Abstract
Apart from the significant progress the scientific community has made during the last few decades, inflammation-mediated kidney-related diseases like chronic and diabetic kidney diseases (CKD and DKD) and glomerulosclerosis still continue to raise mortality rates. Recently, conventional therapeutic interventions have been put aside, since natural vitamin D-derived treatment has gained attention and offered several promising outcomes. Within this article, the utilization of vitamin D and its analogues as potential treatment toward kidney-related diseases, due to their anti-inflammatory, antioxidant and anti-fibrotic activity, is outlined. Vitamin D analogues including calcitriol, paricalcitol and 22-oxacalcitriol have been previously explored for such applications, but their hidden potential has yet to be further elucidated. Several clinical trials have demonstrated that vitamin D analogues’ supplementation is correlated with inflammatory signaling and oxidative stress regulation, immunity/metabolism augmentation and subsequently, kidney diseases and healthcare-related infections’ prevention, and the results of these trials are thoroughly evaluated. The highlighted research outcomes urge further study on a plethora of vitamin D analogues with a view to fully clarify their potential as substantial anti-inflammatory constituents of renal diseases-related treatment and their health-promoting properties in many kidney-associated healthcare complications and infections.
1. Introduction
Nowadays, as a result of a sedentary lifestyle, poor eating habits and abstention from social activities, people are more susceptible to numerous chronic diseases [1,2]. Reportedly, one out of ten people worldwide as of 2022 suffered from chronic kidney disease (CKD), which based on recent population data translates to 800 million people worldwide [1,2,3]. In 2015, 1.2 million people died from CKD that was induced by either hypertension, type II diabetes mellitus, or glomerulonephritis, increasing the corresponding mortality rates by 32% compared to those recorded in 2005 [4].
Kidneys are an organ of utmost importance to every living organism, since their main function lies upon converting metabolic by-products into urea, which is excreted into the urine and then dismissed from the body through the process of nitrogen metabolism urea cycle [5,6]. Normally, urine is free of compounds that the kidneys come into contact with like carbohydrates, protein, glucose and ketones. Other equally important functions kidneys are involved in include their contribution to regulating the balance of electrolytes in the blood and the adjustment of blood pressure [7,8]. In addition, through the secretion of hydrogen cations, kidneys balance the pH value of the blood and are also responsible for the production of hormones such as prostaglandins or renin [9]. The hydroxylation of vitamin D, which concludes to its activation, is also one of the most vital functions of our kidneys [10,11,12]. In general, the kidneys consist of the renal cortex and the renal medulla, while the organelles that carry out all the important processes inside the kidneys are known as nephrons and are located in the renal medulla’s pyramids [6,7]. At this point, it is worth mentioning the kidney’s important role in the production of an important glycoprotein hormone by the peritubular cells that stimulates efficiently red blood cells’ generation as a regulator of erythropoiesis [13,14]. Furthermore, kidneys play a major role in drugs and selected cytokines’ metabolization, as they partake in the intricate interplay between immune and metabolic pathways in kidney diseases and hence promote the efficacy of modern pharmacotherapy [15,16]. Other significant hormones, are secreted and degraded as well by our kidneys, which are in fact crucial for regulating blood pressure and renal flow, including hormones like renin, urodilatin and medullipin, as kidneys normally maintain arterial pressure within a narrow range by employing the mechanism of pressure natriuresis [17,18,19].
Kidneys in general perform complex processes that are crucial for homeostasis, ensuring that our internal environment remains stable and free of toxins [8,20]. The length of the adult kidney ranges from 10 to 20 cm based on the correlation of body height and age, while the right kidney is most of the times slightly longer than the left one [21,22]. Kidneys are divided into renal parenchyma and renal sinus. The renal sinus is hyperechoic and comprises catalyces, the renal pelvis, accumulated fat and major intrarenal vessels. In a normal-functioning kidney, the urinary-collecting system located in the renal sinus, although it is invisible, initiates a heteroechoic appearance with both the intervening fat and vessels [21,22]. On the other hand, the renal parenchyma is more homogenous and hypoechoic and is in fact separated into the innermost and outermost cortex as well as some marginally less echogenic medullary pyramids that consisted of cortical infoldings, namely columns of Bertin [21,22,23,24]. More specifically, the renal parenchyma of kidneys consists of nephrons, collecting tubules, lymphatic vessels, neurons and arteries. A nephron consists of a renal corpuscle, which is attached to a coiled “tubule”. This tubule leads first to the Bowman’s capsule and secondly to the proximal tubule, where the secretion of water and sodium chloride from the pre-urine takes place. The coiled tubule then meets the Henle’s loop, in which the secretion of water molecules and the reabsorption of sodium chloride is initiated. Right after the distal tube, an exchange of sodium ions with potassium ones (excretion) as well as hydrogen ions (absorption) occurs: a situation wrapped up in a collection tube. The renal corpuscle continuously filters blood plasma through the glomerulus, providing filtrate to the renal tubules [25]. As a consequence, several collection tubes merge to form the Bellini ducts, which in turn exit the renal pyramid carrying along body fluids destined to end up in the bladder and then excreted from our body [6]. It is useful to note that the collection tubes play a crucial role in the permittance or the forbidding of the excretion of water and electrolytes in the blood as well as in the desired activation of vitamin D [6,22,26].
Vitamin D, a fat-soluble vitamin traditionally known for its role in calcium homeostasis and bone health, has gathered attention in the recent years for its broader implication in the human health and particularly for its anti-inflammatory properties [27,28]. This vitamin is predominantly produced when the surface of the skin is exposed to sunlight [29,30]. Vitamin D has two equal forms: D3 (“cholecalciferol”) and D2 (“ergocalciferol”), that differ in the one extra double bond of vitamin D2 [30]. Vitamin D’s metabolism is at first initiated by a cholesterol precursor molecule known as 7-dehydrocholesterol, found in the sunlight-exposed skin, and subsequently a series of chained reactions lead to vitamin D3’s generation in the epidermis [12,27,31,32]. As vitamin D3 reaches the liver, it is enzymatically converted into 25-hydroxy vitamin D3, which is then transformed in the kidneys to 1α,25-dihydroxy-vitamin D3, a complex with a crucial role, as a regulator, in several biochemical pathways, that will be further analyzed in the main body of our research [13].
The activated form of vitamin D3, namely calcitriol, participates in multiple functions, interrelated or not inside the human body; thus, it is essential for its proper functioning. First of all, calcitriol aids in the balance of calcium ratio in the blood. Calcium in turn is a trace element present in human bones and teeth in the form of hydroxyapatite, providing them with strength and stability [27,33,34,35]. Regarding its metabolism, three areas of the human body are associated with a regulatory role in maintaining calcium ions in normal levels in the blood serum: the intestine (small and large), kidneys and bones. The hormones that assist in this regulation are calcitriol and parathyroid hormone (PTH). At low recorded levels of calcium ions in the blood, PTH is secreted, which acts on the proximal tubules of kidneys and bone osteoclasts, leading to the reabsorption of calcium ions, the increase in the renal enzyme 1-a-hydroxylase (catalyst of the reaction of vitamin D3’s activation) and finally osteoclasts’ activation so as to initiate the intended release of calcium ions [33,34].
Newly emerging research highlights the potential of vitamin D and its analogues in mitigating inflammatory processes, which are central to the pathogenesis of various chronic diseases, including glomerulosclerosis and kidney-related diseases. Vitamin D analogues like calcitriol halted cell proliferation, glomerular growth, glomerulosclerosis and albuminuria [36,37], while treatment with 22-oxacalcitriol notably suppressed and/or prevented urinary albumin expression, serum creatinine and serum urea nitrogen increase as well as inhibited glomerular cell number/growth/volume and glomerulosclerosis [38,39]. Concurrently, paricalcitol was able to restrain procedures such as macrophage infiltration, mRNA expression of proteins and growth factors including the transforming growth factor-β-1 (TGF-β1) and the phosphorylation of others like the mothers against decapentaplegic homolog-2 (Smad2) [40,41].
Glomerulosclerosis is highly characterized by the scarring of the glomeruli within the kidneys that leads to compromised kidney function and is a common endpoint of many kidney diseases [42,43,44]. The inflammatory milieu in glomerulosclerosis is responsible for the exacerbation of tissue damage and the acceleration of the disease’s progression. Vitamin D, through its active form namely calcitriol, exerts immunomodulatory effects that may help attenuate these inflammatory pathways [42,43,44]. Notably, patients that suffered from CKD exhibited reduced serum calcitriol values in their blood, because the fibroblast growth factor 23 (FGF-23) suppressed the enzyme of 1-a-hydroxylase [45]. As a result, no active transport of calcium ions occurred. Hence, it has been established that the recommended daily dose of calcium for patients with chronic kidney disease is 800 to 1000 mg/d [46], as amounts greater than 1000 mg/d put patients at risk due to the suppression of the process of excretion of calcium ions from the urine [47]. Concurrently, considering glomerulosclerosis treatment, the recommended dose of calcitriol (1α,25-dihydroxy-vitamin D3) that did not cause hypercalcemia in sham-operated and subtotally nephrectomized (SNX) rats was 3 ng/100 g body weight/day [48], while 1–2 μg of paricalcitol and 0.25–0.50 μg of calcitriol were the recommended doses for CKD patients suffering from glomerulosclerosis; however, more clinical trials must be conducted in order to discover the best possible dose [49]. On the contrary, an excess of calcium cations in the blood leads to the calcification of soft tissues and arteries. Some calcium deposits are declared harmless; however, others may be a sign of serious and possibly asymptomatic health conditions that can even be potentially fatal [50,51].
Understanding the anti-inflammatory benefits of vitamin D could open new therapeutic avenues for managing glomerulosclerosis and promoting better outcomes in kidney disease patients. This review aims to elucidate the mechanisms by which vitamin D and its analogues influence inflammatory responses in the kidneys against the progression of glomerulosclerosis by analyzing in vitro research and in vivo studies, including clinical trials, with respect to several inflammation-related renal disorders. Emphasis is given to vitamin D’s potential as a therapeutic agent in the context of glomerulosclerosis, as well as mainly chronic (CKD) and diabetic (DKD) kidney diseases. Vitamin D’s benefits against glomerulosclerosis and kidney diseases are depicted in Figure 1.
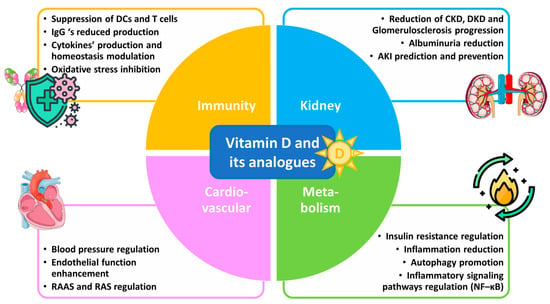
Figure 1.
