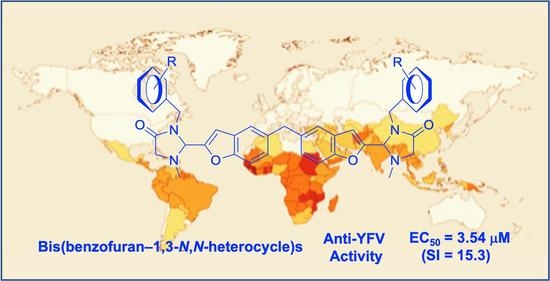
Bis(Benzofuran–1,3-N,N-heterocycle)s as Symmetric and Synthetic Drug Leads against Yellow Fever Virus
Abstract
1. Introduction
2. Results and Discussion
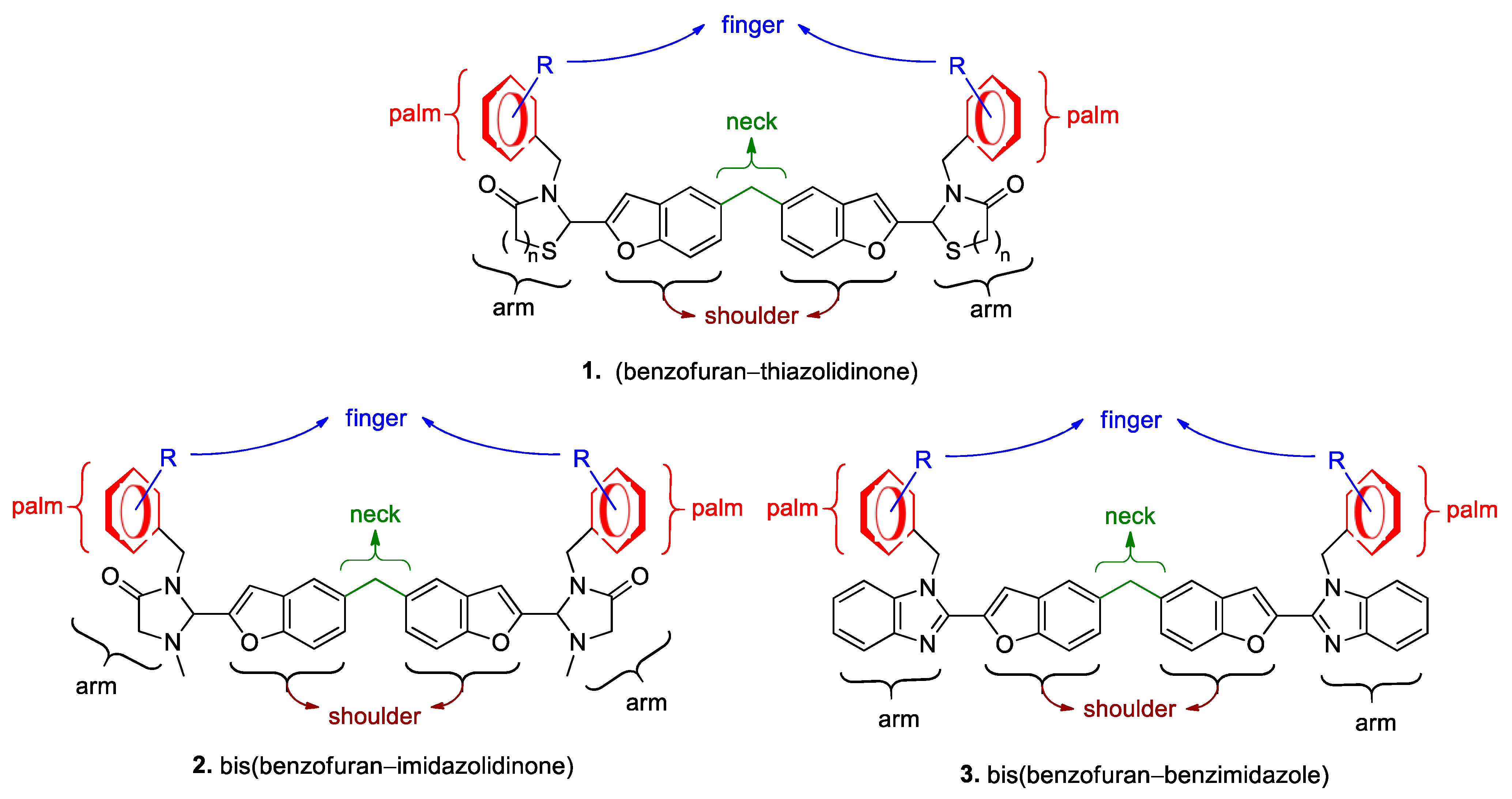
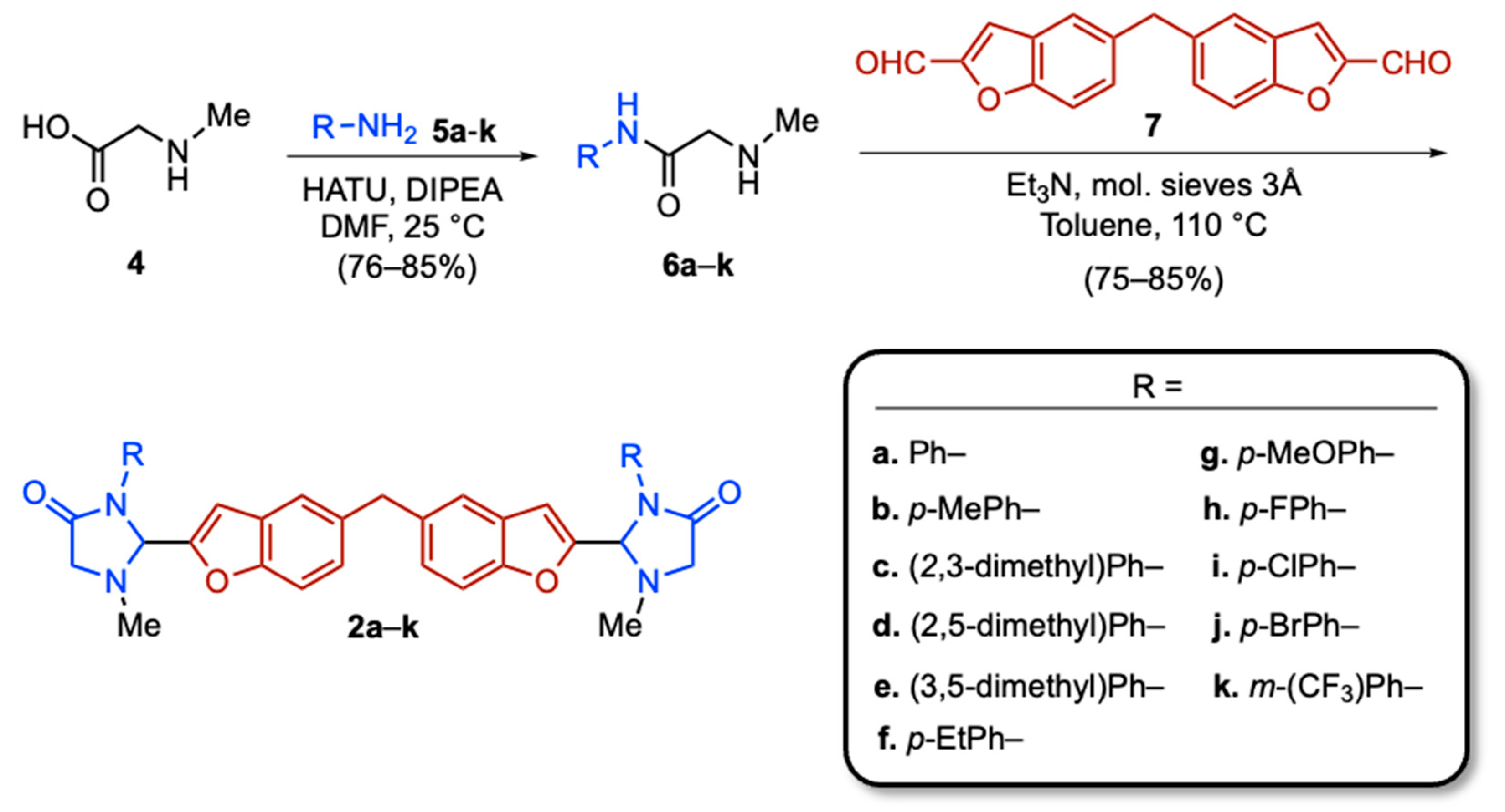
2.1. Syntheses of Bis(Benzofuran–Imidazolidinone) Conjugates 2 (Scheme 1) and Their Structural Identification
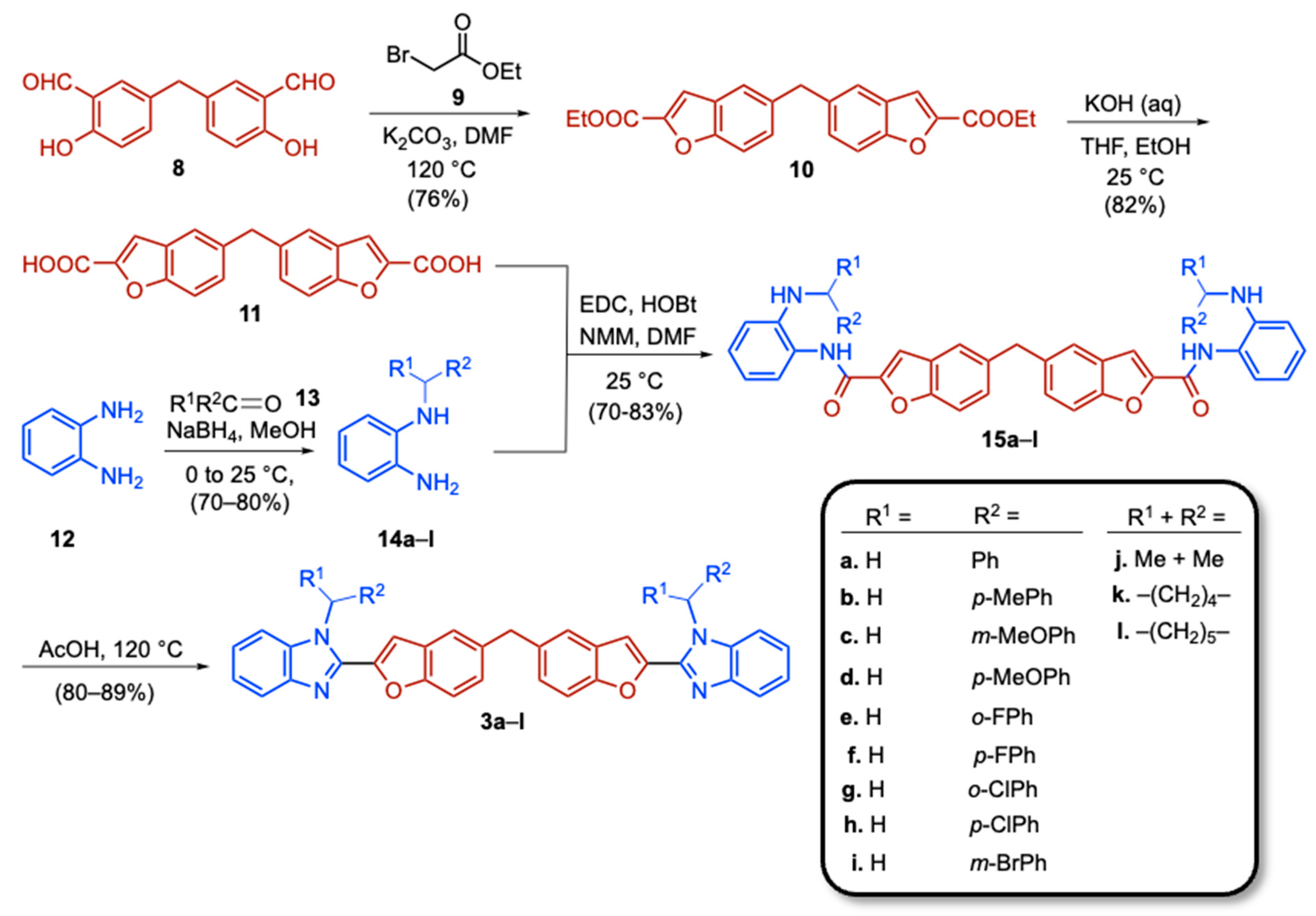
2.2. Synthesis of Bis(Benzofuran–Benzimidazole) Conjugates 3 (Scheme 2) and Their Structural Identification
2.3. Biological Activities of the New Bis-Conjugated Compounds and Their Lipophilicity
2.4. Essential Moieties and Substituents for Anti-YFV Activity
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Bis(Benzofuran–Imidazolidinone)s 2 (Standard Procedure 1)
3.2.1. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis[2-(N-methyl-3″-phenyl-4″-oxo-1″,3″-imidazolidin-2″-yl)benzofuran] (2a)
3.2.2. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis(2-[N-methyl-3″-(4-methylphenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2b)
3.2.3. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis(2-[N-methyl-3″-(2,3-dimethylphenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2c)
