- Review
In Situ and Operando Monitoring Techniques for Carbon- and Silicon-Based Anodes in Lithium-Ion Batteries: A Review
- Mingjie Wang,
- Siqing Chen and
- Yifei Yu
- + 5 authors
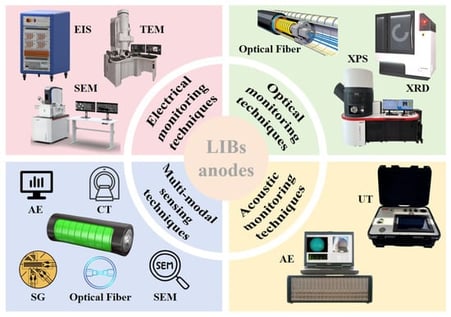
Lithium-ion batteries (LIBs) power devices from portable electronics to electric vehicles and grid storage, yet their reliable operation requires real-time monitoring of battery state, particularly at the anode where complex reactions and structural changes occur. Sensor technologies capable of capturing dynamic physical and chemical signals have therefore gained increasing attention for probing internal battery processes. This review summarizes recent operando and in situ monitoring strategies for carbon-based and silicon-based anodes, highlighting advances in electrical, optical, and acoustic sensing. These methods reveal degradation mechanisms and morphological evolution in real time. Multimodal sensing strategies that integrate multiple signals for improved battery state estimation are also discussed. Finally, future directions are outlined, focusing on real-time anode monitoring and the integration of sensing technologies with next-generation battery designs. This review aims to guide the development of smart battery sensing for artificial-intelligence-assisted and multimodal sensing, providing solutions for battery management system that enable accurate synchronous detection of mechanical, thermal, and electrical signals.
9 February 2026