Advances in Medicinal Chemistry
A topical collection in International Journal of Molecular Sciences (ISSN 1422-0067). This collection belongs to the section "Molecular Pharmacology".
Submission Status: Closed | Viewed by 49886Editors
Interests: drug discovery; medicinal chemistry; molecular pharmacology; neurodegenerative diseases
Special Issues, Collections and Topics in MDPI journals
Interests: anti-cancer; anti-virus; anti-bacteria drugs; nano-biomaterials and nano-polymers; green chemistry
Topical Collection Information
Dear Colleagues,
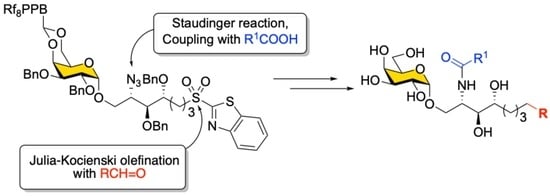
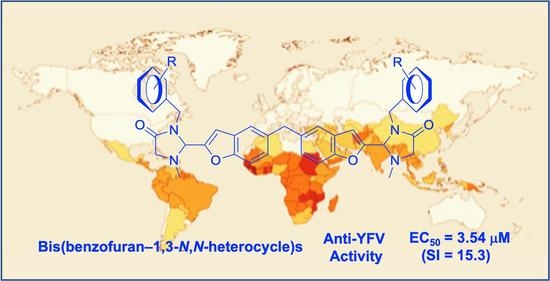
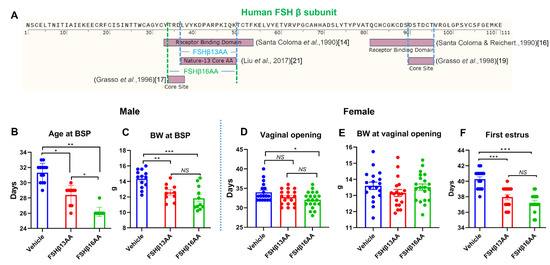
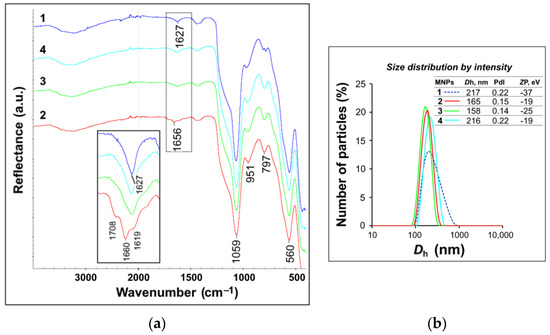
The main goal of this thematic issue is to provide information on the state-of-art in the field of medicinal chemistry in different areas of drug discovery and development. Special attention is focused on new biologically active compounds and synthetic strategies related to design and study of novel potential anti-viral agents, oncolytics, cardiovascular drugs, neuroprotective, cognition-enhancing compounds and some others. Novel synthetic approaches for creation multi-target-directed-ligands (MTDL), as well as monotarget strategy for drug discovery will be discussed. Contemporary approaches for improvement and focusing drug delivery on specific targets and bioavailability of drug-candidates will be provided and analyzed.
Prof. Dr. Sergey O. Bachurin
Prof. Dr. Reuben Jih-Ru Hwu
Prof. Dr. Valery Charushin
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. International Journal of Molecular Sciences is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. There is an Article Processing Charge (APC) for publication in this open access journal. For details about the APC please see here. Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- medicinal chemistry
- anti-viral agents
- oncolytics, cardiovascular drugs
- neuroprotectors
- cognition-enhancers
- drug-delivery
- bioavailability