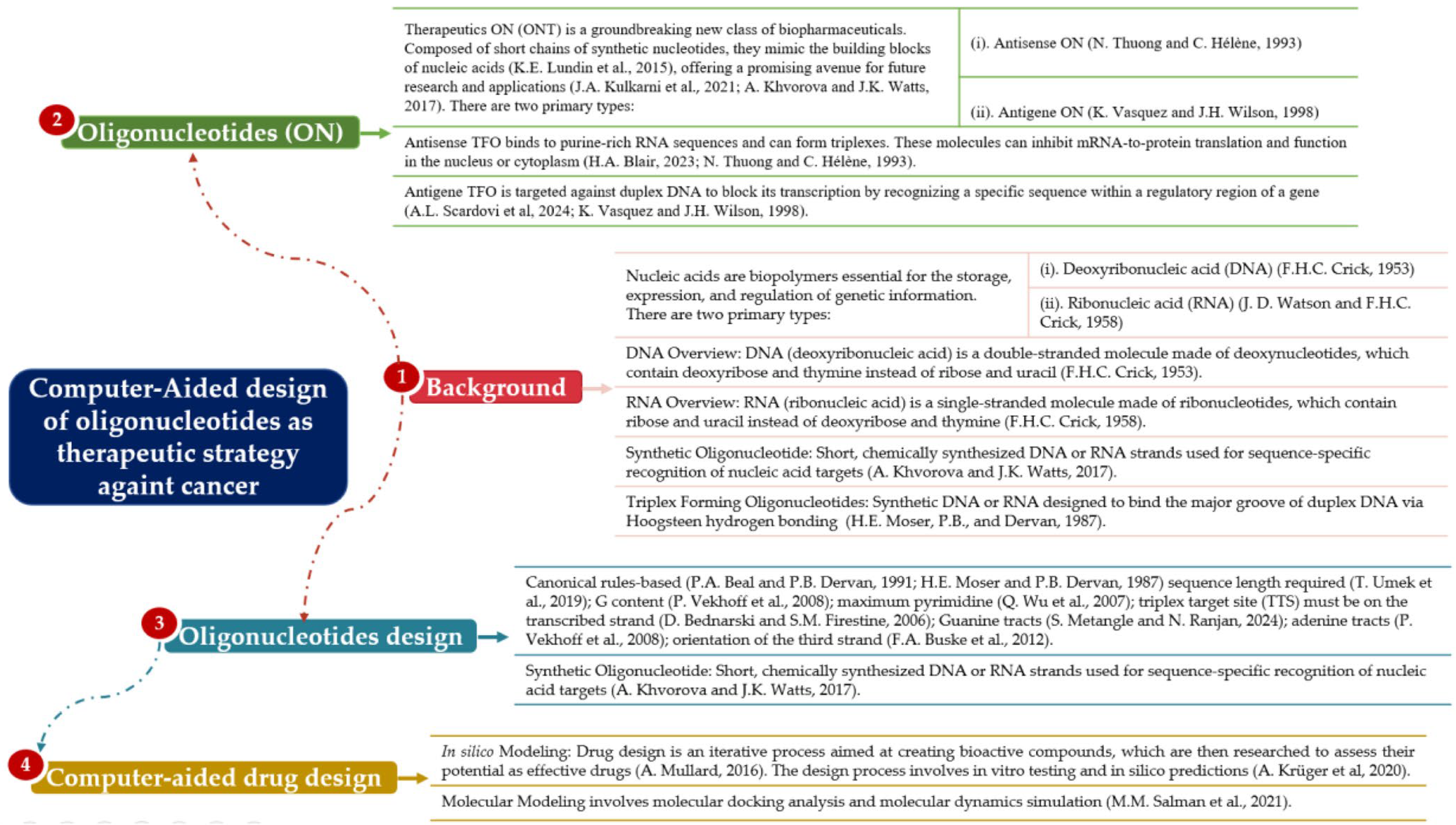
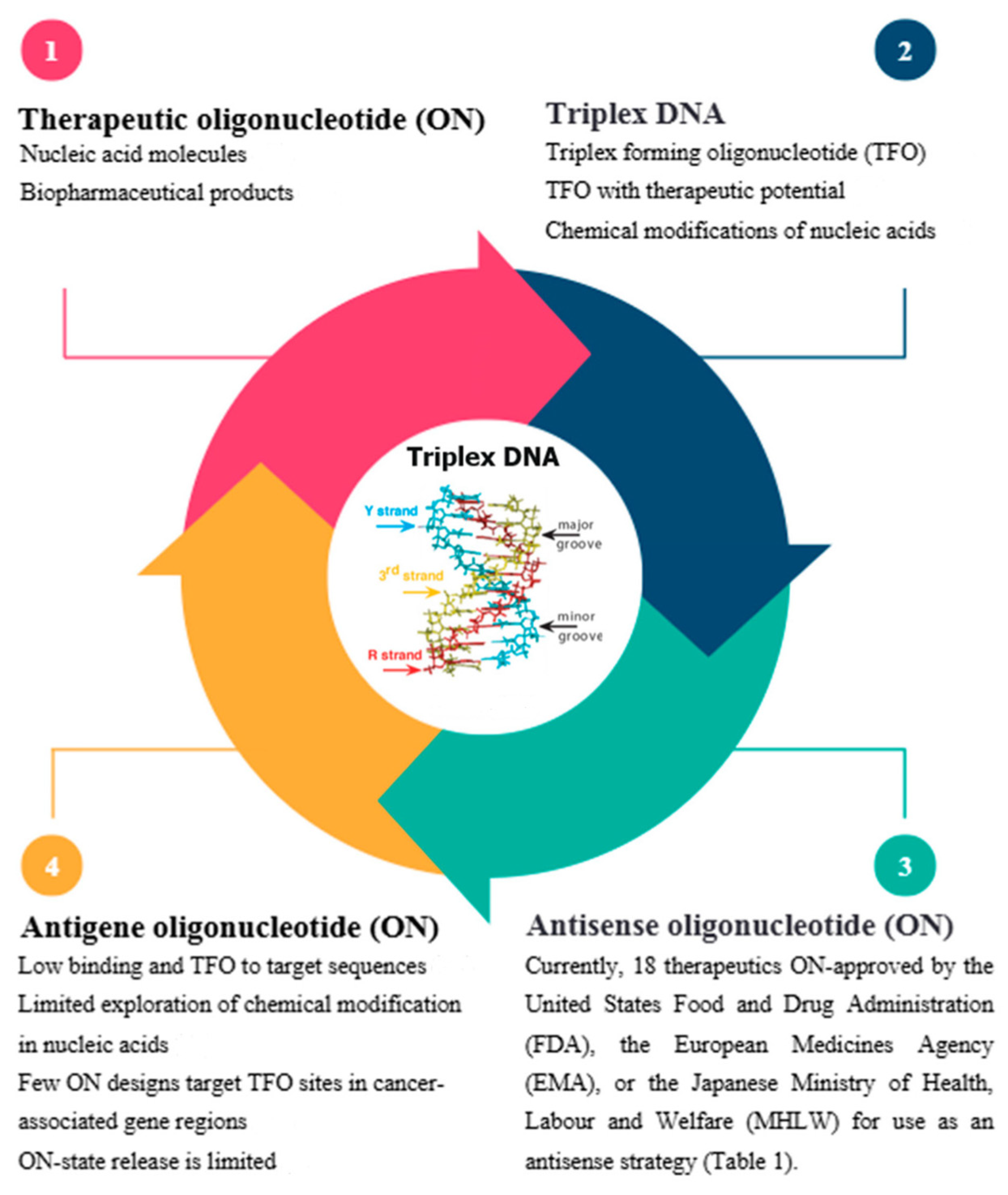
1.5. Conceptual and Theoretical Framework
1.5.1. Nucleic Acids’ Structures
1.5.2. Synthetic Oligonucleotides
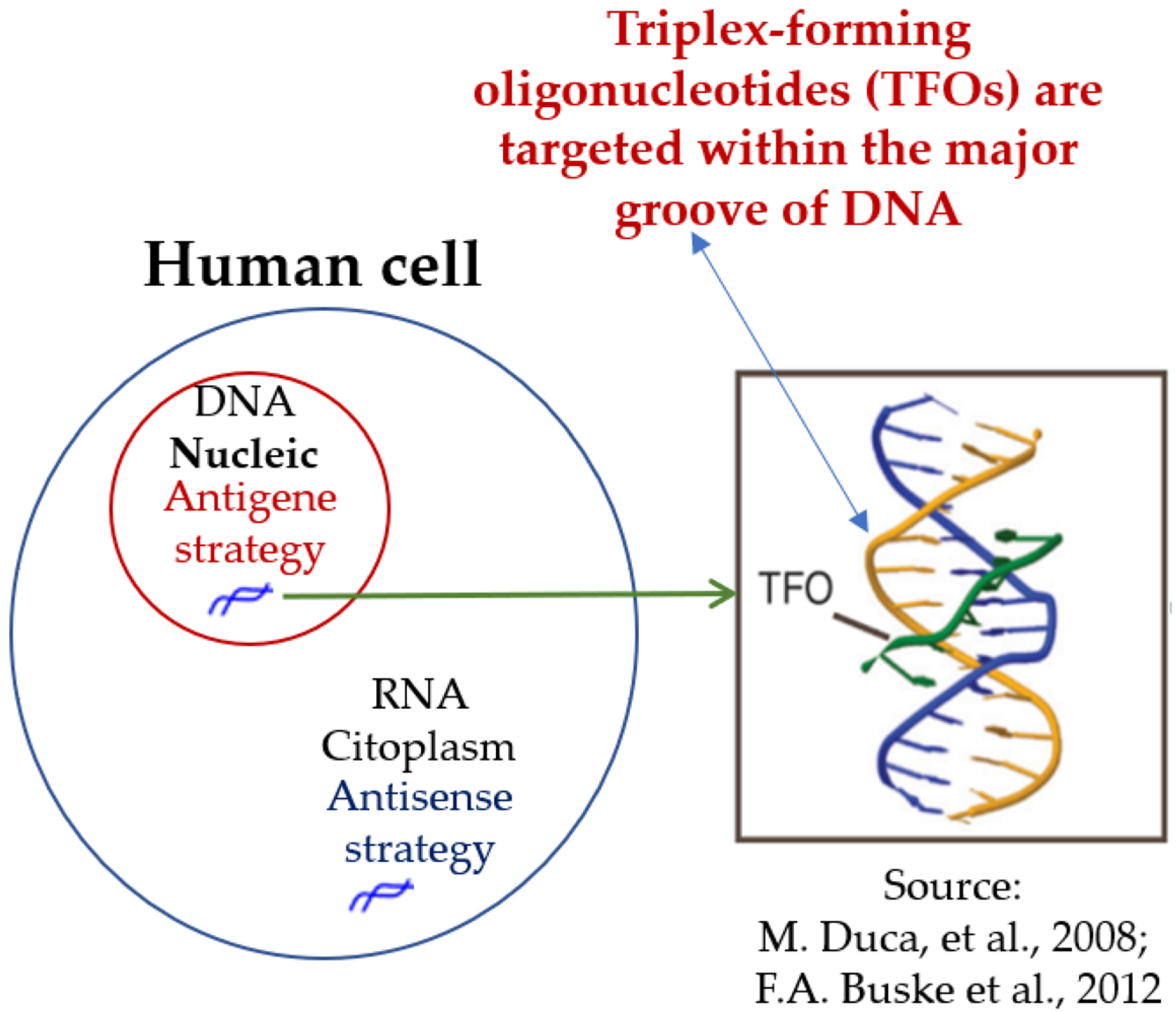
1.5.3. Triplex-Forming Oligonucleotides (TFOs)
1.5.4. Therapeutic Oligonucleotides (ONTs)
1.5.5. Antisense and Antigene Oligonucleotides
- Antisense Oligonucleotide Strategy
- RNase H-mediated degradation: When hybridized to target mRNA, the antisense-based TFO recruits RNase H, an endogenous endonuclease that degrades the RNA strand of DNA–RNA hybrids [78]. It then leaves the ASO intact, allowing for sustained inhibition [67]. The recognition efficiency depends on the TFO’s chemical modifications [28].
- Steric blockade of translation: Antisense-based TFOs can bind to essential mRNA regions like the 5′ cap, ribosome binding site, or start codon, physically blocking ribosome assembly and thereby inhibiting translation [78]. This steric interference also affects alternative splicing and causes mRNA destabilization in the nucleus [79].