3.2.4. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis(2-[N-methyl-3″-(2,5-dimethylphenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2d)
3.2.5. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis[2-(N-methyl-3″-(3,5-dimethylphenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl)benzofuran] (2e)
3.2.6. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis(2-[N-methyl-3″-(2-ethylphenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2f)
3.2.7. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis(2-[N-methyl-3″-(4-methoxyphenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2g)
3.2.8. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebi(2-[N-methyl-3″-(4-fluorophenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2h)
3.2.9. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis(2-[N-methyl-3″-(4-chlorophenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2i)
3.2.10. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis(2-[N-methyl-3″-(4-bromophenyl)-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2j)
3.2.11. (2″R,2‴R)-, (2″S,2‴S)-, and meso-(2″R,2‴S)-5,5′-Methylenebis(2-[N-methyl-3″-(3-trifluoromethyl)phenyl-4″-oxo-1″,3″-imidazolidin-2″-yl]benzofuran) (2k)
3.3. Synthesis of Bis(Benzofuran–Benzimidazole)s 3 (Standard Procedure 2)
3.3.1. 5,5′-Methylenebis[2-(1-benzylbenzimidazol-2-yl)benzofuran] (3a)
3.3.2. 5,5′-Methylenebis(2-[1-(p-methylbenzyl)benzimidazol-2-yl]benzofuran) (3b)
3.3.3. 5,5′-Methylenebis(2-[1-(m-methoxybenzyl)benzimidazol-2-yl]benzofuran) (3c)
3.3.4. 5,5′-Methylenebis(2-[1-(p-methoxybenzyl)benzimidazol-2-yl]benzofuran) (3d)
3.3.5. 5,5′-Methylenebis(2-[1-(o-fluorobenzyl)benzimidazol-2-yl]benzofuran) (3e)
3.3.6. 5,5′-Methylenebis(2-[1-(p-fluorobenzyl)benzimidazol-2-yl]benzofuran) (3f)
3.3.7. 5,5′-Methylenebis(2-[1-(o-chlorobenzyl)benzimidazol-2-yl]benzofuran) (3g)
3.3.8. 5,5′-Methylenebis(2-[1-(p-chlorobenzyl)benzimidazol-2-yl]benzofuran) (3h)
3.3.9. 5,5′-Methylenebis(2-[1-(m-bromobenzyl)benzimidazol-2-yl]benzofuran) (3i)
3.3.10. 5,5′-Methylenebis[2-(1-isopropylbenzimidazol-2-yl)benzofuran] (3j)
3.3.11. 5,5′-Methylenebis[2-(1-cyclopentylbenzimidazol-2-yl)benzofuran] (3k)
3.3.12. 5,5′-Methylenebis[2-(1-cyclohexylbenzimidazol-2-yl)benzofuran] (3l)
3.4. Biological Evaluation
3.5. Study Plans of QSAR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/news-room/fact-sheets/detail/yellow-fever (accessed on 7 May 2019).
