Abstract
In this study, first-principles calculations were employed to systematically investigate the interaction mechanisms between (18-crown-6) potassium (18C6-K+) and six typical defect sites on the SnO2 (110) surface, including Sni + SnO, Oi + OSn, VO + Sni, VSn + SnO, VSn + Sni, and Sni. Six intrinsic or complex defects universally coexist on the SnO2 surface, and the defect states they introduced allow for precise tuning of material performance. The results demonstrated that the 18C6-K+ molecule can stably adsorb on all six defect sites and significantly increase defect formation energies, indicating its thermodynamic capability to suppress defect generation. A subsequent density of states (DOS) analysis revealed that the 18C6-K+ molecule exhibits strong defect passivation effects at Sni + SnO, VO + Sni, VSn + Sni, and Sni sites, and partially mitigated the electronic disturbances induced by Oi + OSn and VSn + SnO defects. Furthermore, the incorporation of 18C6-K+ has been shown to reduce the electronic effective mass of defective systems, thereby enhancing surface carrier transport. A subsequent charge density difference (CDD) analysis revealed that the 18C6-K+ molecule forms Sn-ether and O-ether interactions through its ether bonds (C-O-C) with surface Sn and O atoms, inducing interfacial electronic reconstruction and charge transfer. The Bader charge analysis revealed that the H, C, and O atoms in 18C6-K+ lose electrons, whereas the Sn or O atoms at the surface defect sites gain electrons. This outcome is consistent with the CDD analysis and quantitatively confirms the extent of electron transfer from 18C6-K+ to the SnO2 defect regions. These interactions effectively passivate defect states, thereby enhancing interfacial stability. The present study offers theoretical guidance and design insights for the development of molecular passivation strategies in SnO2-based optoelectronic devices.
1. Introduction
Tin oxide (SnO2), a wide-bandgap semiconductor (∼3.6 eV) [1], has found extensive applications in solar cells, transparent conductive oxide (TCO) coatings, and gas sensors, owing to its excellent electrical conductivity [2], robust chemical adsorption capacity, and high thermal stability [3]. However, SnO2 is typically synthesized via low-temperature processes [4], which, in combination with its small grain size, often results in the formation of abundant surface structural defects such as dangling bonds and under-coordinated atoms [5]. These surface defects have been shown to enhance non-radiative recombination at the interfaces, thereby reducing carrier lifetime and impeding efficient charge transport pathways [6]. This, in turn, has been demonstrated to limit the stability and overall performance of SnO2-based devices to some extent [7]. To alleviate the adverse impact of surface defects on SnO2 performance, a variety of surface passivation strategies have been developed in recent years. These include heteroatom doping [8] and interfacial modification approaches [9]. By modulating the surface chemical environment or the electronic structure of defects [10], these strategies have been shown to effectively reduce defect state densities [11] and suppress non-radiative recombination processes [12], thereby improving carrier dynamics and enhancing device stability [13]. However, conventional doping techniques frequently encounter challenges, including poor selectivity, processing steps, and limited compatibility with underlying substrates, which hinders their practical applicability [14]. Consequently, the development of efficient, controllable, and adaptable passivation strategies has emerged as a primary research focus in the regulation of surface defects in SnO2.
In recent years, molecular passivation has emerged as a promising approach for surface defect control and has attracted considerable attention [15]. This strategy is predicated on the coordination interactions between molecules and surface defect sites [16], thereby enabling precise modulation of the local electronic structure and effective passivation of surface defect states [17]. In comparison with conventional doping methodologies, molecular passivation demonstrated superior selectivity and tunability, enabling targeted interactions with specific defect sites and regulation of their properties [18]. Furthermore, the structural diversity and functional tunability of molecular complexes endow them with strong environmental adaptability, providing new insights and pathways for improving the performance of SnO2-based materials. This includes organic molecules, inorganic salts, polymer modifiers, and organic–inorganic hybrid materials, which can work synergistically to improve the stability of the SnO2 interface. Yuan et al. [19] introduced thiophenol-based ligands (TP-ligands) bearing SH groups and π-conjugated structures into CsPbI3 perovskite precursors, leading to the formation of stable CsPbI3 films with reduced trap densities and enhanced carrier mobilities. Wang et al. [20] utilized the incorporation of cyano-substituted π-conjugated molecules into perovskite films, a strategy that effectively addressed both surface and grain boundary defects. This approach led to a substantial enhancement in device performance and operational stability. Koseki et al. [21] employed organic molecules with extended π-conjugated frameworks to inhibit molecular desorption, optimize interfacial energy level alignment, and simultaneously improve defect passivation and hole transport in perovskite solar cells. Consequently, the efficiency of the device increased from 22.7% to 24.6%, accompanied by a substantial enhancement in device stability.
This study aims to explore the potential application of molecular complexes in defect passivation on the SnO2 (110) surface. To elucidate the underlying mechanism, first-principles calculations were employed to systematically investigate the interactions between the 18-crown-6–potassium complex (18C6-K+) and six typical surface defects of SnO2 (110), namely Sni + SnO, Oi + OSn, VO + Sni, VSn + SnO, VSn + Sni, and Sni. Particular attention was paid to the regulation of adsorption stability, defect formation energies, and electronic effective masses, combined with analyses of band structures, density of states, charge density differences, and Bader charge results, to comprehensively clarify the defect passivation mechanism of 18C6-K+. In Section 2, the computational models, parameter settings, and theoretical methods were detailed; In Section 3, we systematically analyzed the adsorption characteristics of 18C6-K+ on the SnO2 (110) surface, the variations in defect formation energies and electronic effective masses, and revealed its defect passivation mechanism through analyses of band structures, density of states, charge density differences, and Bader charge results. And in Section 4, the main conclusions and highlights the potential application value of the 18C6-K+ passivation strategy in enhancing the performance of SnO2-based optoelectronic devices were made. This study provides a more complete theoretical foundation and technical guidance for optimizing the performance of SnO2-based optoelectronic devices.