- Splice-switching and transcript processing inhibition: Splice-switching ONs (SSOs) can regulate pre-mRNA splicing by preventing the binding of spliceosomal components, leading to exon skipping or intron retention [80]. Antisense-based TFOs can also block polyadenylation and transcript maturation by targeting related signals [81].
- Antigene Oligonucleotide Strategy
1.5.6. Oligonucleotide Design
- Canonical Design Rules
- Minimum TTS Length. The length of the TTS is a key factor that directly affects TFO specificity [51]. It is essential to note that short TTS sequences (e.g., 15 nucleotides) have a less than 1% chance of being unique in the genome [23]. However, the length of ≥21 nt significantly increases uniqueness to over 50% [90], while a TTS ≥ 24 nt achieves approximately 90%, and those ≥ 26 nt are virtually unique [10]. Therefore, longer TTSs are preferred to reduce off-target interactions [51], a key insight for your research on DNA targeting and gene regulation [12].
- Maximum Pyrimidine Interruptions. Then, it is essential to understand that TFOs bind to homopurine DNA strands through Hoogsteen base pairing. Interruptions by pyrimidines within the TTSs markedly decrease triplex stability and binding affinity due to energetic penalties from mismatches [91]. For optimal specificity and triplex stability, it is advisable to limit the number of pyrimidine interruptions to one [10]. This cautionary note should guide your selection process for TTS.
- Location of TTSs on the Transcribed Strand. Triplex formation can suppress gene expression either at the promoter, blocking transcription initiation, or within the coding region, hindering elongation. Although targeting the promoter is conceptually attractive, TFOs often encounter access limitations due to bound transcription factors [92]. In contrast, the transcribed region offers more accessible TTSs and fewer steric hindrances [93]. Therefore, TFOs should ideally target the transcribed strand within coding regions, employing genome-wide alignment to ensure site uniqueness and reduce off-target effects [17,51].
- Guanine Tracts. TTS sequences containing long guanine tracts (>3 G) may form G-quadruplex structures, which compete with triplex formation and decrease binding efficiency [19,94]. Therefore, it is essential to prevent sequences that could form such secondary structures during TTS selection [95]. This awareness will assist your TTS targeting and gene regulation research [51].
- Adenine Tracts. Adenine content of no more than seven nucleotides [95] in a sequence segment.
- Non-Canonical Design Rules
- Sugar modifications, such as locked ribose conformations (e.g., in LNA) or conformational constraints (like in tricyclo-DNA), enhance triplex thermal stability and nuclease resistance [73].
1.5.7. Computer-Aided Drug Design (CADD)
- In Silico Modeling
- Molecular Modeling
- Molecular docking predicts the binding conformation and affinity of a TFO within the major groove of the duplex DNA target. Depending on the structural flexibility, docking protocols are classified as rigid–rigid, flexible–rigid, or flexible–flexible [1]. Scoring functions and ligand sampling algorithms are designed to distinguish between productive and non-productive interactions by evaluating the steric and electrostatic complementarity between TFOs and target DNA. TFO candidates are also evaluated for potential off-target binding [17,51] through homology screening [104] against genomic databases.
- Molecular dynamics simulations (MDS) are essential for gaining atomistic insights into the stability of TFO–DNA triplexes over time under physiological conditions [31]. These simulations model conformational changes, hydrogen bonding, and water-mediated interactions, providing a comprehensive understanding of the key factors that contribute to triplex stability and specificity. This detailed approach to research using MDS should reassure the audience about the reliability of the TFO design [2,105].
2. Methods
2.1. Database Selection and Bibliometric Evaluation Criteria
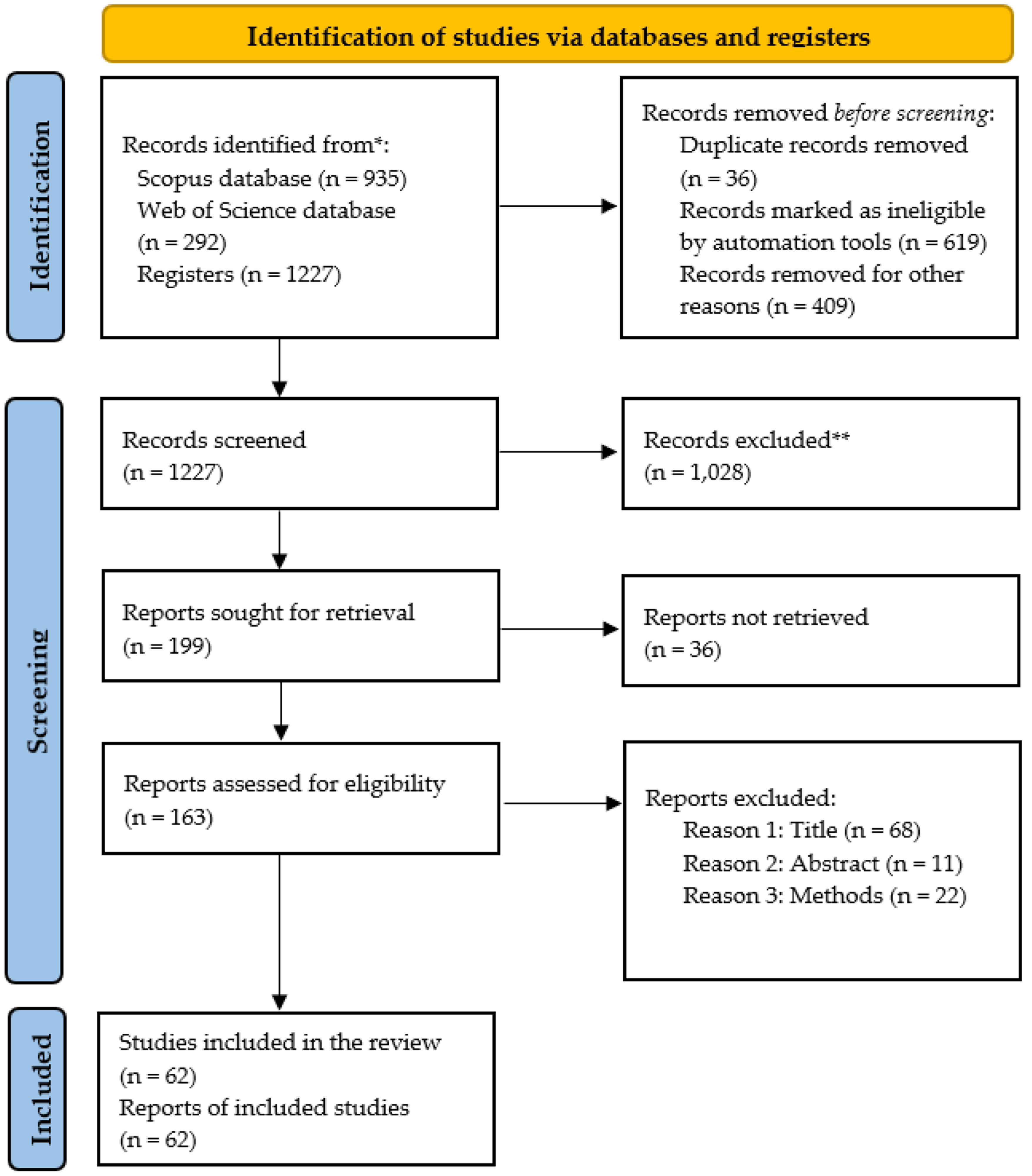
2.2. Search Strategy or Eligibility Criteria
2.3. Bibliometric Analysis
2.4. Systematic Review (PRISMA Framework)
2.4.1. Eligibility Criteria
2.4.2. Information Sources and Search Strategy
- SCOPUS (Advanced Search)
- (TITLE-ABS-KEY(“triplex-forming oligonucleotide” OR “triplex forming oligonucleotide” OR “Triplex-Forming Oligonucleotide” OR “Triplex Forming Oligonucleotide” OR “TFO” OR “antisense oligonucleotide” OR “ASO” OR “antisense strategy” OR “antigene strategy” OR “antigene oligonucleotide”))
- AND
- (TITLE-ABS-KEY(“in silico” OR “in silico model” OR “molecular modeling” OR “molecular dynamics” OR “computer-aided design” OR “computational prediction” OR “rational design” OR “structure-based design”))
- AND NOT
- (TITLE-ABS-KEY(“G-quadruplex” OR “quadruplex DNA” OR “quadruplex structure”))
- Web of Science Core Collection (Advanced Search)
- TS = (“triplex-forming oligonucleotide” OR “triplex forming oligonucleotide” OR “TFO”
- OR “antisense oligonucleotide” OR “ASO” OR “antisense strategy”
- OR “antigene strategy” OR “antigene oligonucleotide”)
- AND
- TS = (“in silico” OR “molecular modeling” OR “molecular dynamics”
- OR “computer-aided design” OR “rational design” OR “structure-based design”)
- NOT
- TS = (“G-quadruplex” OR “quadruplex DNA” OR “quadruplex structure”)
- AND
- LA = (English)
2.4.3. Data Collection Process
2.4.4. Data Items
- Effect Measures. No quantitative meta-analysis was performed due to variability in study designs and outcomes. Instead, results were described narratively, with bibliometric data presented as frequencies, percentages, and network-based measures (e.g., co-occurrence and cluster analyses). Systematic review findings were summarized using counts, proportions, and comparative tables that contrasted antisense and antigene strategies. This descriptive and comparative approach ensured consistency across datasets, providing the rationale for avoiding formal quantitative synthesis and focusing instead on mapping gaps and trends in computer-aided TFO design.
- Study Risk of Bias Assessment. No formal risk of bias tool (e.g., RoB 2.0 or ROBINS-I) [113] was used because the main goal of this study was a descriptive synthesis rather than a critical review of intervention effects. Instead, potential biases were minimized through predefined eligibility criteria (peer-reviewed original articles, English language, open-access, full-text availability), systematic screening of titles and abstracts, and duplicate removal with AteneaSIRES v1.0.3, https://ateneasires.com [55]. All included a single reviewer, who reviewed the studies to ensure consistency. Reporting bias (e.g., missing results or selective reporting) was not formally assessed, as no quantitative synthesis or outcome pooling was conducted.
- Certainty Assessment. No formal framework, such as GRADE [114], was applied to assess certainty in the body of evidence, given the descriptive and non-interventional nature of this dual bibliometric and systematic review. Instead, confidence in the findings was supported through predefined eligibility criteria, systematic screening, and metadata quality control using Bibliometrix, which collectively ensured consistency and transparency of the evidence base.
- Registration and Protocol. This systematic review was not prospectively registered in any public database (e.g., PROSPERO [115], and no pre-specified protocol was archived. Consequently, no protocol amendments were applicable. The review process was instead guided by the PRISMA 2020 statement [106], ensuring structured reporting and methodological transparency.
