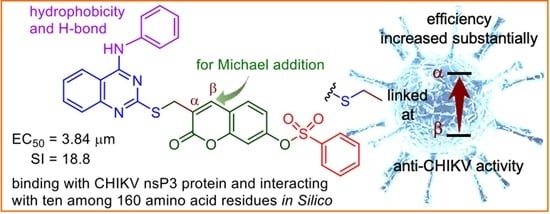
Computer-Aided Design and Synthesis of (Functionalized quinazoline)–(α-substituted coumarin)–arylsulfonate Conjugates against Chikungunya Virus
Abstract
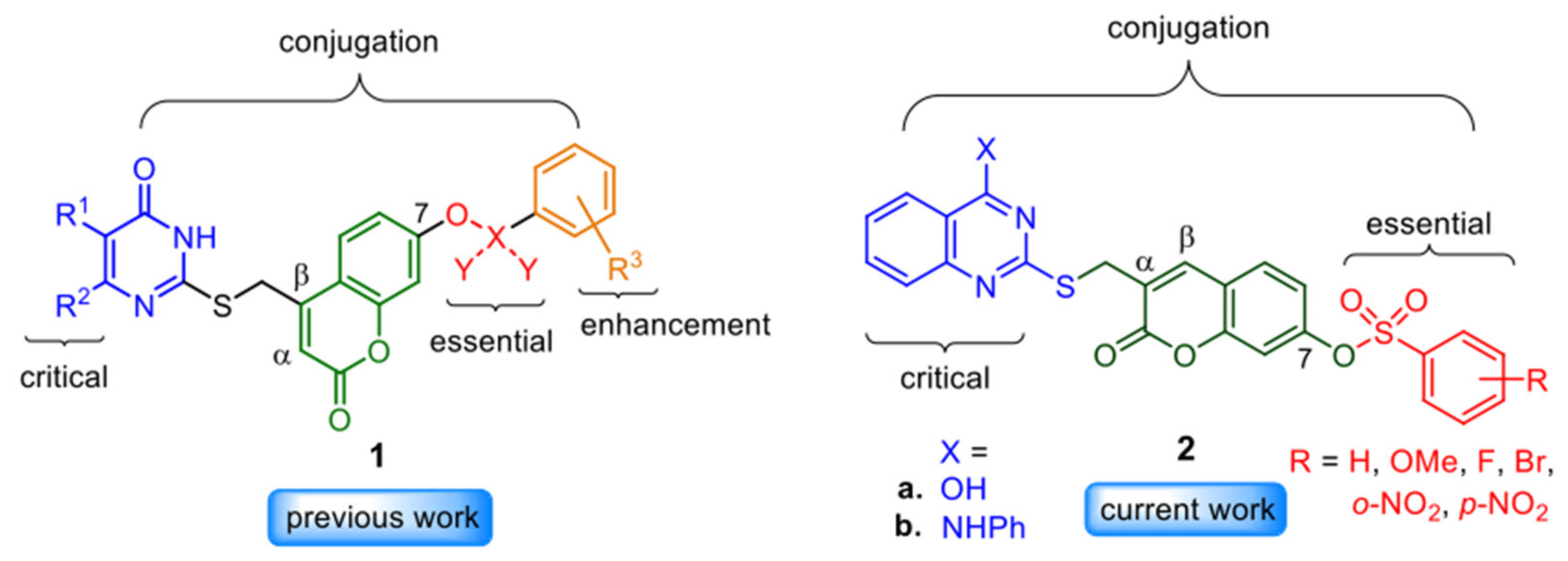
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Quinazolinone-(α-substituted coumarin)-sulfonate Conjugates and Structure Identification
2.2. Anti-CHIKV Activity of New Triple Conjugates
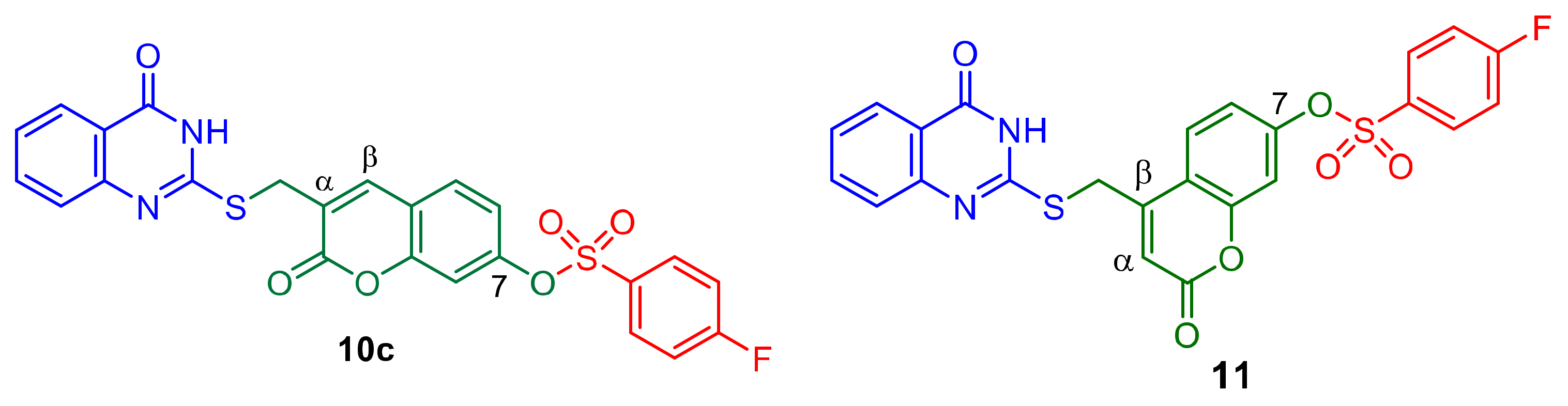
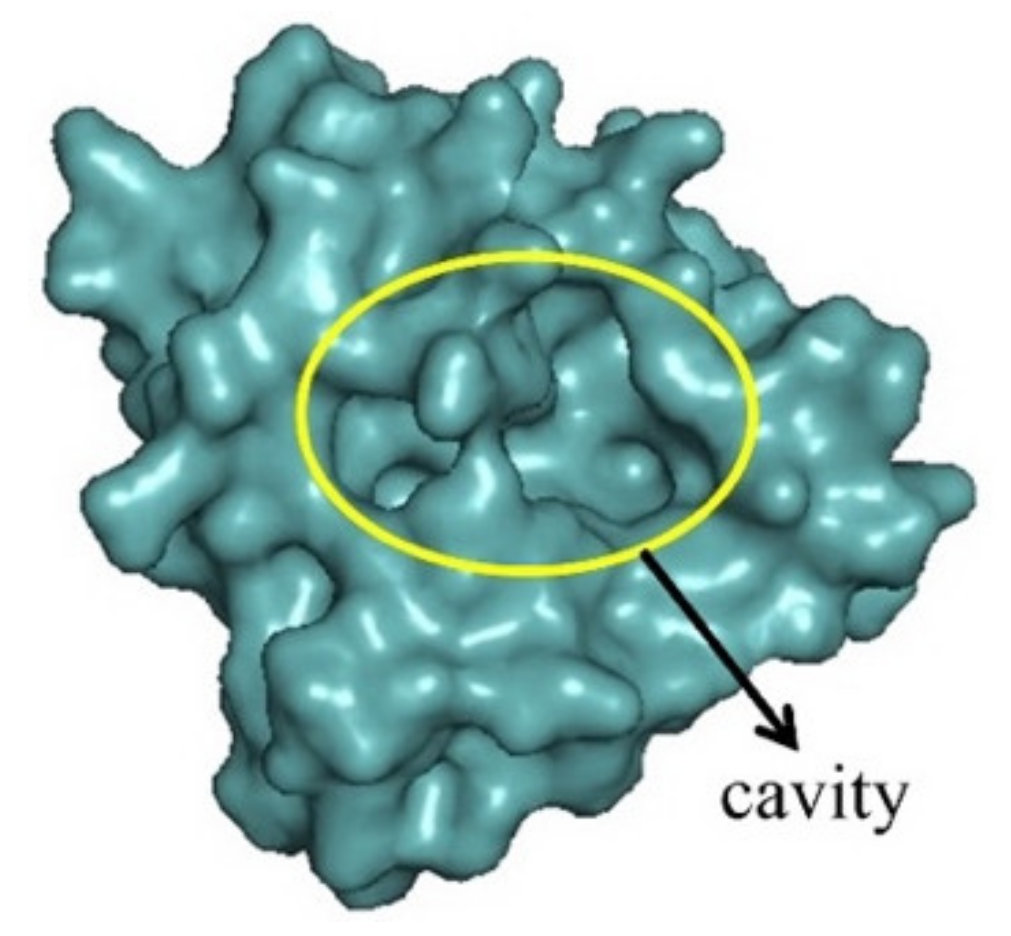
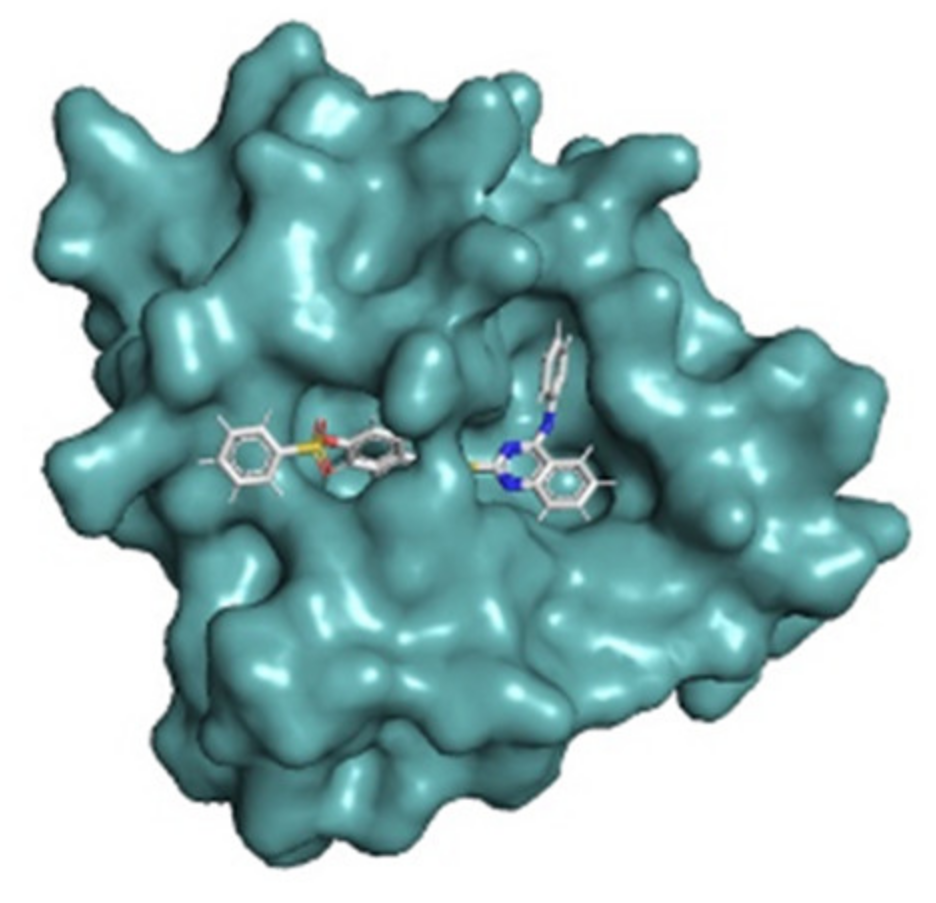
2.3. Design with the Aid of Molecular Docking Computations of a Conjugate with Improved Anti-CHIKV Activity
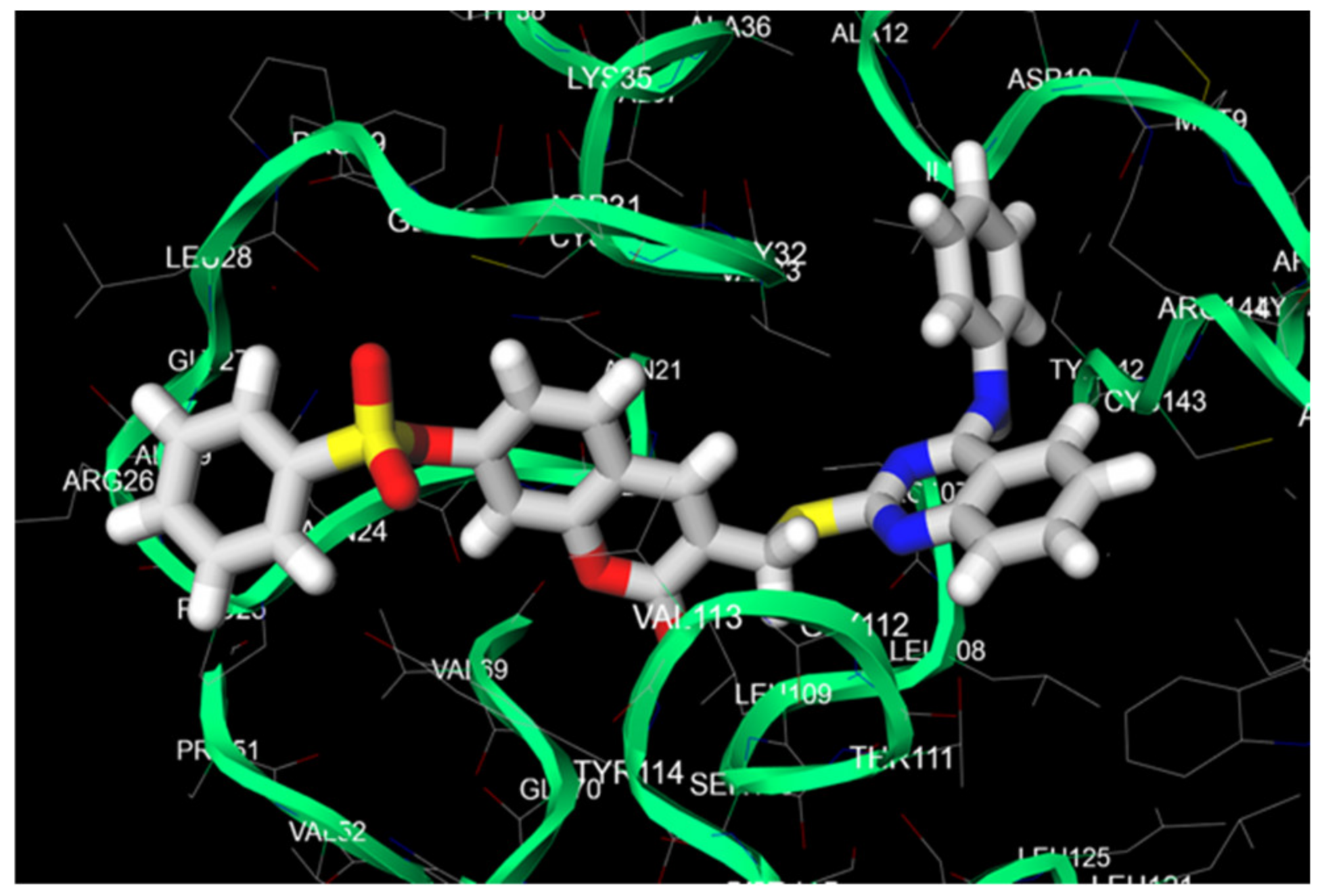
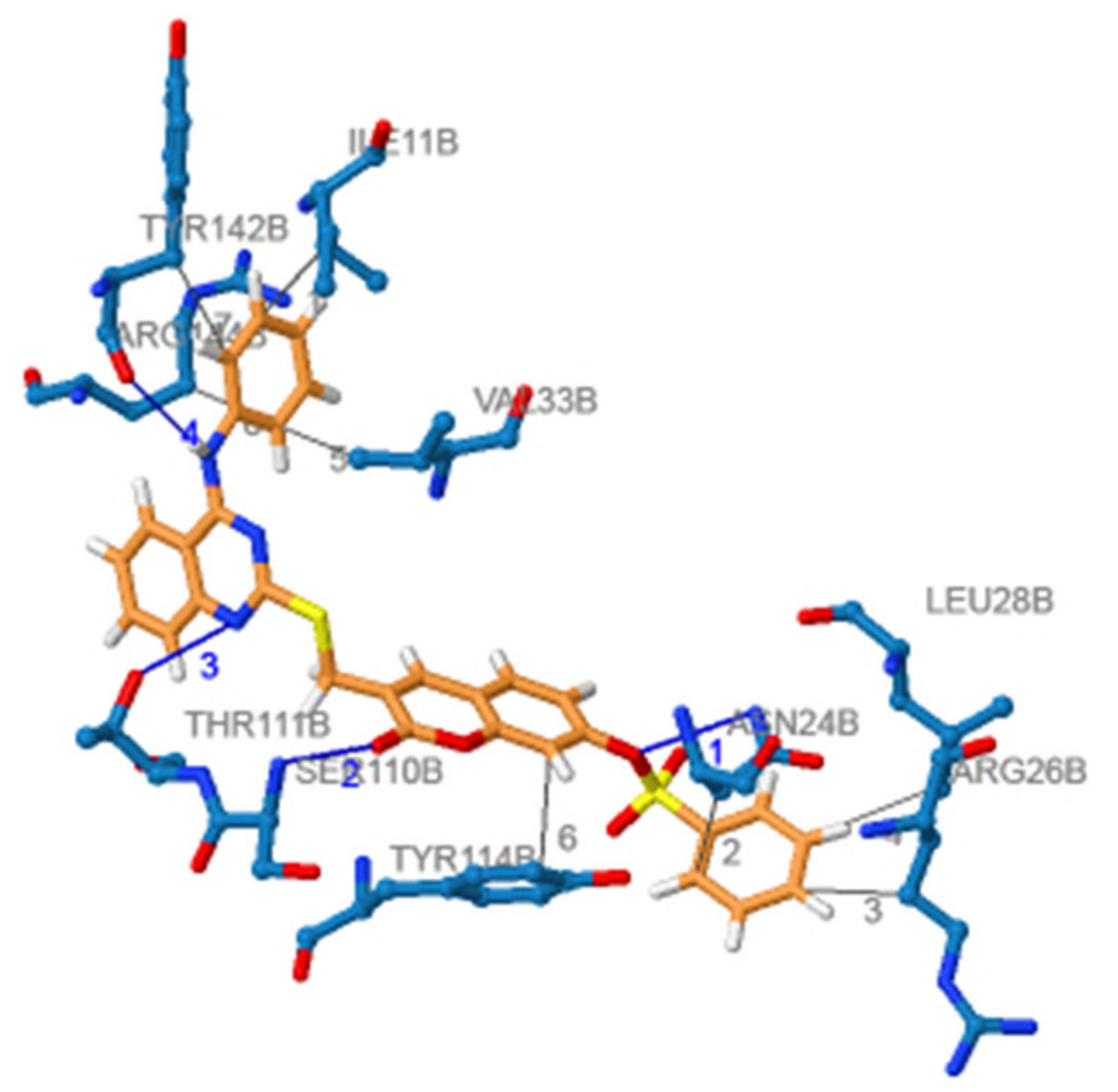
2.4. Molecular Docking Studies and Protein-Substrate Interactions
2.5. Synthesis of 4-Anilinoquinazoline-coumarin-arylsulfonate Conjugate and Its Anti-CHIKV Activity
2.6. Lipophilicity
3. Materials and Methods
3.1. General Information
3.2. Preparation of Coumarin Sulfonate Intermediates 7 (Procedure 1)
3.2.1. 3-Methyl-2-oxo-2H-chromen-7-yl Benzenesulfonate (7a)
3.2.2. 3-Methyl-2-oxo-2H-chromen-7-yl 4-Methoxybenzenesulfonate (7b)
3.2.3. 3-Methyl-2-oxo-2H-chromen-7-yl 4-Fluorobenzenesulfonate (7c)
3.2.4. 3-Methyl-2-oxo-2H-chromen-7-yl 4-Bromobenzenesulfonate (7d)
3.2.5. 3-Methyl-2-oxo-2H-chromen-7-yl 2-Nitrobenzenesulfonate (7e)
3.2.6. 3-Methyl-2-oxo-2H-chromen-7-yl 4-Nitrobenzenesulfonate (7f)
3.3. Preparation of Bromomethyl Coumarin Intermediates 8 (Procedure 2)
3.3.1. 3-Bromomethyl-2-oxo-2H-chromen-7-yl Benzenesulfonate (8a)
3.3.2. 3-Bromomethyl-2-oxo-2H-chromen-7-yl 4-Methoxybenzenesulfonate (8b)
3.3.3. 3-Bromomethyl-2-oxo-2H-chromen-7-yl 4-Fluorobenzenesulfonate (8c)
3.3.4. 3-Bromomethyl-2-oxo-2H-chromen-7-yl 4-Bromobenzenesulfonate (8d)
3.3.5. 3-Bromomethyl-2-oxo-2H-chromen-7-yl 2-Nitrobenzenesulfonate (8e)
3.3.6. 3-Bromomethyl-2-oxo-2H-chromen-7-yl 4-Nitrobenzenesulfonate (8f)
3.4. Preparation of Conjugated Compounds 10 and 2b (Procedure 3)
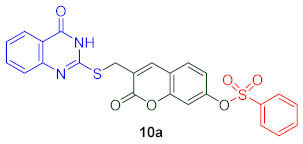
3.4.1. 2′-Oxo-3′-([(4-oxo-3,4-dihydroquinazolin-2-yl)thio]methyl)-2H-chromen-7′-yl Benzenesulfonate (10a)
3.4.2. 2′-Oxo-3′-([(4-oxo-3,4-dihydroquinazolin-2-yl)thio]methyl)-2H-chromen-7′-yl 4″-Methoxybenzenesulfonate (10b)
3.4.3. 2′-Oxo-3′-([(4-oxo-3,4-dihydroquinazolin-2-yl)thio]methyl)-2H-chromen-7′-yl 4″-Fluorobenzenesulfonate (10c)