- Engelmann, F.; Josset, L.; Girke, T.; Park, B.; Barron, A.; Dewane, J.; Hammarlund, E.; Lewis, A.; Axthelm, M.K.; Slifka, M.K.; et al. Pathophysiologic and Transcriptomic Analyses of Viscerotropic Yellow Fever in a Rhesus Macaque Model. PLoS Negl. Trop. Dis. 2014, 8, e3295. [Google Scholar] [CrossRef] [PubMed]
- McNeill, J.R. Yellow Jack and Geopolitics: Environment, Epidemics, and the Struggles for Empire in the American Tropics, 1650–1825. OAH Mag. Hist. 2004, 18, 9–13. [Google Scholar] [CrossRef]
- Gianchecchi, E.; Cianchi, V.; Torelli, A.; Montomoli, E. Yellow Fever: Origin, Epidemiology, Preventive Strategies and Future Prospects. Vaccines 2022, 10, 372. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Pan, Y.; Lyu, Y.; Liang, Y.; Li, J.; Sun, Y.; Dou, X.; Tian, L.; Huo, D.; Chen, L.; et al. Detection of Yellow Fever Virus Genomes from Four Imported Cases in China. Int. J. Infect. Dis. 2017, 60, 93–95. [Google Scholar] [CrossRef]
- Lin, K.; Good, S.S.; Julander, J.G.; Weight, A.E.; Moussa, A.; Sommadossi, J.P. AT-752, a Double Prodrug of a Guanosine Nucleotide Analog, Inhibits Yellow Fever Virus in a Hamster Model. PLoS Negl. Trop. Dis. 2022, 16, e0009937. [Google Scholar] [CrossRef]
- Gawriljuk, V.O.; Foil, D.H.; Puhl, A.C.; Zorn, K.M.; Lane, T.R.; Riabova, O.; Makarov, V.; Godoy, A.S.; Oliva, G.; Ekins, S. Development of Machine Learning Models and the Discovery of a New Antiviral Compound against Yellow Fever Virus. J. Chem. Inf. Model. 2021, 61, 3804–3813. [Google Scholar] [CrossRef]
- Zandi, K.; Amblard, F.; Amichai, S.; Bassit, L.; Tao, S.; Jiang, Y.; Zhou, L.; Russell, O.O.; Mengshetti, S.; Schinazi, R.F. Nucleoside Analogs with Antiviral Activity against Yellow Fever Virus. Antimicrob. Agents Chemother. 2019, 63, e00889-19. [Google Scholar] [CrossRef]
- Pacca, C.C.; Marques, R.E.; Espindola, J.W.P.; Filho, G.B.O.O.; Leite, A.C.L.; Teixeira, M.M.; Nogueira, M.L. Thiosemicarbazones and Phthalyl-thiazoles Compounds Exert Antiviral Activity against Yellow Fever Virus and Saint Louis Encephalitis Virus. Biomed. Pharmacother. 2017, 87, 381–387. [Google Scholar] [CrossRef]
- Saudi, M.; Zmurko, J.; Kaptein, S.; Rozenski, J.; Gadakh, B.; Chaltin, P.; Marchand, A.; Neyts, J.; van Aerschot, A. Synthetic Strategy and Antiviral Evaluation of Diamide Containing Heterocycles Targeting Dengue and Yellow Fever Virus. Eur. J. Med. Chem. 2016, 121, 158–168. [Google Scholar] [CrossRef][Green Version]
- Saudi, M.; Zmurko, J.; Kaptein, S.; Rozenski, J.; Neyts, J.; Van Aerschot, A. Synthesis and Evaluation of Imidazole-4,5- and Pyrazine-2,3-dicarboxamides Targeting Dengue and Yellow Fever Virus. Eur. J. Med. Chem. 2014, 87, 529–539. [Google Scholar] [CrossRef]
- Guo, F.; Wu, S.; Julander, J.; Ma, J.; Zhang, X.; Kulp, J.; Cuconati, A.; Block, T.M.; Du, Y.; Guo, J.T.; et al. A Novel Benzodiazepine Compound Inhibits Yellow Fever Virus Infection by Specifically Targeting NS4B Protein. J. Virol. 2016, 90, 10774–10788. [Google Scholar] [CrossRef]
- Mayhoub, A.S.; Khaliq, M.; Kuhn, R.J.; Cushman, M. Design, Synthesis, and Biological Evaluation of Thiazoles Targeting Flavivirus Envelope Proteins. J. Med. Chem. 2011, 54, 1704–1714. [Google Scholar] [CrossRef]
- Julander, J.G.; Jha, A.K.; Choi, J.A.; Jung, K.H.; Smee, D.F.; Morrey, J.D.; Chu, C.K. Efficacy of 2-C-methylcytidine against Yellow Fever Virus in Cell Culture and in a Hamster Model. Antivir. Res. 2010, 86, 261–267. [Google Scholar] [CrossRef]
- Tonelli, M.; Paglietti, G.; Boido, V.; Sparatore, F.; Marongiu, F.; Marongiu, E.; Colla, P.L.; Loddo, R. Antiviral Activity of Benzimidazole Derivatives. I. Antiviral Activity of 1-Substituted-2-[(benzotriazol-1/2-yl)methyl]benzimidazoles). Chem. Biodivers. 2008, 5, 2386–2401. [Google Scholar] [CrossRef]
- Leyssen, P.; Balzarini, J.; De Clercq, E.; Neyts, J. The Predominant Mechanism by Which Ribavirin Exerts Its Antiviral Activity in Vitro against Faviviruses and Paramyxoviruses is Mediated by Inhibition of IMP Dehydrogenase. J. Virol. 2005, 79, 1943–1947. [Google Scholar] [CrossRef]
- Huggins, J.W. Prospects for Treatment of Viral Hemorrhagic Fevers with Ribavirin, a Broad-spectrum Antiviral Drug. Rev. Infect. Dis. 1989, 11, S750–S761. [Google Scholar] [CrossRef]
