Abstract
The development of multi-target-directed ligands (MTDLs) would provide effective therapy of neurodegenerative diseases (ND) with complex and nonclear pathogenesis. A promising method to create such potential drugs is combining neuroactive pharmacophoric groups acting on different biotargets involved in the pathogenesis of ND. We developed a synthetic algorithm for the conjugation of indole derivatives and methylene blue (MB), which are pharmacophoric ligands that act on the key stages of pathogenesis. We synthesized hybrid structures and performed a comprehensive screening for a specific set of biotargets participating in the pathogenesis of ND (i.e., cholinesterases, NMDA receptor, mitochondria, and microtubules assembly). The results of the screening study enabled us to find two lead compounds (4h and 4i) which effectively inhibited cholinesterases and bound to the AChE PAS, possessed antioxidant activity, and stimulated the assembly of microtubules. One of them (4i) exhibited activity as a ligand for the ifenprodil-specific site of the NMDA receptor. In addition, this lead compound was able to bypass the inhibition of complex I and prevent calcium-induced mitochondrial depolarization, suggesting a neuroprotective property that was confirmed using a cellular calcium overload model of neurodegeneration. Thus, these new MB-cycloalkaneindole conjugates constitute a promising class of compounds for the development of multitarget neuroprotective drugs which simultaneously act on several targets, thereby providing cognitive stimulating, neuroprotective, and disease-modifying effects.
1. Introduction
The growing number of patients with neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) (which are already highly prevalent and increasing) and amyotrophic lateral sclerosis (ALS) (which is low but increasingly prevalent), especially in economically developed countries, makes the search for drugs against these diseases a highly relevant task. The few drugs that are used to treat AD are predominantly symptomatic, i.e., they partially compensate for the decrease in cognitive functions and memory by forced activation of neurotransmitter systems, but they do not influence the neurodegeneration process itself. Currently, the key approach to the development of new-generation drugs for the treatment of AD and ALS is the search and design of substances capable of retarding the progression of neurodegeneration (i.e., disease-modifying agents). It is expected that the development of drugs that act simultaneously on several biological structures participating in neuron death and in the loss of neuron functional activity, or so-called multi-target-directed ligands (MTDLs), would provide more effective therapy of neurodegenerative diseases directed toward the pathogenesis of the disease [1,2,3]. A promising method to address this problem is the design of new pharmaceutical agents that contain distinct neuroactive pharmacophoric groups acting on different biotargets involved in the pathogenesis of neurodegenerative diseases.
Previously, we developed a synthetic algorithm for the conjugation of several pharmacophoric ligands that act on the key stages of pathogenesis, synthesized hybrid structures, and performed screening for a broad range of biotargets participating in the pathogenesis of AD [4,5,6].
The present communication describes the development of another series of conjugates and an evaluation of their possible therapeutic capability. These conjugates comprise indole derivatives and methylene blue, which is a pharmacophore with a unique neuroprotective potential (see Scheme 1).
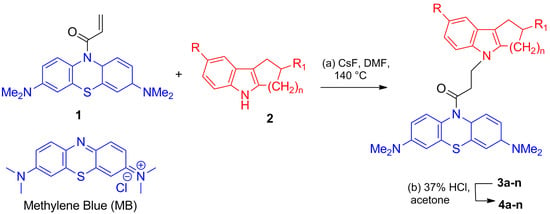
Scheme 1.
Synthesis of compounds 4a–n. Reagents and conditions: (a) CsF, DMF, 140 °C, 4 h, (b) 37% HCl, acetone.
Methylene blue (MB) belongs to the family of phenothiazines, which have a broad range of therapeutic and diagnostic applications. A significant number of MB and derivatives with a wide spectrum of biological activity, including the effect on targets important for the treatment of Alzheimer’s disease, were created [7,8].
Owing to both cationic and lipophilic properties, MB easily penetrates the blood–brain barrier, binds to the mitochondrial membrane, and diffuses to the mitochondrial matrix, where it forms, in low doses, a redox equilibrium with electron transport chain enzymes, thus enhancing mitochondrial respiration. Thus, MB is a potent redox agent that is highly bioavailable for mitochondria, which reduces the frequency of the formation of mitochondrial reactive oxygen species (ROS); hence, it can decrease the rate of cell aging [9,10]. Furthermore, MB modifies the activity of some membrane-bound transporters and ion channels—in particular, the voltage-dependent Na+ channel and the Ca2+-dependent K+ channel—and this is considered to be responsible for the depolarizing effect of MB on the neuronal membrane [11,12]. Correspondingly, MB affects neuronal relationships by changing the activity of cholinergic, monoaminergic, or glutamatergic synaptic neurotransmission [13,14,15]. In addition, it has been shown that MB enhances memory in a normal brain, which can be caused by its effect on neurotransmission. However, many researchers also attribute this effect to the stimulating action of MB on mitochondria [16]. According to recent studies, MB has a high potential for the treatment of ischemic and hypoxic disorders, AD, or some other neurodegenerative diseases, owing to both its neuroprotective potential and its cognitive stimulation effect [17,18,19,20]. It has also been shown that MB considerably decreases the level and the toxicity of abnormal protein aggregates (Huntingtin protein, α-synuclein, tau protein, and β-amyloid). However, the mechanisms of this activity are varied, including redox inhibition of aggregation, decreasing oligomer levels by promoting fibril formation, photodynamic destruction of aggregates, and increasing proteasome activity [21,22,23,24,25]. One more argument for our choice of this pharmacophore is derived from the earlier observation that phenothiazine derivatives, including MB, can efficiently inhibit cholinesterase enzymes [26,27,28,29,30,31].
Meanwhile, many indoles and carbazole derivatives have neuroactive properties. For example, aminopropylcarbazole derivatives exhibit pro-neurogenic and neuroprotective activities without obvious toxicity, with the inhibition of the mitochondrial permeability transition being one of the key mechanisms of such activity [32]. In 2013, Zhu et al. synthesized a series of N-substituted carbazoles. Lead compounds were identified among these products. When present in a concentration of only 3 µM, these compounds had a neuroprotective action on HT-22 neuronal cells against glutamate- or homocysteic acid-induced cell injury. The neuroprotective effect was attributed to the antioxidant activity mediated by a GSH-independent mechanism [33]. Some cyanine compounds based on carbazole were found to prevent Aβ aggregation, down-regulate the activity of glycogen kinase synthase-3β (GSK-3β), and decrease the hyperphosphorylation of tau protein in the triple transgenic mouse model of AD [34]. Therefore, we chose cycloalkaneindoles, a representative of which is tetrahydrocarbazole, as the second pharmacophore for the design of multifunctional conjugates.
2. Results and Discussion
2.1. Chemistry
The target conjugates of MB and cycloalkaneindoles linked with a 1-oxopropylene spacer were synthesized, as shown in Scheme 1, by the reaction of 1-[3,7-bis(dimethylamino)phenothiazin-10-yl]propenone 1 with cycloalkaneindoles 2. Detailed synthesis and NMR spectroscopy data for conjugates 3a–n and 4a–n were reported previously [35].
The prepared conjugates were subjected to primary screening using the methodology relevant to the treatment of neurodegenerative diseases that we proposed previously [6]. The system included neurotransmitter targets related to the compensation of cognitive functions, including: cholinesterases and glutamate receptors; mitochondria and the prevention of the mitochondrial permeability transition, which may provide cyto- and neuroprotection; and microtubules, whose destabilization is a specific feature of certain neurodegenerative diseases, e.g., tauopathies.
2.2. Study of the Esterase Profile of Conjugates 4 and Their Ability to Displace Propidium from the Peripheral Anionic Site of Acetylcholinesterase
We used enzyme kinetics and molecular docking to study the inhibitory activity of the MB-cycloalkaneindole conjugates against acetylcholinesterase (EC 3.1.1.7, AChE), butyrylcholinesterase (EC 3.1.1.8, BChE), and the structurally related carboxylesterase enzyme carboxylesterase (EC 3.1.1.1, CES). In addition, the ability of these compounds to competitively displace propidium iodide (PI), a selective ligand of the AChE peripheral anionic site (PAS) which binds β-amyloid, was studied by a fluorescent method to indirectly evaluate the test compounds as inhibitors of the pro- β-amyloid aggregation activity of AChE.
AChE and BChE can hydrolyze the neurotransmitter acetylcholine, and they are important targets for the amelioration of cognitive symptoms during the development of AD and/or AD-like dementia [36]. BChE inhibition is especially useful against stages of the disease when BChE has taken on part of the role of acetylcholine hydrolysis that has been compromised by a decrease in AChE activity [37,38,39]. For this reason, it is thought that compounds that inhibit both AChE and BChE increase the efficiency of treatment [40,41].
CES is responsible for the hydrolysis of numerous drugs containing ester groups [42], and the inhibition of this enzyme by anticholinesterase drugs taken by AD patients may lead to adverse drug-drug interactions [43,44]. Our approach included the determination of the esterase profiles of compounds, i.e., the comparative determination of their inhibitory activities against a few structurally related serine hydrolases [30,43,44,45,46,47,48,49], making it possible to evaluate the ability of the compounds to inhibit cholinesterases and to reveal their potential adverse effects in an early stage of research.
2.2.1. Inhibitory Activity of Conjugates 4 against Human Erythrocyte AChE, Equine Serum BChE, and Porcine Liver CES
The esterase profile was estimated using the human erythrocyteAChE, the equine serum BChE, and the porcine liver CES. The equine and porcine enzymes have a high degree of sequence identify with their human counterparts, and the applicability of these enzymes for determining the esterase profile of new compounds has been shown in our previous studies [26,35,49,50]. The inhibitory activity was characterized as the percentage of inhibition at 20 µM concentration or by determining the IC50 value, i.e., the inhibitor concentration needed to decrease the enzyme activity by 50%. As reference compounds, we used the base pharmacophores: carbazole, MB, and reduced MB (leuco-MB, MBH2).
The results, summarized in Table 1, show that all conjugates 4 (Figure 1) inhibit CES to a low extent, but they have relatively high inhibitory activities against AChE and BChE. The inhibition of AChE and BChE occurrs in the micromolar range, with pronounces selectivity for AChE. Note that conjugates 4 inhibit AChE and BChE at the level of the parent pharmacophores or somewhat below this level. The inhibitory activity depends little on the ring size.

Table 1.
Inhibitory activity of MB-cycloalkaneindoles conjugates 4 against AChE, BChE, and CES.