3.4.4. 2′-Oxo-3′-([(4-oxo-3,4-dihydroquinazolin-2-yl)thio]methyl)-2H-chromen-7′-yl 4″-Bromobenzenesulfonate (10d)
3.4.5. 2′-Oxo-3′-([(4-oxo-3,4-dihydroquinazolin-2-yl)thio]methyl)-2H-chromen-7′-yl 2″-Nitrobenzenesulfonate (10e)
3.4.6. 2′-Oxo-3′-([(4-oxo-3,4-dihydroquinazolin-2-yl)thio]methyl)-2H-chromen-7′-yl 4″-Nitrobenzenesulfonate (10f)
3.4.7. 2′-Oxo-3′-[([4-(phenylamino) quinazolin-2-yl]thio)methyl]-2H-chromen-7′-yl Benzenesulfonate (2b)
3.5. Biological Assays
3.5.1. Anti-CHIKV Activity Assay
3.5.2. Cytotoxicity Assay
3.6. Molecular Docking Studies and Protein-Ligand Interactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassetto, M.; De Burghgraeve, T.; Delang, L.; Massarotti, A.; Coluccia, A.; Zonta, N.; Gatti, V.; Colombano, G.; Sorba, G.; Silvestri, R.; et al. Computer-aided Identification, Design and Synthesis of a Novel Series of Compounds with Selective Antiviral Activity against Chikungunya Virus. Antivir. Res. 2013, 98, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Gudo, E.S.; Black, J.F.; Cliff, J.L. Chikungunya in Mozambique: A Forgotten History. PLoS Negl. Trop. Dis. 2016, 10, e0005001. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, A.; Cella, E.; Angeletti, S.; Ciccozzi, M. Molecular Epidemiology, Evolution and Phylogeny of Chikungunya Virus: An Updating Review. Infect. Genet. Evol. 2016, 41, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention Website. Available online: https://www.cdc.gov/chikungunya/geo/index.html (accessed on 15 December 2020).
- Hwu, J.R.; Pradhan, T.K.; Tsay, S.-C.; Kapoor, M.; Bachurin, S.O.; Raevsky, O.A.; Neyts, J. Antiviral agents towards chikungunya virus: Structures, syntheses and isolation from natural sources. In New Horizons of Process Chemistry: Scalable Reactions and Technologies; Tomioka, K., Shiori, T., Sajiki, H., Eds.; Springer Nature: Singapore, 2017; pp. 251–274. ISBN 978-981-10-9869-7. [Google Scholar]
- Kaur, P.; Thiruchelvan, M.; Lee, R.C.H.; Chen, H.; Chen, K.C.; Ng, M.L.; Chu, J.J.H. Inhibition of Chikungunya Virus Replication by Harringtonine, a Novel Antiviral that Suppresses Viral Protein Expression. Antimicrob. Agents Chemother. 2013, 57, 155–167. [Google Scholar] [CrossRef] [Green Version]