Vitamin D and its analogues in glomerulosclerosis and kidney disease therapy.
2. Materials and Methods
In order to carry out the necessary literature review, various databases were explored including MDPI, PubMed, Scholar Google, Science Direct, Scopus and the NIST library. The combinations of keywords used were each one word from the first parenthesis AND one of the words of the second parenthesis: (“Vitamin D” OR Vitamin D analogues OR Renal Diseases OR Glomerulosclerosis OR Focal and Segmental Glomerulosclerosis OR Chronic Kidney Disease OR Diabetic Kidney Disease OR Fibrosis OR Acute Kidney Disease OR Diabetic Neuropathy OR Glomerulonephritis OR Kidney Disease Induced by Metals OR Nephrotic Syndrome OR Polycystic Kidney Disease OR Hyperparathyroidism OR End-Stage Renal Disease OR Amyloidosis OR 22-Oxacalcitriol OR Paricalcitol OR Calcipotriol OR Calcitriol OR Doxercalciferol OR Falecalcitriol OR Alfacalcidol OR Calcium OR Lead OR Cadmium) AND (Antioxidant OR Anti-inflammatory OR Inflammation OR Inflammation-Related Signaling Pathways OR Inflammation Biomarkers OR Cytokines OR NF-κΒ Signaling Pathway OR Oxidative stress OR Platelet-Activating Factor OR Thrombin OR Anti-Diabetic OR Renin–Angiotensin System OR Immune Modulation OR Malnutrition OR Atherosclerosis OR Heart Failure OR Mineral and Bone Disorders OR “In vitro” OR “In vivo”).
Initially, the search was focused on the dates 2014–2024, however, the results were not sufficient, especially for the other renal diseases except for CKD and DKD, and thus the search time frame was expanded mainly to 2004–2024. In some cases, some older reported studies were also included, which were not previously thoroughly reviewed.
Articles in other language than English, duplicates and the majority of review articles were excluded, apart from some important recent review articles on general information considering renal diseases.
3. The Multifaceted Role of Vitamin D toward Inflammation, Immune Modulation and Kidney Diseases
3.1. Inflammation and Kidney Diseases
3.1.1. Kidney Function in the Pathophysiology and Symptomatology of Glomerulosclerosis and Kidney-Related Diseases
The kidneys play a vital role in maintaining the body’s overall health by predominantly filtering waste, balancing electrolytes, and regulating blood pressure while participating in the maintenance of homeostasis and the provision of a toxins-free internal environment [8,20]. Glomerulosclerosis is a progressive kidney disease characterized by scarring of the glomeruli, which are the tiny filtering units within the kidney. This scarring disrupts the normal filtration process, leading to a decline in kidney function and ultimately contributing to CKD’s and other kidney-related diseases’ progression [40].
Focal and segmental glomerulosclerosis (FSGS) is a disease with primary damage to podocytes (podocytopathies), which is clinically evident by high proteinuria and the nephrotic syndrome (NS) [52]. FSGS is marked by the presence of sclerosis in parts (segmental) of at least one glomerulus (focal), as apparent by a kidney biopsy specimen, which is examined by ultrasound techniques involving the superb microvascular imaging (SMI) [53] and other microscopic techniques like the light, immunofluorescence and electron microscopy [52]. Severe FSGS, the most frequent idiopathic NS, is defined by high proteinuria, renal impairment during the initial stages, and an adverse outlook on the advancement of renal dysfunction, which may even conclude to an end-stage renal disease (ESRD) [52,54,55]. The onset of FSGS is evident where 25–50% of patients face a decrease in the kidney function, 50% hematuria and 20% arterial hypertension [52].
Several circulating permeability factors (CPFs) are able to damage the glomeruli and cause podocyte injury, leading to proteinuria, loss of podocytes, permeabilization of the glomerular filtration barrier and the induction of FSGS’s recurrence. As a result, proteins affiliated with CPFs’ mechanism of action and/or potential biomarkers for CPF’s presence have been suggested to possibly inhibit their menacing role in FSGS’s progression [56,57]. Recent study results have demonstrated that CPF-containing plasmas from FSGS patients enhance both podocyte lipid droplet accumulation and the expression of perilipin-2, which is consequently considered as a potential FSGS biomarker [56,57]. Additionally, the inhibition of reactive oxygen species’ (ROS) formation or facilitating the rapid ROS scavenging may exert some beneficial effects toward FSGS’s progression and subsequently a framework for regulating CPF activity, which may contribute to the better future monitoring and prognosis of NS-related diseases [57]. Furthermore, differentiating proteins, including apolipoprotein A4 (APOA4), hemopexin, gelsolin, vitronectin, complement C4B protein gene (C4B), retinol and vitamin D-binding proteins, could also be potent FSGS biomarkers against the evolvement of kidney-related diseases [52].
The use of vitamin D3 and its derivatives toward kidney-related diseases’ prevention has been justified in several clinical trials by mainly its vast antioxidant and anti-inflammatory ability to modulate key elements of the inflammatory process, including the reduction of pro-inflammatory cytokines (like interleukin 1 (IL-1β)) that contribute to inflammation, fibrosis, blood pressure elevation and kidney damage progression as well as the abatement of oxidative stress and the renin–angiotensin–aldosterone system’s (RAAS) overactivation [58,59]. Moreover, vitamin D’s anti-fibrotic properties that enhance the reduction of fibrogenic factors and thus fibrosis, along with the immune system modulation (T cells’ activity promotion) and proteinuria decrease, place this hormone between the most promising candidates in the battle against kidney diseases [45].
3.1.2. The Role of Inflammation in the Progression of Kidney Diseases
Although our kidneys have protective mechanisms toward systemic inflammation, CKD’s occurrence or other renal diseases make it vulnerable to damage deriving from pro-inflammatory cytokines and oxidative stress. Unlike other highly vascularized organs (e.g., the liver) that own antioxidants and detoxifying agents, kidneys receive almost 25% of the body’s blood volume without any similar anti-inflammatory defenses [59,60,61]. Thus, despite the fact that regulatory hormones and vasoactive molecules normally prevent damage from the physiologic hypoxic medulla environment, these constituents are disrupted by inflammation-related processes, with CKD causing several intrarenal alterations in the microvasculature, which eventually leads to renal damage [60]. Furthermore, the renal tubules house many regulated inflammatory cytokines, chemokines, and fibrosis mediators that play key roles in renal injuries’ progression, since pre-existing conditions, such as diabetes, incomplete recovery from acute kidney disease (AKI), genetic and epigenetic factors, age, hypertension, chronic and recurrent infections, altered adipose tissue metabolism, acidosis, glomerulonephritis, and uncontrolled inflammatory responses, may enhance kidney dysregulation [58,59,60]. Regarding the proximal tubules, their high energy demands set them prone to inflammation-mediated ischemia, while oxidative damage induced by increased reactive oxygen species (ROS) and decreased nitric oxide (NO) levels due to elevated homocysteine exacerbate CKD. Additionally, when antioxidant systems like the glutathione redox cycle reach their maximum capacity, a higher oxidized-to-reduced glutathione ratio occurs and hence, the ability to combat oxidation in CKD patients is restrained [59,60,62].
Inflammasomes and inflammasome components in general are highly implicated in infectious and non-infectious tissue injury [63]. The inflammasome structurally comprises a sensory component, a mediator of caspase-associated recruitment domains (CARD), apoptosis-associated speck-like protein that contains a CARD (ASC) and procaspase-1. Inflammasome sensors are categorized as nucleotide-binding domain-like receptors (NLRs) that enclose inflammasome-forming NLRs (NLRP1, NLRP3, NLRC4, etc.) and potent inflammasome sensors (NLRP6 and NLRP12) as well as missing in melanoma 2-like receptors (ALRs) and pyrin [58,60,63,64]. The ASC protein may bind with the NLRP3’s amino-terminal pyrin domain; thus, it stimulates procaspase-1 with all three constituents forming a multiprotein inflammasome complex able to activate caspase-1. Activated caspase-1 mediates the conversion of pro-IL-1β and pro-IL-18 to their mature forms, namely IL-1β and interleukin 18 (IL-18), via the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κΒ) signaling (signal 1) and their cleavage by caspase-1 (signal 2) [63,65,66]. Signal 1 is responsible for the regulation of the expression of several inflammasome components and pro-cytokines’ synthesis. In particular, pathogen-associated and danger-associated molecular patterns (PAMPs and DAMPs, respectively), provide signal 1 through the activation of the toll-like receptor (TLR-mediated) NF-κΒ signaling. Signal 2 is concurrently provided by various formed crystal particles via environmental or metabolic pathways and adenosine triphosphate (ATP), and thus, the inflammasome complex is activated and marks cells’ reaction to cellular stress [59,63,64,65,66]. The NLRP3 activation displayed interesting clinical results. NLRP3 expression affects tissue remodeling following chronic trauma and death signaling in epithelial cells, enhances angiogenesis as observed in a rat model and augments the TGF-β1/Smad signaling pathway, leading to tubular–interstitial fibrosis during CKD and kidney fibrosis [63,65,67,68]. The diverse activation of the NLRP3 inflammasome implied its potential detective role of cellular stress or injury. Three activation mechanisms are reported to successfully enhance NLRP3’s activation: cathepsin B after lysosomal injury, potassium efflux and phagocytosis, while caspase-1 and other caspases’ related signaling as well as post-transitional protein modifications (PTMs) still need to be further evaluated in order to fully comprehend their activity in kidney diseases [59,63,64,65,66,67,68].
During CKD specifically, an increase in adipokines and adhesion proteins (e.g., intercellular and vascular cell adhesion protein 1 (ICAM-1 and VCAM-1, respectively) is observed, which triggers uremic toxins’ activity. Uremic toxins subsequently play a vital role in the both the onset and the evolvement of inflammation signaling pathways by activating and increasing c-reactive protein (CPR), NO and NF-κΒ. More specifically, growing evidence further supports the degenerating impact of uremic toxins including indoxyl sulfate, p-cresyl sulfate, hippuric acid, homocysteine, etc. on the progression of CKD-related health complications [69,70]. As a result, increased levels of pro-inflammatory cytokines such as interleukins 1β and 6 (IL-1β and IL-6) that suppress PTH secretion, tumor necrosis factor α (TNF-α) and other pro-inflammatory enzymes and molecules are exhibited. Consequently, the inflammatory state is amplified and complications like malnutrition, atherosclerosis, heart failure, mineral and bone disorders, coronary artery calcification, anemia, etc., are aggravated (Figure 2) [47,58,60].