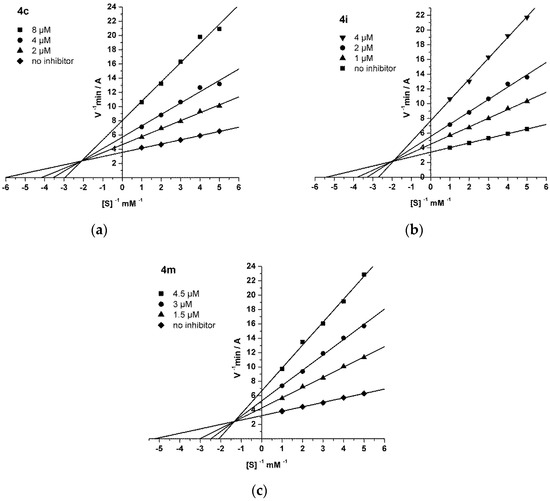
Figure 1.
Steady state inhibition of AChE by compounds 4c (a), 4i (b), and 4m (c). The Lineweaver–Burk reciprocal plots of the initial reaction rate and substrate concentration in the presence of inhibitors 4c, 4i, and 4m (three increasing concentrations) and without inhibitors are presented. The plots (a–c) show mixed type inhibition.
2.2.2. Study of the Mechanism of AChE Inhibition by Conjugates 4
The mechanism of AChE inhibition by MB-cycloalkaneindole conjugates was considered in relation to three of the most active compounds: 4c, 4i, and 4m. The results were analyzed using the Lineweaver–Burk double reciprocal plot, and the graphical analysis of the kinetic data is shown in Figure 1. The binding of 4c (a), 4i (b), or 4m (c) to AChE changed both the Vmax and the Km, which is typical of mixed type inhibition. The AChE inhibition constants by compounds 4c (Ki = 1.98 ± 0.17 µM for the competitive component and αKi = 5.83 ± 0.31 µM for the noncompetitive component), 4i (Ki = 1.12 ± 0.05 µM, αKi = 3.19 ± 0.10 µM), and 4m (Ki = 1.06 ± 0.04 µM, αKi = 4.03 ± 0.15 µM) were determined. These conjugates were found to be effective reversible mixed-type inhibitors of AChE.
2.2.3. Molecular Modeling
The kinetic data were in full agreement with the molecular docking results. Molecular docking into AChE was performed for the same compounds from the kinetics studies, including 4c and 4m, which differed according to the size of the methylene ring (n = 2 and n = 4, respectively), and compound 4i, which contained a CF3O substituent and was the most potent AChE inhibitor.
As was shown in our earlier studies, the choice of the X-ray diffraction structure for molecular docking can significantly affect the modeling results [51]. Based on these previous findings, we used two X-ray structures of hAChE as docking targets in the present study [52]. For the apo-hAChE structure (PDB ID 4EY4), the ligands were found only in the PAS. For the X-ray structure of hAChE co-crystallized with donepezil (PDB ID 4EY7), the ligands were found predominantly in the catalytic active site (CAS). These two main protein structures had different conformations of the Tyr337 side chain which intercepted the enzyme gorge with the apo-state and acted as one of the valves of the bottleneck. In the X-ray structure with donepezil in the gorge, Tyr337 was rotated and moved to the wall, making room for a ligand, which made this structure (PDB ID 4EY7) a preferable target for the molecular docking of bulky ligands.
The positions of compounds in the CAS had a specific π-π interaction between the MB moiety and the Trp86 ring (Figure 2a,b). In both positions of compound 4c, with the methylene blue moiety in the PAS and the CAS, the cycloalkaneindole ring was squeezed between Tyr341 and Trp286 in the PAS, just above the bottleneck. For compound 4m, a deeper position of the cycloalkaneindole ring was found. Thus, the docking results show that the ligands are dual-site inhibitors, which initially bind to the PAS and then move down the gorge after the opening of the bottleneck (according to the mechanism described earlier [53]) to the active site, where their binding is stabilized by π-π interaction between the Trp86 and the MB ring. There are X-ray structures of MB in complex with Torpedo californica AChE (TcAChE) obtained in the presence and absence of polyethylene glycol (PEG), respectively (PDB ID 5E4T and 5DLP, [54]). In these structures, MB is located in the PAS. However, it cannot serve as a reference for MB derivatives due to the significant change in its geometry upon conjugation to the cycloalkaneindole component. MB is completely planar, and joining the linker to the nitrogen atom impairs aromatic conjugation and leads to a loss of planarity in the MB group (Figure 2). This could be seen in the available X-ray structures of the MB conjugates (e.g., PDB ID 1LVJ, 4MA7, 5NUN). QM optimization of the compounds 4 revealed that the angle between the two halves of the MB fragment is 131°, which corresponds with the X-ray data.
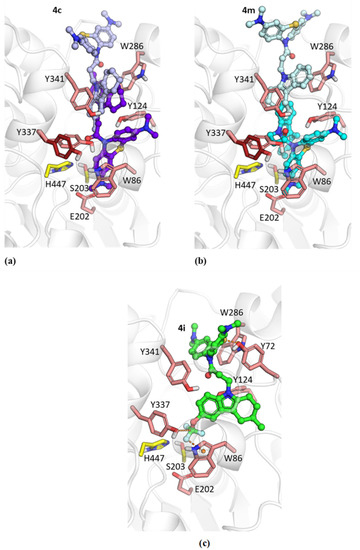
Figure 2.
Docked positions of compounds 4c (a), 4m (b) and 4i (c) in hAChE. The position in the PAS (carbon atoms are colored light purple for 4c and light cyan for 4m) was obtained with the PDB ID 4EY4 structure as a target. The position in the CAS (carbon atoms are colored purple for 4c and cyan for 4m) was obtained with the PDB ID 4EY7 structure as a target. The conformation for Tyr337 in PDB ID 4EY7 differing from the PDB ID 4EY4 conformation is shown with dark red carbon atoms. The catalytic residues His447 and Ser203 are shown with yellow carbon atoms. (c) The position of compound 4i docked into the PDB ID 4EY7 structure (carbon atoms are colored green).
The position of compound 4i is noticeably different from the positions of compounds 4c and 4m due to the CF3O group (Figure 2c). It is surrounded by aromatic residues Tyr337 and Trp86. Because the carbon atom of this moiety is surrounded by electron-withdrawing atoms, its interactions with aromatic residues could be considered as a tetrel bond [55]. Similar configurations were observed for -CF3…π interactions in computational studies [56] and in experimental X-ray structures of protein–ligand complexes [57]. As a result of such interactions of the CF3O group, the MB moiety better occupies the PAS compared to the other compounds. Notably, the sulfur atom of the MB group is directed toward the Trp286 aromatic ring, which, along with the nearby Tyr72 residue, forms an entity called a “3-bridge cluster” [58,59].
2.2.4. Inhibition of EeAChE and Displacement of Propidium Iodide from the EeAChE Peripheral Anionic Site
It is known that AChE plays an important role in β-amyloid processing via the PAS, which interacts with soluble peptides of β-amyloid and promotes their aggregation [60,61,62,63]. Therefore, the development of drugs that block the PAS of AChE, disrupt its interaction with β-amyloid, and decrease the AChE-induced β-amyloid aggregation is a promising trend of the anti-amyloid strategy of AD therapy.
We used a fluorescence method to determine the ability of the compounds to competitively displace propidium iodide (PI) from the AChE PAS. This procedure is widely known and often used as a primary screen to indirectly identify compounds as inhibitors of β-amyloid aggregation mediated the AChE PAS. The method is based on the increase in the fluorescence intensity of PI upon binding to AChE. The decrease in the fluorescence intensity of AChE-bound propidium in the presence of test compounds shows the ability of these compounds to displace propidium and to bind to the PAS of AChE. Donepezil and decamethonium were used as reference compounds.
We selected AChE from electric eels (Electrophorus electricus) for consistency with our other reports and because of its purity, specific activity, and lower cost compared to human AChE [64]. First, the inhibitory activity of conjugates 4 against EeAChE, i.e., against its ability to hydrolyze acetylthiocholine, was investigated. The results are summarized in Table 2 and demonstrate high inhibitory activity of compounds against the catalytic activity of EeAChE.

Table 2.
Inhibitory activity of MB-cycloalkaneindoles conjugates 4 against EeAChE and their ability to displace PI from the EeAChE PAS 1.
Following our confirmation of the inhibitory activity against EeAChE, we then investigated all conjugates 4 for their ability to bind to the AChE PAS and competitively displace the selective ligand (PI). The results are summarized in Table 2. Conjugates 4 in a concentration of 3 µM decreased the fluorescence intensity by 10–20%, while at 20 µM, this decrease was 15–37%. Thus, these compounds displaced PI from the AChE PAS more efficiently than the reference compounds donepezil and decamethonium, and in some cases, they approached the activities of MB and MBH2.
The results indicate that the test compounds can effectively bind to the AChE PAS which mediates for β-amyloid aggregation. Moreover, these findings agree with the kinetics data (Figure 2) demonstrating mixed-type inhibition, as well as the molecular docking results, showing binding to the AChE PAS (Figure 2). These findings suggest that the studied conjugates have the potential to block the AChE-induced aggregation of β-amyloid.
2.3. Studies of the Antioxidant Activity of MB-Cycloalkaneindole Conjugates
2.3.1. Studies of the Primary Antioxidant Activity of Conjugates 4
The primary antioxidant activity of conjugates 4 was determined by their ability to scavenge free radicals in the ORAC-FL and ABTS assays. The ORAC-FL assay is based exclusively on the hydrogen atom transfer (HAT) mechanism, whereas the ABTS assay is a combined method in which both HAT and single electron transfer (SET) mechanisms are possible. Trolox was used as a reference antioxidant (the antioxidant activity of the test compounds was referred to the activity of Trolox). The well-known antioxidant catechol was used as a positive control.
Evaluation of the Antiradical Activity by the ABTS Radical Cation Scavenging Assay
The ABTS assay is based on the direct binding of a model ABTS radical cation (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonate), ABTS•+) by antioxidants [65]. The measurements were performed as previously described in detail [64,66].
The radical scavenging activity was expressed as TEAC values (Trolox equivalent antioxidant capacity) and determined as the ratio between the slopes obtained from the linear correlation of the ABTS radical absorbance with the concentrations of the test compounds and Trolox. For the test compounds, we also determined the IC50 values (the compound concentration [μM] required for a 50% decrease in the concentration of the ABTS radical). The results are summarized in Table 3.

Table 3.
Radical scavenging activity of MB-cycloalkaneindoles conjugates according to ABTS and ORAC-FL assays.
The results indicate that all MB-cycloalkaneindole conjugates have a high ABTS•+- scavenging activity, which is close to or somewhat higher than the activity of the standard antioxidant Trolox (Table 3). The highest activity in this assay was found for compound 4e (R=CH3O, R1=CH3): TEAC = 1.46.
