Influence of Brewing Methods on the Bioactive and Mineral Composition of Coffee Beverages
Abstract
1. Introduction
2. Results
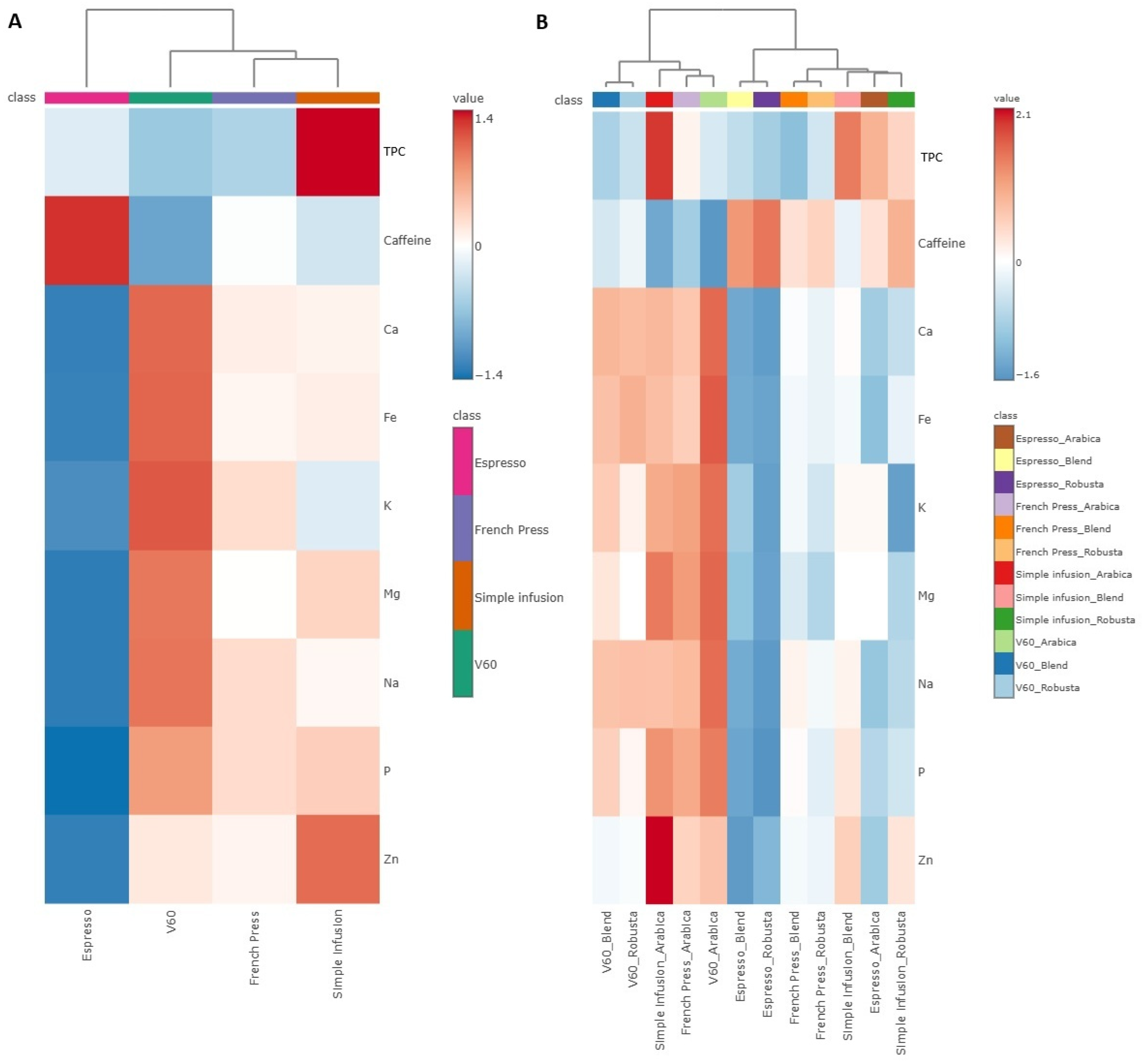
2.1. Caffeine, TPC, and Mineral Content Across Brewing Methods
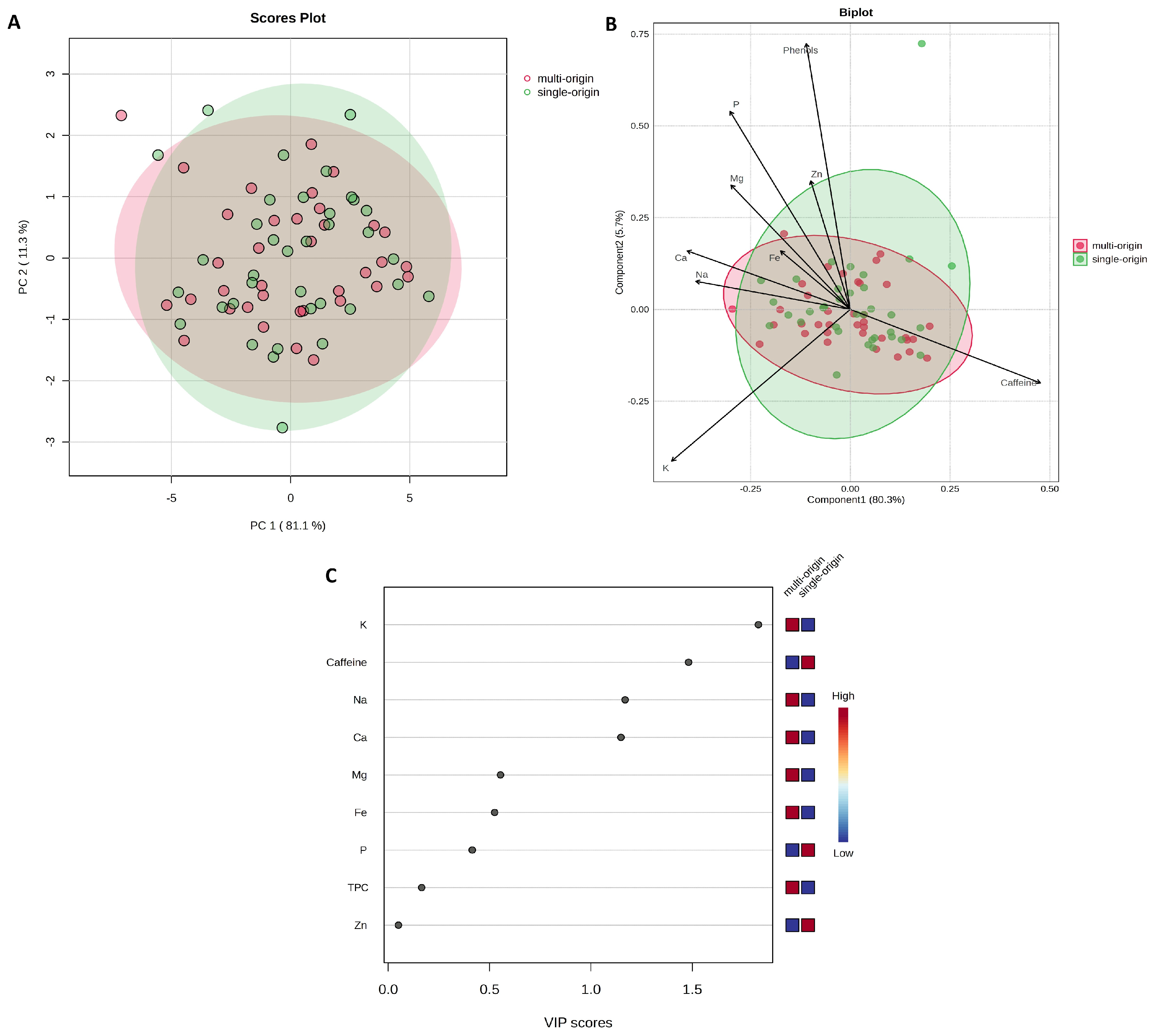
2.2. Content of Caffeine, Total Polyphenols, and Mineral Content Across Coffee Species
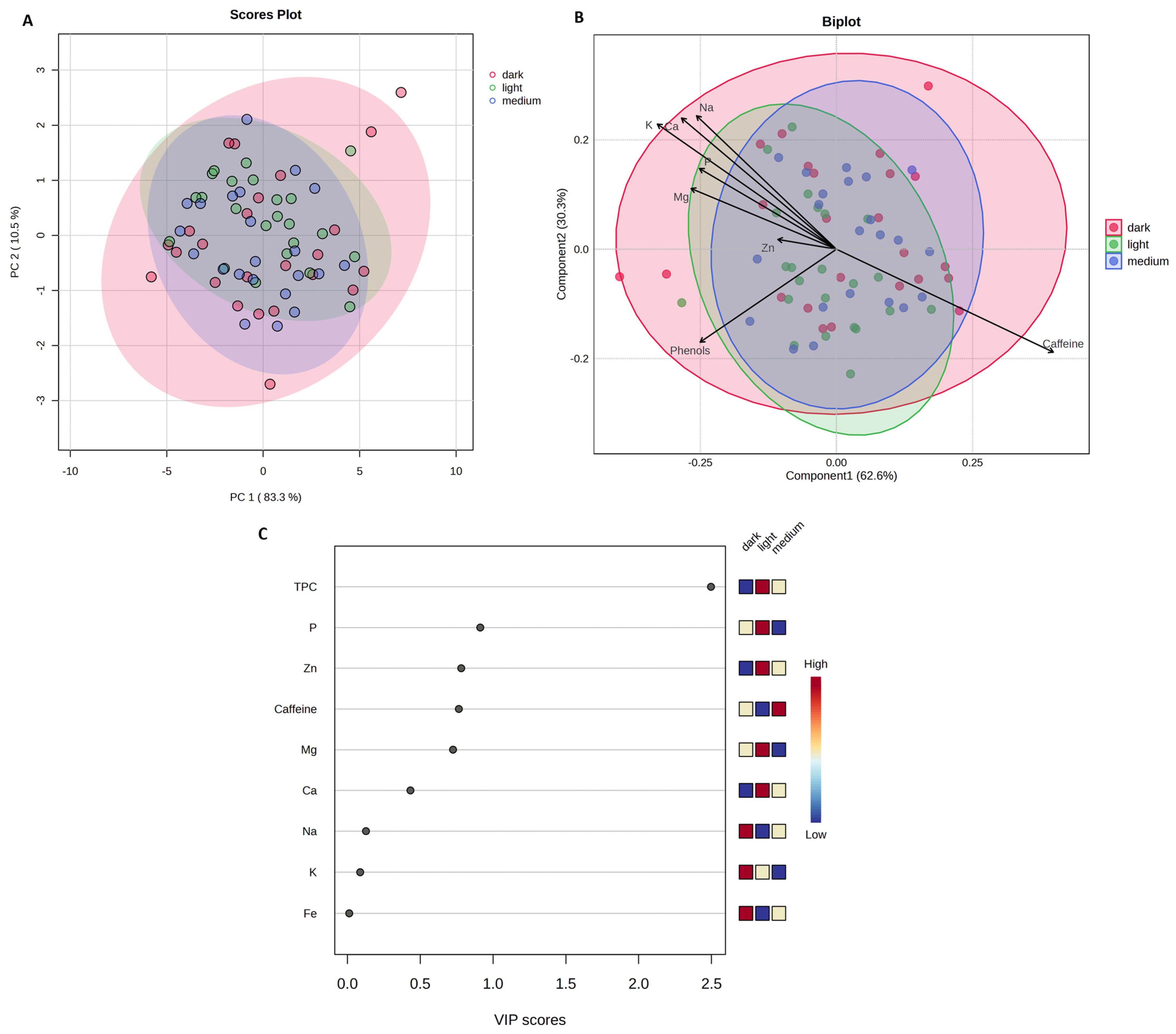
2.3. Content of Caffeine, Total Polyphenols, and Mineral Content Across Degree of Roasting and Origin
2.4. Brewing Method and Coffee Species as Factors for Differentiating the Chemical Profile of Coffees
2.5. Chemical Composition of a Portion of Infused Coffee Depending on Brewing Method and the Percentage of the Daily Requirement Covered
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Brewing Methods
4.3. Assessment of Physicochemical Parameters
4.4. Determination of Caffeine Content
4.5. Determination of TPC
4.6. Determination of Mineral Content
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrea, L.; Pugliese, G.; Frias-Toral, E.; El Ghoch, M.; Castellucci, B.; Chapela, S.P.; Carignano, M.D.L.A.; Laudisio, D.; Savastano, S.; Colao, A.; et al. Coffee Consumption, Health Benefits and Side Effects: A Narrative Review and Update for Dietitians and Nutritionists. Crit. Rev. Food Sci. Nutr. 2023, 63, 1238–1261. [Google Scholar] [CrossRef]
- Haghighatdoost, F.; Hajihashemi, P.; De Sousa Romeiro, A.M.; Mohammadifard, N.; Sarrafzadegan, N.; De Oliveira, C.; Silveira, E.A. Coffee Consumption and Risk of Hypertension in Adults: Systematic Review and Meta-Analysis. Nutrients 2023, 15, 3060. [Google Scholar] [CrossRef]
- Safe, S.; Kothari, J.; Hailemariam, A.; Upadhyay, S.; Davidson, L.A.; Chapkin, R.S. Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 2706. [Google Scholar] [CrossRef]
- Farraj, A.; Akeredolu, T.; Wijeyesekera, A.; Mills, C. Coffee and Cardiovascular Health: A Review of Literature. Nutrients 2024, 16, 4257. [Google Scholar] [CrossRef]
- Karagöz, M.F.; Koçyiğit, E.; Koçak, T.; Özturan Şirin, A.; Icer, M.A.; Ağagündüz, D.; Coreta-Gomes, F. Decoding Coffee Cardiometabolic Potential: Chemical Composition, Nutritional, and Health Relationships. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13414. [Google Scholar] [CrossRef]
- Lopes, C.R.; Cunha, R.A. Impact of Coffee Intake on Human Aging: Epidemiology and Cellular Mechanisms. Ageing Res. Rev. 2024, 102, 102581. [Google Scholar] [CrossRef] [PubMed]
- Wierzejska, R.E.; Gielecińska, I.; Hallmann, E.; Wojda, B. Polyphenols vs. Caffeine in Coffee from Franchise Coffee Shops: Which Serving of Coffee Provides the Optimal Amount of This Compounds to the Body. Molecules 2024, 29, 2231. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N. Chlorogenic Acids and Other Cinnamates—Nature, Occurrence, Dietary Burden, Absorption and Metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Liczbiński, P.; Bukowska, B. Tea and Coffee Polyphenols and Their Biological Properties Based on the Latest in Vitro Investigations. Ind. Crops Prod. 2022, 175, 114265. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Socha, K.; Zujko, M.E. Coffee Brews: Are They a Source of Macroelements in Human Nutrition? Foods 2021, 10, 1328. [Google Scholar] [CrossRef]