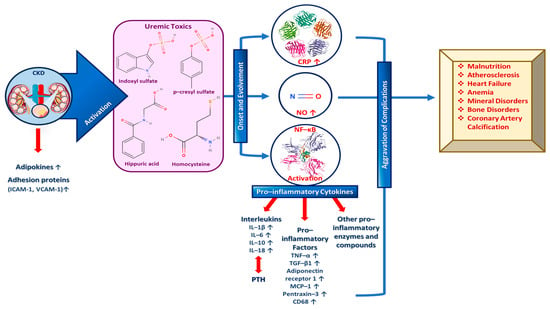
Figure 2.
The role of important inflammation biomarkers in CKD and other kidney-related diseases progression as well as several complications’ enhancement.
A plethora of inflammatory markers produced by the adipose tissue, like IL-1β, fibrinogen and TNF-α, independently predict CKD progression. IL-1β is responsible for activating the adhesion molecule expression in the endothelium and for inducing chemokine expression so as to recruit white blood cells (WBCs), while interleukin 6 (IL-6) may be involved in atherosclerosis and/or acute phases protein response, and IL-18 triggers the upregulation of other inflammatory cytokines and participates in inflammasome formation [60,64]. Moreover, TGF-β1 increases the extracellular matrix (ECM) and induces kidney fibrosis, while TNF-α upregulates other inflammatory biomarkers; thus, it enhances renal fibrosis via decreasing pro-apoptotic signals [71,72]. At this point, it must be noted that IL-1β and IL-6 have been claimed to enhance renal scarring, while TGF-β1 in synergy with IL-6 have been implicated in renal fibrosis [23,58,59,60,62,63]. In addition, other pro-inflammatory cytokines such as adiponectin receptor 1, cluster of differentiation 68 (CD68), monocyte chemoattractant protein-1 (MCP-1), pentraxin-3, CPR, etc., are validated biomarkers of inflammation and predictors of kidney-related diseases and mainly CKD progression, as depicted in Figure 2 [58,60].
Inflammation-induced, progressive oxidative stress is also worth mentioning, since it is implicated in the manifestation of numerous complications such as for example malnutrition, atherosclerosis, heart failure, or mineral and bone disorders [58]. Growing evidence has currently supported that vitamin D and its analogues exhibit beneficial effects in these disturbances, as this anti-oxidant facilitates balanced mitochondrial activities and immune modulation; thus, it prevents oxidative stress-related protein oxidation, lipid peroxidation, autoimmune diseases or diabetes onset and DNA damage [58,73]. Vitamin D, surprisingly, reduces systemic inflammatory cardiovascular diseases (CVDs) oxidative mediators [74], downregulates RAAS activity [58], and counteracts hydrogen peroxide (H2O2)-induced oxidative stress [75], as a potent ROS-targeting treatment [76].
3.2. Vitamin D: Structure, Function and Its Potential Role on the Immune Modulation
3.2.1. Vitamin D’s Structure, Metabolism, Renal Production and Analogues
Since the 17th century and the mass movement from rural to urban, smoke-filled cities, many efforts to combat the public burden of rickets, a childhood disease responsible for softening and weakening children’s bones, had been made. It was only in the 20th century that Mellanby and McCollum claimed that cod liver oil could be a potential cure to rickets. Specifically, McCollum heated this cod liver-derived oil in an attempt to destroy all the vitamin A content; however, some antirachitic properties still remained with the antirachitic factor corresponding to vitamin D [77,78,79]. Shortly thereafter, scientists Steenbock and Black proved that exposing food, especially its non-saponifiable lipids to UV radiation could be a potential cure to rickets, which led to the realization that rickets may be prevented or cured in children exposed to sunlight and/or artificial UV radiation [80,81]. As a result, vitamin D was believed and demonstrated to be produced by irradiation of precursors in vivo [77]. Eventually, Askew et al. [80,82] isolated and established vitamin D2’s structure from irradiated plant sterols (ergosterol), while Windaus et al. [79,83], ascertained both the structures and the specific pathway where 7-dehydrocholesterol (7-DHC), found in the skin, is converted to vitamin D3 [79].
Generally, vitamin D is predominantly produced when the surface of the skin is exposed to plain sunlight. However, it is also obtained from food sources and dietary supplements, while its production is suppressed by the use of sunscreen emulsions and clothing [29,30]. Vitamin D has two equal forms: D3 (“cholecalciferol”) and D2 (“ergocalciferol”). The difference between these two derivatives lies in their side chain [30,77]. Essentially, vitamin D2 has one more double bond in its molecule (C22–23) than its counterpart vitamin D3 [77]. Moreover, vitamin D3 is synthesized de novo in our skin after UV-B radiation exposure or it is obtained from animal source foods, and at the same time, vitamin D2 is acquired from plants like mushrooms and yeast [79,84]. The 2D form of these two vitamin D derivatives is depicted in Figure 3.
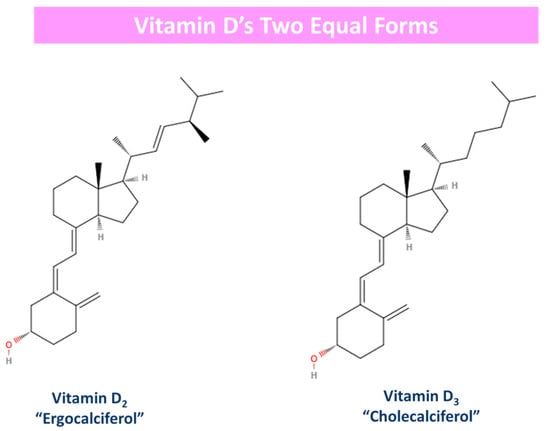
Figure 3.
Vitamin D’s two equal forms: derivatives. Vitamin D2 has one more double bond (c22–23) in its molecule in opposition to vitamin D3.
Vitamin D’s metabolism is at first initiated by a cholesterol precursor molecule known as 7-DHC, which is located in the skin. As aforementioned, Windaus et al. proposed and determined the pathway of 7-DHC’s conversion to vitamin D3. As long as our skin is exposed to sunlight, the precursor molecule is converted into pre-vitamin D3, which is then converted by heat into vitamin D3. This specific reaction is induced in the epidermis (skin), where sunlight (UV-B form, hv) successfully breaks the B ring of 7-DHC with a view to form pre-vitamin D3, which is in turn converted to vitamin D3 after undergoing a thermal-induced rearrangement. Furthermore, the prolonged irradiation of pre-vitamin D3 concludes to the reversible formation of two more vitamin D3 derivatives, namely tachysterol and lumisterol, that are able to be reverted back to pre-vitamin D3 in dark conditions [31,77,79]. Vitamin D3 later on enters the bloodstream and is transported to the liver after binding to vitamin D’s specific binding protein (Figure 4).
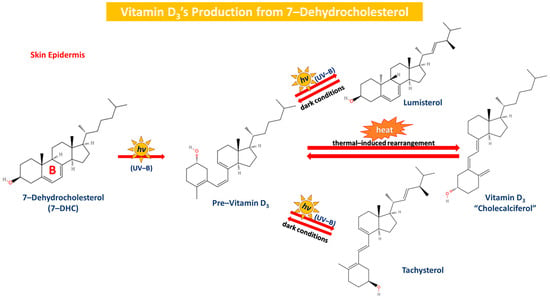
Figure 4.
The production of vitamin D3 from 7-DHC in the skin epidermis. 7-DHC is converted to pre-vitamin D3 via the exposure to UV (UV-B) and then pre-vitamin D3 is thermally rearranged to form D3 reversibly. Lumisterol and tachysterol are formed through continuous exposure to UV-B irradiation, and they are reverted back to pre-vitamin D3 in dark conditions.
Vitamin D3’s metabolism is conducted first in the liver and secondly in the kidneys so as to obtain its active form with both vitamins D2 and D3 being widely acquired from food, plant and animal food sources. Vitamins deriving from food especially bind likewise to a vitamin D carrier protein, and this newly formed binding complex reaches the liver, where it is enzymatically converted to 25-hydroxyvitamin D3 [85].
The first step of this process is 25-hydroxylation, which primarily occurs in the liver, even though other tissues exhibit enzymatic activity as well [77]. The liver converts vitamin D3 to its major circulating form 25-hydroxyvitamin D3 (25OHD3), which is then converted by the kidney (undergoes filtration by the proximal glomerular tubules and absorption by surface receptors of proximal glomerular cells). This enzymatic procedure is induced by the cytochrome CYP27B1, where via the hydroxylation in the 1α (A ring) position, 25OHD3 is converted to 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3) and via the hydroxylation in the 24th position, 25OHD3 is converted to 24,25-dihydroxyvitamin D3 otherwise (24,25(OH)2D3). The conversion of 25OHD3 to 24,25(OH)2D3 increases phosphorus and calcium concentrations, while 1α,25(OH)2D3 on the contrary decreases them, as PTH and FGF-23 production is plus promoted. More specifically, vitamin D’s metabolites of 25OHD3 and 1α,25(OH)2D3 may be hydroxylated at the 24th position, with 1α,25 (OH)2D3 and 1,24(OH)2D3 showing similar behavior. However, the 24–hydroxylation of metabolites with a pre-existing 25OHD3 group leads to further, mostly unfavorable catabolism, since vitamin D3 is preferentially 24-hydroxylated and vitamin D2 is mostly 25-hydroxylated (Figure 5) [31,45,77,86,87,88]. The 1α,25(OH)2D3 complex binds as well to its specific receptor and owns a regulative role in several biochemical pathways [85].
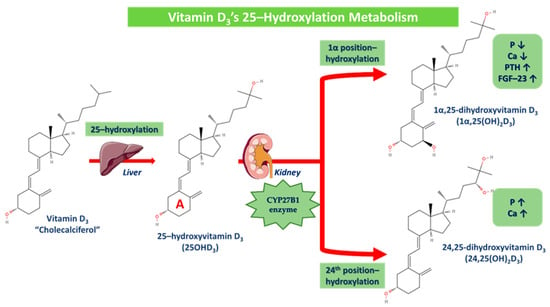
Figure 5.
Vitamin D3’s 25-hydroxylation metabolism. 25-hydroxylation occurs in the liver, where vitamin D3 is converted to 25-hydroxyvitamin D3 (25OHD3), which is then converted in the kidney via the cytochrome CYP27B1 enzyme in two different derivatives. Via the hydroxylation in the 1α (A ring) position, 25OHD3 is converted to 1α,25-dihydroxyvitamin D3 (P and Ca are decreased while PTH and FGF-23 are increased) and via the hydroxylation in the 24th position, 25OHD3 is converted to 24,25-dihydroxyvitamin D3 (P and Ca are increased).
