Rewiring the Lymphatic Landscape: Disorders, Remodeling, and Cancer Progression
Abstract
1. Lymphatic System
1.1. Lymphatic Disorders
1.1.1. Lymphedema: A Major Concern
1.1.1.1. Primary Lymphedema
1.1.1.2. Secondary Lymphedema
1.1.2. Congenital and Developmental Lymphatic Anomalies
1.1.3. Lymphatic Malformations (LMs)
1.1.4. Central Conducting Lymphatic Anomaly (CCLA)
1.1.5. Generalized Lymphatic Anomaly (GLA)
1.1.6. Kaposiform Lymphangiomatosis (KLA)
1.1.7. Lymphatic Neoplasm
1.1.7.1. Hodgkin Lymphoma
1.1.7.2. Non-Hodgkin Lymphoma
1.2. Infectious Lymphatic Disorders
1.2.1. Lymphangitis
1.2.2. Lymphadenitis
1.2.3. Lymphatic Filariasis
1.2.4. Tuberculous Lymphadenitis
1.2.5. Other Rare Systemic Lymphatic Conditions
1.3. Biomarkers in Lymphatic Disorders: Immune Signatures and Molecular Insights
1.4. Lymphatic Remodeling in Pathological Condition
1.4.1. Cancer-Associated Lymphatic Remodeling
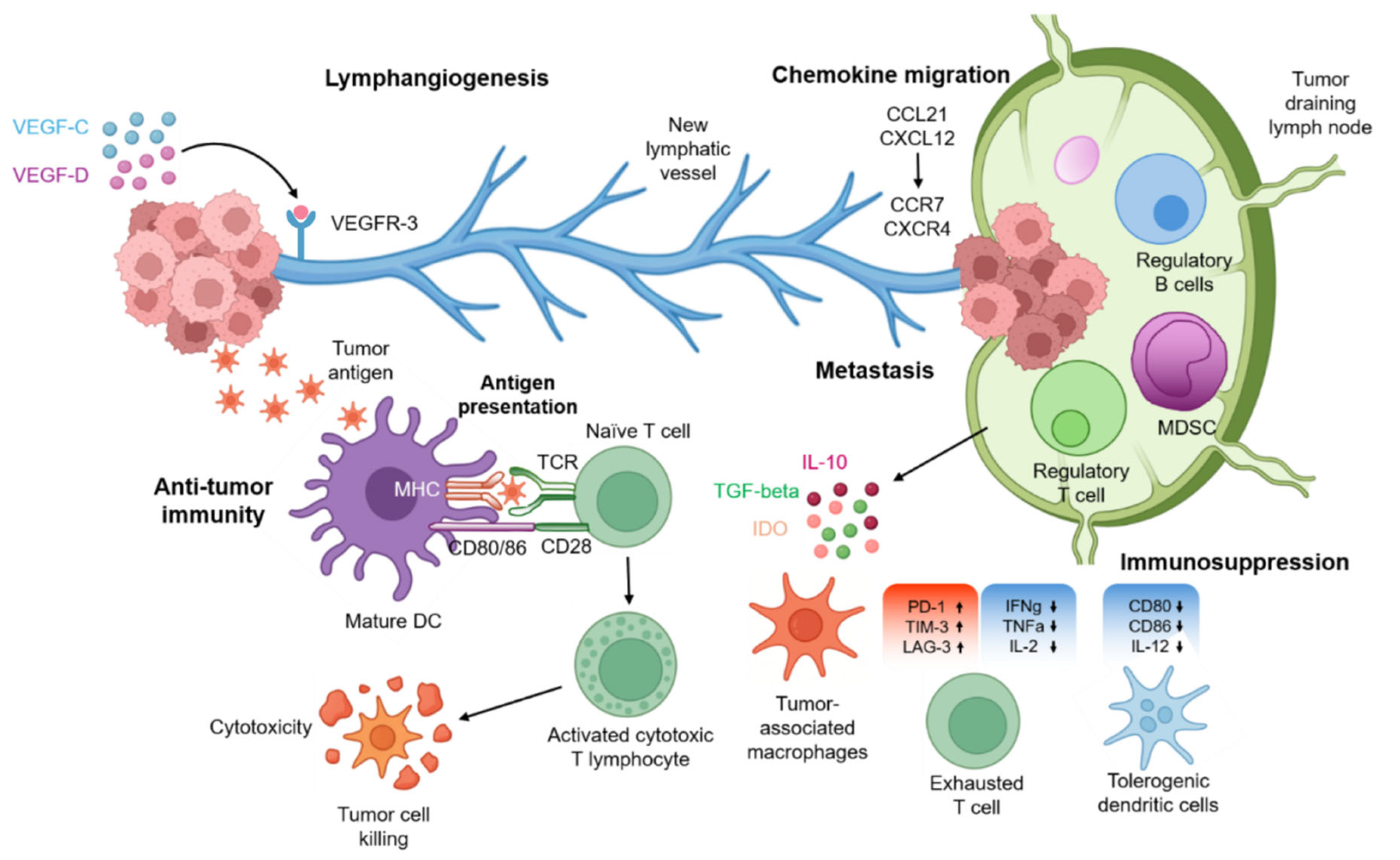
1.4.1.1. Lymphangiogenesis
1.4.1.2. Metastasis Through Lymphatic Vessels
1.4.1.3. Chemokine-Guided Tumor Migration
1.4.1.4. Lymphatics in Anti-Tumor Immunity
1.4.1.5. Immunosuppression Within Tumor-Draining Lymph Nodes
1.5. Clinical and Translational Perspectives
1.5.1. Lymphedema Remodeling
1.5.2. Autoimmune-Associated Remodeling
1.6. Therapeutic Targets: Targeting Lymphatic Remodeling
1.7. Tertiary Lymphoid Structures
1.7.1. Cellular Composition of TLS
1.7.2. Regulatory Mechanisms Related to Immunology
1.7.3. Metabolic Reprogramming in TLSs
1.7.4. TLS, Its Association with Diseases, and Therapeutic Advances
2. Techniques and Advances in Prognosis and Therapy
2.1. Advances in Molecular Imaging
2.1.1. Lymphoscintigraphy (LS)
2.1.2. Fluorescence Imaging
2.1.3. Ultrasonography (US)
2.1.4. Magnetic Resonance Lymphography (MRL)
2.1.5. Computed Tomography
2.1.6. Photoacoustic Imaging (PAI)
2.1.7. Optical Coherence Tomography (OCT)
2.2. Nanobiotechnology in Molecular Imaging
2.3. Advances in Lymph Node-Targeted Nano-Delivery
2.3.1. Route of Administration
2.3.2. Physical Properties
2.4. Current Advances in AI-Assisted Diagnosis
Challenges and Future Perspectives
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Girard, J.P.; Moussion, C.; Förster, R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat. Rev. Immunol. 2012, 12, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.J.; Zawieja, S.D.; King, P.D. Transport and Immune Functions of the Lymphatic System. Annu. Rev. Physiol. 2025, 87, 151–172. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Ozdowski, L.; Gupta, V. Physiology, Lymphatic System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557833/ (accessed on 1 May 2023).
- Null, M.; Arbor, T.C.; Agarwal, M. Anatomy, Lymphatic System. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513247/ (accessed on 6 March 2023).
- Petrova, T.V.; Koh, G.Y. Biological functions of lymphatic vessels. Science 2020, 369, aax4063. [Google Scholar] [CrossRef] [PubMed]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef]
- Singh, B.; Kasam, R.K.; Sontake, V.; Wynn, T.A.; Madala, S.K. Repetitive intradermal bleomycin injections evoke T-helper cell 2 cytokine-driven pulmonary fibrosis. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L796–L806. [Google Scholar] [CrossRef]
- Brouillard, P.; Boon, L.; Vikkula, M. Genetics of lymphatic anomalies. J. Clin. Investig. 2014, 124, 898–904. [Google Scholar] [CrossRef]
- Alders, M.; Hogan, B.M.; Gjini, E.; Salehi, F.; Al-Gazali, L.; Hennekam, E.A.; Holmberg, E.E.; Mannens, M.M.; Mulder, M.F.; Offerhaus, G.J.; et al. Mutations in CCBE1 cause generalized lymph vessel dysplasia in humans. Nat. Genet. 2009, 41, 1272–1274. [Google Scholar] [CrossRef]
- Schaverien, M.V.; Coroneos, C.J. Surgical Treatment of Lymphedema. Plast. Reconstr. Surg. 2019, 144, 738–758. [Google Scholar] [CrossRef]
- Babu, S.; Nutman, T.B. Immunology of lymphatic filariasis. Parasite Immunol. 2014, 36, 338–346. [Google Scholar] [CrossRef]
- Rodriguez-Laguna, L.; Agra, N.; Ibañez, K.; Oliva-Molina, G.; Gordo, G.; Khurana, N.; Hominick, D.; Beato, M.; Colmenero, I.; Herranz, G.; et al. Somatic activating mutations in PIK3CA cause generalized lymphatic anomaly. J. Exp. Med. 2019, 216, 407–418. [Google Scholar] [CrossRef]
- McCormack, L.; Jones, K.; Huang, J.T. Micro- and Macrocystic Lymphatic Malformation. J. Pediatr. 2020, 219, 275–276. [Google Scholar] [CrossRef] [PubMed]
- Garlisi Torales, L.D.; Sempowski, B.A.; Krikorian, G.L.; Woodis, K.M.; Paulissen, S.M.; Smith, C.L.; Sheppard, S.E. Central conducting lymphatic anomaly: From bench to bedside. J. Clin. Investig. 2024, 134, 172839. [Google Scholar] [CrossRef] [PubMed]
- Manevitz-Mendelson, E.; Leichner, G.S.; Barel, O.; Davidi-Avrahami, I.; Ziv-Strasser, L.; Eyal, E.; Pessach, I.; Rimon, U.; Barzilai, A.; Hirshberg, A.; et al. Somatic NRAS mutation in patient with generalized lymphatic anomaly. Angiogenesis 2018, 21, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Ozeki, M.; Hirose, K.; Matsuoka, K.; Matsui, T.; Kohara, M.; Tahara, S.; Toyosawa, S.; Fukao, T.; Morii, E. Analysis of mTOR pathway expression in lymphatic malformation and related diseases. Pathol. Int. 2020, 70, 323–329. [Google Scholar] [CrossRef]
- Ozeki, M.; Nozawa, A.; Yasue, S.; Endo, S.; Asada, R.; Hashimoto, H.; Fukao, T. The impact of sirolimus therapy on lesion size, clinical symptoms, and quality of life of patients with lymphatic anomalies. Orphanet J. Rare Dis. 2019, 14, 141. [Google Scholar] [CrossRef]
- Ozeki, M.; Fujino, A.; Matsuoka, K.; Nosaka, S.; Kuroda, T.; Fukao, T. Clinical Features and Prognosis of Generalized Lymphatic Anomaly, Kaposiform Lymphangiomatosis, and Gorham-Stout Disease. Pediatr. Blood Cancer 2016, 63, 832–838. [Google Scholar] [CrossRef]
- Trenor, C.C., 3rd; Chaudry, G. Complex lymphatic anomalies. Semin. Pediatr. Surg. 2014, 23, 186–190. [Google Scholar] [CrossRef]
- Mugnaini, E.N.; Ghosh, N. Lymphoma. Prim. Care Clin. Off. Pract. 2016, 43, 661–675. [Google Scholar] [CrossRef]
- Jamil, A.; Mukkamalla, S.K.R. Lymphoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560826/ (accessed on 17 July 2023).
- Kaseb, H.; Hodgkin, H.M.B. Lymphoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499969/ (accessed on 26 June 2023).
- Sapkota, S.; Shaikh, H. Non-Hodgkin Lymphoma. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK559328/ (accessed on 24 February 2023).
