Recent Advances in Heterogeneous Hydroformylation at Metal–Oxide Interfaces
Abstract
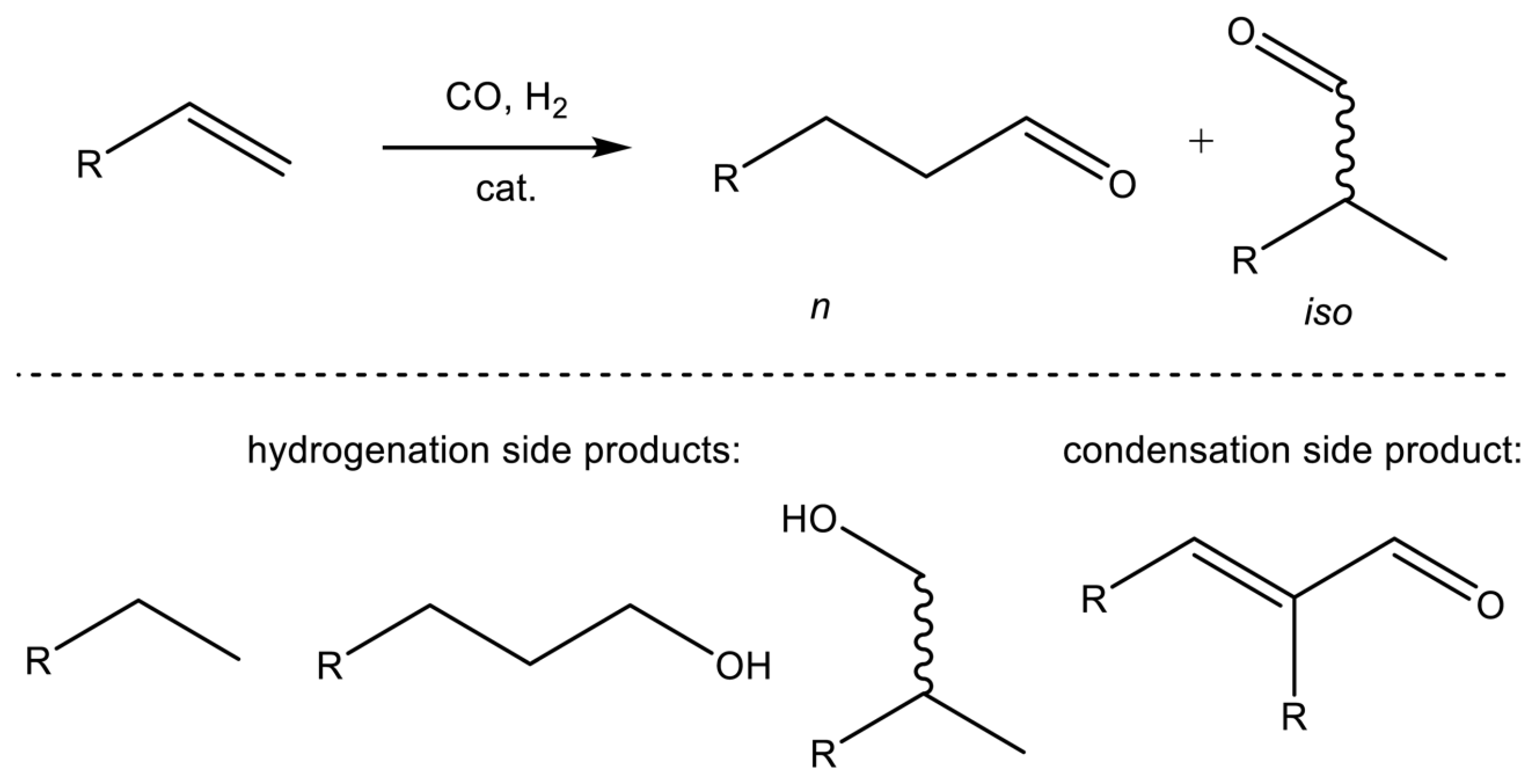
1. Introduction
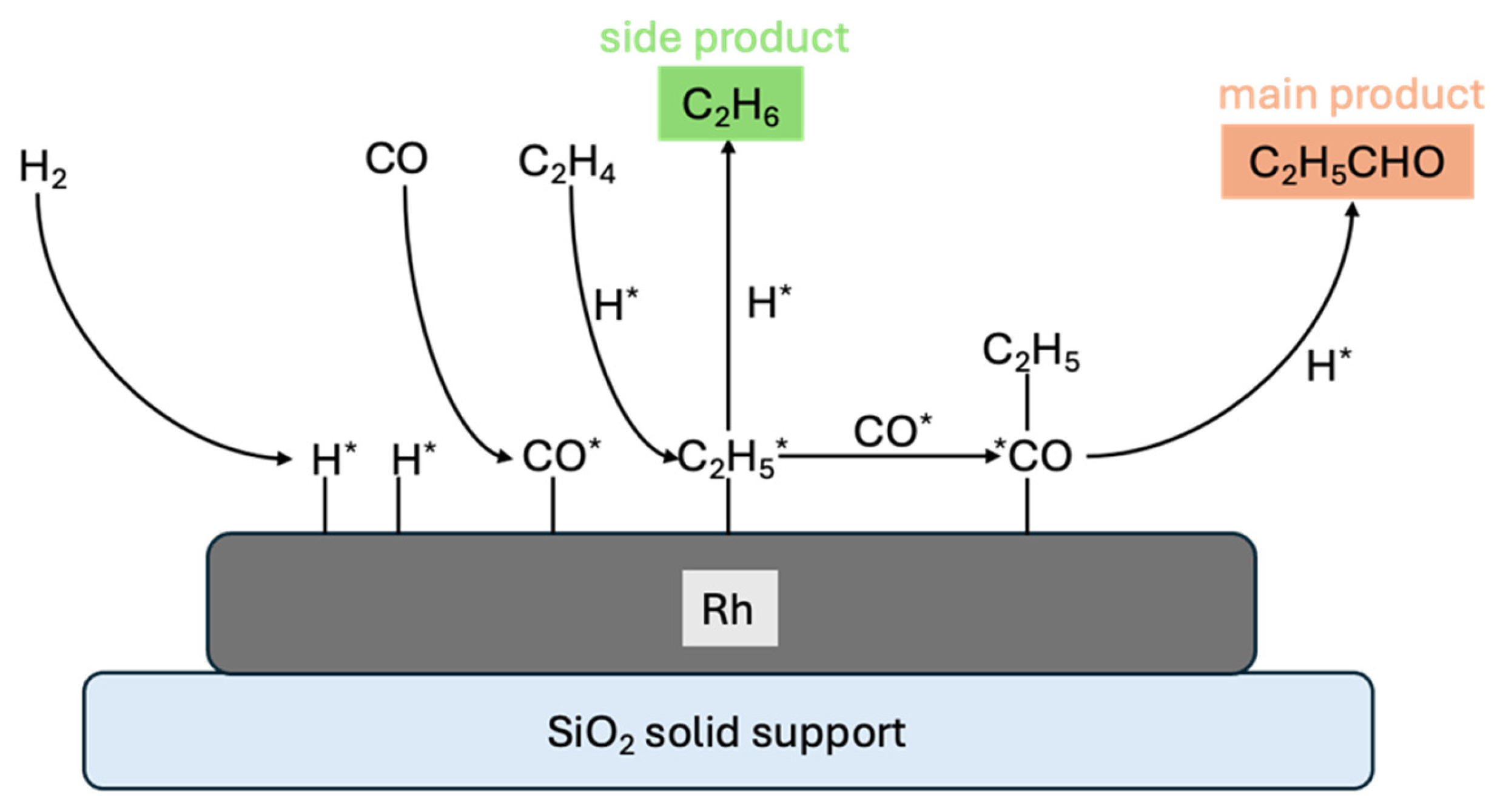
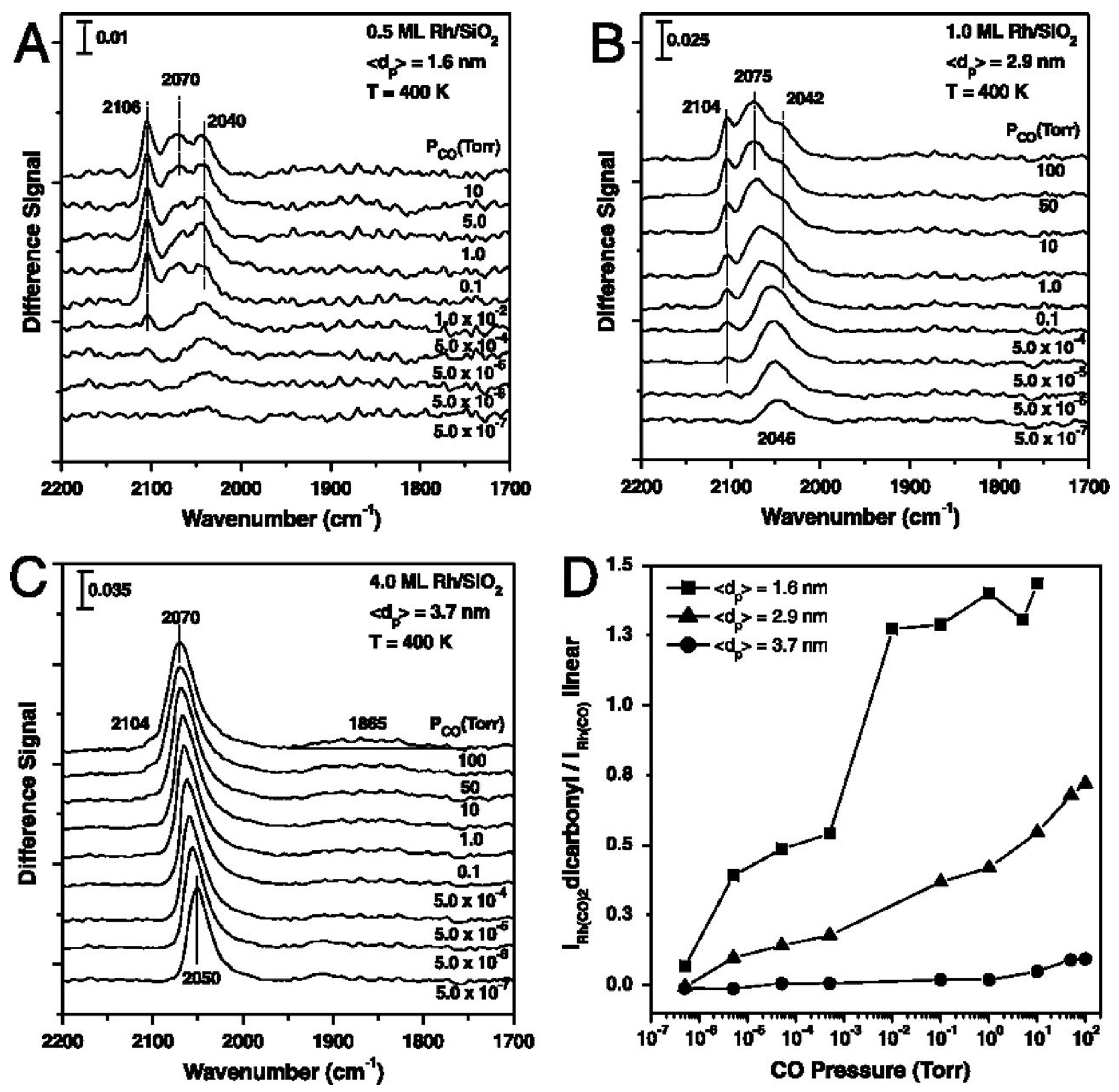
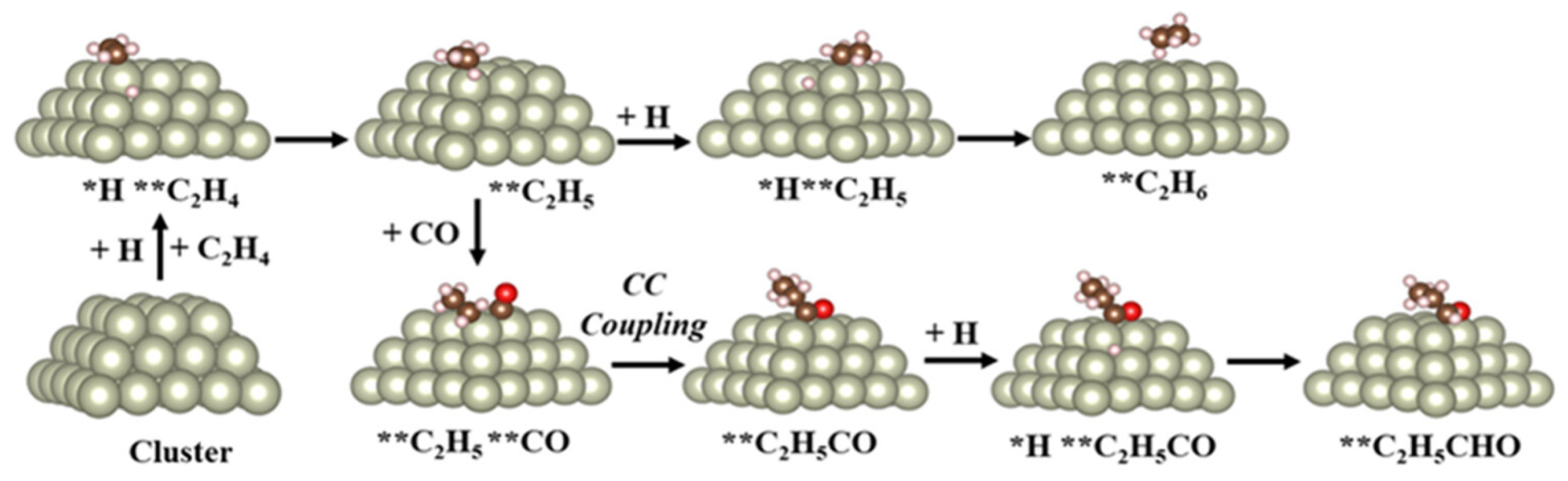
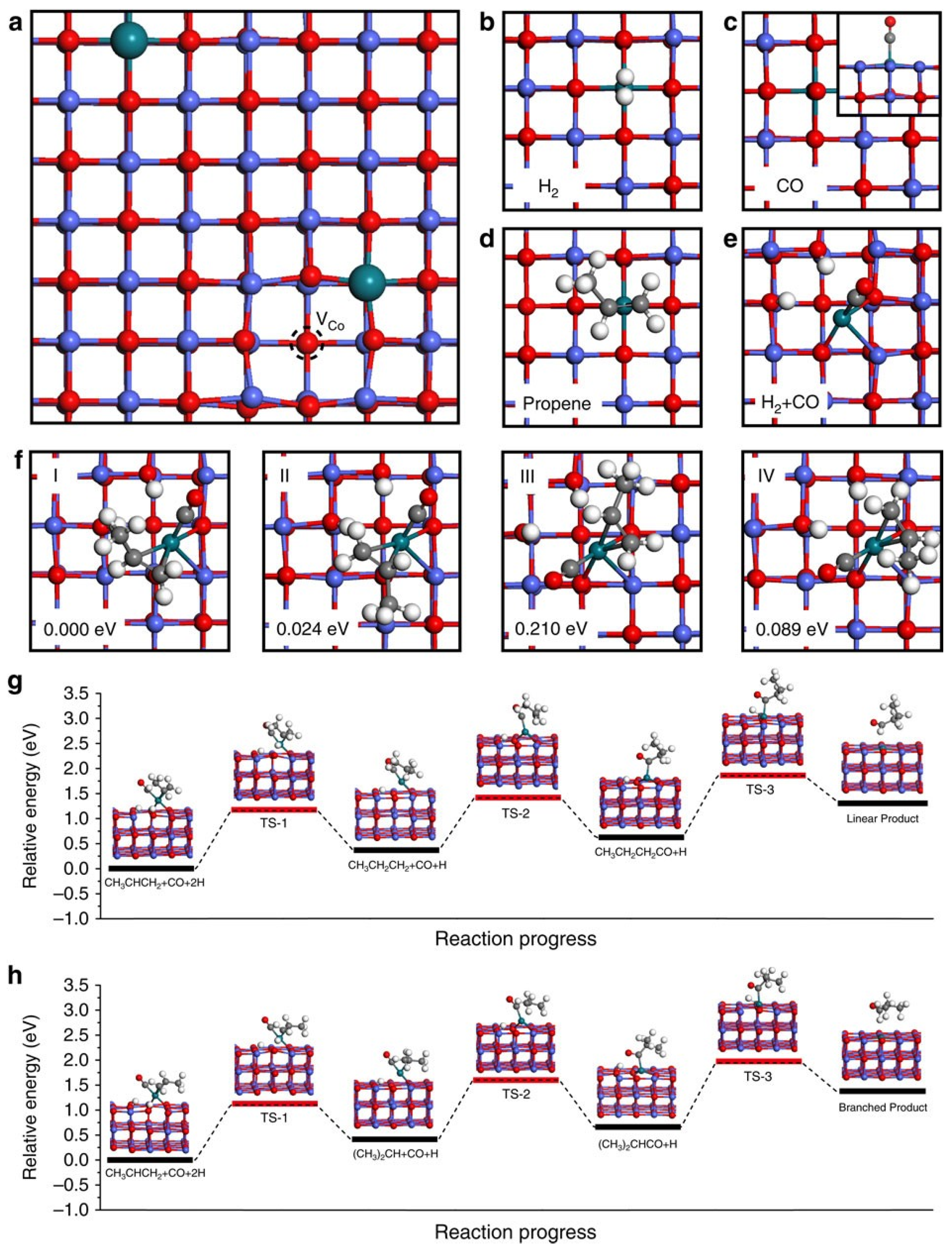
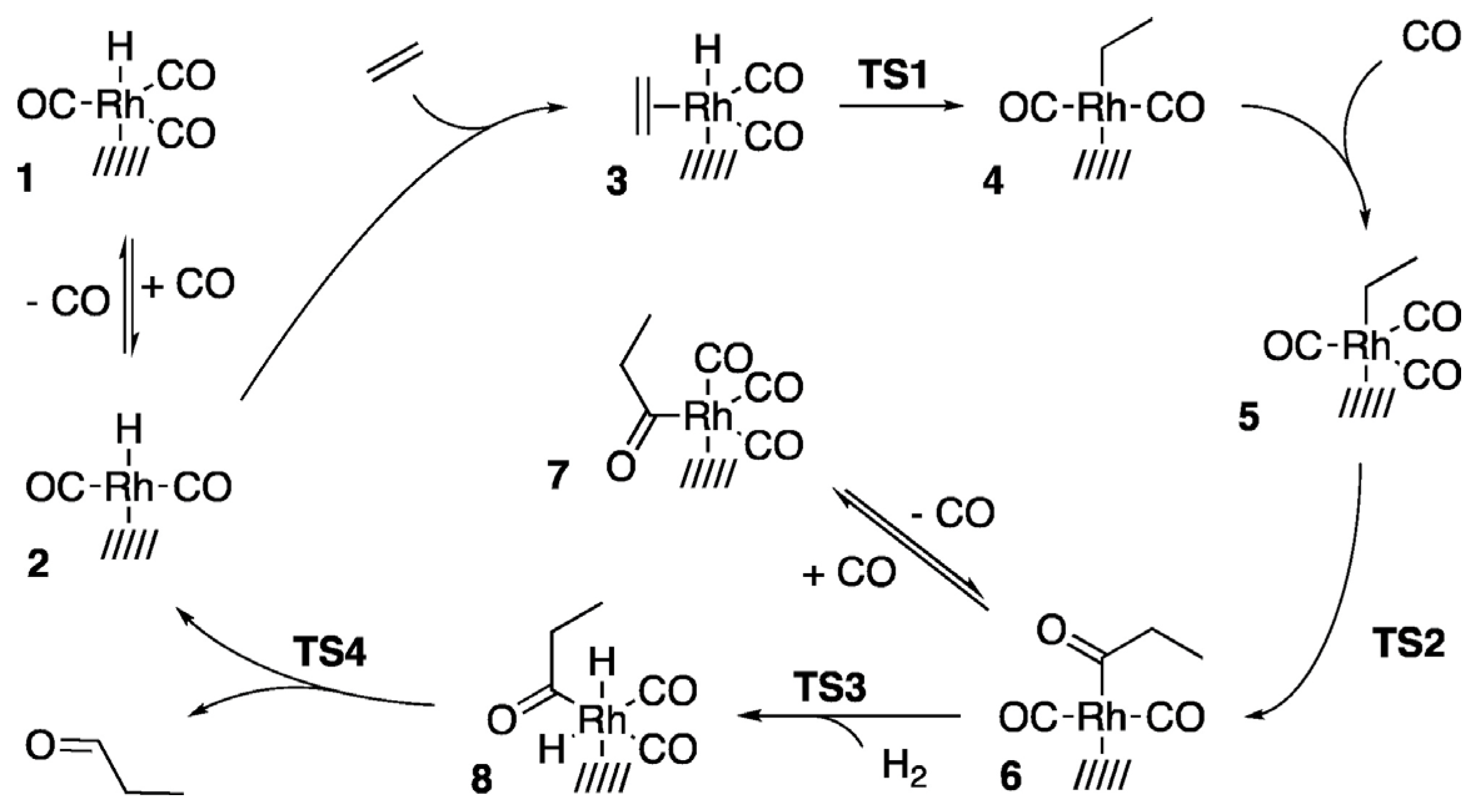
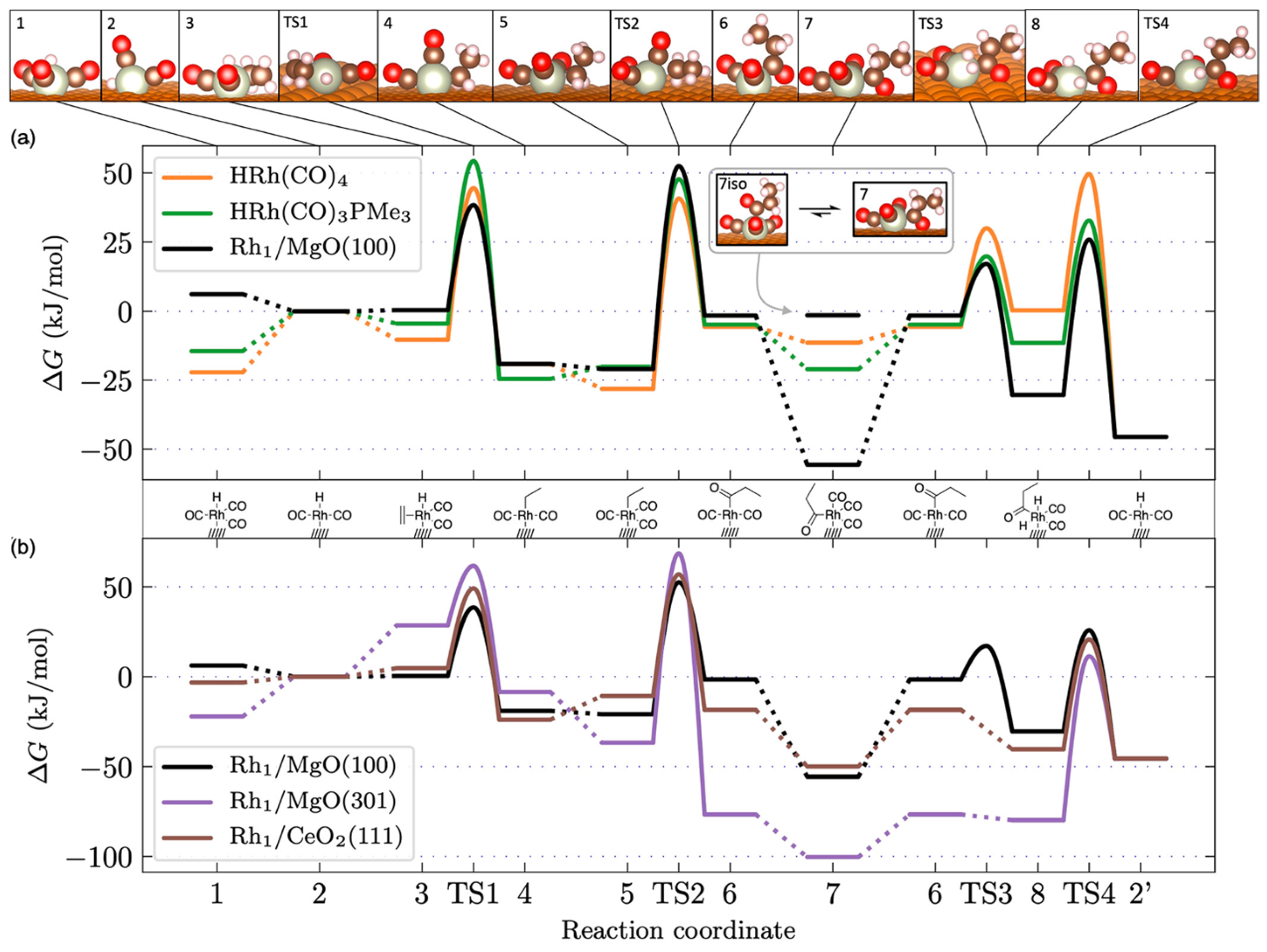
2. Surface Chemistry of Hydroformylation: Model Studies
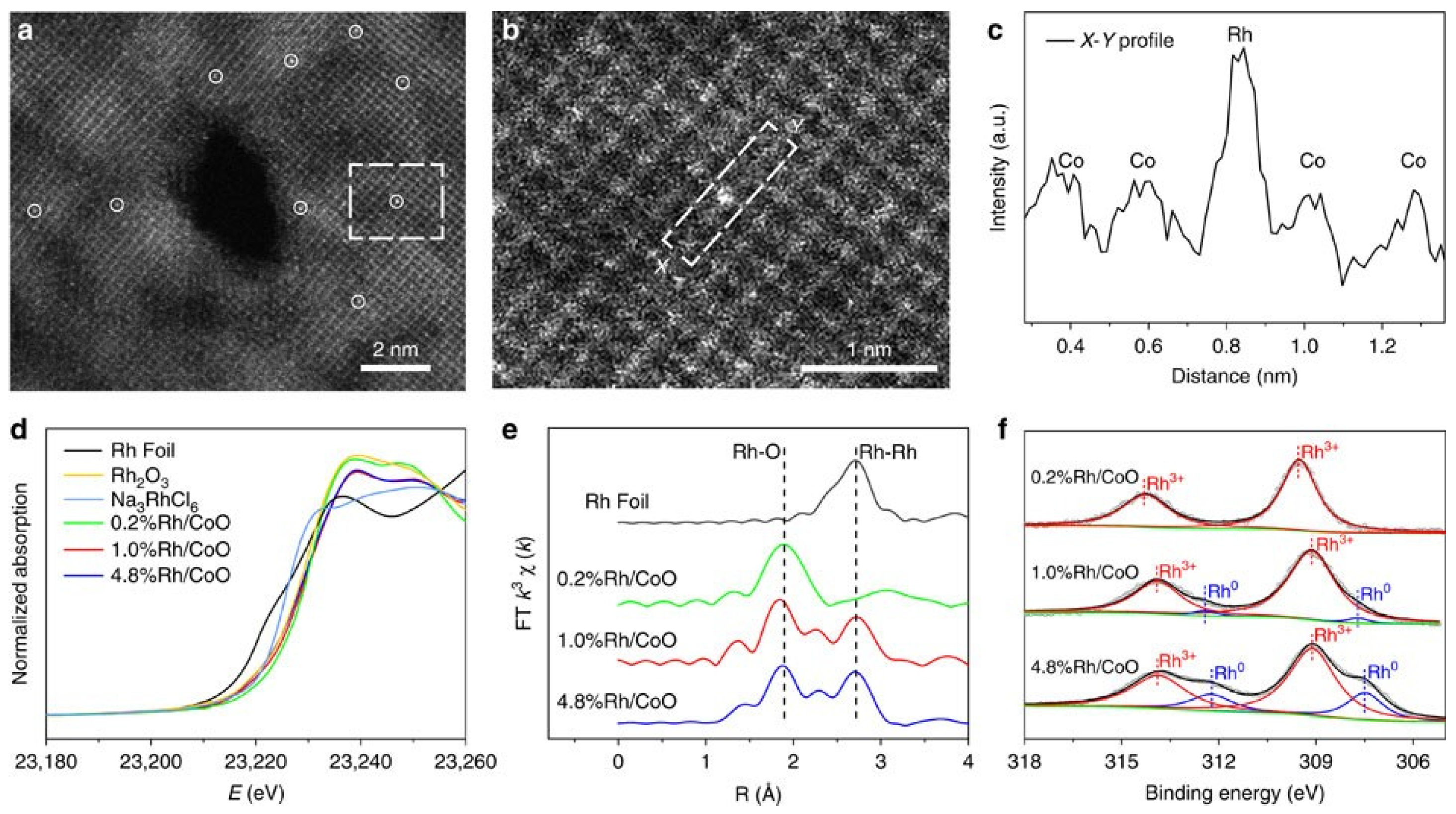
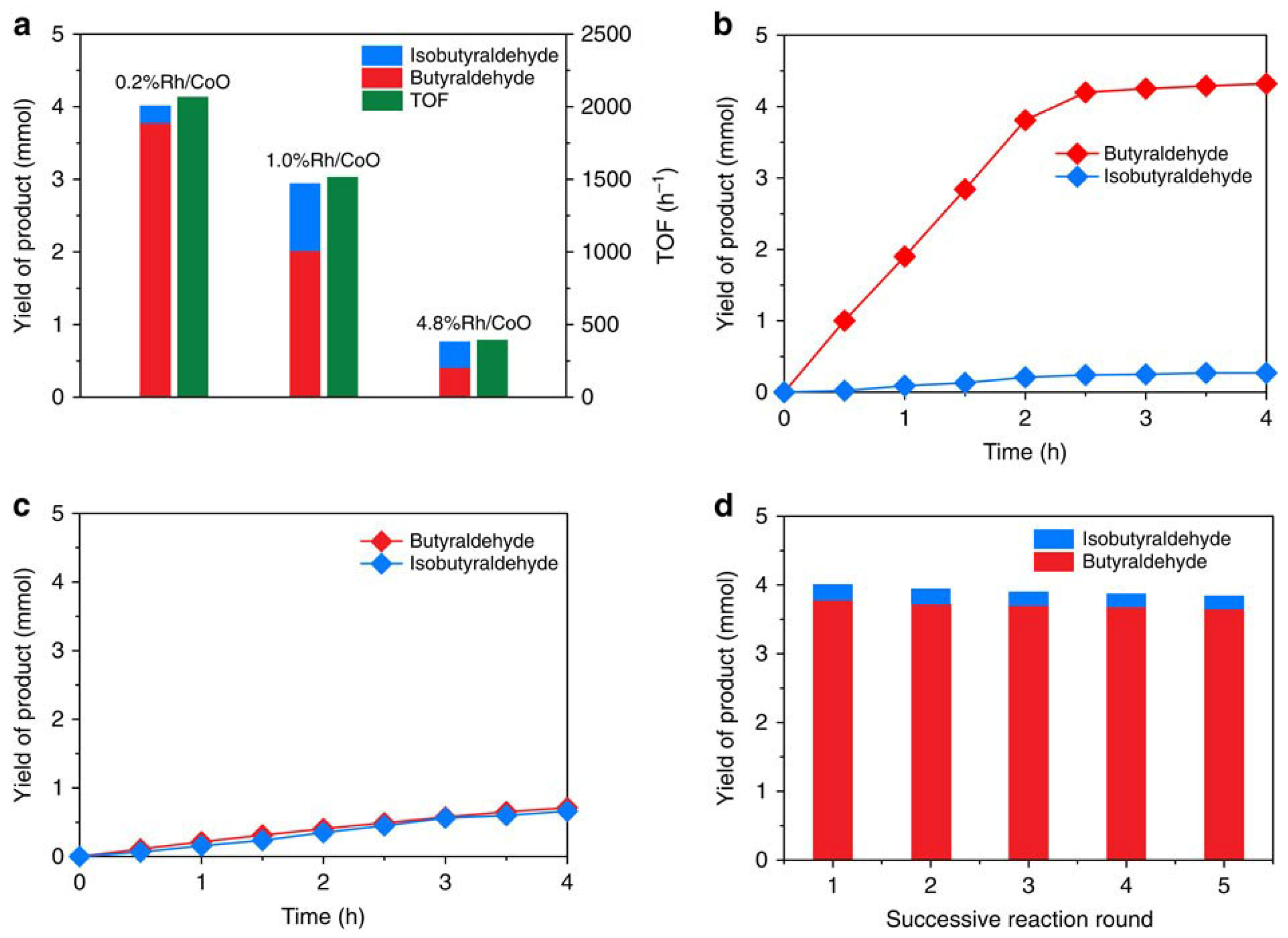
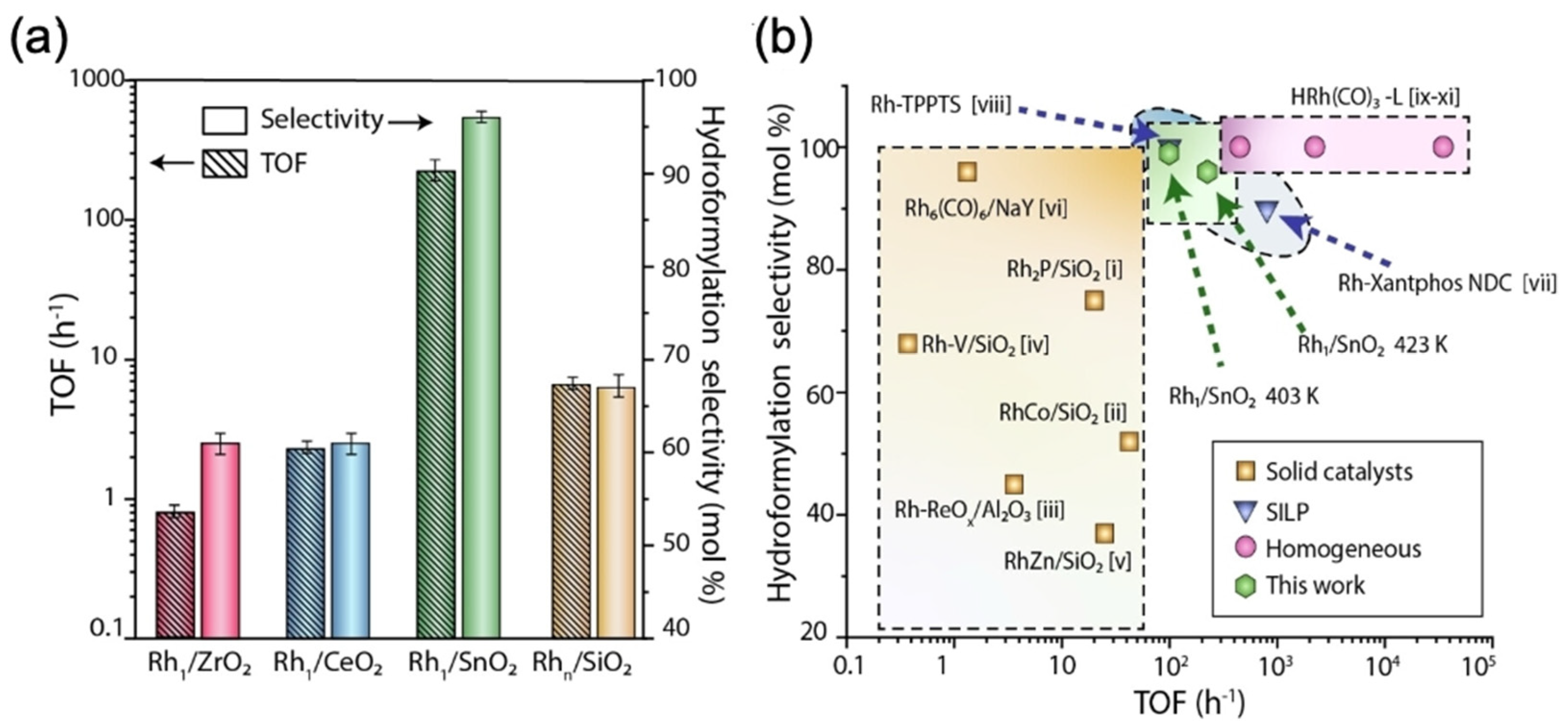
3. Rh Single Atoms and Clusters on Oxide Support
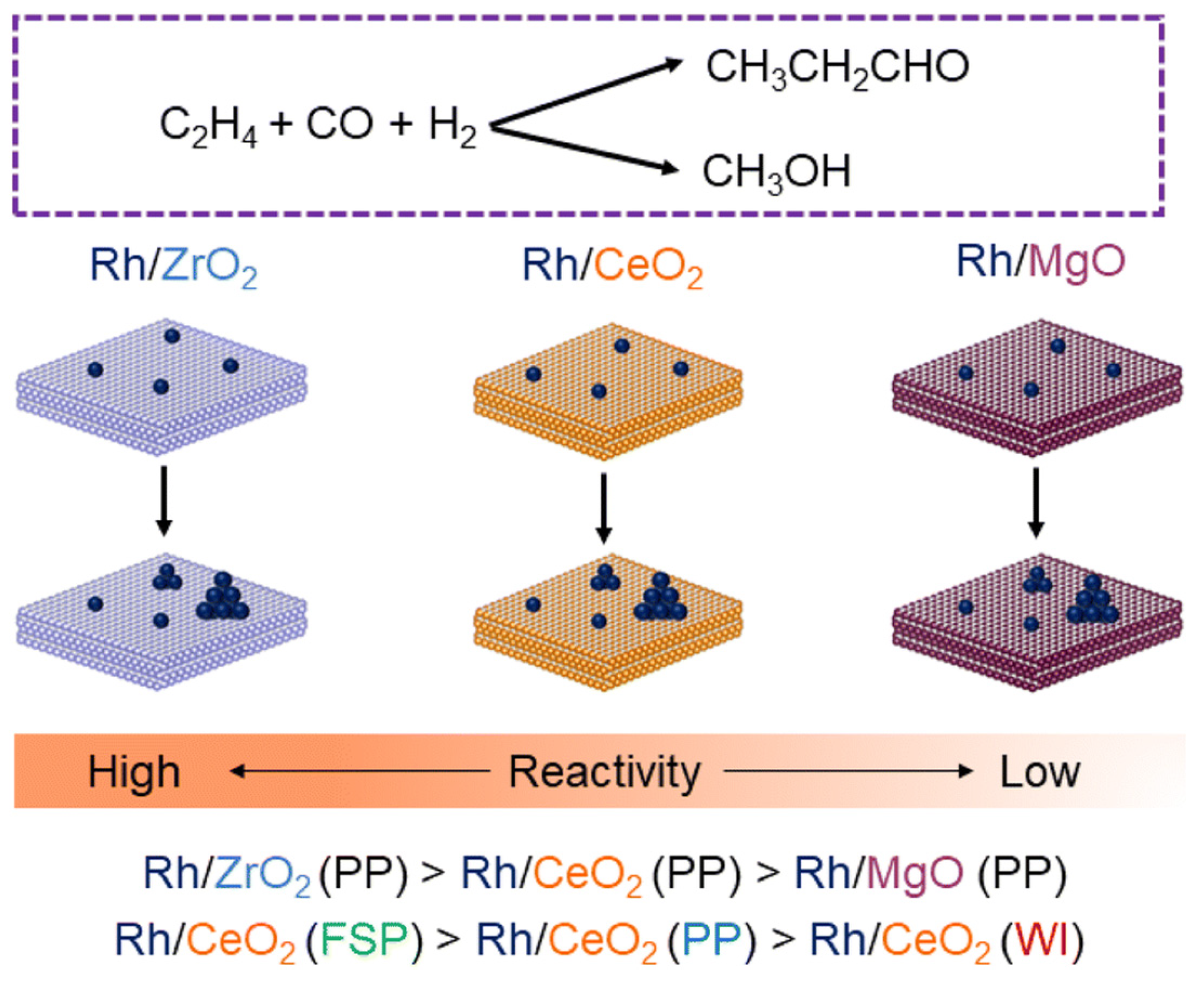
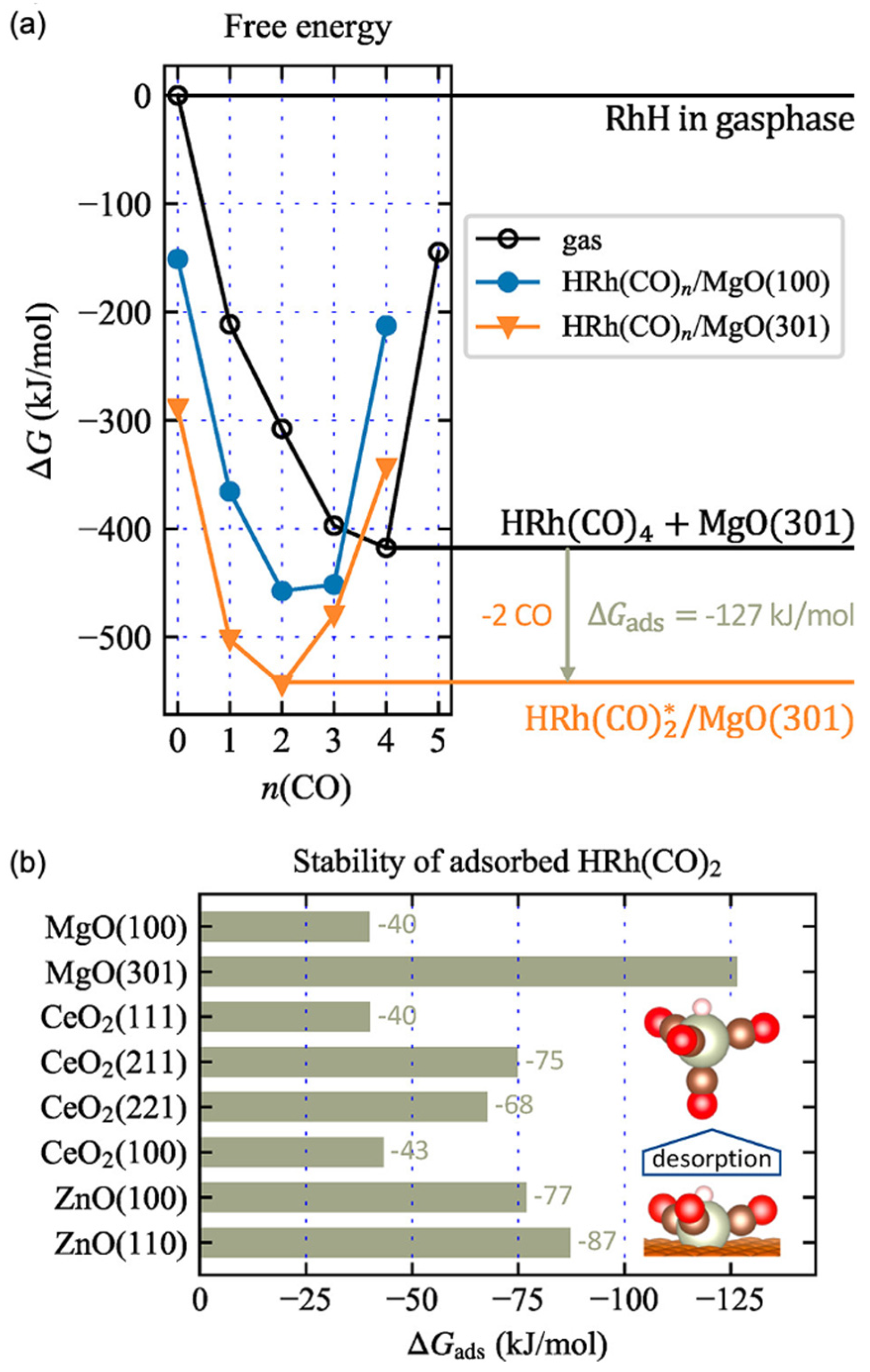
3.1. Effect of Various Oxide Support and Defects
3.2. Effect of Synthesis Procedure
3.3. Strong Metal Support Interaction in SAC
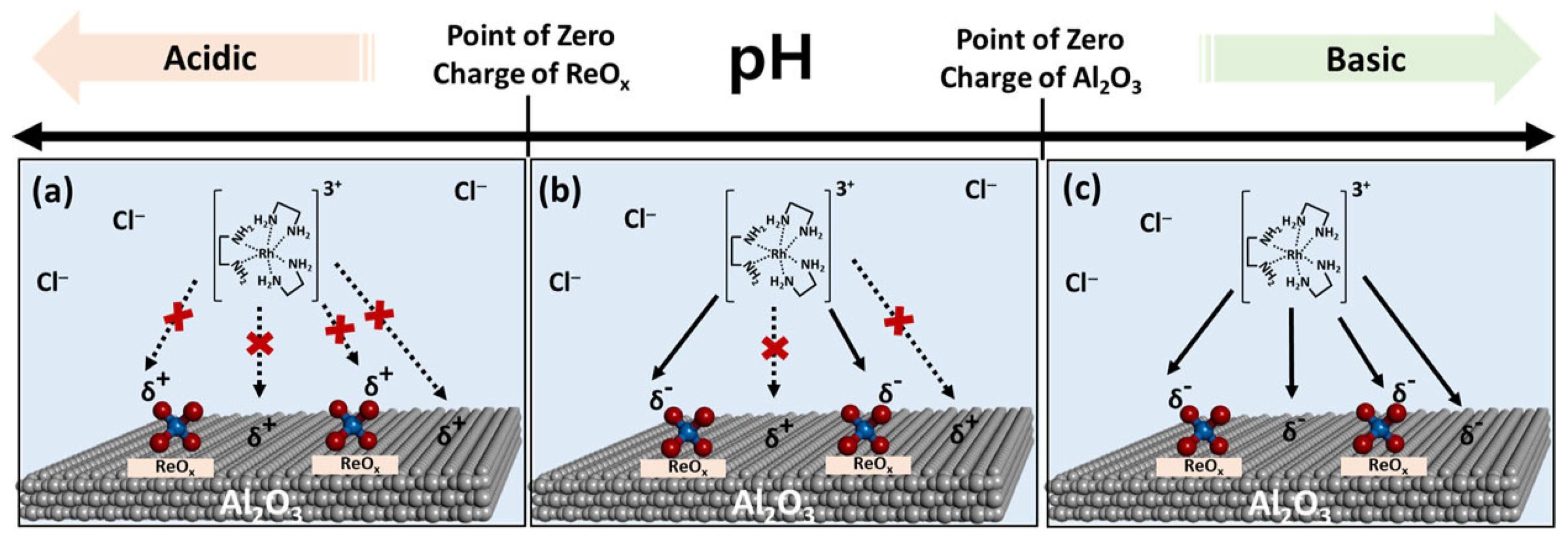
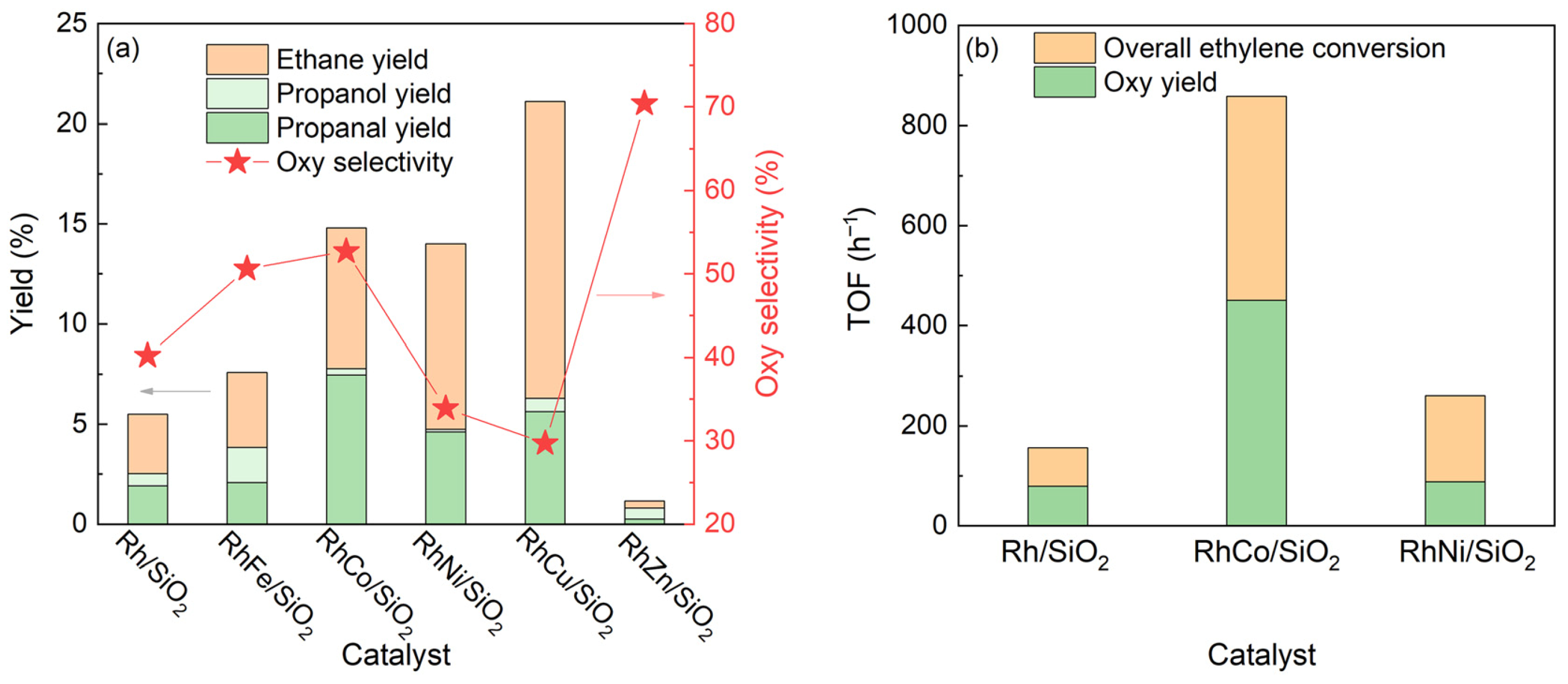
4. Bifunctional Catalysts
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Franke, R.; Selent, D.; Börner, A. Applied hydroformylation. Chem. Rev. 2012, 112, 5675–5732. [Google Scholar] [CrossRef]
- Bohnen, H.-W.; Cornils, B. Hydroformylation of Alkenes: An Industrial View of the Status and Importance; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Hanf, S.; Alvarado Rupflin, L.; Gläser, R.; Schunk, S.A. Current state of the art of the solid Rh-based catalyzed hydroformylation of short-chain olefins. Catalysts 2020, 10, 510. [Google Scholar] [CrossRef]
- Pruchnik, F.P. Organometallic Chemistry of the Transition Elements; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Samanta, P.; Canivet, J. MOF-Supported Heterogeneous Catalysts for Hydroformylation Reactions: A Minireview. ChemCatChem 2024, 16, e202301435. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Y.; Huang, N.; Lan, X.; Xie, Z.; Chen, J.G.; Wang, T. Heterogeneous hydroformylation of alkenes by Rh-based catalysts. Chem 2022, 8, 2630–2658. [Google Scholar] [CrossRef]
- Farpón, M.G.; Henao, W.; Plessow, P.N.; Andrés, E.; Arenal, R.; Marini, C.; Agostini, G.; Studt, F.; Prieto, G. Rhodium Single-Atom Catalyst Design through Oxide Support Modulation for Selective Gas-Phase Ethylene Hydroformylation. Angew. Chem. 2023, 135, e202214048. [Google Scholar] [CrossRef]