Vitamin D is mainly utilized in kidney-related therapeutic interventions in the form of one its several analogues. Calcitriol (1α,25(OH)2D3) is the main utilized vitamin D analogue in kidney diseases’ treatment and has been widely supplemented in many CKD patients globally. Vitamin D in the form of calcitriol has been demonstrated to lessen the severity of proteinuria in CKD patients with low serum 25(OH)D3 levels [89], while recently, research findings claimed that CKD patients could benefit from calcitriol supplementation toward COVID–19’s potent infection [90]. As calcitriol’s deficiency enhances hyperparathyroidism evolvement, a calcitriol treatment strategy could activate the VDR and replenish vitamin stores while avoiding hypercalcemia and hyperphosphatemia in patients suffering from renal diseases [91]. Oral calcitriol treatment has also been pointed out in the past as a potent attributor to lower death and dialysis risk, decreased renal failure incidence, reduced proteinuria and albuminuria, regulated mesangial cell proliferation, increased inflammatory cytokines and oxidative stress modulation and murine lupus attenuation in CKD or renal diseases patients [36,43,92].
One of the most explored vitamin D analogues yet to be discussed is maxacalcitol, which is also known as 22-oxacalcitriol (22-oxa-1α,25(OH)2D3). Maxacalcitol has been revealed to possess protective effects toward the renal structure and function in mesangial cell proliferative and ECM productive nephrosis models. Specifically, the daily administration of either calcitriol or this analogue is able to ameliorate the nephrotic state while protecting podocytes, thus leading to the prevention and/or treatment of podocyte injury [36,93]. Furthermore, maxacalcitol treatment attenuated functional deterioration and histological damage in induced AKI, significantly decreased cell fibrosis and apoptosis and hence further confirmed the renoprotective value of this analogue [38,39]. On the other hand, paricalcitol (19-Nor-1α,25(OH)2D2) is also an important vitamin D analogue with a remedial impact against kidney diseases. Paricalcitol is able to suppress macrophage infiltration, proteinuria, PTH activity, MCP-1 production, renal interstitial fibrosis, TGF-β1 mRNA protein expression and Smad2 phosphorylation [36]. In addition, albuminuria attenuation as well as mesangial matrix expansion and DKD podocyte injury alleviation by the enhancement of podocyte autophagy activity has also been recorded after paricalcitol treatment [94]. Moreover, paricalcitol has been pointed out to provide beneficial effects to hemodialysis patients, as it strongly affects PAF/thrombin activity and metabolism, while it also regulates IL-8, IL-1β and TNF-α circulating levels [61,95]. Paricalcitol improves the inflammatory status of hemodialysis patients, since the produced inflammatory hemodialysis mediator—namely, the platelet-activating factor (PAF), that is implicated in CKD’s progression, is affected by this analogue. Specifically, paricalcitol inhibited in vitro and in vivo the PAF/thrombin-induced platelet aggregation comparably to PAF and thrombin antagonists while strongly affecting PAF/thrombin activities, PAF metabolism, and IL-8, IL-1β, and TNF-α circulating levels [95].
Vitamin D analogues like calcipotriol (1α,24(OH)2-22-ene-24-C3H5-D3) have been recently claimed to manage more effectively uremic pruritus and have been used as adjunctive CKD-associated pruritus (CKD-ap) and ESRD treatment [96,97]. Clinical studies have firmly established alfacalcidol’s (1α(OH)D3) beneficial effects on CKD, bone disease and secondary hyperparathyroidism [98,99]. Doxercalciferol (1α(OH)D2) is a highly effective vitamin D analogue as well, as it suppresses secondary hyperparathyroidism in CKD patients at stage 4 [100], and PTH levels in patients suffering from CKD stages 3 and 4 [101]. Falecalcitriol has also been suggested to be a potent inhibitor of PTH, and in fact, it has been proved to be more effective than alfacalcidol in reducing PTH with similar rates of hypercalcemia, almost as calcitriol’s corresponding impact [102]. As apparent, there are several beneficial effects of vitamin D analogues toward kidney-related diseases, but in order for a more sustained utilization of the related treatment to be promoted, further and newer clinical trials must be conducted worldwide. Vitamin D and its most widely used analogues in kidney-associated diseases treatment are depicted in Figure 6.
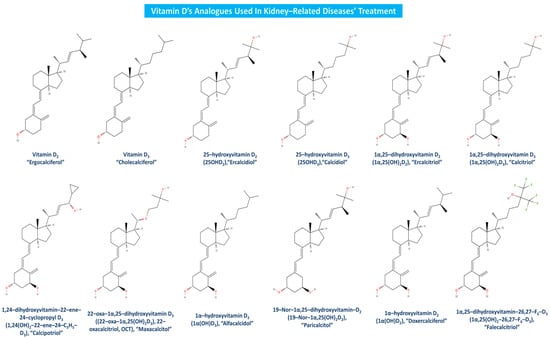
Figure 6.
Vitamin D analogues utilized in kidney-related diseases treatment.
3.2.2. Vitamin D’s Function and Anti-Inflammatory Effects
As apparent, globally, there is a growing interest in vitamin D’s broader health implications and especially, in terms of its regulative role against the observed inflammation in a plethora of different pestering disorders and in our context, against kidney-related diseases’ progression and development [44,59,92].
Vitamin D holds great anti-inflammatory potential since it aids at first in the regulation of mainly pro-inflammatory cytokine production including TNF-α and IL-6 and plus promotes the expression of many anti-inflammatory cytokines like IL-10, thus reducing the overall inflammation [103,104]. Moreover, this vitamin in the form of calcitriol participates in the inhibition of NF-κB by directly interacting with the inhibitory κB kinase β (IKKβ kinase) [105,106]. Vitamin D also participates in the downregulation of the renin–angiotensin system (RAS) and the transformation of renal cells into myofibroblasts, which are often induced by kidney diseases, leading to the reduction of inflammation, scarring in glomerulosclerosis and fibrosis, respectively [107,108,109,110,111]. In terms of fighting both inflammation and kidney diseases, vitamin D plays several roles as it enhances the regulation of T cells [112], protects by enhancing antioxidant defenses against the oxidative stress [73], modulates the immune cell activity of several macrophages and dendritic cells [113], improves the endothelial function [114,115], inhibits the mesangial cell proliferation in the glomeruli, which is connected with glomerulosclerosis and kidney disease progression [87], and promotes autophagy and cell survival in kidneys [86,116].
Vitamin D deficiency has been commonly observed in CKD and ESRD and thus has been implicated in deteriorating renal and podocyte function, proteinuria and neuropathy, as well as in increasing morbidity, acute failure and mortality rates in CKD patients. Vitamin D has attenuated effectively kidney injury by suppressing inflammation, fibrosis and apoptosis via the inhibition of multiple kidney injury pathways such as the RAAS, NF-κΒ, TGF-β/Smad and Wnt/β–catenin signaling pathways [9,44,58,105,106,117]. Vitamin D deficiency was also linked to the exacerbation of renal impairment, hyperparathyroidism and the evolvement of periodontitis, since vitamin D-binding protein (VDBP) polymorphisms that are associated with bioavailable 25(OH)D3 induced CKD and periodontitis severity and progression [43]. More specifically, vitamin D deficiency and especially calcitriol-reduced production were affiliated with a reduction in the renal mass of CKD patients that limited the free and available 1-α-hydroxylase responsible for the production of calcitriol. Furthermore, the decreased estimated glomerular filtration rate (eGFR) levels reduced renal megalin receptors, and the elevated FGF-23 activity and oxidative stress restrained the conversion of 25(OH)D3 to 1-α-hydroxylase, which further attenuated calcitriol’s production [43,73,118,119]. Vitamin D supplementation in overweight and obese populations has also been correlated with a plethora of beneficial effects, since vitamin D intake exerts its anti-inflammatory effect predominantly through decreasing the protective stabile values of IL-10 rather than severely impacting pro-inflammatory cytokines like IL-7 and leukocytes [120]. Additionally, vitamin D has been demonstrated to reduce the expression of TLR-4, IL-6, TNF-α, IL-10, cathelicidin and MCP-1 in monocytes incubated with uremic serum. This uremic pool environment activated an inflammatory response in monocytes, which was successfully reversed by 25(OH)D3 supplementation [121]. Vitamin D deficiency is not only highly affiliated with CKD and CVDs, but it may also accelerate their incontrollable progression; thus, treatment with vitamin D analogues could be therapeutic options for renal and cardiovascular problems due to their role in regulating blood pressure, improving the endothelial function and monitoring RAAS’ activity [122,123].
Vitamin D supplementation has reduced blood inflammatory cytokines’ expression and has improved graft function in kidney transplant recipients by enhancing the regulation of excessive immune inflammation and restoration of immune homeostasis [124]. At the same time, a vitamin D3-related pretreatment has been correlated to renal inflammatory responses’ regulation during lipopolysaccharide-induced AKI [125], while vitamin D3 administration has counteracted diabetes-induced kidney damage primarily via a mediated inhibition of the NF-κΒ activation [126]. Vitamin D deficiency is also linked to the prevalence of albuminuria in the presence of diabetes due to increased microalbuminuria when the serum 25(OH)D3 level was less than 20 ng/mL. Vitamin D seemed to have anti-inflammatory and anti-fibrotic effects in diabetic patients suffering from glomerular hyperfiltration, mesangial expansion, inflammation and finally glomerulosclerosis and tubulointerstitial fibrosis (Table 1) [127]. However, further insight is required in order to fully comprehend vitamin D’s anti-inflammatory potential toward kidney-associated diseases including CKD, DKD and glomerulosclerosis [61,128].
3.2.3. Overview of the Immune System Modulation by Vitamin D
Vitamin D, apart from its aforementioned anti-inflammatory potential impact, is also implicated in immunity (immune cells) and oxidative stress regulation. Pointedly, the mechanisms linking vitamin D and CKD, as well as periodontitis which is a potent risk factor for kidney disease [129], are related to mainly calcitriol’s biofunction and its immunomodulatory properties that impact both the innate and the adaptive immune system [43]. Vitamin D3 via its 25-hydroxylation in the liver is converted into 25(OH)D3, which in turn through 1α-hydroxylation turns into the active form of calcitriol (1α,25 (OH)D3) [87,121,130]. At first, calcitriol binds to VDRs located both on the cell surface (membrane bound) and inside the cytoplasm of immune cells (intracellular) and hence, it activates both innate and adaptive immune cells [43,77,131,132].