- Pohjala, L.; Utt, A.; Varjak, M.; Lulla, A.; Merits, A.; Ahola, T.; Tammela, P. Inhibitors of Alphavirus Entry and Replication Identified with a Stable Chikungunya Replicon Cell Line and Virus-Based Assays. PLoS ONE 2011, 6, e28923. [Google Scholar] [CrossRef]
- Delogu, I.; Pastorino, B.; Baronti, C.; Nougairède, A.; Bonnet, E.; Lamballerie, X.D. In Vitro Antiviral Activity of Arbidol against Chikungunya Virus and Characteristics of a Selected Resistant Mutant. Antivir. Res. 2011, 90, 99–107. [Google Scholar] [CrossRef]
- Hwu, J.R.; Gupta, N.K.; Tsay, S.-C.; Huang, W.-C.; Albulescu, I.C.; Kovacikova, K.; van Hemert, M.J. Bis(benzofuran-thiazolidinone)s and Bis(benzofuran-thiazinanone)s as Inhibiting Agents for Chikungunya Virus. Antivir. Res. 2017, 146, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Ozden, S.; Lucas-Hourani, M.; Ceccaldi, P.E.; Basak, A.; Valentine, M.; Benjannet, S.; Hamelin, J.; Jacob, Y.; Mamchaoui, K.; Mouly, V.; et al. Inhibition of Chikungunya Virus Infection in Cultured Human Muscle Cells by Furin Inhibitors: Impairment of the Maturation of the E2 Surface Glycoprotein. J. Biol. Chem. 2008, 283, 21899–21908. [Google Scholar] [CrossRef] [Green Version]
- Hwu, J.R.; Kapoor, M.; Tsay, S.-C.; Lin, C.-C.; Hwang, K.C.; Horng, J.-C.; Chen, I.-C.; Shieh, F.-K.; Leyssen, P.; Neyts, J. Benzouracil-coumarin-arene Conjugates as Inhibiting Agents for Chikungunya Virus. Antivir. Res. 2015, 118, 103–109. [Google Scholar] [CrossRef]
- Hwu, J.R.; Huang, W.-C.; Lin, S.-Y.; Tan, K.-T.; Hu, Y.-C.; Shieh, F.-K.; Bachurin, S.O.; Ustyugov, A.; Tsay, S.-C. Chikungunya Virus Inhibition by Synthetic Coumarin-Guanosine Conjugates. Eur. J. Med. Chem. 2019, 166, 136–143. [Google Scholar] [CrossRef]
- Khan, M.; Dhanwani, R.; Patro, I.K.; Rao, P.V.L.; Parida, M.M. Cellular IMPDH Enzyme Activity is a Potential Target for the Inhibition of Chikungunya Virus Replication and Virus Induced Apoptosis in Cultured Mammalian Cells. Antivir. Res. 2011, 89, 1–8. [Google Scholar] [CrossRef] [PubMed]
- D’hooghe, M.; Mollet, K.; Vreese, R.D.; Jonckers, T.H.M.; Dams, G.; Kimpe, N.D. Design, Synthesis, and Antiviral Evaluation of Purine-β-lactam and Purine-Aminopropanol Hybrids. J. Med. Chem. 2012, 55, 5637–5641. [Google Scholar] [CrossRef] [PubMed]