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748, Correction in Leukemia 2023, 37, 1944–1951. [Google Scholar] [CrossRef]
- Loghavi, S.; Kanagal-Shamanna, R.; Khoury, J.D.; Medeiros, L.J.; Naresh, K.N.; Nejati, R.; Patnaik, M.M. Fifth Edition of the World Health Classification of Tumors of the Hematopoietic and Lymphoid Tissue: Myeloid Neoplasms. Mod. Pathol. 2024, 37, 100397. [Google Scholar] [CrossRef]
- Marafioti, T.; Hummel, M.; Foss, H.D.; Laumen, H.; Korbjuhn, P.; Anagnostopoulos, I.; Lammert, H.; Demel, G.; Theil, J.; Wirth, T.; et al. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood 2000, 95, 1443–1450. [Google Scholar] [CrossRef]
- Kanzler, H.; Küppers, R.; Hansmann, M.L.; Rajewsky, K. Hodgkin and Reed-Sternberg cells in Hodgkin’s disease represent the outgrowth of a dominant tumor clone derived from (crippled) germinal center B cells. J. Exp. Med. 1996, 184, 1495–1505. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, N. B-cell non-Hodgkin lymphoma: Importance of angiogenesis and antiangiogenic therapy. Angiogenesis 2020, 23, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Bowman, C.; Rockson, S.G. The Role of Inflammation in Lymphedema: A Narrative Review of Pathogenesis and Opportunities for Therapeutic Intervention. Int. J. Mol. Sci. 2024, 25, 3907. [Google Scholar] [CrossRef] [PubMed]
- Alves, F.; Baptista, A.; Brito, H.; Mendonça, I. Necrotising granulomatous lymphadenitis. BMJ Case Rep. 2011, 2011. [Google Scholar] [CrossRef]
- Babu, S.; Nutman, T.B. Immunopathogenesis of lymphatic filarial disease. Semin. Immunopathol. 2012, 34, 847–861. [Google Scholar] [CrossRef]
- Golden, M.P.; Vikram, H.R. Extrapulmonary tuberculosis: An overview. Am. Fam. Physician 2005, 72, 1761–1768. [Google Scholar]
- Kathamuthu, G.R.; Moideen, K.; Baskaran, D.; Banurekha, V.V.; Nair, D.; Sekar, G.; Sridhar, R.; Vidyajayanthi, B.; Gajendraraj, G.; Parandhaman, D.K.; et al. Tuberculous Lymphadenitis Is Associated with Enhanced Baseline and Antigen-Specific Induction of Type 1 and Type 17 Cytokines and Reduced Interleukin-1β (IL-1β) and IL-18 at the Site of Infection. Clin. Vaccine Immunol. CVI 2017, 24. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kim, S.C.; Park, S.B.; Kim, M.J. Hennekam Syndrome: A Case Report. Ann. Rehabil. Med. 2018, 42, 184–188. [Google Scholar] [CrossRef]
- Maldonado, F.; Tazelaar, H.D.; Wang, C.W.; Ryu, J.H. Yellow nail syndrome: Analysis of 41 consecutive patients. Chest 2008, 134, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Mehrara, B.J.; Radtke, A.J.; Randolph, G.J.; Wachter, B.T.; Greenwel, P.; Rovira, I.I.; Galis, Z.S.; Muratoglu, S.C. The emerging importance of lymphatics in health and disease: An NIH workshop report. J. Clin. Investig. 2023, 133, 171582. [Google Scholar] [CrossRef] [PubMed]
- Vargo, M.; Aldrich, M.; Donahue, P.; Iker, E.; Koelmeyer, L.; Crescenzi, R.; Cheville, A. Current diagnostic and quantitative techniques in the field of lymphedema management: A critical review. Med. Oncol. 2024, 41, 241. [Google Scholar] [CrossRef]
- Avraham, T.; Daluvoy, S.; Zampell, J.; Yan, A.; Haviv, Y.S.; Rockson, S.G.; Mehrara, B.J. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 2010, 177, 3202–3214. [Google Scholar] [CrossRef]
- Fink, A.M.; Kaltenegger, I.; Schneider, B.; Frühauf, J.; Jurecka, W.; Steiner, A. Serum level of VEGF-D in patients with primary lymphedema. Lymphology 2004, 37, 185–189. [Google Scholar]
- Lee, C.; Kim, M.J.; Kumar, A.; Lee, H.W.; Yang, Y.; Kim, Y. Vascular endothelial growth factor signaling in health and disease: From molecular mechanisms to therapeutic perspectives. Signal Transduct. Target. Ther. 2025, 10, 170. [Google Scholar] [CrossRef]
- Newman, B.; Lose, F.; Kedda, M.A.; Francois, M.; Ferguson, K.; Janda, M.; Yates, P.; Spurdle, A.B.; Hayes, S.C. Possible genetic predisposition to lymphedema after breast cancer. Lymphat. Res. Biol. 2012, 10, 2–13. [Google Scholar] [CrossRef]
- He, M.; He, Q.; Cai, X.; Chen, Z.; Lao, S.; Deng, H.; Liu, X.; Zheng, Y.; Liu, X.; Liu, J.; et al. Role of lymphatic endothelial cells in the tumor microenvironment-a narrative review of recent advances. Transl. Lung Cancer Res. 2021, 10, 2252–2277. [Google Scholar] [CrossRef]
- Ferrell, R.E.; Levinson, K.L.; Esman, J.H.; Kimak, M.A.; Lawrence, E.C.; Barmada, M.M.; Finegold, D.N. Hereditary lymphedema: Evidence for linkage and genetic heterogeneity. Hum. Mol. Genet. 1998, 7, 2073–2078. [Google Scholar] [CrossRef]
- Rezaie, T.; Ghoroghchian, R.; Bell, R.; Brice, G.; Hasan, A.; Burnand, K.; Vernon, S.; Mansour, S.; Mortimer, P.; Jeffery, S.; et al. Primary non-syndromic lymphoedema (Meige disease) is not caused by mutations in FOXC2. Eur. J. Hum. Genet. EJHG 2008, 16, 300–304. [Google Scholar] [CrossRef]
- Traboulsi, E.I.; Al-Khayer, K.; Matsumoto, M.; Kimak, M.A.; Crowe, S.; Wilson, S.E.; Finegold, D.N.; Ferrell, R.E.; Meisler, D.M. Lymphedema-distichiasis syndrome and FOXC2 gene mutation. Am. J. Ophthalmol. 2002, 134, 592–596. [Google Scholar] [CrossRef]
- Yang, Y.; Oliver, G. Development of the mammalian lymphatic vasculature. J. Clin. Investig. 2014, 124, 888–897. [Google Scholar] [CrossRef]
- Karkkainen, M.J.; Ferrell, R.E.; Lawrence, E.C.; Kimak, M.A.; Levinson, K.L.; McTigue, M.A.; Alitalo, K.; Finegold, D.N. Missense mutations interfere with VEGFR-3 signalling in primary lymphoedema. Nat. Genet. 2000, 25, 153–159. [Google Scholar] [CrossRef]
- Baik, J.E.; Park, H.J.; Kataru, R.P.; Savetsky, I.L.; Ly, C.L.; Shin, J.; Encarnacion, E.M.; Cavali, M.R.; Klang, M.G.; Riedel, E.; et al. TGF-β1 mediates pathologic changes of secondary lymphedema by promoting fibrosis and inflammation. Clin. Transl. Med. 2022, 12, e758. [Google Scholar] [CrossRef] [PubMed]
- Kataru, R.P.; Jung, K.; Jang, C.; Yang, H.; Schwendener, R.A.; Baik, J.E.; Han, S.H.; Alitalo, K.; Koh, G.Y. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood 2009, 113, 5650–5659. [Google Scholar] [CrossRef] [PubMed]
- Gardenier, J.C.; Kataru, R.P.; Hespe, G.E.; Savetsky, I.L.; Torrisi, J.S.; Nores, G.D.; Jowhar, D.K.; Nitti, M.D.; Schofield, R.C.; Carlow, D.C.; et al. Topical tacrolimus for the treatment of secondary lymphedema. Nat. Commun. 2017, 8, 14345. [Google Scholar] [CrossRef] [PubMed]
- Gousopoulos, E.; Proulx, S.T.; Bachmann, S.B.; Dieterich, L.C.; Scholl, J.; Karaman, S.; Bianchi, R.; Detmar, M. An Important Role of VEGF-C in Promoting Lymphedema Development. J. Investig. Dermatol. 2017, 137, 1995–2004. [Google Scholar] [CrossRef]
- Chesnais, C.B.; Vlaminck, J.; Kunyu-Shako, B.; Pion, S.D.; Awaca-Uvon, N.P.; Weil, G.J.; Mumba, D.; Boussinesq, M. Measurement of Circulating Filarial Antigen Levels in Human Blood with a Point-of-Care Test Strip and a Portable Spectrodensitometer. Am. J. Trop. Med. Hyg. 2016, 94, 1324–1329. [Google Scholar] [CrossRef]
- Jaoko, W.G.; Lund, M.; Michael, E.; Simonsen, P.E. A simple and quick method for enhanced detection of specific IgE in serum from lymphatic filariasis patients. Acta Trop. 2001, 80, 51–57. [Google Scholar] [CrossRef]
- Boscolo, E.; Coma, S.; Luks, V.L.; Greene, A.K.; Klagsbrun, M.; Warman, M.L.; Bischoff, J. AKT hyper-phosphorylation associated with PI3K mutations in lymphatic endothelial cells from a patient with lymphatic malformation. Angiogenesis 2015, 18, 151–162. [Google Scholar] [CrossRef]
- Sun, S.; Chen, S.; Liu, F.; Wu, H.; McHugh, J.; Bergin, I.L.; Gupta, A.; Adams, D.; Guan, J.L. Constitutive Activation of mTORC1 in Endothelial Cells Leads to the Development and Progression of Lymphangiosarcoma through VEGF Autocrine Signaling. Cancer Cell 2015, 28, 758–772. [Google Scholar] [CrossRef] [PubMed]
- Biko, D.M.; DeWitt, A.G.; Pinto, E.M.; Morrison, R.E.; Johnstone, J.A.; Griffis, H.; O’Byrne, M.L.; Fogel, M.A.; Harris, M.A.; Partington, S.L.; et al. MRI Evaluation of Lymphatic Abnormalities in the Neck and Thorax after Fontan Surgery: Relationship with Outcome. Radiology 2019, 291, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Allen-Rhoades, W.; Al-Ibraheemi, A.; Kohorst, M.; Tollefson, M.; Hull, N.; Polites, S.; Folpe, A.L. Cellular variant of kaposiform lymphangiomatosis: A report of three cases, expanding the morphologic and molecular genetic spectrum of this rare entity. Hum. Pathol. 2022, 122, 72–81. [Google Scholar] [CrossRef]
- Safaei, F.; Sadeghi, A.; Ketabi Moghadam, P.; Taheri, P. Small Bowel Lymphangiectasia Leading to Massive Gastrointestinal Bleeding: A Case Report. Middle East. J. Dig. Dis. 2023, 15, 60–62. [Google Scholar] [CrossRef]
- Ozeki, M.; Fukao, T. Generalized Lymphatic Anomaly and Gorham-Stout Disease: Overview and Recent Insights. Adv. Wound Care 2019, 8, 230–245. [Google Scholar] [CrossRef]
- Xiang, J.; Zhong, W. The molecular mechanism of Gorham syndrome: An update. Front. Immunol. 2023, 14, 1165091. [Google Scholar] [CrossRef]
- Zenner, K.; Cheng, C.V.; Jensen, D.M.; Timms, A.E.; Shivaram, G.; Bly, R.; Ganti, S.; Whitlock, K.B.; Dobyns, W.B.; Perkins, J.; et al. Genotype correlates with clinical severity in PIK3CA-associated lymphatic malformations. JCI Insight 2019, 4, 129884. [Google Scholar] [CrossRef]