Considering its activity toward the dendritic cells, calcitriol downregulates the expression of the major histocompatibility complex (MHC) class II, which induces antigen recognition and dendritic cell activation [43,133]. At this point, it must be noted that activated dendritic cells stimulate the activity of T-lymphocytes (T-cells), and consequently, the suppression of dendritic cells leads to T-cells’ reduced function, which implies the communicative exchange between innate and adaptive immunity [131,134,135]. Calcitriol also represses pro-inflammatory cytokines including IL-2’s production while encouraging anti-inflammatory cytokines development [135,136]. Concerning macrophages/monocytes on the contrary, calcitriol binds to and activates VDRs in macrophages and their monocyte precursors by forming heterodimers with the RXR in order to produce antibiotic peptides like β-defensin 2 and cathelicidins [43,137,138]. Moreover, in macrophages’ activation, calcitriol enhances epigenetically the immunological memory and differentiation of macrophages and monocytes [139,140]. During the crosstalk of innate and adaptive immunity, T-lymphocytes and B-lymphocytes namely T-cells and B-cells are also heavily affected and suppressed [131,134]. Calcitriol is responsible for reducing T-helper cells’ differentiation into types T helper 1 and T helper 17 (Th1 and Th17) as well as their anti-inflammatory cytokines’ generation. Concurrently, calcitriol stimulates Th2 types’ differentiation and favors their mass production of anti-inflammatory cytokines such as IL-10 [43,77,134]. T cell-derived pro-inflammatory cytokines finally induce B cells’ differentiation, so T cells’ suppression initiates indirect B cells’ action suppression as well [134], and direct naïve B cells differentiation and maturation into memory and plasma cells is thus supported (Figure 7) [43,77,134,138,139,140].
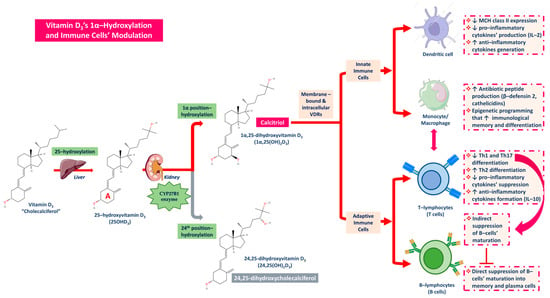
Figure 7.
The roles of Vitamin D3 and its active forms produced in the kidney, calcitriol, on immune cells’ modulation towards activation of anti-inflammatory cell responses and suppression of pro-inflammatory signaling.
Vitamin D deficiency is also affiliated with oxidative stress’ aggravation and CKD’s progression [43,58,73]. Hypovitaminosis D is one of the key controllers of systemic inflammation, the excessive intracellular oxidative stress and mitochondrial respiratory function, and hence, the aging process in humans, while in turn cellular actions that form calcitriol, the active form of vitamin D antioxidant, hinder oxidative stress conditions, cell and tissue damage and subsequently the aging process [73]. Generally, oxidative stress has been linked to the generation of highly reactive inflammatory intermediates, while ROS are able to further boost the inflammatory response by triggering pro-inflammatory mediators (NF-κΒ signaling cascade) [58,73]. Vitamin D supplementation downregulates various intracellular oxidative stress-associated pathways such as the erythroid–2-related factor (Nrf2) and Klotho family proteins that are mainly expressed in the kidney. Especially, α-Klotho protein has been proved to reduce oxidative stress by modulating NF-κΒ signaling cascade’s expression and CKD complications [43,58,73,141].
The progression of CKD is closely linked to oxidative stress and inflammation, while vitamin D and its analogues have been proposed in the outcomes of a plethora of clinical trials to promote the reduction of systemic implications following vitamin D’s deficiency including atherosclerosis and CVDs [43,58,73]. Calcitriol is able to alleviate oxidative stress and fibrosis due to regulating the CXCL12/ERK1/2 pathway, so as to inhibit every inflammatory response and renal cell apoptosis and to induce renal autophagy via the AMPK/mTOR cascade [142]. Moreover, this active vitamin D form is able to decrease inflammation, endothelial damage and oxidative stress as well as increase vascular regeneration markers and antioxidant enzymes’ expression in AKI induced by cisplatin [143]. In addition, Vero cells pre-exposed to iohexol, a low osmolar comprising iodine contrast media, were submitted to iron-enhanced renotoxicity, which was ameliorated by calcitriol’s utility and its prevention of AKI [144].
Vitamin D, as analyzed previously, contributes greatly to pro-inflammatory and anti-inflammatory cytokine regulation as well as in the NF-κΒ pathway’s modulation and inhibition [103,105,106,145]. RAS suppression is also further encouraged by vitamin D or its analogues’ supplementation, since recent study outcomes have supported its role in lowering blood pressure in vitamin D-deficient patients [108]. Calcitriol especially has been proved to restrain renin genes’ expression, inhibiting the RAS system and ameliorating hypertension [109]. Hence, the vitamin D hormone has a major impact on the homeostasis of the renal and cardiovascular system through RAS’ suppression [107].
Inflammation-related cytokine regulation, NF-κΒ cascade’s inhibition, RAS suppression and fibrosis hindering, as well as immunity perseverance and oxidative stress reduction, are only a few of vitamin D’s pleotropic roles in maintaining homeostasis and enhancing our immune system toward various kidney related diseases.
4. Anti-Inflammatory Health-Promoting Effects of Vitamin D and Its Analogues in Glomerulosclerosis and Kidney Diseases
4.1. Vitamin D’s Role in Kidney-Related Diseases
The spotted molecular differences between standard anti-inflammatory treatments for glomerulosclerosis and the anti-inflammatory activities of vitamin D are rooted in their distinct mechanisms of action, molecular targets, and effects on the immune system and inflammatory pathways. Glomerulosclerosis represents the final stage of glomerular injury during kidney-related diseases and may represent a primary disturbance in disorders like FSGS or a secondary response to tubulointerstitial disease, which accounts for 10–20% of patients of all ages who progress to ESRD. Current immunosuppressive therapy and conservative management including RAAS inhibitors and sodium–glucose cotransporter are in fact insufficient and no FDA-approved therapeutic options effectively prevent or delay kidney failure onset. More specifically, existing glomerulosclerosis treatment and FSGS focus on non-specific treatment blood pressure and proteinuria-reductive agents, and they involve corticosteroids (e.g., prednisone), calcineurin inhibitors or immunosuppressants (e.g., cyclosporine), that mainly target the NF-κΒ pathway, cytokine suppression and leukocyte activity [146,147]. Vitamin D and derivatives on the contrary exert their effects by binding to the VDR, and they induce VDR-mediated gene regulation, NF-κΒ and MAPK inhibition, immune cells and RAS modulation, as well as the reduction of oxidative stress without any unfavorable side effects, great selectivity, crucial specificity and a long–term impact [19,52,58].
Vitamin D has anti-inflammatory and anti-fibrotic effects, and it may block the intrarenal RAS system. Adequate vitamin D levels in conjunction with the use of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ACEI/ARB) may restrain CKD’s progression. Moreover, vitamin D3 supplementation presented a significant eGFR decrease in CKD patients and attenuated a compensatory increase in the RAS associated with the use of ACEI/ARB by suppressing renin transcription, reducing interstitial fibrosis, decreasing glomerular and tubulointerstitial injury, improving endothelial function and reducing blood pressure, risk of hypercalcemia, hyperkalemia and proteinuria [148,149]. Additionally, targeting the unbalanced RAS and angiotensin-converting enzyme 2 (ACE2) downregulation with vitamin D in SARS-CoV-2 infection may be a potential therapeutic approach to combat the expansion of COVID-19 and the acute respiratory distress syndrome (ARDS), as it potentially decreases the risk of severe pneumonia, renin and angiotensin plasmatic levels [150,151,152].
At this point, it must be highlighted that there are many factors affecting the response to vitamin D supplementation, ranging from genetic, environmental, and physiological factors. In particular, patient-specific factors could further affect the amount of vitamin D required in order to attain a sufficient concentration and should be extensively evaluated. At first, there is mounting evidence that genetic variations of proteins involved in the vitamin D metabolic pathway may affect tissue response to vitamin D and thus may influence the risks and severity of multiple chronic diseases. Genetic variations in the VDR, enzymes like CYP2R1 and the GC gene (VDR-related gene produced in the liver), namely polymorphisms, may affect the binding affinity of vitamin D to its receptor, the conversion of vitamin D to its active form, vitamin D’s degradation and the availability of this free, bioactive hormone, while appropriate adjustment of this dose may reverse all these outcomes [153,154].
Environmental factors, such as sunlight exposure, also affect vitamin D supplementation as its significantly influences endogenous vitamin D synthesis, and higher treatment doses are recommended [155]. Concurrently, many biological, pathological and demographic characteristics could be determinants of vitamin D-related treatment, including BMI, obesity, smoking, physical activity, aging, ethnicity and calcium intake–dietary status. Body weight has an influence on the blood volume, the amount of muscle and adipose tissue, as higher body fat percentage or body mass index (BMI) have been associated with a smaller increase in 25(OH)D3 concentration in response to vitamin D supplementation in both younger and older adults [156]. In particular, obesity is linked to lower vitamin D bioavailability due to sequestration in adipose tissue and as a result, obese patients require higher vitamin D doses during treatment [157]. Smoking and more specifically tobacco smoke exposure is known to lower serum 25(OH)D3 concentrations [158], while vitamin D status above 75 nmol/L was positively correlated with physical activity [159]. Furthermore, aging, ethnicity and dietary calcium intake are associated with lower levels of 25(OH)D3 in circulation with aging being affiliated with decreased skin synthesis of vitamin D and renal conversion to its active form as well as potential changes in VDR expression. Older patients, therefore, display less response to standard vitamin D doses, and hence, higher ones are required, while increased calcium intake exhibited a slight increase in serum calcium levels and a decrease in PTH ones [155].
Comorbid conditions including hypo- and hyperparathyroidism [160,161], musculoskeletal disorders (e.g., osteoporosis, osteopenia), autoimmune and systemic connective tissue diseases (e.g., rheumatoid arthritis, fibromyalgia, chronic musculoskeletal pain), glucocorticoid-induced osteoporosis, endocrine and metabolic conditions like diabetes mellitus (I or II), the metabolic syndrome, malabsorption–gastrointestinal syndromes, like inflammatory bowel disease [162,163], Crohn’s disease [164], cystic fibrosis [165], ulcerative colitis [166], celiac disease [167], CKD [43,44,47], cancer [168,169,170], immunocompromization caused by HIV infection [171,172], as well as central nervous system diseases, such as multiple sclerosis, epilepsy, dementia, Alzheimer’s and Parkinson’s disease [173,174,175,176], are plus associated with lower levels of 25(OH)D3 in circulation and require higher vitamin D doses most of the time [155,177]. Lastly, certain medications like anticonvulsants, antiepileptic drugs, bile acid sequestrants, lipase inhibitors, glucocorticoids, and antifungals may induce enzymes responsible for the increase in vitamin D’s metabolism, reducing its effectiveness. Higher doses may be consequently required in order to achieve efficient vitamin D-related treatment [155,178,179].
