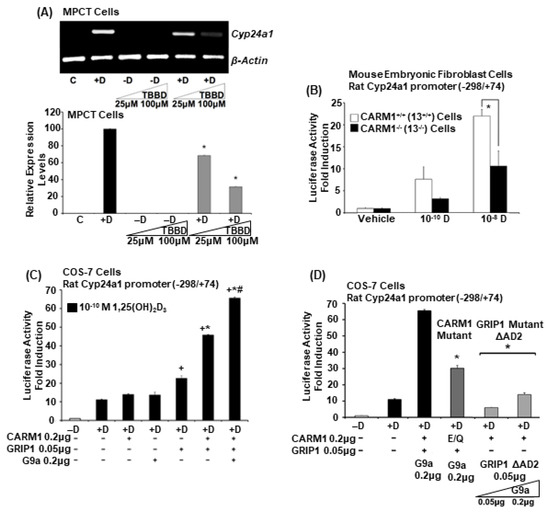
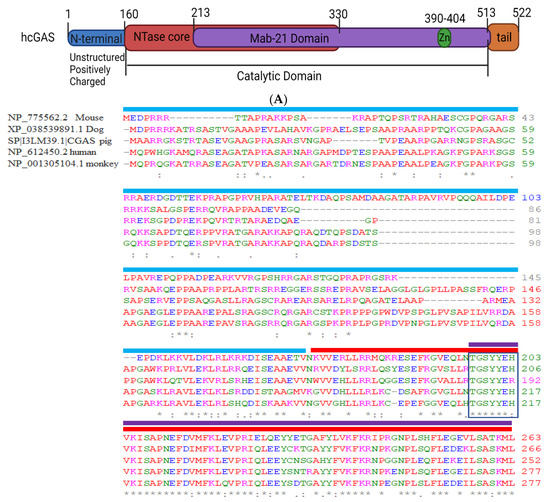
Cell Biology in the United States: Latest Advances and Perspectives
A topical collection in Cells (ISSN 2073-4409).
Viewed by 19254Editor
Interests: TRPC channels; voltage-gated Ca2+ channels; Ca2+ influx; endothelial dysfunction; atherosclerosis
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
This Topic Collection aims to showcase the latest scientific advances in cell biology achieved in the United States. The scope of this Topic Collection is very broad, ranging from cell division regulation and subcellular organelle function to the mechanisms of membrane transport and cell motility. Original research articles, comprehensive reviews and perspectives will be considered. Manuscripts should describe the novel molecular and cellular mechanisms underlying the physiologically relevant phenomena or disease-related cellular processes in all disciplines, such as neuroscience, physiology, immunology, cancer cell biology, developmental biology and beyond. Manuscripts describing organoid research and cellular responses to viral infections, such as SARS-CoV-2 or HIV infection, will also be considered. Potential topics may include, but are not limited to, the following research areas:
- Cancer cell biology and cancer stem cells.
- CAR T-cell and other adoptive cell transfer therapies.
- Cell cycle.
- Cell migration.
- Cell-to-cell communication and cell adhesion.
- Cell growth, differentiation, aging and death.
- Cellular metabolism.
- Cellular mechanosensory elements.
- Cellular mechanisms underlying human diseases.
- Cellular photosensory elements and underlying mechanisms.
- Cellular quality control.
- Cytoskeletal dynamics.
- DNA replication and repair; non-coding RNAs.
- Hematopoiesis and stem cells.
- Ion channel function in health and disease (transient receptor potential channels, store-operated channels, ligand-gated ion channels, second-messenger-gated channels and voltage-gated ion channels).
- Ion channel biophysics.
- Membrane physiology and membrane transport.
- Cellular organelles (mitochondria, lysosomes, peroxisomes, etc.).
- Omics: transcriptomics, genomics, proteomics, metabolomics, glycomics, lipidomics, interactomics, fluxomics and biomics.
- Protein synthesis and trafficking.
- Signal transduction.
Dr. Alexander G. Obukhov
Collection Editor
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cells is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2700 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.