Carborane-Containing Folic Acid bis-Amides: Synthesis and In Vitro Evaluation of Novel Promising Agents for Boron Delivery to Tumour Cells
Abstract
1. Introduction
2. Results
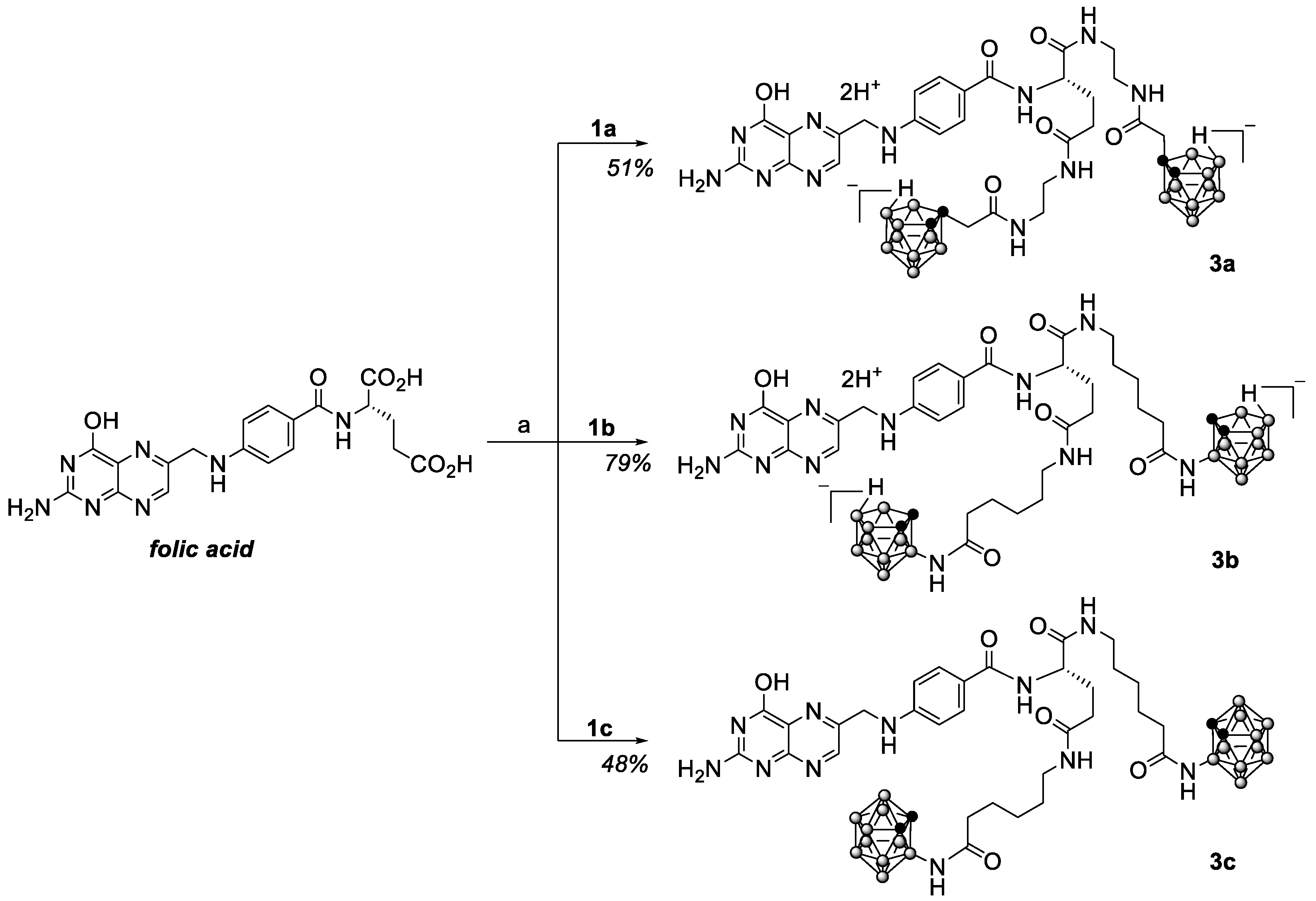
2.1. Synthesis
2.2. Toxicity Assay
2.3. Evaluation of Boron Accumulation by Cells
3. Discussion
4. Materials and Methods
4.1. Chemistry General Section
4.2. Synthesis
4.3. Cell Lines
4.4. MTT Cytotoxicity Assay
4.5. Boron Uptake and Accumulation Assay
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hu, K.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Rev. 2020, 405, 213139. [Google Scholar] [CrossRef]
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Miyatake, S.-I.; Wanibuchi, M.; Hu, N.; Ono, K. Boron neutron capture therapy for malignant brain tumours. J. Neuro-Oncol. 2020, 149, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Coderre, J.A.; Graça, H.; Vicente, M.; Blue, T.E. Boron Neutron Capture Therapy of Cancer: Current Status and Future Prospects. Clin. Cancer Res. 2005, 11, 3987–4002. [Google Scholar] [CrossRef] [PubMed]
- Barth, R.F.; Mi, P.; Yang, W. Boron delivery agents for neutron capture therapy of cancer. Cancer Commun. 2018, 38, 35. [Google Scholar] [CrossRef]
- Lamba, M.; Goswami, A.; Bandyopadhyay, A. A periodic development of BPA and BSH based derivatives in boron neutron capture therapy (BNCT). Chem. Commun. 2021, 57, 827–839. [Google Scholar] [CrossRef]
- Soloway, A.H.; Hatanaka, H.; Davis, M.A. Penetration of Brain and Brain Tumour. VII. Tumour-Binding Sulfhydryl Boron Compounds. J. Med. Chem. 1967, 10, 714–717. [Google Scholar] [CrossRef]
- Snyder, H.R.; Reedy, A.J.; Lennarz, W.J. Synthesis of Aromatic Boronic Acids. Aldehydo Boronic Acids and a Boronic Acid Analog of Tyrosine. J. Am. Chem. Soc. 1958, 80, 835–838. [Google Scholar] [CrossRef]
- Michiue, H.; Sakurai, Y.; Kondo, N.; Kitamatsu, M.; Bin, F.; Nakajima, K.; Hirota, Y.; Kawabata, S.; Nishiki, T.; Ohmori, I.; et al. The acceleration of boron neutron capture therapy using multi-linked mercaptoundecahydrododecaborate (BSH) fused cell-penetrating peptide. Biomaterials 2014, 35, 3396–3405. [Google Scholar] [CrossRef]
- Iguchi, Y.; Michiue, H.; Kitamatsu, M.; Hayashi, Y.; Takenaka, F.; Nishiki, T.; Matsui, H. Tumour-specific delivery of BSH-3R for boron neutron capture therapy and positron emission tomography imaging in a mouse brain tumour model. Biomaterials 2015, 56, 10–17. [Google Scholar] [CrossRef]
- Kikuchi, S.; Kanoh, D.; Sato, S.; Sakurai, Y.; Suzuki, M.; Nakamura, H. Maleimide-functionalized closo-dodecaborate albumin conjugates (MID-AC): Unique ligation at cysteine and lysine residues enables efficient boron delivery to tumour for neutron capture therapy. J. Control. Release 2016, 237, 160–167. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Zhang, Z.; Liu, H.; Lang, L.; Liu, T.; Chen, X.; Liu, Z. A Metabolically Stable Boron-Derived Tyrosine Serves as a Theranostic Agent for Positron Emission Tomography Guided Boron Neutron Capture Therapy. Bioconjugate Chem. 2019, 30, 2870–2878. [Google Scholar] [CrossRef]