Evaluation of the Oxygen Radical Absorbance Capacity by a Fluorescence Method (ORAC-FL)
The ability of conjugates 4 to decrease the amount of peroxyl radicals as an additional characteristic of primary antioxidant activity was determined by a fluorescence assay (ORAC-FL) using fluorescein (FL) as the fluorescence probe. The method is based on the measurement of the fluorescence intensity characterizing the degree of destruction of the fluorescence probe under the action of peroxyl radicals. In the presence of antioxidants, the degree of destruction of the fluorescence probe induced by peroxyl radicals decreased and, hence, the time of fluorescence increased.
The peroxyl radical scavenging capacity of the test compounds was characterized in terms of the Trolox equivalent (TE), which is equal to the ratio of the Trolox concentration to the concentration of a test compound when they show equal fluorescence intensities in the assay. The peroxyl radical scavenging capacity of Trolox was set as 1 [67,68]. The results of the ORAC-FL assay are summarized in Table 3.
It was shown that the MB-cycloalkaneindole conjugates possessed high peroxyl radical scavenging capacity, which markedly exceeded that of Trolox, being in the range from 4 to 15 TE. All conjugates containing 7- or 8-membered cycloalkane moieties were generally more efficient. Compound 4m (n = 4, R=R1=H) was the most active in the ORAC test. Among the conjugates with 6-membered aliphatic rings (tetrahydrocarbazole), the highest activity was characteristic of compound 4e (R=CH3O, R1=CH3), which was also the most efficient in the ABTS test.
The data on the radical scavenging capacity for MB and MBH2 are not shown in Table 3 as the values for MB and MBH2 were not detectable with the ABTS and ORAC methods. This may be attributable to the very low redox potential of MB (11 mV) [69] and the ease of cycling between the oxidized and reduced forms.
Frontier Orbital Calculations
The DFT(B3LYP)/6-31++G ** quantum mechanical calculations carried out for MB- cycloalkaneindole conjugates resulted in the visualization of the frontier orbitals (Figure 3) and confirmed the experimental results of the antioxidant activity of the test compounds. The HOMO location on the cycloalkaneindole moiety (Figure 3) explains why the size of the aliphatic ring influences the antioxidant capacity in the ORAC assay. In particular, higher antiradical activity was demonstrated by compounds with 7- and 8-membered aliphatic rings in the cycloalkaneindole moiety.
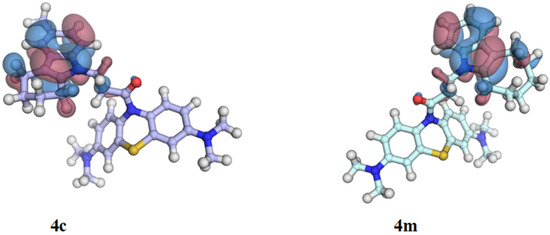
Figure 3.
Highest occupied molecular orbital (HOMO) of conjugates 4c (n = 2) and 4m (n = 4). Areas of positive and negative signs of the molecular orbital wave function are colored red and blue, respectively. The isovalue surface is 0.01 e∙Bohr−3.
2.3.2. Inhibition of the Iron-Induced LP in Rat Brain Homogenate
The influence of MB and its conjugates with indole derivatives on Fe3+-induced lipid peroxidation (LP) in rat brain homogenate was studied over a broad concentration range (from 10 nM to 30 µM).
All the conjugates, as well as MB and MBH2, exhibited no pro-oxidant activity in the concentrations used and under our experimental conditions. However, as shown in Table 4, all compounds showed efficient concentration-dependent inhibition of Fe3+-induced LP in rat brain homogenate. The IC50 values for all the test conjugates were below 1.9 μM or IC50 values for MB and close to IC50 values for MBH2 (Table 4). Effective inhibition of LP in the rat brain homogenate may be particularly connected to the high radical-scavenging activity of the compounds found in the ABTS and ORAC tests (Table 3). However, the lack of direct correlation between the radical scavenging activity in the model ABTS and ORAC assays and the biologically relevant inhibition of LP in rat brain homogenates may be indicative of additional contributions from other mechanisms.

Table 4.
Effect of MB–cycloalcaneindole conjugates on the characteristics of isolated rat liver mitochondria and the inhibition of LP in rat brain homogenates (RBH) induced by 0.5 mM Fe3+ (IC50, μM).
2.4. Effect of MB-Cycloalkaneindoles Conjugates on Mitochondrial Functions
2.4.1. Evaluation of the Effect of Test Compounds on the Membrane Potential and Calcium-Induced Mitochondrial Permeability Transition
The primary screening of new compounds to determine their effect on the mitochondrial membrane potential formed due to respiratory chain (RC) activity after additions of RC substrates was performed on isolated rat liver mitochondria. It can be used to estimate the probability of the potential toxicity of these compounds. Furthermore, the influence of the compounds on the calcium-dependent depolarization of isolated rat liver mitochondria in this assay made it possible to evaluate the effect on the mitochondrial permeability transition.
As shown in Table 4, in the presence of NADH-dependent substrates of the mitochondrial respiratory chain (RC) complex I, none of the newly synthesized conjugates in 1 μM concentration affected the mitochondrial membrane potential or calcium-induced depolarization. However, in the presence of the complex I inhibitor rotenone and a substrate of RC complex II, compound 4m, as well as MB induced mitochondrial depolarization, which increased with time and enhanced calcium-dependent mitochondrial depolarization. When present in a concentration of 30 μM, all compounds depolarized mitochondria both in the presence of the substrates of complex I and, to a larger extent, in the presence of complex II substrate succinate and complex I inhibitor rotenone. The lowest degree of depolarization in the presence of the substrates of complex II and I was found in compounds 4i and 4h. In addition, in a 30 μM concentration, MB and 4i completely suppress, while compound 4h considerably decrease the calcium-induced depolarization, but not influence the depolarization induced by carbonyl cyanide-3-chlorophenylhydrazone (CCCP), which is an uncoupler of the mitochondrial respiratory chain.
Subsequently, a more detailed study of compound 4i was carried out against a number of targets relevant for the selection of potential drugs for the treatment of neurodegenerative diseases. The results are presented in the following sections.
2.4.2. Evaluation of the Effect of the Lead Compound 4i on the Bioenergetic Potential of Rat Liver Mitochondria
MB possesses amphiphilic characteristics as well as unique electron-donating and electron-withdrawing properties. These features account for its ability to transfer electrons in the mitochondrial electron transport chain and its capacity to stimulate mitochondrial respiration, decrease the inhibition of respiratory chain complexes [70], and also provide an alternative electron transfer in the case of a dysfunctional electron transport chain [71].
The ability to bypass complex I inhibition was determined for MB, its reduced form MBH2, and compound 4i (Figure 4).
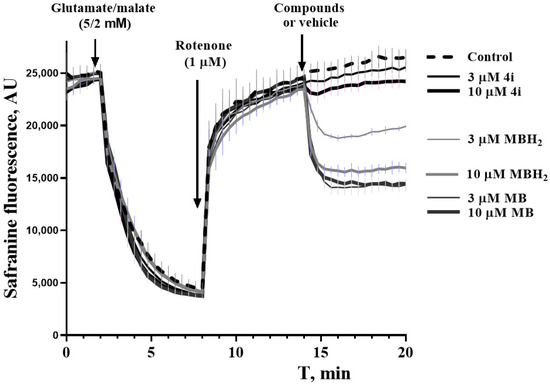
Figure 4.
Bypassing properties of MB, MBH2, and 4i against the rotenone-induced depolarization of isolated rat liver mitochondria.
The addition of rotenone to mitochondria energized by complex I substrates (glutamate/malate) causes considerable depolarization. The subsequent addition of MB or MBH2 induces concentration-dependent repolarization. Compound 4i exhibits this type of activity only in a 10 µM concentration, and the effect is much less pronounced.
Meanwhile, compound 4i can stimulate respiration of isolated rat liver mitochondria, which was estimated using an XFe96 Seahorse extracellular flux analyzer (Figure 5).
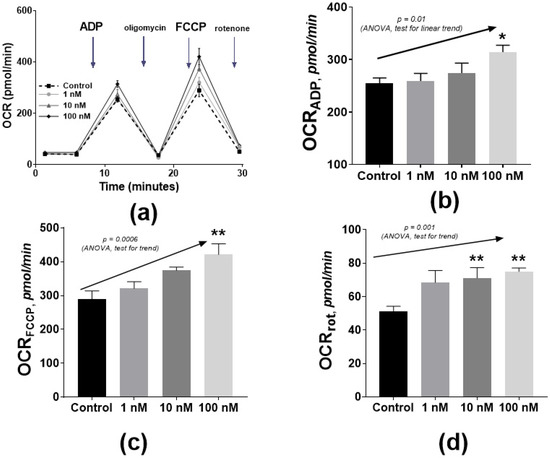
Figure 5.
The influence of 4i on complex I (glutamate–malate)-dependent oxygen consumption rate. (OCR): OCR profile plot (a), ADP-linked respiration (b), maximal FCCP-linked respiration (c), and rotenone-dependent respiration (d). (b–d) plots are means and SEM; * p ≤ 0.05, ** p ≤ 0.01 from ANOVA and post-hoc Dunnett’s test. The concentration dependence was proposed according to the post-hoc linear trend test (arrows with p-values).
Analysis was based on the fluorometric determination of O2 and H+ levels with solid-state probes on a sensor cartridge. The oxygen consumption rate (OCR) was measured successively in the presence of RC substrates and then after the addition of ADP (phosphorylating respiration), ATP synthase Fo-subunit inhibitor oligomycin (proton leak), oxidative phosphorylation uncoupler FCCP (maximal respiration), and the corresponding inhibitors of respiration chain complexes (non-mitochondrial respiration) (Figure 5a).
Even nanomolar concentrations of compound 4i produced a statistically significant increase in the phosphorylating respiration and maximal respiration (Figure 5b,c) in the presence of RC complex I substrates (glutamate and malate) and in the presence of complex I inhibitor (rotenone) (Figure 5d). This finding corresponds to the above-described effect of the repolarization of mitochondria and also reflects the effect of bypassing the rotenone inhibition of ETC (Figure 4). A similar effect was previously observed for isolated brain mitochondria where MB increased the rate of cytochrome reduction using NADH as the electron donor [72,73].
The elucidated ability of compound 4i to stimulate mitochondrial respiration and suppress the Ca2+-induced depolarization of mitochondria allows us to suggest that the new compound may possess cyto-(neuro)-protective properties and stimulate neuronal activity.
