Abstract
Protoplast systems have been proven powerful tools in modern plant biology. However, successful preparation of abundant viable protoplasts remains a challenge for Cymbidium orchids. Herein, we established an efficient protoplast isolation protocol from orchid petals through optimization of enzymatic conditions. It requires optimal D-mannitol concentration (0.5 M), enzyme concentration (1.2 % (w/v) cellulose and 0.6 % (w/v) macerozyme) and digestion time (6 h). With this protocol, the highest yield (3.50 × 107/g fresh weight of orchid tissue) and viability (94.21%) of protoplasts were obtained from flower petals of Cymbidium. In addition, we achieved high transfection efficiency (80%) through the optimization of factors affecting polyethylene glycol (PEG)-mediated protoplast transfection including incubation time, final PEG4000 concentration and plasmid DNA amount. This highly efficient protoplast-based transient expression system (PTES) was further used for protein subcellular localization, bimolecular fluorescence complementation (BiFC) assay and gene regulation studies of flowering related genes in Cymbidium orchids. Taken together, our protoplast isolation and transfection protocol is highly efficient, stable and time-saving. It can be used for gene function and molecular analyses in orchids and other economically important monocot crops.
1. Introduction
Among the 350,000 plant species on earth, Orchidaceae (more than 25,000 species) is one of the largest and the most evolved families of monocot plants (Figure S1) [1,2]. Over the past 2000 years, more than 70,000 orchids have been cultivated as ornamental and medicinal plants, as well as a food flavoring additives [3]. Chinese Cymbidium orchids are regarded as an important symbol of oriental culture. To obtain high yielding quality orchid plants, intense efforts have been put into orchid biology research for the past few decades. Related researches include tissue culture, protoplast fusion and regeneration [4,5]. Recently, the release of orchid complete genome sequences of Phalaenopsis [6], Dendrobium [7] and Apostasia [8] has greatly facilitated gene cloning, genetic evolutionary analysis and synthetic biology. Advancements in plant genomics, transcriptomics, proteomics and metabolomics have renewed interest in orchid protoplasts. The versatility of protoplast-based platform helps biologists to explore intracellular processes involving cell structures [9], synthesis of pharmaceutical products [10] and toxicological assessments [11]. In recent studies, plant protoplasts have attracted great attention, since they can be isolated from specific plant tissues/organs and maintained their cellular identity, which practically provided a unique single cell system to answer specific questions related to cell types [12,13]. However, isolation of sufficient viable protoplasts remains a challenge for orchids and many other monocot crop plants [14].
Generally, young plant organs such as cotyledon, hypocotyl, leaf, root and root hair have been used as source materials for protoplast isolation [15]. Most common source of protoplast isolation is from leaf tissues. Plant mesophyll protoplasts have been successfully isolated from tobacco [16], Arabidopsis [17], maize (Zea mays L.) [18], rice (Oryza sativa L.) [19] and other non-model plant species, such as Medicago sativa [20], Panicum virgatum [21], Elaeis guineensis [22], Hevea brasiliensis [23], Phaseolus vulgaris [24] and Magnolia [25]. However, it is difficult to isolate protoplasts with high yield and viability from mature leaf tissues of many plants. Hence, young leaves of in vitro grown plantlets of Phalaenopsis [26], Dendrobium [27], Tanacetum [28], grape (Vitis vinifera) [29], pepper (Capsicum annuum L.) [30] and pineapple [31] have been used. Nevertheless, callus induction is time consuming and impractical for protoplast isolation. Hence, flower petals have been selected as an alternative for protoplast isolation for ornamental plants such as Rosa rugose [32], Dendrobium [33] and Phalaenopsis [34]. In addition to species types and source materials, enzyme combination and the concentration of osmotic pressure regulating substance D-mannitol also affect the effective release of protoplasts [22,35]. Therefore, an efficient method of protoplast isolation is still lacking in many non-model species. For family Orchidaceae, protoplasts are first successful isolated from leaves of Cymbidium [36] and various tissues of Renantanda, Dendrobium and Paphiopedilum [37,38]. Leaf tissues of in vitro grown seedlings of Dendrobium (3.97 × 105/g fresh weight (FW)) [39] and Cymbidium (maximum 1.1 × 107/g FW) [40], and flower petals of Phalaenopsis (1.1–5.9 × 106/g FW) [34,41] have been used for successfully protoplast isolation. However, the protoplast isolation efficiency is lower than that of Arabidopsis (3.0 × 107) [42], maize (1–5 × 106/g FW) [43], rice (1.0 × 107) [44] and cassava (4.4 × 107/g FW) [45]. Up to now, few efficient protoplast isolation protocols have been established for orchids.
Protoplast-based transient expression system (PTES) has been proven to be a powerful experimental tool in molecular biology. It is widely used for protein localization, protein–protein interactions, identification of the gene function and gene regulation [14,34,44,46,47,48,49]. Transient protoplast transfection has been used due to its advantages in high screening efficiency with gene silencing and genome editing technologies, prior to the development of transgenic plants [43,50,51,52]. Protoplast transfection refers to the introduction of exogenous nucleic acids into protoplasts mediated by polyethylene glycol (PEG) or electroporation [53,54,55]. PEG-mediated transfection method is commonly used to introduce DNA or RNA into protoplasts. To obtain reliable and reproducible results, high transfection efficiency (>50%) is required [14]. In addition to viability and quantity of protoplasts, DNA or RNA amount, final concentration of PEG and incubation time are factors affecting transfection efficiency [41,56]. High level transfection efficiency (>80%) has been achieved using Phalaenopsis petal protoplasts [34]. This is equivalent to or better than that of previously reported transfection efficiencies [14,42]. However, the transfection efficiency dramatically decreases with an increase of plasmid DNA size from 5 to 10 kb [50,57,58,59]. Hence, improving transfection efficiency with a larger size plasmid is necessary for genome editing and other studies.
Limitations of existing protoplast isolation protocols led us to develop a simple and efficient orchid protoplast isolation and transfection method. This method recommends the use of flower petals and buds, which is readily available, sustainable and low cost. Using this protocol with optimal enzymatic conditions, high yielding viable protoplasts were successfully isolated efficiently from petals and buds of Cymbidium orchids. It does not require vacuum infiltration, treatment with high osmotic solution to plasmolyze tissues prior to enzymatic digestion, or special equipment. By optimizing factors affecting PEG-mediated protoplast transfection, we have also established a robust PTES for gene functional analysis. It takes less than 24 h from tissue preparation to confocal laser scanning microscopic observation or expression analysis using qRT-PCR. This PTES can be used for protein subcellular localization, protein–protein interaction and gene regulation analysis in Cymbidium orchids.
2. Results
2.1. Protoplast Isolation from Cymbidium Flower Petals
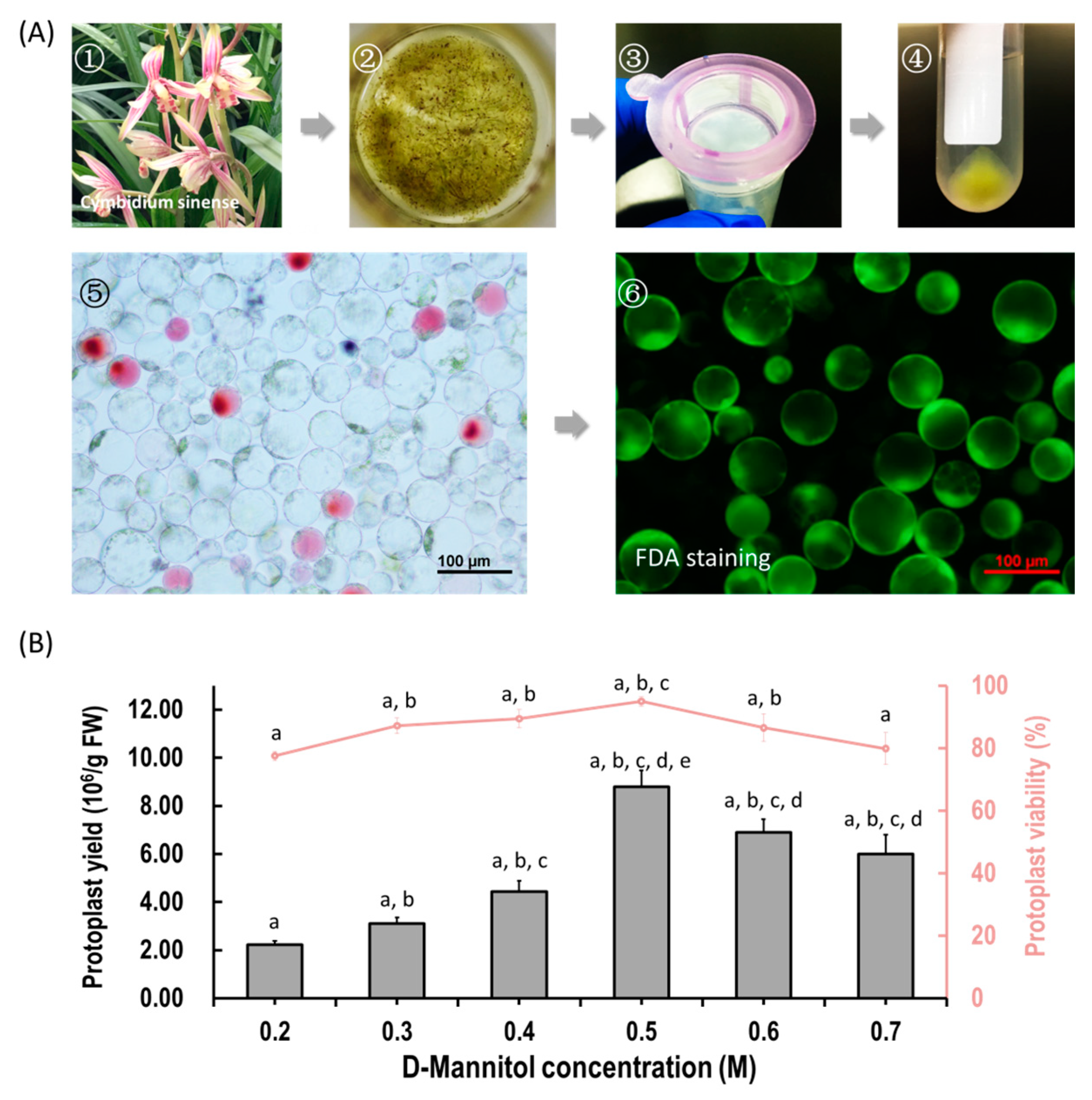
We aimed to establish an efficient protoplast isolation protocol for orchids. Herein, flower petals and buds from Cymbidium orchid plants were used (Figure 1A). To ensure that the released protoplasts do not rupture or collapse during enzyme digestion, different concentrations of D-mannitol (0.2, 0.3, 0.4, 0.5, 0.6 and 0.7 M) were tested. Viable protoplasts were released from petals with variable yield and viability (Figure 1B). Generally, the yields of protoplasts increased with increasing D-mannitol concentrations, and then decreased with increased osmotic pressures. The optimal D-mannitol concentration in enzyme solution for petals was determined as 0.5 M (Figure 1B). With optimal D-mannitol concentrations, orchid petals gave the highest yield (8.80 ± 0.68 × 106/g FW) and viability (94.98% ± 1.51%) of petal protoplasts. In addition, protoplasts isolated from petals remained intact for more than 24 h in W5 solution. To increase the yield and viability of protoplasts, more factors affecting protoplast isolation including enzyme concentration and incubation duration were investigated. Given the combination of optimal D-mannitol concentration (0.5 M), enzyme mixture (1.2% cellulose and 0.6% macerozyme) and digestion time (6 h), the highest yield (3.50 × 107 /g FW) and viability (94.21%) of Cymbidium protoplasts were achieved. The isolated protoplasts ranged from 20 to 100 μm in diameters, and a large proportion of protoplasts isolated from flowers petals were rich in cytoplasm and anthocyanidin. It indicates that our protoplast isolation protocol is suitable for the investigation of intracellular processes in tissues/organs-specific protoplasts of Cymbidium orchids.
