- Article
Insights into Neutral vs. Deprotonated Phenol Adsorption on Graphene Oxide
- Jeton Halili,
- Kledi Xhaxhiu and
- Avni Berisha
- + 4 authors
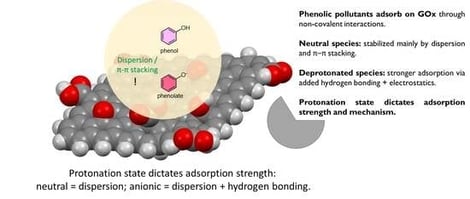
Water pollution from phenols remains a critical concern due to their persistence, toxicity, and industrial prevalence. Graphene oxide (GOx), with its functional groups and large surface area, offers strong adsorption potential. Using density functional theory (DFT), reduced density gradient (RDG), and quantitative structure–activity relationship (QSAR), we examined how protonation and substituents influence phenol adsorption. Deprotonated phenolates bind more strongly to GO than neutral species via electrostatics and H-bonding. Substituents alter affinity: halogens enhance it, bulky alkyls hinder it, and nitro groups show electron-withdrawing effects. Bisphenolate A displayed multidentate binding. QSAR models reproduced DFT energies with R2 > 0.99, enabling fast prediction. These results highlight how pH speciation and substituents govern adsorption on GO, guiding the design of efficient water treatment materials.
6 February 2026



![Electronic energy-resolved dynamic PDF of beryllium [46]. Features below 1 Å are due to self-part (single particle excitations). The negative part below 2 Å signifies the exchange-correlation hole, which is extended at the plasmon energy of 21 eV.](https://mdpi-res.com/cdn-cgi/image/w=281,h=192/https://mdpi-res.com/condensedmatter/condensedmatter-11-00004/article_deploy/html/images/condensedmatter-11-00004-g001-550.jpg)