- Chen, M.; Gupta, G.; Ordonez, C.W.; Lamkins, A.R.; Ward, C.J.; Abolafia, C.A.; Zhang, B.; Roling, L.T.; Huang, W. Intermetallic nanocatalyst for highly active heterogeneous hydroformylation. J. Am. Chem. Soc. 2021, 143, 20907–20915. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.; Wang, S.; Gao, Z.; Luo, Z.; Wang, X.; Zeng, R.; Li, A.; Li, H.; Wang, M. Atomic-level insights in optimizing reaction paths for hydroformylation reaction over Rh/CoO single-atom catalyst. Nat. Commun. 2016, 7, 14036. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Zheng, Y.; He, G.; Zhang, C.; Chen, H.; Liang, L.; Liu, P.; Ma, J.; Dong, Z. Efficient Catalytic Hydroformylation of Alkenes on Rh/CeO2 Catalysts Modulated by CeO2 Morphology. Ind. Eng. Chem. Res. 2024, 63, 2958–2968. [Google Scholar] [CrossRef]
- McClure, S.M.; Lundwall, M.; Goodman, D. Planar oxide supported rhodium nanoparticles as model catalysts. Proc. Natl. Acad. Sci. USA 2011, 108, 931–936. [Google Scholar] [CrossRef]
- Amsler, J.; Sarma, B.B.; Agostini, G.; Prieto, G.; Plessow, P.N.; Studt, F. Prospects of heterogeneous hydroformylation with supported single atom catalysts. J. Am. Chem. Soc. 2020, 142, 5087–5096. [Google Scholar] [CrossRef]
- Gao, P.; Liang, G.; Ru, T.; Liu, X.; Qi, H.; Wang, A.; Chen, F.-E. Phosphorus coordinated Rh single-atom sites on nanodiamond as highly regioselective catalyst for hydroformylation of olefins. Nat. Commun. 2021, 12, 4698. [Google Scholar] [CrossRef]
- Galdeano-Ruano, C.; Lopes, C.W.; Motta Meira, D.; Corma, A.; Oña-Burgos, P. Rh2P nanoparticles stabilized by carbon patches for hydroformylation of olefins. ACS Appl. Nano Mater. 2021, 4, 10743–10753. [Google Scholar] [CrossRef]
- Suarez-Martinez, I.; Ewels, C.P.; Ke, X.; Van Tendeloo, G.; Thiess, S.; Drube, W.; Felten, A.; Pireaux, J.-J.; Ghijsen, J.; Bittencourt, C. Study of the interface between rhodium and carbon nanotubes. ACS Nano 2010, 4, 1680–1686. [Google Scholar] [CrossRef]
- Gao, R.; Tan, C.; Baker, R. Ethylene hydroformylation on graphite nanofiber supported rhodium catalysts. Catal. Today 2001, 65, 19–29. [Google Scholar] [CrossRef]
- Ernst, F. Metal-oxide interfaces. Mater. Sci. Eng. R Rep. 1995, 14, 97–156. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.; Martinez-Arias, A.; Hanson, J.; Rodriguez, J. Nanostructured oxides in chemistry: Characterization and properties. Chem. Rev. 2004, 104, 4063–4104. [Google Scholar] [CrossRef] [PubMed]