- Olechno, E.; Puścion-Jakubik, A.; Socha, K.; Zujko, M.E. Coffee Infusions: Can They Be a Source of Microelements with Antioxidant Properties? Antioxidants 2021, 10, 1709. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Clifford, M.N.; Lean, M.E.J.; Ashihara, H.; Crozier, A. Coffee: Biochemistry and Potential Impact on Health. Food Funct. 2014, 5, 1695–1717. [Google Scholar] [CrossRef]
- Król, K.; Gantner, M.; Tatarak, A.; Hallmann, E. The Content of Polyphenols in Coffee Beans as Roasting, Origin and Storage Effect. Eur. Food Res. Technol. 2020, 246, 33–39. [Google Scholar] [CrossRef]
- Laukalēja, I.; Krūma, Z.; Cinkmanis, I. Impact of The Roast Level on Chemical Composition of Coffee from Colombia. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2022, 76, 145–151. [Google Scholar] [CrossRef]
- Espitia-López, J.; Rogelio-Flores, F.; Angel-Cuapio, A.; Flores-Chávez, B.; Arce-Cervantes, O.; Hernández-León, S.; Garza-López, P.M. Characterization of Sensory Profile by the CATA Method of Mexican Coffee Brew Considering Two Preparation Methods: Espresso and French Press. Int. J. Food Prop. 2019, 22, 967–973. [Google Scholar] [CrossRef]
- Stanek, N.; Zarębska, M.; Biłos, Ł.; Barabosz, K.; Nowakowska-Bogdan, E.; Semeniuk, I.; Błaszkiewicz, J.; Kulesza, R.; Matejuk, R.; Szkutnik, K. Influence of Coffee Brewing Methods on the Chromatographic and Spectroscopic Profiles, Antioxidant and Sensory Properties. Sci. Rep. 2021, 11, 21377. [Google Scholar] [CrossRef]
- Santanatoglia, A.; Caprioli, G.; Cespi, M.; Ciarlantini, D.; Cognigni, L.; Fioretti, L.; Maggi, F.; Mustafa, A.M.; Nzekoue, F.; Vittori, S. A Comprehensive Comparative Study among the Newly Developed Pure Brew Method and Classical Ones for Filter Coffee Production. LWT 2023, 175, 114471. [Google Scholar] [CrossRef]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef]
- Wang, X.; Lim, L.T. Effects of Grind Size, Temperature, and Brewing Ratio on Immersion Cold Brewed and French Press Hot Brewed Coffees. Appl. Food Res. 2023, 3, 100334. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Dietary Reference Values for Nutrients Summary Report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Sagratini, G.; Vittori, S. The Influence of Different Types of Preparation (Espresso and Brew) on Coffee Aroma and Main Bioactive Constituents. Int. J. Food Sci. Nutr. 2015, 66, 505–513. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Bellumori, M.; Daluiso, S.; Parenti, A.; Innocenti, M. What Kind of Coffee Do You Drink? An Investigation on Effects of Eight Different Extraction Methods. Food Res. Int. 2019, 116, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Macrae, R. (Eds.) Coffee; Springer: Dordrecht, The Netherlands, 1985. [Google Scholar] [CrossRef]