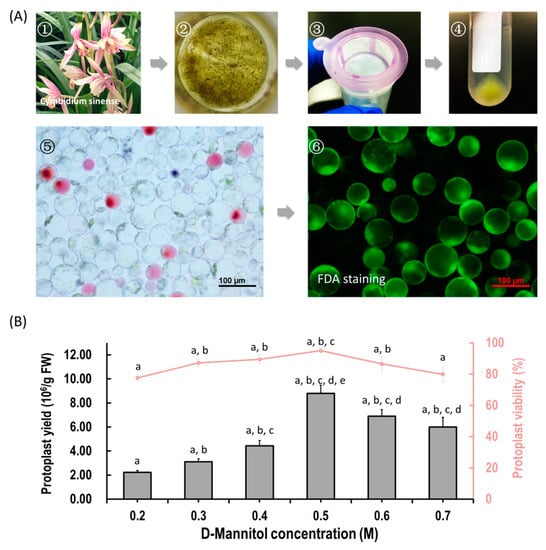
Figure 1.
Protoplast isolation from flower petals of Cymbidium orchids. (A) Schematic illustration of protoplast isolation from Cymbidium orchids. (1) Cymbidium flower petals were collected and cut into 0.5–1.0 mm strips using fresh surgical blades on sterile filter papers; (2) immediately, transfer strips into in a 100 mL flask containing enzymatic solution; (3) after 5 h incubation at 28 °C in dark, the solution containing released protoplasts were filter through a 150 μm nylon mesh into a 50 mL round-bottomed tube; (4) following centrifuge at 200 rpm for 2 min, the protoplast pellet were re-suspended with W5 solution; (5) check the morphology and yield of protoplasts under a microscope and (6) measure the protoplast viability using the FDA staining method. (B) Optimization of Cymbidium protoplast isolation conditions. A series of D-mannitol concentration in the enzyme solutions (0.2, 0.3, 0.4, 0.5, 0.6 and 0.7 M) were tested for different tissues to ensure that released protoplasts do not rupture or collapse during enzyme digestion; data presented as means of three biological replicates with error bars indicating standard deviations (SD), and different letters (a–e) among treatments indicate statistically significant differences at p < 0.05 based on the Tukey–Kramer test.
2.2. PEG-Mediated Transient Expression in Orchid Protoplasts
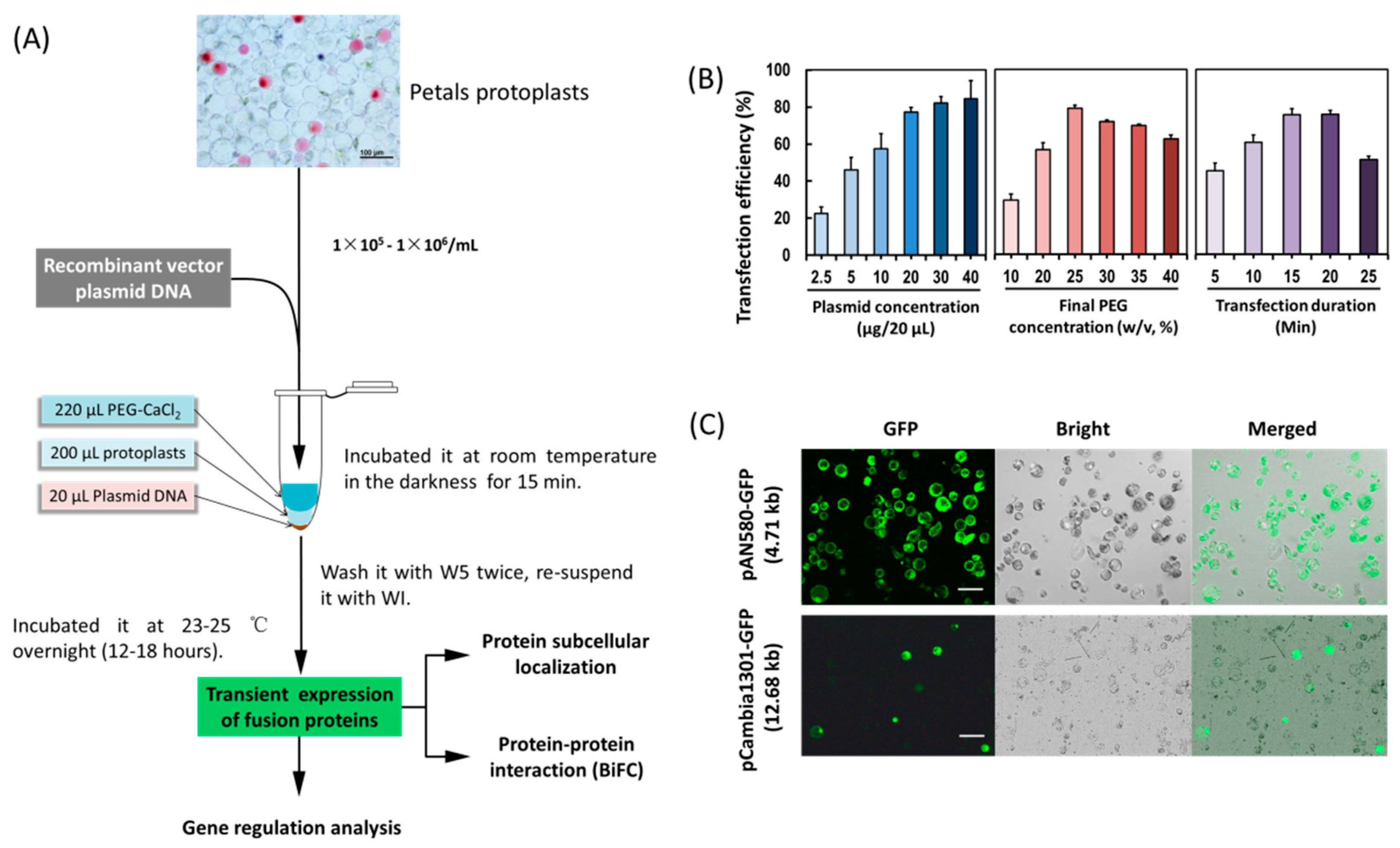
In order to establish an efficient PTES for protein subcellular location, protein–protein interaction and gene regulation studies (Figure 2A), factors affecting PEG-mediated protoplast transfection were investigated. The transfection efficiency increased from 45.26–75.39% within 5–15 min, but decreased from 75.69% to 51.11% within 20–25 min (Figure 2B), indicating that the optimal incubation time was 15 min. The transfection efficiency increased from 29.19% to 79.09% with increasing PEG4000 concentration, and then decreased from 79.09% to 62.42% when the incubation time was 15 min, indicating optimal final PEG4000 concentration was 25%. Moreover, when given the optimal incubation time (15 min) and the final PEG4000 concentration (25%), the transfection efficiency increased significantly when using 1–2 μg/μL of plasmid DNA, and the efficiency remained at high level (80%). To sum up, factors affecting transfection efficiency were optimized as follows: transfection duration (15 min), PEG4000 concentration (25%) and 1 μg/μL of plasmid DNA. A high transfection efficiency of 75–80% was achieved in orchid protoplasts.
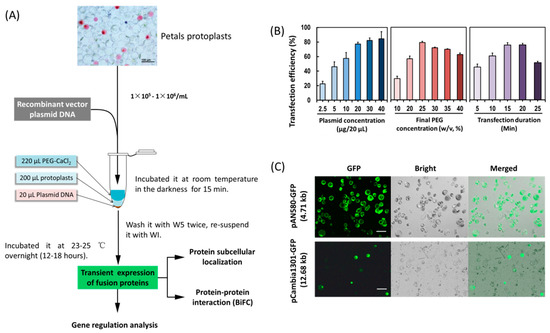
Figure 2.
Establishment of an efficient protoplast-based transient expression system (PTES) for Cymbidium orchids. (A) Schematic illustration of protoplast transfection and application of the PTES; (B) factors affecting the PEG-mediated protoplast transfection, including incubation time, final PEG4000 concentrations and plasmid DNA amount were optimized and (C) the green fluorescence of the green fluorescent protein (GFP) reporter expressed by the two vectors was clearly visible. The maximum transfection efficiency of PAN-580 (4.71 kb in size) and pCambia1301-GFP (12.68 kb) were 80% and 30%, respectively. Data presented as means of three biological replicates with error bars indicating the SD. Bar = 100 μm.
A small amount of plasmid DNA was sufficient to allow fluorescence observation of fusion proteins in protoplasts (Figure 2C). A low concentration of 1 μg/μL of plasmids was used for subsequent protoplasts transfection. Using the optimized transfection method, a larger size plasmid (12 kb) pCambia1301-GFP was transformed, and the fluorescence of green fluorescent protein (GFP) expressed by the vector was detected in 30% of the transfected protoplasts (Figure 2C).