- Fukuo, Y.; Hattori, Y.; Kawabata, S.; Kashiwagi, H.; Kanemitsu, T.; Takeuchi, K.; Futamura, G.; Hiramatsu, R.; Watanabe, T.; Hu, N.; et al. The Therapeutic Effects of Dodecaborate Containing Boronophenylalanine for Boron Neutron Capture Therapy in a Rat Brain Tumour Model. Biology 2020, 9, 437. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Guo, J.; Xu, Y.; Suan, K.; Zheng, J.; Wan, H.; Yuan, Z.; Chen, H. Application of Nitroimidazole–Carborane-Modified Phenylalanine Derivatives as Dual-Target Boron Carriers in Boron Neutron Capture Therapy. Mol. Pharmaceutics 2020, 17, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, Z.; Chen, J.; Li, J.; Li, N.; Yang, Z.; Wang, Y.; Liu, Z. 18F-Boramino acid PET/CT in healthy volunteers and glioma patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3113–3121. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M.B.; Hey-Hawkins, E. New keys for old locks: Carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, F.; Tang, L.; Xu, J.; Zhao, Y.; Wu, X.; Li, M.; Shen, J.; Wen, Q.; HinCho, C.; et al. Carboranes as unique pharmacophores in antitumour medicinal chemistry. Mol. Ther.–Oncolytics 2022, 24, 400–416. [Google Scholar] [CrossRef]
- Gruzdev, D.A.; Levit, G.L.; Krasnov, V.P.; Charushin, V.N. Carborane-containing amino acids and peptides: Synthesis, properties and applications. Coord. Chem. Rev. 2021, 433, 213753. [Google Scholar] [CrossRef]
- Levit, G.L.; Demin, A.M.; Kodess, M.I.; Ezhikova, M.A.; Sadretdinova, L.S.; Ol’shevskaya, V.A.; Kalinin, V.N.; Krasnov, V.P.; Charushin, V.N. Acidic hydrolysis of N-acyl-1-substituted 3-amino-1,2-dicarba-closo-dodecaboranes. J. Organomet. Chem. 2005, 690, 2783–2786. [Google Scholar] [CrossRef]
- Stogniy, M.Y.; Zakharova, M.V.; Sivaev, I.B.; Godovikov, I.A.; Chizov, A.O.; Bregadze, V.I. Synthesis of new carborane-based amino acids. Polyhedron 2013, 55, 117–120. [Google Scholar] [CrossRef]
- Gruzdev, D.A.; Levit, G.L.; Olshevskaya, V.A.; Krasnov, V.P. Synthesis of ortho-Carboranyl Derivatives of (S)-Asparagine and (S)-Glutamine. Russ. J. Org. Chem. 2017, 53, 769–776. [Google Scholar] [CrossRef]
- He, T.; Misuraca, J.C.; Musah, R.A. “Carboranyl-cysteine”—Synthesis, Structure and Self-Assembly Behavior of a Novel α-Amino Acid. Sci. Rep. 2017, 7, 16995. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, D.A.; Ustinova, V.O.; Chulakov, E.N.; Ol’shevskaya, V.A.; Slepukhin, P.A.; Levit, G.L.; Krasnov, V.P.; Charushin, V.N. Preparation of enantiomerically pure derivatives of (3-amino-1,2-dicarba-closo-dodecaboran-1-yl)acetic acid. J. Organomet. Chem. 2018, 876, 50–56. [Google Scholar] [CrossRef]
- Ol’shevskaya, V.A.; Alpatova, V.M.; Makarenkov, A.V.; Kononova, E.G.; Smol’yakov, A.F.; Peregudov, A.S.; Rys, E.G. Synthesis of maleimide-functionalized carboranes and their utility in Michael addition reactions. New J. Chem. 2021, 45, 12159–12167. [Google Scholar] [CrossRef]
- Gruzdev, D.A.; Telegina, A.A.; Levit, G.L.; Krasnov, V.P. N-Aminoacyl-3-amino-nido-carboranes a s a Group of Boron-Containing Derivatives of Natural Amino Acids. J. Org. Chem. 2022, 87, 5437–5441. [Google Scholar] [CrossRef] [PubMed]
- Hilgenbrink, A.R.; Low, P.S. Folate Receptor-Mediated Drug Targeting: From Therapeutics to Diagnostics. J. Pharm. Sci. 2005, 94, 2135–2146. [Google Scholar] [CrossRef]
