Comparison of Chemical and Electrochemical Approaches to Abacavir Oxidative Stability Testing
Abstract
1. Introduction
2. Materials and Methods
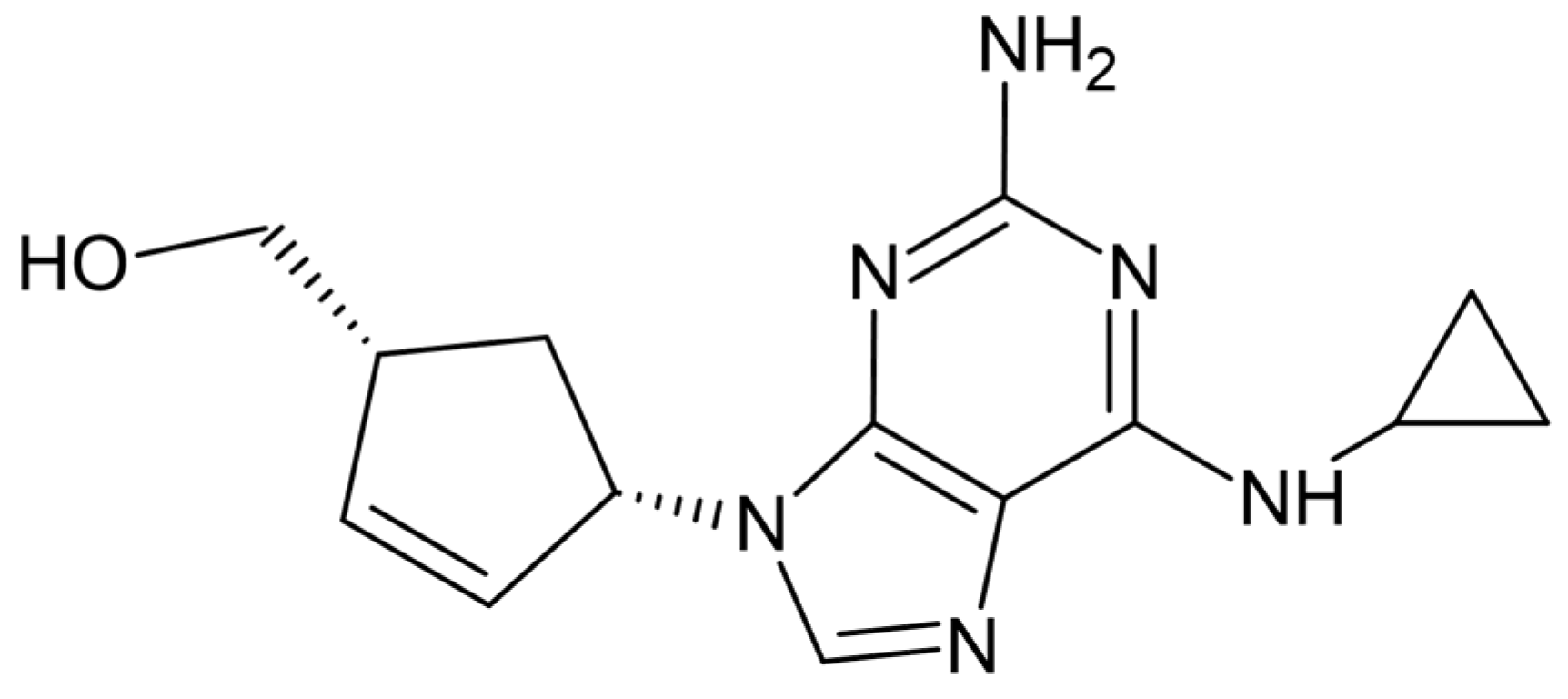
2.1. Chemicals
2.2. Instruments
2.3. Chemical Oxidation
2.4. Electrochemical Oxidation
2.4.1. Apparatus with Platinum Working Electrode
2.4.2. Apparatus with BDD Working Electrode
2.5. UHPLC/MS Method
3. Results and Discussion
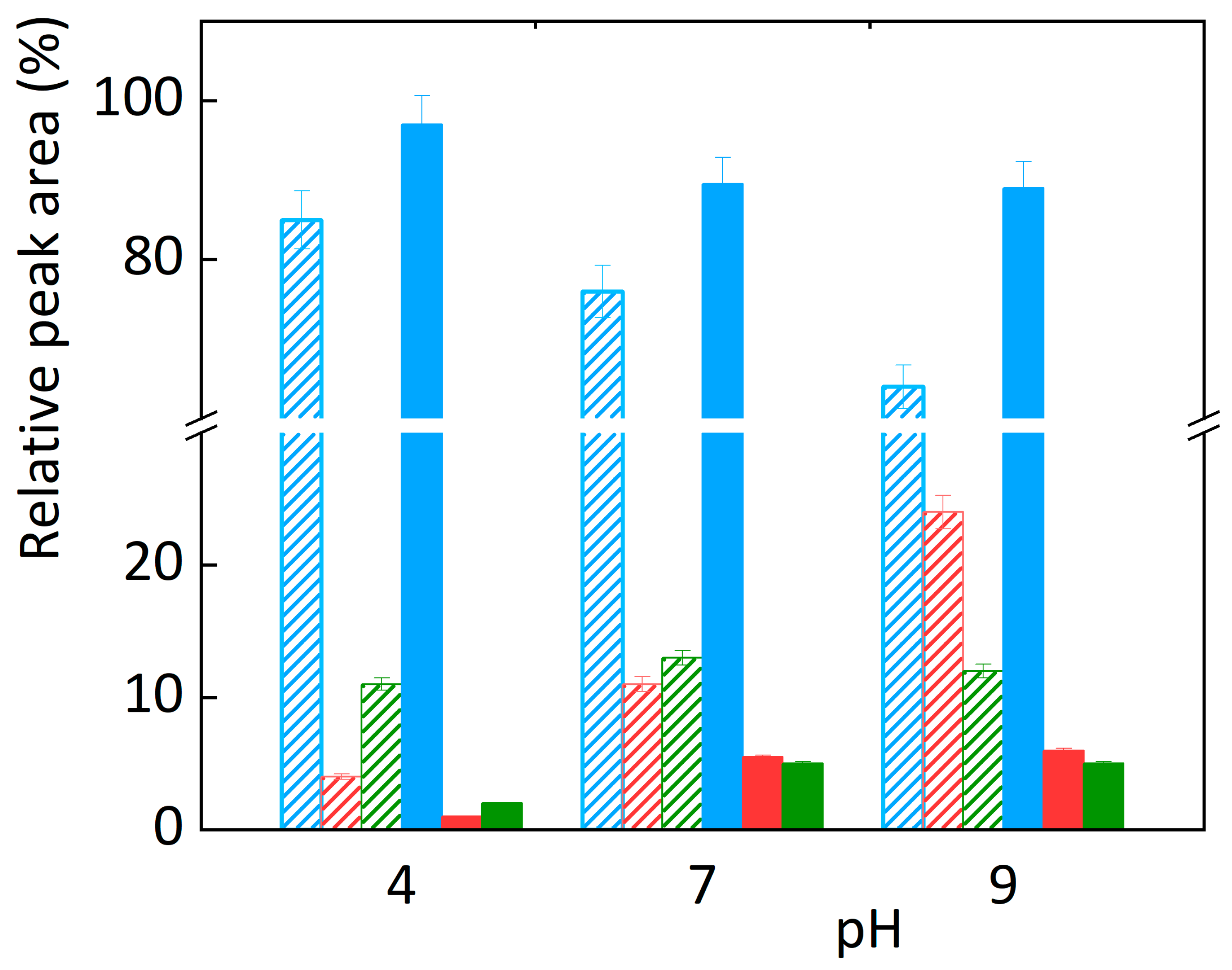
3.1. Chemical Oxidation
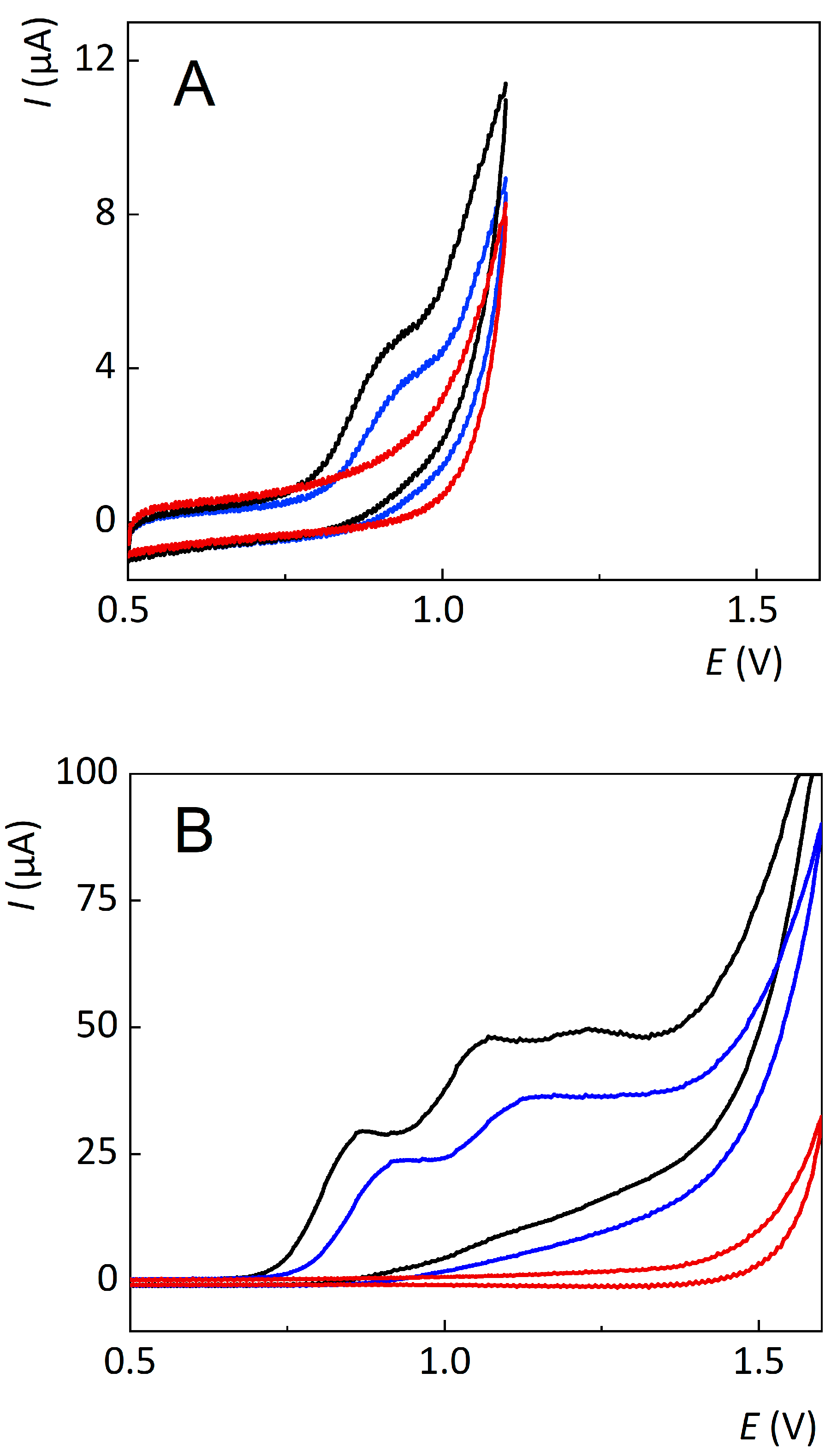
3.2. Electrochemical Oxidation
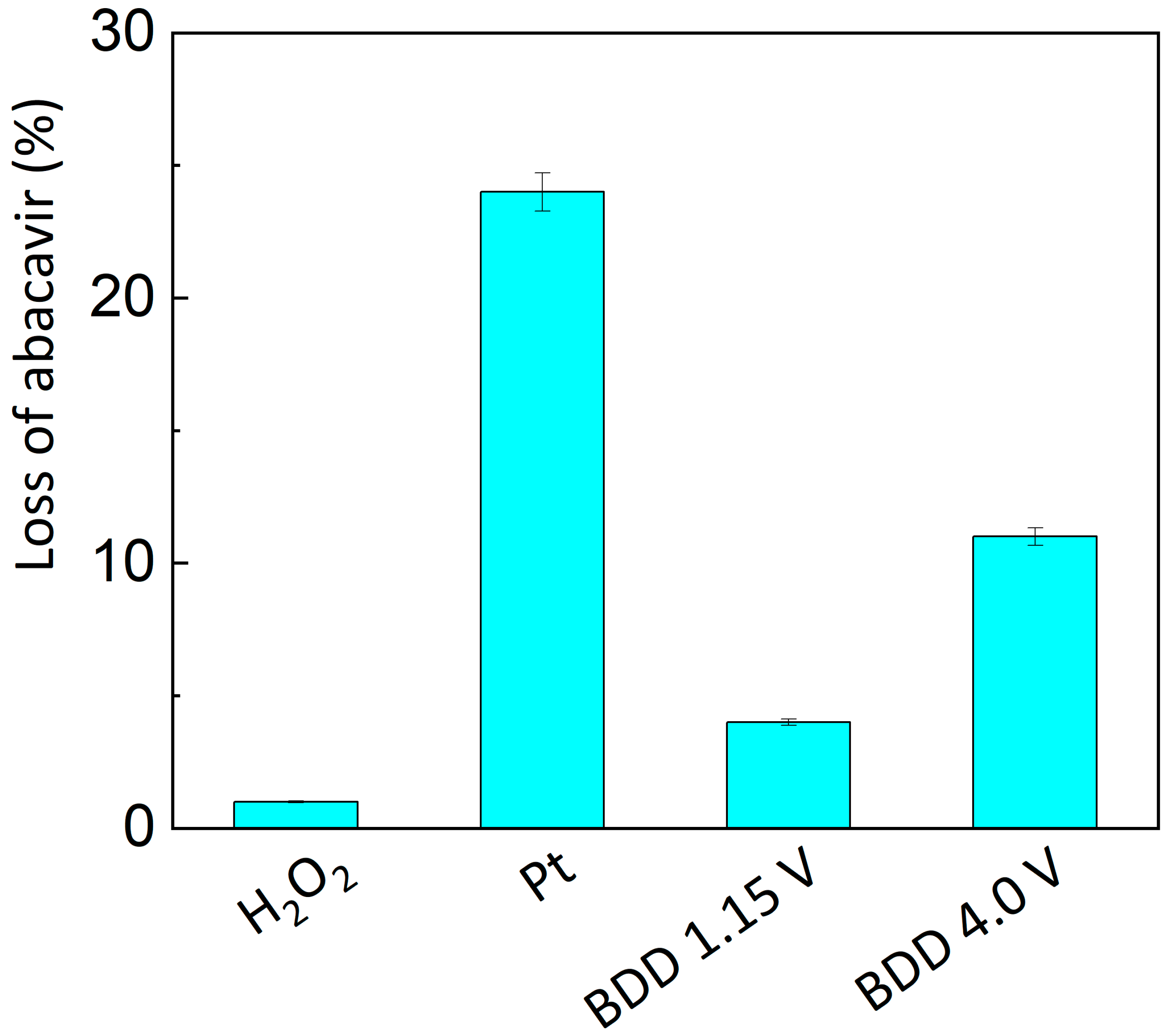
3.3. Comparison of Electrochemical and Chemical Oxidation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Rehman, Z. Current trends in forced degradation study for pharmaceutical product development. J. Pharm. Educ. Res. 2012, 3, 54–63. [Google Scholar]
- Iram, F.; Iram, H.; Iqbal, A.; Husain, A. Forced degradation studies. J. Anal. Pharm. Res. 2016, 3, 73. [Google Scholar] [CrossRef]
- Seshachalam, U.; Haribabu, B.; Chandrasekhar, K.B. Development and validation of a reverse-phase liquid chromatographic method for assay and related substances of abacavir sulfate. J. Sep. Sci. 2007, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. ICH Q1A (R2) Stability testing of new drug substances and drug products. In Note for Guidance on Stability Testing: Stability Testing of New Drug Substances and Products; CPMP/ICH/2736/99; European Medicines Agency: London, UK, 2003. [Google Scholar]
- Venkataraman, S.; Manasa, M. Forced degradation studies: Regulatory guidance, characterization of drugs, and their degradation products—A review. Drug Invention Today 2018, 10, 1–10. [Google Scholar]
