Inflaming and Immune-Resolving: The Ambivalent Role of Eosinophils in Osteoarthritis
Abstract
1. Introduction
2. Overview of Eosinophils in the Immune System
3. Dual Role of Eosinophils: Pro-Inflammatory and Anti-Inflammatory Functions
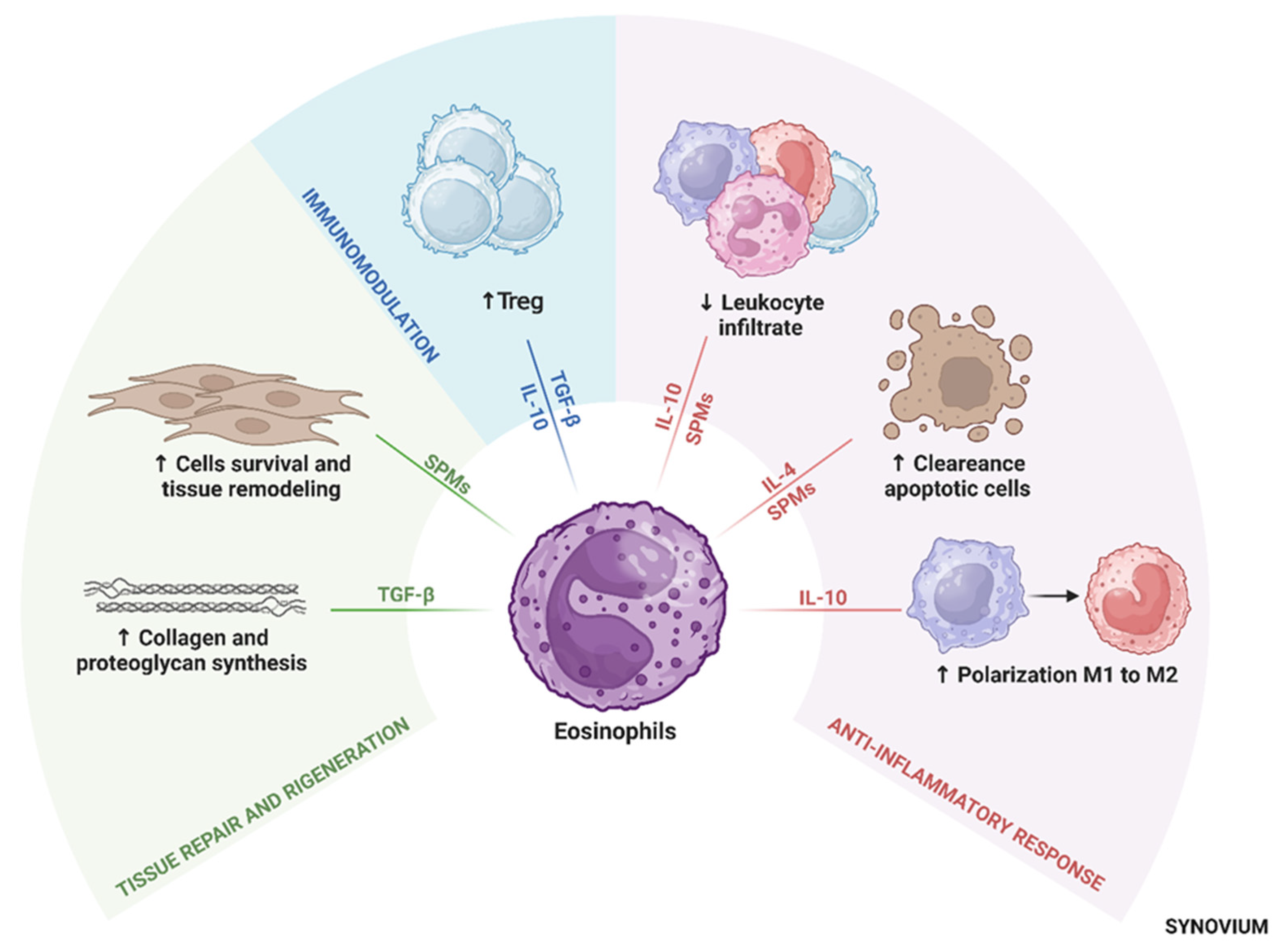
4. Eosinophil-Derived Anti-Inflammatory Mediators
4.1. Interleukin-10
4.2. Interleukin-4 (IL-4)
4.3. Resolvins and Protectins
4.4. Transforming Growth Factor-Beta (TGF-β)
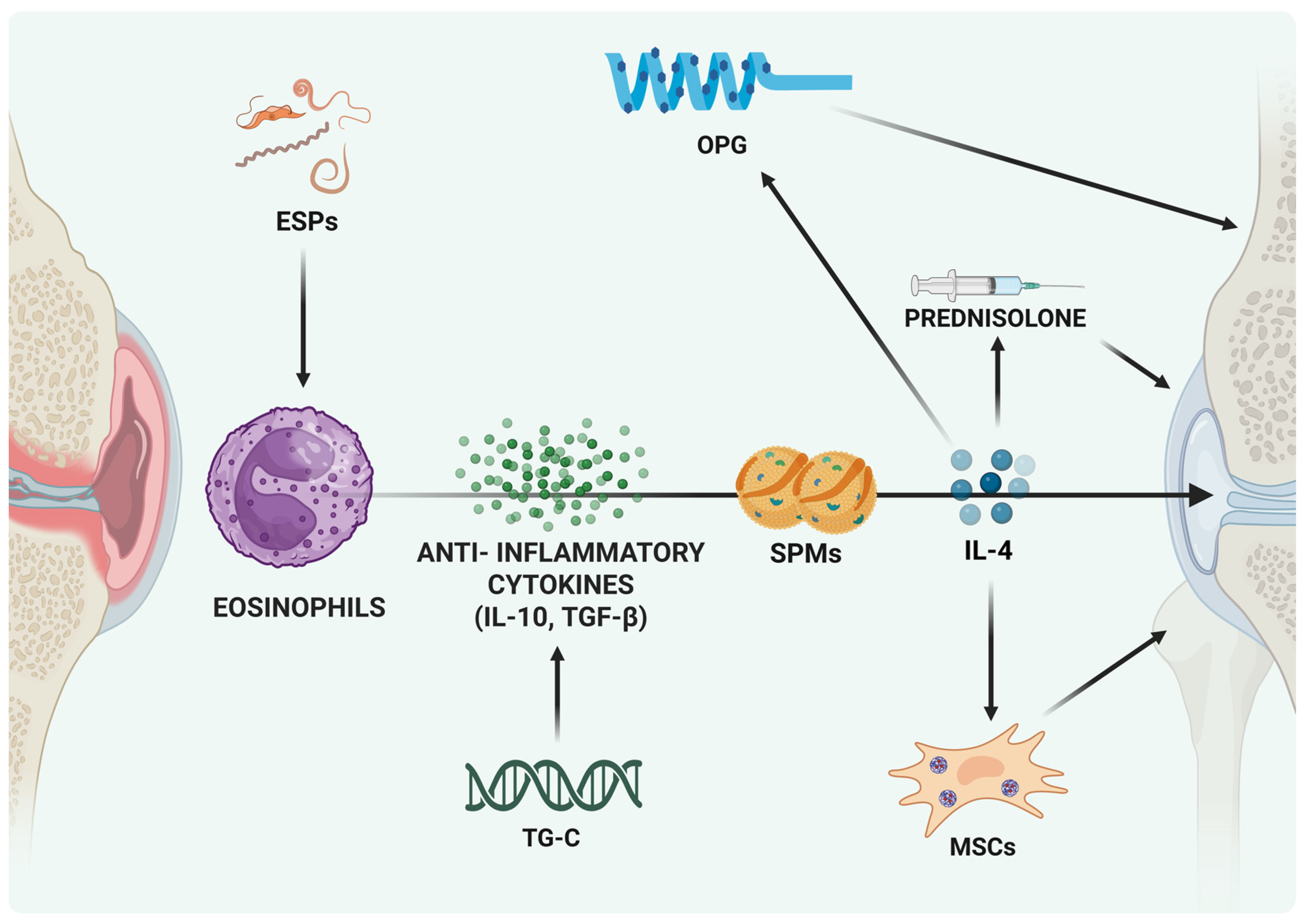
5. Anti-Inflammatory Potential of Eosinophils in OA
6. Protective Versus Harmful Roles: Context-Dependent Effects
7. Potential for Therapeutic Modulation
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perruccio, A.V.; Young, J.J.; Wilfong, J.M.; Denise Power, J.; Canizares, M.; Badley, E.M. Osteoarthritis year in review 2023: Epidemiology & therapy. Osteoarthr. Cartil. 2024, 32, 159–165. [Google Scholar] [CrossRef]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Roelofs, A.J.; De Bari, C. Osteoarthritis year in review 2023: Biology. Osteoarthr. Cartil. 2024, 32, 148–158. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- De Roover, A.; Escribano-Nunez, A.; Monteagudo, S.; Lories, R. Fundamentals of osteoarthritis: Inflammatory mediators in osteoarthritis. Osteoarthr. Cartil. 2023, 31, 1303–1311. [Google Scholar] [CrossRef]
- Cao, F.; Xu, Z.; Li, X.X.; Fu, Z.Y.; Han, R.Y.; Zhang, J.L.; Wang, P.; Hou, S.; Pan, H.F. Trends and cross-country inequalities in the global burden of osteoarthritis, 1990–2019: A population-based study. Ageing Res. Rev. 2024, 99, 102382. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.; van Caam, A.; van der Kraan, P.M. Obesity and osteoarthritis, more than just wear and tear: Pivotal roles for inflamed adipose tissue and dyslipidaemia in obesity-induced osteoarthritis. Rheumatology 2015, 54, 588–600. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Motta, F.; Barone, E.; Sica, A.; Selmi, C. Inflammaging and Osteoarthritis. Clin. Rev. Allergy Immunol. 2023, 64, 222–238. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Jrad, A.I.S.; Trad, M.; Bzeih, W.; El Hasbani, G.; Uthman, I. Role of pro-inflammatory interleukins in osteoarthritis: A narrative review. Connect. Tissue Res. 2023, 64, 238–247. [Google Scholar] [CrossRef]
- Mobasheri, A.; Saarakkala, S.; Finnila, M.; Karsdal, M.A.; Bay-Jensen, A.C.; van Spil, W.E. Recent advances in understanding the phenotypes of osteoarthritis. F1000Research 2019, 8, 2091. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Matta, C.; Zakany, R.; Musumeci, G. Chondrosenescence: Definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 2015, 80, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Cai, L.; Xie, J.; Zhou, X. The role of TGF-beta3 in cartilage development and osteoarthritis. Bone Res. 2023, 11, 2. [Google Scholar] [CrossRef]
- Freund, A.; Orjalo, A.V.; Desprez, P.Y.; Campisi, J. Inflammatory networks during cellular senescence: Causes and consequences. Trends Mol. Med. 2010, 16, 238–246. [Google Scholar] [CrossRef]
- Gigon, L.; Fettrelet, T.; Yousefi, S.; Simon, D.; Simon, H.U. Eosinophils from A to Z. Allergy 2023, 78, 1810–1846. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Munitz, A.; Ackerman, S.J.; Drake, M.G.; Jackson, D.J.; Wardlaw, A.J.; Dougan, S.K.; Berdnikovs, S.; Schleich, F.; Matucci, A.; et al. Eosinophils in Health and Disease: A State-of-the-Art Review. Mayo Clin. Proc. 2021, 96, 2694–2707. [Google Scholar] [CrossRef]
- Chusid, M.J. Eosinophils: Friends or Foes? J. Allergy Clin. Immunol. Pract. 2018, 6, 1439–1444. [Google Scholar] [CrossRef]
- Kanda, A.; Yasutaka, Y.; Van Bui, D.; Suzuki, K.; Sawada, S.; Kobayashi, Y.; Asako, M.; Iwai, H. Multiple Biological Aspects of Eosinophils in Host Defense, Eosinophil-Associated Diseases, Immunoregulation, and Homeostasis: Is Their Role Beneficial, Detrimental, Regulator, or Bystander? Biol. Pharm. Bull. 2020, 43, 20–30. [Google Scholar] [CrossRef]
- Nedunchezhiyan, U.; Varughese, I.; Sun, A.R.; Wu, X.; Crawford, R.; Prasadam, I. Obesity, Inflammation, and Immune System in Osteoarthritis. Front. Immunol. 2022, 13, 907750. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Yuan, W.; Ou, Y.; Lin, H.; He, K.; Qian, X.; Chen, H.; Wang, C.; Lu, J.; et al. Eosinophils-Induced Lumican Secretion by Synovial Fibroblasts Alleviates Cartilage Degradation via the TGF-beta Pathway Mediated by Anxa1 Binding. Adv. Sci. 2025, 12, e2416030. [Google Scholar] [CrossRef]
