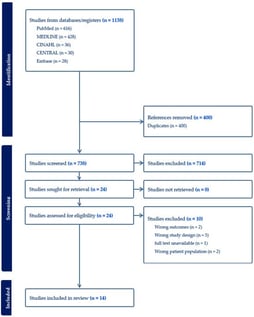
- Systematic Review
Genetic Contributions to Breast Cancer-Related Lymphedema—Does Subclinical Disease Increase Risk? A Systematic Review
- Andrew J. James,
- Quinton L. Carr and
- Ryan Shapiro
- + 3 authors
Breast cancer-related lymphedema (BCRL) is a chronic and debilitating complication of breast cancer treatment, commonly associated with mastectomy, axillary lymph node dissection, and adjuvant radiation therapy. Though demographic and treatment-related risk factors for BCRL are well documented, emerging evidence suggests that certain genetic polymorphisms may predispose some patients to developing the condition. This review aims to summarize the current research regarding the genetic variants implicated in the development and severity of BCRL. Several candidate genes related to lymphangiogenesis, inflammation, immune cell activation, and lymphatic contractility have been identified. Unfortunately, the existing literature remains limited by the small number of manuscripts, modest sample sizes, and heterogeneous methodologies of available studies. However, further research may shed light on screening options and lead to more personalized treatment strategies to mitigate the incidence and severity of secondary lymphedema.
9 February 2026