- Revilla-López, E.; Ruiz de Miguel, V.; López-Meseguer, M.; Berastegui, C.; Boada-Pérez, M.; Mendoza-Valderrey, A.; Arjona-Peris, M.; Zapata-Ortega, M.; Monforte, V.; Bravo, C.; et al. Lymphangioleiomyomatosis: Searching for potential biomarkers. Front. Med. 2023, 10, 1079317. [Google Scholar] [CrossRef]
- Polke, M.; Polke, N.; Piel, S.; Brunnemer, E.; Wälscher, J.; Buschulte, K.; Warth, A.; Heussel, C.P.; Eichinger, M.; Frankenstein, L.; et al. Pulmonary lymphangiomatosis: Insights into an ultra-rare disease. Respir. Res. 2024, 25, 416. [Google Scholar] [CrossRef]
- Pahwa, R.; Hedau, S.; Jain, S.; Jain, N.; Arora, V.M.; Kumar, N.; Das, B.C. Assessment of possible tuberculous lymphadenopathy by PCR compared to non-molecular methods. J. Med. Microbiol. 2005, 54, 873–878. [Google Scholar] [CrossRef]
- Rew, S.Y.; Jang, H.C.; Park, K.H.; Ahn, J.S.; Kim, G.E.; Choi, Y.D.; Jung, S.I. A case of rosai-dorfman disease with highly elevated serum ferritin. Ann. Lab. Med. 2012, 32, 158–161. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; Uldrick, T.S.; Bagg, A.; Frank, D.; Wu, D.; Srkalovic, G.; Simpson, D.; Liu, A.Y.; Menke, D.; Chandrakasan, S.; et al. International, evidence-based consensus diagnostic criteria for HHV-8-negative/idiopathic multicentric Castleman disease. Blood 2017, 129, 1646–1657. [Google Scholar] [CrossRef]
- Weiss, L.M.; O’Malley, D. Benign lymphadenopathies. Mod. Pathol. 2013, 26 (Suppl. 1), S88–S96. [Google Scholar] [CrossRef]
- Shah, S.; Wu, E.; Rao, V.K.; Tarrant, T.K. Autoimmune lymphoproliferative syndrome: An update and review of the literature. Curr. Allergy Asthma Rep. 2014, 14, 462. [Google Scholar] [CrossRef]
- Soares, L.; Rebelo Matos, A.; Mello Vieira, M.; Cruz, R.; Caixas, U. Generalized Lymphadenopathy as the First Manifestation of Systemic Lupus Erythematosus. Cureus 2022, 14, e30089. [Google Scholar] [CrossRef]
- Matson, E.M.; Abyazi, M.L.; Bell, K.A.; Hayes, K.M.; Maglione, P.J. B Cell Dysregulation in Common Variable Immunodeficiency Interstitial Lung Disease. Front. Immunol. 2020, 11, 622114. [Google Scholar] [CrossRef]
- Bisno, A.L.; Stevens, D.L. Streptococcal infections of skin and soft tissues. N. Engl. J. Med. 1996, 334, 240–245. [Google Scholar] [CrossRef]
- Boyd, S.D.; Natkunam, Y.; Allen, J.R.; Warnke, R.A. Selective immunophenotyping for diagnosis of B-cell neoplasms: Immunohistochemistry and flow cytometry strategies and results. Appl. Immunohistochem. Mol. Morphol. AIMM 2013, 21, 116–131. [Google Scholar] [CrossRef]
- Otto, M.; Nagalli, S. Mesenteric Adenitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560822/ (accessed on 8 August 2023).
- Ohta, N.; Okazaki, S.; Fukase, S.; Akatsuka, N.; Aoyagi, M.; Yamakawa, M. Serum concentrations of eosinophil cationic protein and eosinophils of patients with Kimura’s disease. Allergol. Int. 2007, 56, 45–49. [Google Scholar] [CrossRef]
- Lagerstrom, I.T.; Danielson, D.T.; Muir, J.M.; Foss, R.D.; Auerbach, A.; Aguilera, N.S. A Comprehensive Review of Kimura Disease. Head. Neck Pathol. 2025, 19, 75. [Google Scholar] [CrossRef] [PubMed]
- Goyal, G.; Ravindran, A.; Young, J.R.; Shah, M.V.; Bennani, N.N.; Patnaik, M.M.; Nowakowski, G.S.; Thanarajasingam, G.; Habermann, T.M.; Vassallo, R.; et al. Clinicopathological features, treatment approaches, and outcomes in Rosai-Dorfman disease. Haematologica 2020, 105, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Barry, T.S.; Jaffe, E.S.; Sorbara, L.; Raffeld, M.; Pittaluga, S. Peripheral T-cell lymphomas expressing CD30 and CD15. Am. J. Surg. Pathol. 2003, 27, 1513–1522. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Sullivan, H.C.; Edgar, M.A.; Cohen, C.; Kovach, C.K.; HooKim, K.; Reid, M.D. The utility of ERG, CD31 and CD34 in the cytological diagnosis of angiosarcoma: An analysis of 25 cases. J. Clin. Pathol. 2015, 68, 44–50. [Google Scholar] [CrossRef]
- Kojima, N.; Arai, Y.; Satomi, K.; Kubo, T.; Matsushita, Y.; Mori, T.; Matsushita, H.; Ushijima, T.; Yatabe, Y.; Shibata, T.; et al. Co-expression of ERG and CD31 in a subset of CIC-rearranged sarcoma: A potential diagnostic pitfall. Mod. Pathol. 2022, 35, 1439–1448. [Google Scholar] [CrossRef]
- Su, J.L.; Yen, C.J.; Chen, P.S.; Chuang, S.E.; Hong, C.C.; Kuo, I.H.; Chen, H.Y.; Hung, M.C.; Kuo, M.L. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br. J. Cancer 2007, 96, 541–545. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, S.; Zhang, R.; Zhang, L. The role of VEGF-C/D and Flt-4 in the lymphatic metastasis of early-stage invasive cervical carcinoma. J. Exp. Clin. Cancer Res. 2009, 28, 98. [Google Scholar] [CrossRef]
- Sun, R.; Medeiros, L.J.; Young, K.H. Diagnostic and predictive biomarkers for lymphoma diagnosis and treatment in the era of precision medicine. Mod. Pathol. 2016, 29, 1118–1142. [Google Scholar] [CrossRef]
- Ibarra-Ramírez, M.; Campos-Acevedo, L.D.; Martínez de Villarreal, L.E. Chromosomal Abnormalities of Interest in Turner Syndrome: An Update. J. Pediatr. Genet. 2023, 12, 263–272. [Google Scholar] [CrossRef]
- Tartaglia, M.; Zampino, G.; Gelb, B.D. Noonan syndrome: Clinical aspects and molecular pathogenesis. Mol. Syndromol. 2010, 1, 2–26. [Google Scholar] [CrossRef]
- Vignes, S.; Baran, R. Yellow nail syndrome: A review. Orphanet J. Rare Dis. 2017, 12, 42. [Google Scholar] [CrossRef]
- Lakshmi Narendra, B.; Eshvendar Reddy, K.; Shantikumar, S.; Ramakrishna, S. Immune system: A double-edged sword in cancer. Inflamm. Res. 2013, 62, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Karaman, S.; Detmar, M. Mechanisms of lymphatic metastasis. J. Clin. Investig. 2014, 124, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol. Res. 2014, 2, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.S.; Femel, J.; Breazeale, A.P.; Loo, C.P.; Thibault, G.; Kaempf, A.; Mori, M.; Tsujikawa, T.; Chang, Y.H.; Lund, A.W. IFNγ-activated dermal lymphatic vessels inhibit cytotoxic T cells in melanoma and inflamed skin. J. Exp. Med. 2018, 215, 3057–3074. [Google Scholar] [CrossRef]
- Morton, D.L.; Thompson, J.F.; Cochran, A.J.; Mozzillo, N.; Nieweg, O.E.; Roses, D.F.; Hoekstra, H.J.; Karakousis, C.P.; Puleo, C.A.; Coventry, B.J.; et al. Final trial report of sentinel-node biopsy versus nodal observation in melanoma. N. Engl. J. Med. 2014, 370, 599–609. [Google Scholar] [CrossRef]
- Rakha, E.A.; Martin, S.; Lee, A.H.; Morgan, D.; Pharoah, P.D.; Hodi, Z.; Macmillan, D.; Ellis, I.O. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer 2012, 118, 3670–3680. [Google Scholar] [CrossRef]
- Sleeman, J.P.; Thiele, W. Tumor metastasis and the lymphatic vasculature. Int. J. Cancer 2009, 125, 2747–2756. [Google Scholar] [CrossRef]
- Swarz, M.A.; Lund, A.W. Lymphatic and interstitial flow in the tumour microenvironment: Linking mechanobiology with immunity. Nat. Rev. Cancer 2012, 12, 210–219. [Google Scholar] [CrossRef]
- Reticker-Flynn, N.E.; Zhang, W.; Belk, J.A.; Basto, P.A.; Escalante, N.K.; Pilarowski, G.O.W.; Bejnood, A.; Martins, M.M.; Kenkel, J.A.; Linde, I.L.; et al. Lymph node colonization induces tumor-immune tolerance to promote distant metastasis. Cell 2022, 185, 1924–1942.e1923. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Hu, C.; Yang, X.; Liu, Y.; Ji, G.; Ge, S.; Wang, X.; Wang, M. Lymph node metastasis in cancer progression: Molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Chitra, J.; Selvaraj, P. CCL2, CCL3 and CCL4 gene polymorphisms in pulmonary tuberculosis patients of South India. Int. J. Immunogenet. 2014, 41, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D.; Emmett, M.S.; Dunn, D.B.; Joory, K.D.; Sage, L.M.; Rigby, H.; Mortimer, P.S.; Orlando, A.; Levick, J.R.; Bates, D.O. Chemokine-mediated migration of melanoma cells towards lymphatics--A mechanism contributing to metastasis. Oncogene 2007, 26, 2997–3005. [Google Scholar] [CrossRef]
- Tutunea-Fatan, E.; Majumder, M.; Xin, X.; Lala, P.K. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol. Cancer 2015, 14, 35. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef]
- Krishnaswamy, J.K.; Gowthaman, U.; Zhang, B.; Mattsson, J.; Szeponik, L.; Liu, D.; Wu, R.; White, T.; Calabro, S.; Xu, L.; et al. Migratory CD11b(+) conventional dendritic cells induce T follicular helper cell-dependent antibody responses. Sci. Immunol. 2017, 2, 9169. [Google Scholar] [CrossRef]
- Esensten, J.H.; Helou, Y.A.; Chopra, G.; Weiss, A.; Bluestone, J.A. CD28 Costimulation: From Mechanism to Therapy. Immunity 2016, 44, 973–988. [Google Scholar] [CrossRef]
- Fransen, M.F.; van der Sluis, T.C.; Ossendorp, F.; Arens, R.; Melief, C.J. Controlled local delivery of CTLA-4 blocking antibody induces CD8+ T-cell-dependent tumor eradication and decreases risk of toxic side effects. Clin. Cancer Res. 2013, 19, 5381–5389. [Google Scholar] [CrossRef] [PubMed]
- Piersiala, K.; da Silva, P.F.N.; Lagebro, V.; Kolev, A.; Starkhammar, M.; Elliot, A.; Marklund, L.; Munck-Wikland, E.; Margolin, G.; Georén, S.K.; et al. Tumour-draining lymph nodes in head and neck cancer are characterized by accumulation of CTLA-4 and PD-1 expressing Treg cells. Transl. Oncol. 2022, 23, 101469. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.F.; Schoonderwoerd, M.; Knopf, P.; Camps, M.G.; Hawinkels, L.J.; Kneilling, M.; van Hall, T.; Ossendorp, F. Tumor-draining lymph nodes are pivotal in PD-1/PD-L1 checkpoint therapy. JCI Insight 2018, 3, 124507. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- He, Y.; Qian, H.; Liu, Y.; Duan, L.; Li, Y.; Shi, G. The roles of regulatory B cells in cancer. J. Immunol. Res. 2014, 2014, 215471. [Google Scholar] [CrossRef]