- Mendes, É.A.; Pilger, D.R.B.; Santos Nastri, A.C.S.; Malta, F.M.; Pascoalino, B.D.S.; Carneiro D’Albuquerque, L.A.; Balan, A.; Freitas, L.H.G., Jr.; Durigon, E.L.; Carrilho, F.J.; et al. Sofosbuvir Inhibits Yellow Fever Virus in Vitro and in Patients with Acute Liver Failure. Ann. Hepatol. 2019, 18, 816–824. [Google Scholar] [CrossRef]
- Qian, X.; Wu, B.; Tang, H.; Luo, Z.; Xu, Z.; Ouyang, S.; Li, X.; Xie, J.; Yi, Z.; Leng, Q.; et al. Rifapentine is an Entry and Replication Inhibitor against Yellow Fever Virus both in Vitro and in Vivo. Emerg. Microbes Infect. 2022, 11, 873–884. [Google Scholar] [CrossRef]
- Chand, K.; Rajeshwari; Hiremathad, A.; Singh, M.; Santos, M.A.; Keri, R.S. A Review on Antioxidant Potential of Bioactive Heterocycle Benzofuran: Natural and Synthetic Derivatives. Pharmacol. Rep. 2017, 69, 281–295. [Google Scholar] [CrossRef]
- Li, Y.; Bao, L.; Song, B.; Han, J.; Li, H.; Zhao, F.; Liu, H. A New Benzoquinone and a New Benzofuran from the Edible Mushroom Neolentinus lepideus and Their Inhibitory Activity in NO Production Inhibition Assay. Food Chem. 2013, 141, 1614–1618. [Google Scholar] [CrossRef]
- Yun, B.S.; Kang, H.-C.; Koshino, H.; Yu, S.-H.; Yoo, I.-D. Suillusin, a Unique Benzofuran from the Mushroom Suillusgranulatus. J. Nat. Prod. 2001, 64, 1230–1231. [Google Scholar] [CrossRef]
- Bhovia, V.K.; Bodke, Y.D.; Biradar, S.; Kumaraswamy, B.E.; Umesh, S. A Facile Synthesis of Bromo-substituted Benzofuran Containing Thiazolidinone Nucleus Bridged with Quinoline Derivatives: Potent Analgesic and Antimicrobial Agents. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 110–116. [Google Scholar] [CrossRef]
- Zha, X.; Lamba, D.; Zhang, L.; Lou, Y.; Xu, C.; Kang, D.; Chen, L.; Xu, Y.; Zhang, L.; De Simone, A.; et al. Novel Tacrine-benzofuran Hybrids as Potent Multitarget-directed Ligands for the Treatment of Alzheimer’s Disease: Design, Synthesis, Biological Evaluation, and X-ray Crystallography. J. Med. Chem. 2016, 59, 114–131. [Google Scholar] [CrossRef]
- Spaniol, M.; Bracher, R.; Ha, H.R.; Follath, F.; Krähenbühl, S. Toxicity of Amiodarone and Amiodarone Analogues on Isolated Rat Liver Mitochondria. J. Hepatol. 2001, 35, 628–636. [Google Scholar] [CrossRef]
- Yadav, P.; Singh, P.; Tewari, A.K. Design Synthesis, Docking and Anti-inflammatory Evaluation of Novel Series of Benzofuran Based Prodrugs. Bioorg. Med. Chem. Lett. 2014, 24, 2251–2255. [Google Scholar] [CrossRef]
- Hiremathad, A.; Patil, M.R.; Chetna, K.R.; Chand, K.; Santos, M.A.; Keri, R.S. Benzofuran: An Emerging Scaffold for Antimicrobial Agents. RSC Adv. 2015, 5, 96809–96828. [Google Scholar] [CrossRef]
- Manna, K.; Agrawal, Y.K. Design, Synthesis, and Antitubercular Evaluation of Novel Series of 3-Benzofuran-5-aryl-1-pyrazolyl-pyridylmethanone and 3-Benzofuran-5-aryl-1-pyrazolylcarbonyl-4-oxo-naphthyridin Analogs. Eur. J. Med. Chem. 2010, 45, 3831–3839. [Google Scholar] [CrossRef]
- Abdelhafez, M.O.; Amin, K.M.; Ali, H.I.; Abdallad, M.M.; Ahmed, E.Y. Design, Synthesis and Anticancer Activity of Benzofuran Derivatives Targeting VEGFR-2 Tyrosine Kinase. RSC Adv. 2014, 4, 11569–11579. [Google Scholar] [CrossRef]
- Hwu, J.R.; Gupta, N.K.; Tsay, S.-C.; Huang, W.-C.; Albulescu, I.C.; Kovacikova, K.; van Hemert, M.J. Bis(benzofuran-thiazolidinone)s and bis(benzofuran-thiazinanone)s as inhibiting agents for chikungunya virus. Antivir. Res. 2017, 146, 96–101. [Google Scholar] [CrossRef]
- Naik, R.; Harmalkar, D.S.; Xu, X.; Jang, K.; Lee, K. Bioactive Benzofuran Derivatives: Moracins A–Z in Medicinal Chemistry. Eur. J. Med. Chem. 2015, 90, 379–393. [Google Scholar] [CrossRef]
- He, S.; Jain, P.; Lin, B.; Ferrer, M.; Hu, Z.; Southall, N.; Hu, X.; Zheng, W.; Neuenswander, B.; Cho, C.H.; et al. High-Throughput Screening, Discovery, and Optimization to Develop a Benzofuran Class of Hepatitis C Virus Inhibitors. ACS Comb. Sci. 2015, 17, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Moorkoth, S.; Srinivasan, K.K.; Kutty, N.G.; Joseph, A.; Naseer, M. Synthesis and Evaluation of a Series of Novel Imidazolidinone Analogues of 6-Aminoflavone as Anticancer and Anti-inflammatory Agents. Med. Chem. Res. 2013, 22, 5066–5075. [Google Scholar] [CrossRef]