2.4.5. Data Cleaning and Software Tools (AteneaSIRES)
2.4.6. Data Merging and Quality Control Procedures
2.4.7. Software and Analytical Tools
3. Results and Discussion
3.1. Bibliometric Findings and Discussion
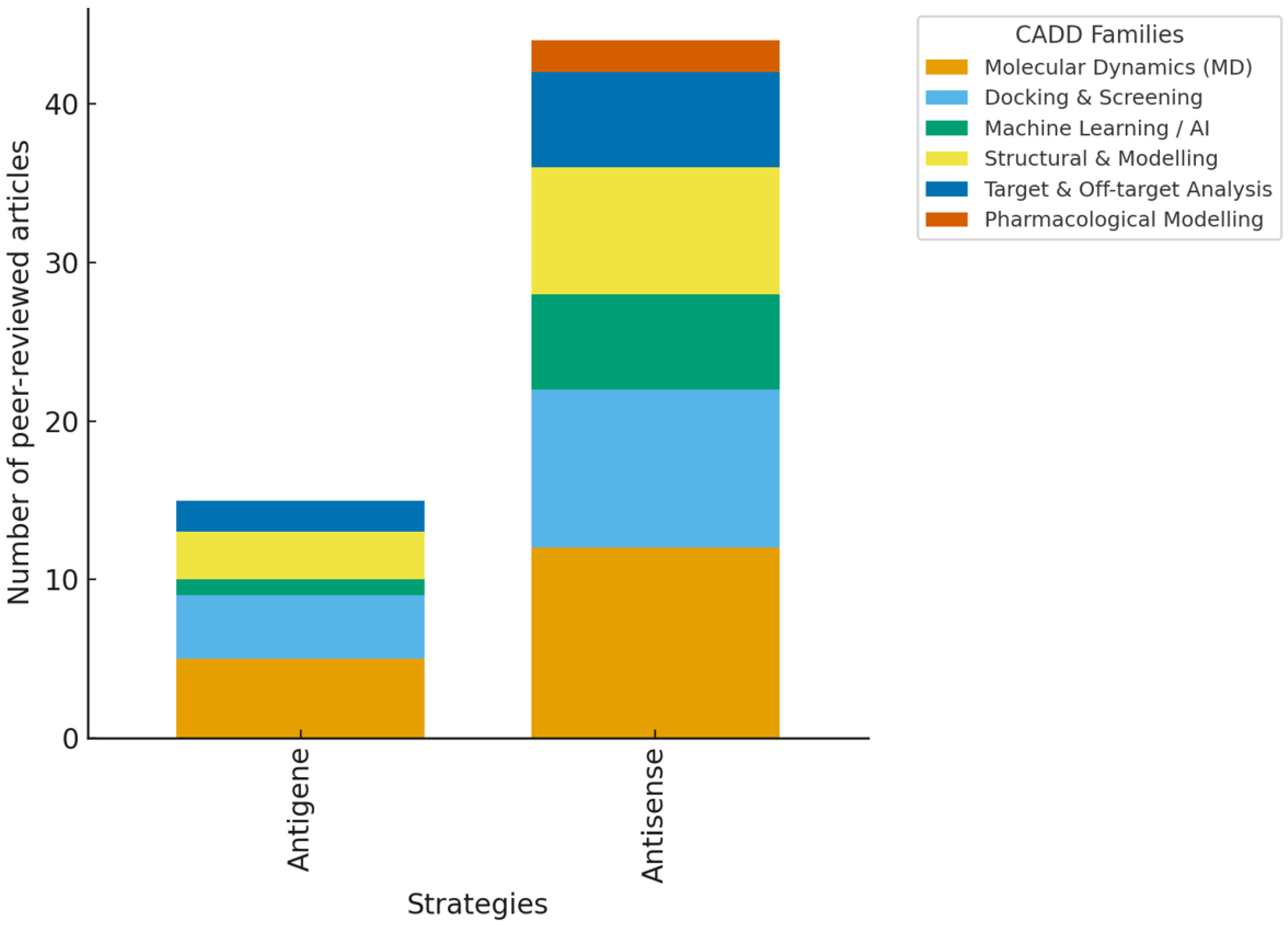
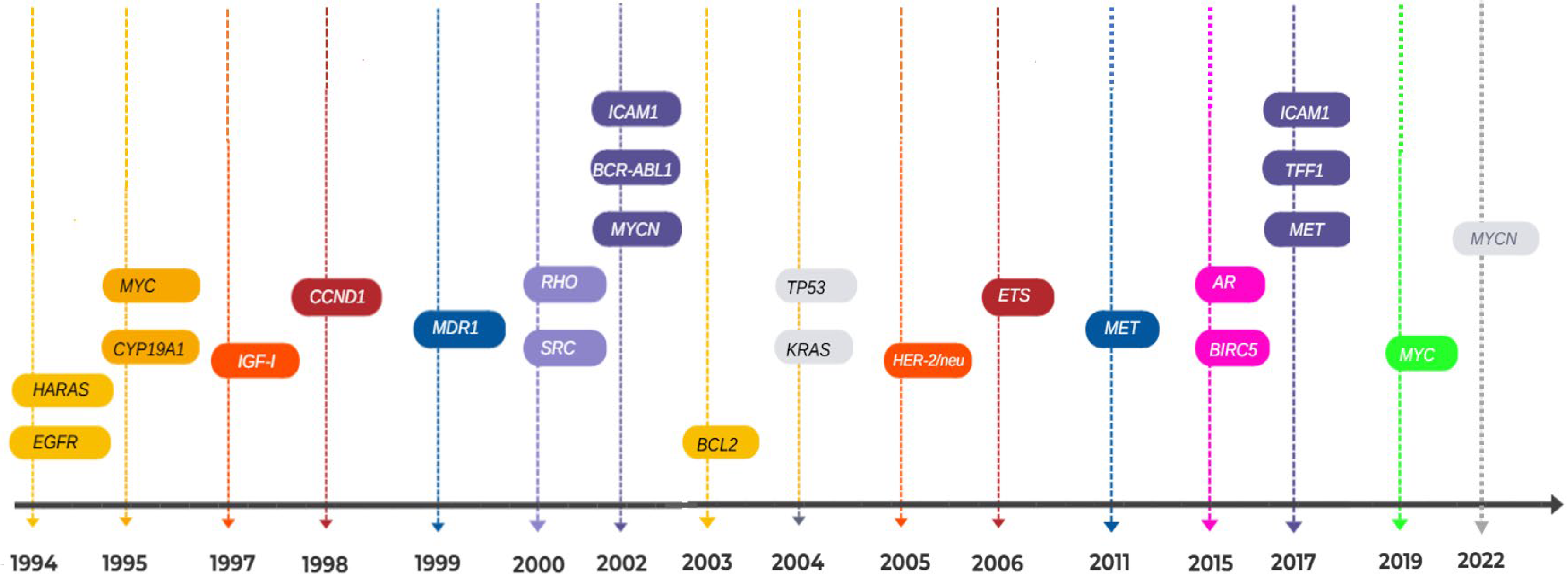
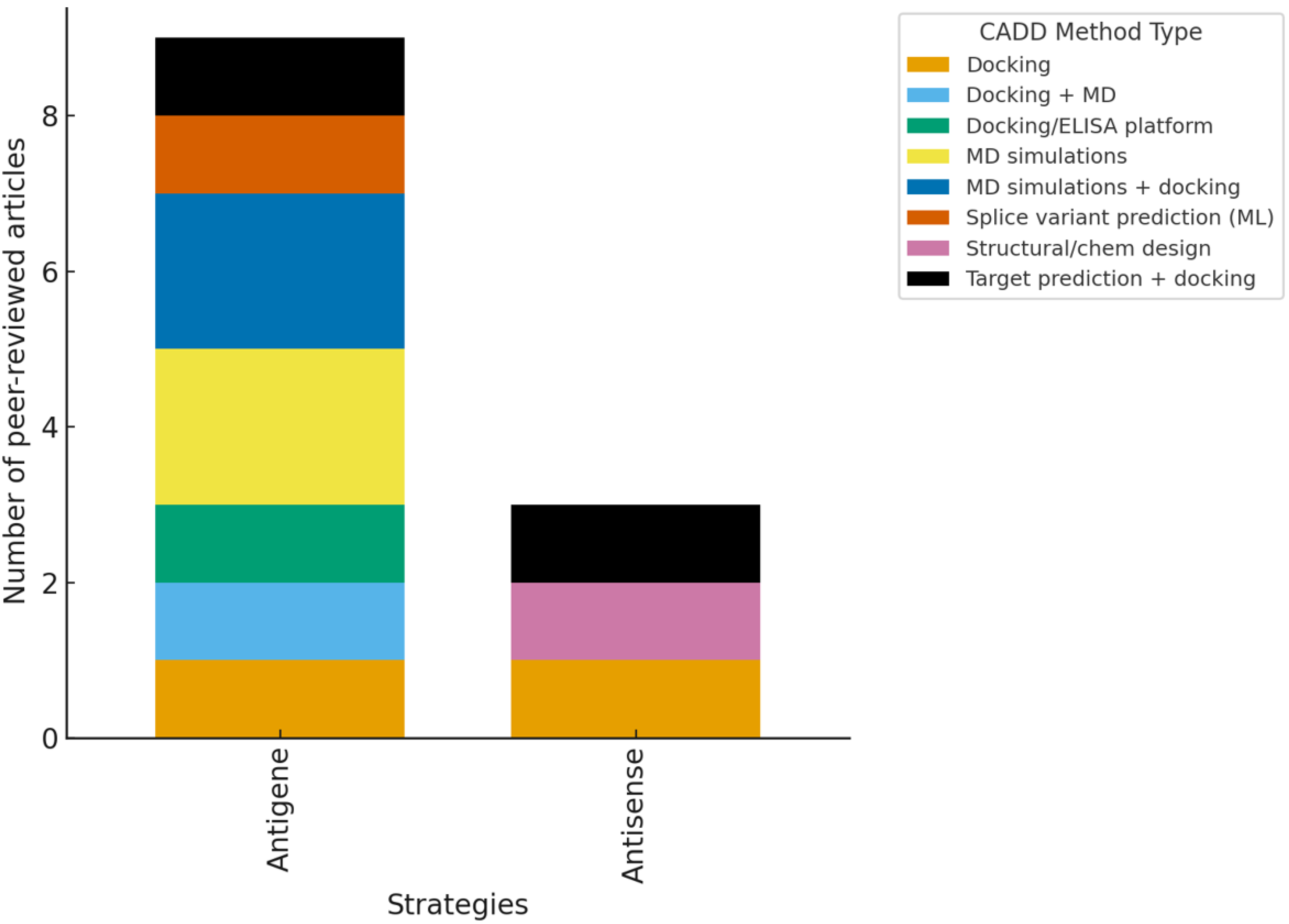
3.2. Systematic Review Findings and Discussion
3.3. Integrative Insight Across Methods
3.4. Implications for Design Practice and Future Research
3.5. Limitations of the Present Study
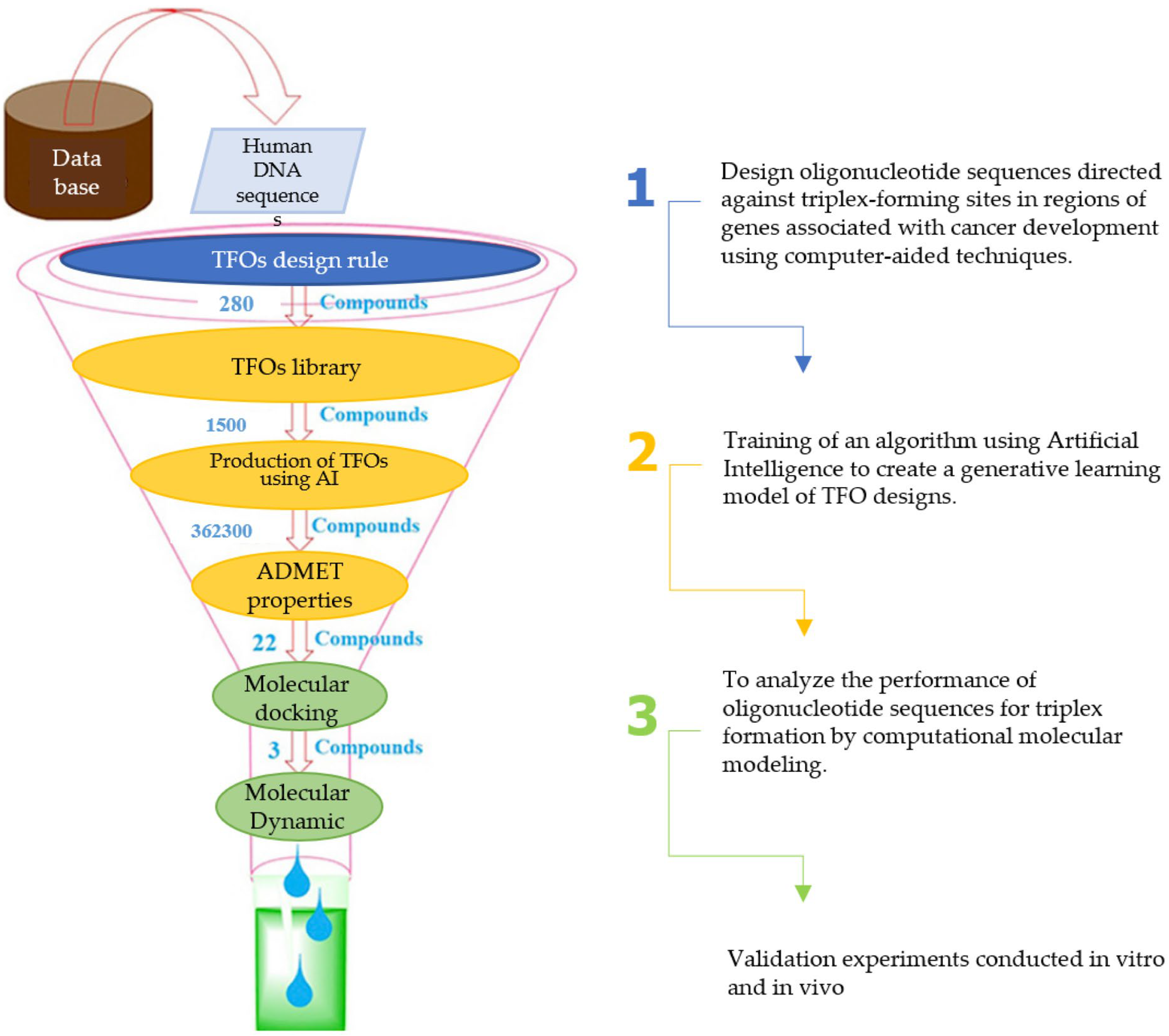
3.6. Integrated Forward Path: Toward Therapeutic TFOs
3.7. Final Contributions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hasselgren, C.; Oprea, T.I. Artificial Intelligence for Drug Discovery: Are We There Yet? Annu. Rev. Pharmacol. Toxicol. 2024, 64, 527–550. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design; Springer: Berlin/Heidelberg, Germany, 2023; Volume 15, ISBN 4474710010. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Laub, V.; Devraj, K.; Elias, L.; Schulte, D. Bioinformatics for wet-lab scientists: Practical application in sequencing analysis. BMC Genom. 2023, 24, 382. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. Parsing clinical success rates. Nat. Rev. Drug Discov. 2016, 15, 447. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Khan, M.; Zhang, Y.; Shafiq, M.; Ullah, M.; Abbas, A.; Xianxiang, X.; Chen, G.; Diao, Y. Advancing Therapeutic Strategies with Polymeric Drug Conjugates for Nucleic Acid Delivery and Treatment. Int. J. Nanomed. 2025, 20, 25–52. [Google Scholar] [CrossRef]
- Moumné, L.; Marie, A.C.; Crouvezier, N. Oligonucleotide Therapeutics: From Discovery and Development to Patentability. Pharmaceutics 2022, 14, 260. [Google Scholar] [CrossRef]
- Vasquez, K.M.; Glazer, P.M. Triplex-forming oligonucleotides: Principles and applications. Q. Rev. Biophys. 2002, 35, 89–107. [Google Scholar] [CrossRef]
- Mikame, Y.; Toyama, H.; Dohno, C.; Wada, T.; Yamayoshi, A. Development and functional evaluation of a psoralen-conjugated nucleoside mimic for triplex-forming oligonucleotides. Commun. Chem. 2025, 8, 18. [Google Scholar] [CrossRef]
- Wu, Q.; Gaddis, S.S.; MacLeod, M.C.; Walborg, E.F.; Thames, H.; DiGiovanni, J.; Vasquez, K.M. High-Affinity Triplex-Forming Oligonucleotide Target Sequences in Mammalian Genomes. Mol. Carcinog. 2007, 46, 15–27. [Google Scholar] [CrossRef]
- Suzuki, T.; Katayama, Y.; Komatsu, Y.; Kamiya, H. Analysis of large deletion mutations induced by abasic site analog in human cells. Genes Environ. 2018, 40, 24. [Google Scholar] [CrossRef]
- Scardovi, A.L.; Bartolucci, D.; Montemurro, L.; Bortolotti, S.; Angelucci, S.; Amadesi, C.; Nieddu, G.; Oosterholt, S.; Cerisoli, L.; Della Pasqua, O.; et al. Preclinical Pharmacokinetics in Tumors and Normal Tissues of the Antigene PNA Oligonucleotide MYCN-Inhibitor BGA002. Nucleic Acid. Ther. 2024, 34, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Wang, G.; Vasquez, K.M. DNA triple helices: Biological consequences and therapeutic potential. Biochimie 2008, 90, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Pabón-Martínez, Y.V. Targeting Double-Stranded DNA with Locked Nucleic Acid-Based (LNA) Oligonucleotides (ON) and DNA-Binding Peptide Conjugates. Ph.D. Thesis, Karolinska Institutet, Stockholm, Sweden, 2016. [Google Scholar]
- Hoogsteen, K. The crystal and molecular structure of a hydrogen-bonded complex between 1-methylthymine and 9-methyladenine. Acta Crystallogr. 1963, 16, 907–916. [Google Scholar] [CrossRef]
- Hoogsteen, K. The structure of crystals containing a hydrogen-bonded complex of 1-methylthymine and 9-methyladenine. Acta Crystallogr. 1959, 12, 822–823. [Google Scholar] [CrossRef]
- Mikame, Y.; Yamayoshi, A. Recent Advancements in Development and Therapeutic Applications of Genome-Targeting Triplex-Forming Oligonucleotides and Peptide Nucleic Acids. Pharmaceutics 2023, 15, 2515. [Google Scholar] [CrossRef]
- Mikame, Y.; Eshima, H.; Toyama, H.; Nakao, J.; Matsuo, M.; Yamamoto, T.; Hari, Y.; Komano, J.A.; Yamayoshi, A. Development and Crosslinking Properties of Psoralen-Conjugated Triplex-Forming Oligonucleotides as Antigene Tools Targeting Genome DNA. ChemMedChem 2023, 18, e202300348. [Google Scholar] [CrossRef]
- Wang, G.; Vasquez, K.M. Dynamic alternative DNA structures in biology and disease. Nat. Rev. Genet. 2023, 24, 211–234. [Google Scholar] [CrossRef]
- Pandya, N.; Bhagwat, S.R.; Kumar, A. Regulatory role of Non-canonical DNA Polymorphisms in human genome and their relevance in Cancer. Biochim. Biophys. Acta—Rev. Cancer. 2021, 1876, 188594. [Google Scholar] [CrossRef]
- Blair, H.A. Tofersen: First Approval. Drugs 2023, 83, 1039–1043. [Google Scholar] [CrossRef]
- Leclair, N.K.; Brugiolo, M.; Park, S.H.; Devoucoux, M.; Urbanski, L.; Angarola, B.L.; Yurieva, M.; Anczuków, O. Antisense oligonucleotide-mediated TRA2β poison exon inclusion induces the expression of a lncRNA with anti-tumor effects. Nat. Commun. 2025, 16, 1670. [Google Scholar] [CrossRef]
- Pabon-Martinez, Y.V.; Xu, Y.; Villa, A.; Lundin, K.E.; Geny, S.; Nguyen, C.H.; Pedersen, E.B.; Jørgensen, P.T.; Wengel, J.; Nilsson, L.; et al. LNA effects on DNA binding and conformation: From single strand to duplex and triplex structures. Sci. Rep. 2017, 7, 11043. [Google Scholar] [CrossRef] [PubMed]