- Zhuang, Q.; Cai, H.; Cao, Q.; Li, Z.; Liu, S.; Ming, Y. Tolerogenic Dendritic Cells: The Pearl of Immunotherapy in Organ Transplantation. Front. Immunol. 2020, 11, 552988. [Google Scholar] [CrossRef]
- Taylor, A.; Verhagen, J.; Blaser, K.; Akdis, M.; Akdis, C.A. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: The role of T regulatory cells. Immunology 2006, 117, 433–442. [Google Scholar] [CrossRef]
- Mbongue, J.C.; Nicholas, D.A.; Torrez, T.W.; Kim, N.S.; Firek, A.F.; Langridge, W.H. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines 2015, 3, 703–729. [Google Scholar] [CrossRef]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2020, 11, 622509. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Sun, M.; Angelillo, J.; Hugues, S. Lymphatic transport in anti-tumor immunity and metastasis. J. Exp. Med. 2025, 222. Correction in J. Exp. Med. 2025, 222, e2023195403262025c. [Google Scholar] [CrossRef] [PubMed]
- Saddawi-Konefka, R.; Schokrpur, S.; Gutkind, J.S. Let it be: Preserving tumor-draining lymph nodes in the era of immuno-oncology. Cancer Cell 2024, 42, 930–933. [Google Scholar] [CrossRef]
- Karakousi, T.; Mudianto, T.; Lund, A.W. Lymphatic vessels in the age of cancer immunotherapy. Nat. Rev. Cancer 2024, 24, 363–381. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.-H.; Li, N. Immune evasion in cancer: Mechanisms and cutting-edge therapeutic approaches. Signal Transduct. Target. Ther. 2025, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xie, Y.Q.; Gao, M.; Zhao, Y.; Franco, F.; Wenes, M.; Siddiqui, I.; Bevilacqua, A.; Wang, H.; Yang, H.; et al. Metabolic reprogramming of terminally exhausted CD8(+) T cells by IL-10 enhances anti-tumor immunity. Nat. Immunol. 2021, 22, 746–756. [Google Scholar] [CrossRef]
- Rivas, J.R.; Liu, Y.; Alhakeem, S.S.; Eckenrode, J.M.; Marti, F.; Collard, J.P.; Zhang, Y.; Shaaban, K.A.; Muthusamy, N.; Hildebrandt, G.C.; et al. Interleukin-10 suppression enhances T-cell antitumor immunity and responses to checkpoint blockade in chronic lymphocytic leukemia. Leukemia 2021, 35, 3188–3200. [Google Scholar] [CrossRef]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Lee, H.; Park, S.-H.; Shin, E.-C. IL-15 in T-Cell Responses and Immunopathogenesis. Immune Netw. 2024, 24, e11. [Google Scholar] [CrossRef]
- Ihim, S.A.; Abubakar, S.D.; Zian, Z.; Sasaki, T.; Saffarioun, M.; Maleknia, S.; Azizi, G. Interleukin-18 cytokine in immunity, inflammation, and autoimmunity: Biological role in induction, regulation, and treatment. Front. Immunol. 2022, 13, 919973. [Google Scholar] [CrossRef]
- Li, Z.; Wang, T.; Liu, J.; Qi, W.; Lv, Q.; Xu, Y.; Tian, L. The mechanism and research progress of PD-1/PD-L1 on immune escape of lung cancer. Transl. Cancer Res. 2025, 14, 6041–6051. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Liu, Y.; Zhao, J.; Wang, G.; Chen, H.; Tang, Y.; Ouyang, D.; Xie, S.; You, J.; et al. Tumor vascular endothelial cells promote immune escape by upregulating PD-L1 expression via crosstalk between NF-κB and STAT3 signaling pathways in nasopharyngeal carcinoma. Cell Death Dis. 2025, 16, 129. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, D.C.; Theocharopoulos, C.; Lialios, P.P.; Foteinou, D.; Koumprentziotis, I.A.; Xynos, G.; Gogas, H. Beyond CTLA-4 and PD-1 Inhibition: Novel Immune Checkpoint Molecules for Melanoma Treatment. Cancers 2023, 15, 2718. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Patel, M.R.; Blumenschein, G.R.; Hamilton, E.; Chmielowski, B.; Ulahannan, S.V.; Connolly, R.M.; Santa-Maria, C.A.; Wang, J.; Bahadur, S.W.; et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: A phase 1 trial. Nat. Med. 2023, 29, 2814–2824. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nixon, M.J.; Wang, Y.; Wang, D.Y.; Castellanos, E.; Estrada, M.V.; Ericsson-Gonzalez, P.I.; Cote, C.H.; Salgado, R.; Sanchez, V.; et al. Tumor-specific MHC-II expression drives a unique pattern of resistance to immunotherapy via LAG-3/FCRL6 engagement. JCI Insight 2018, 3, 120360. [Google Scholar] [CrossRef]
- Kandel, S.; Adhikary, P.; Li, G.; Cheng, K. The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy. Cancer Lett. 2021, 510, 67–78. [Google Scholar] [CrossRef]
- Mattila, M.M.-T.; Ruohola, J.K.; Karpanen, T.; Jackson, D.G.; Alitalo, K.; Härkönen, P.L. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int. J. Cancer 2002, 98, 946–951. [Google Scholar] [CrossRef]
- Hirakawa, S.; Kodama, S.; Kunstfeld, R.; Kajiya, K.; Brown, L.F.; Detmar, M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J. Exp. Med. 2005, 201, 1089–1099. [Google Scholar] [CrossRef]
- Suzuki, H.; Kaneko, M.K.; Kato, Y. Roles of Podoplanin in Malignant Progression of Tumor. Cells 2022, 11, 575. [Google Scholar] [CrossRef]
- Vimalraj, S.; Hariprabu, K.N.G.; Rahaman, M.; Govindasami, P.; Perumal, K.; Sekaran, S.; Ganapathy, D. Vascular endothelial growth factor-C and its receptor-3 signaling in tumorigenesis. 3 Biotech 2023, 13, 326. [Google Scholar] [CrossRef]
- de Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef]
- Azhar, S.H.; Lim, H.Y.; Tan, B.K.; Angeli, V. The Unresolved Pathophysiology of Lymphedema. Front. Physiol. 2020, 11, 137. [Google Scholar] [CrossRef]
- Yuan, Y.; Arcucci, V.; Levy, S.M.; Achen, M.G. Modulation of Immunity by Lymphatic Dysfunction in Lymphedema. Front. Immunol. 2019, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Bryan, C.; Sleigh, B.M. Lymphedema. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537239/ (accessed on 19 April 2023).
- Schwartz, N.; Chalasani, M.L.S.; Li, T.M.; Feng, Z.; Shipman, W.D.; Lu, T.T. Lymphatic Function in Autoimmune Diseases. Front. Immunol. 2019, 10, 519. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Williams, S.P.; Karnezis, T.; Shayan, R.; Fox, S.B.; Achen, M.G. Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 2014, 14, 159–172. [Google Scholar] [CrossRef] [PubMed]
- Lodha, P.; Acari, A.; Rieck, J.; Hofmann, S.; Dieterich, L.C. The Lymphatic Vascular System in Extracellular Vesicle-Mediated Tumor Progression. Cancers 2024, 16, 4039. [Google Scholar] [CrossRef]
- Cheminant, J.R.; Deering-Rice, C.E.; Massa, C.B.; Adhikari, U.; Noll, J.; Reilly, C.A.; Venosa, A. Parenchymal and inflammatory responses to ozone exposure in the aging healthy and surfactant protein C mutant lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2025, 328, L334–L349. [Google Scholar] [CrossRef]
- Sato, Y.; Silina, K.; van den Broek, M.; Hirahara, K.; Yanagita, M. The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol. 2023, 19, 525–537. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Randall, T.D.; Silva-Sanchez, A. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front. Immunol. 2016, 7, 258. [Google Scholar] [CrossRef]
- Zhao, R.; Gao, D. Innate Immunity and Tertiary Lymphoid Structures. Immunol. Rev. 2025, 332, e70052. [Google Scholar] [CrossRef]
- Kang, W.; Feng, Z.; Luo, J.; He, Z.; Liu, J.; Wu, J.; Rong, P. Tertiary Lymphoid Structures in Cancer: The Double-Edged Sword Role in Antitumor Immunity and Potential Therapeutic Induction Strategies. Front. Immunol. 2021, 12, 689270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, M.; Ren, Y.; Ba, Y.; Liu, S.; Zuo, A.; Xu, H.; Weng, S.; Han, X.; Liu, Z. Tertiary lymphoid structural heterogeneity determines tumour immunity and prospects for clinical application. Mol. Cancer 2024, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Y.; Luan, S.; Xu, W.; Gao, Y. Tertiary lymphoid structures in gliomas: Impact on tumour immunity and progression. J. Transl. Med. 2025, 23, 528. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Wen, Y.; Sun, Z.J. The impact of metabolic reprogramming on tertiary lymphoid structure formation: Enhancing cancer immunotherapy. BMC Med. 2025, 23, 217. [Google Scholar] [CrossRef]
- Jones, G.W.; Hill, D.G.; Jones, S.A. Understanding Immune Cells in Tertiary Lymphoid Organ Development: It Is All Starting to Come Together. Front. Immunol. 2016, 7, 401. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, S.; Wang, S.; Zhang, Z.; Wang, X.; Chen, Z.; Wang, X.; Huang, S.; Zhang, D.; Wu, H. Tertiary lymphoid structures in diseases: Immune mechanisms and therapeutic advances. Signal Transduct. Target. Ther. 2024, 9, 225. [Google Scholar] [CrossRef]
- Nayar, S.; Campos, J.; Chung, M.M.; Navarro-Nunez, L.; Chachlani, M.; Steinthal, N.; Gardner, D.H.; Rankin, P.; Cloake, T.; Caamano, J.H.; et al. Bimodal Expansion of the Lymphatic Vessels Is Regulated by the Sequential Expression of IL-7 and Lymphotoxin alpha1beta2 in Newly Formed Tertiary Lymphoid Structures. J. Immunol. 2016, 197, 1957–1967. [Google Scholar] [CrossRef]
- Meier, D.; Bornmann, C.; Chappaz, S.; Schmutz, S.; Otten, L.A.; Ceredig, R.; Acha-Orbea, H.; Finke, D. Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 2007, 26, 643–654. [Google Scholar] [CrossRef]