- Araújo, M.J.; Bom, J.; Capela, R.; Casimiro, C.; Chambel, P.; Gomes, P.; Iley, J.; Lopes, F.; Morais, J.; Moreira, R.; et al. Imidazolidin-4-one Derivatives of Primaquine as Novel Transmission-blocking Antimalarials. J. Med. Chem. 2005, 48, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, T.-Y.; Worley, S.D.; Sun, G. Novel Refreshable N-Halamine Polymeric Biocides Containing Imidazolidin-4-one Derivatives. J. Polym. Sci. A Polym. Chem. 2001, 39, 3073–3084. [Google Scholar] [CrossRef]
- Chern, J.H.; Shia, K.S.; Chang, C.M.; Lee, C.C.; Lee, Y.C.; Tai, C.L.; Lin, Y.T.; Chang, C.S.; Tseng, H.Y. Synthesis and in Vitro Cytotoxicity of 5-Substituted 2-Cyanoimino-4-imidazodinone and 2-Cyanoimino-4-pyrimidinone Derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1169–1172. [Google Scholar] [CrossRef]
- Al-Madi, S.H.; Al-Obaid, A.M.; El-Subbagh, H.I. The in Vitro Antitumor Assay of 5-(Z)-Arylidene-4-imidazolidinones in Screens of AIDS-related Leukemia and Lymphomas. Anticancer Drugs 2001, 12, 835–839. [Google Scholar] [CrossRef]
- Pinza, M.; Farina, C.; Cerri, A.; Pfeiffer, U.; Riccaboni, M.T.; Banfi, S.; Biagetti, R.; Pozzi, O.; Magnani, M.; Dorigotti, L. Synthesis and Pharmacological Activity of a Series of Dihydro-1H-pyrrolo[1,2-a]imidazole-2,5(3H,6H)-diones, a Novel Class of Potent Cognition Enhancers. J. Med. Chem. 1993, 36, 4214–4220. [Google Scholar] [CrossRef]
- Sutherland, R.; Robinson, O.P. Laboratory and Pharmacological Studies in Man with Hetacillin and Ampicillin. Brit. Med. J. 1967, 2, 804–808. [Google Scholar] [CrossRef][Green Version]
- Thomsen, C.; Hohlweg, R. (8-Naphthalen-1-ylmethyl-4-oxo-1-phenyl-1,3,8-triaza-spiro[4.5]-dec-3-yl)-acetic Acid Methyl Ester (NNC 63-0532) is a Novel Potent Nociceptin Receptor Agonist. Br. J. Pharmacol. 2000, 131, 903–908. [Google Scholar] [CrossRef]
- Zheng, L.T.; Hwang, J.; Ock, J.; Lee, M.G.; Lee, W.H.; Suk, K. The Antipsychotic Spiperone Attenuates Inflammatory Response in Cultured Microglia via the Reduction of Proinflammatory Cytokine Expression and Nitric Oxide Production. J. Neurochem. 2008, 107, 1225–1235. [Google Scholar] [CrossRef]
- Terrón, J.A.; Ibarra, M.; Ransanz, V.; Hong, E.; Villalón, C.M. The Alpha-antiadrenergic Properties of Spiroxatrine, a Ligand of Serotonergic 5-HT1A Receptors. Arch. Inst. Cardiol. Mex. 1993, 63, 289–295. [Google Scholar]
- Andrzejewska, M.; Yépez-Mulia, L.; Cedillo-Rivera, R.; Tapia, A.; Vilpo, L.; Vilpo, J.; Kazimierczuk, Z. Synthesis, Antiprotozoal and Anticancer Activity of Substituted 2-Trifluoromethyl- and 2-Pentafluoroethylbenzimidazoles. Eur. J Med. Chem. 2002, 37, 973–978. [Google Scholar] [CrossRef]
- Garuti, L.; Roberti, M.; Malagoli, M.; Rossi, T.; Castelli, M. Synthesis and Antiproliferative Activity of Some Benzimidazole-4,7-dione Derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 2193–2195. [Google Scholar] [CrossRef]
- Lukevics, E.; Arsenyan, P.; Shestakova, I.; Domracheva, I.; Nesterova, A.; Pudova, O. Synthesis and Antitumour Activity of Trimethylsilylpropyl Substituted Benzimidazoles. Eur. J. Med. Chem. 2001, 36, 507–515. [Google Scholar] [CrossRef]
- Hwu, J.R.; Singha, R.; Hong, S.C.; Chang, Y.H.; Das, A.R.; Vliegen, I.; De Clercq, E.; Neyts, J. Synthesis of New Benzimidazole-coumarin Conjugates as Anti-hepatitis C Virus Agents. Antiviral Res. 2008, 77, 157–162. [Google Scholar] [CrossRef]
- Komazin, G.; Ptak, R.G.; Emmer, B.T.; Townsend, L.B.; Drach, J.C. Resistance of Human Cytomegalovirus to D- and L-Ribosyl Benzimidazoles as a Tool to Identify Potential Targets for Antiviral Drugs. Nucleosides Nucleotides Nucleic Acids 2003, 22, 1725–1727. [Google Scholar] [CrossRef]
- Biron, K.K. Antiviral Drugs for Cytomegalovirus Diseases. Antivir. Res. 2006, 71, 154–163. [Google Scholar] [CrossRef]
- Tonelli, M.; Simone, M.; Tasso, B.; Novelli, F.; Boido, V.; Sparatore, F.; Paglietti, G.; Pricl, S.; Giliberti, G.; Blois, S.; et al. Antiviral Activity of Benzimidazole Derivatives. II. Antiviral Activity of 2-Phenylbenzimidazole Derivatives. Bioorg. Med. Chem. 2010, 18, 2937–2953. [Google Scholar] [CrossRef]
- Neyts, J.; De Clercq, E.; Singha, R.; Chang, Y.H.; Das, A.R.; Chakraborty, S.K.; Hong, S.C.; Tsay, S.C.; Hsu, M.H.; Hwu, J.R. Structure-Activity Relationship of New Anti-Hepatitis C Virus Agents: Heterobicycle-Coumarin Conjugates. J. Med. Chem. 2009, 52, 1486–1490. [Google Scholar] [CrossRef]