- Escudé, C.; Sun, J.S. DNA major Groove Binders: Triple helix-forming oligonucleotides, triple helix-specific DNA ligands and cleaving agents. Top. Curr. Chem. 2005, 253, 109–148. [Google Scholar]
- Gissberg, O.; Zain, R.; Lundin, K.E. Oligonucleotide—Based Therapies; Springer Nature: Stockholm, Sweden, 2019; Volume XI, p. 344. [Google Scholar]
- Duca, M.; Vekhoff, P.; Oussedik, K.; Halby, L.; Arimondo, P.B. The triple helix: 50 years later, the outcome. Nucleic Acids Res. 2008, 36, 5123–5138. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.I.E.; Zain, R. Therapeutic oligonucleotides: State of the art. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 605–630. [Google Scholar] [CrossRef]
- Khvorova, A.; Watts, J.K. The chemical evolution of oligonucleotide therapies of clinical utility. Nat. Biotechnol. 2017, 35, 238–248. [Google Scholar] [CrossRef]
- Yang, X.; Xu, Y.; Fu, J.; Shen, Z. Nanoparticle delivery of TFOs is a novel targeted therapy for HER2 amplified breast cancer. BMC Cancer 2023, 23, 680. [Google Scholar] [CrossRef]
- Singh, S.K.; Nielsen, P.; Koshkin Aa Wengel, J. LNA (locked nucleic acids): Synthesis and high-affinity nucleic acid recognition. Chem Commun. 1998, 4, 455–456. [Google Scholar] [CrossRef]
- Xu, Y.; Gissberg, O.; Pabon-Martinez, Y.V.; Wengel, J.; Lundin, K.E.; Edvard Smith, C.I.; Zain, R.; Nilsson, L.; Villa, A. The ability of locked nucleic acid oligonucleotides to pre-structure the double helix: A molecular simulation and binding study. PLoS ONE 2019, 14, e0211651. [Google Scholar] [CrossRef]
- Brooks, B.J.; Et, A. CHARMM: The Biomolecular Simulation Program B. J. Comput. Chem. 2009, 30, 1545–1614. [Google Scholar] [CrossRef]
- Paik, J.; Duggan, S. Volanesorsen: First Global Approval. Drugs 2019, 79, 1349–1354. [Google Scholar] [CrossRef]
- Keam, S.J. Inotersen: First Global Approval. Drugs 2018, 78, 1371–1376. [Google Scholar] [CrossRef]
- Hair, P.; Cameron, F.; McKeage, K. Mipomersen sodium: First global approval. Drugs 2013, 73, 487–493. [Google Scholar] [CrossRef]
- Scott, L.J.; Keam, S.J. Lumasiran: First Approval. Drugs 2021, 81, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. Inclisiran: First Approval. Drugs 2021, 81, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J. Givosiran: First Approval. Drugs 2020, 80, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Patisiran: First Global Approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- Morgan, L. Defitelio (Defibrotide sodium): First drug approved for patients with hepatic veno-occlusive disease. Am. Health Drug Benefits 2017, 10, 51–53. [Google Scholar]
- Keam, S.J. Vutrisiran: First Approval. Drugs 2022, 82, 1419–1425. [Google Scholar] [CrossRef]
- Shirley, M. Casimersen: First Approval. Drugs 2021, 81, 875–879. [Google Scholar] [CrossRef]
- Dhillon, S. Viltolarsen: First Approval. Drugs 2020, 80, 1027–1031. [Google Scholar] [CrossRef]
- Heo, Y.A. Golodirsen: First Approval. Drugs 2020, 80, 329–333. [Google Scholar] [CrossRef]
- Kim, J.; Hu, C.; Moufawad El Achkar, C.; Black, L.E.; Douville, J.; Larson, A.; Pendergast, M.K.; Goldkind, S.F.; Lee, E.A.; Kuniholm, A.; et al. Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. N. Engl. J. Med. 2019, 381, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Nusinersen: First Global Approval. Drugs 2017, 74, 473–479. [Google Scholar] [CrossRef]
- Syed, Y.Y. Eteplirsen: First Global Approval. Drugs 2016, 76, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Adamis, A.P.; Cunningham, E.T.; Feinsod, M.; Guyer, D.R. Pegaptanib for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2004, 351, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Vitravene Study Group. A randomized controlled clinical trial of intravitreous fomivirsen for treatment of newly diagnosed peripheral cytomegalovirus retinitis in patients with aids. Am. J. Ophthalmol. 2002, 133, 467–474. [Google Scholar] [CrossRef]
- Casey, B.P.; Glazer, P.M. Gene targeting via triple-helix formation. Prog. Nucleic Acid Res. Mol. Biol. 2001, 67, 163–192. [Google Scholar] [CrossRef]
- Jenjaroenpun, P.; Chew, C.S.; Yong, T.P.; Choowongkomon, K.; Thammasorn, W.; Kuznetsov, V.A. The TTSMI database: A catalog of triplex target DNA sites associated with genes and regulatory elements in the human genome. Nucleic Acids Res. 2015, 43, D110–D116. [Google Scholar] [CrossRef]
- Theillet, F.X.; Binolfi, A.; Frembgen-Kesner, T.; Hingorani, K.; Sarkar, M.; Kyne, C.; Li, C.; Crowley, P.B.; Gierasch, L.; Pielak, G.J.; et al. Physicochemical properties of cells and their effects on intrinsically disordered proteins (IDPs). Chem. Rev. 2014, 114, 6661–6714. [Google Scholar] [CrossRef]
- Takahashi, S.; Sugimoto, N. Stability prediction of canonical and non-canonical structures of nucleic acids in various molecular environments and cells. Chem. Soc. Rev. 2020, 49, 8439–8468. [Google Scholar] [CrossRef]
- James, P.L.; Brown, T.; Fox, K.R. Thermodynamic and kinetic stability of intermolecular triple helices containing different proportions of C+·GC and T·AT triplets. Nucleic Acids Res. 2003, 31, 5598–5606. [Google Scholar] [CrossRef]
- Galeano-Barrera, C.J.; Barrera, E.; Romero-Riaño, E.; AteneaSIRES. Sistema Para la Identificación y Remoción de Documentos Duplicados en Revisiones Sistemáticas. 2023. Available online: https://ateneasires.com/ (accessed on 7 July 2025).