- Amisaki, M.; Zebboudj, A.; Yano, H.; Zhang, S.L.; Payne, G.; Chandra, A.K.; Yu, R.; Guasp, P.; Sethna, Z.M.; Ohmoto, A.; et al. IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer. Nature 2025, 638, 1076–1084. [Google Scholar] [CrossRef]
- Menk, A.V.; Scharping, N.E.; Moreci, R.S.; Zeng, X.; Guy, C.; Salvatore, S.; Bae, H.; Xie, J.; Young, H.A.; Wendell, S.G.; et al. Early TCR Signaling Induces Rapid Aerobic Glycolysis Enabling Distinct Acute T Cell Effector Functions. Cell Rep. 2018, 22, 1509–1521. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Perez-Tomas, R.; Perez-Guillen, I. Lactate in the Tumor Microenvironment: An Essential Molecule in Cancer Progression and Treatment. Cancers 2020, 12, 3244. [Google Scholar] [CrossRef]
- Tatum, J.L.; Kelloff, G.J.; Gillies, R.J.; Arbeit, J.M.; Brown, J.M.; Chao, K.S.; Chapman, J.D.; Eckelman, W.C.; Fyles, A.W.; Giaccia, A.J.; et al. Hypoxia: Importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int. J. Radiat. Biol. 2006, 82, 699–757. [Google Scholar] [CrossRef]
- Serafini, B.; Rosicarelli, B.; Franciotta, D.; Magliozzi, R.; Reynolds, R.; Cinque, P.; Andreoni, L.; Trivedi, P.; Salvetti, M.; Faggioni, A.; et al. Dysregulated Epstein-Barr virus infection in the multiple sclerosis brain. J. Exp. Med. 2007, 204, 2899–2912. [Google Scholar] [CrossRef] [PubMed]
- Manzo, A.; Bombardieri, M.; Humby, F.; Pitzalis, C. Secondary and ectopic lymphoid tissue responses in rheumatoid arthritis: From inflammation to autoimmunity and tissue damage/remodeling. Immunol. Rev. 2010, 233, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Hugaboom, M.B.; Wirth, L.V.; Street, K.; Ruthen, N.; Jegede, O.A.; Schindler, N.R.; Shah, V.; Zaemes, J.P.; El Ahmar, N.; Matar, S.; et al. Presence of Tertiary Lymphoid Structures and Exhausted Tissue-Resident T Cells Determines Clinical Response to PD-1 Blockade in Renal Cell Carcinoma. Cancer Discov. 2025, 15, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Colbeck, E.J.; Jones, E.; Hindley, J.P.; Smart, K.; Schulz, R.; Browne, M.; Cutting, S.; Williams, A.; Parry, L.; Godkin, A.; et al. Treg Depletion Licenses T Cell-Driven HEV Neogenesis and Promotes Tumor Destruction. Cancer Immunol. Res. 2017, 5, 1005–1015. [Google Scholar] [CrossRef]
- Lin, J.; Jiang, S.; Chen, B.; Du, Y.; Qin, C.; Song, Y.; Peng, Y.; Ding, M.; Wu, J.; Lin, Y.; et al. Tertiary Lymphoid Structures are Linked to Enhanced Antitumor Immunity and Better Prognosis in Muscle-Invasive Bladder Cancer. Adv. Sci. 2025, 12, e2410998. [Google Scholar] [CrossRef]
- Kinmonth, J.B. Lymphangiography in man; a method of outlining lymphatic trunks at operation. Clin. Sci. 1952, 11, 13–20. [Google Scholar]
- Sherman, A.I.; Ter-Pogossian, M. Lymph-node concentration of radioactive colloidal gold following interstitial injection. Cancer 1953, 6, 1238–1240. [Google Scholar] [CrossRef]
- Rockson, S.G. Advances in Lymphedema. Circ. Res. 2021, 128, 2003–2016. [Google Scholar] [CrossRef]
- Hassanein, A.H.; Maclellan, R.A.; Grant, F.D.; Greene, A.K. Diagnostic Accuracy of Lymphoscintigraphy for Lymphedema and Analysis of False-Negative Tests. Plast. Reconstr. Surgery. Glob. Open 2017, 5, e1396. [Google Scholar] [CrossRef]
- Iimura, T.; Fukushima, Y.; Kumita, S.; Ogawa, R.; Hyakusoku, H. Estimating Lymphodynamic Conditions and Lymphovenous Anastomosis Efficacy Using (99m)Tc-phytate Lymphoscintigraphy with SPECT-CT in Patients with Lower-limb Lymphedema. Plast. Reconstr. Surgery. Glob. Open 2015, 3, e404. [Google Scholar] [CrossRef]
- Naaman, Y.; Pinkas, L.; Roitman, S.; Ikher, S.; Oustinov, N.; Vaisbuch, E.; Yachnin, A.; Ben-Arie, A. The Added Value of SPECT/CT in Sentinel Lymph Nodes Mapping for Endometrial Carcinoma. Ann. Surg. Oncol. 2016, 23, 450–455. [Google Scholar] [CrossRef]
- Saad, Z.Z.; Omorphos, S.; Michopoulou, S.; Gacinovic, S.; Malone, P.; Nigam, R.; Muneer, A.; Bomanji, J. Investigating the role of SPECT/CT in dynamic sentinel lymph node biopsy for penile cancers. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Tew, K.; Farlow, D. SPECT/CT in Melanoma Lymphoscintigraphy. Clin. Nucl. Med. 2016, 41, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, A.; Jäger, K.; Sgier, F.; Seglias, J. Fluorescence microlymphography. Circulation 1981, 64, 1195–1200. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M.; Sharma, R.; Rasmussen, J.C.; Marshall, M.V.; Wendt, J.A.; Pham, H.Q.; Bonefas, E.; Houston, J.P.; Sampath, L.; Adams, K.E.; et al. Imaging of Lymph Flow in Breast Cancer Patients after Microdose Administration of a Near-Infrared Fluorophore: Feasibility Study. Radiology 2008, 246, 734–741. [Google Scholar] [CrossRef]
- Granoff, M.D.; Johnson, A.R.; Lee, B.T.; Padera, T.P.; Bouta, E.M.; Singhal, D. A Novel Approach to Quantifying Lymphatic Contractility during Indocyanine Green Lymphangiography. Plast. Reconstr. Surg. 2019, 144, 1197–1201. [Google Scholar] [CrossRef]
- Groenlund, J.H.; Telinius, N.; Skov, S.N.; Hjortdal, V. A Validation Study of Near-Infrared Fluorescence Imaging of Lymphatic Vessels in Humans. Lymphat. Res. Biol. 2017, 15, 227–234. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yoshimatsu, H.; Yamamoto, N. Complete lymph flow reconstruction: A free vascularized lymph node true perforator flap transfer with efferent lymphaticolymphatic anastomosis. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2016, 69, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Farias-Cisneros, E.; Chilton, P.M.; Palazzo, M.D.; Ozyurekoglu, T.; Hoying, J.B.; Williams, S.K.; Baughman, C.; Jones, C.M.; Kaufman, C.L. Infrared imaging of lymphatic function in the upper extremity of normal controls and hand transplant recipients via subcutaneous indocyanine green injection. SAGE Open Med. 2019, 7, 2050312119862670. [Google Scholar] [CrossRef] [PubMed]
- Garza, R.M.; Chang, D.W. Lymphovenous bypass for the treatment of lymphedema. J. Surg. Oncol. 2018, 118, 743–749, Correction in J. Surg. Oncol. 2019, 119, 1031. [Google Scholar] [CrossRef] [PubMed]
- Burnier, P.; Niddam, J.; Bosc, R.; Hersant, B.; Meningaud, J.P. Indocyanine green applications in plastic surgery: A review of the literature. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2017, 70, 814–827. [Google Scholar] [CrossRef]
- Gashev, A.A.; Nagai, T.; Bridenbaugh, E.A. Indocyanine Green and Lymphatic Imaging: Current Problems. Lymphat. Res. Biol. 2010, 8, 127–130. [Google Scholar] [CrossRef]
- Hayashi, A.; Giacalone, G.; Yamamoto, T.; Belva, F.; Visconti, G.; Hayashi, N.; Handa, M.; Yoshimatsu, H.; Salgarello, M. Ultra High-frequency Ultrasonographic Imaging with 70 MHz Scanner for Visualization of the Lymphatic Vessels. Plast. Reconstr. Surgery. Glob. Open 2019, 7, e2086. [Google Scholar] [CrossRef]
- Russo, A.; Reginelli, A.; Lacasella, G.V.; Grassi, E.; Karaboue, M.A.A.; Quarto, T.; Busetto, G.M.; Aliprandi, A.; Grassi, R.; Berritto, D. Clinical Application of Ultra-High-Frequency Ultrasound. J. Pers. Med. 2022, 12, 1733. [Google Scholar] [CrossRef]
- Lahtinen, O.; Vanninen, R.; Rautiainen, S. Contrast-enhanced ultrasound: A new tool for imaging the superficial lymphatic vessels of the upper limb. Eur. Radiol. Exp. 2022, 6, 18. [Google Scholar] [CrossRef]
- Jang, S.; Lee, C.U.; Hesley, G.K.; Knudsen, J.M.; Brinkman, N.J.; Tran, N.V. Lymphatic Mapping Using US Microbubbles before Lymphaticovenous Anastomosis Surgery for Lymphedema. Radiology 2022, 304, 218–224. [Google Scholar] [CrossRef]
- Mohos, B.; Czedik-Eysenberg, M.; Steinbacher, J.; Tinhofer, I.; Meng, S.; Tzou, C.J. Long-term Use of Ultrasound for Locating Optimal LVA Sites: A Descriptive Data Analysis. J. Reconstr. Microsurg. 2022, 38, 238–244. [Google Scholar] [CrossRef]
- Czedik-Eysenberg, M.; Steinbacher, J.; Obermayer, B.; Yoshimatsu, H.; Hara, H.; Mihara, M.; Tzou, C.J.; Meng, S. Exclusive use of ultrasound for locating optimal LVA sites-A descriptive data analysis. J. Surg. Oncol. 2020, 121, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Pons, G.; Clavero, J.A.; Alomar, X.; Rodríguez-Bauza, E.; Tom, L.K.; Masia, J. Preoperative planning of lymphaticovenous anastomosis: The use of magnetic resonance lymphangiography as a complement to indocyanine green lymphography. J. Plast. Reconstr. Aesthetic Surg. JPRAS 2019, 72, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Mihara, M.; Hara, H.; Araki, J.; Kikuchi, K.; Narushima, M.; Yamamoto, T.; Iida, T.; Yoshimatsu, H.; Murai, N.; Mitsui, K.; et al. Indocyanine green (ICG) lymphography is superior to lymphoscintigraphy for diagnostic imaging of early lymphedema of the upper limbs. PLoS ONE 2012, 7, e38182. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.; van Zanten, M.; Borri, M.; Mortimer, P.S.; Gordon, K.; Ostergaard, P.; Howe, F.A. Systematic Review of Magnetic Resonance Lymphangiography From a Technical Perspective. J. Magn. Reson. Imaging JMRI 2021, 53, 1766–1790. [Google Scholar] [CrossRef]
- Neligan, P.C.; Kung, T.A.; Maki, J.H. MR lymphangiography in the treatment of lymphedema. J. Surg. Oncol. 2017, 115, 18–22. [Google Scholar] [CrossRef]