- Bajaj, S.; Singla, D.; Sakhuja, N. Stability testing of pharmaceutical products. J. Appl. Pharm. Sci. 2012, 2, 129–138. [Google Scholar] [CrossRef]
- Brummer, H. How to approach a forced degradation study. Life Sci. Tech. Bull. 2011, 31, 1–4. [Google Scholar]
- Venkatesh, D.N.; Kumar, S.D.S. Forced Degradation—A Review. Biomed. J. Sci. Tech. Res. 2022, 47, 38381–38394. [Google Scholar] [CrossRef]
- Mullani, A.K.; Nargatti, P.I. Forced degradation study—A new approach for stress testing of drug substances and drug products. Int. J. Pharm. Sci. 2021, 12, 2683–2691. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Alsante, K.M.; Reed, R.A. Pharmaceutical Stress Testing, Predicting Drug Degradation, 2nd ed.; Taylor and Francis: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Martinez-Huitle, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Yu, X.M.; Zhou, M.H.; Hu, Y.S.; Serrano, K.G.; Yu, F.K. Recent updates on electrochemical degradation of bio-refractory organic pollutants using BDD anode: A mini review. Environ. Sci. Pollut. Res. 2014, 21, 8417–8431. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.A.; Calzadilla, W.; Salazar, R. Influence of reactor design on the electrochemical oxidation and disinfection of wastewaters using boron-doped diamond electrodes. Curr. Opin. Electrochem. 2022, 33, 100939. [Google Scholar] [CrossRef]
- Qiao, J.; Xiong, Y.Z. Electrochemical oxidation technology: A review of its application in high-efficiency treatment of wastewater containing persistent organic pollutants. J. Water Process. Eng. 2021, 44, 102308. [Google Scholar] [CrossRef]