- Parastaev, A.; Muravev, V.; Osta, E.H.; Kimpel, T.F.; Simons, J.F.; van Hoof, A.J.; Uslamin, E.; Zhang, L.; Struijs, J.J.; Burueva, D.B. Breaking structure sensitivity in CO2 hydrogenation by tuning metal–oxide interfaces in supported cobalt nanoparticles. Nat. Catal. 2022, 5, 1051–1060. [Google Scholar] [CrossRef]
- Chuang, S.S.; Pien, S.-I. Infrared spectroscopic studies of ethylene hydroformylation on Rh/SiO2: An investigation of the relationships between homogeneous and heterogeneous hydroformylation. J. Mol. Catal. 1989, 55, 12–22. [Google Scholar] [CrossRef]
- Chuang, S.S.; Pien, S.I. Infrared study of the CO insertion reaction on reduced, oxidized, and sulfided Rh/SiO2 catalysts. J. Catal. 1992, 135, 618–634. [Google Scholar] [CrossRef]
- Huang, L.; Xu, Y.; Guo, W.; Liu, A.; Li, D.; Guo, X. Study on catalysis by carbonyl cluster-derived SiO2-supported rhodium for ethylene hydroformylation. Catal. Lett. 1995, 32, 61–81. [Google Scholar] [CrossRef]
- Basu, P.; Panayotov, D.; Yates, J.T. Rhodium-carbon monoxide surface chemistry: The involvement of surface hydroxyl groups on alumina and silica supports. J. Am. Chem. Soc. 1988, 110, 2074–2081. [Google Scholar] [CrossRef]
- Sarma, B.B.; Neukum, D.; Doronkin, D.E.; Nilayam, A.R.L.; Baumgarten, L.; Krause, B.; Grunwaldt, J.-D. Understanding the role of supported Rh atoms and clusters during hydroformylation and CO hydrogenation reactions with in situ/operando XAS and DRIFT spectroscopy. Chem. Sci. 2024, 15, 12369–12379. [Google Scholar] [CrossRef] [PubMed]
- Berkó, A.; Solymosi, F. Adsorption-induced structural changes of Rh supported by TiO2(110)-(1 × 2): An STM study. J. Catal. 1999, 183, 91–101. [Google Scholar] [CrossRef]
- Solymosi, F.; Bánsági, T. Low reactivity of rhodium clusters produced in the presence of carbon monoxide. J. Phys. Chem. 1993, 97, 10133–10138. [Google Scholar] [CrossRef]
- Solymosi, F.; Pasztor, M. Infrared study of the effect of hydrogen on carbon monoxide-induced structural changes in supported rhodium. J. Phys. Chem. 1986, 90, 5312–5317. [Google Scholar] [CrossRef]
- Yang, C.; Garl, C.W. Infrared studies of carbon monoxide chemisorbed on rhodium. J. Phys. Chem. 1957, 61, 1504–1512. [Google Scholar] [CrossRef]
- Wang, C.; Sombut, P.; Puntscher, L.; Ulreich, M.; Pavelec, J.; Rath, D.; Balajka, J.; Meier, M.; Schmid, M.; Diebold, U. A Multitechnique Study of C2H4 Adsorption on a Model Single-Atom Rh1 Catalyst. J. Phys. Chem. C 2024, 128, 15404–15411. [Google Scholar] [CrossRef]