2. Results and Discussion
2.1. Atomic Structures of the 18C6-K+ and SnO2
Figure 1a presents the crystallographic configuration of the 18C6-K+ molecular complex. The complex under consideration consists of a crown ether (18C6) composed of 12 saturated carbon atoms and 6 oxygen atoms, forming a ring that encapsulates a K+ ion. The potassium ion is located at the geometric center of the ring and forms a stable structure through coordination interactions with the inner-ring oxygen atoms. The selection of 18C6-K+ as the passivation molecule is primarily based on its structural advantages, charge regulation capability, and novelty in the field of SnO2 defect passivation. To highlight its uniqueness, a comparison with representative passivation molecules for SnO2 reported in recent years, along with 18C6-K+, is summarized (as shown in Table 1). As depicted in Figure 1b, pristine SnO2 adopts a rutile-type crystal structure belonging to the tetragonal space group P42/mnm [22], characterized by typical tetragonal symmetry [23]. Within this lattice, each Sn atom is octahedrally coordinated by six oxygen atoms, while each O atom forms bonds with three Sn atoms, collectively forming a stable three-dimensional crystal framework. Following the structural optimization of the SnO2 crystal utilizing the GGA + U method, the obtained lattice parameters were determined to be a = b = 4.689 Å and c = 3.131 Å. These results closely match the experimental lattice constants of a = b = 4.737 Å and c = 3.185 Å [24], confirming the accuracy and reliability of the computational method in describing geometric structures. Additionally, the calculated bandgap is 2.631 eV (as shown in Figure 2), representing a significant enhancement compared to the conventional PBE result of 0.64 eV and closer to the experimental benchmark of 3.6 eV [25]. The underestimation of the SnO2 bandgap mainly arises from the systematic errors of the PBE functional in wide-bandgap oxides, including the absence of derivative discontinuity and the incomplete cancelation of electron self-interaction, which result in an artificially lowered conduction band energy. This error has a greater impact on unoccupied states but only a minor effect on occupied states; therefore, the structural parameters, relative energetics, and defect thermodynamics remain reliable. This study focuses on the relative trends of defect formation energies, defect state distributions, and electronic effective masses before and after passivation, which are insensitive to the choice of U values and thus reliable. Compared with PBE, the GGA + U method more reasonably corrects the correlation between Sn-4d and O-2p states, significantly improving the accuracy of describing the electronic structure of SnO2, making the results closer to experimental observations and more physically rigorous.
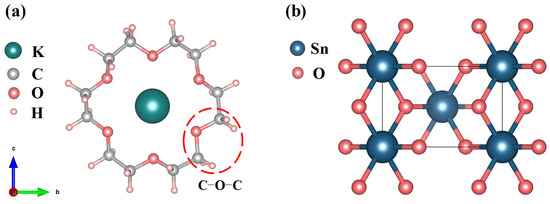
Figure 1.
(a) Crystal structure of 18C6-K+; (b) crystal structure of SnO2.

Table 1.
Comparison of passivation mechanisms in passivation molecules for SnO2.
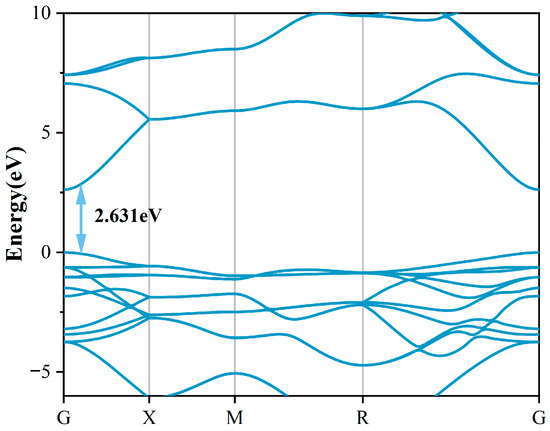
Figure 2.
Band structure of pristine SnO2.
2.2. Adsorption Behavior of 18C6-K+ on the SnO2 (110) Surface
2.2.1. Stability of the Adsorption System
Extensive experimental and theoretical studies have demonstrated that the (110) surface, owing to its lowest surface formation energy, is the most thermodynamically stable and most commonly exposed facet [31]. In SnO2 films prepared by solution processing, ALD, or sputtering, the (110) plane is consistently the dominant exposed surface, and the (110) diffraction peak remains the most prominent in XRD scans for films with a thickness ≤ 100 nm [32]. Consequently, the SnO2 (110) surface was selected in this study, and a slab with 3 × 2 × 1 supercell was constructed for surface structure. To eliminate artificial interactions between periodic images along the surface normal, a vacuum region of 15 Å was introduced. During the process of structural optimization, the bottom atomic layer was constrained, while all other atoms were fully relaxed to obtain a realistic and stable surface structure. Subsequent to surface relaxation, the 18C6-K+ was positioned on the optimized SnO2 (110) surface, and additional relaxation was executed to attain a stable adsorption configuration (see Figure 3a). The thermal stability of the adsorption configuration was assessed via ab initio molecular dynamics (AIMD) simulations conducted under the canonical (NVT) ensemble. The time step was set to one fs to ensure the precise capture of phonon vibrations, with the total simulation time fixed at 10,000 fs. As demonstrated in Figure 3b, the total energy of the system fluctuated marginally without any substantial energy drift at both 300 K and 600 K. Furthermore, the overall geometry manifested only negligible distortions, with no occurrence of bond breakage or molecular desorption. These results indicate that the constructed 18C6-K+/SnO2 (110) adsorption model exhibits excellent thermodynamic stability across a range of temperatures, suggesting that it can maintain structural integrity under practical working conditions and holds great potential for real-world applications.
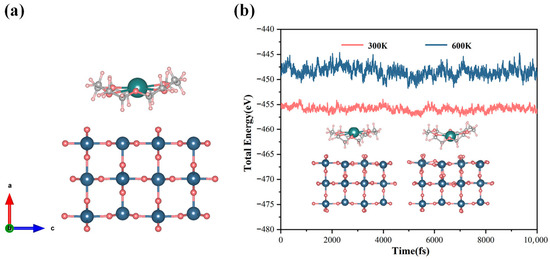
Figure 3.
(a) Optimized adsorption model of 18C6-K+ on the SnO2 (110) surface; (b) AIMD simulations of the adsorption system performed at 300 K and 600 K within the NVT ensemble framework.