2.5. Evaluation of the Effect of Test Compounds on Binding of Specific Ligands of the Intrachannel and Peripheral Sites of the NMDA Receptor
The binding of all the new synthesized compounds as well as MB and MBH2 to glutamate receptors was studied by considering their ability to compete for binding with specific ligands of the NMDA receptor intrachannel and peripheral sites, which were radiolabeled [3H]MK-801 and [3H]ifenprodil, respectively. The experiments were carried out using a rat brain membrane fraction. Almost all the compounds showed no activity toward the NMDA receptor as the IC50 values were above 100 µM for both specific ligands. Methylene blue had a very low ability to interfere with MK-801 binding to the NMDA receptor (Table 5), but it effectively blocked the binding of [3H]ifenprodil. Only one compound of the whole series, 4i, showed activity toward the NMDA receptor approaching that of MB.

Table 5.
Effect of the MB–cycloalkaneindole conjugates on the binding of [3H]MK-801 and [3H]ifenprodil ligands to different sites of the NMDA receptor.
2.6. Evaluation of the Effect of Lead Compounds 4i and 4h on the Assembly of Microtubules
Another important type of activity to evaluate in the test compounds is the stimulation of tubulin polymerization to give microtubules a normal structure. The compensation of the ability of tau protein to stabilize the microtubular structure, which has been lost as a result of pathological hyperphosphorylation and aggregation, may serve to normalize axonal transport and promote the growth of axons, thereby exerting therapeutic effects [74].
The MB-cycloalkaneindole conjugates were studied for their effect on tubulin polymerization. The anti-aggregation properties of MB toward tau protein have been ascribed to a relatively nonspecific oxidative mechanism involving the formation of covalent bonds between tau protein cysteine SH groups [75]. On the other hand, the tubulin dimer contains more than 20 cysteine residues, and a considerable fraction of them are significant for tubulin polymerization with an oxidation-dependent decrease of polymerization competence [76]. However, in our experiments, MB and MBH2 induced a considerable concentration-dependent increase in the rate of polymerization, which was more than 1.5-fold at 100 µM (Figure 6). Therefore, the mechanism of potentiation of microtubule assembly by MB and MBH2 might not be exclusively oxidative.
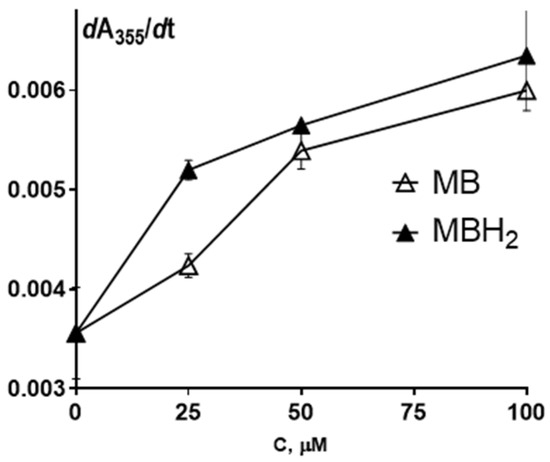
Figure 6.
The dependence of the tubulin polymerization rate on MB and MBH2 concentration. Tubulin polymerization was monitored by recording the change in absorbance at λ = 355 nm. The slope of the linear portion of the curve, dA355/dt, is plotted vs. MB and MBH2 concentration. Each point is the mean ± SEM (n = 5).
The same effect on tubulin polymerization seen with MB and MBH2 was observed for lead compounds 4i and 4h (Table 6), although it was somewhat more pronounced.

Table 6.
The effect of 100 µM MB, MBH2, and lead compounds 4i and 4h on the tubulin polymerization rate (dA355/dt).
It is important to note that straight, long microtubules with a normal structure were produced in the presence of MB compounds 4i and 4h.
2.7. Neuroprotective Effect of Lead Compound 4i under Calcium Overload Conditions on Primary Cultures of Rat Cerebellar Granule Cells
The above-described ability of the lead compounds to suppress the development of calcium-induced mitochondrial depolarization allows us to suggest that these compounds should have cyto(neuro)protective activity. For the validation of this suggestion, the influence of compound 4i on calcium overload-induced death (in the presence of a calcium ionophore, ionomycin, where the cytoprotective effect may be connected only with intracellular targets, mainly with the mitochondria) was studied using a primary culture of rat cerebellar granule cells. This neurotoxicity model reflects the calcium stress involved with several types of insults, such as amyloid toxicity, excitotoxicity, and ischemic damage. The viability of the primary culture of rat cerebellar granule cells (CGC) and brain cortical neurons (BCN) was assessed by the MTT assay in the presence of various concentrations of compound 4i. It was found that this compound did not affect formazan formation in the MTT assay. Thus, it could be inferred that it is not toxic to these cells and does not directly affect MTT conversion to formazan.
Compound 4i produced a statistically significant protection from ionomycin-induced toxicity for CGC (Figure 7a) and BCN (Figure 7b).
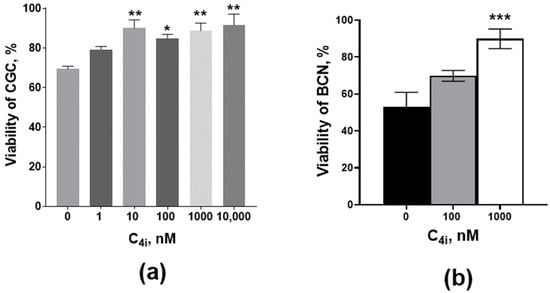
Figure 7.
The effect of compound 4i on ionomycin-induced toxicity in a primary culture of rat (a) cerebellar granule cells (CGC) and (b) brain cortex neurons (BCN). Cell viability was estimated by MTT assay. The ionomycin concentration in all samples of CGC was 3 µM, and it was 1 µM in all samples of BCN. Control samples, in addition to ionomycin, also contain DMSO solvent (≤1% v/v). * p < 0.05, ** p < 0.01, *** p < 0.001 in comparison with the control (one-way nonparametric Kruskal–Wallis ANOVA with Dunn’s multiple comparisons test).
3. Materials and Methods
3.1. In Vitro AChE, BChE, and CES Inhibition
Human erythrocyte AChE, equine serum BChE, porcine liver CES, acetylthiocholine iodide (ATCh), butyrylthiocholine iodide (BTCh), 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), 4-nitrophenol acetate (4-NPA), and BNPP were purchased from Sigma-Aldrich (St. Louis, MO, USA).
AChE and BChE activities were measured via the Ellman method [77]. The assay solution consisted of 0.1 M K/Na phosphate buffer pH 7.5, 25 °C with the addition of 0.33 mM DTNB, 0.02 unit/mL of AChE or BChE and 1 mM of substrate (ATCh or BTCh, respectively). The assays were carried out with a reagent blank containing all components (except AChE orBChE) in order to account for the non-enzymatic hydrolysis of the substrate. In addition, an enzyme blank was included that contained all components except substrate to account for non-substrate sulfhydryl groups.
The activity of CES was determined spectrophotometrically by monitoring the release of 4-nitrophenol at 405 nm [78]. The assay solution consisted of 0.1 M K/Na phosphate buffer pH 8.0, 25 °C, with the addition of 1 mM 4-nitrophenyl acetate and 0.02 unit/mL of CES. The assays were carried out with a blank containing all components, except CES.
The test compounds were dissolved in DMSO, and the incubation mixture contained 2% (v/v) of the solvent. Enzyme inhibition was first assessed at a single concentration of 20 µM for each compound after a 5 min incubation at 25 °C in three separate experiments. Compounds that inhibited the enzyme by more than 30% were then selected for the determination of the IC50 (the inhibitor concentration resulting in a 50% inhibition of control enzyme activity). Eight different concentrations of the test compounds in the range from 10−11 to 10−4 M were selected in order to obtain inhibition of AChE and BChE activity between 20% and 80%. The test compounds were added to the assay solution and preincubated at 25 °C with the enzymes for 5 min, followed by the addition of substrate. A parallel control was made for the assay solution with no inhibitor. Measurements were performed with a FLUOStar Optima microplate reader (BMG Labtech, Ortenberg, Germany). Each experiment was performed in triplicate. The results were expressed as the mean ± SEM. The reaction rates in the presence and absence of the inhibitor were compared, and the percent of residual enzyme activity due to the presence of test compounds was calculated. The IC50 values (the concentration of inhibitor required to decrease the enzyme activity by 50%) were determined graphically from the inhibition curves (the log inhibitor concentration vs. the percent residual enzyme activity) using Origin 6.1 software (OriginLab, Northampton, MA, USA).
3.2. Kinetic Analysis of AChE Inhibition. Determination of Steady-State Inhibition Constants
To elucidate the inhibition mechanisms for the active compounds, the residual activity of AChE was determined in the presence of three increasing concentrations of the test compounds and six decreasing concentrations of the substrates. The test compounds were preincubated with the enzyme at 25 °C for 5 min, followed by the addition of the substrates. Parallel controls were made for an assay of the rate of hydrolysis of the same concentrations of substrates in the solutions with no inhibitor. The kinetic parameters of substrate hydrolysis were determined. Measurements were performed with a FLUOStar Optima microplate reader. Each experiment was performed in triplicate. The results were fitted into Lineweaver–Burk double-reciprocal kinetic plots of 1/V versus 1/[S], and the inhibition constants Ki (competitive component) and αKi (noncompetitive component) were calculated using Origin 6.1 software.
3.3. Propidium Iodide Displacement Studies
PIand donepezil were purchased from Sigma-Aldrich. The ability of the test compounds to competitively displacePI, which is a selective ligand of the peripheral anionic site of AChE, was evaluated with the fluorescent method [79,80]. As the source of the enzyme, electric eel (Elecrophorus electricus) AChE (EeAChE, type VI-S, lyophilized powder, Sigma-Aldrich, Saint Louis, MO, USA) was used. The applicability of this enzyme was shown earlier [64]
To determine the percentage of displacement of PI from the EeAChE PAS, EeAChE (with a final concentration of 7 μM) was incubated with the test compound at a concentration of 3 and 20 μM in 1 mM Tris-HCl buffer pH 8.0, 25 °C for 15 min. Next, PIsolution (with a final concentration of 8 μM) was added, the samples were incubated for 15 min, and the fluorescence spectrum (530 nm (exc.) and 600 nm (emiss.)) was taken. Donepezil and decamethonium were used as reference compounds. The blank contained PI of the same concentration in 1 mM Tris-HCl buffer pH 8.0. The measurements were carried out in triplicate on a FLUOStar Optima microplate reader).
The percentage of displacement of PIfrom the peripheral anionic site of AChE was calculated using the following formula:
where IFAChE+Propidium is the fluorescence intensity of the propidium associated with AChE in the absence of the test compound (taken as 100%) and IFAChE+Propidium+inhibitor is the fluorescence intensity of the propidium associated with AChE in the presence of the test compound.
