Electrochemical Sensors and Platforms: Design and Application
A topical collection in Sensors (ISSN 1424-8220). This collection belongs to the section "Chemical Sensors".
Viewed by 54161Editors
Interests: electrochemistry; voltammetry; electrochemical impedance spectroscopy; antioxidants; macrocyclic chemistry; metal complexes; modified electrodes
Special Issues, Collections and Topics in MDPI journals
Interests: bioinorganic chemistry; inorganic chemistry/spontaneous precipitation; precipitation in vitro; precipitation with additives; pathological biomineralization; biomineralization; mineralogy; material science; crystallization
Special Issues, Collections and Topics in MDPI journals
Interests: electrochemistry; voltammetry; redox mechanisms; bioactive compounds
Topical Collection Information
Dear Colleagues,
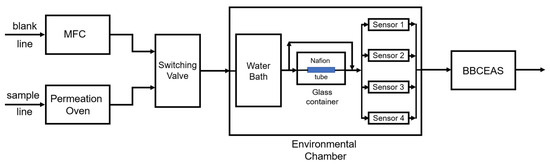
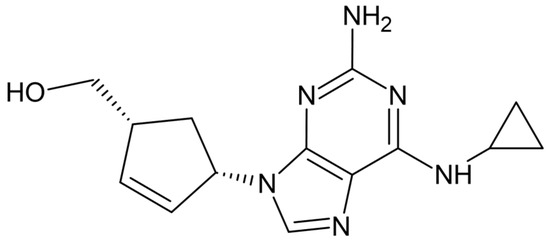
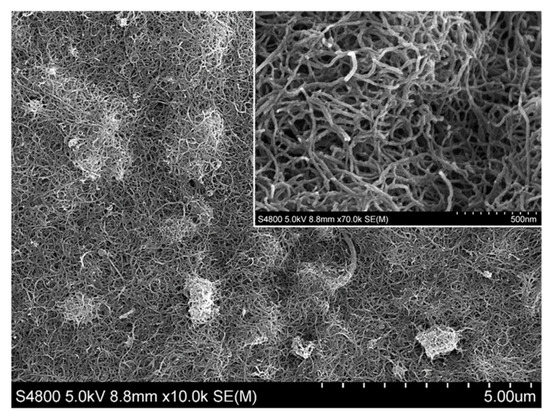
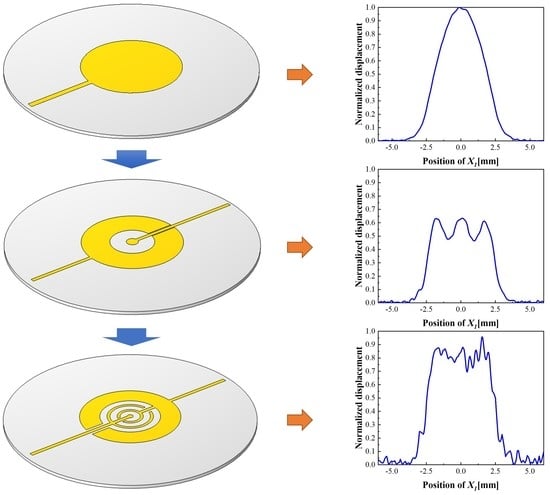
At present, electrochemical sensors are widely used in routine analytical measurements as well as in different areas of scientific research. Thanks to the long-lasting devotion and work of a large number of pioneering research groups, certain electrochemical sensors excel in terms of a short response time, high selectivity, sensitivity, lower limit of detection, etc. The flexibility of sensors’ structure and size allow their application in solving reaction mechanisms, in studying the fine details of biological processes or corrosion steps, etc. This has obviously led to a growing amount of interest in the field of electrochemical sensor development. Applications of new mechanisms and materials (such as ceramics, conductive polymers, carbon nanomaterials, semiconductors, metals, etc.) are being tested to improve selective analyte recognition, as well as for signal formation or transmission. New manufacturing methods have been developed or tested for the fabrication of advanced sensors with improved measuring properties.
This Special Issue entitled “Electrochemical Sensors and Platforms: Design and Application” aims to highlight the current state-of-the-art in the field of sensor design as well as the application of sensors in various analytical tasks (such as environmental monitoring, food analysis, medical applications, and other fields). We invite you to contribute to this Special Issue. Review articles, short communications, and full-size research papers are all welcome.
Dr. Martina Medvidović-Kosanović
Dr. Anamarija Stanković
Dr. Ivana Novak Jovanović
Dr. Géza Nagy
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Sensors is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2600 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- Electrochemical sensors
- Sensor design
- Sensor characterization techniques
- Intelligent processing of sensor materials
- Chemical change monitoring
- Electroanalytical techniques