- Kelemen, L.E. The role of folate receptor alpha in cancer development, progression and treatment: Cause, consequence or innocent bystander? Int. J. Cancer 2006, 119, 243–250. [Google Scholar] [CrossRef]
- Ebrahimnejad, P.; Taleghani, A.S.; Asare-Addo, K.; Nokhodchi, A. An updated review of folate-functionalized nanocarriers: A promising ligand in cancer. Drug Discov. Today 2022, 27, 471–489. [Google Scholar] [CrossRef]
- Martín-Sabroso, C.; Torres-Suárez, A.I.; Alonso-González, M.; Fernández-Carballido, A.; Fraguas-Sánchez, A.I. Active Targeted Nanoformulations via Folate Receptors: State of the Art and Future Perspectives. Pharmaceutics 2022, 14, 14. [Google Scholar] [CrossRef]
- Leamon, C.P.; Vlahov, I.R.; Reddy, J.A.; Vetzel, M.; Santhapuram, H.K.R.; You, F.; Bloomfield, A.; Dorton, R.; Nelson, M.; Kleindl, P.; et al. Folate-Vinca Alkaloid Conjugates for Cancer Therapy: A Structure-Activity Relationship. Bioconjugate Chem. 2014, 25, 560–568. [Google Scholar] [CrossRef]
- Dcona, M.M.; Sheldon, J.E.; Mitra, D.; Hartman, M.C.T. Light induced drug release from a folic acid-drug conjugate. Bioorg. Med. Chem. Lett. 2017, 27, 466–469. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Geersing, A.; de Vries, R.H.; Jansen, G.; Rots, M.G.; Roelfes, G. Folic acid conjugates of a bleomycin mimic for selective targeting of folate receptor positive cancer cells. Bioorg. Med. Chem. Lett. 2019, 29, 1922–1927. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-M.; Lu, Q.-Q.; Yao, S.; Su, H.-F.; Liu, H.-J.; Wang, Z.-J.; Wu, F.-S.; Wang, K. N-Methylpyridylporphyrin tailed with folate conjugate as a potential lysosomal-targeted photosensitizer: Synthesis, DNA interaction, singlet oxygen and subcellular localization. J. Porphyr. Phthalocyanines 2019, 23, 679–684. [Google Scholar] [CrossRef]
- Reddy, J.A.; Nelson, M.; Dircksen, C.; Vetzel, M.; Johnson, T.; Cross, V.; Westrick, E.; Qi, L.W.; Hahn, S.; Santhapuram, H.K.; et al. Pre-clinical studies of EC2629, a highly potent folate-receptor-targeted DNA crosslinking. Sci. Rep. 2020, 10, 12772. [Google Scholar] [CrossRef] [PubMed]
- Marverti, G.; Marraccini, C.; Martello, A.; D’Arca, D.; Pacifico, S.; Guerrini, R.; Spyrakis, F.; Gozzi, G.; Lauriola, A.; Santucci, M.; et al. Folic Acid–Peptide Conjugates Combine Selective Cancer Cell Internalization with Thymidylate Synthase Dimer Interface Targeting. J. Med. Chem. 2021, 64, 3204–3221. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Henriksen, J.R.; Jørgensen, J.T.; Amitay, Y.; Shmeeda, H.; Gabizon, A.A.; Kjær, A.; Andersen, T.L.; Hansen, A.E. Folate receptor targeting of radiolabeled liposomes reduces intratumoural liposome accumulation in human KB carcinoma xenografts. Int. J. Nanomed. 2018, 13, 7647–7656. [Google Scholar] [CrossRef] [PubMed]
- Handali, S.; Moghimipour, E.; Kouchak, M.; Ramezani, Z.; Amini, M.; Angali, K.A.; Saremy, S.; Dorkoosh, F.A.; Rezaei, M. New folate receptor targeted nano liposomes for delivery of 5-fluorouracil to cancer cells: Strong implication for enhanced potency and safety. Life Sci. 2019, 227, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Huo, P.; Liu, B. Formulation Strategies for Folate-Targeted Liposomes and Their Biomedical Applications. Pharmaceutics 2019, 11, 381. [Google Scholar] [CrossRef]