4.1.1. Vitamin D’s Role in Glomerulosclerosis and Chronic Kidney Disease
Without overlooking its function in regulating calcium homeostasis in the blood, vitamin D has recently been reported via cellular, animal and human studies to interact through complex pathways with the kidneys in the context of glomerulosclerosis. Diseases such as diabetes, lupus nephritis, proteinuria and CKD cause injury to the glomerulus and/or podocytes. As a result, such conditions lead to glomerulosclerosis through a plethora of different pathways. The activation of vitamin D in the kidneys occurs mainly in the proximal renal tubules, while the binding of the active form of vitamin D requires a specific vitamin D receptor (VDR) as well. Wang et al. [180] used a very specific antibody (D-6) to detect vitamin D receptors in different types of renal cells. Finally, VDRs were found in both distal and proximal tubules and were also detected in macula densa cells, suggesting a possible role of vitamin D in the regulation of renin’s production. Continuing on this path, Mihajlovic et al. [181] observed that by exposing ciPTEC–OAT1 cells—human renal cell lines that mimic the proximal tubule cells of the kidney to uremic conditions—vitamin D was predominantly catabolized. In the cells where vitamin D was administered, the enzymes responsible for its metabolism were traced and plus observed to remain unaffected by uremic toxins [181]. In this recent research case study, ROS were reduced when vitamin D was administered to patients, suggesting that this hormone offers a protective role against inflammatory responses and oxidative stress induced by uremic toxins [181].
Vitamin D supplementation offers a plethora of benefits, many of which are crucial to our well-being. Prior to the previously mentioned research outcomes, a scientific team consisting of Finch et al. [182] showed that the combination of two drugs, enalapril and paricalcitol, improved renal dysfunction and mitigated oxidative stress in uremic rats. Paricalcitol as a vitamin D derivative upregulated the RAAS system, while this type of treatment mitigated oxidative stress by inhibiting the expression of NADPH oxidase, promoting the expression of endothelial nitric oxide synthase (eNOS) and maintaining glutathione (GSH) cycle’s activity [182]. Needless to say, many other drugs have been and could be used to treat glomerulosclerosis as well. For example, Gonçalves et al. [183] treated losartan and erlotinib to rats that suffered from vitamin D deficiency and CKD. Such treatment was responsible for the suppression of renal fibrosis formation (RFF)-related elements’ expression, including TGF-β1, trans-arterial chemoembolization (TACE), TGF-α, epidermal growth factor (EGF) and epidermal growth factor receptor (EGFR). In addition, attenuated inflammatory cell infiltration was observed, suggesting that such treatment, most likely, targeted the TGF-β and EGFR pathways [23]. Adding to our previous statements, it is acclaimed that under proteinuria conditions, the transient receptor potential cation channel (TRPC6) receptor is increased, where overexpression of the TRPC6 receptor injures renal podocytes. Sonneveld et al. [184] demonstrated both in vivo and in vitro findings that suggested that calcitriol administration reduces glomerular TRPC6 expression in injured podocytes. In particular, calcitriol inhibits TRPC6 promoter’s activity through binding to its specific region [184]. In addition, another enzyme, namely heparinase, is involved in the development of proteinuria in various glomerular diseases. In a study conducted by Garsen et al. (2015), calcitriol reduced heparinase’s expression in podocytes both in vitro and in vivo findings. More specifically, vitamin D treatment inhibited the heparinase promoter’s activity through binding to the promoter, thus protecting podocytes from injury [185]. This protective mechanism of vitamin D resembles to a great extent the pre-mentioned mechanism described by Sonneveld et al. [184].
Foot cell injury generally leads to autophagy and apoptosis, where deregulated autophagy and apoptosis of specifically podocytes is a pathogenic process taking part in glomerulosclerosis. These processes are activated when podocytes are exposed to various damaging stimuli. Vitamin D is claimed to be responsible for the regulation of the process of autophagy and apoptosis. Yu et al. [186] discovered that vitamin D supplementation in patients suffering from Lupus Nephritis (LN) reduces autophagy. During the LN disease, patients’ autoantibodies stimulate in fact podocyte autophagy. Podocytes also expressed VDRs and retinoid X receptors, while Hamano et al. (2009) previously noticed that calcitriol and 22-oxacalcitriol analogues (OCTs) owned a protective effect against podocyte damage as well [86,186].
Several research studies over the years have proved that by administrating vitamin D analogues, nephrin and podocin expression is restored and thus, proteinuria is reduced. Nephrin and podocin are generally recognized markers of renal podocyte protection [187,188]. Wang et al. [93] investigated the effect of high-dose vitamin D3 in rats with renal fibrosis that was induced by CKD. The supplementation of vitamin D effectively normalized serum calcitriol levels while reducing at the same time serum creatinine, urea and proteinuria levels. Moreover, vitamin D decreased the expression of α-smooth muscle actin (α-SMA) proteins, collagen I, vimentin and E-canterine. As a consequence, the signaling biochemical pathway TGF-β1/Smad3, a key factor of fibrosis, was inhibited [93]. In full agreement with the results of Wang et al. [93], Sari et al. [189] found that vitamin D’s administration to mice with CKD inhibited the activation of the NF-κB and TGF β–Smad3 biochemical pathways, leading to reduced expression of pro-inflammatory mediators and profibrotic pathways [189]. Chebotareva et al. [52], by comparing the urinary proteomic profiles of patients with focal segmental glomerulosclerosis (FSGS), proved that patients with severe FSGS displayed elevated levels of specific proteins associated with podocyte damage, such as apolipoprotein A-1 (ApoA-1), hemopexin, vitronectin and gelsolin. Apo-A1, in fact, was functional as a marker able to distinguish stereo-sensitive FSGS from stereo-sensitive-resistant FSGS. It was further established that regarding the severe FSGS the extracellular wall matrix’s (ECM) deposition in the glomeruli increased, and as a result, the ECM could partake both in the progression and further development of the disease and patient’s unresponsiveness to treatment [52].
At this point, it is worth mentioning the possible negative effects of vitamin D’s deficiency in the mother of the newborn. Nascimento et al. [190] pointed out that maternal vitamin D deficiency causes harmful alterations in proteins such as podocin or renin and the angiotensin II type 1 (AT1) receptor. These proteins play critical roles in renal function, including podocin’s role toward the structural organization of the renal filtration system, renin’s action in the regulation of blood pressure and AT1 receptors’ participation in the renin–angiotensin system. These proteins’ possible deregulation may initiate important changes in kidney structure and function [190]. As a part of their research, Denburg et al. [191] evaluated bioavailable 25(OH)D3 and 24,25(OH)2D3 concentrations in children that faced CKD. Regardless of proteinuria, lower concentrations of bioavailable 25(OH)D3 were found in children with CKD compared to healthy ones, suggesting impaired vitamin D’s metabolism. As the severity of CKD increased, the concentrations of 24,25(OH)D3 in patients lowered [191]. Furthermore, Zaniew and Jarmoliński [192] showed that the supplementation of 1300 ± 772 IU/d of vitamin D and calcium in bone health in children suffering from glomerulopathy increased the levels of vitamin D in patients’ blood but unfortunately did not optimize their condition. Hence, it is suggested to reconsider the dose of cholecalciferol due to the high frequency of bone deficiencies [192]. All of the aforementioned clinical trials on vitamin D’s role against glomerulosclerosis and CKD are depicted in Table 1.
4.1.2. Vitamin D’s Role in Diabetic Kidney Disease Compared to Chronic Kidney Disease
Diabetes is a disease that is inextricably linked to modern lifestyle. In particular, type II diabetes, which is common in Western developed societies, initiates the alleged DKD due to constantly elevated blood glucose levels. Wang et al. [193] had originally discovered that in type II diabetes conditions, the amount of 1-a-hydroxylase and calcitriol increased, indicating elevated 1-a-hydroxylation of vitamin D, while it was observed in vivo that fibronectin and collagen IV’s production was inhibited. Furthermore, the vitamin D receptor activator (VDRA) is upregulated under high glucose conditions. Early studies have demonstrated that vitamin D might have an indirect protective effect against DKD [193]. Subsequently, in 2009, Petchey et al. [130] conducted a controlled trial on CKD patients, in which the association of vitamin D with insulin resistance and markers of adverse cardiac risk was studied. Notably, it was the first controlled trial to correlate these two variables. All outcomes pointed that vitamin D could partake in a safer drug pathway, as it offered a plethora of different benefits beyond these related to bone and mineral homeostasis [130].
The autophagic capacity of podocytes plays a critical role in the progression of DKD as well, as podocytes act as a barrier to the passage of proteins during the filtration process. Song et al. [194] demonstrated that DKD affects the autophagic function of podocytes by reducing autophagosomes. Proteins like nephrin and podocin are also reduced in diabetic nephropathy. Markedly, the treatment of diabetic rats with calcitriol was found to restore autophagy activity in podocytes as well as to maintain the expression of nephrin and podocin proteins, thus to mitigate podocyte damage [194]. Ristov et al. [195] used the “ShGlom Assay”, a semi-automated and high-throughput version of the “Glom Assay”, so as to analyze vitamin D’s effect on podocyte differentiation considering glomerulopathies. In accordance with the findings of the above-mentioned study, it was indicated that calcipotriol upregulated the expression of specific podocyte genes and proteins, especially the essential for sustaining podocyte morphology and function at the glomerular filtration barrier (GFB) of nephrin [195].
The epithelial–mesenchymal transition (EMT) is a process where epithelial cells undergo a series of molecular changes resulting in mesenchymal cells. During this process, cells become more flexible, resist apoptosis and as a result, many pathological conditions are induced via the TGF-β1 pathway. In particular, Souza [118] exhibited in vivo that the EMT process was accelerated by vitamin D’s deficiency in diabetic mice. Diabetes combined with vitamin D’s deficiency may lead to an increased expression of mesenchymal markers like the ZEB1 and ZEB2 transcription factors, which regulate the EMT process. Their increase along with the decreased expression of microRNA, miR-200b, and increased levels of TGF-β1 as well inflamed glomerulosclerosis [118].
Furthermore, the dysregulation of autophagy in podocytes is another key factor in the progression of DKD. Zhang et al. [94] revealed recently the protective effect of vitamin D in DKD. In these research outcomes, it was observed in agreement with previous results that the autophagic activity of podocytes was restored both in vitro and in vivo through the regulation of its associated proteins. However, it is worth noting that the exact mechanism underlying this process is not yet fully elucidated [94]. OCTs as other vitamin D analogues were deemed by Hamzawy et al. [196] to reduce blood glucose, while also enhancing autophagy, suppressing podocyte apoptosis and disrupting the G1 cell cycle. Specifically, OCTs activate the VDR receptors, leading to the direct expression of genes such as beclin-1 and the microtubule-associated protein 1A/1B-light chain 3 (LC3). Also, OCTs could affect calcium’s metabolism, the activation of cyclin-dependent kinase (CDK) and the activation of calcium/calmodulin-dependent protein kinase 2, which are involved in the regulation of autophagy. In this way, autophagosomes accumulate and autophagy is effectively regulated [196].