- Ishida, T.; Suzuki, T.; Hirashima, S.; Mizutani, K.; Yoshida, A.; Ando, I.; Ikeda, S.; Adachi, T.; Hashimoto, H. Benzimidazole Inhibitors of Hepatitis C Virus NS5B Polymerase: Identification of 2-[(4-Diarylmethoxy)phenyl]-benzimidazole. Bioorg. Med. Chem. Lett. 2006, 16, 1859–1863. [Google Scholar] [CrossRef]
- Hu, Y.; Jo, H.; DeGrado, W.F.; Wang, J. Brilacidin, a COVID-19 Drug Candidate, Demonstrates Broad-spectrum Antiviral Activity against Human Coronaviruses OC43, 229E, and NL63 through Targeting both the Virus and the Host Cell. J. Med. Virol. 2022, 94, 2188–2200. [Google Scholar] [CrossRef]
- Mirsadraee, M.; Sabbagh, S.Z.; Ghafari, S.; Tavakoli, A.; Sabbagh, S.S. Cromolyn, a New Hope for Limited Treatment of Neutrophilic Asthma: A Phase II Randomized Clinical Trial. Tanaffos 2019, 18, 208–214. [Google Scholar]
- Gandhi, Y.; Eley, T.; Fura, A.; Li, W.; Bertz, R.J.; Garimella, T. Daclatasvir: A Review of Preclinical and Clinical Pharmacokinetics. Clin. Pharmacokinet. 2018, 57, 911–928. [Google Scholar] [CrossRef]
- Gentile, I.; Buonomo, A.R.; Borgia, G. Ombitasvir: A Potent Pan-Genotypic enotypic Inhibitor of NS5A for the Treatment of Hepatitis C Virus Infection. Expert Rev. Anti-Infect. Ther. 2014, 12, 1033–1043. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Lee, W.; Kris, M.G.; Miller, V.A.; Krug, L.M.; Azzoli, C.G.; Senturk, E.; Calcutt, M.W.; Rizvi, N.A. A phase I Trial of SJG-136 (NSC#694501) in Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2010, 65, 833–838. [Google Scholar]
- Valeur, E.; Bradley, M. Amide Bond Formation: Beyond the Myth of Coupling Reagents. Chem. Soc. Rev. 2009, 38, 606–631. [Google Scholar] [CrossRef]
- Marx, M.A.; Grillot, A.-L.; Louer, C.T.; Beaver, K.A.; Bartlett, P.A. Synthetic Design for Combinatorial Chemistry. Solution and Polymer-Supported Synthesis of Polycyclic Lactams by Intramolecular Cyclization of Azomethine Ylides. J. Am. Chem. Soc. 1997, 119, 6153–6167. [Google Scholar] [CrossRef]
- Plaskon, A.S.; Ryabukhin, S.V.; Volochnyuk, D.M.; Shivanyuk, A.N.; Tolmachev, A.A. A Synthesis of 5-Hetaryl-3-(2-hydroxybenzoyl)pyrroles. Tetrahedron 2008, 64, 5933–5943. [Google Scholar] [CrossRef]
- Delogu, G.; Podda, G.; Corda, M.; Fadda, M.B.F.; Fais, A.; Era, B. Synthesis and Biological Evaluation of a Novel Series of Bis-salicylaldehydes as Mushroom Tyrosinase Inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 6138–6140. [Google Scholar] [CrossRef]
- Korthals, K.A.; Wulff, W.D. Traceless Stereoinduction in the One-Pot Assembly of All Three Rings of Hexahydrodibenzopyrans. J. Am. Chem. Soc. 2008, 130, 2898–2899. [Google Scholar] [CrossRef]
- Mahboobi, S.; Uecker, A.; Cénac, C.; Sellmer, A.; Eichhorn, E.; Elz, S.; Böhmerb, F.-D.; Dove, S. Inhibition of FLT3 and PDGFR Tyrosine Kinase Activity by Bis(benzo[b]furan-2-yl)methanones. Bioorg. Med. Chem. 2007, 15, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Micklitsch, C.M.; Yu, Q.; Schneider, J.P. Unnatural Multidentate Metal Ligating α-Amino Acids. Tetrahedron Lett. 2006, 47, 6277–6280. [Google Scholar] [CrossRef]
- Mitry, M.M.A.; El-Araby, A.M.; Neyts, J.; Kaptein, S.J.F.; Serya, R.A.T.; Samir, N. Molecular Dynamic Study and Synthesis of 1H-benzo[d]imidazole-5-carboxamide Derivatives as Inhibitors for Yellow Fever and Zika Virus Replication. Arch. Pharm. Sci. ASU 2020, 4, 145–180. [Google Scholar] [CrossRef]
- Kraszni, M.; Bányai, I.; Noszál, B. Determination of Conformer-specific Partition Coefficients in Octanol/Water Systems. J. Med. Chem. 2003, 46, 2241–2245. [Google Scholar] [CrossRef] [PubMed]
- Hann, M.M.; Keserü, G.M. Finding the Sweet Spot: The Role of Nature and Nurture in Medicinal Chemistry. Nat. Rev. 2012, 11, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A Knowledge-based Approach in Designing Combinatorial or to be Medicinal Chemistry Libraries for Drug Discovery. 1. A Qualitative and Quantitative Characterization of Known Drug Databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Khalifa, N.M.; Srour, A.M.; Abd El-Karim, S.S.; Saleh, D.O.; Al-Omar, M.A. Synthesis and 2D-QSAR Study of Active Benzofuran-Based Vasodilators. Molecules 2017, 22, 1820. [Google Scholar] [CrossRef]
- The CODESSA PRO Project Website. Available online: http://www.codessa-pro.com/index.htm (accessed on 20 July 2017).