- Jakub, Z.; Hulva, J.; Ryan, P.T.; Duncan, D.A.; Payne, D.J.; Bliem, R.; Ulreich, M.; Hofegger, P.; Kraushofer, F.; Meier, M. Adsorbate-induced structural evolution changes the mechanism of CO oxidation on a Rh/Fe3O4 (001) model catalyst. Nanoscale 2020, 12, 5866–5875. [Google Scholar] [CrossRef]
- Mahapatra, M.; Sharp, M.A.; Lee, C.J.; Zhu, Y.; Gutiérrez, O.Y.; Kay, B.D.; Dohnálek, Z. The evolution of model Rh/Fe3O4 (001) catalysts in hydrogen environments. Surf. Sci. 2025, 751, 122617. [Google Scholar] [CrossRef]
- Ghoshal, S.; Yuan, Y.; Ezeakunne, C.; Priyadarsini, A.; Chen, J.G.; Kattel, S. Unraveling the Structural Sensitivity of Metal Catalysts in Ethylene Hydroformylation: Insights from Theory and Experiments. ACS Catal. 2025, 15, 12325–12339. [Google Scholar] [CrossRef]
- Sheng, W.; Kattel, S.; Yao, S.; Yan, B.; Liang, Z.; Hawxhurst, C.J.; Wu, Q.; Chen, J.G. Electrochemical reduction of CO2 to synthesis gas with controlled CO/H 2 ratios. Energy Environ. Sci. 2017, 10, 1180–1185. [Google Scholar] [CrossRef]
- Wang, C.; Gu, X.-K.; Yan, H.; Lin, Y.; Li, J.; Liu, D.; Li, W.-X.; Lu, J. Water-mediated Mars–van Krevelen mechanism for CO oxidation on ceria-supported single-atom Pt1 catalyst. Acs Catal. 2017, 7, 887–891. [Google Scholar] [CrossRef]
- Yan, H.; Zhao, X.; Guo, N.; Lyu, Z.; Du, Y.; Xi, S.; Guo, R.; Chen, C.; Chen, Z.; Liu, W. Atomic engineering of high-density isolated Co atoms on graphene with proximal-atom controlled reaction selectivity. Nat. Commun. 2018, 9, 3197. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-F.; Wang, A.; Qiao, B.; Li, J.; Liu, J.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res. 2013, 46, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, G.; Shi, L.; Ye, J. Single-atom catalysts: Emerging multifunctional materials in heterogeneous catalysis. Adv. Energy Mater. 2018, 8, 1701343. [Google Scholar] [CrossRef]
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- Gao, C.; Low, J.; Long, R.; Kong, T.; Zhu, J.; Xiong, Y. Heterogeneous single-atom photocatalysts: Fundamentals and applications. Chem. Rev. 2020, 120, 12175–12216. [Google Scholar] [CrossRef]
- Wang, B.; Cai, H.; Shen, S. Single metal atom photocatalysis. Small Methods 2019, 3, 1800447. [Google Scholar] [CrossRef]