- Caprioli, G.; Cortese, M.; Maggi, F.; Minnetti, C.; Odello, L.; Sagratini, G.; Vittori, S. Quantification of Caffeine, Trigonelline and Nicotinic Acid in Espresso Coffee: The Influence of Espresso Machines and Coffee Cultivars. Int. J. Food Sci. Nutr. 2014, 65, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Ciaramelli, C.; Palmioli, A.; Airoldi, C. Coffee Variety, Origin and Extraction Procedure: Implications for Coffee Beneficial Effects on Human Health. Food Chem. 2019, 278, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Tfouni, S.A.V.; Carreiro, L.B.; Teles, C.R.A.; Furlani, R.P.Z.; Cipolli, K.M.V.A.B.; Camargo, M.C.R. Caffeine and Chlorogenic Acids Intake from Coffee Brew: Influence of Roasting Degree and Brewing Procedure. Int. J. Food Sci. Technol. 2014, 49, 747–752. [Google Scholar] [CrossRef]
- Mehaya, F.M.; Mohammad, A.A. Thermostability of Bioactive Compounds during Roasting Process of Coffee Beans. Heliyon 2020, 6, e05508. [Google Scholar] [CrossRef]
- Alamri, E.; Rozan, M.; Bayomy, H. A Study of Chemical Composition, Antioxidants, and Volatile Compounds in Roasted Arabic Coffee. Saudi J. Biol. Sci. 2022, 29, 3133–3139. [Google Scholar] [CrossRef]
- Cwiková, O.; Komprda, T.; Šottníková, V.; Svoboda, Z.; Simonová, J.; Slováček, J.; Jůzl, M. Effects of Different Processing Methods of Coffee Arabica on Colour, Acrylamide, Caffeine, Chlorogenic Acid, and Polyphenol Content. Foods 2022, 11, 3295. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.J.; Crozier, A. Variations in Caffeine and Chlorogenic Acid Contents of Coffees: What Are We Drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef]
- Górecki, M.; Hallmann, E. The Antioxidant Content of Coffee and Its In Vitro Activity as an Effect of Its Production Method and Roasting and Brewing Time. Antioxidants 2020, 9, 308. [Google Scholar] [CrossRef]
- Grządka, E.; Starek-Wójcicka, A.; Krajewska, M.; Matusiak, J.; Orzeł, J.; Studziński, M.; Bonczyk, M.; Chmielewska, I.; Mieczkowska, A.; Ronda, O.; et al. Chemical Insight into Pros and Cons of Coffees from Different Regions. Sci. Rep. 2025, 15, 455. [Google Scholar] [CrossRef] [PubMed]
- Olechno, E.; Puścion-Jakubik, A.; Zujko, M.E.; Socha, K. Influence of Various Factors on Caffeine Content in Coffee Brews. Foods 2021, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, I.A.; Sanchez, L.; Caemmerer, B.; Kroh, L.W.; De Peña, M.P.; Cid, C. Extraction of Coffee Antioxidants: Impact of Brewing Time and Method. Food Res. Int. 2012, 48, 57–64. [Google Scholar] [CrossRef]
- Derossi, A.; Ricci, I.; Caporizzi, R.; Fiore, A.; Severini, C. How Grinding Level and Brewing Method (Espresso, American, Turkish) Could Affect the Antioxidant Activity and Bioactive Compounds in a Coffee Cup. J. Sci. Food Agric. 2018, 98, 3198–3207. [Google Scholar] [CrossRef]
- Caporaso, N.; Genovese, A.; Canela, M.D.; Civitella, A.; Sacchi, R. Neapolitan Coffee Brew Chemical Analysis in Comparison to Espresso, Moka and American Brews. Food Res. Int. 2014, 61, 152–160. [Google Scholar] [CrossRef]