- Luiz, T.; Dutra, J.A.P.; de Cássia Ribeiro, T.; Carvalho, G.C.; Sábio, R.M.; Marchetti, J.M.; Chorilli, M. Folic acid-modified curcumin-loaded liposomes for breast cancer therapy. Colloids Surf. A 2022, 645, 128935. [Google Scholar] [CrossRef]
- Jing, D.; Wu, W.; Chen, X.; Xiao, H.; Zhang, Z.; Chen, F.; Zhang, Z.; Liu, J.; Dhao, Z.; Pu, F. Quercetin encapsulated in folic acid-modified liposomes is therapeutic against osteosarcoma by non-covalent binding to the JH2 domain of JAK2 via the JAK2-STAT3-PDL1. Pharmacol. Res. 2022, 182, 106287. [Google Scholar] [CrossRef]
- Roger, E.; Kalscheuer, S.; Kirtane, A.; Gurum, B.R.; Grill, A.E.; Whittum-Hudson, J.; Panyam, J. Folic Acid Functionalized Nanoparticles for Enhanced Oral Drug Delivery. Mol. Pharm. 2012, 9, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Gayam, S.R.; Wu, S.-P. Redox responsive Pd(II) templated rotaxane nanovalve capped mesoporous silica nanoparticles: A folic acid mediated biocompatible cancer-targeted drug delivery system. J. Mater. Chem. B 2014, 2, 7009–7016. [Google Scholar] [CrossRef]
- Brazzale, C.; Canaparo, R.; Racca, L.; Foglietta, F.; Durando, G.; Fantozzi, R.; Caliceti, P.; Salmaso, S.; Serpe, L. Enhanced selective sonosensitizing efficacy of ultrasound-based anticancer treatment by targeted gold nanoparticles. Nanomedicine 2016, 11, 3053–3070. [Google Scholar] [CrossRef]
- Koirala, N.; Das, D.; Fayazzadeh, E.; Sen, S.; McClain, A.; Puskas, J.E.; Drazba, J.A.; McLennan, G. Folic acid conjugated polymeric drug delivery vehicle for targeted cancer detection in hepatocellular carcinoma. J. Biomed. Mater. Res. 2019, 107, 2522–2535. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Yu, N.; Jiao, Y.; Hong, W.; Zhou, K.; Ji, X.; Yuan, H.; Wang, H.; Li, A.; Wang, G.; et al. Star polyester-based folate acid-targeting nanoparticles for doxorubicin and curcumin codelivery. Drug Deliv. 2021, 28, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarian, F.; Rastegari, B.; Zeinali, S.; Tohidi, M.; Karbalaei-Heidari, H.R. Theranostic Effect of Folic Acid Functionalized MIL-100(Fe) for Delivery of Prodigiosin and Simultaneous Tracking-Combating Breast Cancer. J. Nanomater. 2022, 2022, 1108865. [Google Scholar] [CrossRef]
- Achilli, C.; Jadhav, S.A.; Guidetti, G.F.; Ciana, A.; Abbonante, V.; Malara, A.; Fagnoni, M.; Torti, M.; Balduini, A.; Balduini, C.; et al. Folic acid-conjugated 4-amino-phenylboronate, a boron-containing compound designed for boron neutron capture therapy, is an unexpected agonist for human neutrophils and platelets. Chem. Biol. Drug Des. 2014, 83, 532–540. [Google Scholar] [CrossRef]
- Kettenbach, K.; Schieferstein, H.; Grunewald, C.; Iffland, D.; Reffert, L.M.; Hampel, G.; Schütz, C.L.; Bings, N.H.; Ross, T.L. Synthesis and evaluation of boron folates for Boron-Neutron-Capture-Therapy (BNCT). Radiochim. Acta 2015, 103, 799–809. [Google Scholar] [CrossRef]
- Nakagawa, F.; Kawashima, H.; Morita, T.; Nakamura, H. Water-Soluble closo-Dodecaborate-Containing Pteroyl Derivatives Targeting Folate Receptor-Positive Tumours for Boron Neutron Capture Therapy. Cells 2020, 9, 1615. [Google Scholar] [CrossRef]