- Zhao, C.; Dai, X.; Yao, T.; Chen, W.; Wang, X.; Wang, J.; Yang, J.; Wei, S.; Wu, Y.; Li, Y. Ionic exchange of metal–organic frameworks to access single nickel sites for efficient electroreduction of CO2. J. Am. Chem. Soc. 2017, 139, 8078–8081. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Shi, Q.; Du, D.; Lin, Y. Single-atom electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 13944–13960. [Google Scholar] [CrossRef]
- Tao, S.; Yang, D.; Wang, M.; Sun, G.; Xiong, G.; Gao, W.; Zhang, Y.; Pan, Y. Single-atom catalysts for hydroformylation of olefins. iScience 2023, 26, 106183. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Li, M.; Chen, X.; Sun, Y.; Wang, W.; Fan, Z.; Lan, X.; Wei, F.; Yan, B.; Zhong, Q. Oxidative Strong Metal–Support Interaction Stabilized Single-Atom Catalysts for Heterogeneous Hydroformylation. J. Am. Chem. Soc. 2025, 147, 15859–15866. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shin, D.; Oh, D.; Jeong, B.; Kim, K.Y.; Hur, C.; Han, J.W.; An, K. Atomically dispersed Rh catalysts formed on defective CeO2 surfaces with hydroformylation activity. Chem. Eng. J. 2024, 496, 153758. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, Q.; Yang, Q.; Wang, S.; Hulsey, M.J.; Ding, S.; Furukawa, S.; Li, M.; Yan, N.; Ma, X. Boosting the hydroformylation activity of a Rh/CeO2 single-atom catalyst by tuning surface deficiencies. ACS Catal. 2023, 13, 7243–7255. [Google Scholar] [CrossRef]
- Yang, Q.; Zheng, Y.; Feng, Y.; Ding, J.; Li, M.; Huang, S.; Wang, M.Y.; Ma, X. Balanced Rh+-Rh0 Sites over Rh Clusters Enhance Heterogeneous Hydroformylation of Aldehyde. ChemCatChem 2025, 17, e202401392. [Google Scholar] [CrossRef]
- Li, X.; Pereira-Hernández, X.I.; Chen, Y.; Xu, J.; Zhao, J.; Pao, C.-W.; Fang, C.-Y.; Zeng, J.; Wang, Y.; Gates, B.C. Functional CeOx nanoglues for robust atomically dispersed catalysts. Nature 2022, 611, 284–288. [Google Scholar] [CrossRef]
- Lang, R.; Li, T.; Matsumura, D.; Miao, S.; Ren, Y.; Cui, Y.T.; Tan, Y.; Qiao, B.; Li, L.; Wang, A. Hydroformylation of olefins by a rhodium single-atom catalyst with activity comparable to RhCl (PPh3)3. Angew. Chem. Int. Ed. 2016, 55, 16054–16058. [Google Scholar] [CrossRef]
- Li, T.; Chen, F.; Lang, R.; Wang, H.; Su, Y.; Qiao, B.; Wang, A.; Zhang, T. Styrene hydroformylation with in situ hydrogen: Regioselectivity control by coupling with the low-temperature water–gas shift reaction. Angew. Chem. 2020, 132, 7500–7504. [Google Scholar] [CrossRef]