- Chavez, S.G.; Mendoza, M.M.; Caetano, A.C. Antioxidants, Phenols, Caffeine Content and Volatile Compounds in Coffee Beverages Obtained by Different Methods. Food Sci. Technol. 2022, 42. [Google Scholar] [CrossRef]
- Fărcaş, A.C.; Socaci, S.A.; Bocăniciu, I.; Pop, A.; Tofană, M.; Muste, S.; Feier, D. Evaluation of Biofunctional Compounds Content from Brewed Coffee. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2014, 71, 114–118. [Google Scholar] [CrossRef]
- Hečimović, I.; Belščak-Cvitanović, A.; Horžić, D.; Komes, D. Comparative Study of Polyphenols and Caffeine in Different Coffee Varieties Affected by the Degree of Roasting. Food Chem. 2011, 129, 991–1000. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic Compounds in Coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Pan, L.; Xiao, Y.; Jiang, F.; Jiang, T.; Zhu, J.; Tang, W.; Liu, X.; Zhou, Y.; Yu, L. Comparison of Characterization of Cold Brew and Hot Brew Coffee Prepared at Various Roasting Degrees. J. Food Process. Preserv. 2023, 2023, 1–15. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. Chemical Characterization and Antioxidant Properties of Coffee Melanoidins. J. Agric. Food Chem. 2002, 50, 6527–6533. [Google Scholar] [CrossRef] [PubMed]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee Extraction: A Review of Parameters and Their Influence on the Physicochemical Characteristics and Flavour of Coffee Brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Vandeponseele, A.; Draye, M.; Piot, C.; Chatel, G. Study of Influential Parameters of the Caffeine Extraction from Spent Coffee Grounds: From Brewing Coffee Method to the Waste Treatment Conditions. Clean Technol. 2021, 3, 335–350. [Google Scholar] [CrossRef]
- Moroney, K.M.; Lee, W.T.; O׳Brien, S.B.G.; Suijver, F.; Marra, J. Modelling of Coffee Extraction during Brewing Using Multiscale Methods: An Experimentally Validated Model. Chem. Eng. Sci. 2015, 137, 216–234. [Google Scholar] [CrossRef]
- Stelmach, E.; Pohl, P.; Szymczycha-Madeja, A. The Suitability of the Simplified Method of the Analysis of Coffee Infusions on the Content of Ca, Cu, Fe, Mg, Mn and Zn and the Study of the Effect of Preparation Conditions on the Leachability of Elements into the Coffee Brew. Food Chem. 2013, 141, 1956–1961. [Google Scholar] [CrossRef]
- Janda, K.; Jakubczyk, K.; Baranowska-Bosiacka, I.; Kapczuk, P.; Kochman, J.; Rębacz-Maron, E.; Gutowska, I. Mineral Composition and Antioxidant Potential of Coffee Beverages Depending on the Brewing Method. Foods 2020, 9, 121. [Google Scholar] [CrossRef]
- Özdestan, Ö. Evaluation of Bioactive Amine and Mineral Levels in Turkish Coffee. Food Res. Int. 2014, 61, 167–175. [Google Scholar] [CrossRef]
- Gogoaşă, I.; Sipos, L.; Negrea, A.; Alda, L.M.; Costescu, C.; Rada, M.; Velimirovici, D.; Draghici, G.A.; Ostan, M.; Bordean, D.M. Study Regarding Coffee Brew Metal Content. In Proceedings of the 22nd International Symposium on Analytical and Environmental Problems, Szeged, Hungary, 10 October 2016; Available online: https://acta.bibl.u-szeged.hu/55893/1/proceedings_of_isaep_2016.pdf (accessed on 18 August 2025).