- Dominguez, J.R.; Gonzalez, T.; Palo, P.; Sanchez-Martin, J.; Rodrigo, M.A.; Saez, C. Electrochemical degradation of a real pharmaceutical effluent. Water Air Soil Pollut. 2012, 223, 2685–2694. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, Y.Y.; Yao, B.; Yang, J.; Zhi, D. Current progress in electrochemical anodic-oxidation of pharmaceuticals: Mechanisms, influencing factors, and new technique. J. Hazard. Mater. 2021, 418, 126313. [Google Scholar] [CrossRef]
- Brillas, E.; Sires, I. Electrochemical removal of pharmaceuticals from water streams: Reactivity elucidation by mass spectrometry. Trends Anal. Chem. 2015, 70, 112–121. [Google Scholar] [CrossRef]
- Zhou, C.Z.; Wang, Y.P.; Chen, J.; Xu, L.; Huang, H.M.; Niu, J.F. High-efficiency electrochemical degradation of antiviral drug abacavir using a penetration flux porous Ti/SnO2-Sb anode. Chemosphere 2019, 225, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Vymyslicky, F.; Krizek, T.; Kozlik, P.; Kubickova, A.; Hert, J.; Bartosinska, E. Alternative method for canagliflozin oxidation analysis using an electrochemical flow cell—Comparative study. J. Pharm. Biomed. Anal. 2022, 207, 114341. [Google Scholar] [CrossRef]
- Bartosinska, E.; Kozlik, P.; Kubickova, A.; Hert, J.; Fischer, J.; Krizek, T. Comparison of static and dynamic mode in the electrochemical oxidation of fesoterodine with the use of experimental design approach. Talanta 2021, 226, 122141. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.P.; Ma, C.A.; Feng, H. A novel PbO2 electrode preparation and its application in organic degradation. Electrochim. Acta 2008, 53, 3002–3006. [Google Scholar] [CrossRef]