- Pelissier, A.; Laragione, T.; Gulko, P.S.; Rodriguez Martinez, M. Cell-specific gene networks and drivers in rheumatoid arthritis synovial tissues. Front. Immunol. 2024, 15, 1428773. [Google Scholar] [CrossRef]
- Rothenberg, M.E.; Hogan, S.P. The eosinophil. Annu. Rev. Immunol. 2006, 24, 147–174. [Google Scholar] [CrossRef]
- Mack, E.A.; Pear, W.S. Transcription factor and cytokine regulation of eosinophil lineage commitment. Curr. Opin. Hematol. 2020, 27, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Farahi, N.; Singh, N.R.; Heard, S.; Loutsios, C.; Summers, C.; Solanki, C.K.; Solanki, K.; Balan, K.K.; Ruparelia, P.; Peters, A.M.; et al. Use of 111-Indium-labeled autologous eosinophils to establish the in vivo kinetics of human eosinophils in healthy subjects. Blood 2012, 120, 4068–4071. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, H.F.; Dyer, K.D.; Foster, P.S. Eosinophils: Changing perspectives in health and disease. Nat. Rev. Immunol. 2013, 13, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.D.; Marleau, S.; Griffiths-Johnson, D.A.; Jose, P.J.; Williams, T.J. Cooperation between interleukin-5 and the chemokine eotaxin to induce eosinophil accumulation in vivo. J. Exp. Med. 1995, 182, 1169–1174. [Google Scholar] [CrossRef]
- Fulkerson, P.C.; Schollaert, K.L.; Bouffi, C.; Rothenberg, M.E. IL-5 triggers a cooperative cytokine network that promotes eosinophil precursor maturation. J. Immunol. 2014, 193, 4043–4052. [Google Scholar] [CrossRef]
- Gurtner, A.; Crepaz, D.; Arnold, I.C. Emerging functions of tissue-resident eosinophils. J. Exp. Med. 2023, 220, e20221435. [Google Scholar] [CrossRef] [PubMed]
- Boneva, B.; Ralchev, N.; Ganova, P.; Tchorbanov, A.; Mihaylova, N. Collagenase-Induced Mouse Model of Osteoarthritis—A Thorough Flow Cytometry Analysis. Life 2022, 12, 1938. [Google Scholar] [CrossRef]
- Rankin, S.M.; Conroy, D.M.; Williams, T.J. Eotaxin and eosinophil recruitment: Implications for human disease. Mol. Med. Today 2000, 6, 20–27. [Google Scholar] [CrossRef]
- Marques, R.E.; Guabiraba, R.; Russo, R.C.; Teixeira, M.M. Targeting CCL5 in inflammation. Expert Opin. Ther. Targets 2013, 17, 1439–1460. [Google Scholar] [CrossRef]
- Shin, J.S.; Lee, H.; Kim, S.H.; Noh, K.C.; Kim, S.J.; Kim, H.N.; Choi, J.Y.; Song, S.Y. Identification of plasma and urinary inflammatory markers in severe knee osteoarthritis: Relations with synovial fluid markers. Knee Surg. Relat. Res. 2024, 36, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.P.; Lee, N.A. Eosinophils in health and disease: The LIAR hypothesis. Clin. Exp. Allergy 2010, 40, 563–575. [Google Scholar] [CrossRef]
- Winqvist, I.; Olofsson, T.; Olsson, I.; Persson, A.M.; Hallberg, T. Altered density, metabolism and surface receptors of eosinophils in eosinophilia. Immunology 1982, 47, 531–539. [Google Scholar]
- Prin, L.; Capron, M.; Tonnel, A.B.; Bletry, O.; Capron, A. Heterogeneity of human peripheral blood eosinophils: Variability in cell density and cytotoxic ability in relation to the level and the origin of hypereosinophilia. Int. Arch. Allergy Appl. Immunol. 1983, 72, 336–346. [Google Scholar] [CrossRef]
- Kuo, H.P.; Yu, T.R.; Yu, C.T. Hypodense eosinophil number relates to clinical severity, airway hyperresponsiveness and response to inhaled corticosteroids in asthmatic subjects. Eur. Respir. J. 1994, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Abdala Valencia, H.; Loffredo, L.F.; Misharin, A.V.; Berdnikovs, S. Phenotypic plasticity and targeting of Siglec-F(high) CD11c(low) eosinophils to the airway in a murine model of asthma. Allergy 2016, 71, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Mesnil, C.; Raulier, S.; Paulissen, G.; Xiao, X.; Birrell, M.A.; Pirottin, D.; Janss, T.; Starkl, P.; Ramery, E.; Henket, M.; et al. Lung-resident eosinophils represent a distinct regulatory eosinophil subset. J. Clin. Investig. 2016, 126, 3279–3295. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, A.; Borrelli, C.; Gonzalez-Perez, I.; Bach, K.; Acar, I.E.; Nunez, N.G.; Crepaz, D.; Handler, K.; Vu, V.P.; Lafzi, A.; et al. Active eosinophils regulate host defence and immune responses in colitis. Nature 2023, 615, 151–157. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, L.; Li, Z.; Wang, X.Y.; Yi, H. Understanding the Multifaceted Role of Neutrophils in Cancer and Autoimmune Diseases. Front. Immunol. 2018, 9, 2456. [Google Scholar] [CrossRef] [PubMed]