- Ashu, R.; Singh Chandravanshi, B. Concentration Levels of Metals in Commercially Available Ethiopian Roasted Coffee Powders and Their Infusions. Bull. Chem. Soc. Ethiop. 2011, 25, 11–24. [Google Scholar] [CrossRef]
- Sano, Y.; Kubota, S.; Kawarazaki, A.; Kawamura, K.; Kashiwai, H.; Kuwahara, F. Mathematical Model for Coffee Extraction Based on the Volume Averaging Theory. J. Food Eng. 2019, 263, 1–12. [Google Scholar] [CrossRef]
- Jaganyi, D.; Madlala, S.P. Kinetics of Coffee Infusion: A Comparative Study on the Extraction Kinetics of Mineral Ions and Caffeine from Several Types of Medium Roasted Coffees. J. Sci. Food. Agric. 2000, 80, 85–90. [Google Scholar] [CrossRef]
- Spiro, M.; Selwood, R.M. The Kinetics and Mechanism of Caffeine Infusion from Coffee: The Effect of Particle Size. J. Sci. Food Agric. 1984, 35, 915–924. [Google Scholar] [CrossRef]
- Spiro, M.; Toumi, R.; Kandiah, M. The Kinetics and Mechanism of Caffeine Infusion from Coffee: The Hindrance Factor in Intra-bean Diffusion. J. Sci. Food Agric. 1989, 46, 349–356. [Google Scholar] [CrossRef]
- Grembecka, M.; Malinowska, E.; Szefer, P. Differentiation of Market Coffee and Its Infusions in View of Their Mineral Composition. Sci. Total Environ. 2007, 383, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Fabrícia, Q.M.; Marcelo, R.R.; Flavia, R.P.; Andre, M.X.D.C.; Katia, R.D.O.R.; Frederico, G.P. Determination of Heavy Metals in the Roasted and Ground Coffee Beans and Brew. Afr. J. Agric. Res. 2017, 12, 221–228. [Google Scholar] [CrossRef]
- Martín, M.J.; Pablos, F.; González, A.G. Characterization of Green Coffee Varieties According to Their Metal Content. Anal. Chim. Acta 1998, 358, 177–183. [Google Scholar] [CrossRef]
- Dippong, T.; Dan, M.; Kovacs, M.H.; Kovacs, E.D.; Levei, E.A.; Cadar, O. Analysis of Volatile Compounds, Composition, and Thermal Behavior of Coffee Beans According to Variety and Roasting Intensity. Foods 2022, 11, 3146. [Google Scholar] [CrossRef]
- Laviola, B.G.; Martinez, H.E.P.; Salomão, L.C.C.; Cruz, C.D.; Mendonça, S.M. Nutrient accumulation in coffee fruits at four plantations altitude: Calcium, magnesium and sulfur. Rev. Bras. Ciênc. Solo 2007, 31, 1451–1462. [Google Scholar] [CrossRef]