- Sarma, B.B.; Jelic, J.; Neukum, D.; Doronkin, D.E.; Huang, X.; Studt, F.; Grunwaldt, J.-D. Tracking and understanding dynamics of atoms and clusters of late transition metals with in-situ DRIFT and XAS spectroscopy assisted by DFT. J. Phys. Chem. C 2023, 127, 3032–3046. [Google Scholar] [CrossRef]
- Jones, J.; Xiong, H.; DeLaRiva, A.T.; Peterson, E.J.; Pham, H.; Challa, S.R.; Qi, G.; Oh, S.; Wiebenga, M.H.; Pereira Hernández, X.I. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science 2016, 353, 150–154. [Google Scholar] [CrossRef]
- Sarma, B.B.; Kim, J.; Amsler, J.; Agostini, G.; Weidenthaler, C.; Pfänder, N.; Arenal, R.; Concepción, P.; Plessow, P.; Studt, F. One-pot cooperation of single-atom Rh and Ru solid catalysts for a selective tandem olefin isomerization-hydrosilylation process. Angew. Chem. Int. Ed. 2020, 59, 5806–5815. [Google Scholar] [CrossRef]
- Koirala, R.; Pratsinis, S.E.; Baiker, A. Synthesis of catalytic materials in flames: Opportunities and challenges. Chem. Soc. Rev. 2016, 45, 3053–3068. [Google Scholar] [CrossRef]
- Tauster, S.; Fung, S.; Garten, R.L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Lin, L.; Li, R.; Li, D.; Song, T.; Mu, R.; Fu, Q.; Bao, X. Overturning CO2 hydrogenation selectivity with high activity via reaction-induced strong metal–support interactions. J. Am. Chem. Soc. 2022, 144, 4874–4882. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.X.; Qian, K.; Jia, A.; Li, D.; Shi, L.; Hu, J.; Zhu, J.; Huang, W. Structure sensitivity of Au-TiO2 strong metal–support interactions. Angew. Chem. Int. Ed. 2021, 60, 12074–12081. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, Y.; Wang, J. Oxidative-atmosphere-induced strong metal–support interaction and its catalytic application. Acc. Chem. Res. 2023, 56, 911–923. [Google Scholar] [CrossRef]
- Liu, S.; Qi, H.; Zhou, J.; Xu, W.; Niu, Y.; Zhang, B.; Zhao, Y.; Liu, W.; Ao, Z.; Kuang, Z. Encapsulation of platinum by titania under an oxidative atmosphere: Contrary to classical strong metal–support interactions. ACS Catal. 2021, 11, 6081–6090. [Google Scholar] [CrossRef]
- Tang, H.; Su, Y.; Guo, Y.; Zhang, L.; Li, T.; Zang, K.; Liu, F.; Li, L.; Luo, J.; Qiao, B. Oxidative strong metal–support interactions (OMSI) of supported platinum-group metal catalysts. Chem. Sci. 2018, 9, 6679–6684. [Google Scholar] [CrossRef]