2.3. Protein Subcellular Localization
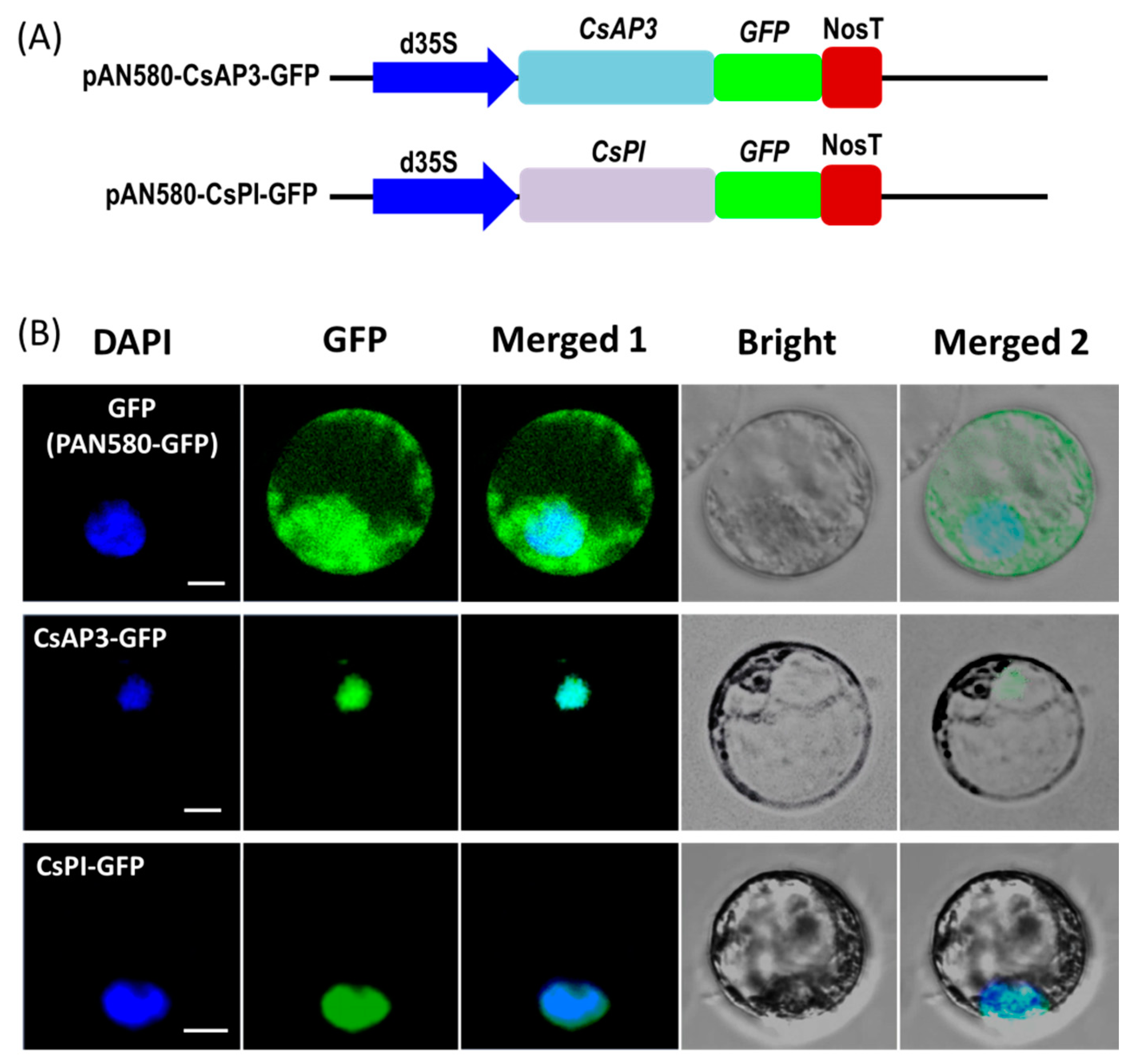
To test the feasibility of the protoplast transient expression system, we investigated the subcellular localization of the two flowering related homologous genes APETALA3 (CsAP3) and PISTILLATA (CsPI) in Cymbidium orchid using our PTES. Recombinant vectors used for subcellular localization of fusion proteins were obtained by cloning the full-length coding sequences (CDSs) of CsAP3 and CsPI into the transient expression vector PAN580-GFP (Figure 3A). Recombinant vectors pAN580-CsAP3-GFP and pAN580-CsPI-GFP, as well as empty control vector (expressing GFP) were transformed into Cymbidium protoplasts. Twelve to sixteen hours post transfection (hpt), green fluorescence was observed in intracellular compartments of transfected protoplasts. The vector control was distributed throughout the entire cell, while fusion proteins CsAP3-GFP and CsPI-GFP were both colocalized with the 4’-6’-diamidino-2-phenylindole (DAPI) signal, indicating nuclear localization of both fusion proteins (Figure 3B).
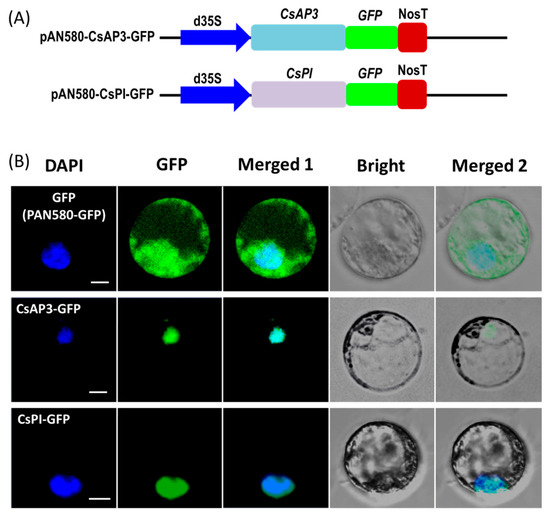
Figure 3.
Protein subcellular localization studies using the PTES. (A) Vectors used for transient expression were obtained by cloning the full-length coding sequences (CDSs) of CsAP3 and CsPI into the vector PAN580-GFP. (B) Recombinant vectors pAN580-CsAP3-GFP and pAN580-CsPI-GFP, as well as empty control vector (expressing GFP) were transformed into Cymbidium protoplasts to test feasibility of the PTES for protein subcellular localization; fusion proteins CsAP3-GFP and CsPI-GFP were both colocalized with the DAPI signal, indicating nuclear localization of both fusion proteins; bar = 10 μm.
2.4. Protein–Protein Interaction Studies in Cymbidium Protoplasts
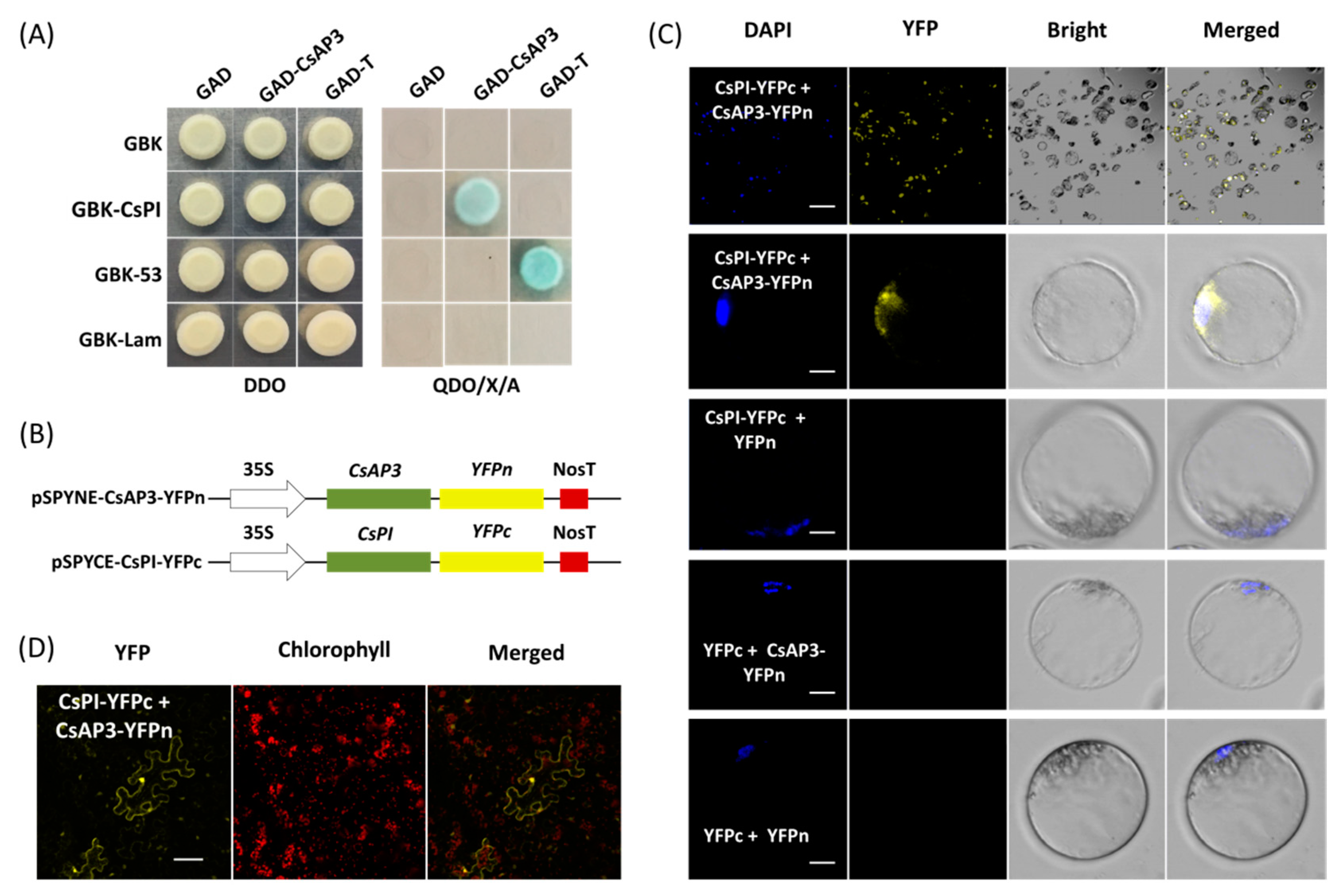
Yeast cells cotransformed with vectors GAD-CeAP3 and GBK-CePI gave blue colonies growth on QDO/X/A medium consistent with that of positive control rather than negative control (Figure 4A), which indicated that CsAP3 and CsPI proteins interact with each other (Figure 4A). Using this PTES protocol, protein–protein interaction was confirmed by bimolecular fluorescence complementation (BiFC) assay. Vectors used for BiFC assays were obtained by cloning the full-length CDSs of CsAP3 and CsPI into vectors pSPYNE-35S and pSPYCE-35S, respectively (Figure 4B). Fusion proteins CsAP3-YFPn and CsPI-YFPc were co-expressed in Cymbidium protoplasts and yellow fluorescent protein (YFP) signals were detected in the nuclei where the DAPI signal was also present. As negative controls, combinations of empty YFPn + CsPI-YFPc, CsAP3-YFPn + empty YFPc and empty YFPn + YFPc did not produce any BiFC fluorescence (Figure 4C). In addition, BiFC assays were also carried out using another transient expression method in N. benthamiana leaves. As expected, strong YFP signals were observed in N. benthamiana mesophyll cells, especially nuclei (Figure 4D), which confirmed the interaction between proteins encoded by CsAP3 and CsPI. Taken together, the PTES protocol is suitable for protein subcellular localization and protein–protein interaction studies.