- Tagliaferro, F.S.; De Nadai Fernandes, E.A.; Bacchi, M.A.; Bode, P.; Joacir De França, E. Can Impurities from Soil-Contaminated Coffees Reach the Cup? J. Radioanal. Nucl. Chem. 2007, 271, 371–375. [Google Scholar] [CrossRef]
- Angeloni, G.; Guerrini, L.; Masella, P.; Innocenti, M.; Bellumori, M.; Parenti, A. Characterization and Comparison of Cold Brew and Cold Drip Coffee Extraction Methods. J. Sci. Food Agric. 2019, 99, 391–399. [Google Scholar] [CrossRef]
- Almeida, F.S.; Dias, F.F.G.; Ford, M.W.; Bogusz Junior, S.; Sato, A.C.K.; De Moura Bell, J.M.L.N. Exploring the Nutritional and Biological Properties of Green Coffee Extracts: A Comparative Study of Aqueous and Enzymatic Extraction Processes. Curr. Res. Food Sci. 2024, 9, 100890. [Google Scholar] [CrossRef]
- Bratthäll, T.; Figueira, J.; Nording, M.L. Influence of Divalent Cations on the Extraction of Organic Acids in Coffee Determined by GC-MS and NMR. Heliyon 2024, 10, e26625. [Google Scholar] [CrossRef]
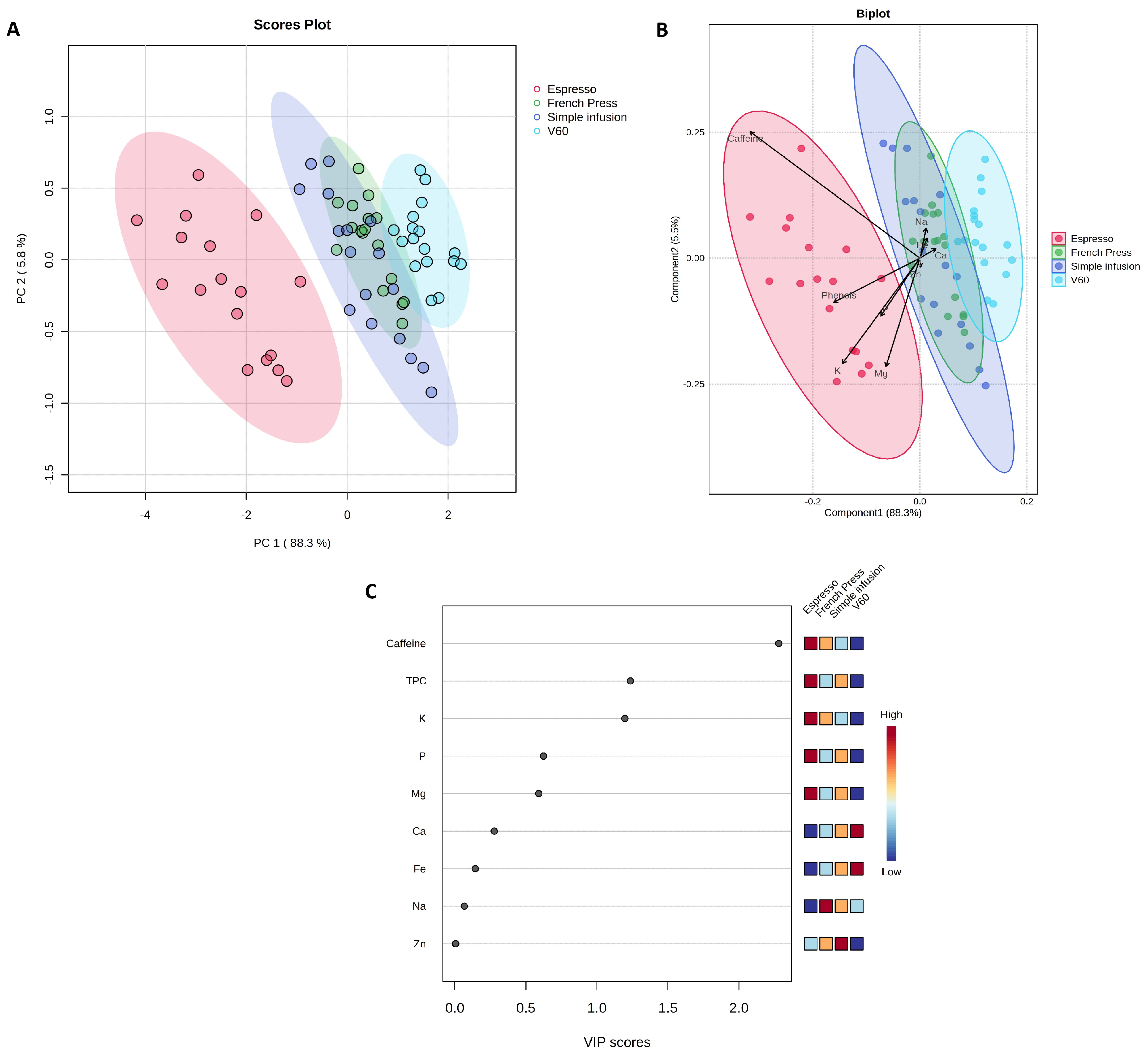
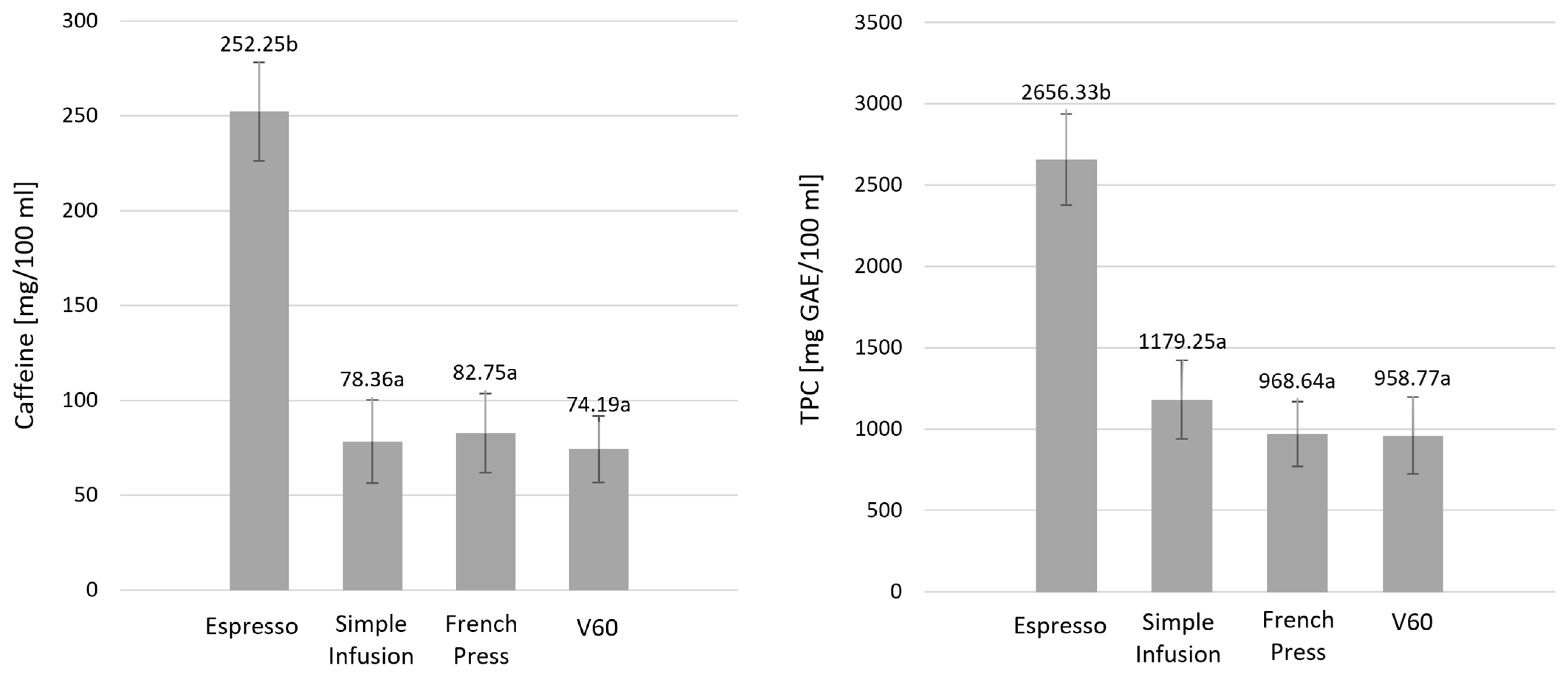

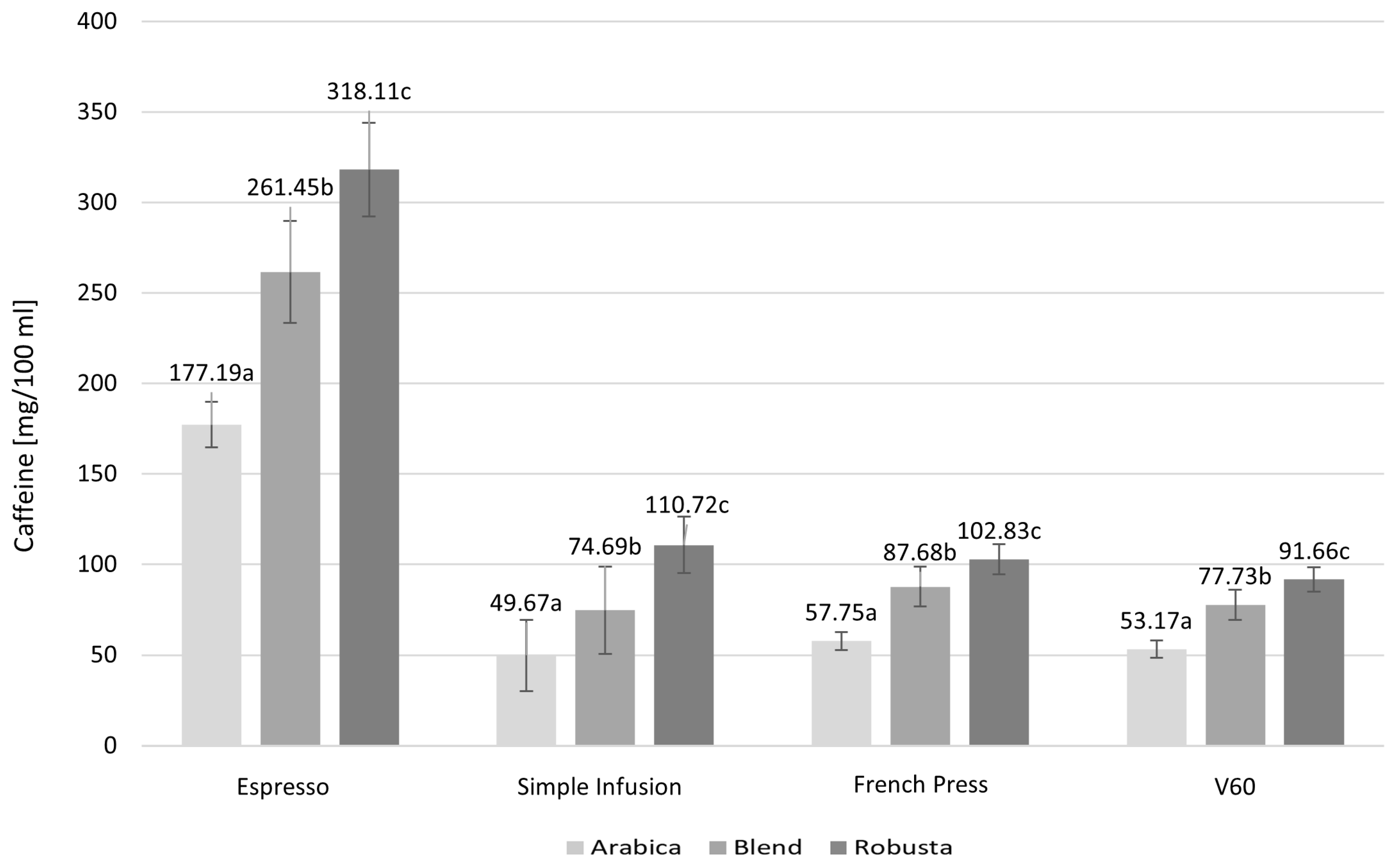
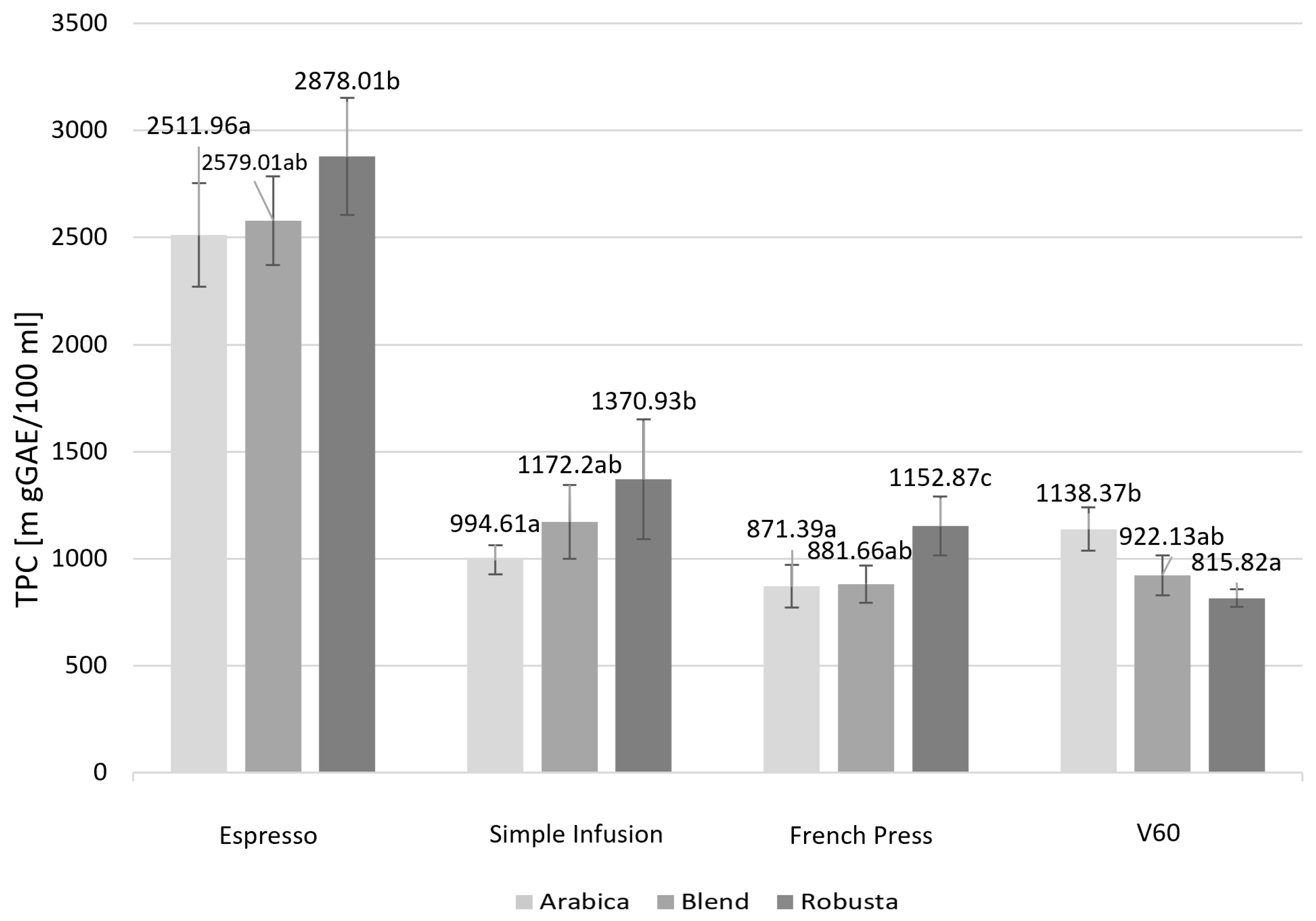

| Parameter [mg/100 mL] | Espresso | Simple Infusion | French Press | V60 | p Value |
|---|---|---|---|---|---|
| Ca | 4.35 ± 0.48 b | 3.76 ± 0.73 a | 4.22 ± 0.67 ab | 6.53 ± 0.75 c | <0.001 |
| Fe | 0.16 ± 0.02 a | 0.15 ± 0.05 a | 0.16 ± 0.03 a | 0.27 ± 0.05 b | <0.001 * |
| K | 37.63 ± 3.44 d | 15.43 ± 2.91 a | 18.53 ± 2.25 ab | 21.62 ± 1.27 bc | <0.001 * |
| Mg | 4.21 ± 0.55 c | 2.24 ± 0.51 a | 2.27 ± 0.42 a | 2.83 ± 0.41 b | <0.001 |
| Na | 4.48 ± 0.54 b | 3.60 ± 0.73 a | 4.34 ± 0.43 b | 5.97 ± 0.63 c | <0.001 * |
| P | 5.67 ± 0.54 c | 3.43 ± 0.65 a | 3.67 ± 0.30 ab | 4.18 ± 0.35 b | <0.001 * |
| Zn | 0.04 ± 0.01 a | 0.06 ± 0.03 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.0604 * |
| Parameter [mg/100 mL] | Arabica | Blend | Robusta | p Value | |
|---|---|---|---|---|---|
| Espresso | Ca | 4.65 ± 0.50 a | 4.16 ± 0.31 a | 4.24 ± 0.53 a | 0.1653 |
| Fe | 0.15 ± 0.03 a | 0.15 ± 0.01 a | 0.17 ± 0.03 a | 0.4199 | |