Oxidative stress may also induce DKD, as demonstrated in Yang et al.’s study [117]. ROS were identified as key contributors to diabetic nephropathy’s onset. In the examined cells, ROS were reduced after vitamin D’s administration, while a link between the JAK/STAT signaling pathway and specific microRNAs in mesovascular cells was also revealed. Essentially, it was claimed that vitamin D inhibited the JAK/STAT pathway and thus, it could be used as a treatment for diabetic nephropathy [117]. On the contrary, many research groups claimed that vitamin D supplementation in adults with type II diabetes does not enhance kidney function. A clinical trial conducted by de Boer et al. [197] showed that the administration of vitamin D and omega-3 fatty acids did not significantly slow the decrease in estimated glomerular filtration rate. Moreover, no significant differences in urinary albumin excretion were found between patients who ingested vitamin D and those who did not. Although previous studies have pointed out many benefits, the long-term findings of this study came into disagreement with the previous results claiming vitamin D’s protective role [197]. Recently, da Silva et al. [122] asserted that vitamin D supplementation in patients with CKD reduced the risk of healthcare-associated infections by 59%. The conservative treatment patients undergo needs to ensure the necessary prevention from infections, which is a case where vitamin D may act as an effective preventive agent [122]. Thus, it is understood that the potential of vitamin D is multidimensional and needs further investigation. All of the aforementioned clinical trials on vitamin D’s role against DKD are summarized in Table 1.
4.1.3. Vitamin D’s Role in Kidney Disease Induced by Metals
In addition to diseases that may enhance pathogenicity in the kidneys, various elements such as cadmium (Cd) and lead (Pb) traced in water may also induce it. Obaid [198] examined the impact of vitamin D supplementation in Cd2+-induced CKD rats. Cd2+ at elevated levels impaired calcium’s homeostasis and vitamin D’s regulation in the serum, kidney tissue and urine due to its high toxicity and, thus, exacerbated oxidative stress and inflammation. Treatment with vitamin D and Ca2+, either separately or combined, reduced the built-up oxidative stress and inflammation, modulated Ca2+ regulatory molecules and re-regulated vitamin D’s metabolism [198]. Similar findings were presented by Refaat et al. [199], who investigated the connection between chronic lead exposure and vitamin D supplementation, highlighting the antioxidant and anti-inflammatory mechanisms involved. Lead in a similar way to cadmium leads to tissue damage, oxidative stress and inflammation. Studies declared that calcitriol treatment increased the expression of many antioxidant enzymes such as glutathione peroxidase-1 (Gpx1) and peroxiredoxin-1 (Prdx1), boosted the expression of the anti-inflammatory cytokine IL-10 and decreased the levels of IL-4 and TNF-α [199].
Heavy metal exposure, along with other potential environmental hazards, are reportedly a potent CKD cause. Toxic elements including Cd, aluminum (Al), silica (Al2O3) and strontium (Sr) have been proved to be nephrotoxic factors of kidney-related diseases and dysfunction as well as several complications, CVDs and elevated blood pressure following CKD patients [200]. Recent study trial findings pointed out improved alleviations against CKD and aging by supplementing vitamin D or its analogues, possibly by a regulation of Ca-dependent, antioxidative and anti-inflammatory actions [201]. Furthermore, vitamin D has been demonstrated to mitigate Cd-induced liver and kidney damage; thus, its protective role against Cd toxicity and its potential assistance in the preservation of organ function under toxin-induced stress has been highlighted [202].
Consequently, it is well understood that vitamin D has protective properties against the toxicity of heavy metals like Pb and Cd. The details of the aforementioned clinical trials on vitamin D’s role against kidney disease induced by metals are thoroughly analyzed and further examined in Table 1.
4.1.4. Vitamin D’s Role in the Neuphrotic Syndrome (NS) and Other Kidney Diseases
FSGS and MCD are widely known diseases with primary podocyte damage clinically manifested by the NS; however, the pathogenesis of such podocytopathies and their biomarkers are yet to be discovered [52,57]. Previous studies have claimed that the daily administration of calcitriol or 22-oxacalcitriol ameliorated the nephrotic state by protecting podocytes (Table 1) [93]. In addition, Ca homeostasis derangement is common during NS’ occurrence, as possibly, low total Ca and vitamin D levels are attributed to the loss of protein-bound Ca and vitamin D. Recent study outcomes revealed that vitamin D and Ca bone derangement observed during NS had trended toward normalization and thus, Ca and vitamin D replacement was not indicated regarding early-phase NS, but it may be a great treatment solution in the prolonged form of this renal disease [203].
Children with prevalent NS have reportedly displayed medium to high 25(OH)D3 deficiency rates, a state that was reversed by vitamin D supplementation, which supported a role for supplementation in incident NS [204]. Maji et al. [205] also suggested the immunomodulatory property of vitamin D and the urgent need to introduce this supplementation routinely in all NS cases [205]. Moreover, total 25(OH)D3 levels are low during the nephrotic state condition and are related to a degree of proteinuria increase. In proteinuric renal diseases, free rather than total 25(OH)D3 levels should be used so as to diagnose the vitamin D’s deficiency and to guide efficiently NS’s therapy, while vitamin D administration from steroid-sensitive NS patients is further supported by the study’s outcomes [206].
Except for its regulative role in skeletal homeostasis, RAAS endocrine activity, CKD, DKD, NS, ESRD, AKI, glomerulosclerosis, etc., vitamin D has also a crucial impact on glomerulonephritis (GN). Considering GN, vitamin D supplementation notably reduced proteinuria, impeded kidney disease development, inhibited the onset of kidney inflammation and protected podocytes from injury. More specifically, treatment with calcitriol via the VDR action regulated heparanase promoter activity and monitored podocyte distribution by modulating mRNA synthesis and subsequently the protein expression of nephrin and podocin, thus promoting podocyte protection. Maxacalcitol during the same trial showed more promising results, since it overcame hypercalcemia, hyperphosphatemia and calcification enhancement risk, while paricalcitol and doxercalciferol also exhibited lower hypercalcemia and hypercalciuria and partook in RAAS genes’ expression, proteinuria and renal damage prevention [42,207,208].
Vitamin D deficiency is common; however, no data were obtained concerning vitamin D levels in light chain (AL) amyloidosis (rare B cell-secreting clonal disorder) until recently. A clinical trial conducted by Muchtar et al. [209] demonstrated that hypovitaminosis is frequent in AL amyloidosis, mainly among patients suffering from heavy proteinuria and confirmed that severe 25(OH)D3 deficiency during the diagnosis period could predict ESRD progression [209]. Furthermore, regarding polycystic kidney disease (PKD), chronic vitamin deficiency had a negative long-term impact on proteinuria, interstitial inflammation, renal function, and CVDs in PKD, which invalidated its mild inhibitory effect on kidney enlargement [210]. Similarly, low 25(OH)D3 and VDR levels are linked with a higher kidney volume, supporting that vitamin D’s daily administration in the right doses is not only recommended but also needed toward autosomal dominant PKD (ADPKD) and other kidney diseases [211]. Lastly, in kidney stone disease, many different monogenic polymorphisms have been suggested to play a crucial role for calcium nephrolithiasis during hypercalciuria, like the VDR gene. A balanced Ca consumption via an individual’s nutritional choices has been thus projected as a protective mean toward kidney stone risk prevention due to the fact that intestinal oxalate availability, its urinate expression and subsequently the risk for stone formation are reduced; however, further research is once again required [34].
Although many clinical trials have been conducted with the aim to fully elucidate vitamin D’s specific role in kidney-related diseases, the scientific community is still far from reaching its overall hidden potential. CKD as well as DKD have been extensively studied, but still inadequate data have been collected, while glomerulosclerosis, NS, GN, ESRD, AKI, hyperparathyroidism, PKD, etc. have been less studied, and the little gathered information is insufficient in supporting vitamin D’s exact protective, regulative, modulatory, anti-inflammatory, anti-fibrotic, antioxidant and preventive role.

Table 1.
Experimental data from corresponding clinical trials concerning vitamin D supplementation/administration of drugs containing vitamin D and its analogues and their renal/kidney health-promoting effects against glomerulosclerosis and kidney diseases.
Table 1.
Experimental data from corresponding clinical trials concerning vitamin D supplementation/administration of drugs containing vitamin D and its analogues and their renal/kidney health-promoting effects against glomerulosclerosis and kidney diseases.