- Briolant, S.; Garin, D.; Scaramozzino, N.; Jouan, A.; Crance, J.M. In Vitro Inhibition of Chikungunya and Semliki Forest Viruses Replication by Antiviral Compounds: Synergistic Effect of Interferon-Alpha and Ribavirin Combination. Antivir. Res. 2004, 61, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Albulescu, I.C.; van Hoolwerff, M.; Wolters, L.A.; Bottaro, E.; Nastruzzi, C.; Yang, S.C.; Tsay, S.-C.; Hwu, J.R.; Snijder, E.J.; van Hemert, M.J. Suramin Inhibits Chikungunya Virus Replication Through Multiple Mechanisms. Antivir. Res. 2015, 121, 39–46. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.-J.; Delang, L.; Ng, L.F.P.; Priego, E.-M. Chikungunya Virus Drug Discovery: Still a Long Way to Go? Expert Opin. Drug Discov. 2019, 14, 855–866. [Google Scholar] [CrossRef]
- Tsay, S.-C.; Lin, S.-Y.; Huang, W.-C.; Hsu, M.-H.; Hwang, K.C.; Lin, C.-C.; Horng, J.-C.; Chen, I.-C.; Hwu, J.R.; Shieh, F.-K. Synthesis and Structure-Activity Relationships of Imidazole-Coumarin Conjugates against Hepatitis C Virus. Molecules 2016, 21, 228. [Google Scholar] [CrossRef] [Green Version]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Bonazzi, S.; Eidam, O.; Guettinger, S.; Wach, J.-Y.; Zemp, I.; Kutay, U.; Gademann, K. Anguinomycins and Derivatives: Total Syntheses, Modelling, and Biological Evaluation of the Inhibition of Nucleocytoplasmic Transport. J. Am. Chem. Soc. 2010, 132, 1432–1442. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishitaq, M.; Khan, K.M. Quinazoline and Quinazolinone as Important Medicinal Scaffolds: A Comparative Patent Review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef]
- Auti, P.S.; George, G.; Paul, A.T. Recent Advances in the Pharmacological Diversification of Quinazoline/quinazolinone Hybrids. RSC Adv. 2020, 10, 41353–41392. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, L.; Gao, H.; Wu, Y.; Wang, Y.; Fang, F.; Lan, T.; Lou, Z.; Rao, Y. Discovery, Optimization and Target Identification of Novel Potent Broad-Spectrum Antiviral Inhibitors. J. Med. Chem. 2019, 62, 4056–4073. [Google Scholar] [CrossRef] [PubMed]
- Persoons, L.; Vanderlinden, E.; Vangeel, L.; Wang, X.; Do, N.D.T.; Foo, S.-Y.C.; Leyssen, P.; Neyts, J.; Jochmans, D.; Schols, D.; et al. Broad Spectrum Anti-Coronavirus Activity of a Series of Anti-Malaria Quinoline Analogues. Antivir. Res. 2021, 193, 105127. [Google Scholar] [CrossRef] [PubMed]