- Crick, F.; Watson, J. Genetical implications of the structure of deoxyribonucleic acid. Nature 1953, 171, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Cech, T.R. The chemical repertoire of natural ribozymes. Nature 2002, 418, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Leontis, N.B.; Westhof, E. Geometric nomenclature and classification of RNA base pairs. RNA 2001, 7, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Rich, A.; Nordheim, A.; Wang, A.H.-J. The chemistry and biology of left-handed Z-DNA. Ann. Rev. Biochem. 1984, 53, 791–846. [Google Scholar] [CrossRef]
- Felsenfeld, G.; Davies, D.R.; Rich, A. Formation of a three-stranded polynucleotide molecule. J. Am. Chem. Soc. 1957, 79, 2023–2024. [Google Scholar] [CrossRef]
- Davis, J.T. G-Quartets 40 Years Later: From 5′-GMP to Molecular Biology and Supramolecular Chemistry. Angew. Chem.—Int. Ed. 2004, 43, 668–698. [Google Scholar] [CrossRef]
- Draper, D.E. RNA Folding: Thermodynamic and Molecular Descriptions of the Roles of Ions. Biophys. J. 2008, 95, 5489–5495. [Google Scholar] [CrossRef]
- Zadeh, J.N.; Steenberg, C.D.; Bois, J.S.; Wolfe, B.R.; Pierce, M.B.; Khan, A.R.; Dirks, R.M.; Pierce, N.A. NUPACK: Analysis and Design of Nucleic Acid Systems. J. Comput. Chem. 2012, 32, 170–173. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.G.; Yun, G.; Lee, C.; Kim, Y.J.; Kim, K.S.; Kim, T.H.; Kim, D.N. Rapid Computational Analysis of DNA Origami Assemblies at Near-Atomic Resolution. ACS Nano 2021, 15, 1002–1015. [Google Scholar] [CrossRef]
- Hörberg, J.; Carlesso, A.; Reymer, A. Mechanistic insights into ASO-RNA complexation: Advancing antisense oligonucleotide design strategies. Mol. Ther. Nucleic Acids 2024, 35, 102351. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.K.; Deleavey, G.F.; Damha, M.J. Chemically modified siRNA: Tools and applications. Drug Discov. Today 2008, 13, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Jia, Q.; Li, D.; Li, S.; Li, X.J.; Yin, P. Application of antisense oligonucleotide drugs in amyotrophic lateral sclerosis and Huntington’s disease. Transl. Neurodegener. 2025, 14, 4. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, D.; Pession, A.; Hrelia, P.; Tonelli, R. Precision Anti-Cancer Medicines by Oligonucleotide Therapeutics in Clinical Research Targeting Undruggable Proteins and Non-Coding RNAs. Pharmaceutics 2022, 14, 1453. [Google Scholar] [CrossRef]
- Buske, F.A.; Mattick, J.S.; Bailey, T.L. Potential in vivo roles of nucleic acid triple-helices. RNA Biol. 2011, 8, 427–439. [Google Scholar] [CrossRef]
- Tonelli, R.; Purgato, S.; Camerin, C.; Fronza, R.; Bologna, F.; Alboresi, S.; Franzoni, M.; Corradini, R.; Sforza, S.; Faccini, A.; et al. Antigene peptide nucleic acid specifically inhibits MYCN expression in human neuroblastoma cells leading to cell growth inhibition and apoptosis. Mol. Cancer Ther. 2005, 4, 779–786. [Google Scholar] [CrossRef]
- Thuong, N.T.; Hélène, C. Sequence-Specific Recognition and Modification of Double-Helical DNA by Oligonucleotides. Angew. Chem. Int. Ed. 1993, 32, 666–690. [Google Scholar] [CrossRef]
- Bortolotti, S.; Angelucci, S.; Montemurro, L.; Bartolucci, D.; Raieli, S.; Lampis, S.; Amadesi, C.; Scardovi, A.; Nieddu, G.; Cerisoli, L.; et al. Antigene MYCN Silencing by BGA002 Inhibits SCLC Progression Blocking mTOR Pathway and Overcomes Multidrug Resistance. Cancers 2023, 15, 990. [Google Scholar] [CrossRef]
- Genna, V.; Portella, G.; Sala, A.; Terrazas, M.; Serrano-Chacón, I.; González, J.; Villegas, N.; Mateo, L.; Castellazzi, C.; Labrador, M.; et al. Systematic study of hybrid triplex topology and stability suggests a general triplex-mediated regulatory mechanism. Nucleic Acids Res. 2025, 53, gkaf170. [Google Scholar] [CrossRef]
- Khalil, A.S.; Collins, J.J. Synthetic biology: Applications come of age. Nat. Rev. Genet. 2010, 11, 367–379. [Google Scholar] [CrossRef]
- Liu, A.P.; Appel, E.A.; Ashby, P.D.; Baker, B.M.; Franco, E.; Gu, L.; Haynes, K.; Joshi, N.S.; Kloxin, A.M.; Kouwer, P.H.J.; et al. The living interface between synthetic biology and biomaterial design. Nat. Mater. 2022, 21, 390–397. [Google Scholar] [CrossRef]
- Shchaslyvyi, A.Y.; Antonenko, S.V.; Tesliuk, M.G.; Telegeev, G.D. Current State of Human Gene Therapy: Approved Products and Vectors. Pharmaceuticals 2023, 16, 1416. [Google Scholar] [CrossRef]
- Mendes, B.B.; Conniot, J.; Avital, A.; Yao, D.; Jiang, X.; Zhou, X.; Sharf-Pauker, N.; Xiao, Y.; Adir, O.; Liang, H.; et al. Nanodelivery of nucleic acids. Nat. Rev. Methods Prim. 2022, 2, 24. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu. Rev. Med. 2019, 70, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Kurreck, J. Antisense technologies Improvement through novel chemical modifications. Eur. J. Biochem. 2003, 270, 1628–1644. [Google Scholar] [CrossRef]
- Arechavala-Gomeza, V.; Aartsma-Rus, A. Splicing modulation therapy in the treatment of genetic diseases. Appl. Clin. Genet. 2014, 7, 245–252. [Google Scholar] [CrossRef]
- Le, B.T.; Raguraman, P.; Kosbar, T.R.; Fletcher, S.; Wilton, S.D.; Veedu, R.N. Antisense Oligonucleotides Targeting Angiogenic Factors as Potential Cancer Therapeutics. Mol. Ther. Nucleic Acid 2019, 14, 142–157. [Google Scholar] [CrossRef]
- Nishida, Y.; Ishizawa, J.; Ayoub, E.; Montoya, R.H.; Ostermann, L.B.; Muftuoglu, M.; Ruvolo, V.R.; Patsilevas, T.; Scruggs, D.A.; Khazaei, S.; et al. Enhanced TP53 reactivation disrupts MYC transcriptional program and overcomes venetoclax resistance in acute myeloid leukemias. Sci. Adv. 2023, 9, eadh1436. [Google Scholar] [CrossRef]
- Shen, C.; Rattat, D.; Buck, A.; Mehrke, G.; Polat, B.; Ribbert, H.; Schirrmeister, H.; Mahren, B.; Matuschek, C.; Reske, S.N. Targeting bcl-2 by Triplex-Forming Oligonucleotide—A Promising Carrier for Gene—Radiotherapy. Cancer Biother. Radiopharm. 2003, 18, 17–26. [Google Scholar] [CrossRef]
- Okada, T.; Yamaguchi, K.; Yamashita, J. Triplex-forming oligonucleotide binding represses transcription of the human c-erbB gene in glioma. Growth Factors 1994, 11, 259–270. [Google Scholar] [CrossRef]
- Re, R.N.; Cook, J.L.; Giardina, J.F. The inhibition of tumor growth by triplex-forming oligonucleotides. Cancer Lett. 2004, 209, 51–53. [Google Scholar] [CrossRef]
- Cogoi, S.; Ballico, M.; Bonora, G.M.; Xodo, L.E. Antiproliferative activity of a triplex-forming oligonucleotide recognizing a Ki-ras polypurine/polypyrimidine motif correlates with protein binding. Cancer Gene Ther. 2004, 11, 465–476. [Google Scholar] [CrossRef]
- Wells, R.D.; Dere, R.; Hebert, M.L.; Napierala, M.; Son, L.S. Advances in mechanisms of genetic instability related to hereditary neurological diseases. Nucleic Acids Res. 2005, 33, 3785–3798. [Google Scholar] [CrossRef]
- Moser, H.E.; Dervan, P.B. Sequence—Specific Cleavage of Double Helical DNA by Triple Helix Formation. Science 1987, 238, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Beal, P.A.; Dervan, P.B. Second structural motif for recognition of DNA by oligonucleotide-directed triple-helix formation. Science 1991, 251, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Catapano, C.V.; McGuffie, E.M.; Pacheco, D.; Carbone, G.M.R. Inhibition of gene expression and cell proliferation by triple helix- forming oligonucleotides directed to the c-myc gene. Biochemistry 2000, 39, 5126–5138. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.W.; Crothers, D.M. Specificity and stringency in DNA triplex formation. Proc. Natl. Acad. Sci. USA 1991, 88, 9397–9401. [Google Scholar] [CrossRef]
- Maher, L.J. Inhibition of T7 RNA polymerase initiation by triple-helical DNA complexes: A model for artificial gene repression. Biochemistry 1992, 31, 7587–7594. [Google Scholar] [CrossRef]
- Ebbinghaus, S.W.; Fortinberry, H.; Gamper, H.B. Inhibition of transcription elongation in the HER-2/neu coding sequence by triplex-directed covalent modification of the template strand. Biochemistry 1999, 38, 619–628. [Google Scholar] [CrossRef]
- Metangle, S.; Ranjan, N. Preferential Binding of a Red Emissive Julolidine Derivative to a Promoter G-Quadruplex. ChemBioChem 2024, 23, e202300527. [Google Scholar] [CrossRef]
- Vekhoff, P.; Ceccaldi, A.; Polverari, D.; Pylouster, J.; Pisano, C.; Arimondo, P.B. Triplex formation on DNA targets: How to choose the oligonucleotide. Biochemistry 2008, 47, 12277–12289. [Google Scholar] [CrossRef] [PubMed]
- Hartono, Y.D.; Pabon-Martinez, Y.V.; Uyar, A.; Wengel, J.; Lundin, K.E.; Zain, R.; Smith, C.I.E.; Nilsson, L.; Villa, A. Role of Pseudoisocytidine Tautomerization in Triplex-Forming Oligonucleotides: In Silico and in Vitro Studies. ACS Omega 2017, 2, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Kunkler, C.N.; Schiefelbein, G.E.; O’Leary, N.J.; Mccown, P.J.; Brown, J.A. A single natural RNA modification can destabilize a U • A-T-rich RNA • DNA-DNA triple helix. RNA 2022, 28, 1172–1184. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Pradeep, S.P.; Kumar, V.; Xiao, Y.; Deng, Y.; Fan, R.; Vasquez, J.C.; Singh, V.; Bahal, R. Antitumor efficacy of a sequence-specific DNA-targeted γPNA-based c-Myc inhibitor. Cell Rep. Med. 2024, 5, 101354. [Google Scholar] [CrossRef]
- Muangkaew, P.; Vilaivan, T. Modulation of DNA and RNA by PNA. Bioorg. Med. Chem. Lett. 2020, 30, 127064. [Google Scholar] [CrossRef]
- Imbert, M.; Blandel, F.; Leumann, C.; Garcia, L.; Goyenvalle, A. Lowering Mutant Huntingtin Using Tricyclo-DNA Antisense for Huntington’ s Disease. Nucleic Acid. Ther. 2019, 29, 256–265. [Google Scholar] [CrossRef]
- Wengel, J.; Petersen, M.; Frieden, M.; Kock, T. Chemistry of locked nucleic acids (LNA): Design, synthesis, and bio-physical properties. Lett. Pept. Sci. 2004, 10, 237–253. [Google Scholar] [CrossRef]
- Toropov, A.A.; Toropova, A.P. QSAR as a random event: Criteria of predictive potential for a chance model. Struct. Chem. 2019, 30, 1677–1683. [Google Scholar] [CrossRef]
- Juneja, S.; Dhankhar, A.; Juneja, A.; Bali, S. An Approach to DNA Sequence Classification Through Machine Learning. Int. J. Reliab. Qual. E-Healthc. 2022, 11, 1–15. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, Z.; Liu, H.; Davuluri, R.V. DNABERT: Pre-trained Bidirectional Encoder Representations from Transformers model for DNA-language in genome. Bioinformatics 2021, 37, 2112–2120. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Baas, J.; Schotten, M.; Plume, A.; Côté, G.; Karimi, R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant. Sci. Stud. 2020, 1, 377–386. [Google Scholar] [CrossRef]
- Birkle, C.; Pendlebury, D.A.; Schnell, J.; Adams, J. Web of Science as a data source for research on scientific and scholarly activity. Quant. Sci. Stud. 2020, 1, 363–376. [Google Scholar] [CrossRef]
- Elsevier. Cit0eScore, SNIP and SJR Metrics are Published Annually and Accessible via Scopus [Internet]. Available online: https://www.scopus.com/sources (accessed on 7 July 2025).