Furthermore, to further verify the stability of the adsorption system, the adsorption energy of the 18C6-K+ on the SnO2 (110) surface was determined using the following equation:
where represents the total energy of 18C6-K+ adsorbed on the SnO2 (110) surface, refers to the energy of the pristine SnO2 (110) surface, and corresponds to the energy of the isolated 18C6-K+ in vacuum. The detailed energy values are listed in Table 2. The calculated adsorption energy of 18C6-K+ on the SnO2 (110) surface is −3.884 eV, indicating a strong thermodynamic favorability and robust binding interaction at the interface. This result further verifies that 18C6-K+ can form a thermodynamically stable adsorption configuration on the SnO2 (110) surface.

Table 2.
Numerical values of energy terms corresponding to adsorption energy (eV).
2.2.2. Electronic Properties of Adsorption System
To gain deeper insights into the interaction between 18C6-K+ and the SnO2 (110) surface, a systematic analysis of the projected density of states (PDOS) and charge density difference (CDD) of the adsorption system was performed. As illustrated in Figure 4a, the adsorption process leads to a noticeable electronic overlap between the Sn and O atoms on the SnO2 (110) surface and the hydrogen atoms of 18C6-K+, indicating a strong electronic interaction between 18C6-K+ and the substrate. Furthermore, as illustrated in Figure 4b, there is a depletion of charge around the H, C, and O atoms of 18C6-K+, accompanied by evident charge accumulation on the Sn and O atoms of the SnO2 (110) surface. This redistribution of charge suggests electron transfer from the ether linkages (C-O-C) in 18C6-K+ to the Sn and O atoms on the surface. This transfer results in the formation of stable Sn–ether and O–ether interactions, which, in turn, enhance the adsorption stability of the molecule on the surface.
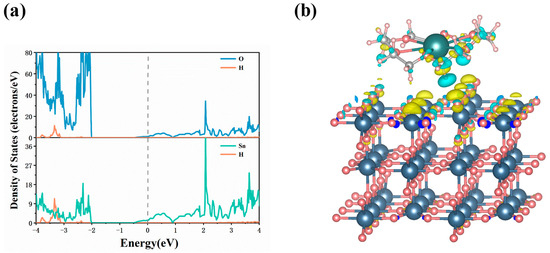
Figure 4.
(a) PDOS of the 18C6-K+ adsorbed on the SnO2 (110) surface; (b) CDD map of the adsorption system. The isosurface was set at 0.001 bohr−3, where the cyan and yellow regions represent charge depletion and accumulation, respectively.
2.3. Passivation Effect of 18C6-K+ on Surface Defects of SnO2
In this study, defective SnO2 (110) surface models were constructed (Figure 5), and 18C6-K+ was introduced onto the defect regions to investigate its passivation effect on surface defects. Six representative surface defects, Sni + SnO, Oi + OSn, VO + Sni, VSn + SnO, VSn + Sni, and Sni were selected for passivation due to their prevalence and significant impact on material properties as intrinsic or complex defect types. These defects introduce surface states that affect the electrical and optical performance of SnO2. From an energetic perspective, isolated point defects (such as single oxygen vacancies) typically exhibit high formation energies and chemical reactivity. The system tends to reduce its overall free energy—and thereby achieve greater stability—through vacancy aggregation, complexation with other defects, or by inducing surface reconstructions. So, this study focused on defect complexes, namely VO + Sni, instead of single VO. An initial evaluation was performed on the adsorption energies of 18C6-K+ across different defect sites on SnO2 surface. The definition of adsorption energy is based on the total energy difference of the supercell rather than the energy difference of a single atom; therefore, its value usually falls within the energy range of forming one or two chemical bonds. Under different defect environments, the relative magnitude of the adsorption energy can effectively reflect the differences and trends in binding strength and structural stability. More negative values reflect stronger interactions with defect sites and enhanced thermodynamic stability of the corresponding adsorption configurations. The detailed energy values are listed in Table 2. As demonstrated in Table 3, All adsorption configurations exhibit negative adsorption energies, thereby substantiating the thermodynamic favorability of the interactions. Among them, the Oi + OSn defect site is the most favorable adsorption site, with an energy of −6.117 eV, suggesting the strongest interaction with 18C6-K+.
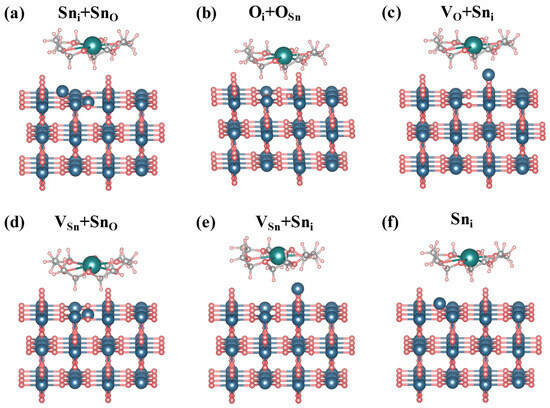
Figure 5.
Adsorption model of 18C6-K+ on defective SnO2 (110) surface. Here, “V” and “i” stand for vacancy and interstitial, respectively.

Table 3.
Adsorption energies (in eV) of 18C6-K+ on defective SnO2 (110) surface.
2.3.1. Defect Formation Energy
To assess the defect passivation effect induced by 18C6-K+ on SnO2, the formation energies of six representative surface defects of SnO2 (110), i.e., Sni + SnO, Oi + OSn, VO + Sni, VSn + SnO, VSn + Sni, and Sni, were calculated before and after 18C6-K+ adsorption under both Snrich and Orich growth conditions. The total energies of the related systems are summarized in Table 4, while the calculated defect formation energies are listed in Table 5. The defect formation energy (Ef) was determined according to the following expression:
where denotes the total energy of the system containing the defect, while refers to the total energy of the pristine (defect-free) structure. represents the number of atoms of species i that are removed from (positive ) or added to (negative ) the system, representing the chemical potential in equilibrium with the growth environment, and corresponds to the total energy of element i in its standard reference state. Although already includes the total energy of the system after structural and compositional changes, it does not account for the exchange energy with external atomic reservoirs. The term explicitly incorporates the effect of stoichiometric variation and environmental conditions, thereby ensuring the comparability of supercells with different compositions. In this work, the reference energies were set as follows: = −19.835 (eV), = −3.975 (eV), and = −9.694 (eV). The chemical potentials were determined under the thermodynamic constraint + 2=. By imposing the upper bounds, the Sn-rich and O-rich limiting conditions were defined, and the corresponding chemical potential values were obtained. These values were then substituted into the defect formation energy formula for the final calculations (see Table 5). A lower defect formation energy suggests a higher probability of defect formation under the specified chemical potential conditions.