% Displacement = 100 − (IFAChE+Propidium+inhibitor/IFAChE+Propidium) × 100,
3.4. ABTS Radical Cation Scavenging Assay
The radical scavenging activity of the compounds was assessed using an ABTS radical decolorization assay. The procedure followed the reported protocol [65] with minor modifications [66]. ABTS (2,2ʹ-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) was purchased from TCI (Tokyo, Japan), while potassium persulfate (di-potassium peroxodisulfate), Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), catechol, and DMSO were received from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The ethanol was HPLC grade. Aqueous solutions were prepared using deionized water.
Trolox was used as the standard reference compound. Catechol was used as the positive control. All test and reference compounds were dissolved in DMSO. The final concentration of DMSO in the reaction mixture was 4% (v/v).
The solution of ABTS radical cation (ABTS•+) was produced by mixing a 7 mM ABTS stock solution with a 2.45 mM aqueous solution of potassium persulfate in equal quantities and allowing them to react for 12–16 h at room temperature in the dark. At the time of activity determination, the ABTS•+ solution was diluted with ethanol to adjust it to an absorbance value of about 0.80 ± 0.05 at 734 nm. A fresh working solution of ABTS•+ was prepared for each assay.
The radical scavenging capacity of the compounds was analyzed by mixing 10 μL of test compound with 240 μL of ABTS•+ working solution. The reduction in absorbance was measured spectrophotometrically at 734 nm using an xMark microplate UV/VIS spectrophotometer (Bio-Rad, Hercules, CA, USA). The reaction was monitored for an hour at 10 min intervals. Data were given for 1 h of incubation of compounds with ABTS•+. EtOH blanks were run in each assay. The results were obtained from three replicates of each sample and three independent experiments.
The antioxidant capacity as a Trolox equivalent (TEAC values) was determined as the ratio between the slopes obtained from the linear correlation of concentrations of test compounds and Trolox with absorbance of ABTS radical. For the test compounds, we also determined the IC50 values (the compound concentration required for a 50% reduction of ABTS radical). The compounds were tested in the concentration range of 1 × 10−6–1 × 10−4 M. The IC50 values were calculated using Origin 6.1 for Windows software (OriginLab, Northampton, MA, USA).
3.5. Oxygen Radical Absorbance Capacity Assay
The ORAC-FL method of Ou et al. [68], partially modified by Dávalos et al. [67], was followed, using a FluoStar Optima microplate reader with 485-P excitation and 520-P emission filters. 2,2´-Azobis(amidinopropane) dihydrochloride (AAPH), (±)-6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and fluorescein (FL) were purchased from Sigma-Aldrich. The reaction was carried out at 37 °C in 75 mM K,Na phosphate buffer (pH 7.4), and the final reaction mixture was 200 µL. The test compounds and the Trolox standard were dissolved in DMSO to 10 mM and further diluted in a 75 mM K,Na phosphate buffer (pH 7.4). The final concentrations were 0.1–1 µM for the test compounds and 1–6 µM for the Trolox. The blank was composed of 20 µL of a 75 mM K,Na phosphate buffer (pH 7.4) containing 20% (v/v) DMSO, 120 µL of FL and 60 µL of AAPH, and it was added in each assay. The antioxidant (20 µL) and FL (120 µL, final concentration: 70 nM) solutions were placed in a black 96-well microplate and were preincubated for 15 min at 37 °C. A solution of AAPH (60 µL, final concentration: 12 mM) was then added rapidly using a multichannel pipette. The microplate was immediately placed in the reader, and the fluorescence was recorded every minute for 100 min. The microplate was automatically shaken prior to each reading. The Trolox standard curve was also obtained in each assay. All reactions were carried out in triplicate, and at least three different assays were performed for each sample.
The antioxidant curves (fluorescence vs. time) were first normalized to the curve of the blank (without the antioxidant) corresponding to the same assay, and the area under the fluorescence decay curve (AUC) was calculated. The net AUC corresponding to a sample was calculated by subtracting the AUC corresponding to the blank. Regression equations were calculated by plotting the net AUC against the antioxidant concentration. The ORAC Tolox equivalent value (TE) was obtained by dividing the slope of the latter curve by the slope of the Trolox curve obtained in the same assay. The ability of Trolox to scavenge the peroxyl radical was equal to 1 [64,65]. Final ORAC values were expressed as μmol of test compounds per μmol of Trolox where the value of Trolox was taken as 1 [67,68]. The data are expressed as means ± SEM.
3.6. Lipid Peroxidation of Rat Brain Homogenate
On the day of the experiment, adult Wistar male rats that fasted overnight were euthanized in a CO2 chamber, followed by decapitation. The procedure was in compliance with the Guidelines for Animal Experiments at the Institute of Physiologically Active Compounds of the Russian Academy of Sciences. The brains were rapidly removed and homogenized in 0.12 M HEPES/0.15M NaCl, pH 7.4 buffer (HBS) (10 mg/g wet weight) and used immediately for the assay.
The protein concentrations in rat brain homogenate (RBH) were determined by the biuret assay using bovine serum albumin as the standard [81].
The effect of the compounds on LP of the RBH was studied at 30 °C for 40 min in 0.25 mL of RBH (2 mg of protein·mL−1) in the presence of the compounds or the vehicle (DMSO). Lipid peroxidation was induced by Fe3+ (0.5 mM Fe(NH4)(SO4)2) as an oxidant [82].
The reaction mixture was incubated for 30 min at 37 °C, then quenched by adding 0.4 mL of quench medium containing 250 mM HCl and 15% (w/v) trichloroacetic acid. The samples were then centrifuged for 10 min at 10,000× g, and 75 µL of the supernatant was transferred to a 96-well plate. Next, 75 µL of 0.8% (w/v) 2-thiobarbituric acid (TBA) was added. The plate was then sealed, heated at 95 °C for 15 min, cooled to 4 °C, and the absorbance at 530–620 nm was measured using a Wallac Victor 3 1420 Multilabel Counter (Perkin Elmer, Turku, Finland).
All the experiments were performed as four independent runs with different brain homogenate preparations and three repeated probes in each run. The average results for each run were normalized between the positive control with the vehicle and Fe3+ and negative control with only the vehicle. They were presented as the IC50 ± SD values for the antioxidant activities of the compounds which were calculated using GraphPad Prism 7.00 software (San Diego, CA, USA).
3.7. Rat Liver Mitochondria Isolation
Rat liver mitochondria were isolated by conventional differential centrifugation from the livers of adult Wistar strain rats that fasted overnight, pH 7.6 [83,84]. The mitochondrial protein concentration was determined using a biuret procedure with bovine serum albumin as the standard [81].
3.8. Mitochondrial Potential
Safranine O (10 µM) was used as a membrane potential probe [85]. Fluorescence intensity at 580 nm (excitation at 520 nm) was measured with a Wallac Victor 3 1420 Multilabel Counter (Perkin Elmer, Turku, Finland). The mitochondrial protein concentration was 0.2 mg/mL. The medium for measurements contained 75 mM sucrose, 225 mM mannitol, 10 mM K-HEPES (pH 7.4), 0.02 mM EGTA, and 1 mM KH2PO4. After a 5-min incubation, 5 mM glutamate/malate or 5 mM succinate in the presence of 0.5 µM rotenone were added to produce the mitochondrial potential. Then, the compounds (30 µM) or the same volume of the vehicle (DMSO) were injected into the mitochondrial suspension. After 15–20 min, 12.5 µM CaCl2 was added to each sample to induce the depolarization of mitochondria, and after 5 min, 0.5 µM CCCP was added for maximum depolarization of the mitochondria. The level of depolarization (ΔΨm) was calculated using the fluorescence value after 10-min incubation with 30 µM of compounds (or vehicle) normalized between the fluorescence measurements after substrate and CCCP addition, where fluorescence intensity at 580 nm was 100% after substrate addition and was 0% after CCCP addition.
3.9. Measurements of Oxygen Consumption in Rat Liver Mitochondria
The oxygen consumption was measured using a Seahorse XF96 flux analyzer on mitochondria respiring on glutamate (5 mM) and malate (5 mM), according the same protocol as [86] with a correction to a 96-well instead of a 24-well plate. Respiration by rat liver mitochondria (5 µg/well) was sequentially measured in a coupled state with substrates of complex I (basal respiration); followed by phosphorylating respiration after the addition of ADP; non-phosphorylating or resting respiration induced with the addition of oligomycin; uncoupled respiration stimulated by the addition of FCCP; and finally, non-mitochondrial respiration after the addition of the inhibitor of complex I rotenone.
3.10. Primary Screening of the Action of Compounds on Tubulin Polymerization
The assembly of tubulin into microtubules was carried out using pure tubulin from an HTS-Tubulin polymerization assay kit (Cytoskeleton, Inc., Denver, CO, USA). A standard polymerization reaction used 100 μL of 4 mg/mL tubulin in 80 mM PIPES (pH 6.9), 0.5 mM EGTA, 2 mM MgCl2, and 1 mM GTP. Polymerization was monitored by recording the change in absorbance on a Wallac Victor 3 1420 Multilabel Counter (Perkin Elmer, Turku, Finland) at λ = 3 55 nm. Electron microscopic monitoring was carried out with a Carl Zeiss Libra 120 electron microscope (Carl Zeiss Meditec AG, Jena, Germany) at 120 kV using negative contrasting.
3.11. Effect of Compounds on Ionomycin-Induced Toxicity in Primary Culture of Rat Cerebellar Granule Cells and Rat Brain Cortical Neurons
Cerebellar granule cells (CGC) and brain cortical neurons (BCN) were isolated from P-7 or P0-2 rat’s pups, respectively, using a previously described protocol [87]. Once removed, cerebella or brain cortex were digested for 15 min in 0.025% type II trypsin (w/v) at 37 °C. Ice-cold DMEM containing 5% (w/v) fetal bovine serum was added to inactivate the trypsin. The crude cell suspension was centrifuged, and the cell pellet was resuspended in Neurobasal with 2% (v/v) B27 supplemented with 15% (v/v) fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin. The cells were cultured in a humidified, 5% (v/v) CO2-controlled environment at 37 °C. After 8-10 day in culture the cells were incubated with a test compound or an equal volume of the DMSO (<1% (v/v) of the whole volume of the medium under the layer of cells and 3 µM ionomycin for 24 h. The cell viability was then evaluated as the dehydrogenase activity with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The absorbance was measured at 570 nm using a Victor microplate reader (Perkin Elmer), and data were normalized with intact control assumed 100%.
3.12. Radioligand Study of Interaction of the Compounds with NMDA-Receptor Binding Sites
The effect of test compounds on the radioligand binding to NMDA receptors was determined as previously described [4]. Two radioactive ligands were used: [3H] MK-801 (dizocilpine) with a specific activity of 210 Ci/mmol, binding to all isolated NMDA receptors, and [3H] ifenprodil with a specific activity of 79 Ci/mmol, binding only to NMDA receptors containing the NR2B subunit. A pellet of rat brain membrane specimen was resuspended in a working buffer (5 mM HEPES/4.5 mM Tris buffer, pH 7.6) in a ratio of 1:5 and stored in liquid nitrogen. The reaction mixture (with a final volume of 0.5 mL) contained 200 µL of the working buffer, 50 µL of 50 nM radioligand solution, and 250 µL of the membrane suspension. Nonspecific binding was determined in the presence of 50 µL of 1 M of unlabeled ligand.