- Johnson, D.C.; Feng, J.; Houk, L.L. Direct electrochemical degradation of organic wastes in aqueous media. Electrochim. Acta 2000, 46, 323–330. [Google Scholar] [CrossRef]
- Zhu, X.Y.; Chen, X.J.; Yang, Z.M.; Liu, Y.; Zhou, Z.Y.; Ren, Z.Q. Investigating the influences of electrode material property on degradation behavior of organic wastewaters by iron-carbon micro-electrolysis. Chem. Eng. J. 2018, 338, 46–54. [Google Scholar] [CrossRef]
- Abdel-Hamid, R.; Newair, E.F. Electrochemical behavior of antioxidants: I. Mechanistic study on electrochemical oxidation of gallic acid in aqueous solutions at glassy-carbon electrode. J. Electroanal. Chem. 2011, 657, 107–112. [Google Scholar] [CrossRef]
- Musilova, J.; Barek, J.; Peckova, K. The use of boron-doped diamond film electrodes for detection of organic compounds. Chem. Listy 2009, 103, 469–478. [Google Scholar]
- McDowell, J.A.; Chittick, G.E.; Ravitch, J.R.; Polk, R.E.; Kerkering, T.M.; Stein, D.S. Pharmacokinetics of C-14 abacavir, a human immunodeficiency virus type 1 (HIV-1) reverse transcriptase inhibitor, administered in a single oral dose to HIV-1-infected adults: A mass balance study. Antimicrob. Agents Chemother. 1999, 43, 2855–2861. [Google Scholar] [CrossRef]
- Fernandez, J.V.; Munir, A. Abacavir; StatPearls Publishing: Treasure Island, CA, USA, 2002. [Google Scholar]
- Vukkum, P.; Deshpande, G.R.; Babu, J.M.; Muralikrishna, R.; Jagu, P. Stress degradation behavior of abacavir sulfate and development of a suitable stability-indicating UHPLC method for the determination of abacavir, its related substances, and degradation products. Sci. Pharm. 2012, 80, 903–922. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.N.; Vali, R.M.; Ramachandra, B.; Raju, S.S. Separation and characterization of forced degradation products of abacavir sulphate by LC-MS/MS. J. Pharm. Biomed. Anal. 2011, 54, 279–285. [Google Scholar] [CrossRef]