| K | 37.89 ± 2.55 a | 36.98 ± 1.63 a | 38.10 ± 5.92 a | 0.8590 | |
| Mg | 4.79 ± 0.34 b | 4.03 ± 0.29 a | 3.82 ± 0.44 a | 0.0008 | |
| Na | 4.43 ± 0.60 a | 4.54 ± 0.59 a | 4.45 ± 0.53 a | 0.8304 * | |
| P | 5.56 ± 0.51 a | 5.53 ± 0.41 a | 5.92 ± 0.66 a | 0.4004 | |
| Zn | 0.04 ± 0.01 b | 0.03 ± 0.00 a | 0.04 ± 0.01 b | 0.0031 * | |
| Simple infusion | Ca | 4.22 ± 0.48 a | 3.63 ± 0.54 a | 3.43 ± 0.94 a | 0.1427 * |
| Fe | 0.16 ± 0.07 a | 0.13 ± 0.05 a | 0.17 ± 0.05 a | 0.4049 * | |
| K | 16.32 ± 2.38 a | 16.47 ± 2.06 a | 13.51 ± 3.48 a | 0.1372 | |
| Mg | 2.61 ± 0.37 a | 2.06 ± 0.38 a | 2.05 ± 0.61 a | 0.0907 | |
| Na | 3.81 ± 0.32 a | 3.78 ± 0.82 a | 3.23 ± 0.87 a | 0.3137 | |
| P | 3.47 ± 0.45 a | 3.24 ± 0.38 a | 3.55 ± 0.99 a | 0.7441 | |
| Zn | 0.07 ± 0.05 a | 0.05 ± 0.02 a | 0.05 ± 0.02 a | 0.5539 * | |
| French Press | Ca | 4.51 ± 0.64 a | 3.95 ± 0.25 a | 4.21 ± 0.93 a | 0.3734 |
| Fe | 0.17 ± 0.03 a | 0.15 ± 0.02 a | 0.16 ± 0.03 a | 0.3310 | |
| K | 19.21 ± 0.91 a | 17.72 ± 1.91 a | 18.67 ± 3.37 a | 0.2621 * | |
| Mg | 2.76 ± 0.24 b | 2.03 ± 0.09 a | 2.01 ± 0.33 a | 0.0001 | |
| Na | 4.54 ± 0.50 a | 4.23 ± 0.33 a | 4.22 ± 0.43 a | 0.3802 | |
| P | 3.75 ± 0.18 a | 3.56 ± 0.26 a | 3.70 ± 0.42 a | 0.3857 * | |
| Zn | 0.04 ± 0.02 a | 0.04 ± 0.01 a | 0.04 ± 0.01 a | 0.9011 | |
| V60 | Ca | 6.74 ± 0.71 a | 6.10 ± 0.94 a | 6.74 ± 0.47 a | 0.2469 |
| Fe | 0.28 ± 0.06 ab | 0.23 ± 0.02 a | 0.29 ± 0.02 b | 0.0159 * | |
| K | 21.90 ± 1.34 | 21.30 ± 1.17 | 21.65 ± 1.44 | 0.6300 * | |
| Mg | 3.24 ± 0.30 b | 2.60 ± 0.32 a | 2.67 ± 0.31 a | 0.0049 | |
| Na | 6.21 ± 0.70 a | 5.42 ± 0.18 a | 6.29 ± 0.51 a | 0.0519 * | |
| P | 4.30 ± 0.42 a | 4.12 ± 0.23 a | 4.12 ± 0.38 a | 0.5808 | |
| Zn | 0.04 ± 0.01 a | 0.03 ± 0.01 a | 0.04 ± 0.01 a | 0.1794 |
| Parameter (mg per Serving) | Espresso | Simple Infusion | French Press | V60 |
|---|---|---|---|---|
| Caffeine | 63.06 | 195.90 | 206.88 | 185.48 |
| TPC | 664.08 | 2948.13 | 2421.60 | 2396.93 |
| Ca | 1.09 | 9.40 | 10.55 | 16.33 |
| Mg | 1.05 | 5.60 | 5.68 | 7.08 |
| K | 9.41 | 38.58 | 46.33 | 54.05 |
| Na | 1.12 | 9.00 | 10.85 | 14.93 |
| P | 1.42 | 8.58 | 9.18 | 10.45 |
| Fe | 0.04 | 0.38 | 0.40 | 0.68 |
| Zn | 0.01 | 0.15 | 0.10 | 0.10 |