- Kellert, M.; Friedrichs, J.-S.J.; Ullrich, N.A.; Feinhals, A.; Tepper, J.; Lönnecke, P.; Hey-Hawkins, E. Modular Synthetic Approach to Carboranyl–Biomolecules Conjugates. Molecules 2021, 26, 2057. [Google Scholar] [CrossRef]
- Kanemitsu, T.; Kawabata, S.; Fukumura, M.; Futamura, G.; Hiramatsu, R.; Nonoguchi, N.; Nakagawa, F.; Takata, H.; Suzuki, M.; Masunaga, S.-I.; et al. Folate receptor-targeted novel boron compound for boron neutron capture therapy of F98 glioma-bearing rats. Radiat. Environ. Biophys. 2019, 58, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.Q.; Wang, H.; Shukla, S.; Sekido, M.; Adams, D.M.; Tjarks, W.; Barth, R.F.; Lee, R.J. Boron-Containing Folate Receptor-Targeted Liposomes as Potential Delivery Agents for Neutron Capture Therapy. Bioconjugate Chem. 2002, 13, 435–442. [Google Scholar] [CrossRef]
- Sudimack, J.J.; Adams, D.; Rotaru, J.; Shukla, S.; Yan, J.; Sekido, M.; Barth, R.F.; Tjarks, W.; Lee, R.J. Folate Receptor-Mediated Liposomal Delivery of a Lipophilic Boron Agent to Tumour Cells in vitro for Neutron Capture Therapy. Pharm. Res. 2002, 19, 1502–1508. [Google Scholar] [CrossRef]
- Shukla, S.; Wu, G.; Chatterjee, M.; Yang, W.; Sekido, M.; Diop, L.A.; Müller, R.; Sudimack, J.J.; Lee, R.J.; Barth, R.F.; et al. Synthesis and Biological Evaluation of Folate Receptor-Targeted Boronated PAMAM Dendrimers as Potential Agents for Neutron Capture Therapy. Bioconjugate Chem. 2003, 14, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Thirumamagal, B.T.S.; Zhao, X.B.; Bandyopadhyaya, A.K.; Narayanasamy, S.; Johnsamuel, J.; Tiwari, R.; Golightly, D.W.; Patel, V.; Jehning, B.T.; Backer, M.V.; et al. Receptor-Targeted Liposomal Delivery of Boron-Containing Cholesterol Mimics for Boron Neutron Capture Therapy (BNCT). Bioconjugate Chem. 2006, 17, 1141–1150. [Google Scholar] [CrossRef]
- Ciofani, G.; Raffa, V.; Menciassi, A.; Cuschieri, A. Folate Functionalized Boron Nitride Nanotubes and their Selectrive Uptake by Glioblastoma Multiforme Cells: Implications for their Use as Boron Carriers in Clinical Boron Neutron Capture Therapy. Nanoscale Res. Lett. 2009, 4, 113–121. [Google Scholar] [CrossRef]
- Hwang, K.C.; Lai, P.D.; Chiang, C.-S.; Wang, P.-J.; Yuan, C.-J. Neutron capture nuclei-containing carbon nanoparticles for destruction of cancer cells. Biomaterials 2010, 31, 8419–8425. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.X.; Cai, F.; Hwang, K.C.; Zhou, Y.M.; Zhang, Z.Z.; Liu, X.H.; Ma, S.H.; Yang, Y.K.; Yao, Y.; Feng, M.; et al. Folate receptor-mediated boron-10 containing carbon nanoparticles as potential delivery vehicles for boron neutron capture therapy of nonfunctional pituitary adenomas. Sci. China Life Sci. 2013, 56, 163–173. [Google Scholar] [CrossRef]
- Achilli, C.; Grandi, S.; Ciana, A.; Guidetti, G.F.; Malara, A.; Abbonante, V.; Cansolino, L.; Tomasi, C.; Balduini, A.; Fagnoni, M.; et al. Biocompatibility of functionalized boron phosphate (BPO4) nanoparticles for boron neutron capture therapy (BNCT) application. Nanomedicine 2014, 10, 589–597. [Google Scholar] [CrossRef]