- Lipinski, C.A. Lead- and Drug-like Compounds: The Rule-of-five Revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Prasanth Kumar, S.; Jasrai, Y.T.; Pandya, H.A.; Rawal, R.M. Pharmacophore-similarity-based QSAR (PS-QSAR) for Group-specific Biological Activity Predictions. J. Biomol. Struct. Dyn. 2015, 33, 56–69. [Google Scholar] [CrossRef]
- Sheng, C.; Che, X.; Wang, W.; Wang, S.; Cao, Y.; Yao, J.; Miao, Z.; Zhang, W. Structure-based Design, Synthesis, and Antifungal Activity of New Triazole Derivatives. Chem. Biol. Drug Des. 2013, 78, 309–313. [Google Scholar] [CrossRef]
- Mulliez, M. Etude D’Un Schema de Synthese Peptidique Intramoleculaire a L’Aide de Derives du Phosphore Tetraedrique. Tetrahedron 1981, 37, 2027–2041. [Google Scholar] [CrossRef]
- Pilkington, L.I.; Haverkate, N.A.; van Rensburg, M.; Reynisson, J.; Leung, E.; Barker, D. Synthesis of 3-Amino-2-carboxamide Tetrahydropyrrolo[2,3-b]quinolines. Synlett 2016, 27, 2811–2814. [Google Scholar]
- Sykes, B.M.; Atwell, G.J.; Hogg, A.; Wilson, W.R.; O’Connor, C.J.; Denny, W.A. N-Substituted 2-(2,6-Dinitrophenylamino)propanamides: Novel Prodrugs that Release a Primary Amine via Nitroreduction and Intramolecular Cyclization. J. Med. Chem. 1999, 42, 346–355. [Google Scholar] [CrossRef]
- Wei, C.; Wang, C.E.; Hwan, K.M.; Ronald, K.R.; Le, D.T.; Lew, A.; Nuss, J.M.; Wei, X. P70s6 Kinase Modulators and Method of Use. WO2005039506A2, 6 May 2005. [Google Scholar]
- Banik, A.; Ahmed, J.; Sil, S.; Mandal, S.K. Mimicking Transition Metals in Borrowing Hydrogen from Alcohols. Chem. Sci. 2021, 12, 8353–8361. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, D.; Li, G.; Li, L.; Zhao, G.; Tang, Z. Biomimetic Alloxan-catalyzed Intramolecular Redox Reaction with O2: One-Pot Atom-Economic Synthesis of Sulfinyl-Functionalized Benzimidazoles. Tetrahedron Lett. 2021, 62, 152688. [Google Scholar] [CrossRef]
- Kallmeier, F.; Fertig, R.; Irrgang, T.; Kempe, R. Chromium-catalyzed Alkylation of Amines by Alcohols. Angew. Chem. Int. Ed. 2020, 59, 11789–11793. [Google Scholar] [CrossRef]
- Ryu, H.J.; Yang, S.J.; Lee, G.H.; Gong, Y.D. Construction of Druglike 2-Amido Benzo[d]imidazole Analogues via Desulfurative Cyclization of Thiourea Intermediate Resin on Solid-phase. ACS Comb. Sci. 2018, 20, 282–291. [Google Scholar] [CrossRef]
- Tian, J.; Vandermosten, L.; Peigneur, S.; Moreels, L.; Rozenski, J.; Tytgat, J.; Herdewijn, P.; den Steen, P.E.V.; De Jonghe, S. Astemizole Analogues with Reduced hERG Inhibition as Potent Antimalarial Compounds. Bioorg. Med. Chem. 2017, 25, 6332–6344. [Google Scholar] [CrossRef]
- Heydari, A.; Khaksar, S.; Esfandyari, M.; Tajbakhsh, M. A Novel One-Pot Reductive Amination of Aldehydes and Ketones with Lithium Perchlorate and Zirconium Borohydride–Piperazine Complexes. Tetrahedron 2007, 63, 3363–3366. [Google Scholar] [CrossRef]
| Bis-Conjugate | CC50 (μM) | EC50 (μM) | SI | log p |
|---|---|---|---|---|
| 2a | – | >133 | – | 4.75 |
| 2b | − | >160 | − | − |
| 2c | 41.0 | 6.75 | 6.07 | 3.77 |
| 2d | 18.5 | 7.7 | 2.40 | 4.38 |
| 2e | 54.1 | 3.54 | 15.3 | 4.15 |
| 2f | 28.5 | 8.51 | 3.35 | 4.68 |
| 2g | – | >152 | – | 4.25 |
| 2h | 38.5 | 23.5 | 1.63 | 4.60 |
| 2i | 12.3 | 14.4 | 0.85 | – |
| 2j | – | >133 | − | – |
| 2k | 75.0 | 8.89 | 8.43 | 5.16 |
| 3a | – | >114 | – | – |
| 3b | – | >145 | – | – |
| 3c | – | >139 | – | – |
| 3d | – | 27.7 | – | – |
| 3e | – | >21.5 | – | 4.65 |
| 3f | – | 28.7 | – | – |
| 3g | – | >137 | – | – |
| 3h | – | >103 | – | – |
| 3i | – | 91.6 | – | – |
| 3j | 69.3 | 6.14 | 11.3 | 3.71 |
| 3k | – | >162 | – | – |
| 3l | – | >28.7 | – | – |
| 7 | – | >329 | – | 3.75 |
| 10 | – | >255 | – | – |
| 11 | – | >297 | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.K.; Jayakumar, S.; Huang, W.-C.; Leyssen, P.; Neyts, J.; Bachurin, S.O.; Hwu, J.R.; Tsay, S.-C. Bis(Benzofuran–1,3-N,N-heterocycle)s as Symmetric and Synthetic Drug Leads against Yellow Fever Virus. Int. J. Mol. Sci. 2022, 23, 12675. https://doi.org/10.3390/ijms232012675
Gupta NK, Jayakumar S, Huang W-C, Leyssen P, Neyts J, Bachurin SO, Hwu JR, Tsay S-C. Bis(Benzofuran–1,3-N,N-heterocycle)s as Symmetric and Synthetic Drug Leads against Yellow Fever Virus. International Journal of Molecular Sciences. 2022; 23(20):12675. https://doi.org/10.3390/ijms232012675
Chicago/Turabian StyleGupta, Nitesh K., Srinivasan Jayakumar, Wen-Chieh Huang, Pieter Leyssen, Johan Neyts, Sergey O. Bachurin, Jih Ru Hwu, and Shwu-Chen Tsay. 2022. "Bis(Benzofuran–1,3-N,N-heterocycle)s as Symmetric and Synthetic Drug Leads against Yellow Fever Virus" International Journal of Molecular Sciences 23, no. 20: 12675. https://doi.org/10.3390/ijms232012675
APA StyleGupta, N. K., Jayakumar, S., Huang, W.-C., Leyssen, P., Neyts, J., Bachurin, S. O., Hwu, J. R., & Tsay, S.-C. (2022). Bis(Benzofuran–1,3-N,N-heterocycle)s as Symmetric and Synthetic Drug Leads against Yellow Fever Virus. International Journal of Molecular Sciences, 23(20), 12675. https://doi.org/10.3390/ijms232012675