- Masia, J.; Pons, G.; Nardulli, M.L. Combined Surgical Treatment in Breast Cancer-Related Lymphedema. J. Reconstr. Microsurg. 2016, 32, 16–27. [Google Scholar] [CrossRef]
- Kajita, H.; Oh, A.; Urano, M.; Takemaru, M.; Imanishi, N.; Otaki, M.; Yagi, T.; Aiso, S.; Kishi, K. Photoacoustic lymphangiography. J. Surg. Oncol. 2020, 121, 48–50. [Google Scholar] [CrossRef]
- Kajita, H.; Kishi, K. High-Resolution Imaging of Lymphatic Vessels with Photoacoustic Lymphangiography. Radiology 2019, 292, 35. [Google Scholar] [CrossRef]
- Vu, T.; Razansky, D.; Yao, J. Listening to tissues with new light: Recent technological advances in photoacoustic imaging. J. Opt. 2019, 21, 103001. [Google Scholar] [CrossRef]
- Deán-Ben, X.L.; Razansky, D. Optoacoustic image formation approaches—A clinical perspective. Phys. Med. Biol. 2019, 64, 18TR01. [Google Scholar] [CrossRef]
- Zackrisson, S.; van de Ven, S.; Gambhir, S.S. Light in and sound out: Emerging translational strategies for photoacoustic imaging. Cancer Res. 2014, 74, 979–1004. [Google Scholar] [CrossRef] [PubMed]
- Catherine, M.; Junjie, Y.; Jun, Z.; Chih-Hsien, H.; Gwendalyn, J.R.; Lihong, V.W. Photoacoustic lymphatic imaging with high spatial-temporal resolution. J. Biomed. Opt. 2014, 19, 116009. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Agollah, G.D.; Wu, G.; Sevick-Muraca, E.M. Spatio-Temporal Changes of Lymphatic Contractility and Drainage Patterns following Lymphadenectomy in Mice. PLoS ONE 2014, 9, e106034. [Google Scholar] [CrossRef]
- Bachmann, S.B.; Proulx, S.T.; He, Y.; Ries, M.; Detmar, M. Differential effects of anaesthesia on the contractility of lymphatic vessels in vivo. J. Physiol. 2019, 597, 2841–2852. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Lanning, R.M.; Tyrrell, J.A.; Padera, T.P.; Bartlett, L.A.; Stylianopoulos, T.; Munn, L.L.; Tearney, G.J.; Fukumura, D.; Jain, R.K.; et al. Three-dimensional microscopy of the tumor microenvironment in vivo using optical frequency domain imaging. Nat. Med. 2009, 15, 1219–1223. [Google Scholar] [CrossRef]
- Zhi, Z.; Jung, Y.; Wang, R.K. Label-free 3D imaging of microstructure, blood, and lymphatic vessels within tissue beds in vivo. Opt. Lett. 2012, 37, 812–814. [Google Scholar] [CrossRef]
- Siavash, Y.; Jia, Q.; Zhongwei, Z.; Ruikang, K.W. Label-free optical lymphangiography: Development of an automatic segmentation method applied to optical coherence tomography to visualize lymphatic vessels using Hessian filters. J. Biomed. Opt. 2013, 18, 086004. [Google Scholar] [CrossRef]
- Horstmann, J.; Schulz-Hildebrandt, H.; Bock, F.; Siebelmann, S.; Lankenau, E.; Hüttmann, G.; Steven, P.; Cursiefen, C. Label-Free In Vivo Imaging of Corneal Lymphatic Vessels Using Microscopic Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5880–5886. [Google Scholar] [CrossRef]
- Gong, P.; Yu, D.Y.; Wang, Q.; Yu, P.K.; Karnowski, K.; Heisler, M.; Francke, A.; An, D.; Sarunic, M.V.; Sampson, D.D. Label-free volumetric imaging of conjunctival collecting lymphatics ex vivo by optical coherence tomography lymphangiography. J. Biophotonics 2018, 11, e201800070. [Google Scholar] [CrossRef]
- Cousins, A.; Tsopelas, C.; Balalis, G.; Thompson, S.K.; Bartholomeusz, D.; Wedding, A.B.; Thierry, B. Hybrid (99m)Tc-magnetite tracer for dual modality sentinel lymph node mapping. J. Mater. Science. Mater. Med. 2018, 29, 76. [Google Scholar] [CrossRef]
- Zalewski, K.; Benke, M.; Mirocha, B.; Radziszewski, J.; Chechlinska, M.; Kowalewska, M. Technetium-99m-based Radiopharmaceuticals in Sentinel Lymph Node Biopsy: Gynecologic Oncology Perspective. Curr. Pharm. Des. 2018, 24, 1652–1675. [Google Scholar] [CrossRef] [PubMed]
- Bouché, M.; Hsu, J.C.; Dong, Y.C.; Kim, J.; Taing, K.; Cormode, D.P. Recent Advances in Molecular Imaging with Gold Nanoparticles. Bioconjugate Chem. 2020, 31, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Xie, J.; Chen, X. Peptide-based probes for targeted molecular imaging. Biochemistry 2010, 49, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Amersi, F.; Hansen, N.M. The benefits and limitations of sentinel lymph node biopsy. Curr. Treat. Options Oncol. 2006, 7, 141–151. [Google Scholar] [CrossRef]
- Li, F.; Cheng, T.; Dong, Q.; Wei, R.; Zhang, Z.; Luo, D.; Ma, X.; Wang, S.; Gao, Q.; Ma, D.; et al. Evaluation of (99m)Tc-HYNIC-TMTP1 as a tumor-homing imaging agent targeting metastasis with SPECT. Nucl. Med. Biol. 2015, 42, 256–262. [Google Scholar] [CrossRef]
- Wu, Y.; Shang, J.; Zhang, X.; Li, N. Advances in molecular imaging and targeted therapeutics for lymph node metastasis in cancer: A comprehensive review. J. Nanobiotechnol. 2024, 22, 783. [Google Scholar] [CrossRef]
- Han, N.; Zhou, D.; Ruan, M.; Yan, M.; Zhang, C. Cancer cell-derived extracellular vesicles drive pre-metastatic niche formation of lymph node via IFNGR1/JAK1/STAT1-activated-PD-L1 expression on FRCs in head and neck cancer. Oral. Oncol. 2023, 145, 106524. [Google Scholar] [CrossRef]
- Riedel, A.; Helal, M.; Pedro, L.; Swietlik, J.J.; Shorthouse, D.; Schmitz, W.; Haas, L.; Young, T.; da Costa, A.S.H.; Davidson, S.; et al. Tumor-Derived Lactic Acid Modulates Activation and Metabolic Status of Draining Lymph Node Stroma. Cancer Immunol. Res. 2022, 10, 482–497. [Google Scholar] [CrossRef]
- Da Silva, C.G.; Rueda, F.; Löwik, C.W.; Ossendorp, F.; Cruz, L.J. Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials 2016, 83, 308–320. [Google Scholar] [CrossRef]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Huynh, C.; Huang, X.; Desai, T.A. Bioinspired nanotopographical design of drug delivery systems. Nat. Rev. Bioeng. 2023, 1, 139–152. [Google Scholar] [CrossRef]
- Getts, D.R.; Shea, L.D.; Miller, S.D.; King, N.J.C. Harnessing nanoparticles for immune modulation. Trends Immunol. 2015, 36, 419–427. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Taghipour, B. PLGA micro and nanoparticles in delivery of peptides and proteins; problems and approaches. Pharm. Dev. Technol. 2015, 20, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Senti, G.; Kündig, T.M. Intralymphatic immunotherapy. World Allergy Organ. J. 2015, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Van Lint, S.; Goyvaerts, C.; Maenhout, S.; Goethals, L.; Disy, A.; Benteyn, D.; Pen, J.; Bonehill, A.; Heirman, C.; Breckpot, K.; et al. Preclinical evaluation of TriMix and antigen mRNA-based antitumor therapy. Cancer Res. 2012, 72, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Schudel, A.; Chapman, A.P.; Yau, M.K.; Higginson, C.J.; Francis, D.M.; Manspeaker, M.P.; Avecilla, A.R.C.; Rohner, N.A.; Finn, M.G.; Thomas, S.N. Programmable multistage drug delivery to lymph nodes. Nat. Nanotechnol. 2020, 15, 491–499, Correction in Nat. Nanotechnol. 2020, 15, 724. [Google Scholar] [CrossRef]
- Li, M.; Zhou, H.; Fang, M.; Qu, K.; Wang, Y. Cationic Lipids-Mediated Dual-Targeting of Both Dendritic Cells and Tumor Cells for Potent Cancer Immunotherapy. Adv. Funct. Mater. 2023, 33, 2306752. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Liang, R.; Chen, W.; Li, Q.; Xu, J.; Zhao, H.; Xing, D. Targeting lymph nodes for enhanced cancer vaccination: From nanotechnology to tissue engineering. Mater. Today. Bio 2024, 26, 101068. [Google Scholar] [CrossRef]
- Liu, M.; Feng, Y.; Lu, Y.; Huang, R.; Zhang, Y.; Zhao, Y.; Mo, R. Lymph-targeted high-density lipoprotein-mimetic nanovaccine for multi-antigenic personalized cancer immunotherapy. Sci. Adv. 2024, 10, eadk2444. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Pan, S.; Gao, P.; Sheng, H.; Li, L.; Shi, L.; Zhang, Y.; Cai, X. Stimuli-responsive charge-reversal nano drug delivery system: The promising targeted carriers for tumor therapy. Int. J. Pharm. 2020, 575, 118841. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wu, J.; Yan, Y.; Wang, Y.; Zhao, H.; Wang, X.; Chang, S.; Li, S. Charge-Reversal Nano-Drug Delivery Systems in the Tumor Microenvironment: Mechanisms, Challenges, and Therapeutic Applications. Int. J. Mol. Sci. 2024, 25, 9779. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Hwang, S.Y.; Lee, E.K. Imaging-based analysis of liposome internalization to macrophage cells: Effects of liposome size and surface modification with PEG moiety. Colloids Surfaces. B Biointerfaces 2015, 136, 786–790. [Google Scholar] [CrossRef]
- Raghuvanshi, R.S.; Katare, Y.K.; Lalwani, K.; Ali, M.M.; Singh, O.; Panda, A.K. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. Int. J. Pharm. 2002, 245, 109–121. [Google Scholar] [CrossRef]
- Moghimi, S.M. The effect of methoxy-PEG chain length and molecular architecture on lymph node targeting of immuno-PEG liposomes. Biomaterials 2006, 27, 136–144. [Google Scholar] [CrossRef]
- Zhan, X.; Tran, K.K.; Shen, H. Effect of the poly(ethylene glycol) (PEG) density on the access and uptake of particles by antigen-presenting cells (APCs) after subcutaneous administration. Mol. Pharm. 2012, 9, 3442–3451. [Google Scholar] [CrossRef]
- De Koker, S.; Cui, J.; Vanparijs, N.; Albertazzi, L.; Grooten, J.; Caruso, F.; De Geest, B.G. Engineering Polymer Hydrogel Nanoparticles for Lymph Node-Targeted Delivery. Angew. Chem. (Int. Ed. Engl.) 2016, 55, 1334–1339. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhu, D.; Wang, Y.; Qing, G.; Zhang, Y.; Liu, X.; Liang, X.J. Effect of physicochemical properties on in vivo fate of nanoparticle-based cancer immunotherapies. Acta Pharm. Sinica. B 2021, 11, 886–902. [Google Scholar] [CrossRef]