- Matsubu, J.C.; Zhang, S.; DeRita, L.; Marinkovic, N.S.; Chen, J.G.; Graham, G.W.; Pan, X.; Christopher, P. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 2017, 9, 120–127. [Google Scholar]
- Breslow, D.; Heck, R. Mechanism of the Hydroformylation of Olefins; Soc Chemical Industry 14 Belgrave Square: London, UK, 1960; p. 467.
- Heck, R.F.; Breslow, D.S. The reaction of cobalt hydrotetracarbonyl with olefins. J. Am. Chem. Soc. 1961, 83, 4023–4027. [Google Scholar] [CrossRef]
- Campos, J. Bimetallic cooperation across the periodic table. Nat. Rev. Chem. 2020, 4, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Hetterscheid, D.G.; Chikkali, S.H.; de Bruin, B.; Reek, J.N. Binuclear cooperative catalysts for the hydrogenation and hydroformylation of olefins. ChemCatChem 2013, 5, 2785–2793. [Google Scholar] [CrossRef]
- Huang, N.; Ma, Y.; Liu, B.; Yang, L.; Lan, X.; Wu, X.; Wang, T. Designing a Rh-based bimetallic catalyst for heterogeneous ethylene hydroformylation: Combining theoretical predictions and experimental screening. Catal. Sci. Technol. 2025, 15, 1113–1121. [Google Scholar] [CrossRef]
- Ro, I.; Qi, J.; Lee, S.; Xu, M.; Yan, X.; Xie, Z.; Zakem, G.; Morales, A.; Chen, J.G.; Pan, X. Bifunctional hydroformylation on heterogeneous Rh-WOx pair site catalysts. Nature 2022, 609, 287–292. [Google Scholar]
- Ro, I.; Xu, M.; Graham, G.W.; Pan, X.; Christopher, P. Synthesis of heteroatom Rh–ReO x atomically dispersed species on Al2O3 and their tunable catalytic reactivity in ethylene hydroformylation. ACS Catal. 2019, 9, 10899–10912. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Zhao, Y.; Jiang, P. Cu Decorated Rh/TiO2 Catalyst for Selective Hydrogenation of Phenylacetylene to Styrene. ChemNanoMat 2021, 7, 1213–1220. [Google Scholar]
- Kettner, M.; Stumm, C.; Schwarz, M.; Schuschke, C.; Libuda, J. Pd model catalysts on clean and modified HOPG: Growth, adsorption properties, and stability. Surf. Sci. 2019, 679, 64–73. [Google Scholar] [CrossRef]
- Motin, M.A.; Steiger-Thirsfeld, A.; Stöger-Pollach, M.; Rupprechter, G. Model Catalysis with HOPG-Supported Pd Nanoparticles and Pd Foil: XPS, STM and C2H4 Hydrogenation. Catal. Lett. 2022, 152, 2892–2907. [Google Scholar]
- Ellis, L.D.; Trottier, R.M.; Musgrave, C.B.; Schwartz, D.K.; Medlin, J.W. Controlling the surface reactivity of titania via electronic tuning of self-assembled monolayers. ACS Catal. 2017, 7, 8351–8357. [Google Scholar] [CrossRef]