- Alberti, A.; Toppino, A.; Geninatti Crich, S.; Meraldi, C.; Prandi, C.; Protti, N.; Bortolussi, S.; Altieri, S.; Aime, S.; Deagostino, A. Synthesis of a carborane-containing cholesterol derivative and evaluation as a potential dual agent for MRI/BNCT applications. Org. Biomol. Chem. 2014, 12, 2457–2467. [Google Scholar] [CrossRef]
- Singh, A.; Kim, B.K.; Mackeyev, Y.; Rohani, P.; Mahajan, S.D.; Swihart, M.T.; Krishnan, S.; Prasad, P.N. Boron-Nanoparticle-Loaded Folic-Acid-Functionalized Liposomes to Achieve Optimum Boron Concentration for Boron Neutron Capture Therapy of Cancer. J. Biomed. Nanotechnol. 2019, 15, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Gruzdev, D.A.; Telegina, A.A.; Ol’shevskaya, V.A.; Andronova, V.L.; Galegov, G.A.; Zarubaev, V.V.; Levit, G.L.; Krasnov, V.P. New nido-carborane-containing conjugates of purine: Synthesis and antiviral activity. Russ. Chem. Bull. 2022, in press. [Google Scholar]
- Omran, Z.; Kay, G.; Hector, E.E.; Knott, R.M.; Cairns, D. Folate pro-drug of cystamine as an enhanced treatment for nephropathic cystinosis. Bioorg. Med. Chem. Lett. 2011, 21, 2502–2504. [Google Scholar] [CrossRef] [PubMed]
- Pignatello, R.; Spampinato, G.; Sorrenti, V.; Vicari, L.; Di Giacomo, C.; Vanella, C.; Puglisi, G. Aliphatic α,γ-bis(Amides) of Methotrexate. Influence of Chain Length in In-vitro Activity Against Sensitive and Resistant Tumour Cells. Pharm. Pharmacol. Commun. 1999, 5, 299–305. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sánchez-del-Campo, L.; Montenegro, M.F.; Cabezas-Herrera, J.; Rodríguez-López, J.N. The critical role of alpha-folate receptor in the resistance of melanoma to methotrexate. Pigment Cell Melanoma Res. 2009, 22, 588–600. [Google Scholar] [CrossRef]
- Chen, K.G.; Valencia, J.C.; Lai, B.; Zhang, G.; Paterson, J.K.; Rouzaud, F.; Berens, W.; Wincovitch, S.M.; Garfield, S.H.; Leapman, R.D.; et al. Melanosomal sequestration of cytotoxic drugs contributes to the intractability of malignant melanomas. Proc. Natl. Acad. Sci. USA 2006, 103, 9903–9907. [Google Scholar] [CrossRef]
- Chen, N.; Shao, C.; Qu, Y.; Li, S.; Gu, W.; Zheng, T.; Ye, L.; Yu, C. Folic Acid-Conjugated MnO Nanoparticles as a T1 Contrast Agent for Magnetic Resonance Imaging of Tiny Brain Gliomas. ACS Appl. Mater. Interfaces 2014, 6, 19850–19857. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Chen, Y.-C. Targeting delivery of etoposide to inhibit the growth of human glioblastoma multiforme using lactoferrin- and folic acid-grafted poly(lactide-co-glycolide) nanoparticles. Int. J. Pharm. 2015, 479, 138–149. [Google Scholar] [CrossRef]
- Fan, L.; Yang, Q.; Tan, J.; Qiao, Y.; Wang, Q.; He, J.; Wu, H.; Zhang, Y. Dual loading miR-218 mimics and Temozolomide using AuCOOH@FA-CS drug delivery system: Promising targeted antitumour drug delivery system with sequential release functions. J. Exp. Clin. Cancer Res. 2015, 34, 106. [Google Scholar] [CrossRef]
- Marfavi, Z.H.; Farhadi, M.; Jameie, S.B.; Zahmatkeshan, M.; Pirhajati, V.; Jameie, M. Glioblastoma U-87MG tumour cells suppressed by ZnO folic acid-conjugated nanoparticles: An in vitro study. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2783–2790. [Google Scholar] [CrossRef]