- Zou, D.; Wu, Z.; Yi, X.; Hui, Y.; Yang, G.; Liu, Y.; Wang, H.; Brooks, A.; Wang, H.; Liu, X.; et al. Nanoparticle elasticity regulates the formation of cell membrane-coated nanoparticles and their nano-bio interactions. Proc. Natl. Acad. Sci. USA 2023, 120, e2214757120. [Google Scholar] [CrossRef]
- Song, T.; Xia, Y.; Du, Y.; Chen, M.W.; Qing, H.; Ma, G. Engineering the Deformability of Albumin-Stabilized Emulsions for Lymph-Node Vaccine Delivery. Adv. Mater. 2021, 33, 2100106. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Yan, X.; Zong, X.; Li, X.; Yang, C.; Chen, X.; Li, Y.; Wen, Y.; Zhu, T.; Xue, W.; et al. Modulating Elasticity of Liposome for Enhanced Cancer Immunotherapy. ACS Nano 2024, 18, 23797–23811. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, Y.; Cabral, H.; Tong, A.; Yue, Q.; Zhao, L.; Sun, X.; Mi, P. Glucosylated Nanovaccines for Dendritic Cell-Targeted Antigen Delivery and Amplified Cancer Immunotherapy. ACS Nano 2024, 18, 25826–25840. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Le, X.T.; Lee, W.T.; Lim, Y.T.; Oh, K.T.; Lee, E.S.; Choi, H.G.; Youn, Y.S. STING-activating dendritic cell-targeted nanovaccines that evoke potent antigen cross-presentation for cancer immunotherapy. Bioact. Mater. 2024, 42, 345–365. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, M.; Pang, L.; Wang, S.; Kong, Y.; Zhu, X.; Zhou, X.; Wang, X.; Chen, C.; Ning, H.; et al. Identification of a novel DEC-205 binding peptide to develop dendritic cell-targeting nanovaccine for cancer immunotherapy. J. Control Release 2024, 373, 568–582. [Google Scholar] [CrossRef]
- Lei, J.; Qi, S.; Yu, X.; Gao, X.; Yang, K.; Zhang, X.; Cheng, M.; Bai, B.; Feng, Y.; Lu, M.; et al. Development of Mannosylated Lipid Nanoparticles for mRNA Cancer Vaccine with High Antigen Presentation Efficiency and Immunomodulatory Capability. Angew. Chem. Int. Ed. 2024, 63, e202318515. [Google Scholar] [CrossRef]
- Li, X.; Khorsandi, S.; Wang, Y.; Santelli, J.; Huntoon, K.; Nguyen, N.; Yang, M.; Lee, D.; Lu, Y.; Gao, R.; et al. Cancer immunotherapy based on image-guided STING activation by nucleotide nanocomplex-decorated ultrasound microbubbles. Nat. Nanotechnol. 2022, 17, 891–899. [Google Scholar] [CrossRef]
- Jung, C.; Fichter, M.; Oberländer, J.; Schunke, J.; Bolduan, V.; Schneider, P.; Kang, J.; Koynov, K.; Mailänder, V.; Landfester, K. Nanobodies Outperform Antibodies—Rapid Functionalization with Equal In Vivo Targeting Properties. Adv. Mater. 2024, 36, 2412563. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Poon, W.; Sefton, E.; Chan, W.C.W. Suppressing Subcapsular Sinus Macrophages Enhances Transport of Nanovaccines to Lymph Node Follicles for Robust Humoral Immunity. ACS Nano 2020, 14, 9478–9490. [Google Scholar] [CrossRef]
- Alimohammadvand, S.; Kaveh Zenjanab, M.; Mashinchian, M.; Shayegh, J.; Jahanban-Esfahlan, R. Recent advances in biomimetic cell membrane–camouflaged nanoparticles for cancer therapy. Biomed. Pharmacother. 2024, 177, 116951. [Google Scholar] [CrossRef]
- Wang, T.; Han, M.; Han, Y.; Jiang, Z.; Zheng, Q.; Zhang, H.; Li, Z. Antigen Self-Presented Personalized Nanovaccines Boost the Immunotherapy of Highly Invasive and Metastatic Tumors. ACS Nano 2024, 18, 6333–6347. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Liu, Q.; Li, H.; Li, S.; Liu, Z.; Tang, F.; Feng, H. Macrophage membrane-coated Eucommia ulmoides polysaccharides-loaded PLGA nanoparticles as an effective antigen-targeted delivery system. Appl. Mater. Today 2024, 38, 102173. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Wu, J.; Lv, X.; Yang, N.; Wei, Q.; Wang, C.; Chen, J. Surgically Derived Cancer Cell Membrane-Coated R837-Loaded Poly(2-Oxazoline) Nanoparticles for Prostate Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2023, 15, 7878–7886. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Sun, Y.; Xie, L.; Liu, Y.; You, Y.; Xu, J.; Ma, F.; Huang, Y.; Song, Q.; Xiao, W.; et al. Biologically Self-Assembled Tumor Cell-Derived Cancer Nanovaccines as an All-in-One Platform for Cancer Immunotherapy. ACS Nano 2024, 18, 6702–6717. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Li, K.; Huang, F.; Dai, Y.; Zhang, T.; Muaibati, M.; Abuduyilimu, A.; Huang, X. Extracellular vesicles hybrid plasmid-loaded lipid nanovesicles for synergistic cancer immunotherapy. Mater. Today Bio 2023, 23, 100845. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Niu, Z.; Galli, V.; Howe, N.; Zhao, Y.; Wiklander, O.P.B.; Zheng, W.; Wiklander, R.J.; Corso, G.; Davies, C.; et al. Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models. J. Extracell. Vesicles 2022, 11, e12248. [Google Scholar] [CrossRef]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef]
- Noorbakhsh-Sabet, N.; Zand, R.; Zhang, Y.; Abedi, V. Artificial Intelligence Transforms the Future of Health Care. Am. J. Med. 2019, 132, 795–801. [Google Scholar] [CrossRef]
- Zanagnolo, V.; Minig, L.; Rollo, D.; Tomaselli, T.; Aletti, G.; Bocciolone, L.; Landoni, F.; Cardenas Rebollo, J.M.; Maggioni, A. Clinical and Oncologic Outcomes of Robotic Versus Abdominal Radical Hysterectomy for Women With Cervical Cancer: Experience at a Referral Cancer Center. Int. J. Gynecol. Cancer 2016, 26, 568–574. [Google Scholar] [CrossRef]
- Krill, L.S.; Bristow, R.E. Robotic surgery: Gynecologic oncology. Cancer J. 2013, 19, 167–176. [Google Scholar] [CrossRef]
- Vicentini, P.; Carmen, M.d.; Perez, J.; Araujo, E. Fuzzy lymphedema assessment based on clinical and functional criteria. In Proceedings of the 2011 IEEE International Conference on Fuzzy Systems (FUZZ-IEEE 2011), Taipei, Taiwan, 27–30 June 2011; pp. 2708–2713. [Google Scholar]
- Moreira, R.; Magalhães, A.; Oliveira, H.P. A Kinect-Based System to Assess Lymphedema Impairments in Breast Cancer Patients. In Proceedings of the Pattern Recognition and Image Analysis, Cham, Switzerland, 17–19 June 2015; pp. 228–236. [Google Scholar]
- Hou, D.; Her, H.; Han, W.; Ge, X. Artificial Intelligence in CT for Predicting Lymph Node Metastasis in Rectal Cancer Patients: A Meta-Analysis. Clin. Radiol. 2025, 107001. [Google Scholar] [CrossRef]
- Bai, Z.; Xu, L.; Ding, Z.; Cao, Y.; Wang, Z.; Yang, W.; Xu, W.; Li, H. Artificial intelligence in magnetic resonance imaging for predicting lymph node metastasis in rectal cancer patients: A meta-analysis. Eur. Radiol. 2025, 35, 6193–6206. [Google Scholar] [CrossRef]
- Liu, C.-J.; Zhang, L.; Sun, Y.; Geng, L.; Wang, R.; Shi, K.-M.; Wan, J.-X. Application of CT and MRI images based on an artificial intelligence algorithm for predicting lymph node metastasis in breast cancer patients: A meta-analysis. BMC Cancer 2023, 23, 1134. [Google Scholar] [CrossRef]
- Wu, S.; Hong, G.; Xu, A.; Zeng, H.; Chen, X.; Wang, Y.; Luo, Y.; Wu, P.; Liu, C.; Jiang, N.; et al. Artificial intelligence-based model for lymph node metastases detection on whole slide images in bladder cancer: A retrospective, multicentre, diagnostic study. Lancet Oncol. 2023, 24, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Bedrikovetski, S.; Dudi-Venkata, N.N.; Kroon, H.M.; Seow, W.; Vather, R.; Carneiro, G.; Moore, J.W.; Sammour, T. Artificial intelligence for pre-operative lymph node staging in colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2021, 21, 1058. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Hu, J.; Tang, P.; Xu, T.; He, L.; Zeng, Z.; Sheng, J. Application of CT and MRI images based on artificial intelligence to predict lymph node metastases in patients with oral squamous cell carcinoma: A subgroup meta-analysis. Front. Oncol. 2024, 14, 1395159. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.F.; Lin, J.; Huang, Y.L.; Chen, S.T.; Chou, C.T.; Chen, D.R.; Wu, W.P. Deep learning-based breast MRI for predicting axillary lymph node metastasis: A systematic review and meta-analysis. Cancer Imaging 2025, 25, 44. [Google Scholar] [CrossRef]
- Wang, X.; Qi, Y.; Zhang, X.; Liu, F.; Li, J. Ultrasound-based artificial intelligence for predicting cervical lymph node metastasis in papillary thyroid cancer: A systematic review and meta-analysis. Front. Endocrinol. 2025, 16, 1570811. [Google Scholar] [CrossRef]
- Trinh, X.-T.; Chien, P.N.; Long, N.-V.; Van Anh, L.T.; Giang, N.N.; Nam, S.-Y.; Myung, Y. Development of predictive models for lymphedema by using blood tests and therapy data. Sci. Rep. 2023, 13, 19720. [Google Scholar] [CrossRef]
- Agarwal, R.; Rajanbabu, A.; Goel, G.; Unnikrishnan, U.G. A Comparison of the Clinical Outcomes in Uterine Cancer Surgery After the Introduction of Robotic-Assisted Surgery. J. Obstet. Gynaecol. India 2019, 69, 284–291. [Google Scholar] [CrossRef]
- Schwarz, G. Precision-and-Progress-Two-First-of-Their-Kind-Robotic-Assisted-Lymphatic-Surgeries. Cleveland Clinic, Case Study. Available online: https://consultqd.clevelandclinic.org/precision-and-progress-two-first-of-their-kind-robotic-assisted-lymphatic-surgeries (accessed on 27 February 2025).