- Kurmi, M.; Sahu, A.; Singh, S. Stability behaviour of antiretroviral drugs and their combinations. 5: Characterization of novel degradation products of abacavir sulfate by mass and nuclear magnetic resonance spectrometry. J. Pharm. Biomed. Anal. 2017, 134, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Teotia, A.K.; Farooqi, J.A.; Singh, G.N. Forced degradation study of abacavir sulfate under the frame of genotoxic impurity. Indian J. Chem. 2016, 55, 213–219. [Google Scholar]
- Uslu, B.; Ozkan, S.A. Anodic voltammetry of abacavir and its determination in pharmaceuticals and biological fluids. Electrochim. Acta 2004, 49, 4321–4329. [Google Scholar] [CrossRef]
- Oliveira-Brett, A.M.; Diculescu, V.; Piedade, J.A.P. Electrochemical oxidation mechanism of guanine and adenine using a glassy carbon microelectrode. Bioelectrochemistry 2002, 55, 61–62. [Google Scholar] [CrossRef]
- Torres, S.; Brown, R.; Szucs, R.; Hawkins, J.M.; Zelesky, T.; Scrivens, G.; Pettman, A.; Taylor, M.R. The application of electrochemistry to pharmaceutical stability testing—Comparison with in silico prediction and chemical forced degradation approaches. J. Pharm. Biomed. Anal. 2015, 115, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.M.; Kurbanoglu, S.; Vytras, K.; Uslu, B.; Ozkan, S.A. Electrochemical mechanism and sensitive assay of antiretroviral drug Abacavir in biological sample using multiwalled carbon nanotube modified pyrolytic graphite electrode. J. Electroanal. Chem. 2014, 712, 178–184. [Google Scholar] [CrossRef]