- Clarivate Analytics. Metrics Published Annually in Journal Citation Reports (JCR). [Internet]. Available online: https://clarivate.com/academia-government/scientific-and-academic-research/research-funding-analytics/journal-citation-reports/ (accessed on 7 July 2025).
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Tomlinson, E.; Cooper, C.; Davenport, C.; Rutjes, A.W.S.; Leeflang, M.; Mallett, S.; Whiting, P. Common challenges and suggestions for risk of bias tool development: A systematic review of methodological studies. J. Clin. Epidemiol. 2024, 171, 111370. [Google Scholar] [CrossRef]
- Prasad, M. Introduction to the GRADE tool for rating certainty in evidence and recommendations. Clin. Epidemiol. Glob. Health 2024, 25, 101484. [Google Scholar] [CrossRef]
- Booth, A.; Clarke, M.; Dooley, G.; Ghersi, D.; Moher, D.; Petticrew, M.; Stewart, L. The nuts and bolts of PROSPERO: An international prospective register of systematic reviews. Syst. Rev. 2012, 1, 2. [Google Scholar] [CrossRef]
- Waltman, L.; Van Eck, N.J. A smart local moving algorithm for large-scale modularity-based community detection. Eur. Phys. J. B 2013, 86, 471–481. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Kwoh, C.K. Deep learning based DNA:RNA triplex forming potential prediction. BMC Bioinform. 2020, 21, 522. [Google Scholar] [CrossRef]
- Rusling, D.A.; Powers, V.E.C.; Ranasinghe, R.T.; Wang, Y.; Osborne, S.D.; Brown, T.; Fox, K.R. Four base recognition by triplex-forming oligonucleotides at physiological pH. Nucleic Acids Res. 2005, 33, 3025–3032. [Google Scholar] [CrossRef] [PubMed]
- Marquez, V.E.; Siddiqui, M.A.; Ezzitouni, A.; Russ, P.; Wang, J.; Wagner, R.W.; Matteucci, M.D. Nucleosides with a twist. Can fixed forms of sugar ring pucker influence biological activity in nucleosides and oligonucleotides? J. Med. Chem. 1996, 39, 3739–3747. [Google Scholar] [CrossRef]
- Wang, Y.; Macke, J.P.; Abella, B.S.; Andreasson, K.; Worley, P.; Gilbert, D.J.; Copeland, N.G.; Jenkins, N.A.; Nathans, J. A large family of putative transmembrane receptors homologous to the product of the Drosophila tissue polarity gene frizzled. J. Biol. Chem. 1996, 271, 4468–4476. [Google Scholar] [CrossRef] [PubMed]
- Seth, P.P.; Siwkowski, A.; Allerson, C.R.; Vasquez, G.; Lee, S.; Prakash, T.P.; Kinberger, G.; Migawa, M.T.; Gaus, H.; Bhat, B.; et al. Design, synthesis and evaluation of constrained methoxyethyl (cMOE) and constrained ethyl (cEt) nucleoside analogs. Nucleic Acids Symp. Ser. 2008, 52, 553–554. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Yu, J.; Migawa, M.T.; Kinberger, G.A.; Wan, W.B.; Østergaard, M.E.; Carty, R.L.; Vasquez, G.; Low, A.; Chappell, A.; et al. Comprehensive Structure-Activity Relationship of Triantennary N-Acetylgalactosamine Conjugated Antisense Oligonucleotides for Targeted Delivery to Hepatocytes. J. Med. Chem. 2016, 59, 2718–2733. [Google Scholar] [CrossRef]
- Wang, H.Y.; Gopalan, V.; Aksentijevich, I.; Yeager, M.; Ma, C.A.; Mohamoud, Y.A.; Quinones, M.; Matthews, C.; Boland, J.; Niemela, J.E.; et al. A custom 148 gene-based resequencing chip and the SNP explorer software: New tools to study antibody deficiency. Hum. Mutat. 2010, 31, 1080–1088. [Google Scholar] [CrossRef]
- Li, Y.; Laue, K.; Temtamy, S.; Aglan, M.; Kotan, L.D.; Yigit, G.; Canan, H.; Pawlik, B.; Nürnberg, G.; Wakeling, E.L.; et al. Temtamy preaxial brachydactyly syndrome is caused by loss-of-function mutations in chondroitin synthase 1, a potential target of BMP signaling. Am. J. Hum. Genet. 2010, 87, 757–767. [Google Scholar] [CrossRef]
- Zhang, K.; Zheludev, I.N.; Hagey, R.J.; Haslecker, R.; Hou, Y.J.; Kretsch, R.; Pintilie, G.D.; Rangan, R.; Kladwang, W.; Li, S.; et al. Cryo-EM and antisense targeting of the 28-kDa frameshift stimulation element from the SARS-CoV-2 RNA genome. Nat. Struct. Mol. Biol. 2021, 28, 747–754. [Google Scholar] [CrossRef]
- Lavery, R.; Zakrzewska, K.; Beveridge, D.; Bishop, T.C.; Case, D.A.; Cheatham, T.; Dixit, S.; Jayaram, B.; Lankas, F.; Laughton, C.; et al. A systematic molecular dynamics study of nearest-neighbor effects on base pair and base pair step conformations and fluctuations in B-DNA. Nucleic Acids Res. 2010, 38, 299–313. [Google Scholar] [CrossRef]
- Zhang, J.; Thakuri, B.K.C.; Zhao, J.; Nguyen, L.N.; Nguyen, L.N.T.; Cao, D.; Dang, X.; Khanal, S.; Schank, M.; Lu, Z.; et al. Long noncoding RNA HOTAIRM1 promotes myeloid-derived suppressor cell expansion and suppressive functions through up-regulating HOXA1 expression during latent HIV infection. Aids 2020, 34, 2211–2221. [Google Scholar] [CrossRef]
- DHsu, P.; Scott, D.A.; Weinstein, J.A.; Ran, F.A.; Konermann, S.; Agarwala, V.; Li, Y.; Fine, E.J.; Wu, X.; Shalem, O.; et al. DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 2013, 31, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing sequence intrinsic composition to classify protein-coding and long non-coding transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34 (Suppl. 1), D668–D672. [Google Scholar] [CrossRef]
- Ejlersen, M.; Langkjær, N.; Wengel, J. 3′-Pyrene-modified unlocked nucleic acids: Synthesis, fluorescence properties and a surprising stabilization effect on duplexes and triplexes. Org. Biomol. Chem. 2017, 15, 2073–2085. [Google Scholar] [CrossRef]
- Kasuya, T.; Kugimiya, A. Role of Computationally Evaluated Target Specificity in the Hepatotoxicity of Gapmer Antisense Oligonucleotides. Nucleic Acid. Ther. 2018, 28, 312–317. [Google Scholar] [CrossRef]
- Zaniani, N.R.; Oroujalian, A.; Valipour, A.; Peymani, M. LAMTOR5 expression level is a biomarker for colorectal cancer and lncRNA LAMTOR5-AS1 predicting miRNA sponging effect. Mol. Biol. Rep. 2021, 48, 6093–6101. [Google Scholar] [CrossRef]
- Liang, X.H.; Sun, H.; Nichols, J.G.; Crooke, S.T. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol. Ther. 2017, 25, 2075–2092. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, P.; Ren, M.; Li, D.; Lin, J.; Sun, T.; Wang, Y.; Yang, S.; Nenopoulos, C.; Oetheimer, C.; et al. Bottlebrush Polymers with Sequence-Controlled Backbones for Enhanced Oligonucleotide Delivery. J. Am. Chem. Soc. 2024, 146, 34763–34770. [Google Scholar] [CrossRef]
- Chao, H.H.; Luo, J.L.; Hsu, M.H.; Chen, L.H.; Lu, T.P.; Tsai, M.H.; Chuang, E.Y.; Chuang, L.L.; Lai, L.C. Regulatory mechanisms and function of hypoxia-induced long noncoding RNA NDRG1-OT1 in breast cancer cells. Cell Death Dis. 2022, 13, 807. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Naito, Y.; Yasuhara, H.; Sasaki, K.; Kawaji, H.; Kawai, J.; Naito, M.; Okuda, H.; Obika, S.; Inoue, T. Evaluation of off-target effects of gapmer antisense oligonucleotides using human cells. Genes. to Cells 2019, 24, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ni, D.; Huang, K.; Wang, H.; Zhou, W.; Guo, M.; Baimanov, D.; Xue, Y.; Chen, Y.; Liu, Y. Two-in-one combination therapy of fluoronucleoside analogues and triplex forming oligonucleotides. Nano Today 2023, 48, 101699. [Google Scholar] [CrossRef]
- Boehm, B.J.; Whidborne, C.; Button, A.L.; Pukala, T.L.; Huang, D.M. DNA triplex structure, thermodynamics, and destabili- sation: Insight from molecular simulations. Phys. Chem. Chem. Phys. 2018, 20, 14013–14023. [Google Scholar] [CrossRef]