- Dolitzky, A.; Shapira, G.; Grisaru-Tal, S.; Hazut, I.; Avlas, S.; Gordon, Y.; Itan, M.; Shomron, N.; Munitz, A. Transcriptional Profiling of Mouse Eosinophils Identifies Distinct Gene Signatures Following Cellular Activation. Front. Immunol. 2021, 12, 802839. [Google Scholar] [CrossRef] [PubMed]
- Wedemeyer, J.; Vosskuhl, K. Role of gastrointestinal eosinophils in inflammatory bowel disease and intestinal tumours. Best Pract. Res. Clin. Gastroenterol. 2008, 22, 537–549. [Google Scholar] [CrossRef]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]
- de Waal Malefyt, R.; Yssel, H.; Roncarolo, M.G.; Spits, H.; de Vries, J.E. Interleukin-10. Curr. Opin. Immunol. 1992, 4, 314–320. [Google Scholar] [CrossRef]
- Spits, H.; de Waal Malefyt, R. Functional characterization of human IL-10. Int. Arch. Allergy Immunol. 1992, 99, 8–15. [Google Scholar] [CrossRef]
- Cassatella, M.A.; Meda, L.; Bonora, S.; Ceska, M.; Constantin, G. Interleukin 10 (IL-10) inhibits the release of proinflammatory cytokines from human polymorphonuclear leukocytes. Evidence for an autocrine role of tumor necrosis factor and IL-1 beta in mediating the production of IL-8 triggered by lipopolysaccharide. J. Exp. Med. 1993, 178, 2207–2211. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [CrossRef]
- McPeek, M.K.; Gomez, J.C.; Doerschuk, C.M. Neutrophils sing “IL[-10] be seeing you” in the lungs during pneumonia. J. Leukoc. Biol. 2024, 115, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Molofsky, A.B.; Liang, H.E.; Ricardo-Gonzalez, R.R.; Jouihan, H.A.; Bando, J.K.; Chawla, A.; Locksley, R.M. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science 2011, 332, 243–247. [Google Scholar] [CrossRef]
- Yamada, T.; Tani, Y.; Nakanishi, H.; Taguchi, R.; Arita, M.; Arai, H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J. 2011, 25, 561–568. [Google Scholar] [CrossRef]
- Souza, P.P.; Brechter, A.B.; Reis, R.I.; Costa, C.A.; Lundberg, P.; Lerner, U.H. IL-4 and IL-13 inhibit IL-1beta and TNF-alpha induced kinin B1 and B2 receptors through a STAT6-dependent mechanism. Br. J. Pharmacol. 2013, 169, 400–412. [Google Scholar] [CrossRef]
- Fenn, A.M.; Henry, C.J.; Huang, Y.; Dugan, A.; Godbout, J.P. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain Behav. Immun. 2012, 26, 766–777. [Google Scholar] [CrossRef]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef]
- Goh, Y.P.; Henderson, N.C.; Heredia, J.E.; Red Eagle, A.; Odegaard, J.I.; Lehwald, N.; Nguyen, K.D.; Sheppard, D.; Mukundan, L.; Locksley, R.M.; et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 9914–9919. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, C.; Liu, T.; Deng, Z.; Fang, W.; Zhang, X.; Li, J.; Huang, Q.; Liu, C.; Wang, Y.; et al. Eosinophils improve cardiac function after myocardial infarction. Nat. Commun. 2020, 11, 6396. [Google Scholar] [CrossRef]
- Lee, S.H.; Chaves, M.M.; Kamenyeva, O.; Gazzinelli-Guimaraes, P.H.; Kang, B.; Pessenda, G.; Passelli, K.; Tacchini-Cottier, F.; Kabat, J.; Jacobsen, E.A.; et al. M2-like, dermal macrophages are maintained via IL-4/CCL24-mediated cooperative interaction with eosinophils in cutaneous leishmaniasis. Sci. Immunol. 2020, 5, eaaz4415. [Google Scholar] [CrossRef] [PubMed]
- Kolbinger, A.; Schaufele, T.J.; Steigerwald, H.; Friedel, J.; Pierre, S.; Geisslinger, G.; Scholich, K. Eosinophil-derived IL-4 is necessary to establish the inflammatory structure in innate inflammation. EMBO Mol. Med. 2023, 15, e16796. [Google Scholar] [CrossRef]
- Serhan, C.N.; Savill, J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. 2005, 6, 1191–1197. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Van Dyke, T.E. Resolving inflammation: Dual anti-inflammatory and pro-resolution lipid mediators. Nat. Rev. Immunol. 2008, 8, 349–361. [Google Scholar] [CrossRef]
- Isobe, Y.; Kato, T.; Arita, M. Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front. Immunol. 2012, 3, 270. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Masterson, J.C.; McNamee, E.N.; Fillon, S.A.; Hosford, L.; Harris, R.; Fernando, S.D.; Jedlicka, P.; Iwamoto, R.; Jacobsen, E.; Protheroe, C.; et al. Eosinophil-mediated signalling attenuates inflammatory responses in experimental colitis. Gut 2015, 64, 1236–1247. [Google Scholar] [CrossRef]