| Parameter (mg per Serving) | EFSA Recommendation | Espresso [% RDI] | Simple Infusion [% RDI] | French Press [% RDI] | V60 [% RDI] |
|---|---|---|---|---|---|
| Caffeine | 400 | 15.77 | 48.98 | 51.72 | 46.37 |
| Ca | 1000 (18–24 years); 950 (≥25 years) | 0.11 * | 0.99 * | 1.11 * | 1.72 * |
| Mg | M: 350 F: 300 | 0.30 0.35 | 1.60 1.87 | 1.62 1.89 | 2.02 2.36 |
| K | 3500 | 0.27 | 1.10 | 1.32 | 1.54 |
| Na | 2000 | 0.06 | 0.45 | 0.54 | 0.75 |
| P | 550 | 0.26 | 1.56 | 1.67 | 1.90 |
| Fe | M: 11 F: 16 (premenopausal); 11 (postmenopausal) | M: 0.36 F: 0.25 | M: 3.45 F: 2.38 | M: 3.64 F: 2.50 | M: 6.18 F: 4.25 |
| Zn | M: 9.4–16.3 F: 7.5–12.7 | M: 0.11 F: 0.13 | M: 1.60 F: 2.00 | M: 1.06 F: 1.33 | M: 1.06 F: 1.33 |
| Brewing Methods | Espresso | Simple Infusion | French Press | V60 |
|---|---|---|---|---|
| pH | 5.48 ± 0.22 | 5.84 ± 0.22 | 5.84 ± 0.23 | 5.78 ± 0.26 |
| TDS (%) | 3.65 ± 0.48 | 1.40 ± 0.13 | 1.35 ± 0.24 | 1.18 ± 0.10 |
| Extraction yield (%) | 11.65 ± 1.49 | 18.97 ± 1.78 | 18.88 ± 2.83 | 16.11 ± 1.26 |
| Brew Mass (g) | 22.43 ± 1.43 | 192.11 ± 4.69 | 221.61 ± 2.52 | 204.26 ± 2.99 |
| Brew volume (mL) | 24.47 ± 1.42 | 204.10 ± 5.32 | 214.93 ± 2.43 | 208.17 ± 3.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sijko-Szpańska, M.; Mystkowska, I.; Dmitrowicz, A. Influence of Brewing Methods on the Bioactive and Mineral Composition of Coffee Beverages. Molecules 2025, 30, 4080. https://doi.org/10.3390/molecules30204080
Sijko-Szpańska M, Mystkowska I, Dmitrowicz A. Influence of Brewing Methods on the Bioactive and Mineral Composition of Coffee Beverages. Molecules. 2025; 30(20):4080. https://doi.org/10.3390/molecules30204080
Chicago/Turabian StyleSijko-Szpańska, Monika, Iwona Mystkowska, and Aleksandra Dmitrowicz. 2025. "Influence of Brewing Methods on the Bioactive and Mineral Composition of Coffee Beverages" Molecules 30, no. 20: 4080. https://doi.org/10.3390/molecules30204080
APA StyleSijko-Szpańska, M., Mystkowska, I., & Dmitrowicz, A. (2025). Influence of Brewing Methods on the Bioactive and Mineral Composition of Coffee Beverages. Molecules, 30(20), 4080. https://doi.org/10.3390/molecules30204080