Table 4.
Total energies (eV) of the pristine and various defective SnO2 (110) surface with 18C6-K+ adsorption.

Table 5.
Defect formation energy (Ef) values for various defect types on the SnO2 (110) surface, with/without 18C6-K+ adsorption, under Snrich and Orich growth conditions, respectively.
As demonstrated in Figure 6, the Sni + SnO, VO + Sni, VSn + SnO, VSn + Sni, and Sni defects manifest lower formation energies under Snrich condition compared to Orich condition, suggesting that these defects are more prone to form under Snrich environment. Conversely, the Oi + OSn defect exhibits a lower formation energy under Orich condition. Moreover, following the introduction of the 18C6-K+, the formation energies of all defects showed a significant increase under both Snrich and Orich conditions. These results imply that the interaction between 18C6-K+ and defect sites increases the formation energy of defects, thereby effectively inhibiting their generation. These findings suggest that 18C6-K+ has the potential to passivate interface defects, which is expected to enhance the structural stability of SnO2 materials and improve device performance.
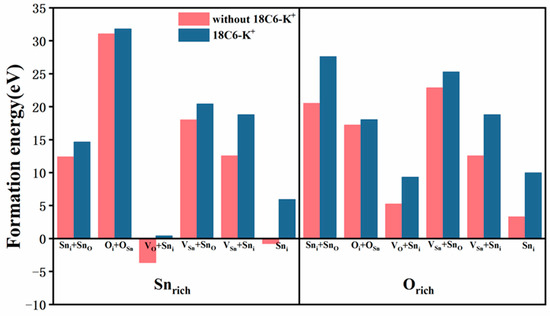
Figure 6.
Defect formation energies (Ef) of various defect configurations on the SnO2 (110) surface, with and without 18C6-K+ adsorption, under Snrich and Orich growth conditions, respectively.
2.3.2. Electron Effective Mass
This study focuses on the primary role of SnO2 as an n-type electron transport layer (ETL) in solar cells. In this system, regardless of whether a passivation layer is introduced, minority carrier holes mainly participate through recombination with electrons, and their contribution can generally be neglected. Therefore, to further evaluate the impact of the 18C6-K+ on the electronic transport characteristics of defective SnO2 (110) surface, the electron effective masses () were computed. The corresponding values are presented in Table 6. is defined by the following equation:
where h denotes Planck’s constant, k represents the electron wave-vector (momentum), and E(k) stands for the electronic energy as a function the wave vector k. The calculations were performed using a Monkhorst–Pack grid, and the electron effective mass was obtained at the conduction band minimum (CBM) at the Γ point, with the specific values determined along the Γ→R direction. The results demonstrate that, following the introduction of the 18C6-K+ to various defect sites, there is a consistent reduction in m ⁎ suggesting that the presence of the 18C6-K+ could facilitate carrier migration and enhances the overall charge transport capability on the surface. The coordination between K+ ions and the 18C6 ligand facilitates electron donation to the SnO2 (110) surface, thereby partially filling defect states and mitigating charge carrier localization effects. Concurrently, the 18C6-K+ molecule has the capacity to passivate surface defects through either physisorption or the formation of weak chemical bonds, thereby effectively reducing the density of surface states. This passivation has been demonstrated to reduce electron scattering, thereby optimizing transport performance. Furthermore, the incorporation of K+ may modulate the surface band structure to a slight extent, enhancing band delocalization and further promoting electron mobility. In summary, the 18C6-K+ could effectively reduce the electron effective mass of the SnO2 (110) surface through a synergistic combination of charge transfer and band structure modulation. In this work, the reduction in is considered only indicative evidence of improved transport trends, rather than direct proof of an absolute increase in mobility. These improvements have been shown to enhance the electron transport properties of SnO2 significantly, and they offer a novel interfacial engineering strategy for the development of high-performance electronic devices.

Table 6.
Electron effective mass () of defective SnO2 (110) surface with/without 18C6-K+ adsorption.
2.3.3. Density of States and Band Structure
A systematic evaluation was performed to further investigate the defect healing ability of 18C6-K+ on the SnO2 (110) surface. Accordingly, total density of states (DOS) analyses was carried out for six representative surface defects before and after adsorption of 18C6-K+. As shown in Figure 7a,c, after introducing 18C6 K+ to the SnO2 (110) surface containing Sni + SnO and VO + Sni defects, the defect-induced electronic states initially located in the bandgap undergo a complete disappearance. As illustrated in Figure 7b,d, for the Oi + OSn and VSn + SnO defects, the incorporation of the 18C6-K+ results in a significant reduction in the peaks of defect-related states within the bandgap, close to the valence band maximum (VBM), indicating that the 18C6-K+ displays a moderate healing effect on these defects, thereby mitigating their impact on the electronic structure to a certain extent, though not completely eliminating the associated defect states. A subsequent examination of Figure 7e,f indicates that for surfaces containing VSn + Sni and Sni defects, the defect-related peaks near the CBM are significantly reduced or nearly eliminated upon adsorption of the 18C6-K+, suggesting that the adsorption of the 18C6-K+ effectively repairs these defects and restores the electronic structure of the SnO2 (110) surface, particularly in the conduction band region, thereby significantly enhancing the material’s electron transport properties. In summary, the adsorption of 18C6-K+ on the SnO2 (110) surface can eliminate the defect states of all six types of surface defects.