- Bannenberg, G.L.; Chiang, N.; Ariel, A.; Arita, M.; Tjonahen, E.; Gotlinger, K.H.; Hong, S.; Serhan, C.N. Molecular circuits of resolution: Formation and actions of resolvins and protectins. J. Immunol. 2005, 174, 4345–4355. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, X.; Wan, J.Y.; Zhang, L.; Zhang, Z.; Li, H.Z.; Min, S. Resolvin D1 protects mice from LPS-induced acute lung injury. Pulm. Pharmacol. Ther. 2011, 24, 434–441. [Google Scholar] [CrossRef]
- Dalli, J.; Serhan, C.N. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012, 120, e60–e72. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Jiang, W.; Wang, X.; Du, S.; Qi, J.; Jia, Q.; Song, H. Resolvin D1 inhibits the proliferation of osteoarthritis fibroblast-like synoviocytes through the Hippo-YAP signaling pathway. BMC Musculoskelet. Disord. 2022, 23, 149. [Google Scholar] [CrossRef]
- Zhu, C.; Weng, Q.; Gao, S.; Li, F.; Li, Z.; Wu, Y.; Wu, Y.; Li, M.; Zhao, Y.; Han, Y.; et al. TGF-beta signaling promotes eosinophil activation in inflammatory responses. Cell Death Dis. 2024, 15, 637. [Google Scholar] [CrossRef]
- Li, M.O.; Wan, Y.Y.; Sanjabi, S.; Robertson, A.K.; Flavell, R.A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 2006, 24, 99–146. [Google Scholar] [CrossRef]
- Tzavlaki, K.; Moustakas, A. TGF-beta Signaling. Biomolecules 2020, 10, 487. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-beta signaling in health, disease and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Sanjabi, S.; Oh, S.A.; Li, M.O. Regulation of the Immune Response by TGF-beta: From Conception to Autoimmunity and Infection. Cold Spring Harb. Perspect. Biol. 2017, 9, a022236. [Google Scholar] [CrossRef]
- Brabletz, T.; Pfeuffer, I.; Schorr, E.; Siebelt, F.; Wirth, T.; Serfling, E. Transforming growth factor beta and cyclosporin A inhibit the inducible activity of the interleukin-2 gene in T cells through a noncanonical octamer-binding site. Mol. Cell. Biol. 1993, 13, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ma, Y.; Li, X.; Deng, Z.; Zheng, M.; Zheng, Q. The Immune Cell Landscape in Different Anatomical Structures of Knee in Osteoarthritis: A Gene Expression-Based Study. Biomed Res. Int. 2020, 2020, 9647072. [Google Scholar] [CrossRef]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef]
- Chiurchiu, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Serhan, C.N. Lipoxins and new lipid mediators in the resolution of inflammation. Curr. Opin. Pharmacol. 2006, 6, 414–420. [Google Scholar] [CrossRef]
- Andreev, D.; Liu, M.; Kachler, K.; Llerins Perez, M.; Kirchner, P.; Kolle, J.; Giessl, A.; Rauber, S.; Song, R.; Aust, O.; et al. Regulatory eosinophils induce the resolution of experimental arthritis and appear in remission state of human rheumatoid arthritis. Ann. Rheum. Dis. 2021, 80, 451–468. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, X.; Su, S.; Du, S.; Song, H. Identifying the shared genes and KEGG pathways of Resolvin D1-targeted network and osteoarthritis using bioinformatics. Bioengineered 2022, 13, 9839–9854. [Google Scholar] [CrossRef] [PubMed]
- Sano, Y.; Toyoshima, S.; Miki, Y.; Taketomi, Y.; Ito, M.; Lee, H.; Saito, S.; Murakami, M.; Okayama, Y. Activation of inflammation and resolution pathways of lipid mediators in synovial fluid from patients with severe rheumatoid arthritis compared with severe osteoarthritis. Asia Pac. Allergy 2020, 10, e21. [Google Scholar] [CrossRef]
- Benabdoune, H.; Rondon, E.P.; Shi, Q.; Fernandes, J.; Ranger, P.; Fahmi, H.; Benderdour, M. The role of resolvin D1 in the regulation of inflammatory and catabolic mediators in osteoarthritis. Inflamm. Res. 2016, 65, 635–645. [Google Scholar] [CrossRef]
- Kariminezhad, Z.; Rahimi, M.; Fernandes, J.; Maltais, R.; Sanceau, J.Y.; Poirier, D.; Fahmi, H.; Benderdour, M. Development of New Resolvin D1 Analogues for Osteoarthritis Therapy: Acellular and Computational Approaches to Study Their Antioxidant Activities. Antioxidants 2024, 13, 386. [Google Scholar] [CrossRef]
- Savill, J.; Dransfield, I.; Gregory, C.; Haslett, C. A blast from the past: Clearance of apoptotic cells regulates immune responses. Nat. Rev. Immunol. 2002, 2, 965–975. [Google Scholar] [CrossRef]