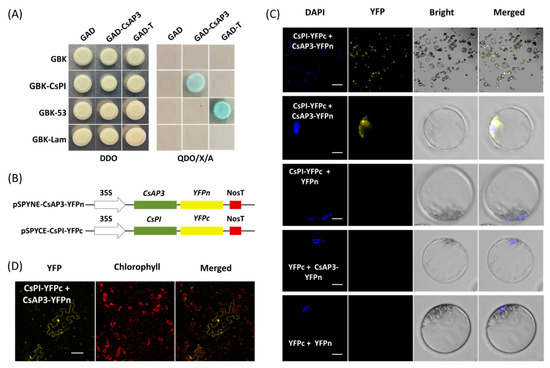
Figure 4.
Protein–protein interaction studies using the PTES. (A) Yeast two-hybrid assay (Y2H) of proteins encoded by CsPI and CsAP3. (B) Vectors used for bimolecular fluorescence complementation (BiFC) assays were obtained by cloning the full-length CDSs of CsAP3 and CsPI into the vectors pSPYNE-35S and pSPYCE-35S, respectively. (C) Fusion proteins CsAP3-YFPn and CsPI-YFPc were co-expressed in Cymbidium protoplasts and yellow fluorescent protein (YFP) signals were detected in nuclei where the DAPI signal presented, while negative controls did not produce any BiFC fluorescence; (D) BiFC assays were also carried out in N. benthamiana leaves and strong YFP signals were observed in the protoplasts, especially in the nuclei. Bar = 10 μm.
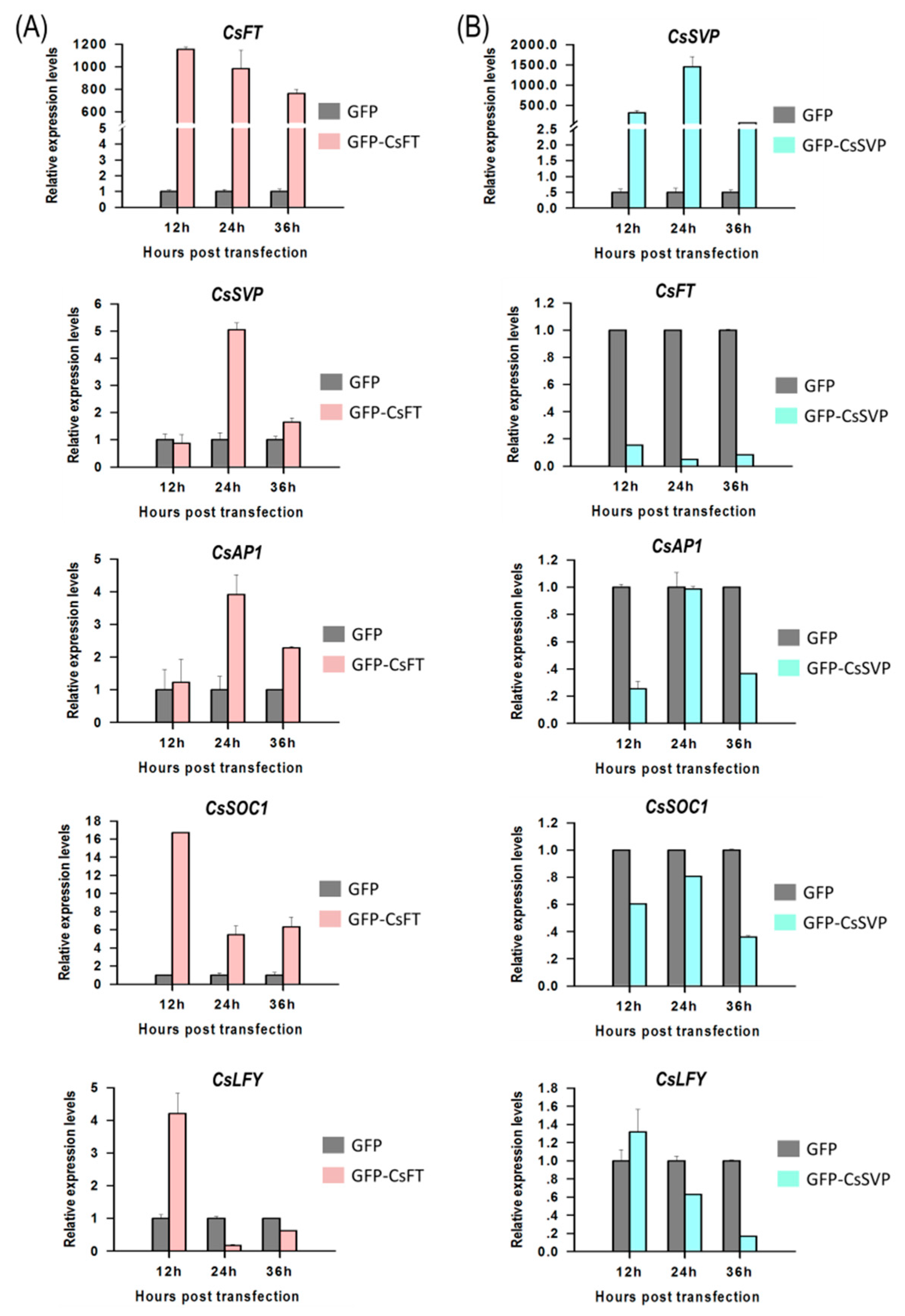
2.5. Gene Regulation Analysis in Cymbidium Protoplasts Using qRT-PCR
To identify functions of flowering pathway integrators, expression of Cymbidium homologous of FLOWERING LOCUS T (CsFT) and SHORT VEGETATIVE PHASE (CsSVP) to their downstream genes were investigated in Cymbidium protoplasts by qRT-PCR. Using the PTES, CsFT was successfully transient overexpressed in Cymbidium protoplasts, which in turn enhanced the expression of SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (CsSOC1), CsAP1, LEAFY (CsLFY) and CsSVP expression (Figure 5A). Among the four genes, CsSOC1 was the only significantly up-regulated gene with 5–16 folds at 12–36 hours post transfection (hpt). Similarly, CsSVP and CsAP1 were both significantly upregulated for 2–5 folds from 24–36 hpt. The expression of CsLFY was significantly upregulated only at 12 hpt, but was significantly downregulated from 12–36 hpt. These results suggested that the transient overexpressed CsFT enhanced the expression of CsSOC1, CsAP1 and CsSVP. In addition, CsSVP was also successfully overexpressed in Cymbidium protoplasts 12–36 hpt, and the expression levels of CsSOC1, CsAP1, CsLFY and CsFT were all significantly suppressed (Figure 5B). The expression of CsSOC1 and CsFT were both significantly downregulated 12–36 hpt. The expression levels of CsAP1 were downregulated at 12–36 hpt, while that of CsLFY were downregulated from 24–36 hpt, respectively. These results indicated that CsSVP suppressed the expression of CsSOC1, CsAP1, CsLFY and CsFT. The above results indicated that our PTES could be used to explore the inter-regulation relationship among orchid genes.
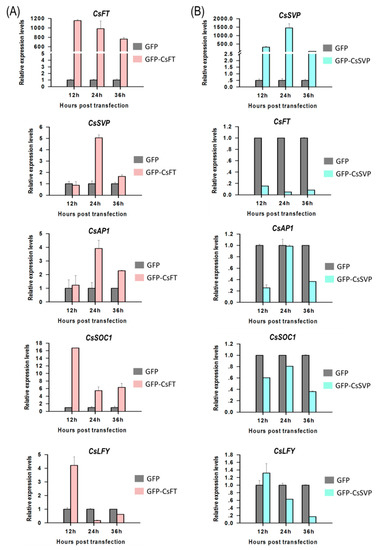
Figure 5.
Gene regulation analysis using the PTES and qRT-PCR. (A) CsFT was successfully overexpressed in Cymbidium protoplasts using the PTES, which in turn resulted in upregulated expression of CsSOC1, CsAP1, CsLFY and CsSVP and (B) CsSVP was also successfully overexpressed in Cymbidium protoplasts 12–36 hpt, and the expression levels of CsSOC1, CsAP1, CsLFY and CsFT were significantly reduced. Y-axes indicate the relative expression levels of controls and transfected protoplasts at different time points (12, 24 and 36 hpt). Data were expressed as the mean of three biological replicates with error bars indicating the SD.
3. Discussion
Previous attempts to isolate higher yield and viability of protoplasts from different tissues of numerous species have been made separately under different conditions [14,34,42]. Though vacuum infiltration or treatment with high osmotic solution of plasmolyzed tissue prior to the enzymatic digestion has been recommended [14], high yielding viable protoplasts were obtained from flower petals of Cymbidium orchids without those treatments. In this study, we developed a simple, efficient and low cost orchid protoplast isolation method through optimizing enzymatic conditions (Figure 1). Osmotic conditions significantly influenced the yield of viable protoplasts (Figure 1B), which is in agreement with previous reports [14,16,18,46,60]. Plant cells are surrounded by cell walls and cytoplasm maintains an osmotic balance through vacuoles. Protoplasts are plant cell whose cell walls are enzymatically removed using cellulose-R10 combined with macerozyme-R10 [14,34]. We noted that increase in total concentration of enzymes led to an increase in protoplast yield, while excess enzymes led to a decrease in protoplast yield and viability (data not shown), probably due to phytotoxicity of enzymes on the membrane of protoplasts [61,62]. To obtain a higher number of viable protoplasts, a suitable concentration of additional enzymes such as pectolyase Y-23 [63] or/and hemicellulose may be added [24,29]. The efficiency of protoplast isolation from orchids was greatly increased from 106 to 107/g FW (almost ten folds) with this protocol. Given the optimal digestion conditions, sufficient protoplasts were obtained from flower petals and buds of Cymbidium, which is higher efficient than that of past.
In addition to protoplast fusion, the PTES is also an important experimental tool in plant molecular biology (Figure 2A). Protoplast transfection efficiency >50% is required for reliable and reproducible experimental data [14]. By optimizing factors affecting PEG-mediated transfection (Figure 2B), we achieved high protoplasts transfection efficiency (80%), which is equivalent or greater than that reported in other systems [34,41]. Previously PEG and calcium ion (Ca2+) are added to increase the fluidity of plasma membrane [53] to facilitate plasmid DNA uptake in protoplasts. Although the transfection efficiency increased with higher PEG4000 concentration and incubation period, the number of ruptured protoplasts increased after the threshold was reached (Figure 2B). Hence, suitable concentration of PEG and incubation time should be fully considered when establishing an efficient protoplasts transfection protocol. Higher transfection efficiency was obtained with a higher quantity of plasmid DNA (Figure 2B), which is consistent with other protocols [41,49]. In consideration of simple small-scale plasmid DNA isolation for fluorescence observation of fusion proteins in protoplasts (Figure 2C), a lower concentration of 1 μg/μL was used for subsequent protoplasts transfection. In addition, a larger size plasmid (12 kb) was transformed using the optimized transfection method, and the fluorescence of GFP reporter expressed by the vector was detected in 30% of protoplasts (Figure 2C). It provides wide application of our protoplast transfection system for rapid screening of targets of genome editing and gene silencing.