- Visscher, M.; Pouw, J.J.; Baarlen, J.v.; Klaase, J.M.; Haken, B.t. Quantitative Analysis of Superparamagnetic Contrast Agent in Sentinel Lymph Nodes Using Ex Vivo Vibrating Sample Magnetometry. IEEE Trans. Biomed. Eng. 2013, 60, 2594–2602. [Google Scholar] [CrossRef]
- Johnson, L.; Pinder, S.E.; Douek, M. Deposition of superparamagnetic iron-oxide nanoparticles in axillary sentinel lymph nodes following subcutaneous injection. Histopathology 2013, 62, 481–486. [Google Scholar] [CrossRef]
- Vangijzegem, T.; Lecomte, V.; Ternad, I.; Van Leuven, L.; Muller, R.N.; Stanicki, D.; Laurent, S. Superparamagnetic Iron Oxide Nanoparticles (SPION): From Fundamentals to State-of-the-Art Innovative Applications for Cancer Therapy. Pharmaceutics 2023, 15, 236. [Google Scholar] [CrossRef]
- Qiu, M.Z.; Qiu, H.J.; Wang, Z.Q.; Ren, C.; Wang, D.S.; Zhang, D.S.; Luo, H.Y.; Li, Y.H.; Xu, R.H. The tumor-log odds of positive lymph nodes-metastasis staging system, a promising new staging system for gastric cancer after D2 resection in China. PLoS ONE 2012, 7, e31736. [Google Scholar] [CrossRef]
- Robb, J.A.; Gulley, M.L.; Fitzgibbons, P.L.; Kennedy, M.F.; Cosentino, L.M.; Washington, K.; Dash, R.C.; Branton, P.A.; Jewell, S.D.; Lapham, R.L. A Call to Standardize Preanalytic Data Elements for Biospecimens. Arch. Pathol. Lab. Med. 2013, 138, 526–537. [Google Scholar] [CrossRef]
- Warnat-Herresthal, S.; Schultze, H.; Shastry, K.L.; Manamohan, S.; Mukherjee, S.; Garg, V.; Sarveswara, R.; Händler, K.; Pickkers, P.; Aziz, N.A.; et al. Swarm Learning for decentralized and confidential clinical machine learning. Nature 2021, 594, 265–270. [Google Scholar] [CrossRef]
- Klontzas, M.E.; Gatti, A.A.; Tejani, A.S.; Kahn, C.E. AI Reporting Guidelines: How to Select the Best One for Your Research. Radiol. Artif. Intell. 2023, 5, e230055. [Google Scholar] [CrossRef]
- Vaidya, A.; Chen, R.J.; Williamson, D.F.K.; Song, A.H.; Jaume, G.; Yang, Y.; Hartvigsen, T.; Dyer, E.C.; Lu, M.Y.; Lipkova, J.; et al. Demographic bias in misdiagnosis by computational pathology models. Nat. Med. 2024, 30, 1174–1190. [Google Scholar] [CrossRef]
- Murdoch, B. Privacy and artificial intelligence: Challenges for protecting health information in a new era. BMC Med. Ethics 2021, 22, 122. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, M.R.; Cockerill, R.G.; Mirza, O.F.; Appel, J.M. Ethical considerations for the use of artificial intelligence in medical decision-making capacity assessments. Psychiatry Res. 2023, 328, 115466. [Google Scholar] [CrossRef] [PubMed]
| Lymphatic Disorder | Immune Cell Features | Key Cytokines | Molecular Markers | References |
|---|---|---|---|---|
| Primary and Secondary Lymphedema | ||||
| Primary Lymphedema | CD4+ T cells (Th2 bias), M2 macrophages, impaired dendritic trafficking | TNF-α,IL-1β, IL-6, TGF-β1 | VEGF-C/VEGFR-3 upregulation, TGF-b1, fibrosis markers (collagens I and III) | [7,39,40] |
| Milroy Disease | Abnormal lymphatic vessel development | VEGF-C, VEGFR-3 | FLT4 mutation | [44] |
| Meige Disease | Impaired lymph drainage | VEGF-C | FOXC2 mutation | [45] |
| Lymphedema-Distichiasis | Distichiasis, lymphedema onset in puberty | Unknown | FOXC2 | [46] |
| Hypoplasia of Lymphatics | Underdeveloped lymph vessels | VEGF-C | Various | [47,48] |
| Secondary Lymphedema | CD4+ T cells, M2 macrophages, Tregs accumulation, chronic fibrosis | IL-4, IL-6, IL-13, TGF-β, PDGF, CTGF | TGF-b, PDGF, CTGF, VEGF-C in tissue | [49,50,51] |
| Post-surgical Lymphedema | Local immune dysregulation | VEGF-A, TNF-α | D2-40 | [43] |
| Post-cancer Treatment Lymphedema | T cell infiltration, fibrosis | TGF-β, IL-6 | PROX1, LYVE-1 | [42] |
| Radiation-induced Lymphedema | Chronic inflammation, macrophages | TNF-α, IL-1β | CD68 | [52] |
| Lymphatic Filariasis | Th2 dominant, eosinophils, Tregs expansion | IL-4, IL-5, IL-10, TGF-β | CFA antigen, microfilariae, high IgE | [12,53,54] |
| Congenital and developmental lymphatic anomalies | ||||
| Lymphatic Malformations (LMs) | Mild local inflammation, mast cells, scattered T cells (if infected) | VEGF-C, VEGF-D, IL-6, TNF-α | PIK3CA mutations, mTOR activation (pS6, pAKT) | [55,56] |
| Central Conduction Lymphatic Anomaly | Reduced lymphocyte recirculation, lymphocyte loss in chyle | No characteristic cytokines; possible local inflammatory mediators | Abnormal lymphatic imaging, no known genetic mutations | [57] |
| Generalized Lymphatic Anomaly | Macrophages and T cells around lymphatic proliferations | VEGF-C, VEGF-D, IL-6, IL-1β | PIK3CA and NRAS mutations, mTOR pathway activation, VEGFR-3, Prox1 | [13,58] |
| Kaposiform Lymphangiomatosis | Spindle cells, mononuclear infiltration, platelet trapping | IL-6, TNF-α, elevated D-dimer related factors | NRAS mutations, VEGF, D-dimer, fibrinogen consumption | [19,20] |
| Intestinal Lymphangiectasia | Dilated intestinal lymphatics | Albumin loss | CD31, D2-40 | [59] |
| Gorham–Stout Disease | Lymphatic bone invasion | VEGF-A, IL-6 | RANKL | [60,61] |
| Cystic Hygroma | Lymph fluid-filled cysts | VEGF-C | PIK3CA | [62] |
| Lymphangioleiomyomatosis | - | - | VEGF-D | [63] |
| Lymphangiomatosis | - | - | VEGF-C/D, D2-40 | [64] |
| Lymphadenitis and lymph node-related disorders | ||||
| Lymphadenitis | Germinal center activation, granulomas (TB), T and B cell expansion | IL-1β, IL-6, IFN-g, TNF-α | PCR/culture for pathogens, high ferritin (systemic), IGRA (TB) | [65,66,67] |
| Castleman Disease | Plasma cell proliferation | IL-6 | HHV-8 LANA | [68] |
| Tuberculous Lymphadenitis | Granulomas with macrophages, Langhans giant cells, strong Th1 | IFN-γ, TNF-α, IL-12 | Mycobacterial PCR, AFB staining, IGRA | [33] |
| Reactive Lymphadenopathy | Follicular hyperplasia | IL-2, IFN-γ | CD3, CD20 | [69] |
| Autoimmune Lymphadenopathy | T/B cell hyperplasia | IL-6, TNF-α | ANA, RF | [70,71] |
| CVID with Lymphoid Hyperplasia | Decreased B cells | BAFF, IL-21 | Low IgG | [72] |
| Lymphangitis | Neutrophil-dominated acute response, strong chemokine signaling | IL-1b, IL-6, TNF-α, CXCL8 | Elevated CRP, procalcitonin, bacterial cultures | [73] |
| Neoplastic Lymphadenopathy | Clonal cell expansion | Various | BCL2, CD30 | [74] |
| Mesenteric lymphadenitis | - | - | C-Reactive protein | [75] |
| Kimura Disease | - | - | Eosinophilia, serum IgE, GM-CSF, IL-4, IL-5 | [76,77] |
| Rosai–Dorfman Disease (RDD) | - | - | Histiocytes (CD68+ve, S100+ve, CD1a-ve) | [78] |
| Lymphatic cancers and neoplasms | ||||
| Hodgkin Lymphoma | Reed–Sternberg cells | IL-13, IL-5 | CD30, CD15 | [79] |
| Non-Hodgkin Lymphoma | B-cell or T-cell expansion | IL-6, IL-10 | CD19, CD20 | [80] |
| Lymphangiosarcoma | Endothelial malignancy | VEGF-A | CD31, ERG | [81,82] |
| Lymphatic metastasis | - | - | VEGF-C/VEGF-D and their receptor VEGFR-3 (FLT4) | [83,84] |
| Lymphoma | - | - | Pax-5, CD10, TIA-1, granzyme B, perforin, LMO2, Bcl-6, CD30, SOX-11 | [85] |
| Rare Genetic Syndromes (with lymphatic involvement) | ||||
| Turner Syndrome | Lymphedema at birth | Unknown | X chromosome abnormalities | [86] |
| Noonan Syndrome | Peripheral lymphedema | SHOC2 pathway | PTPN11 | [87] |
| Yellow Nail Syndrome | Nail dystrophy, pleural effusion | - | - | [88] |
| Hennekam Syndrome | Possible lymphopenia, hypoalbuminemia | No clear systemic cytokines; possible local inflammatory signals | CCBE1, FAT4 mutations, Î ± 1-antitrypsin clearance | [10] |
| Category | Biomarker | Functional Role in Tumor Immunity/Evasion | Major Pathways/Mechanisms | References |
|---|---|---|---|---|
| Inflammatory Cytokines | IL-6 | Promotes inflammation, tumor growth, and immune suppression | JAK/STAT3, NF-κB | [119,120] |
| TNF-α | Triggers inflammation: chronic levels support invasion | NF-κB, MAPK | [119,120] | |
| TGF-β | Induces Tregs and suppresses effector T cells | SMAD, PI3K/AKT | [120,121] | |
| IL-10 | Inhibits antigen presentation and T-cell activity | STAT3, SOCS3 | [122,123] | |
| Pro-Immunogenic Cytokine | IFN-γ | Activates cytotoxic T cells, upregulates MHC, induces PD-L1 | JAK/STAT1, IRF1 | [119,124] |
| IL-2 | Stimulates proliferation and activation of cytotoxic T cells and NK cells | JAK/STAT5, PI3K/AKT | [120] | |
| IL-12 | Promotes Th1 differentiation, IFN-γ production, and cytotoxic immune response | STAT4, NF-κB | [119,120] | |
| IL-15 | Enhances NK cell and CD8+ T-cell survival and memory formation | JAK1/3–STAT5, PI3K/AKT | [125] | |
| IL-18 | Synergizes with IL-12 to boost IFN-γ release and antitumor activity | MyD88, NF-κB, MAPK | [126] | |
| Chemokine Axes | CCL21/CCR7 | Guides immune cells; hijacked by tumors for LN migration | Chemokine, MAPK | [119] |
| CXCL12/CXCR4 | Mediates tumor homing and lymphatic metastasis | PI3K/AKT, ERK | [120] | |
| Immune Checkpoints | PD-1 | Suppresses T-cell activation upon PD-L1 binding | PD-1/PD-L1 axis | [127,128] |
| PD-L1 | Inhibits cytotoxic T cells; induced by hypoxia and IFN-γ | JAK/STAT, HIF-1α | [127,128] | |
| CTLA-4 | Blocks T-cell priming and proliferation | CD80/86–CTLA-4 | [120,129] | |
| LAG-3 | Restrains T-cell proliferation under chronic antigen load | LAG-3/MHC-II | [120,130,131] | |
| TIM-3 | Promotes T-cell exhaustion and tolerance | TIM-3/Gal-9 | [120,132] | |
| Lymphangiogenic/Angiogenic Factors | VEGF-C | Induces lymphangiogenesis and metastasis | VEGF-C/VEGFR-3 | [133] |
| VEGF-D | Works with VEGF-C to remodel lymphatics | VEGFR-3 | [133] | |
| VEGF-A | Stimulates angiogenesis and vascular permeability | VEGF-A/VEGFR-2 | [134] | |
| Lymphatic Markers | LYVE-1 | Marker of active lymphatics; linked to metastasis | Lymphatic endothelial | [119] |
| D2-40 (Podoplanin) | Marker of lymphatic invasion; enhances motility | Rho-GTPase, PI3K | [135] | |
| Genetic/Oncogenic Pathways | FLT4 (VEGFR-3) | Mediates VEGF-C/D signaling; promotes metastasis | PI3K/AKT, MAPK | [136] |
| PIK3CA | Activates PI3K pathway; drives lymphatic proliferation | PI3K/AKT/mTOR | [120] | |
| PTEN | Tumor suppressor; loss enhances immune evasion | PI3K/AKT | [120] | |
| HIF-1α | Hypoxia-induced PD-L1 and VEGF expression | HIF-1α/VEGF | [127,128] | |
| mTOR | Integrates growth and immune signals | PI3K/AKT/mTOR | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, S.; Adhikari, U.; Singh, B. Rewiring the Lymphatic Landscape: Disorders, Remodeling, and Cancer Progression. Lymphatics 2025, 3, 37. https://doi.org/10.3390/lymphatics3040037
Kumar S, Adhikari U, Singh B. Rewiring the Lymphatic Landscape: Disorders, Remodeling, and Cancer Progression. Lymphatics. 2025; 3(4):37. https://doi.org/10.3390/lymphatics3040037
Chicago/Turabian StyleKumar, Sudeep, Ujjwal Adhikari, and Brijendra Singh. 2025. "Rewiring the Lymphatic Landscape: Disorders, Remodeling, and Cancer Progression" Lymphatics 3, no. 4: 37. https://doi.org/10.3390/lymphatics3040037
APA StyleKumar, S., Adhikari, U., & Singh, B. (2025). Rewiring the Lymphatic Landscape: Disorders, Remodeling, and Cancer Progression. Lymphatics, 3(4), 37. https://doi.org/10.3390/lymphatics3040037