- von Kaeppler, E.P.; Wang, Q.; Raghu, H.; Bloom, M.S.; Wong, H.; Robinson, W.H. Interleukin 4 promotes anti-inflammatory macrophages that clear cartilage debris and inhibits osteoclast development to protect against osteoarthritis. Clin. Immunol. 2021, 229, 108784. [Google Scholar] [CrossRef]
- Dolitzky, A.; Hazut, I.; Avlas, S.; Grisaru-Tal, S.; Itan, M.; Zaffran, I.; Levi-Schaffer, F.; Gerlic, M.; Munitz, A. Differential regulation of Type 1 and Type 2 mouse eosinophil activation by apoptotic cells. Front. Immunol. 2022, 13, 1041660. [Google Scholar] [CrossRef]
- Herrero-Beaumont, G.; Castro-Dominguez, F.; Migliore, A.; Naredo, E.; Largo, R.; Reginster, J.Y. Systemic osteoarthritis: The difficulty of categorically naming a continuous condition. Aging Clin. Exp. Res. 2024, 36, 45. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.Y.; Chin, K.Y. The Role of Inflammation in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2020, 2020, 8293921. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef]
- Ambarus, C.A.; Noordenbos, T.; de Hair, M.J.; Tak, P.P.; Baeten, D.L. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 2012, 14, R74. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.M.; Scanzello, C.R. Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S120), 57–63. [Google Scholar]
- Nussbaum, J.C.; Van Dyken, S.J.; von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef]
- Yang, F.; Luo, X.; Zhu, W.; Li, J.; Zheng, Z.; Zhu, P. Dysregulation of Innate Lymphoid Cells in Patients with Active Rheumatoid Arthritis and Mice with Collagen-Induced Arthritis. Mediat. Inflamm. 2021, 2021, 1915068. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Chen, Z. Neuromedin U Suppresses Collagen-Induced Arthritis through ILC2-Th2 Activation. J. Immunol. Res. 2021, 2021, 5599439. [Google Scholar] [CrossRef]
- Reichman, H.; Moshkovits, I.; Itan, M.; Pasmanik-Chor, M.; Vogl, T.; Roth, J.; Munitz, A. Transcriptome profiling of mouse colonic eosinophils reveals a key role for eosinophils in the induction of s100a8 and s100a9 in mucosal healing. Sci. Rep. 2017, 7, 7117. [Google Scholar] [CrossRef]
- Ge, Q.; Shi, Z.; Zou, K.A.; Ying, J.; Chen, J.; Yuan, W.; Wang, W.; Xiao, L.; Lin, X.; Chen, D.; et al. Protein phosphatase PPM1A inhibition attenuates osteoarthritis via regulating TGF-beta/Smad2 signaling in chondrocytes. J. Clin. Investig. 2023, 8, e166688. [Google Scholar] [CrossRef]
- Che, X.; Jin, X.; Park, N.R.; Kim, H.J.; Kyung, H.S.; Kim, H.J.; Lian, J.B.; Stein, J.L.; Stein, G.S.; Choi, J.Y. Cbfbeta Is a Novel Modulator against Osteoarthritis by Maintaining Articular Cartilage Homeostasis through TGF-beta Signaling. Cells 2023, 12, 1064. [Google Scholar] [CrossRef] [PubMed]
- Scharstuhl, A.; Glansbeek, H.L.; van Beuningen, H.M.; Vitters, E.L.; van der Kraan, P.M.; van den Berg, W.B. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J. Immunol. 2002, 169, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Zhen, G.; Wen, C.; Jia, X.; Li, Y.; Crane, J.L.; Mears, S.C.; Askin, F.B.; Frassica, F.J.; Chang, W.; Yao, J.; et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat. Med. 2013, 19, 704–712. [Google Scholar] [CrossRef]
- He, J.; Cao, W.; Azeem, I.; Shao, Z. Epigenetics of osteoarthritis: Histones and TGF-beta1. Clin. Chim. Acta 2020, 510, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Xu, L.; Li, Y.; Zhao, Z. Roles of TGF-beta 1 signaling in the development of osteoarthritis. Histol. Histopathol. 2016, 31, 1161–1167. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Zhuang, W.; Yin, L.; Chen, C.; Li, J.; Chi, F.; Bai, Y.; Chen, X.P. The inhibitory effect against collagen-induced arthritis by Schistosoma japonicum infection is infection stage-dependent. BMC Immunol. 2010, 11, 28. [Google Scholar] [CrossRef]
- Osada, Y.; Shimizu, S.; Kumagai, T.; Yamada, S.; Kanazawa, T. Schistosoma mansoni infection reduces severity of collagen-induced arthritis via down-regulation of pro-inflammatory mediators. Int. J. Parasitol. 2009, 39, 457–464. [Google Scholar] [CrossRef]
- Osada, Y.; Horie, Y.; Nakae, S.; Sudo, K.; Kanazawa, T. STAT6 and IL-10 are required for the anti-arthritic effects of Schistosoma mansoni via different mechanisms. Clin. Exp. Immunol. 2019, 195, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Andreev, D.; Oeser, K.; Krljanac, B.; Hueber, A.; Kleyer, A.; Voehringer, D.; Schett, G.; Bozec, A. Th2 and eosinophil responses suppress inflammatory arthritis. Nat. Commun. 2016, 7, 11596. [Google Scholar] [CrossRef]