- Saul, S.; Huang, P.-T.; Einav, S.; Asquith, C.R.M. Identification and Evaluation of 4-Anilinoquin(az)olines as Potent Inhibitors of Both Dengue Virus (DENV) and Venezuelan Equine Encephalitis Virus (VEEV). Bioorg. Med. Chem. Lett. 2021, 52, 128407. [Google Scholar] [CrossRef] [PubMed]
- Saul, S.; Pu, S.-Y.; Zuercher, W.J.; Einav, S.; Asquith, C.R.M. Potent Antiviral Activity of Novel Multi-substituted 4-Anilinoquin(az)olines. Bioorg. Med. Chem. Lett. 2020, 30, 127284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-Y.; Patel, S.J.; Vangrevelinghe, E.; Xu, H.Y.; Rao, R.; Jaber, D.; Schul, W.; Gu, F.; Heudi, O.; Ma, N.L.; et al. A Small-Molecule Dengue Virus Entry Inhibitor. Antimicrob. Agents Chemother. 2009, 53, 1823–1831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavrini, F.; Gaibani, P.; Pierro, A.M.; Rossini, G.; Landini, M.P.; Sambri, V. Chikungunya: An Emerging and Spreading Arthropod-Borne Viral Disease. J. Infect. Dev. Ctries. 2009, 3, 744–752. [Google Scholar] [CrossRef] [Green Version]
- Ahola, T.; Merits, A. Functions of chikungunya virus nonstructural proteins. In Chikungunya Virus: Advances in Biology, Pathogenesis and Treatment; Okeoma, C., Ed.; Springer: Cham, Switzerland, 2016; pp. 75–98. ISBN 978-3-319-82704-9. [Google Scholar]
- Kovacikova, K.; van Hemert, M.J. Small-molecule Inhibitors of Chikungunya Virus: Mechanisms of Action and Antiviral Drug Resistance. Antimicrob. Agents Chemother. 2020, 64, e01788-20. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Shukri, M.; Hassandarvish, P.; Oo, A.; Shankar, E.M.; Abubakar, S.; Zandi, K. Computational Approach Towards Exploring Potential Anti-Chikungunya Activity of Selected Flavonoids. Sci. Rep. 2016, 6, 24027. [Google Scholar] [CrossRef]
- McPherson, R.L.; Abraham, R.; Sreekumar, E.; Ong, S.-E.; Cheng, S.-J.; Baxter, V.K.; Kistemaker, H.A.V.; Filippov, D.V.; Griffin, D.E.; Leung, A.K.L. ADP-ribosylhydrolase Activity of Chikungunya Virus Macro Domain is Critical for Virus Replication and Virulence. Proc. Natl. Acad. Sci. USA 2017, 114, 1666–1671. [Google Scholar] [CrossRef] [Green Version]
- Malet, H.; Coutard, B.; Jamal, S.; Dutartre, H.; Papageorgiou, N.; Neuvonen, M.; Ahola, T.; Forrester, N.; Gould, E.A.; Lafitte, D.; et al. The Crystal Structures of Chikungunya and Venezuelan Equine Encephalitis Virus Nsp3 Macro Domains Define a Conserved Adenosine Binding Pocket. J. Virol. 2009, 83, 6534–6545. [Google Scholar] [CrossRef] [Green Version]