- Peckova, K.; Musilova, J.; Barek, J. Boron-doped diamond film electrodes-new tool for voltammetric determination of organic substances. Crit. Rev. Anal. Chem. 2009, 39, 148–172. [Google Scholar] [CrossRef]


| Author | Method of Oxidation | Temperature | Time of Oxidation | Reference |
|---|---|---|---|---|
| Vukkum et al. | 3% H2O2 | Laboratory | 7 days | [28] |
| Rao et al. | 6% H2O2 | Laboratory | 7 days | [29] |
| Kurmi et al. | 15% H2O2 | 30 °C | 10 days | [30] |
| Prakash et al. | 30% H2O2 | 80 °C | 30 min | [31] |
| Zhou et al. | 0.2 mA cm−2, Ti/SnO2-Sb anode | Laboratory | 10 min | [18] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pražáková, L.; Fischer, J.; Taylor, A.; Kubíčková, A. Comparison of Chemical and Electrochemical Approaches to Abacavir Oxidative Stability Testing. Sensors 2023, 23, 2776. https://doi.org/10.3390/s23052776
Pražáková L, Fischer J, Taylor A, Kubíčková A. Comparison of Chemical and Electrochemical Approaches to Abacavir Oxidative Stability Testing. Sensors. 2023; 23(5):2776. https://doi.org/10.3390/s23052776
Chicago/Turabian StylePražáková, Lucie, Jan Fischer, Andrew Taylor, and Anna Kubíčková. 2023. "Comparison of Chemical and Electrochemical Approaches to Abacavir Oxidative Stability Testing" Sensors 23, no. 5: 2776. https://doi.org/10.3390/s23052776
APA StylePražáková, L., Fischer, J., Taylor, A., & Kubíčková, A. (2023). Comparison of Chemical and Electrochemical Approaches to Abacavir Oxidative Stability Testing. Sensors, 23(5), 2776. https://doi.org/10.3390/s23052776