- Sewell, D.; Qing, Z.; Reinke, E.; Elliot, D.; Weinstock, J.; Sandor, M.; Fabry, Z. Immunomodulation of experimental autoimmune encephalomyelitis by helminth ova immunization. Int. Immunol. 2003, 15, 59–69. [Google Scholar] [CrossRef]
- Burford, N.T.; Clark, M.J.; Wehrman, T.S.; Gerritz, S.W.; Banks, M.; O’Connell, J.; Traynor, J.R.; Alt, A. Discovery of positive allosteric modulators and silent allosteric modulators of the mu-opioid receptor. Proc. Natl. Acad. Sci. USA 2013, 110, 10830–10835. [Google Scholar] [CrossRef]
- Manetti, D.; Dei, S.; Arias, H.R.; Braconi, L.; Gabellini, A.; Teodori, E.; Romanelli, M.N. Recent Advances in the Discovery of Nicotinic Acetylcholine Receptor Allosteric Modulators. Molecules 2023, 28, 1270. [Google Scholar] [CrossRef] [PubMed]
- Marichal, T.; Mesnil, C.; Bureau, F. Homeostatic Eosinophils: Characteristics and Functions. Front. Med. 2017, 4, 101. [Google Scholar] [CrossRef] [PubMed]
- Dravid, A.A.; Dhanabalan, K.M.; Naskar, S.; Vashistha, A.; Agarwal, S.; Padhan, B.; Dewani, M.; Agarwal, R. Sustained release resolvin D1 liposomes are effective in the treatment of osteoarthritis in obese mice. J. Biomed. Mater. Res. A 2023, 111, 765–777. [Google Scholar] [CrossRef]
- Valdes, A.M.; Ravipati, S.; Menni, C.; Abhishek, A.; Metrustry, S.; Harris, J.; Nessa, A.; Williams, F.M.K.; Spector, T.D.; Doherty, M.; et al. Association of the resolvin precursor 17-HDHA, but not D- or E- series resolvins, with heat pain sensitivity and osteoarthritis pain in humans. Sci. Rep. 2017, 7, 10748. [Google Scholar] [CrossRef]
- Gruber, H.E.; Fisher, E.C., Jr.; Desai, B.; Stasky, A.A.; Hoelscher, G.; Hanley, E.N., Jr. Human intervertebral disc cells from the annulus: Three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp. Cell Res. 1997, 235, 13–21. [Google Scholar] [CrossRef]
- Hara, K.; Hasegawa, T.; Ooi, H.; Koya, T.; Tanabe, Y.; Tsukada, H.; Igarashi, K.; Suzuki, E.; Arakawa, M.; Gejyo, F. Inhibitory role of eosinophils on cell surface plasmin generation by bronchial epithelial cells: Inhibitory effects of transforming growth factor beta. Lung 2001, 179, 9–20. [Google Scholar] [CrossRef]
- Dravid, A.A.; Dhanabalan, K.M.; Agarwal, S.; Agarwal, R. Resolvin D1-loaded nanoliposomes promote M2 macrophage polarization and are effective in the treatment of osteoarthritis. Bioeng. Transl. Med. 2022, 7, e10281. [Google Scholar] [CrossRef] [PubMed]
- Gruber, H.E.; Hoelscher, G.L.; Leslie, K.; Ingram, J.A.; Hanley, E.N., Jr. Three-dimensional culture of human disc cells within agarose or a collagen sponge: Assessment of proteoglycan production. Biomaterials 2006, 27, 371–376. [Google Scholar] [CrossRef]
- Abdala-Valencia, H.; Coden, M.E.; Chiarella, S.E.; Jacobsen, E.A.; Bochner, B.S.; Lee, J.J.; Berdnikovs, S. Shaping eosinophil identity in the tissue contexts of development, homeostasis, and disease. J. Leukoc. Biol. 2018, 104, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, H.; Seo, J.; Choi, K.; Lee, Y.; Park, K.; Kim, S.; Mobasheri, A.; Choi, H. TissueGene-C promotes an anti-inflammatory micro-environment in a rat monoiodoacetate model of osteoarthritis via polarization of M2 macrophages leading to pain relief and structural improvement. Inflammopharmacology 2020, 28, 1237–1252. [Google Scholar] [CrossRef] [PubMed]
- AbuJabal, R.; Ramakrishnan, R.K.; Bajbouj, K.; Hamid, Q. Role of IL-5 in asthma and airway remodelling. Clin. Exp. Allergy 2024, 54, 538–549. [Google Scholar] [CrossRef]
- Dean, N.J.; Clifton, I.J.; Salman, R.; Bridgewood, C.; Nam, J.; Macleod, T.; McGonagle, D.G. Anti-IL-5 biologics and rheumatoid arthritis: A single-centre 500 patient year exposure analysis. RMD Open 2023, 9, e003583. [Google Scholar] [CrossRef]
- Dupin, C.; Valery, S.; Guilleminault, L.; Devouassoux, G.; Merveilleau, M.; Russier, M.; Mourin, G.; Pradelli, J.; Bonniaud, P.; Le Brun, M.; et al. Articular manifestations related to anti-interleukin-5 therapies in severe asthma: A case series. ERJ Open Res. 2024, 10, 00935-2023. [Google Scholar] [CrossRef]
- Bridgewood, C.; Wittmann, M.; Macleod, T.; Watad, A.; Newton, D.; Bhan, K.; Amital, H.; Damiani, G.; Giryes, S.; Bragazzi, N.L.; et al. T Helper 2 IL-4/IL-13 Dual Blockade with Dupilumab Is Linked to Some Emergent T Helper 17-Type Diseases, Including Seronegative Arthritis and Enthesitis/Enthesopathy, but Not to Humoral Autoimmune Diseases. J. Investig. Dermatol. 2022, 142, 2660–2667. [Google Scholar] [CrossRef]