- Leonetti, F.; Favia, A.; Rao, A.; Aliano, R.; Paluszcak, A.; Hartmann, R.W.; Carotti, A. Design, Synthesis, and 3D QSAR of Novel Potent and Selective Aromatase Inhibitors. J. Med. Chem. 2004, 47, 6792–6803. [Google Scholar] [CrossRef] [PubMed]
- Incremona, J.H.; Martin, J.C. N-Bromosuccinimide. Mechanisms of Allylic Bromination and Related Reactions. J. Am. Chem. Soc. 1970, 92, 627–634. [Google Scholar] [CrossRef]
- Begum, S.; Subramanian, R. Reaction of Chlorine Radical with Tetrahydrofuran: A Theoretical Investigation on Mechanism and Reactivity in Gas Phase. J. Mol. Model. 2014, 20, 2262. [Google Scholar] [CrossRef] [PubMed]
- López-Castillo, N.N.; Rojas-Rodríguez, A.D.; Porta, B.M.; Cruz-Gómez, M.J. Process for the Obtention of Coumaric Acid from Coumarin: Analysis of the Reaction Conditions. Adv. Chem. Eng. Sci. 2013, 3, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Somers, F.; Ouedraogo, R.; Antoine, M.H.; Tullio, P.D.; Becker, B.; Fontaine, J.; Damas, J.; Dupont, L.; Rigo, B.; Delarge, J.; et al. Original 2-Alkylamino-6-halogenoquinazolin-4(3H)-ones and KATP Channel Activity. J. Med. Chem. 2001, 44, 2575–2585. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Kapoor, M.; Gupta, N.K.; Tsay, S.-C.; Huang, W.-C.; Tan, K.-T.; Hu, Y.-C.; Lyssen, P.; Neyts, J. Synthesis and Antiviral Activities of Quinazolinamine-coumarin Conjugates Toward Chikungunya and Hepatitis C Viruses. Eur. J. Med. Chem. 2022, 232, 114164. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.H. Reversible Michael Additions: Covalent Inhibitors and Prodrugs. Mini Rev. Med. Chem. 2012, 12, 1330–1344. [Google Scholar]
- Lengauer, T.; Rarey, M. Computational Methods for Biomolecular Docking. Curr. Opin. Struct. Biol. 1996, 6, 402–406. [Google Scholar] [CrossRef]
- Kitchen, D.B.; Decornez, H.; Furr, J.R.; Bajorath, J. Docking and Scoring in Virtual Screening for Drug Discovery: Methods and Applications. Nat. Rev. Drug Discov. 2004, 3, 935–949. [Google Scholar] [CrossRef]
- Sasaki-Tanaka, R.; Venkata, K.C.N.; Okamoto, H.; Moriyama, M.; Kanda, T. Evaluation of Potential Anti-Hepatitis a Virus 3C Protease Inhibitors Using Molecular Docking. Int. J. Mol. Sci. 2022, 23, 6044–6056. [Google Scholar] [CrossRef]