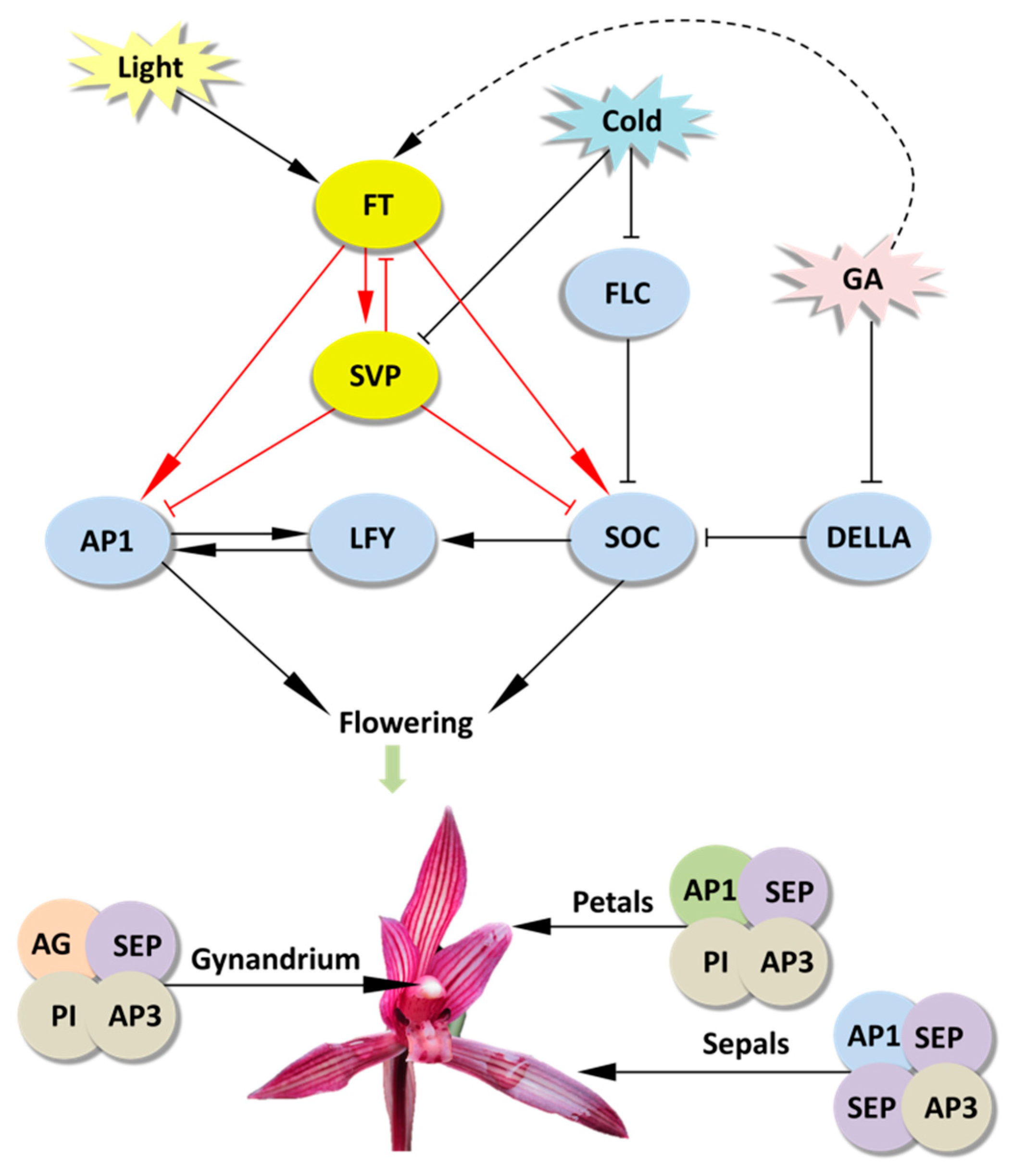
The efficient PTES provides a great platform for gene function identification. It relies on transient expression of transgenes rather than stable transgene in a given plant species. Hence, high throughput PTES are used for rapid screening of transactivation of hundreds of transcription factors [64] and rapid screening of numerous transgenic expression cassettes prior to stable plant transformation [49]. Here, the PTES was demonstrated for protein subcellular localization, BiFC assay and gene regulation analyses of flowering related genes (Figure 3, Figure 4 and Figure 5). As protoplast isolated from certain plant organs or tissues maintain their cellular identity, protoplasts isolated from petals and buds exhibit greatly feasibilities in functional characterization of flowering related genes in orchids and other ornamental plants. The B-class genes AP3 and PI are of particular importance in the specification of petal and stamen identity [65,66,67]. In the present study, both CsAP3 and CsPI were co-localized in nuclei of Cymbidium protoplasts (Figure 3). The result was consistent with that of Y2H assay (Figure 4A), and was confirmed with the BiFC assay in N. benthamiana leaves. In addition, we investigated the regulation of the downstream genes of CsFT and CsSVP in Cymbidium protoplasts (Figure 5) and the results were consistent with previous published results [68,69,70]. It indicated that genetic pathways mediated by FT and SVP (Figure 6) were conserved in orchids and other plant species [70]. These results reinforced the high reliability of the PTES in cellular and molecular biology research for orchids.
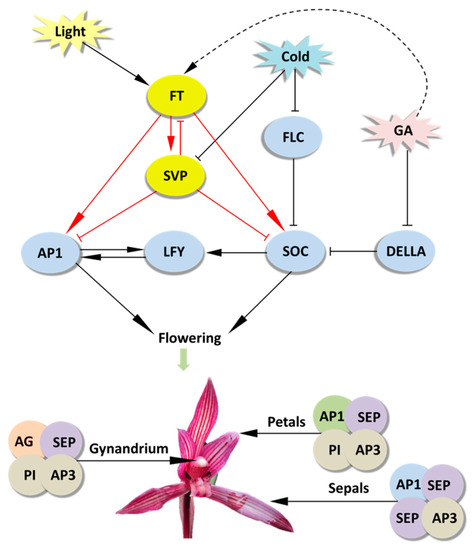
Figure 6.
Conserved putative genetic regulatory networks of flowering in plants. Genes are represented by ovals. Lines with an arrow represent promotion, and those with a perpendicular bar represent repression. Red lines indicate regulation analyzed in this study.
4. Materials and Methods
4.1. Plant Materials
Cymbidium orchid plants were obtained from the orchid breeding base of Environmental Horticulture Research Institute, Guangdong Academy of Agricultural Sciences, China. They were maintained in plastic pots (20 × 20 cm) in greenhouses with environmental conditions as previously described [71]. In addition, tobacco (N. benthamiana) plants were also included. Tobacco seeds were obtained from the National Key Laboratory for Crop Genetics and Germplasm Enhancement, China. The tobacco seedlings were grown in plant growth rooms at 27 °C under a 16 h light/8 h dark cycle with 60% humidity.
4.2. Protoplast Isolation
The protoplast isolation was developed from modifying protocols previously established [14,34,40,41]. Flower petals and buds from Cymbidium were collected for protoplast isolation. The tissues were cut into 0.51 mm strips using fresh surgical blades on sterile filter papers, and were immediately transferred into the freshly prepared enzyme-solution in a 100 mL sterile flask. Generally, 10 mL enzyme solution would be prepared for approximately 0.5 g tissues FW for each treatment. The enzyme solution was prepared as follows: 20 mM KCl (Sigma, St. Louis, MO, USA), 20 mM 2-(N-Morpholino)ethanesulfonic acid (MES, pH = 5.7; Sigma, St. Louis, MO, USA) with different concentrations of D-mannitol (0.2, 0.3, 0.4, 0.5, 0.6 and 0.7 M; Sigma, St. Louis, MO, USA), cellulose R-10 (1.2%, w/v; Yakult Pharmaceutical Industry Ltd., Nishinomiya, Japan) and macerozyme R-10 (Yakult Pharmaceutical Industry Ltd., Nishinomiya, Japan; 0.6%, w/v). The solution was then warmed up to 55 °C for 10 min, and cool to room temperature (25 °C). After that, 10 mM CaCl2 (Sigma, St. Louis, MO, USA) and 0.1% (w/v) bovine serum albumin (BSA; Sigma St. Louis, MO, USA) were added.
The released protoplasts were harvested after incubation at 28 °C in dark with a rotation of 30 rpm for different period of time (2, 4, 6 and 8 h). The enzyme mixture was diluted with equal volume of wash solution (W5) that containing 154 mM NaCl (Sigma, St. Louis, MO, USA), 125 mM CaCl2, 5 mM KCl and 4 mM MES (pH = 5.7). The protoplast-containing solution was filtered through a 150 μm nylon mesh into a 50 mL round-bottomed centrifuge tube. The flow through was centrifuged at 100× g for 2 min (at room temperature) to pellet the protoplasts, and the supernatant was removed carefully with a sterile syringe. The protoplasts pellet was resuspended gently in 10 mL W5 solution [72] and re-centrifuged at 100× g for 2 min (at room temperature), followed by resuspending in 5 mL W5 solution and incubated on ice for 30 min, the protoplasts were settled at the bottom of the tube by gravity. Finally, the supernatant was carefully decanted and discarded, and the purified protoplasts were adjusted to a density of 1 × 105–1 × 106/mL with prechilled MMG solution [73] containing 0.4 M mannitol, 15 mM MgCl2 and 4 mM MES at pH 5.7.
The protoplasts were counted and photographed by a Leica DM2500 microscope (Leica, Wetzlar, Germany) with a hemocytometer for calculation of estimated yield of protoplasts. The protoplasts yield was measured as the total number of protoplasts released in the enzyme mixture divided by the fresh weight of the tissues used for protoplast isolation (protoplasts/g FW). Fluorescein diacetate (FDA; Sigma, St. Louis, MO, USA) stain was used to determine the viability of protoplasts as follows: 9 μL of the protoplasts were put onto a glass slide followed by 1 μL of 0.2% FDA solution (dissolved in acetone (Solarbio Science and Technology Co., Ltd. Beijing, China)), and incubated at room temperature for 1–2 min. The viable protoplasts with green fluorescence were visualized and photographed by LSM 710 confocal laser microscope (Carl Zeiss, Inc., Jena, Germany) with blue excitation block. The protoplasts viability was measured as green cells/ total cells × 100%. Three images were selected for yield and viability measurement of each sample. Each experiment was repeated three times.