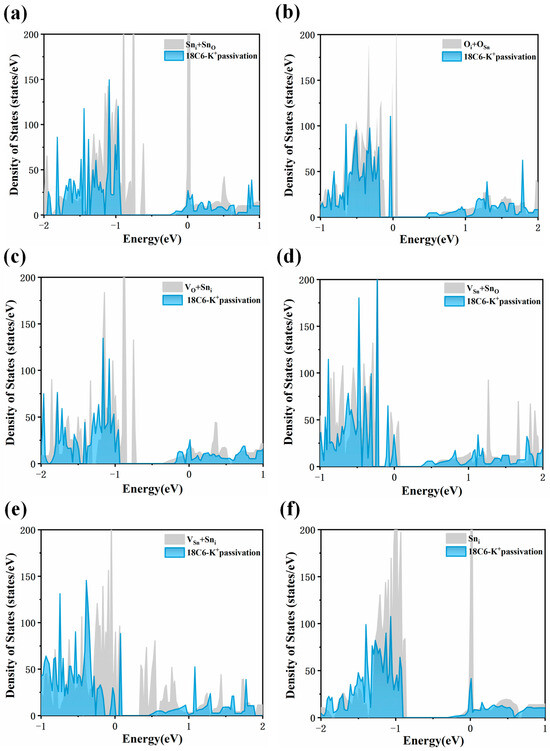
Figure 7.
DOS of defective SnO2 (110) surface with (a) Sni + SnO, (b) Oi + OSn, (c) VO + Sni, (d) VSn + SnO, (e) VSn + Sni, and (f) Sni before and after 18C6-K+ adsorption. The gray areas represent the density of states prior to adsorption, whereas the blue areas indicate the DOS following adsorption.
In addition, we calculated the band structure of the system after 18C6-K+ adsorption (Figure 8) to further verify the accuracy and reliability of the DOS results. The results show that the band curvature is highly consistent with the density of states distribution, and the defect states within the bandgap are significantly suppressed or completely eliminated after passivation. This not only agrees well with the DOS analysis, but also demonstrates that 18C6-K+ effectively eliminates defect-induced deep levels, restores a band structure close to that of ideal SnO2, and reduces the electron effective mass, thereby facilitating improved carrier transport. Therefore, with the dual verification of DOS and band structures, it can be clearly confirmed that the adsorption of 18C6-K+ effectively repairs band distortions caused by multiple defects and significantly improves the electronic structure and transport properties of the SnO2 (110) surface. In particular, strong passivation effects were observed for the Sni + SnO, VO + Sni, VSn + Sni, and Sni defects, thereby effectively enhancing the electronic structure and interfacial characteristics.
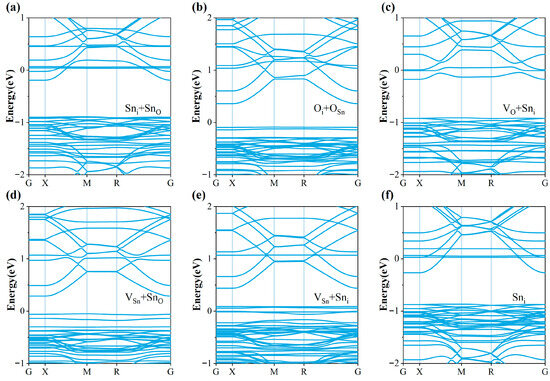
Figure 8.
Band structures of SnO2 (110) surfaces with (a) Sni + SnO, (b) Oi + OSn, (c) VO + Sni, (d) VSn + SnO, (e) VSn + Sni, (f) Sni defects after 18C6-K+ adsorption.
2.3.4. Charge Density Difference
To better understand the 18C6-K+ adsorption mechanism and charge transfer on SnO2 (110), charge density difference (CDD) analyses were performed. The CDD is computed using the following equation:
where and represent the charge densities of the 18C6-K+ adsorbed on the SnO2 (110) surface, the bare SnO2 (110) surface, and the isolated 18C6-K+ molecule, respectively. As shown in Figure 9, the 18C6-K+ molecule exhibits significant charge transfer behavior across all investigated defect configurations, indicating its strong capability for defect passivation. Specifically, when 18C6-K+ is adsorbed at the Sni + SnO defect site as shown in Figure 9a, pronounced charge depletion occurs around the hydrogen, carbon, and oxygen atoms of the molecule, suggesting that electrons are transferred from the molecule to the SnO2 surface. Simultaneously, charge accumulation is observed around the adjacent tin atoms and a portion of the oxygen atoms near the defect region, indicating the formation of interfacial interactions dominated by tin ether coordination, complemented by oxygen ether coordination. This electron redistribution enhances the coupling between the molecule and the defect site, thereby enabling effective passivation of the Sni + SnO defect. In the VO + Sni (Figure 9c), VSn + SnO (Figure 9d), and Sni (Figure 9f) defect configurations, comparable charge depletion occurs around the hydrogen, carbon, and oxygen atoms of the 18C6-K+ molecule, while charge accumulation is primarily located near the tin atoms on the SnO2 surface. These findings suggest that electron transfer predominantly occurs from the molecule’s ether groups (C–O–C) to the surface tin atoms, facilitating the formation of tin–ether bonds which effectively passivate surface defects and enhance interfacial stability. For the Oi + OSn defect site shown in Figure 9b, charge depletion remains concentrated within the 18C6-K+ molecule, whereas charge accumulation predominantly occurs around the surface oxygen atoms of SnO2. This suggests that electrons are transferred from the ether groups to surface oxygen atoms, forming oxygen ether interactions that contribute to the repair of oxygen related defects. In the VSn + Sni defect configuration shown in Figure 9e, the hydrogen, carbon, and oxygen atoms of 18C6-K+ continue to show charge depletion, while charge accumulation simultaneously appears around both tin and oxygen atoms near the defect site. This observation reflects the complexity of this particular defect environment, where the coexistence of tin ether and oxygen ether interactions enables synergistic passivation of the complex defect structure. In summary, the 18C6-K+ donates electrons from its ether (C-O-C) groups to Sn and O atoms at the defect sites on the SnO2 (110) surface, forming Sn-ether and O-ether interactions. These interactions enable effective passivation of diverse surface defects and substantially enhance the electronic structure of the SnO2 (110) surface.