- Eckei, L.; Krieg, S.; Bütepage, M.; Lehmann, A.; Gross, A.; Lippok, B.; Grimm, A.R.; Kümmerer, B.M.; Rossetti, G.; Lüscher, B.; et al. The Conserved Macrodomains of the Non-Structural Proteins of Chikungunya Virus and Other Pathogenic Positive Strand RNA Viruses Function as Mono-ADP-ribosylhydrolases. Sci. Rep. 2017, 7, 41746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, D.; Meena, M.K.; Kumari, K.; Patel, R.; Jayaraj, A.; Singh, P. In-silico Prediction of Novel Drug-Target Complex of Nsp3 of CHIKV Through Molecular Dynamic Simulation. Heliyon 2020, 6, e04720. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Matsumori, N.; Taoka, H.; Fujiwara, D.; Schreiner, E.P.; Wolff, B.; Yoshida, M.; Horinouchi, S. Leptomycin B Inactivates CRM1/Exportin 1 by Covalent Modification at a Cysteine Residue in the Central Conserved Region. Proc. Natl. Acad. Sci. USA 1999, 96, 9112–9117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Carrasco, Y.P.; Hu, Y.; Guo, X.; Mirzaei, H.; MacMillan, J.; Chook, Y.M. Nuclear Export Inhibition Through Covalent Conjugation and Hydrolysis of Leptomycin B by CRM1. Proc. Natl. Acad. Sci. USA 2013, 110, 1303–1308. [Google Scholar] [CrossRef] [Green Version]
- Ando, R.; Amano, Y.; Nakamura, H.; Arai, N.; Kuwajima, I. Design, Synthesis, and Evaluation of Novel Kazusamycin A Derivatives as Potent Antitumor Agents. Bioorg. Med. Chem. Lett. 2006, 16, 3315–3318. [Google Scholar] [CrossRef]
- Atanassov, P.K.; Linden, A.; Heimgartner, H. Synthesis of 4-(Phenylamino)quinazoline-2(1H)-selones and Diselenides from Isoselenocyanates: Dimroth Rearrangement of an Intermediate. Helv. Chim. Acta 2004, 87, 1873–1887. [Google Scholar] [CrossRef]
- Kraszni, M.; Banyai, I.; Noszal, B. Determination of Conformer-specific Partition Coefficients in Octanol/water Systems. J. Med. Chem. 2003, 46, 2241–2245. [Google Scholar] [CrossRef]
- Hann, M.M.; Keserü, G.M. Finding the Sweet Spot: The Role of Nature and Nurture in Medicinal Chemistry. Nat. Rev. 2012, 11, 355–365. [Google Scholar] [CrossRef]



| Compound | CC50 1 (μM) | EC50 2 (μM) | SI 3 | log p |
|---|---|---|---|---|
| 10a | 40.1 | 7.84 | 5.11 | 3.52 |
| 10b | - | >186 | - | 3.45 |
| 10c | 59.7 | 6.46 | 9.24 | 3.68 |
| 10d | 62.6 | 18.4 | 3.40 | 3.94 |
| 10e | 64.3 | 12.7 | 5.06 | 3.48 |
| 10f | - | >186 | - | 3.43 |
| 11 | 30.0 | 18.4 | 1.63 | 3.77 |
| Item | Amino Acid Residue | Moiety of Conjugate 2b | Distance of Hydrophobic Interaction (Å) |