4.3. PEG-Mediated Protoplast Transfection
Protoplast transfection was carried out following a modified PEG-mediated protocol [14] (Figure 2A). The protoplasts were adjusted to 1 × 105–1 × 106 /mL in density with MMG solution. For each transformation, 20 μL prechilled plasmid DNA with different concentrations (0.25, 0.50, 1.00, 2.00, 3.00 and 4.00 μg/μL) was gently mixed with 200 μL protoplasts in 2 mL round-bottomed centrifuge tubes. Then equal volume (220 μL) freshly prepared PEG-CaCl2 solution was immediately added and mixed completely by gently inversion. The PEG-CaCl2 solution was comprised of 100 mM CaCl2 and 0.2 M D-mannitol with different final concentrations of PEG4000 (Sigma, St. Louis, MO, USA; 10%, 20%, 30% and 40%, w/v). The mixture was incubated at room temperature in the darkness with different durations (5, 10, 15, 20 and 25 min). The transfection was stopped by adding two volumes (880 μL) of W5 solution followed by centrifugation at 100 × g for 2 min. The transfected protoplasts were then washed with W5 solution and re-suspended with 1 mL WI solution in each of the 6-well tissue culture plate. The WI solution was comprised of 0.4 M D-mannitol, 20 mM KCl and 4 mM MES (pH 5.7). For transient expression of proteins (genes), the protoplasts mixture was incubated for 12–36 h at 23 °C in the darkness.
Transformation efficiency of the protoplasts was detected according to the expression of GFP reporter using the transient expression vector pAN580-GFP and the binary expression vector pCambia1301-GFP. The GFP fluorescence was observed and 3–5 images were taken in random distribution under LSM780 fluorescent microscope (Carl Zeiss, Inc., Jena, Germany) or LSM710 confocal laser scanning microscope. The transformation efficiency was measured as bright green fluorescent protoplast number in view/total protoplast number in view (%). At least three photographs were taken for each sample, and these experiments were independently conducted at least three times.
4.4. Protein Subcellular Localization
Cellular characters of proteins encoded by CsAP3 and CsPI were investigated using protein localization with this orchid PTES. Vectors used were obtained by cloning the coding sequences (CDSs) of CsAP3 (GenBank accession number: JQ326260.1) and CsPI (JQ326259.1) into the vector PAN580-GFP. Briefly, total RNA was extracted from freshly collected young leaves of Cymbidium sinense using an RNA Simple Total RNA Kit (Tiangen, Beijing, China). The DNA-free RNA was used for first-strand cDNA synthesis with oligo (dT) primers and a PrimeScript™ 1st strand cDNA Synthesis Kit (Takara, Dalian, China), following the manufacturer’s instructions. The specific primers with overlapping homologous ends were designed using Primer Premier 5.0 software (Premier, Palo Alto, CA, USA) according to the full-length CDSs of CsAP3 and CsPI, respectively (Table S1). Subsequently, the fragments for each gene were amplified using PrimerSTAR Max DNA Polymerase (Takara, Dalian, China) with the cDNA and the specific primers. The PCR products were purified and full-length CDSs without termination codon were cloned into certain vectors by recombination using the Seamless Assembly Cloning Kit (CloneSmarter, Houston, USA) following the manufacturer’s instructions. Recombined vectors were transformed into Escherichia coli DH5α competent cells (Tiangen, Beijing, China) according to the manufacturer’s instructions and confirmed by sequencing. Ultimately, CDSs of CsAP3 and CsPI were inserted between the dual cauliflower mosaic virus (CaMV) 35S promoter and GFP gene of pAN580-GFP for subcellular localization analysis, resulting in the recombination plasmids of pAN580-CsAP3-GFP and pAN580-CsPI-GFP expressing GFP fusion-proteins, respectively.
The above mentioned vectors including pAN580-GFP, pAN580-CsAP3-GFP and pAN580-CsPI-GFP were transformed into Escherichia coli DH5α competent cells (Tiangen, Beijing, China) according to the manufacturer’s instructions. Following mass replication of the bacterium, plasmid DNA was extracted by Endo-Free Plasmid Maxi Kit (Omega Bio-tek, Norcross, USA). The concentrated plasmid DNA was prepared with different concentrations (up to 2.0 μg/μL), and was transformed into the orchid protoplasts. After incubation at 23 °C in the darkness for 12–16 h, the fluorescence of GFP or GFP-proteins fusions were viewed under LSM710 confocal laser scanning microscope. To detect cell nucleus, the transfected protoplasts were stained with 50 μg/mLDAPI (Sigma–Aldrich Chemie, Steinheim, Germany) at 37 °C for 10 min. DAPI signals were excited with a blue diode laser (405 nm excitation line with 485-nm long-pass barrier filter).
4.5. Yeast Two-Hybrid Assay
The interaction between proteins encoded by CsAP3 and CsPI was investigated by a yeast two-hybrid assay. In the same way, the full-length CDSs of CsAP3 and CsPI were cloned to vectors pGADT7 and pGBKT7, resulting into recombination plasmids GAD-CsAP3 and GBK-CsPI, respectively. Fusion plasmids GAD-CsAP3 and GBK-CsPI were transformed into Y187 and Y2HGold yeast strains, respectively. Diploids containing GAD-CsAP3 and GBK-CsPI were obtained according to the small-scale protocol (Clontech, Palo Alto, USA). After selection of cotransformants on DDO (SD/-Trp/-Leu) medium, they were transferred to QDO/X/A (SD/-Trp/-His/-Trp/-Ade/X-α-gal/AbA) medium. Yeast cells carrying the pGBKT7-53 and pGADT7-T plasmids were served as the positive control, while yeast cells harboring the pGBKT7-Lam and pGADT7-T plasmids were used as the negative controls. Blue colonies were observed on QDO/X/A plates within 3–5 days, once the two proteins (encoded by CsAP3 and CsPI) were interacted with each other.
4.6. BiFC Assay in Orchid Protoplasts and N. benthamiana Leaves
The vectors used for BiFC assay were prepared accordingly. Empty vectors pSPYNE-35S (harboring the nitrogen terminal of the yellow fluorescent protein (YFP)) and pSPYCE-35S (harboring the carbon terminal of the YFP) were both driven by the CaMV 35S promoter. Full-length CDSs of CsAP3 and CsPI were cloned into pSPYNE-35S and pSPYCE-35S, resulting in recombinant plasmids pSPYNE-35S-CsAP3-YFPn and pSPYNE-35S-CsPI-YFPc, respectively (Figure 4B). For BiFC essay, pSPYNE-35S-CsAP3-YFPn and pSPYNE-35S-CsPI-YFPc were cotransfected into protoplasts. As negative controls, vector combinations pSPYNE-35S-CsPI-YFPc + pSPYNE-35S, pSPYCE-35S + pSPYNE-35S-CsAP3-YFPn and pSPYNE-35S + pSPYCE-35S were also cotransfected into the protoplasts, respectively. BiFC assays were carried out in N. benthamiana leaves to confirm the interaction of proteins encoded by CsAP3 and CsPI. Briefly, the vectors pSPYNE-35S-CsAP3-YFPn and pSPYNE-35S-CsPI-YFPc were transfected into Agrobacterium tumefaciens strain GV301, respectively. Positive agrobacteria which fused with reciprocal halves of YFP were co-infiltrated into N. benthamiana leaves, and the N. benthamiana were cultured in the darkness for two days. The transfected protoplasts and the infiltrated leaves were examined under LSM710 confocal laser scanning microscope. The interaction was confirmed using both combinations of reciprocal YFPn/YFPc fusion proteins in two separate experiments (with three replicates per experiment).
4.7. Gene Regulation Analysis by qRT-PCR
The gene FT is the "flowering element" of plants that induces them to bloom through forming flowering complexes with a series of proteins with transcriptional activity [68,69,70]. However, SVP genes suppress the expression of flowering integration factors SOC1 (MF474250.1), FT and LFY (KC138806.1) [68,69,70]. Therefore, these two key flowering regulatory genes CsFT (accession number: HM803115.1) and CsSVP (MF462098.1) were chosen to investigate the regulations to their downstream genes in orchid protoplasts. Full–length CDSs of CsFT and CsSVP were cloned into vector pAN580, and replaced with GFP gene, resulting in plasmids pAN580-CsAP3 and pAN580-CsPI, respectively (Figure S2). Both plasmids transiently expressing proteins encoded by CsFT and CsSVP were transfected into Cymbidium protoplasts, respectively. The protoplasts were incubated at 23 °C in the dark and harvested at 12, 24 and 36 hpt. The relative expression levels of CsFT and CsSVP along with their downstream genes CsLFY, CsSOC1 and CsAP1 were determined by qRT-PCR.
4.8. qRT-PCR
The transfected protoplasts (5 × 105 protoplasts) were independently collected with three biological replicates and stored at -80 °C immediately after freezing into liquid nitrogen. Total RNA was extracted and treated with RNase-free DNase according to the manufacturer’s instruction. RNA was reverse-transcribed using PrimeScript™ RT reagent Kit with gDNA Eraser (Takara, Dalian, China) following the manufacturer’s instructions. The resulting first-strand cDNA was subjected to relative qPCR as previously described [71]. Gene-specific primers were designed from transcript sequences of CsFT, CsSVP, CsLFY, CsSOC1 and CsAP1 [74], using Primer-BLAST software (https://www.ncbi.nlm.nih.gov/tools/primer-blast/; Table S1). The gene ubiquitin in Cymbidium (referred to as CsUBQ, accession No: AY907703) was used as an internal reference control to normalize the total amount of cDNA in each reaction (Table S1). PCR was conducted, and only primers that amplified a single product were selected for qRT-PCR. The qRT-PCR was performed in a 20 μL reaction volume comprising 2.0 μL (20 ng) of 5× diluted first-strand cDNA, 0.8 μL of each primers (10.0 μM), 10.0 μL of 2× SYBR Green I Master Mix (Takara, Dalian, China) and 6.4 μL of sterile distilled H2O. All reactions were performed in 96-well reaction plates using a Bio-Rad CFX-96 Real-time PCR System (Bio-Rad, Hercules, Canada) with three technical replicates. The following PCR conditions were used: 95 °C for 5 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 30 s and 72 °C for 30 s, and then at 68 °C for 5 min. The expression of candidate genes was quantified using the relative quantification (2−ΔΔCT) method [75].
4.9. Statistical Analysis
The statistical analysis was performed with SPSS Version 18.0 software (SPSS Inc., Chicago, IL, USA). All experiments were replicated three times. Data are presented as mean–standard error from three independent experiments. Significant differences among treatments were determined at p ≤ 0.05 based on the Tukey–Kramer test.