- Elechalawar, C.K.; Bhattacharya, D.; Ahmed, M.T.; Gora, H.; Sridharan, K.; Chaturbedy, P.; Sinha, S.H.; Jaggarapu, M.M.C.S.; Narayan, K.P.; Chakravarty, S.; et al. Dual targeting of folate receptor-expressing glioma tumour-associated macrophages and epithelial cells in the brain using a carbon nanosphere–cationic folate nanoconjugate. Nanoscale Adv. 2019, 1, 3555–3567. [Google Scholar] [CrossRef]
- Kucheryavykh, Y.V.; Davila, J.; Ortiz-Rivera, J.; Inyushin, M.; Almodovar, L.; Mayol, M.; Morales-Cruz, M.; Cruz-Montañez, A.; Barcelo-Bovea, V.; Griebenow, K.; et al. Targeted Delivery of Nanoparticulate Cytochrome C into Glioma Cells Through the Proton-Coupled Folate Transporter. Biomolecules 2019, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lee, R.J.; Mathias, C.J.; Green, M.A.; Low, P.S. Synthesis, Purification, and Tumour Cell Uptake of 67Ga-Deferoxamine-Folate, a Potential Radiopharmaceutical for Tumour Imaging. Bioconjugate Chem. 1996, 7, 56–62. [Google Scholar] [CrossRef]
- Wang, S.; Luo, J.; Lantrip, D.A.; Waters, D.J.; Mathias, C.J.; Green, M.A.; Fuchs, P.S.; Low, P.S. Design and Synthesis of [111In]DTPA-Folate for Use as a Tumour-Targeted Radiopharmaceutical. Bioconjugate Chem. 1997, 8, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Low, P.S. Folate-mediated targeting of antineoplastic drugs, imaging agents and nucleic acids to cancer cells. J. Control. Release 1998, 53, 59–68. [Google Scholar] [CrossRef]
- Leamon, C.P.; Deprince, R.B.; Hendren, R.W. Folate-mediated Drug Delivery: Effect of Alternative Conjugation Chemistry. J. Drug Targeting 1999, 7, 157–169. [Google Scholar] [CrossRef]
- Müller, C.; Hohn, A.; Schubiger, P.A.; Schibli, R. Preclinical evaluation of novel organometallic 99mTc-folate and 99mTc-pteroate radiotracers for folate receptor-positive tumour targeting. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1007–1016. [Google Scholar] [CrossRef]
- Bettio, A.; Honer, M.; Müller, C.; Brühlmeier, M.; Müller, U.; Schibli, R.; Groehn, V.; Schubiger, A.P.; Ametamey, S.M. Synthesis and Preclinical Evaluation of a Folic Acid Derivative Labeled with 18F for PET Imaging of Folate Receptor-Positive Tumours. J. Nucl. Med. 2006, 47, 1153–1160. [Google Scholar]
- Boss, S.D.; Betzel, T.; Müller, C.; Fischer, C.R.; Haller, S.; Reber, J.; Groehn, V.; Schibli, R.; Ametamey, S.M. Comparative Studies of Three Pairs of α- and γ-Conjugated Folic Acid Derivatives Labeled with Fluorine-18. Bioconjugate Chem. 2015, 27, 74–86. [Google Scholar] [CrossRef]
- Xie, J.; Zhu, Z.-H. Synthesis and Imaging Study on the FITC Labeled Folic Derivative. Chem. J. Chin. Univ. 2011, 32, 1532–1536. [Google Scholar]
- Doi, A.; Kawabata, S.; Iida, K.; Yokoyama, K.; Kajimoto, Y.; Kuroiwa, T.; Shirakawa, T.; Kirihata, M.; Kasaoka, S.; Maruyama, K.; et al. Tumour-specific targeting of sodium borocaptate (BSH) to malignant glioma by transferrin-PEG liposomes: A modality for boron neutron capture therapy. J. Neuro-Oncol. 2008, 87, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Tomizawa, K.; Michiue, H.; Miyatake, S.; Han, X.-J.; Fujimura, A.; Seno, M.; Kirihata, M.; Matsui, H. Delivery of codiumborocaptate to glioma cells using immunoliposome conjugated with anti-EGFR antibodies by ZZ-His. Biomaterials 2009, 30, 1746–1755. [Google Scholar] [CrossRef]