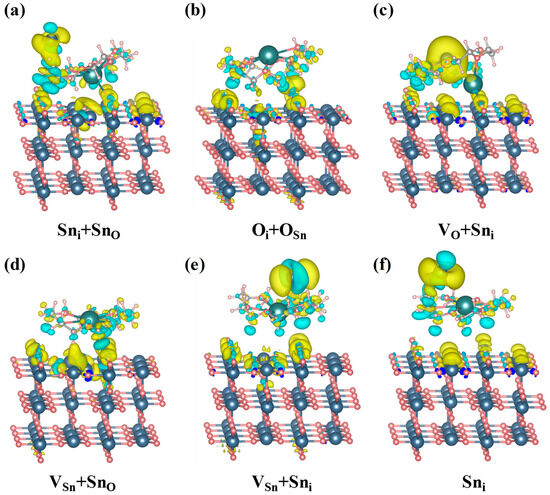
Figure 9.
CDD maps of the 18C6-K+ adsorbed on defective SnO2 (110) surface with (a) Sni + SnO, (b) Oi + OSn, (c) VO + Sni, (d) VSn + SnO, (e) VSn + Sni, and (f) Sni. The isosurface was set at 0.001 bohr−3, where the cyan and yellow regions represent charge depletion and accumulation, respectively.
To quantitatively evaluate the charge transfer results revealed by CDD, Bader charge analysis was performed (see Table 7). The results showed that the H, C, and O atoms in the 18C6-K+ molecule generally lose electrons, while the Sn or O atoms at the defect sites on the surface gain electrons, which is highly consistent with the charge density redistribution trend observed in CDD. Specifically, in Figure 9a, the C and O atoms lose approximately 0.49 e and 1.14 e, respectively, while the Sn and O atoms on the SnO2 (110) surface gain about 2.5 e and 1.29 e, respectively. In Figure 9c, the C and O atoms lose about 0.52 e and 1.09 e, whereas the Sn and surface O atoms gain about 1.69 e and 1.17 e, respectively. Other defect models exhibit the same electron transfer pattern. In summary, the Bader charge analysis and CDD results mutually corroborate each other, quantitatively confirming the mechanism of electron transfer from 18C6-K+ to the defect regions of SnO2.

Table 7.
Bader charge analysis of the adsorption systems (“+” indicates electron gain; “−” indicates electron loss; “surface” refers to atoms of the SnO2 (110) surface).
Meanwhile, 18C6-K+ effectively suppresses deep defect states within the bandgap through coordination interactions and charge compensation. Such defect states typically act as recombination centers that shorten carrier lifetimes. Their suppression implies reduced recombination pathways, thereby potentially extending carrier lifetimes. The density of states and charge density difference results support the reasonable inference that 18C6-K+ contributes to the dual improvement of both effective mass and carrier lifetime.
3. Computational Methods
In this study, structural optimizations, total energy computations, and evaluations of interfacial characteristics were conducted through first-principles methods based on density functional theory (DFT) [33], utilizing the Vienna Ab initio Simulation Package (VASP) (Version 6.4.1) [34]. The projector augmented wave (PAW) method was employed to model the electron–ion interactions [35], the exchange and correlation contributions were addressed by the Perdew–Burke–Ernzerhof (PBE) functional under the generalized gradient approximation (GGA) framework [36]. However, the GGA method often falls short in accurately describing the properties of strongly correlated systems, especially those involving highly localized d or f electrons [37]. To overcome this shortcoming, the GGA + U approach, incorporating the Hubbard U correction, was utilized in this study to better capture the behavior of localized electronic states [38]. While the valence electrons of Sn atoms in SnO2 are mainly derived from s and p orbitals, the 4d orbitals also play a significant role in shaping the material’s electronic structure [39]. To better account for the localized behavior of these 4d states and their effects on the band structure, optical response, and charge transport, the generalized gradient approximation with Hubbard U correction (GGA + U) was applied in this work. The Hubbard U values were obtained via the linear response approach, resulting in 4.17 eV for Sn 4d states and 8.4 eV for O 2p states. Consequently, on-site Coulomb interaction parameters of U(Sn,d) = 4.17 eV and U(O,p) = 8.4 eV were assigned to the 4d105s25p2 electron configuration of Sn and the 2s22p4 configuration of O, respectively. A 4 × 4 × 1 k-point mesh generated by the Monkhorst–Pack scheme was used for Brillouin zone sampling, and the plane-wave basis set was truncated at an energy cut-off of 500 eV. Convergence thresholds were set as 0.02 eV/nm for atomic forces, 1 × 10−5 eV/atom for the total energy in self-consistent field (SCF) calculations, and 0.02 GPa for the residual stress.
4. Conclusions
This study employed first-principles calculations to systematically explore the defect passivation mechanisms of 18C6-K+ at six representative defect sites (i.e., Sni + SnO, Oi + OSn, VO + Sni, VSn + SnO, VSn + Sni, and Sni) of the SnO2 (110) surface. These intrinsic and complex defects are commonly present and significantly influence the material’s performance by creating surface defect states that alter the electrical and optical behavior of SnO2. The results confirmed that the 18C6-K+ molecule can stably adsorb at all six defect sites, exhibiting favorable thermodynamic stability. Moreover, inducing 18C6-K+ on SnO2 (110) surface could increase defect formation energies, indicating a strong potential for suppressing defect generation. Furthermore, the presence of 18C6-K+ has been shown to reduce the electron effective mass of defective systems, thereby enhancing electron mobility and interfacial charge transport efficiency. DOS analyses revealed that 18C6-K+ exhibits strong passivation effects on Sni + SnO, VO + Sni, VSn + Sni, and Sni defects, while also partially mitigating the electronic disturbances caused by Oi + OSn and VSn + SnO defects. Consequently, this results in improved structural stability and electronic performance of SnO2. The corresponding band structure results are highly consistent with the DOS analysis, further confirming its effective defect-healing capability. CDD analysis substantiated that the 18C6-K+ molecule forms Sn-ether and O-ether interactions via its ether bonds (C-O-C) with surface Sn and O atoms, effectively passivating defect states and suppressing SnO2 decomposition. The Bader charge results indicate that the H, C, and O atoms in the 18C6-K+ molecule generally lose electrons, while the Sn or O atoms at the surface defect sites gain electrons. The Bader charge analysis is consistent with the CDD results, quantitatively confirming the mechanism of electron transfer from 18C6-K+ to the SnO2 defect regions. In summary, 18C6-K+ functions as an efficient molecular passivator, providing a promising interfacial engineering strategy and theoretical basis for enhancing the performance of SnO2-based materials and devices.