- Zhang, J.; Deo, S.; Janik, M.J.; Medlin, J.W. Control of molecular bonding strength on metal catalysts with organic monolayers for CO2 reduction. J. Am. Chem. Soc. 2020, 142, 5184–5193. [Google Scholar] [CrossRef] [PubMed]
- Meduna, Z.W.; Schwartz, D.K.; Medlin, J.W. Support modification by phosphonic acid ligands controls ethylene hydroformylation on single-atom rhodium sites. Catal. Sci. Technol. 2025, 15, 5491–5499. [Google Scholar] [CrossRef]
- Chen, L.; Ali, I.S.; Sterbinsky, G.E.; Zhou, X.; Wasim, E.; Tait, S.L. Ligand-coordinated Ir single-atom catalysts stabilized on oxide supports for ethylene hydrogenation and their evolution under a reductive atmosphere. Catal. Sci. Technol. 2021, 11, 2081–2093. [Google Scholar] [CrossRef]
- Manna, S.; Wang, Y.; Hernandez, A.; Lile, P.; Liu, S.; Mueller, T. A database of low-energy atomically precise nanoclusters. Sci. Data 2023, 10, 308. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Guan, D.; Zhou, J.; Huang, Y.-C.; Dong, C.-L.; Wang, J.-Q.; Zhou, W.; Shao, Z. Screening highly active perovskites for hydrogen-evolving reaction via unifying ionic electronegativity descriptor. Nat. Commun. 2019, 10, 3755. [Google Scholar] [CrossRef]
- Kumar, R.; Chikkali, S.H. Hydroformylation of olefins by metals other than rhodium. J. Organomet. Chem. 2022, 960, 122231. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillum, M.; Ariyaratne, G.K.P.A.; Tawny, C.; Alimenti, P.; Krawczykowski, K.; Polik, E.; Mahapatra, M. Recent Advances in Heterogeneous Hydroformylation at Metal–Oxide Interfaces. Molecules 2025, 30, 4078. https://doi.org/10.3390/molecules30204078
Gillum M, Ariyaratne GKPA, Tawny C, Alimenti P, Krawczykowski K, Polik E, Mahapatra M. Recent Advances in Heterogeneous Hydroformylation at Metal–Oxide Interfaces. Molecules. 2025; 30(20):4078. https://doi.org/10.3390/molecules30204078
Chicago/Turabian StyleGillum, Maxwell, Gallage K. P. A. Ariyaratne, Charbel Tawny, Paul Alimenti, Kyle Krawczykowski, Erik Polik, and Mausumi Mahapatra. 2025. "Recent Advances in Heterogeneous Hydroformylation at Metal–Oxide Interfaces" Molecules 30, no. 20: 4078. https://doi.org/10.3390/molecules30204078
APA StyleGillum, M., Ariyaratne, G. K. P. A., Tawny, C., Alimenti, P., Krawczykowski, K., Polik, E., & Mahapatra, M. (2025). Recent Advances in Heterogeneous Hydroformylation at Metal–Oxide Interfaces. Molecules, 30(20), 4078. https://doi.org/10.3390/molecules30204078