For the binding study, the reaction mixture was incubated at room temperature for 2 h. After incubation, the samples were filtered through GF/B glass-fiber filters (Whatman), washed with the working buffer, dried, and transferred to scintillation vials, and then 5 mL of scintillation fluid was added to the vials containing 4 g of diphenyloxazole (PPO), 0.2 g of diphenyloxazoil benzene (POPOP), and 1 L of toluene. The radioactivity was determined in a TriCarb2800 TR scintillation counter (Perkin Elmer, Packard, Downers Grove, Illinois, USA) with a counting efficiency of about 65%.
The effect of the test compounds on the binding of [3H] MK-801 and [3H] ifenprodil to rat brain membranes was studied by adding 50 µL of the test compounds in the concentration range of 10−8–10−3 M to the incubation medium. From the results of inhibition, IC50 values were calculated for the test compounds using GraphPad Prism 4.
3.13. Molecular Modeling
3.13.1. Structure Preparation
Geometries of the ligands were quantum-mechanically (QM) optimized in the Gamess-US package [88] using the B3LYP DFT method and the 6-31G * basis set (and 6-31++G ** for frontier orbital calculations). The partial atomic charges were taken from the QM results according to the Mulliken scheme [89]. These optimized geometries and partial charges were used for molecular docking.
There are several structures of human AChE apo-state and with several ligands available (PDB ID 4EY4-4EY8, [52]). All X-ray diffraction structures and a water-saturated optimized structure of apo-hAChE [51] were used for molecular docking (with water molecules removed). Similar to the results obtained in [51], the best binding affinities were obtained with structure PDB ID 4EY7 (hAChE co-crystallized with Donepezil, 2.35 Å), and they were similar to the results obtained with the water-saturated optimized structure of apo-hAChE.
3.13.2. Molecular Docking
Molecular docking with a Lamarckian Genetic Algorithm (LGA) [90] was performed with Autodock 4.2.6 [91]. The grid box for docking included the whole active site and the gorge of AChE (22.5 Å × 22.5 Å × 22.5 Å grid box dimensions) with a grid spacing of 0.375 Å. The main LGA parameters were 256 runs, 25 × 106 evaluations, 27 × 104 generations, and a population size of 300. Figures were prepared with PyMOL (Schrödinger, New York, NY, USA).
4. Conclusions
The studied compounds effectively inhibited AChE and were shown to be potential blockers of AChE-induced β-amyloid aggregation. The high radical-scavenging activity of MB-cycloalcaneindole conjugates displayed in the ABTS and ORAC tests may be one of the main reasons for their high inhibitory activity of Fe3+-induced LP in rat brain homogenate.
Compound 4i (R=CF3O, R1=CH3) showed properties similar to those of MB toward the ifenprodil-specific site of the NMDA receptor, ligands of which exert cognition-enhancing effects.
As a result of assessing the effect of conjugates 4 on the functions of mitochondria, two lead compounds, 4i (R=CF3O, R1=CH3) and 4h (R=CF3O, R1=H), were identified.
Mitochondrial functions studies showed that like MB, lead compound 4i was able to decrease the efficiency of the inhibition of respiratory chain complex I, and, even in nanomolar concentrations, it stimulated the respiratory chain activity for isolated rat liver mitochondria. In addition, it could prevent calcium-induced mitochondrial depolarization, which suggests inhibition of the mitochondrial permeability transition and, accordingly, the possible presence of cyto(neuro)protective properties. The neuroprotective effect was confirmed using a cellular calcium overload model of neurodegeneration. An equally important feature of compounds 4i and 4h was their ability to stimulate the assembly of microtubules. This finding suggests a compensatory effect on the system of microtubules in the case of tauopathy, which is one of the most pronounced pathogenic characteristics of neurodegenerative diseases.
Thus, new MB-cycloalkaneindoles conjugates form a promising class of compounds for the development of multitarget neuroprotective drugs, which simultaneously act on several targets, thereby providing cognitive stimulating, neuroprotective, and disease-modifying effects.
Author Contributions
All authors contributed to the study conception and design. Conceptualization, S.O.B., E.F.S., G.F.M. and V.V.G.; formal analysis, N.V.K., N.P.B., S.V.L., E.V.R., D.V.V., P.N.S. and I.M.V.; investigation, T.V.G., T.A.E., N.V.K., N.P.B., S.V.L., E.V.R., D.V.V., P.N.S., E.A.P., L.G.D., T.P.S. and I.M.V.; methodology, E.F.S., G.F.M., A.Y.A. and V.V.G.; supervision, S.O.B., V.P.F. and R.J.R.; writing—original draft, A.Y.A., V.V.G. and I.M.V.; writing—review & editing, E.F.S., G.F.M. and R.J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded within the framework of the grant agreement (Moscow, 1 October 2020, Grant No. 075-15-2020-777) as government subsidies from the Federal budget of the Russian Federation in accordance with paragraph 4 of article 78.1 of the Budget Code of the Russian Federation.
Institutional Review Board Statement
This study was performed in line with the principles of the Declaration of Helsinki and was carried out in compliance with the ARRIVE guidelines. All animal procedures were approved by the Bioethics Committee of IPAC RAS (Approval No. 41, 26 November 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the shared research facilities of the HPC computing resources at Lomonosov Moscow State University [92] for supercomputer time and the “Centre for Collective Use of IPAC RAS” (IPAC research topic FFSN-2021-0005) for use of equipment cited in the Methods.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Albertini, C.; Salerno, A.; Sena Murteira Pinheiro, P.; Bolognesi, M.L. From combinations to multitarget-directed ligands: A continuum in Alzheimer’s disease polypharmacology. Med. Res. Rev. 2020, 41, 2606–2633. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O. Medicinal and chemical approaches to focused search of agents for treatment and therapy of Alzheimer disease. Vopr. Med. Khim. 2001, 47, 155–197. [Google Scholar] [PubMed]
- Oliveira Pedrosa, M.; Duarte da Cruz, R.; Oliveira Viana, J.; de Moura, R.; Ishiki, H.; Barbosa Filho, J.; Diniz, M.; Scotti, M.; Scotti, L.; Bezerra Mendonca, F. Hybrid Compounds as Direct Multitarget Ligands: A Review. Curr. Top. Med. Chem. 2017, 17, 1044–1079. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O.; Shevtsova, E.F.; Makhaeva, G.F.; Grigoriev, V.V.; Boltneva, N.P.; Kovaleva, N.V.; Lushchekina, S.V.; Shevtsov, P.N.; Neganova, M.E.; Redkozubova, O.M.; et al. Novel conjugates of aminoadamantanes with carbazole derivatives as potential multitarget agents for AD treatment. Sci. Rep. 2017, 7, 45627. [Google Scholar] [CrossRef]
- Angelova, P.R.; Vinogradova, D.; Neganova, M.E.; Serkova, T.P.; Sokolov, V.V.; Bachurin, S.O.; Shevtsova, E.F.; Abramov, A.Y. Pharmacological Sequestration of Mitochondrial Calcium Uptake Protects Neurons Against Glutamate Excitotoxicity. Mol. Neurobiol. 2019, 56, 2244–2255. [Google Scholar] [CrossRef]
- Sokolov, V.B.; Makhaeva, G.F.; Aksinenko, A.Y.; Grigoriev, V.V.; Shevtsova, E.F.; Bachurin, S.O. Targeted synthesis and biological activity of polypharmacophoric agents for the treatment of neurodegenerative diseases. Russ. Chem. Bull. 2017, 66, 1821–1831. [Google Scholar] [CrossRef]
- Padnya, P.L.; Khadieva, A.I.; Stoikov, I.I. Current Achievements and Perspectives in Synthesis and Applications of 3,7-Disubstituted Phenothiazines as Methylene Blue Analogues. Dyes Pigments 2022, 208, 110806. [Google Scholar] [CrossRef]
- Posso, M.C.; Domingues, F.C.; Ferreira, S.; Silvestre, S. Development of Phenothiazine Hybrids with Potential Medicinal Interest: A Review. Molecules 2022, 27, 276. [Google Scholar] [CrossRef]
- Atamna, H.; Nguyen, A.; Schultz, C.; Boyle, K.; Newberry, J.; Kato, H.; Ames, B.N. Methylene blue delays cellular senescence and enhances key mitochondrial biochemical pathways. FASEB J. 2008, 22, 703–712. [Google Scholar] [CrossRef]
- Gabrielli, D.; Belisle, E.; Severino, D.; Kowaltowski, A.J.; Baptista, M.S. Binding, aggregation and photochemical properties of methylene blue in mitochondrial suspensions. Photochem. Photobiol. 2007, 79, 227–232. [Google Scholar] [CrossRef]
- Kress, M.; Petersen, M.; Reeh, P.W. Methylene blue induces ongoing activity in rat cutaneous primary afferents and depolarization of DRG neurons via a photosensitive mechanism. Naunyn-Schmiedeb. Arch. Pharmacol. 1997, 356, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Saitow, F.; Nakaoka, Y. The Photodynamic Action of Methylene Blue on the Ion Channels of Paramecium Causes Cell Damage. Photochem. Photobiol. 1997, 65, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Pfaffendorf, M.; Bruning, T.A.; Batnik, H.D.; van Zwieten, P.A. The interaction between methylene blue and the cholinergic system. Br. J. Pharmacol. 1997, 122, 95–98. [Google Scholar] [CrossRef][Green Version]
- Ramsay, R.R.; Dunford, C.; Gillman, P.K. Methylene blue and serotonin toxicity: Inhibition of monoamine oxidase A (MAO A) confirms a theoretical prediction. Br. J. Pharmacol. 2007, 152, 946–951. [Google Scholar] [CrossRef] [PubMed]