- Schmid, A.S.; Hemmerle, T.; Pretto, F.; Kipar, A.; Neri, D. Antibody-based targeted delivery of interleukin-4 synergizes with dexamethasone for the reduction of inflammation in arthritis. Rheumatology 2018, 57, 748–755. [Google Scholar] [CrossRef]
- Haldar, P.; Brightling, C.E.; Hargadon, B.; Gupta, S.; Monteiro, W.; Sousa, A.; Marshall, R.P.; Bradding, P.; Green, R.H.; Wardlaw, A.J.; et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 2009, 360, 973–984, Erratum in N. Engl. J. Med. 2011, 364, 588. [Google Scholar] [CrossRef]
- Tao, Z.; Zhu, H.; Zhang, J.; Huang, Z.; Xiang, Z.; Hong, T. Recent advances of eosinophils and its correlated diseases. Front. Public Health 2022, 10, 954721. [Google Scholar] [CrossRef]
- Abonia, J.P.; Blanchard, C.; Butz, B.B.; Rainey, H.F.; Collins, M.H.; Stringer, K.; Putnam, P.E.; Rothenberg, M.E. Involvement of mast cells in eosinophilic esophagitis. J. Allergy Clin. Immunol. 2010, 126, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Haikal, S.M.; Abdeltawab, N.F.; Rashed, L.A.; Abd El-Galil, T.I.; Elmalt, H.A.; Amin, M.A. Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model. Cells 2019, 8, 823. [Google Scholar] [CrossRef]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4, 0020-2015. [Google Scholar] [CrossRef]
- Lombardi, C.; Berti, A.; Cottini, M. The emerging roles of eosinophils: Implications for the targeted treatment of eosinophilic-associated inflammatory conditions. Curr. Res. Immunol. 2022, 3, 42–53. [Google Scholar] [CrossRef]
- Carrera Silva, E.A.; Puyssegur, J.; Errasti, A.E. Coevolutionary interplay: Helminths-trained immunity and its impact on the rise of inflammatory diseases. Elife 2025, 14, e105393. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, A.; Everts, B.; Williams, A.R.; Nejsum, P. Antigens from the parasitic nematode Trichuris suis induce metabolic reprogramming and trained immunity to constrain inflammatory responses in macrophages. Cytokine 2022, 156, 155919. [Google Scholar] [CrossRef] [PubMed]
- Quinteros, S.L.; von Krusenstiern, E.; Snyder, N.W.; Tanaka, A.; O’Brien, B.; Donnelly, S. The helminth derived peptide FhHDM-1 redirects macrophage metabolism towards glutaminolysis to regulate the pro-inflammatory response. Front. Immunol. 2023, 14, 1018076. [Google Scholar] [CrossRef]
- Harnett, M.M.; Doonan, J.; Tarafdar, A.; Pineda, M.A.; Duncombe-Moore, J.; Buitrago, G.; Pan, P.; Hoskisson, P.A.; Selman, C.; Harnett, W. The parasitic worm product ES-62 protects against collagen-induced arthritis by resetting the gut-bone marrow axis in a microbiome-dependent manner. Front. Trop. Dis. 2024, 4, 1334705. [Google Scholar] [CrossRef]
- Wang, H.; Yan, Y.; Pathak, J.L.; Hong, W.; Zeng, J.; Qian, D.; Hao, B.; Li, H.; Gu, J.; Jaspers, R.T.; et al. Quercetin prevents osteoarthritis progression possibly via regulation of local and systemic inflammatory cascades. J. Cell. Mol. Med. 2023, 27, 515–528. [Google Scholar] [CrossRef]
- Li, H.; Yuan, S.; Yue, Z.; Zhang, L.; Chen, S.; Qian, Q.; Fu, Q.; Chen, Y. Suppressive effect of curcumin on apoptosis of articular chondrocytes via regulation on NF-kappaB pathway and NLRP3 inflammasome. Cytotechnology 2025, 77, 52. [Google Scholar] [CrossRef]
- Yi, H.; Zhang, W.; Cui, Z.M.; Cui, S.Y.; Fan, J.B.; Zhu, X.H.; Liu, W. Resveratrol alleviates the interleukin-1beta-induced chondrocytes injury through the NF-kappaB signaling pathway. J. Orthop. Surg. Res. 2020, 15, 424. [Google Scholar] [CrossRef]
- Franco-Trepat, E.; Alonso-Perez, A.; Guillan-Fresco, M.; Lopez-Fagundez, M.; Pazos-Perez, A.; Crespo-Golmar, A.; Belen Bravo, S.; Lopez-Lopez, V.; Jorge-Mora, A.; Ceron-Carrasco, J.P.; et al. beta Boswellic Acid Blocks Articular Innate Immune Responses: An In Silico and In Vitro Approach to Traditional Medicine. Antioxidants 2023, 12, 371. [Google Scholar] [CrossRef]
- Daglia, M. New advances in biotechnology of phytochemicals. Biotechnol. Adv. 2020, 38, 107445. [Google Scholar] [CrossRef]
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar] [CrossRef]
- Ishihara, K. Eosinophil cell lines. Methods Mol. Biol. 2014, 1178, 45–51. [Google Scholar] [CrossRef]