- Pyne, A.L.B.; Noy, A.; Main, K.H.S.; Velasco-Berrelleza, V.; Piperakis, M.M.; Mitchenall, L.A.; Cugliandolo, F.M.; Beton, J.G.; Stevenson, C.E.M.; Hoogenboom, B.W.; et al. Base-pair resolution analysis of the effect of supercoiling on DNA flexibility and major groove recognition by triplex-forming oligonucleotides. Nat. Commun. 2021, 12, 1053. [Google Scholar] [CrossRef]
- Goldsmith, G.; Rathinavelan, T.; Yathindra, N. Selective preference of parallel DNA triplexes is due to the disruption of Hoogsteen hydrogen bonds caused by the severe nonisostericity between the G∗GC and T∗AT triplets. PLoS ONE 2016, 11, e0152102. [Google Scholar] [CrossRef]
- Mall, V.S.; Ojha, R.P.; Tiwari, R.K. Recombinant triplex formed by PNA-TFO: A molecular dynamics simulation study. Orient. J. Chem. 2018, 34, 2440–2446. [Google Scholar] [CrossRef]
- Praseuth, D.; Guieysse, A.L.; Hélène, C. Triple helix formation and the antigene strategy for sequence-specific control of gene expression. Biochim. Biophys. Acta—Gene Struct. Expr. 1999, 1489, 181–206. [Google Scholar] [CrossRef]
- Loeffler, H.H.; He, J.; Tibo, A.; Janet, J.P.; Voronov, A.; Mervin, L.H.; Engkvist, O. Reinvent 4: Modern AI–driven generative molecule design. J. Cheminform. 2024, 16, 20. [Google Scholar] [CrossRef]
- Wen, Y.; Wu, Y.; Xu, B.; Lin, J.; Zhu, H. Fasim-LongTarget enables fast and accurate genome-wide lncRNA/DNA binding prediction. Comput. Struct. Biotechnol. J. 2022, 20, 3347–3350. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular docking: Shifting paradigms in drug discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef]
- Hincapié-López, M.; Vrebosch, J.; Garcia-Zapirain, B.; Pinzón-Reyes, E.; Pabón-Martínez, Y.V. Comparison of classical Machine Learning-based algorithms to predict Triplex Forming Oligonucleotides. Comput. Struct. Biotechnol. Rep. 2024, 1, 100013. [Google Scholar] [CrossRef]
- Chiba, S.; Lim, K.R.Q.; Sheri, N.; Anwar, S.; Erkut, E.; Shah, M.N.A.; Aslesh, T.; Woo, S.; Sheikh, O.; Maruyama, R.; et al. ESkip-Finder: A machine learning-based web application and database to identify the optimal sequences of antisense oligonucleotides for exon skipping. Nucleic Acids Res. 2021, 49, W193–W198. [Google Scholar] [CrossRef]
- Hwang, G.; Kwon, M.; Seo, D.; Kim, D.H.; Lee, D.; Lee, K.; Kim, E.; Kang, M.; Ryu, J.H. ASOptimizer: Optimizing antisense oligonucleotides through deep learning for IDO1 gene regulation. Mol. Ther. Nucleic Acids 2024, 35, 102186. [Google Scholar] [CrossRef]
- Kuroda, M.; Kasahara, Y.; Hirose, M.; Yamaguma, H.; Oda, M.; Nagao, C.; Mizuguchi, K. Construction of a Tm-value prediction model and molecular dynamics study of AmNA-containing gapmer antisense oligonucleotide. Mol. Ther. Nucleic Acids 2024, 35, 102272. [Google Scholar] [CrossRef]
- Hincapié-López, M.; Pinzón-Reyes, E.; Garcia-Zapirain, B.; Pabón-Martínez, Y.V. Comparative study of the computer-aided design of MYCN-specific antigene oligonucleotides. Comput. Struct. Biotechnol. J. Rep. 2025, 2, 100030. [Google Scholar] [CrossRef]
- Buske, F.A.; Bauer, D.C.; Mattick, J.S.; Bailey, T.L. Triplexator: Detecting nucleic acid triple helices in genomic and transcriptomic data. Genome Res. 2012, 22, 1372–1381. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Zhang, H.; Liu, H.; Zhu, H. LongTarget: A tool to predict lncRNA DNA-binding motifs and binding sites via Hoogsteen base-pairing analysis. Bioinformatics 2015, 31, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Warwick, T.; Seredinski, S.; Krause, N.M.; Bains, J.K.; Althaus, L.; Oo, J.A.; Bonetti, A.; Dueck, A.; Engelhardt, S.; Schwalbe, H.; et al. A universal model of RNA.DNA:DNA triplex formation accurately predicts genome-wide RNA–DNA interactions. Brief. Bioinform. 2022, 23, bbac445. [Google Scholar] [CrossRef]
- Murawski, M.; Jagodziński, A.; Bielawska-Pohl, A.; Klimczak, A. Complexity of the Genetic Background of Oncogenesis in Ovarian Cancer—Genetic Instability and Clinical Implications. Cells 2024, 13, 345. [Google Scholar] [CrossRef]
- Alanazi, M.M.; Eissa, I.H.; Alsaif, N.A.; Obaidullah, A.J.; Alanazi, W.A.; Alasmari, A.F.; Albassam, H.; Elkady, H.; Elwan, A. Design, synthesis, docking, ADMET studies, and anticancer evaluation of new 3-methylquinoxaline derivatives as VEGFR-2 inhibitors and apoptosis inducers. J. Enzyme Inhib. Med. Chem. 2021, 36, 1760–1782. [Google Scholar] [CrossRef] [PubMed]
- Chaurasia, R.; Ghose, U. Human DNA/RNA motif mining using deep-learning methods: A scoping review. Netw. Model. Anal. Health Inform. Bioinform. 2023, 12, 20. [Google Scholar] [CrossRef]
- Goldberg, Y. A Primer on Neural Network Models for Natural Language Processing. J. Artif. Intell. Res. 2016, 57, 345–420. [Google Scholar] [CrossRef]
- Gunasekaran, H.; Ramalakshmi, K.; Rex Macedo Arokiaraj, A.; Kanmani, S.D.; Venkatesan, C.; Dhas, C.S.G. Analysis of DNA Sequence Classification Using CNN and Hybrid Models. Comput. Math. Methods Med. 2021, 2021, 1835056. [Google Scholar] [CrossRef]
- Vaswani, A.; Shazeer, N.; Parmar, N.; Uszkoreit, J.; Jones, L.; Gomez, A.N.; Kaiser, L. Attention Is All You Need. In Advances in Neural Information Processing Systems, Proceedings of the 31st Conference on Neural Information Processing Systems, Long Beach, CA, USA, 4–9 December 2017; Guyon, I., Von Luxburg, U., Bengio, S., Wallach, H., Fergus, R., Vishwanathan, S., Garnett, R., Eds.; Curran Associates, Inc.: Red Hook, NY, USA, 2017; Available online: https://proceedings.neurips.cc/paper_files/paper/2017/file/3f5ee243547dee91fbd053c1c4a845aa-Paper.pdf (accessed on 15 September 2025).
- Chaurasia, R.; Ghose, U. XDeMo: A novel deep learning framework for DNA motif mining using transformer models. Netw. Model. Anal. Health Inform. Bioinform. 2024, 13, 25. [Google Scholar] [CrossRef]
- Zhuang, D.; Ibrahim, A.K. Deep learning for drug discovery: A study of identifying high efficacy drug compounds using a cascade transfer learning approach. Appl. Sci. 2021, 11, 7772. [Google Scholar] [CrossRef]
- Holgersen, E.M.; Gandhi, S.; Zhou, Y.; Kim, J.; Vaz, B.; Bogojeski, J.; Bugno, M.; Shalev, Z.; Cheung-Ong, K.; Gonçalves, J.; et al. Transcriptome-Wide Off-Target Effects of Steric-Blocking Oligonucleotides. Nucleic Acid. Ther. 2021, 31, 392–403. [Google Scholar] [CrossRef]
- Chou, W.C.; Lin, Z. Machine learning and artificial intelligence in physiologically based pharmacokinetic modeling. Toxicol. Sci. 2023, 191, 1–14. [Google Scholar] [CrossRef]
- SantaLucia, J.; Hicks, D. The thermodynamics of DNA structural motifs. Annu. Rev. Biophys. Biomol. Struct. 2004, 33, 415–440. [Google Scholar] [CrossRef]

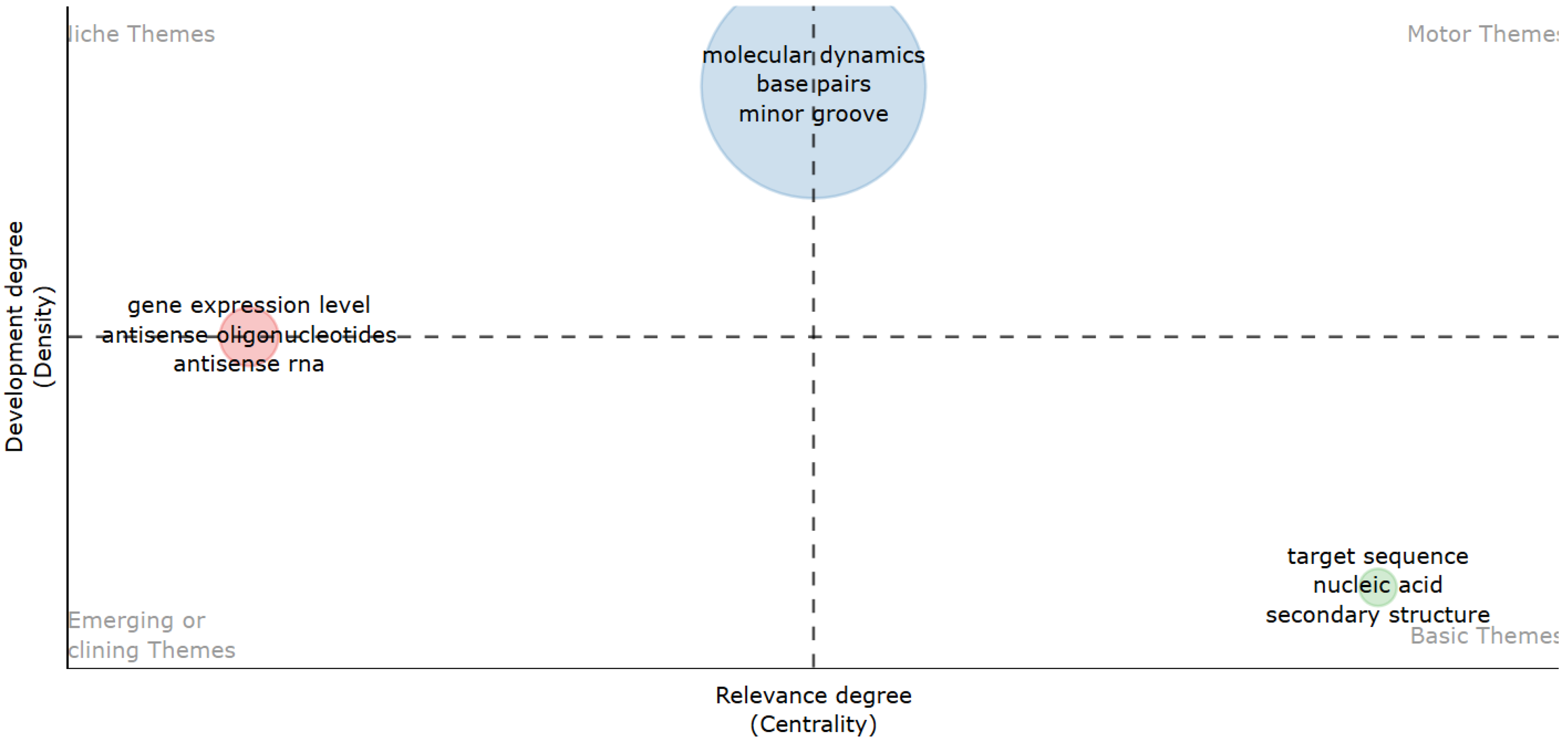
| Feature | Scopus (Elsevier) | Web of Science (Clarivate) |
| Use | Bibliometric analysis and PRISMA-based systematic review | PRISMA-based systematic review |
| Coverage | Broad multidisciplinary (biomedicine, pharmacology, chemistry, bioinformatics) | Highly curated, multidisciplinary; emphasis on high-impact journals |
| Peer-review filtering | Yes—by document type and journal source | Yes—by category and inclusion in JCR |