- Wang, P.; Zhen, H.; Jiang, X.; Zhang, W.; Cheng, X.; Guo, G.; Mao, X.; Zhang, X. Boron neutron capture therapy induces apoptosis of glioma cells through Bcl-2/Bax. BMC Cancer 2010, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Henning, T.D.; Boddington, S.; Tavri, S.; Arora, S.; Piontek, G.; Rudelius, M.; Corot, C.; Daldrup-Link, H.E. Breast Cancers: MR Imaging of Folate-Receptor Expression with the Folate-Specific Nanoparticle P1133. Radiology 2010, 255, 527–535. [Google Scholar] [CrossRef]
- Yang, T.; Xu, F.; Fang, D.; Chen, Y. Targeted Proteomics Enables Simultaneous Quantification of Folate Receptor Isoforms and Potential Isoform-based Diagnosis in Breast Cancer. Sci. Rep. 2015, 5, 16733. [Google Scholar] [CrossRef] [PubMed]
- Godlewska, M.; Majkowska-Pilip, A.; Stachurska, A.; Biernat, J.F.; Gaweł, D.; Nazaruk, E. Voltammetric and biological studies of folate-targeted non-lamellar lipid mesophases. Electrochim. Acta 2019, 299, 1–11. [Google Scholar] [CrossRef]
- Sambi, M.; DeCarlo, A.; Malardier-Jugroot, C.; Szewczuk, M.R. Next-Generation Multimodality of Nanomedicine Therapy: Size and Structure Dependence of Folic Acid Conjugated Copolymers Actively Target Cancer Cells in Disabling Cell Division and Inducing Apoptosis. Cancers 2019, 11, 1698. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 6th ed.; Butterworth Heinemann: Burlington, MA, USA, 2009. [Google Scholar]
- Tsygankova, A.R.; Kanygin, V.V.; Kasatova, A.I.; Zav’yalov, E.L.; Gusel’nikova, T.Y.; Kichigin, A.I.; Mukhamadiyarov, R.A. Determination of boron by inductively coupled plasma atomic emission spectroscopy. Biodistribution of 10B in tumour-bearing mice. Russ. Chem. Bull. 2020, 69, 601–607. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruzdev, D.A.; Telegina, A.A.; Levit, G.L.; Solovieva, O.I.; Gusel’nikova, T.Y.; Razumov, I.A.; Krasnov, V.P.; Charushin, V.N. Carborane-Containing Folic Acid bis-Amides: Synthesis and In Vitro Evaluation of Novel Promising Agents for Boron Delivery to Tumour Cells. Int. J. Mol. Sci. 2022, 23, 13726. https://doi.org/10.3390/ijms232213726
Gruzdev DA, Telegina AA, Levit GL, Solovieva OI, Gusel’nikova TY, Razumov IA, Krasnov VP, Charushin VN. Carborane-Containing Folic Acid bis-Amides: Synthesis and In Vitro Evaluation of Novel Promising Agents for Boron Delivery to Tumour Cells. International Journal of Molecular Sciences. 2022; 23(22):13726. https://doi.org/10.3390/ijms232213726
Chicago/Turabian StyleGruzdev, Dmitry A., Angelina A. Telegina, Galina L. Levit, Olga I. Solovieva, Tatiana Ya. Gusel’nikova, Ivan A. Razumov, Victor P. Krasnov, and Valery N. Charushin. 2022. "Carborane-Containing Folic Acid bis-Amides: Synthesis and In Vitro Evaluation of Novel Promising Agents for Boron Delivery to Tumour Cells" International Journal of Molecular Sciences 23, no. 22: 13726. https://doi.org/10.3390/ijms232213726
APA StyleGruzdev, D. A., Telegina, A. A., Levit, G. L., Solovieva, O. I., Gusel’nikova, T. Y., Razumov, I. A., Krasnov, V. P., & Charushin, V. N. (2022). Carborane-Containing Folic Acid bis-Amides: Synthesis and In Vitro Evaluation of Novel Promising Agents for Boron Delivery to Tumour Cells. International Journal of Molecular Sciences, 23(22), 13726. https://doi.org/10.3390/ijms232213726