- Gachanja, N.N.; Dorward, D.A.; Rossi, A.G.; Lucas, C.D. Assays of Eosinophil Apoptosis and Phagocytic Uptake. Methods Mol. Biol. 2021, 2241, 113–132, Erratum in Methods Mol. Biol. 2021, 2241, C1. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Jacobsen, E.A.; Ochkur, S.I.; McGarry, M.P.; Condjella, R.M.; Doyle, A.D.; Luo, H.; Zellner, K.R.; Protheroe, C.A.; Willetts, L.; et al. Human versus mouse eosinophils: “That which we call an eosinophil, by any other name would stain as red”. J. Allergy Clin. Immunol. 2012, 130, 572–584. [Google Scholar] [CrossRef] [PubMed]
- de Andres, B.; Rakasz, E.; Hagen, M.; McCormik, M.L.; Mueller, A.L.; Elliot, D.; Metwali, A.; Sandor, M.; Britigan, B.E.; Weinstock, J.V.; et al. Lack of Fc-epsilon receptors on murine eosinophils: Implications for the functional significance of elevated IgE and eosinophils in parasitic infections. Blood 1997, 89, 3826–3836. [Google Scholar] [CrossRef] [PubMed]
- Haruna, N.F.; Berdnikovs, S.; Nie, Z. Eosinophil biology from the standpoint of metabolism: Implications for metabolic disorders and asthma. J. Leukoc. Biol. 2024, 116, 288–296. [Google Scholar] [CrossRef]
- Jackson, D.J.; Pavord, I.D. Living without eosinophils: Evidence from mouse and man. Eur. Respir. J. 2023, 61, 2201217. [Google Scholar] [CrossRef]
- Wilson, G.E.; Gautam, S.; Chupp, G.L. Does Eosinophil Heterogeneity Translate into Functional Diversity? A Review of the Evolving Paradigm of Eosinophil Heterogeneity in Asthma. Biomedicines 2024, 12, 2011. [Google Scholar] [CrossRef] [PubMed]
- Erjefalt, J.S. Spatial Eosinophil Phenotypes as Immunopathogenic Determinants in Inflammatory Diseases. Cells 2025, 14, 847. [Google Scholar] [CrossRef] [PubMed]
- Hamour, A.F.; Lee, J.J.; Wasilewski, E.; Monteiro, E.; Lee, J.M.; Vescan, A.; Kotra, L.P. Murine model for chronic rhinosinusitis: An interventional study. J. Otolaryngol. Head Neck Surg. 2023, 52, 32. [Google Scholar] [CrossRef] [PubMed]

| Tissue/Cells | Mediators Involved | Effects Observed | Experimental Model | Ref. |
|---|---|---|---|---|
| Fat tissues | IL-4 | Eo are the major IL-4-expressing | Murine | [54,58] |
| Liver | IL-4 | Eo promote proliferation of quiescent hepatocytes | Murine | [59] |
| Cell lines | IL-4 | Eo support resolution of inflammation | Murine | [62] |
| Cell lines | IL-4/IL-13 | Decrease kinin receptors in a STAT6-dependent mechanism | Human/Murine | [56] |
| Dermis | IL-4/CCL24 | Eo maintain dermis-resident macrophages as replicative niches for Leishmania major | Murine | [61] |
| Myocardium | IL-4/Cationic protein mEar1 | IL-4 establishes a cardioprotective role of Eo in post-MI hearts | Humans/Murine | [60] |
| Blood | SPMs, Resolvin D1/D2/E2 | Resolution of acute injury and bacterial infection | Human PBMCs | [72] |
| Peritoneum | SPMs/Protectin D1 | Resolution of acute peritonitis | Murine | [55,66] |
| Lung | Resolvin D1 | Resolution of LPS-induced lung injury | Murine | [71] |
| Synovium | Resolvin D1 | RvD1 inhibited OA-FLS proliferation and reduced MMP-13 and IL-1β secretion | OA patients | [74] |
| Colon | Chemokines CXCL1/CXCL2 Protectin D1 | Eo exert a protective effect via production of anti-inflammatory lipid mediators | Murine | [69] |
| Lung/Colon | TGF-β | TGF-β signaling regulates eosinophil behavior | Murine | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costantini, S.; Dolzani, P.; Panichi, V.; Borzì, R.M.; Balaji, P.; Daglia, M.; Arciola, C.R. Inflaming and Immune-Resolving: The Ambivalent Role of Eosinophils in Osteoarthritis. Int. J. Mol. Sci. 2025, 26, 10948. https://doi.org/10.3390/ijms262210948
Costantini S, Dolzani P, Panichi V, Borzì RM, Balaji P, Daglia M, Arciola CR. Inflaming and Immune-Resolving: The Ambivalent Role of Eosinophils in Osteoarthritis. International Journal of Molecular Sciences. 2025; 26(22):10948. https://doi.org/10.3390/ijms262210948
Chicago/Turabian StyleCostantini, Silvia, Paolo Dolzani, Veronica Panichi, Rosa Maria Borzì, Paulraj Balaji, Maria Daglia, and Carla Renata Arciola. 2025. "Inflaming and Immune-Resolving: The Ambivalent Role of Eosinophils in Osteoarthritis" International Journal of Molecular Sciences 26, no. 22: 10948. https://doi.org/10.3390/ijms262210948
APA StyleCostantini, S., Dolzani, P., Panichi, V., Borzì, R. M., Balaji, P., Daglia, M., & Arciola, C. R. (2025). Inflaming and Immune-Resolving: The Ambivalent Role of Eosinophils in Osteoarthritis. International Journal of Molecular Sciences, 26(22), 10948. https://doi.org/10.3390/ijms262210948