- Vutskits, L.; Briner, A.; Klauser, P.; Gascon, E.; Dayer, A.G.; Kiss, J.Z.; Muller, D.; Licker, M.J.; Morel, D.R. Adverse effects of methylene blue on the central nervous system. Anesthesiology 2008, 108, 684–692. [Google Scholar] [CrossRef]
- Auchter, A.M.; Shumake, J.; Gonzalez-Lima, F.; Monfils, M.H. Preventing the return of fear using reconsolidation updating and methylene blue is differentially dependent on extinction learning. Sci. Rep. 2017, 7, 46071. [Google Scholar] [CrossRef]
- Schirmer, R.H.; Adler, H.; Pickhardt, M.; Mandelkow, E. Lest we forget you—methylene blue…. Neurobiol. Aging 2011, 32, 2325.e7–2325.e16. [Google Scholar] [CrossRef]
- Stack, C.; Jainuddin, S.; Elipenahli, C.; Gerges, M.; Starkova, N.; Starkov, A.A.; Jove, M.; Portero-Otin, M.; Launay, N.; Pujol, A.; et al. Methylene blue upregulates Nrf2/ARE genes and prevents tau-related neurotoxicity. Hum. Mol. Genet. 2014, 23, 3716–3732. [Google Scholar] [CrossRef]
- Tucker, D.; Lu, Y.; Zhang, Q. From Mitochondrial Function to Neuroprotection-an Emerging Role for Methylene Blue. Mol. Neurobiol. 2018, 55, 5137–5153. [Google Scholar] [CrossRef]
- Wilcock, G.K.; Gauthier, S.; Frisoni, G.B.; Jia, J.; Hardlund, J.H.; Moebius, H.J.; Bentham, P.; Kook, K.A.; Schelter, B.O.; Wischik, D.J.; et al. Potential of Low Dose Leuco-Methylthioninium Bis(Hydromethanesulphonate) (LMTM) Monotherapy for Treatment of Mild Alzheimer’s Disease: Cohort Analysis as Modified Primary Outcome in a Phase III Clinical Trial. J. Alzheimers Dis. 2018, 61, 435–457. [Google Scholar] [CrossRef]
- Lee, B.I.; Suh, Y.S.; Chung, Y.J.; Yu, K.; Park, C.B. Shedding Light on Alzheimer’s beta-Amyloidosis: Photosensitized Methylene Blue Inhibits Self-Assembly of beta-Amyloid Peptides and Disintegrates Their Aggregates. Sci. Rep. 2017, 7, 7523. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.X.; Caccamo, A.; Oddo, S. Methylene blue reduces abeta levels and rescues early cognitive deficit by increasing proteasome activity. Brain Pathol. 2011, 21, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Necula, M.; Breydo, L.; Milton, S.; Kayed, R.; van der Veer, W.E.; Tone, P.; Glabe, C.G. Methylene blue inhibits amyloid Abeta oligomerization by promoting fibrillization. Biochemistry 2007, 46, 8850–8860. [Google Scholar] [CrossRef] [PubMed]
- Schwab, K.; Frahm, S.; Horsley, D.; Rickard, J.E.; Melis, V.; Goatman, E.A.; Magbagbeolu, M.; Douglas, M.; Leith, M.G.; Baddeley, T.C.; et al. A Protein Aggregation Inhibitor, Leuco-Methylthioninium Bis(Hydromethanesulfonate), Decreases alpha-Synuclein Inclusions in a Transgenic Mouse Model of Synucleinopathy. Front. Mol. Neurosci. 2017, 10, 447. [Google Scholar] [CrossRef] [PubMed]
- Sontag, E.M.; Lotz, G.P.; Agrawal, N.; Tran, A.; Aron, R.; Yang, G.; Necula, M.; Lau, A.; Finkbeiner, S.; Glabe, C.; et al. Methylene blue modulates huntingtin aggregation intermediates and is protective in Huntington’s disease models. J. Neurosci. 2012, 32, 11109–11119. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O.; Makhaeva, G.F.; Shevtsova, E.F. Conjugates of methylene blue with γ-carboline derivatives as new multifunctional agents for neurodegenerative diseases treatment. Sci. Rep. 2019, 9, 4873. [Google Scholar] [CrossRef]
- Biberoglu, K.; Tek, M.Y.; Ghasemi, S.T.; Tacal, O. Toluidine blue O is a potent inhibitor of human cholinesterases. Arch. Biochem. Biophys. 2016, 604, 57–62. [Google Scholar] [CrossRef]
- Darvesh, S.; Pottie, I.R.; Darvesh, K.V.; McDonald, R.S.; Walsh, R.; Conrad, S.; Penwell, A.; Mataija, D.; Martin, E. Differential binding of phenothiazine urea derivatives to wild-type human cholinesterases and butyrylcholinesterase mutants. Bioorg. Med. Chem. 2010, 18, 2232–2244. [Google Scholar] [CrossRef]
- Kundaikar, H.; Agre, N.; Degani, M. Pharmacophore Based 3DQSAR of Phenothiazines as Specific Human Butyrylcholinesterase Inhibitors for Treatment of Alzheimer’s Disease. Curr. Comput.-Aided Drug Des. 2015, 10, 335–348. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Lushchekina, S.V.; Boltneva, N.P.; Sokolov, V.B.; Grigoriev, V.V.; Serebryakova, O.G.; Vikhareva, E.A.; Aksinenko, A.Y.; Barreto, G.E.; Aliev, G.; et al. Conjugates of gamma-Carbolines and Phenothiazine as new selective inhibitors of butyrylcholinesterase and blockers of NMDA receptors for Alzheimer Disease. Sci. Rep. 2015, 5, 13164. [Google Scholar] [CrossRef]
- Sezgin, Z.; Biberoglu, K.; Chupakhin, V.; Makhaeva, G.F.; Tacal, O. Determination of binding points of methylene blue and cationic phenoxazine dyes on human butyrylcholinesterase. Arch. Biochem. Biophys. 2013, 532, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.J.; Kong, S.Y.; Park, M.H.; Cho, Y.; Kim, S.E.; Shin, J.Y.; Jung, S.; Lee, J.; Farhanullah; Kim, H.J.; et al. Aminopropyl carbazole analogues as potent enhancers of neurogenesis. Bioorg. Med. Chem. 2013, 21, 7165–7174. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Chen, M.; Li, M.; Luo, B.; Zhao, Y.; Huang, P.; Xue, F.; Rapposelli, S.; Pi, R.; Wen, S. Discovery of novel N-substituted carbazoles as neuroprotective agents with potent anti-oxidative activity. Eur. J. Med. Chem. 2013, 68, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Kosaraju, J.; Zhou, W.; Tam, K.Y. SLOH, a carbazole-based fluorophore, mitigates neuropathology and behavioral impairment in the triple-transgenic mouse model of Alzheimer’s disease. Neuropharmacology 2018, 131, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Bachurin, S.O.; Sokolov, V.B.; Aksinenko, A.Y.; Epishina, T.A.; Goreva, T.V.; Gabrel’yan, A.V.; Grigor´ev, V.V. Molecular construction of multitarget neuroprotectors 1. Synthesis and biological activity of conjugates of substituted indoles and bis(dimethylamino)phenothiazine. Russ. Chem. Bull. 2015, 64, 1354–1361. [Google Scholar] [CrossRef]
- Martinez, A.; Castro, A. Novel cholinesterase inhibitors as future effective drugs for the treatment of Alzheimer’s disease. Expert Opin. Investig. Drugs 2006, 15, 1–12. [Google Scholar] [CrossRef]
- Furukawa-Hibi, Y.; Alkam, T.; Nitta, A.; Matsuyama, A.; Mizoguchi, H.; Suzuki, K.; Moussaoui, S.; Yu, Q.S.; Greig, N.H.; Nagai, T.; et al. Butyrylcholinesterase inhibitors ameliorate cognitive dysfunction induced by amyloid-beta peptide in mice. Behav. Brain Res. 2011, 225, 222–229. [Google Scholar] [CrossRef]
- Kamal, M.A.; Klein, P.; Luo, W.; Li, Y.; Holloway, H.W.; Tweedie, D.; Greig, N.H. Kinetics of human serum butyrylcholinesterase inhibition by a novel experimental Alzheimer therapeutic, dihydrobenzodioxepine cymserine. Neurochem. Res. 2008, 33, 745–753. [Google Scholar] [CrossRef]
- Wang, L.; Esteban, G.; Ojima, M.; Bautista-Aguilera, O.M.; Inokuchi, T.; Moraleda, I.; Iriepa, I.; Samadi, A.; Youdim, M.B.; Romero, A.; et al. Donepezil + propargylamine + 8-hydroxyquinoline hybrids as new multifunctional metal-chelators, ChE and MAO inhibitors for the potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014, 80, 543–561. [Google Scholar] [CrossRef]
- Greig, N.H.; Utsuki, T.; Ingram, D.K.; Wang, Y.; Pepeu, G.; Scali, C.; Yu, Q.S.; Mamczarz, J.; Holloway, H.W.; Giordano, T.; et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA 2005, 102, 17213–17218. [Google Scholar] [CrossRef]
- Lane, R.M.; Potkin, S.G.; Enz, A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int. J. Neuropsychopharmacol. 2006, 9, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, M.J.; Potter, P.M. Carboxylesterase inhibitors. Expert Opin. Ther. Pat. 2011, 21, 1159–1171. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Radchenko, E.V.; Palyulin, V.A.; Rudakova, E.V.; Aksinenko, A.Y.; Sokolov, V.B.; Zefirov, N.S.; Richardson, R.J. Organophosphorus compound esterase profiles as predictors of therapeutic and toxic effects. Chem. Biol. Interact. 2013, 203, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Tsurkan, L.G.; Hatfield, M.J.; Edwards, C.C.; Hyatt, J.L.; Potter, P.M. Inhibition of human carboxylesterases hCE1 and hiCE by cholinesterase inhibitors. Chem. Biol. Interact. 2013, 203, 226–230. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Boltneva, N.P.; Lushchekina, S.V.; Serebryakova, O.G.; Stupina, T.S.; Terentiev, A.A.; Serkov, I.V.; Proshin, A.N.; Bachurin, S.O.; Richardson, R.J. Synthesis, molecular docking and biological evaluation of N,N-disubstituted 2-aminothiazolines as a new class of butyrylcholinesterase and carboxylesterase inhibitors. Bioorg. Med. Chem. 2016, 24, 1050–1062. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Grigoriev, V.V.; Proshin, A.N.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Serkov, I.V.; Bachurin, S.O. Novel conjugates of tacrine with 1,2,4,-thiadiazole as highly effective cholinesterase inhibitors, blockers of NMDA receptors, and antioxidants. Dokl. Biochem. Biophys. 2017, 477, 405–409. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Radchenko, E.V.; Baskin, I.I.; Palyulin, V.A.; Richardson, R.J.; Zefirov, N.S. Combined QSAR studies of inhibitor properties of O-phosphorylated oximes toward serine esterases involved in neurotoxicity, drug metabolism and Alzheimer’s disease. SAR QSAR Environ. Res. 2012, 23, 627–647. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Rudakova, E.V.; Serebryakova, O.G.; Aksinenko, A.Y.; Lushchekina, S.V.; Bachurin, S.O.; Richardson, R.J. Esterase profiles of organophosphorus compounds in vitro predict their behavior in vivo. Chem. Biol. Interact. 2016, 259 Pt B, 332–342. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Shevtsova, E.F.; Boltneva, N.P.; Lushchekina, S.V.; Kovaleva, N.V.; Rudakova, E.V.; Bachurin, S.O.; Richardson, R.J. Overview of novel multifunctional agents based on conjugates of γ-carbolines, carbazoles, tetrahydrocarbazoles, phenothiazines, and aminoadamantanes for treatment of Alzheimer’s disease. Chem. Biol. Interact. 2019, 308, 224–234. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Sokolov, V.B.; Shevtsova, E.F.; Kovaleva, N.V.; Lushchekina, S.V.; Boltneva, N.P.; Rudakova, E.V.; Aksinenko, A.Y.; Shevtsov, P.N.; Neganova, M.E.; et al. Focused design of polypharmacophoric neuroprotective compounds: Conjugates of γ-carbolines with carbazole derivatives and tetrahydrocarbazole. Pure Appl. Chem. 2017, 89, 1167–1184. [Google Scholar] [CrossRef]