Author Contributions
Conceptualization, S.Y. and Y.Z.; Methodology, S.Y., Q.Z. and Q.S.; Software, S.Y., Q.Z. and X.Z. (Xihua Zhang); Validation, S.W., X.Z. (Xihua Zhang) and X.J.; Formal analysis, S.Y., Y.W. and Q.Z.; Investigation, S.Y., Q.Z., J.W., Y.W. and H.Z.; Resources, Q.S., Y.Z. and S.W.; Data curation, Y.D. and W.L.; Writing—original draft, S.Y. and Y.Z.; Writing—review & editing, Q.S. and Y.Z.; Visualization, W.L., J.W., X.Z. (Xintong Zhao), L.Z., H.Z. and X.J.; Supervision, Y.Z., S.W. and Q.S.; Funding acquisition, Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC, Grant No. 62104209).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Das, S.; Jayaraman, V. SnO2: A comprehensive review on structures and gas sensors. Prog. Mater. Sci. 2014, 66, 112–255. [Google Scholar] [CrossRef]
- Ye, P.; Li, M.; Fu, W.; Wei, H.; E, W.; Xiao, X.; He, Y. Nb-doped ZrxSn1−xO2: Experimental and first-principles study. J. Appl. Phys. 2021, 130, 015702. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Lee, Y.; Paek, S.; Cho, K.T.; Oveisi, E.; Gao, P.; Lee, S.; Park, J.-S.; Zhang, Y.; Humphry-Baker, R.; Asiri, A.M.; et al. Enhanced charge collection with passivation of the tin oxide layer in planar perovskite solar cells. J. Mater. Chem. A 2017, 5, 12729–12734. [Google Scholar] [CrossRef]
- Zhao, W.; Lin, H.; Li, Y.; Wang, D.; Wang, J.; Liu, Z.; Yuan, N.; Ding, J.; Wang, Q.; Liu, S. Symmetrical Acceptor–Donor–Acceptor Molecule as a Versatile Defect Passivation Agent toward Efficient FA0.85MA0.15PbI3 Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2112032. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, T.; Chang, Q.; Ma, C. Study on the intrinsic defects in tin oxide with first-principles method. J. Phys. Chem. Solids 2018, 115, 228–232. [Google Scholar] [CrossRef]
- Soe, K.T.; Thansamai, S.; Thongprong, N.; Ruengsrisang, W.; Muhammad, I.A.; Ketsombun, E.; Supruangnet, R.; Kaewprajak, A.; Kumnorkaew, P.; Saetang, V.; et al. Simultaneous Surface Modification and Defect Passivation on Tin Oxide–Perovskite Interfaces Using Pseudohalide Salt of Sodium Tetrafluoroborate. Sol. RRL 2023, 7, 2200964. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Wang, M.; Liu, C.; Liu, Q.; Wang, W.; Zhang, Z.; Han, R.; Ji, N. Computational design and experimental validation of monometallic-doped SnO2 catalysts for selective catalytic oxidation of ammonia. Appl. Catal. B Environ. 2024, 340, 123256. [Google Scholar] [CrossRef]
- Wang, K.-P.; Dong, X.; Yuan, J.-Z.; Wen, B.; He, J.; Tong, C.-J.; Prezhdo, O.V. Self-Passivation at the SnO2/Perovskite Interface. ACS Energy Lett. 2025, 10, 1466–1473. [Google Scholar] [CrossRef]
- Geng, X.; Luo, G.; Zhang, Y.; Ren, P.; Zhang, L.; Ling, X.; Zeng, J.; Wu, X.; Xu, L.; Lin, P.; et al. Multifunctional Regulation of Chemical Bath Deposition Based SnO2 for Efficient Perovskite Solar Cells. Small 2025, 21, 2406696. [Google Scholar] [CrossRef]
- Fang, D.; Niu, T.; Chen, Z.; Zhang, J.; Zhang, Z.; Zhou, S.; Liu, H.; Chen, G.; Fu, N.; Xue, Q.; et al. Lignin carbon dots as effective dopants and passivators for SnO2 electron transport layers to achieve high-performance perovskite solar cells. J. Power Sources 2025, 634, 236497. [Google Scholar] [CrossRef]
- Zhang, F.; Duan, L.; Zhu, X.; Zhou, Z.; Yu, D.; Zhang, Z.; Yang, D. Magnesium Acetate Key Enhancers for Electron Transport Layers in Highly Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2025, 17, 32566–32574. [Google Scholar] [CrossRef]
- Adnan, M.; Lee, W.; Irshad, Z.; Kim, S.; Yun, S.; Han, H.; Chang, H.S.; Lim, J. Managing Interfacial Defects and Charge-Carriers Dynamics by a Cesium-Doped SnO2 for Air Stable Perovskite Solar Cells. Small 2024, 20, 2402268. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Lumbreras, M.; Siadat, M.; van Landschoot, R.C.; Schoonman, J. Electrostatic sprayed SnO2 and Cu-doped SnO2 films for H2S detection. Sens. Actuators B Chem. 2008, 133, 694–698. [Google Scholar] [CrossRef]
- Feng, X.; Lv, X.; Cao, J.; Tang, Y. Continuous Modification of Perovskite Film by a Eu Complex to Fabricate the Thermal and UV-Light-Stable Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 55538–55547. [Google Scholar] [CrossRef] [PubMed]
- Grini, S.; Sopiha, K.V.; Ross, N.; Liu, X.; Bjørheim, T.S.; Platzer-Björkman, C.; Persson, C.; Vines, L. Strong Interplay between Sodium and Oxygen in Kesterite Absorbers: Complex Formation, Incorporation, and Tailoring Depth Distributions. Adv. Energy Mater. 2019, 9, 1900740. [Google Scholar] [CrossRef]
- Skara, G.; Gimferrer, M.; De Proft, F.; Salvador, P.; Pinter, B. Scrutinizing the Noninnocence of Quinone Ligands in Ruthenium Complexes: Insights from Structural, Electronic, Energy, and Effective Oxidation State Analyses. Inorg. Chem. 2016, 55, 2185–2199. [Google Scholar] [CrossRef]