|---|---|---|---|
| 1 | Ile11 | 4-anilinoquinazoline | 3.74 |
| 2 | Asn24 | benzenesulfonate | 3.71 |
| 3 | Arg26 | benzenesulfonate | 3.80 |
| 4 | Leu28 | benzenesulfonate | 3.61 |
| 5 | Val33 | 4-anilinoquinazoline | 3.40 |
| 6 | Tyr114 | coumarin | 3.61 |
| 7 | Tyr142 | 4-anilinoquinazoline | 3.89 |
| 8 | Arg144 | 4-anilinoquinazoline | 3.67 |
| Item | Amino Acid Residue | Moiety of Conjugate 2b | Distance of H-Residue (Å) | H-Donor |
|---|---|---|---|---|
| 1 | Asn24 | benzenesulfonate | 2.76 | nsP3 |
| 2 | Ser110 | coumarin | 2.35 | nsP3 |
| 3 | Thr111 | 4-anilinoquinazoline | 2.58 | nsP3 |
| 4 | Tyr142 | 4-anilinoquinazoline | 2.69 | 2b |
| Compound | CC50 1 (μM) | EC50 2 (μM) | SI 3 | log p |
|---|---|---|---|---|
 | 40.1 | 7.84 | 5.11 | 3.52 |
 | 72.3 | 3.84 | 18.8 | 5.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwu, J.R.; Roy, A.; Tsay, S.-C.; Huang, W.-C.; Lin, C.-C.; Hwang, K.C.; Hu, Y.-C.; Shieh, F.-K.; Leyssen, P.; Neyts, J. Computer-Aided Design and Synthesis of (Functionalized quinazoline)–(α-substituted coumarin)–arylsulfonate Conjugates against Chikungunya Virus. Int. J. Mol. Sci. 2022, 23, 7646. https://doi.org/10.3390/ijms23147646
Hwu JR, Roy A, Tsay S-C, Huang W-C, Lin C-C, Hwang KC, Hu Y-C, Shieh F-K, Leyssen P, Neyts J. Computer-Aided Design and Synthesis of (Functionalized quinazoline)–(α-substituted coumarin)–arylsulfonate Conjugates against Chikungunya Virus. International Journal of Molecular Sciences. 2022; 23(14):7646. https://doi.org/10.3390/ijms23147646
Chicago/Turabian StyleHwu, Jih Ru, Animesh Roy, Shwu-Chen Tsay, Wen-Chieh Huang, Chun-Cheng Lin, Kuo Chu Hwang, Yu-Chen Hu, Fa-Kuen Shieh, Pieter Leyssen, and Johan Neyts. 2022. "Computer-Aided Design and Synthesis of (Functionalized quinazoline)–(α-substituted coumarin)–arylsulfonate Conjugates against Chikungunya Virus" International Journal of Molecular Sciences 23, no. 14: 7646. https://doi.org/10.3390/ijms23147646
APA StyleHwu, J. R., Roy, A., Tsay, S.-C., Huang, W.-C., Lin, C.-C., Hwang, K. C., Hu, Y.-C., Shieh, F.-K., Leyssen, P., & Neyts, J. (2022). Computer-Aided Design and Synthesis of (Functionalized quinazoline)–(α-substituted coumarin)–arylsulfonate Conjugates against Chikungunya Virus. International Journal of Molecular Sciences, 23(14), 7646. https://doi.org/10.3390/ijms23147646