5. Conclusions
The protoplast isolation and transfection system described in this study was proven to be robust, reliable and sustainable. As a time-saving tool-kit, it has a wide application, which includes virus replication, protein localization, protein–protein interactions, gene regulation and gene function identification in orchids.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/7/2264/s1, Table S1 Primer sequences used in this study; Figure S1. Schematic diagram of genetic evolutionary relationships between orchids and other plant species; Figure S2. Plasmid vectors used for gene regulation analysis in this study.
Author Contributions
Conceptualization, G.Z., F.Y.; Investigation, R.R., Y.W., S.A., J.J., J.G., C.L.; Formal Analysis, R.R., S.-M.W., Y.W.; Data Curation, J.J.; Writing—Original Draft, R.R.; Writing—Review and Editing, R.R., S.-M.W., Y.W., G.Z., F.Y.; Supervision, R.R.; Project Administration, G.Z., F.Y.; Funding Acquisition, G.Z., F.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Key Technologies R&D Program (2019YFD1001003, 2018YFD1000404), Chinese postdoctoral science foundation (2018M643033), National natural science foundation of China (31672184,31902065), R&D plan in key areas of Guangdong Province (2018B020202001), Natural Science Foundation of Guangdong province (2017A030312004), Public welfare research and capacity building project (2017B020201004) and Modern agricultural industrial technology system project (2019KJ121) of Guangdong Province, Guangzhou scientific research project (201904020026, 201707010307), Guangdong academy of agricultural sciences project (201612TD, R2016PY-QF015, 2017A070702008, President fund 201721, 201804b), Discipline team Building projects of Guangdong Academy of Agricultural Sciences in the 13th Five-Year Period (201612TD), and Guangdong academy of agricultural sciences project (2017A070702008, President fund 201721, 201834, 201804b).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PEG | Polyethylene glycol |
| PTES | Protoplast-based transient expression system |
| BiFC | Bimolecular fluorescence complementation |
| FW | Fresh weight |
| GFP | Green fluorescent protein |
| CDS | Coding sequences |
| DAPI | 4’-6’-diamidino-2-phenylindole |
| hpt | Hours post transfection |
| FT | FLOWERING LOCUS T |
| SVP | SHORT VEGETATIVE PHASE |
| CsSOC1 | SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 |
| Y2H | Yeast two-hybrid assay |
| Ca2+ | Calcium ion |
| FDA | Fluorescein diacetate |
| YFP | Yellow fluorescent protein |
References
- Hsiao, Y.Y.; Tsai, W.C.; Kuoh, C.S.; Huang, T.H.; Wang, H.C.; Wu, T.S.; Leu, Y.L.; Chen, W.H.; Chen, H.H. Comparison of transcripts in Phalaenopsis bellina and Phalaenopsis equestris (Orchidaceae) flowers to deduce monoterpene biosynthesis pathway. BMC Plant Biol. 2006, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.L.; Dixon, K.W. Orchids. Curr. Biol. 2008, 18, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.M. Orchid viruses–A compendium. In Orchid Biology: Reviews and Perspectives, VIII; Kull, T., Arditti, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 505–546. [Google Scholar]
- Shrestha, B.R.; Tokuhara, K.; Mii, M. Plant regeneration from cell suspension-derived protoplasts of Phalaenopsis. Plant Cell Rep. 2007, 26, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Yam, T.W.; Arditti, J. History of orchid propagation: A mirror of the history of biotechnology. Plant Biotechnol. Rep. 2009, 3, 1. [Google Scholar] [CrossRef]
- Cai, J.; Liu, X.; Vanneste, K.; Proost, S.; Tsai, W.C.; Liu, K.W.; Chen, L.J.; He, Y.; Xu, Q.; Bian, C. The genome sequence of the orchid Phalaenopsis equestris. Nat. Genet. 2015, 47, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.Q.; Xu, Q.; Bian, C.; Tsai, W.C.; Yeh, C.M.; Liu, K.W.; Yoshida, K.; Zhang, L.S.; Chang, S.B.; Chen, F. The Dendrobium catenatum Lindl. genome sequence provides insights into polysaccharide synthase, floral development and adaptive evolution. Sci. Rep. 2016, 6, 19029. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Liu, K.W.; Li, Z.J.; Lohaus, R.; Hsiao, Y.Y.; Niu, S.C.; Wang, J.Y.; Lin, Y.C.; Xu, Q.; Chen, L.J. The Apostasia genome and the evolution of orchids. Nature 2017, 549, 379–383. [Google Scholar] [CrossRef]
- Brisson, L.F.; Tenhaken, R.; Lamb, C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 1994, 6, 1703–1712. [Google Scholar] [CrossRef]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef]
- Pereira, S.P.; Fernandes, M.A.; Martins, J.D.; Santos, M.S.; Moreno, A.J.; Vicente, J.A.; Videirae, R.A.; Jurado, A.S. Toxicity assessment of the herbicide metolachlor comparative effects on bacterial and mitochondrial model systems. Toxicol. In Vitro 2009, 23, 1585–1590. [Google Scholar] [CrossRef]
- Petersson, S.V.; Lindén, P.; Moritz, T.; Ljung, K. Cell-type specific metabolic profiling of Arabidopsis thaliana protoplasts as a tool for plant systems biology. Metabolomics 2015, 11, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Denyer, T.; Ma, X.; Klesen, S.; Scacchi, E.; Nieselt, K.; Timmermans, M.C. Spatiotemporal developmental trajectories in the Arabidopsis root revealed using high-throughput single-cell RNA sequencing. Dev. Cell 2019, 48, 840–852. [Google Scholar] [CrossRef]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.R.; Anthony, P.; Power, J.B.; Lowe, K.C. Plant protoplast technology: Current status. Acta Physiol. Plant 2005, 27, 117–130. [Google Scholar] [CrossRef]
- Nagata, T.; Takebe, I. Plating of isolated tobacco mesophyll protoplasts on agar medium. Planta 1971, 99, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Menczel, L. Improved protoplast culture and plant regeneration from protoplast-derived callus in Arabidopsis thaliana. Z. Pflanzenphysiol. 1980, 96, 77–80. [Google Scholar]
- Kanai, R.; Edwards, G.E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973, 51, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Toriyama, K.; Hinata, K. Cell suspension and protoplast culture in rice. Plant Sci. 1985, 41, 179–183. [Google Scholar] [CrossRef]
- Song, J.; Sorensen, E.L.; Liang, G.H. Direct embryogenesis from single mesophyll protoplasts in alfalfa (Medicago sativa L.). Plant Cell Rep. 1990, 9, 21–25. [Google Scholar] [CrossRef]
- Mazarei, M.; Al-Ahmad, H.; Rudis, M.R.; Stewart, C.N., Jr. Protoplast isolation and transient gene expression in switchgrass, Panicum virgatum L. Biotechnol. J. 2008, 3, 354–359. [Google Scholar] [CrossRef]
- Masani, M.Y.A.; Noll, G.A.; Parveez, G.K.A.; Sambanthamurthi, R.; Prüfer, D. Efficient transformation of oil palm protoplasts by PEG-mediated transfection and DNA microinjection. PLoS ONE 2014, 9, e96831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.D.; Wang, L.J.; He, C.Z.; Luo, H.L. An efficient transient mesophyll protoplast system for investigation of the innate immunity responses in the rubber tree (Hevea brasiliensis). Plant Cell Tissue Organ Cult. 2016, 126, 281–290. [Google Scholar] [CrossRef]
- Nanjareddy, K.; Arthikala, M.K.; Blanco, L.; Arellano, E.S.; Lara, M. Protoplast isolation, transient transformation of leaf mesophyll protoplasts and improved Agrobacterium-mediated leaf disc infiltration of Phaseolus vulgaris: Tools for rapid gene expression analysis. BMC Biotechnol. 2016, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.M.; Meng, D.; McGrouther, K.; Zhang, J.H.; Cheng, L.L. Efficient isolation of Magnolia protoplasts and the application to subcellular localization of MdeHSF1. Plant Methods 2017, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Kameya, T.; Ichihashi, S. Plant regeneration from protoplasts derived from callus of Phalaenopsis. Plant Tissue Cult. Lett. 1993, 10, 267–270. [Google Scholar] [CrossRef]
- Kanchanapoom, K.; Jantaro, S.; Rakchad, D. Isolation and fusion of protoplasts from mesophyll cells of Dendrobium pompadour. Sci. Asia 2001, 27, 29–34. [Google Scholar] [CrossRef]
- Keskitalo, M.; Pehu, E.; Simon, J.E. Variation in volatile compounds from tansy (Tanacetum vulgare L.) related to genetic and morphological differences of genotypes. Biochem. Syst. Ecol. 2001, 29, 267–285. [Google Scholar] [CrossRef]
- Mliki, A.; Jardak, R.; Reustle, G.M.; Ghorbel, A. Isolation and culture of leaf protoplasts from Tunisian grapes. OENO One 2003, 37, 145–153. [Google Scholar] [CrossRef]
- Jeon, J.M.; Ahn, N.Y.; Son, B.H.; Kim, C.Y.; Han, C.D.; Kim, G.D.; Gal, S.W.; Wan, S.; Lee, S.H. Efficient transient expression and transformation of PEG-mediated gene uptake into mesophyll protoplasts of pepper (Capsicum annuum L.). Plant Cell Tissue Organ Cult. 2007, 88, 225–232. [Google Scholar] [CrossRef]
- Priyadarshan, S.V.G.N.; Hu, B.Y.; Li, W.M.; Ali, H.; Jia, H.F.; Zhao, L.H.; Ojolo, S.P.; Azam, S.M.; Xiong, J.J.; Yan, M. Simple protoplast isolation system for gene expression and protein interaction studies in pineapple (Ananas comosus L.). Plant Methods 2018, 14, 95. [Google Scholar] [CrossRef]