- Yang, B.; Suo, J.; Bogachuk, D.; Kaiser, W.; Baretzky, C.; Er-Raji, O.; Loukeris, G.; Alothman, A.A.; Mosconi, E.; Kohlstädt, M.; et al. A universal ligand for lead coordination and tailored crystal growth in perovskite solar cells. Energy Environ. Sci. 2024, 17, 1549–1558. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, D.; Deng, B.; Du, J.; Choy, W.C.H.; Tian, J. High Efficiency Inorganic Perovskite Solar Cells Based on Low Trap Density and High Carrier Mobility CsPbI3 Films. Adv. Funct. Mater. 2022, 32, 2209070. [Google Scholar] [CrossRef]
- Wang, K.; Liu, J.; Yin, J.; Aydin, E.; Harrison, G.T.; Liu, W.; Chen, S.; Mohammed, O.F.; De Wolf, S. Defect Passivation in Perovskite Solar Cells by Cyano-Based π-Conjugated Molecules for Improved Performance and Stability. Adv. Funct. Mater. 2020, 30, 2002861. [Google Scholar] [CrossRef]
- Koseki, D.; Senevirathne, C.A.M.; Senba, D.; Fujita, Y.; Lin, J.; Zhai, M.; Shang, J.; Raju, T.B.; Ida, S.; Watanabe, M.; et al. Elucidating the mechanism of perovskite surface passivation with organic molecules: The impact of π-conjugation length. J. Mater. Chem. A 2025, 13, 17783–17798. [Google Scholar] [CrossRef]
- Xiao, W.-Z.; Wang, L.-L.; Xu, L.; Wan, Q.; Zou, B.S. Magnetic properties in Nitrogen-doped SnO2 from first-principle study. Solid State Commun. 2009, 149, 1304–1307. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Y.-F.; Li, Y.; Ding, K.-N.; Li, J.-Q.; Xu, Y.-J. Structural characterizations and electronic properties of Ti-doped SnO2 surface: A first-principles study. J. Chem. Phys. 2006, 124, 054704. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Huang, S.; Wang, Z. First principle study on the electronic structure of fluorine-doped SnO2. Solid State Commun. 2009, 149, 527–531. [Google Scholar] [CrossRef]
- Chen, Q.; Zeng, G.; Song, H.; Zheng, J.; Feng, L. Influence of SnO2 films with high resistance on the performance of CdTe solar cells. J. Mater. Sci. Mater. Electron. 2009, 20, 661–665. [Google Scholar]
- Duan, M.; Mi, X.; Xia, J.; Yang, Y.; Hu, R.; Li, X.; Zhang, Y.; Huang, F.; Qin, P. Oriented molecular modulation of SnO2 nanoparticles enabled bilateral passivation toward efficient and stable perovskite solar cells. J. Energy Chem. 2025, 109, 109–119. [Google Scholar] [CrossRef]
- Cheng, W.; Huang, P.; Gao, Z.; Chen, Y.; Ren, L.; Feng, Q.; Liu, X.; Ahmad, S.; Zhou, Z. Molecular Bridging of Buried Interface Flattens Grain Boundary Grooves and Imparts Stress Relaxation for Performance Enhancement and UV Stability in Perovskite Solar Cells. Adv. Energy Mater. 2025, 15, 2501296. [Google Scholar] [CrossRef]
- Dong, J.; Li, S.; Ji, W.; Li, K.; Yin, Z.; Liu, Y.; Xu, L.; Li, Y.; Ma, R. Modification of SnO2 buried interface with thiourea to enhance the performance of perovskite solar cells. Opt. Mater. 2024, 154, 115737. [Google Scholar] [CrossRef]
- Mohammed, M.K.A. Highly Efficient Perovskite Photovoltaics Enabled by Molecular Bridging at the SnO2/Perovskite Interface. Langmuir 2025, 41, 16960–16969. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Zhao, Y.; Zhang, Z.; Zheng, Y.; Zhang, S. Modifying the buried interface by a sulfamate enable efficient perovskite solar cells with high stability. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135622. [Google Scholar] [CrossRef]
- Zou, X.; Ding, K.; Zhang, Y.; Yao, S. Acetonitrile adsorption and decomposition on the SnO2 (110) surface: A first-principles computation. Theor. Chem. Acc. 2011, 128, 63–67. [Google Scholar] [CrossRef]
- Batzill, M. Surface Science Studies of Gas Sensing Materials: SnO2. Sensors 2006, 6, 1345–1366. [Google Scholar] [CrossRef]
- Lipsky, F.; Gouveia, A.F.; Sensato, F.R.; Oliva, M.; Longo, E.; San-Miguel, M.A.; Andrés, J. Effective sensing mechanisms of O2 and CO on SnO2 (110) surface: A DFT study. J. Mater. Chem. A 2025, 13, 918–927. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, G.; Zhu, Y.; Yin, W.-J.; Yan, Y.; Al-Jassim, M.; Pennycook, S.J. LDA+U/GGA+U calculations of structural and electronic properties of CdTe: Dependence on the effective U parameter. Comput. Mater. Sci. 2015, 98, 18–23. [Google Scholar] [CrossRef]
- Shi, L.-B.; Dong, H.-K.; Qi, G.-Q. Density functional theory description of origin of ferromagnetism in Cu doped SnO2. J. Magn. Magn. Mater. 2013, 345, 215–221. [Google Scholar]
- De Padova, P.; Fanfoni, M.; Larciprete, R.; Mangiantini, M.; Priori, S.; Perfetti, P. A synchrotron radiation photoemission study of the oxidation of tin. Surf. Sci. 1994, 313, 379–391. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).