- Semenov, V.E.; Zueva, I.V.; Mukhamedyarov, M.A.; Lushchekina, S.V.; Kharlamova, A.D.; Petukhova, E.O.; Mikhailov, A.S.; Podyachev, S.N.; Saifina, L.F.; Petrov, K.A.; et al. 6-Methyluracil Derivatives as Bifunctional Acetylcholinesterase Inhibitors for the Treatment of Alzheimer’s Disease. Chemmedchem 2015, 10, 1863–1874. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, A.D.; Lushchekina, S.V.; Petrov, K.A.; Kots, E.D.; Nachon, F.; Villard-Wandhammer, M.; Zueva, I.V.; Krejci, E.; Reznik, V.S.; Zobov, V.V.; et al. Slow-binding inhibition of acetylcholinesterase by an alkylammonium derivative of 6-methyluracil: Mechanism and possible advantages for myasthenia gravis treatment. Biochem. J. 2016, 473, 1225–1236. [Google Scholar] [CrossRef]
- Dym, O.; Song, W.; Felder, C.; Roth, E.; Shnyrov, V.; Ashani, Y.; Xu, Y.; Joosten, R.P.; Weiner, L.; Sussman, J.L.; et al. The Impact of Crystallization Conditions on Structure-Based Drug Design: A Case Study on the Methylene Blue/Acetylcholinesterase Complex. Protein Sci. 2016, 25, 1096–1114. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Origins and properties of the tetrel bond. Phys. Chem. Chem. Phys. 2021, 23, 5702–5717. [Google Scholar] [CrossRef] [PubMed]
- Esterhuysen, C.; Heßelmann, A.; Clark, T. Trifluoromethyl: An Amphiphilic Noncovalent Bonding Partner. Chemphyschem 2017, 18, 772–784. [Google Scholar] [CrossRef] [PubMed]
- García-Llinás, X.; Bauzá, A.; Seth, S.K.; Frontera, A. Importance of R–CF3···O Tetrel Bonding Interactions in Biological Systems. J. Phys. Chem. A 2017, 121, 5371–5376. [Google Scholar] [CrossRef]
- Gibbs, C.A.; Weber, D.S.; Warren, J.J. Clustering of Aromatic Amino Acid Residues around Methionine in Proteins. Biomolecules 2021, 12, 6. [Google Scholar] [CrossRef]
- Weber, D.S.; Warren, J.J. The interaction between methionine and two aromatic amino acids is an abundant and multifunctional motif in proteins. Arch. Biochem. Biophys. 2019, 672, 108053. [Google Scholar] [CrossRef]
- Bartolini, M.; Bertucci, C.; Cavrini, V.; Andrisano, V. beta-Amyloid aggregation induced by human acetylcholinesterase: Inhibition studies. Biochem. Pharmacol. 2003, 65, 407–416. [Google Scholar] [CrossRef]
- De Ferrari, G.V.; Canales, M.A.; Shin, I.; Weiner, L.M.; Silman, I.; Inestrosa, N.C. A Structural Motif of Acetylcholinesterase That Promotes Amyloid β-Peptide Fibril Formation. Biochemistry 2001, 40, 10447–10457. [Google Scholar] [CrossRef] [PubMed]
- Inestrosa, N.C.; Alvarez, A.; Pérez, C.A.; Moreno, R.D.; Vicente, M.; Linker, C.; Casanueva, O.I.; Soto, C.; Garrido, J. Acetylcholinesterase Accelerates Assembly of Amyloid-β-Peptides into Alzheimer’s Fibrils: Possible Role of the Peripheral Site of the Enzyme. Neuron 1996, 16, 881–891. [Google Scholar] [CrossRef]
- Lushchekina, S.V.; Kots, E.D.; Novichkova, D.A.; Petrov, K.A.; Masson, P. Role of Acetylcholinesterase in β-Amyloid Aggregation Studied by Accelerated Molecular Dynamics. Bionanoscience 2016, 7, 396–402. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Rudakova, E.V.; Boltneva, N.P.; Lushchekina, S.V.; Faingold, I.I.; Poletaeva, D.A.; Soldatova, Y.V.; Kotelnikova, R.A.; Serkov, I.V.; et al. New Multifunctional Agents Based on Conjugates of 4-Amino-2,3-polymethylenequinoline and Butylated Hydroxytoluene for Alzheimer’s Disease Treatment. Molecules 2020, 25, 5891. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Lushchekina, S.V.; Boltneva, N.P.; Serebryakova, O.G.; Rudakova, E.V.; Ustyugov, A.A.; Bachurin, S.O.; Shchepochkin, A.V.; Chupakhin, O.N.; Charushin, V.N.; et al. 9-Substituted acridine derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors possessing antioxidant activity for Alzheimer’s disease treatment. Bioorg. Med. Chem. 2017, 25, 5981–5994. [Google Scholar] [CrossRef] [PubMed]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Xiong, Z.M.; O’Donovan, M.; Sun, L.; Choi, J.Y.; Ren, M.; Cao, K. Anti-Aging Potentials of Methylene Blue for Human Skin Longevity. Sci. Rep. 2017, 7, 2475. [Google Scholar] [CrossRef]
- Sváb, G.; Kokas, M.; Sipos, I.; Ambrus, A.; Tretter, L. Methylene Blue Bridges the Inhibition and Produces Unusual Respiratory Changes in Complex III-Inhibited Mitochondria. Studies on Rats, Mice and Guinea Pigs. Antioxidants 2021, 10, 305. [Google Scholar] [CrossRef]
- Gureev, A.P.; Sadovnikova, I.S.; Popov, V.N. Molecular Mechanisms of the Neuroprotective Effect of Methylene Blue. Biochemistry (Moscow) 2022, 87, 940–956. [Google Scholar] [CrossRef] [PubMed]
- Duicu, O.M.; Privistirescu, A.; Wolf, A.; Petrus, A.; Danila, M.D.; Ratiu, C.D.; Muntean, D.M.; Sturza, A. Methylene blue improves mitochondrial respiration and decreases oxidative stress in a substrate-dependent manner in diabetic rat hearts. Can. J. Physiol. Pharmacol. 2017, 95, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.L.; Poteet, E.; Du, F.; Gourav, R.C.; Liu, R.; Wen, Y.; Bresnen, A.; Huang, S.; Fox, P.T.; Yang, S.H.; et al. Methylene blue as a cerebral metabolic and hemodynamic enhancer. PLoS ONE 2012, 7, e46585. [Google Scholar] [CrossRef] [PubMed]
- Brunden, K.R.; Lee, V.M.Y.; Smith, A.B.; Trojanowski, J.Q.; Ballatore, C. Altered microtubule dynamics in neurodegenerative disease: Therapeutic potential of microtubule-stabilizing drugs. Neurobiol. Dis. 2017, 105, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.; James, M.J.; Lee, V.M.; Smith, A.B., 3rd; Trojanowski, J.Q.; Ballatore, C.; Brunden, K.R. Aminothienopyridazines and methylene blue affect Tau fibrillization via cysteine oxidation. J. Biol. Chem. 2013, 288, 11024–11037. [Google Scholar] [CrossRef]
- Britto, P.J.; Knipling, L.; McPhie, P.; Wolff, J. Thiol–disulphide interchange in tubulin: Kinetics and the effect on polymerization. Biochem. J. 2005, 389, 549–558. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Sterri, S.H.; Johnsen, B.A.; Fonnum, F. A radiochemical assay method for carboxylesterase, and comparison of enzyme activity towards the substrates methyl [1-14C] butyrate and 4-nitrophenyl butyrate. Biochem. Pharmacol. 1985, 34, 2779–2785. [Google Scholar] [CrossRef]
- Taylor, P.; Lappi, S. Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry 1975, 14, 1989–1997. [Google Scholar] [CrossRef]
- Taylor, P.W.; Lwebuga-Mukusa, J.; Lappi, S.; Rademacher, J. Propidium—a Fluorescence Probe for a Peripheral Anionic Site on Acetylcholinesterase. Mol. Pharmacol. 1974, 10, 703–708. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Callaway, J.K.; Beart, P.M.; Jarrott, B. A reliable procedure for comparison of antioxidants in rat brain homogenates. J. Pharmacol. Toxicol. Methods 1998, 39, 155–162. [Google Scholar] [CrossRef]
- Iglesias-Gonzalez, J.; Sanchez-Iglesias, S.; Beiras-Iglesias, A.; Soto-Otero, R.; Mendez-Alvarez, E. A simple method for isolating rat brain mitochondria with high metabolic activity: Effects of EDTA and EGTA. J. Neurosci. Methods 2013, 213, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Shevtsova, E.; Vinogradova, D.; Kireeva, E.; Reddy, V.; Aliev, G.; Bachurin, S. Dimebon Attenuates the Aβ-Induced Mitochondrial Permeabilization. Curr. Alzheimer Res. 2014, 11, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Åkerman, K.E.O.; Wikström, M.K.F. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976, 68, 191–197. [Google Scholar] [CrossRef]
- Rogers, G.W.; Brand, M.D.; Petrosyan, S.; Ashok, D.; Elorza, A.A.; Ferrick, D.A.; Murphy, A.N. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS ONE 2011, 6, e21746. [Google Scholar] [CrossRef]
- Marty, M.S.; Atchison, W.D. Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methyl mercury. Toxicol. Appl. Pharmacol. 1997, 147, 319–330. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.J.; et al. General Atomic and Molecular Electronic-Structure System. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Voevodin, V.; Antonov, A.; Nikitenko, D.; Shvets, P.; Sobolev, S.; Sidorov, I.; Stefanov, K.; Voevodin, V.; Zhumatiy, S. Supercomputer Lomonosov-2: Large scale, deep monitoring and fine analytics for the user community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).