- Hirata, H.; Ohnishi, T.; Ishida, H.; Tomida, K.; Sakai, M.; Hara, M.; Watanabe, N. Functional characterization of aromatic amino acid aminotransferase involved in 2-phenylethanol biosynthesis in isolated rose petal protoplasts. J. Plant Physiol. 2012, 169, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.W.; Wong, S.M.; Loh, C.S.; Goh, C.J. Synergism in replication of cymbidium mosaic potexvirus (CymMV) and odontoglossum ringspot tobamovirus (ORSV) RNA in orchid protoplasts. Arch. Virol. 1998, 143, 1265–1275. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Chen, J.C.; Fang, S.C. A protoplast transient expression system to enable molecular, cellular, and functional studies in Phalaenopsis orchids. Front. Plant Sci. 2018, 9, 843. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, R.; Niedz, R.P. Protoplast isolation and plant regeneration of guava (Psidium guajava L.) using experiments in mixture-amount design. Plant Cell Tissue Organ Cult. 2015, 122, 585–604. [Google Scholar] [CrossRef]
- Capesius, I.; Meyer, Y. Isolation of nuclei from protoplasts of orchids. Cytobiologie 1977, 15, 485–490. [Google Scholar]
- Teo, C.K.H.; Neumann, K.H. Gewinnung, Kultur and fusion von Orchideenprotoplasten. Orchidee 1978, 29, 90–92. [Google Scholar]
- Teo, C.K.H.; Neumann, K.H. The culture of protoplasts isolated from Renantanda Rosalind Cheok. Orchid Rev. 1978, 86, 156–158. [Google Scholar]
- Khentry, Y.; Paradornuvat, A.; Tantiwiwat, S.; Phansiri, S.; Thaveechai, N. Protoplast isolation and culture of Dendrobium Sonia “Bom 17”. Kasetsart J. 2006, 40, 361–369. [Google Scholar]
- Pindel, A. Optimization of isolation conditions of Cymbidium protoplasts. Folia Hortic. 2007, 19, 79–88. [Google Scholar]
- Li, J.L.; Liao, X.Z.; Zhou, S.S.; Liu, S.; Jiang, L.; Wang, G. Efficient protoplast isolation and transient gene expression system for Phalaenopsis hybrid cultivar ‘Ruili Beauty’. In Vitro Cell. Dev. Biol. Plant 2018, 54, 87–93. [Google Scholar] [CrossRef]
- Wu, F.H.; Shen, S.C.; Lee, L.Y.; Lee, S.H.; Chan, M.T.; Lin, C.S. Tape-Arabidopsis Sandwich-a simpler Arabidopsis protoplast isolation method. Plant Methods 2009, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.M.; Yao, D.M.; Lin, F.; Jiang, M.Y. PEG-mediated transient gene expression and silencing system in maize mesophyll protoplasts: A valuable tool for signal transduction study in maize. Acta Physiol. Plant 2014, 36, 1271–1281. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, J.B.; Duan, S.; Ao, Y.; Dai, J.R.; Liu, J.; Wang, P.; Li, Y.G.; Liu, B.; Wang, H.B. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods 2011, 7, 30–38. [Google Scholar] [CrossRef]
- Wu, J.Z.; Liu, Q.; Geng, X.S.; Li, K.M.; Luo, L.J.; Liu, J.P. Highly efficient mesophyll protoplast isolation and PEG-mediated transient gene expression for rapid and large-scale gene characterization in cassava (Manihot esculenta Crantz). BMC Biotechnol. 2017, 17, 29. [Google Scholar] [CrossRef]
- Chen, S.; Tao, L.; Zeng, L.; Vega-Sanchez, M.E.; Umemura, K.; Wang, G.L. A highly efficient transient protoplast system for analyzing defence gene expression and protein–protein interactions in rice. Mol. Plant Pathol. 2006, 7, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Müller, B.; Sheen, J. Cytokinin and auxin interaction in root stem-cell specification during early embryogenesis. Nature 2008, 453, 1094–1097. [Google Scholar] [CrossRef]
- Zulkarnain, Z.; Tapingkae, T.; Taji, A. Applications of in vitro techniques in plant breeding. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Springer: Cham, Switzerland, 2015; pp. 293–328. [Google Scholar]
- Page, M.T.; Parry, M.A.; Carmo-Silva, E. A high-throughput transient expression system for rice. Plant Cell Environ. 2019. [Google Scholar] [CrossRef]
- Bart, R.; Chern, M.; Park, C.J.; Bartley, L.; Ronald, P.C. A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods 2006, 2, 13. [Google Scholar] [CrossRef]
- Tang, X.; Zheng, X.L.; Qi, Y.P.; Zhang, D.W.; Cheng, Y.; Tang, A.T.; Voytas, D.F.; Zhang, Y. A single transcript CRISPR-Cas9 system for efficient genome editing in plants. Mol. Plant 2016, 9, 1088–1091. [Google Scholar] [CrossRef]
- Lin, C.S.; Hsu, C.T.; Yang, L.H.; Lee, L.Y.; Fu, J.Y.; Cheng, Q.W.; Wu, F.H.; Hsiao, H.C.W.; Zhang, Y.S.; Zhang, R. Application of protoplast technology to CRISPR/Cas9 mutagenesis: From single-cell mutation detection to mutant plant regeneration. Plant Biotechnol. J. 2018, 16, 1295–1310. [Google Scholar] [CrossRef]
- Krens, F.A.; Molendijk, L.; Wullems, G.J.; Schilperoort, R.A. In vitro transformation of plant protoplasts with Ti-plasmid DNA. Nature 1982, 296, 72. [Google Scholar] [CrossRef]
- Hauptmann, R.M.; Ozias-Akins, P.; Vasil, V.; Tabaeizadeh, Z.; Rogers, S.G.; Horsch, R.B.; Vasil, I.K.; Fraley, R.T. Transient expression of electroporated DNA in monocot and dicotyledonous species. Plant Cell Rep. 1987, 6, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Hayashimoto, A.; Li, Z.; Murai, N. A polyethylene glycol-mediated protoplast transformation system for production of fertile transgenic rice plants. Plant Physiol. 1990, 93, 857–863. [Google Scholar] [CrossRef]
- Huang, H.Y.; Wang, Z.Y.; Cheng, J.T.; Zhao, W.C.; Li, X.; Wang, H.Y.; Zhang, Z.X.; Sui, X. An efficient cucumber (Cucumis sativus L.) protoplast isolation and transient expression system. Sci. Hortic. Amst. 2013, 150, 206–212. [Google Scholar] [CrossRef]
- Hong, S.Y.; Seo, P.J.; Cho, S.H.; Park, C.M. Preparation of leaf mesophyll protoplasts for transient gene expression in Brachypodium distachyon. J. Plant Biol. 2012, 55, 390–397. [Google Scholar] [CrossRef]
- Burris, K.P.; Dlugosz, E.M.; Collins, A.G.; Stewart, C.N.; Lenaghan, S.C. Development of a rapid, low-cost protoplast transfection system for switchgrass (Panicum virgatum L.). Plant Cell Rep. 2016, 35, 693–704. [Google Scholar] [CrossRef]
- Zhao, F.L.; Li, Y.J.; Hu, Y.; Gao, Y.R.; Zang, X.W.; Ding, Q.; Wang, Y.J.; Wen, Y.Q. A highly efficient grapevine mesophyll protoplast system for transient gene expression and the study of disease resistance proteins. Plant Cell Tissue Organ Cult. 2016, 125, 43–57. [Google Scholar] [CrossRef]
- Sheen, J. Signal transduction in maize and Arabidopsis mesophyll protoplasts. Plant Physiol. 2001, 127, 1466–1475. [Google Scholar] [CrossRef]
- Mathieu, Y.; Lapous, D.; Thomine, S.; Laurie´re, C.; Guern, J. Cytoplasmic acidification as an early phosphorylation-dependent response of tobacco cells to elicitors. Planta 1996, 199, 416–424. [Google Scholar] [CrossRef]
- Raikar, S.V.; Braun, R.H.; Bryant, C.; Conner, A.J.; Christey, M.C. Efficient isolation, culture and regeneration of Lotus corniculatus protoplasts. Plant Biotechnol. Rep. 2008, 2, 171. [Google Scholar] [CrossRef]
- Yao, L.P.; Liao, X.; Gan, Z.Z.; Peng, X.; Wang, P.; Li, S.J.; Li, T.H. Protoplast isolation and development of a transient expression system for sweet cherry (Prunus avium L.). Sci. Hortic. Amst. 2016, 209, 14–21. [Google Scholar] [CrossRef]
- Wehner, N.; Hartmann, L.; Ehlert, A.; Böttner, S.; Oñate-Sánchez, L.; Dröge-Laser, W. High-throughput protoplast transactivation (PTA) system for the analysis of Arabidopsis transcription factor function. Plant J. 2011, 68, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Schultz, E.A.; Pickett, F.B.; Haughn, G.W. The FLO10 gene product regulates the expression domain of homeotic genes AP3 and PI in Arabidopsis flowers. Plant Cell 1991, 3, 1221–1237. [Google Scholar] [CrossRef] [PubMed]
- Pelaz, S.; Ditta, G.S.; Baumann, E.; Wisman, E.; Yanofsky, M.F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 2000, 405, 200. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.C.; Ao, X.; Liu, G.X.; Cheng, F.Y.; He, C.Y. Duplication and whorl-specific down-regulation of the obligate AP3–PI heterodimer genes explain the origin of Paeonia lactiflora plants with spontaneous corolla mutation. Plant Cell Physiol. 2017, 58, 411–425. [Google Scholar]
- Huang, W.T.; Fang, Z.M.; Zeng, S.J.; Zhang, J.X.; Wu, K.L.; Chen, Z.L.; Teixeira da Silva, J.A.; Duan, J. Molecular cloning and functional analysis of three FLOWERING LOCUS T (FT) homologous genes from Chinese Cymbidium. Int. J. Mol. Sci. 2012, 13, 11385–11398. [Google Scholar] [CrossRef]
- Mondragón Palomino, M. Perspectives on MADS-box expression during orchid flower evolution and development. Front. Plant Sci. 2013, 4, 377. [Google Scholar] [CrossRef]
- Wang, H.M.; Tong, C.G.; Jang, S. Current progress in orchid flowering/flower development research. Plant Signal. Behav. 2017, 12, e1322245. [Google Scholar] [CrossRef]
- Yang, F.X.; Zhu, G.F.; Wei, Y.L.; Gao, J.; Liang, G.; Peng, L.Y.; Lu, C.Q.; Jin, J.P. Low-temperature-induced changes in the transcriptome reveal a major role of CgSVP genes in regulating flowering of Cymbidium goeringii. BMC Genom. 2019, 20, 53. [Google Scholar] [CrossRef]
- Negrutiu, I.; de Brouwer, D.; Watts, J.W.; Sidorov, V.I.; Dirks, R.; Jacobs, M. Fusion of plant protoplasts: A study using auxotrophic mutants of Nicotiana plumbaginifolia, Viviani. Theor. Appl. Genet. 1986, 72, 279–286. [Google Scholar] [CrossRef]
- Negrutiu, I.; Shillito, R.; Potrykus, I.; Biasini, G.; Sala, F. Hybrid genes in the analysis of transformation conditions. Plant Mol. Biol. 1987, 8, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Rui, R.; Liu, S.C.; Karthikeyan, A.; Wang, T.; Niu, H.P.; Yin, J.L.; Yang, Y.H.; Wang, L.Q.; Yang, Q.H.; Zhi, H.J. Fine-mapping and identification of a novel locus Rsc15 underlying soybean resistance to Soybean mosaic virus. Theor. Appl. Genet. 2017, 130, 2395–2410. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).