| Peer-review language | English | English |
| Publication year | 2015–2025 | 2015–2025 |
| Primary journal metric | CiteScore: Average citations per document over 4 years | Impact Factor (IF): Avg. citations to articles from the previous 2 years |
| Normalized metric | SNIP: Field-normalized impact per paper | JCI: Journal Citation Indicator (baseline = 1.0) |
| Prestige-weighted metric | SJR: Source prestige + network influence | Eigenfactor Score: Network influence, excluding self-citations |
| Per-article influence | Not available | Article influence score: Derived from Eigenfactor |
| Immediacy metric | Not available | Immediacy index: Same-year citations |
| Quartile ranking | Q1–Q4 based on CiteScore or SJR | Q1–Q4 based on JCR categories |
| Years covered | From 1996 onwards | From 1900 onwards |
| Open access indicator | Integrated filters and metric display | Integrated in JCR with OA tags |
| Citation database source | Scopus Citation Index | Web of Science Core Collection |
| Relevance for TFO design | High—Ideal for in silico design and bioinformatics applications | High—Strong for both historical and current experimental TFO research |
| Source | Originally Downloaded Data | Deleted Data | Merged Data | % Merged Data |
|---|---|---|---|---|
| Scopus | 128 | 36 | 92 | 56.4 |
| Web of Science (WoS) | 71 | 0 | 71 | 43.6 |
| Total | 199 | 36 | 163 | 100.0 |
| Metadata | Description | a Scopus | b Web of Science (WoS) | c Meged Data | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Missing Counts | Missing % | Status | Missing Counts | Missing % | Status | Missing Counts | Missing % | Status | ||
| AB | Abstract | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| C1 | Affiliation | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| AU | Author | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| DI | DOI | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| DT | Document Type | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| SO | Journal | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| LA | Language | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| PY | Publication Year | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| TI | Title | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| TC | Total Citation | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent | 0 | 0.00 | Excellent |
| ID | Keywords Plus | 2 | 1.56 | Good | 4 | 5.63 | Good | 5 | 3.07 | Good |
| DE | Keywords | 36 | 28.12 | Poor | 31 | 43.66 | Poor | 56 | 33.74 | Poor |
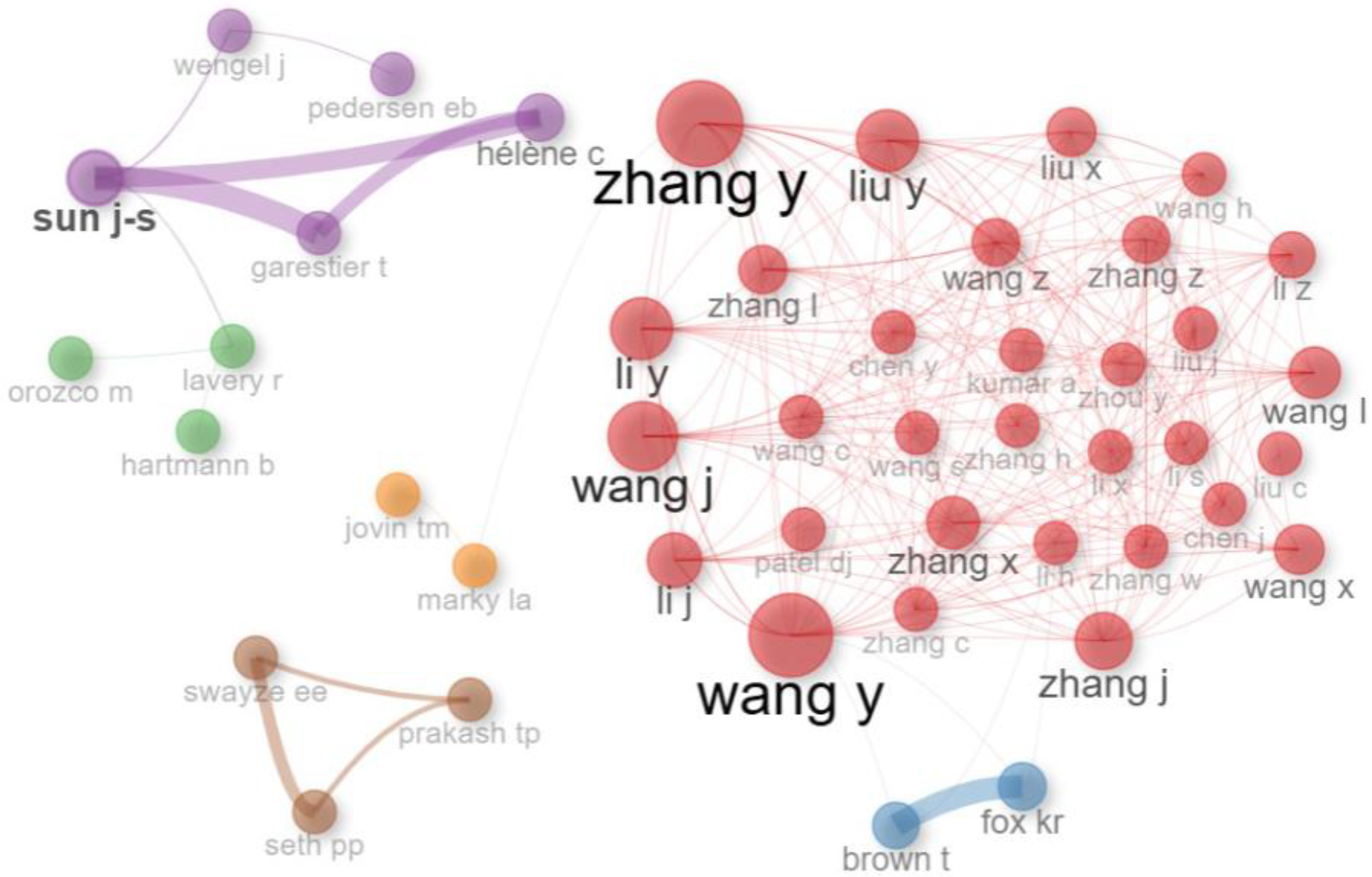
| Author | Country | Institution | Number of Articles Issued | Global Citations | Average Co-Authorship | a Cluster |
|---|---|---|---|---|---|---|
| Zhang Yu | Singapore | Nanyang Technological University | 51 | 283 | 5 | Red |
| Wang Y | England | University of Southampton | 47 | 530 | 5 | Red |
| Wang J | USA | University of California | 42 | 146 | 8 | Red |
| Liu Y | Singapore | Nanyang Technological University | 36 | 145 | 4 | Red |
| Seth, PP | USA | Isis Pharmaceuticals | 34 | 355 | 10 | Brown |
| Li Y | China | Peking University | 34 | 186 | 6 | Red |
| Zhang X | USA | University of California | 31 | 291 | 3 | Red |
| Wang L | China | Peking University | 30 | 116 | 6 | Red |
| Sun J-S | France | Muséum National d’Histoire Naturelle | 27 | 314 | 2 | Purple |
| Hélène C | France | Muséum National d’Histoire Naturelle | 24 | 55 | 2 | Purple |
| Wengel J | Denmark | University of Southern | 23 | 43 | 3 | Purple |
| Swayze, EE | USA | Isis Pharmaceuticals | 15 | 323 | 7 | Brown |
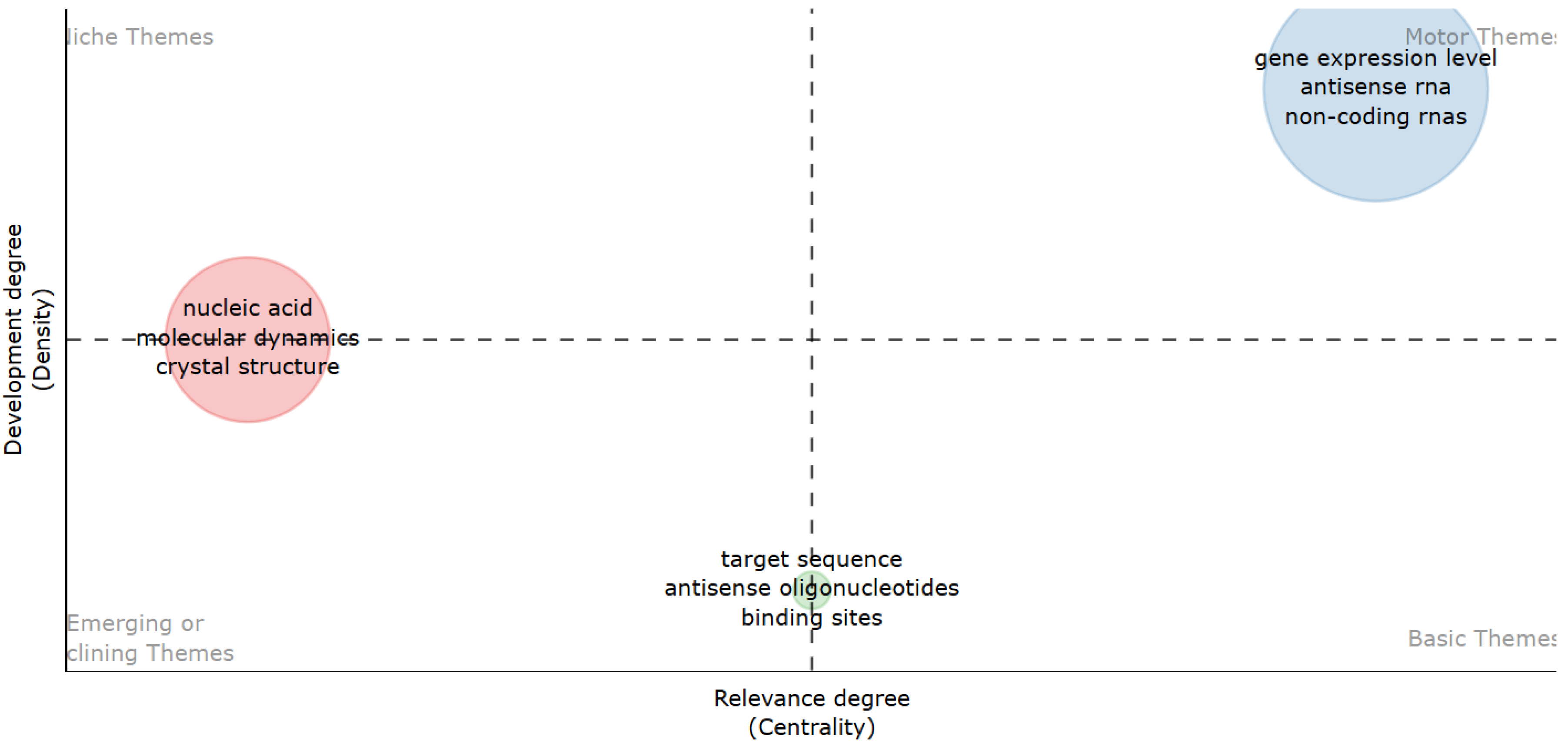
| a Unigrams | Occurrences | Pagerank_Centrality | b Bigrams | Occurrences | Pagerank_Centrality | c Trigrams | Occurrences | Pagerank_Centrality |
|---|---|---|---|---|---|---|---|---|
| DNA | 2386 | 0.013 | Target sequence | 580 | 0.018 | Molecular dynamics simulations | 146 | 0.048 |
| Sequence | 2132 | 0.012 | Gene expression level | 488 | 0.020 | Polymerase Chain Reaction | 129 | 0.019 |
| Structure | 1848 | 0.011 | Molecular dynamics | 362 | 0.017 | Nuclear Magnetic Resonance | 94 | 0.019 |
| Antisense | 1771 | 0.009 | Nucleic acid | 307 | 0.011 | Amino acid residues | 79 | 0.008 |
| Target | 1770 | 0.010 | Mirror groove | 273 | 0.013 | Nucleic acid research | 52 | 0.008 |
| Gene | 1673 | 0.009 | Antisense oligonucleotides | 271 | 0.007 | Antisense oligonucleotides ASOs | 47 | 0.004 |
| Binding | 1575 | 0.009 | Crystal structure | 247 | 0.009 | Peptide nucleic acids | 44 | 0.007 |
| Analysis | 1548 | 0.009 | Secondary structure | 232 | 0.008 | RNA interference RNAi | 41 | 0.004 |
| Molecular | 1493 | 0.009 | Antiparallel stretch | 222 | 0.008 | Non-coding RNAs lncRNAs | 40 | 0.007 |
| Expression | 1383 | 0.008 | Hydrogen bonds | 201 | 0.009 | Isothermal titration calorimetry | 38 | 0.006 |
| Data | 1253 | 0.007 | Circular dichroism | 197 | 0.009 | Transcription start sites | 36 | 0.004 |
| Antiparallel | 1110 | 0.006 | Binding sites | 188 | 0.008 | Single nucleotide polymorphisms | 34 | 0.006 |
| Cells | 1044 | 0.006 | Mayor groove | 187 | 0.009 | Triple helix formation | 33 | 0.003 |
| Proteins | 988 | 0.006 | DNA binding | 168 | 0.008 | Human immunodeficiency virus | 33 | 0.004 |
| Model | 978 | 0.006 | Modeling study | 153 | 0.006 | Northern blot analysis | 33 | 0.004 |
| Interactions | 896 | 0.006 | Dynamic simulations | 148 | 0.006 | Magnetic Resonance Spectroscopy | 32 | 0.007 |
| Oligonucleotides | 777 | 0.004 | Triple helix | 130 | 0.006 | Natural antisense transcripts | 31 | 0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hincapié-López, M.; Marín-Alfonso, J.; Romero-Riaño, E.; Núñez-Rodríguez, R.A.; Pabón-Martínez, Y.V. Antisense Versus Antigene in the Computer-Aided Design of Triplex-Forming Oligonucleotides (TFO): Insights from a Dual-Method Review, Combining Bibliometric and Systematic Review. Int. J. Mol. Sci. 2025, 26, 10936. https://doi.org/10.3390/ijms262210936
Hincapié-López M, Marín-Alfonso J, Romero-Riaño E, Núñez-Rodríguez RA, Pabón-Martínez YV. Antisense Versus Antigene in the Computer-Aided Design of Triplex-Forming Oligonucleotides (TFO): Insights from a Dual-Method Review, Combining Bibliometric and Systematic Review. International Journal of Molecular Sciences. 2025; 26(22):10936. https://doi.org/10.3390/ijms262210936
Chicago/Turabian StyleHincapié-López, Martha, Jeison Marín-Alfonso, Efrén Romero-Riaño, Rafael Augusto Núñez-Rodríguez, and Yarley Vladimir Pabón-Martínez. 2025. "Antisense Versus Antigene in the Computer-Aided Design of Triplex-Forming Oligonucleotides (TFO): Insights from a Dual-Method Review, Combining Bibliometric and Systematic Review" International Journal of Molecular Sciences 26, no. 22: 10936. https://doi.org/10.3390/ijms262210936
APA StyleHincapié-López, M., Marín-Alfonso, J., Romero-Riaño, E., Núñez-Rodríguez, R. A., & Pabón-Martínez, Y. V. (2025). Antisense Versus Antigene in the Computer-Aided Design of Triplex-Forming Oligonucleotides (TFO): Insights from a Dual-Method Review, Combining Bibliometric and Systematic Review. International Journal of Molecular Sciences, 26(22), 10936. https://doi.org/10.3390/ijms262210936