| In Vivo (Animal Studies) | |||||
|---|---|---|---|---|---|
| Hypothesis–Intervention | Study Design | Main Findings | Specific Benefits—Mechanisms of Action—Conclusions | Year of Study * | Ref. |
| This study was conducted to measure the alleviating effects of vitamin D and/or Ca as single and as dual therapies against pre-established nephrotoxicity enhanced by chronic cadmium (Cd) toxicity prior to treatment initiation |
|
|
| 2023 | [198] |
| “ShGlomAssay” was applied to a semi-automatic, high-throughput process combined with analysis techniques. Screening of potential drugs and identification of specific pathways, including the calcitriol and vitamin D pathway by using a minimum number of animals, was demonstrated |
|
|
| 2022 | [195] |
| The aim of the present study was to shed a light on the potential impact of a vitamin D analogue, namely 22-oxacalcitriol (OCT), on different cell responses during diabetic neuropathy (DN), as well as the positive interplay between glucose, the immune system and vitamin D in the determination of the cell’s fate |
|
|
| 2017 | [196] |
| The present study was designed to measure the effects of vitamin D3 supplementation on renal and testicular damage during chronic lead intoxication in rats along with the expression profiles of vitamin D-related molecules, oxidative stress markers and a panel of pro- and anti-inflammatory cytokines in tissues of interest |
|
|
| 2018 | [199] |
| The podocyte-protective effects of active vitamin D or its analogue 22-oxacalcitriol, in puromycin amino-nucleoside (PAN) nephrotic rats were examined. Plus, the preventative effects of vitamin D treatment on podocyte injury were further analyzed |
|
|
| 2009 | [93] |
| This study aimed to characterize a model of DN progression in vitamin D as well as the EMT role in these procedures |
|
|
| 2023 | [118] |
| In this study, SELDI–TOF and LC-MS were applicated so as to explore potential serum biomarkers for mesangial proliferation and kidney injury in anti-Thy1 nephritis with a view to monitoring better the progress of mesangial cell proliferation (MesPGN) |
|
|
| 2016 | [212] |
| The aim of this study was to use a large group of db/db and db/+ mice to investigate genes potentially relevant in the pathophysiology of DN, by the use of high-density and oligonucleotide microarrays, to examine glomerular transcription. The observed upregulation in the number of genes in the glomeruli of db/db mice involved in vitamin D and Ca+2 metabolism follow-up studies on these genes, their protein-related products and potent downstream effects was also conducted |
|
|
| 2006 | [193] |
| This study aimed to explore the effects of maternal vitamin D deficiency on glomerular development in early postnatal life and its effects on the renal structure while in the maternity phase, focusing predominantly on the F1 and F2 generations after F0 maternal vitamin D restriction |
|
|
| 2012 | [190] |
| The aim of this study was to confirm the several locations of VDR in our body except for the confirmed one proximal renal tubule. The biological effects of 1α,25 (OH)2D3 were mediated via the VDR, which was present in distal renal convoluted tubule cells; however, whether it is present in other kidney cell types was uncertain, case examined by immunohistochemistry |
|
|
| 2012 | [180] |
| This study aimed to investigate the impact of molecular mechanisms in high-dose vitamin D3 treatment on renal fibrosis |
|
|
| 2021 | [213] |
| This study aimed to investigate the effects of a double treatment with losartan potassium (L), AT1R antagonist, and the tyrosine kinase inhibitor erlotinib € on the alternative pathway of RFF related to TACE–dependent activation of eGFR in 5/6 SN rats that suffered from vitamin D deficiency (D) |
|
|
| 2021 | [183] |
| The aim of this study was to investigate the way ACE inhibitors ameliorate renal disease progression |
|
|
| 2012 | [182] |
| The renal protective effects of vitamin D in a CKD rat model was examined. Also, this study aimed to prove that calcitriol ameliorates kidney injury via reducing podocytopathy, tubular injury, inflammation and fibrosis through 5/6 subtotal neuropathy assay examination |
|
|
| 2020 | [92] |
| In vivo (Human studies) | |||||
| This study was conducted so as to evaluate the effectiveness of the supplementation of vitamin D as a protective agent against the infection of patients with CKD on conservative treatment |
|
|
| 2018 | [122] |
| The main study hypothesis is that oral vitamin D supplementation could possibly ameliorate insulin resistance to patients with CKD at the 3rd stage compared to placebo. The secondary examined hypothesis will test whether this is affiliated with decreased inflammation and bone/ adipocyte–endocrine dysregulation in this single-centered, double-blinded, randomized, placebo-controlled trial |
|
|
| 2009 | [130] |
| This study aimed to evaluated whether vitamin D3 or omega–3 fatty acids (FAs) prevent the development or progression of CKD in type II diabetes mellitus |
|
|
| 2019 | [197] |
| This study’s objective was to expand the assessment of vitamin D’s metabolism in this cohort to include measures of serum DBP, FGF-23 and 24,25(OH)2D3 so as to identify bioavailable 25(OH)D3 concentrations and vitamin D’s catabolism in children’s CKD |
|
|
| 2013 | [191] |
| The aim of this study was to assess vitamin D status and bone density in steroid-treated children with glomerulopathies and to evaluate the effect of prophylactic vitamin D and Ca supplementation |
|
|
| 2012 | [192] |
| The proteomic profile of urine from patients with FSGS along with minimal change disease (MCD) was evaluated |
|
|
| 2022 | [52] |
| In vivo + In vitro (Animal studies) | |||||
| The protective effect and potential mechanism of vitamin D on the podocyte injury of DKD was thoroughly investigated |
|
|
| 2023 | [94] |
| This study aimed to evaluate the effect of vitamin D and VDR signaling on podocyte autophagy in DN |
|
|
| 2021 | [194] |
| In the present study, the effects of vitamin D on glomerular heparanase and heparan sulfate (HS) in animal models of FSGS or 1α,25(OH)2D3 deficiency and in addition, whether vitamin D was able to directly effectively regulate heparanase expression in selected cultured mouse glomerular endothelial cells and podocytes |
|
|
| 2015 | [185] |
| In this study, it was hypothesized that vitamin D reduced proteinuria by affecting TRPC6 expression in podocytes |
|
|
| 2013 | [184] |
| In vivo + In vitro (Human studies) | |||||
| The aim of this study was to investigate whether vitamin D plays a protective role in podocyte injury induced by autoantibodies purified from the serum of lupus nephritis (LN) patients through reducing aberrant autophagy |
|
|
| 2019 | [186] |
| In vitro | |||||
| The present study’s design was to examine the ciPTEC–OAT1 for the expression of genes responsible for vitamin D’s metabolism and function as well as its activation to the vital form 1α,25(OH)2D3. Additionally, the effect of a specific mixture of eight anionic uremic toxins, mimicking uremic conditions of CKD and ESRD, on vitamin D activation and function. Moreover, the beneficial impact of vitamin D on oxidative stress, inflammation, cell viability and the epithelial monolayer barrier function of ciPTEC–OAT1 cultured on biofunctionalized polyethersulfone hollow fiber membranes (HFMs) |
|
|
| 2017 | [181] |
| The function of vitamin D on the JAK/STAT signaling, TGF-β production and fibronectin expression in glomerular mesangial cells was examined |
|
|
| 2021 | [117] |
* Data obtained from clinical trials conducted over the past two decades.
5. Conclusions
The present study examined the several benefits of vitamin D including its anti-inflammatory promise against glomerulosclerosis and kidney diseases. Vitamin D has a variety of protective effects on kidney diseases, including glomerulosclerosis, CKD, DKD, kidney disease induced by metals like cadmium (Cd) and lead (Pb), NS and other kidney-related diseases. Vitamin D and its analogues are believed to hold great anti-inflammatory, anti-fibrotic and antioxidant potential toward kidney-associated diseases, since their supplementation is linked to kidney diseases prevention, inflammatory signaling pathways’ modulation, immunity system/metabolism’s enhancement and other related diseases, like CVDs proliferation reduction.
Toward glomerulosclerosis, vitamin D and its analogues regulate many pathways involved in podocyte damage, inflammation and oxidative stress, and they reduce the expression of TRPC6, inhibit heparinase activity and regulate autophagy and apoptosis in podocytes; thus, renal well-being is preserved. Moreover, considering DKD, vitamin D restores autophagy activity, maintains podocyte gene expression and mitigates EMT while combating oxidative stress and inflammation. Despite some conflicting evidence, vitamin D supplementation holds promise for improving outcomes in CKD and other kidney diseases potentially by reducing healthcare-associated infections. As for kidney disease caused by heavy metals such as Cd and Pb, vitamin D supplementation also successfully alleviates inflammation and oxidative stress; hence, its protective role against environmental nephrotoxicity is projected. Overall, the multifaceted effects of vitamin D highlight its therapeutic potential in renal diseases, paving the way to further research in order to elucidate its mechanisms and to develop more effective future treatment strategies toward kidney-related diseases.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; software, All Authors; validation, A.T.; investigation, All Authors; writing—original draft preparation, All Authors; writing—review and editing, A.T.; visualization, A.T.; supervision, A.T.; project administration, A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to thank the School of Chemistry of the Faculty of Science of the Democritus University of Thrace for the continuous support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Liu, L.; Yan, Y.; Qiu, J.; Chen, Q.; Zhang, Y.; Liu, Y.; Zhong, X.; Liu, Y.; Tan, R. Association between Sedentary Behavior and Depression in US Adults with Chronic Kidney Disease: NHANES 2007–2018. BMC Psychiatry 2023, 23, 148. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, C.; Gao, X.; Sun, Y.; Han, X. Positive Associations between Different Circulating Trans Fatty Acids (TFAs) and Urinary Albumin Excretion among Adults in the U.S.: A Population-Based Study. Lipids Health Dis. 2023, 22, 152. [Google Scholar] [CrossRef]
- Kovesdy, C.P. Epidemiology of Chronic Kidney Disease: An Update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Mortality and Causes of Death Collaborators Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [CrossRef]
- Colombo, G.; Altomare, A.; Astori, E.; Landoni, L.; Garavaglia, M.L.; Rossi, R.; Giustarini, D.; Lionetti, M.C.; Gagliano, N.; Milzani, A.; et al. Effects of Physiological and Pathological Urea Concentrations on Human Microvascular Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 691. [Google Scholar] [CrossRef]
- Feher, J. 7.2—Functional Anatomy of the Kidneys and Overview of Kidney Function. In Quantitative Human Physiology; Feher, J., Ed.; Academic Press: Boston, MA, USA, 2012; pp. 626–632. ISBN 978-0-12-382163-8. [Google Scholar]
- Valenzuela Fuenzalida, J.J.; Vera-Tapia, K.; Urzúa-Márquez, C.; Yáñez-Castillo, J.; Trujillo-Riveros, M.; Koscina, Z.; Orellana-Donoso, M.; Nova-Baeza, P.; Suazo-Santibañez, A.; Sanchis-Gimeno, J.; et al. Anatomical Variants of the Renal Veins and Their Relationship with Morphofunctional Alterations of the Kidney: A Systematic Review and Meta-Analysis of Prevalence. J. Clin. Med. 2024, 13, 3689. [Google Scholar] [CrossRef]
- Dybiec, J.; Szlagor, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Structural and Functional Changes in Aging Kidneys. Int. J. Mol. Sci. 2022, 23, 15435. [Google Scholar] [CrossRef]
- Tibi, S.; Zeynalvand, G.; Mohsin, H. Role of the Renin Angiotensin Aldosterone System in the Pathogenesis of Sepsis-Induced Acute Kidney Injury: A Systematic Review. J. Clin. Med. 2023, 12, 4566. [Google Scholar] [CrossRef]
- Finco, D.R. Chapter 17—Kidney Function. In Clinical Biochemistry of Domestic Animals, 5th ed.; Kaneko, J.J., Harvey, J.W., Bruss, M.L., Eds.; Academic Press: San Diego, CA, USA, 1997; pp. 441–484. ISBN 978-0-12-396305-5. [Google Scholar]
- Christakos, S.; Ajibade, D.V.; Dhawan, P.; Fechner, A.J.; Mady, L.J. Vitamin D: Metabolism. Endocrinol. Metab. Clin. N. Am. 2010, 39, 243–253. [Google Scholar] [CrossRef]
- Okuda, K.-I.; Ohyama, Y. The Enzymes Responsible for Metabolizing Vitamin D. In Vitamin D: Molecular Biology, Physiology, and Clinical Applications; Holick, M.F., Ed.; Humana Press: Totowa, NJ, USA, 1999; pp. 85–99. ISBN 978-1-4757-2861-3. [Google Scholar]
- Tsiftsoglou, A.S. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells 2021, 10, 2140. [Google Scholar] [CrossRef]
- Migliaccio, A.R. Erythropoietin: A Personal Alice in Wonderland Trip in the Shadow of the Giants. Biomolecules 2024, 14, 408. [Google Scholar] [CrossRef]
- Qu, L.; Jiao, B. The Interplay between Immune and Metabolic Pathways in Kidney Disease. Cells 2023, 12, 1584. [Google Scholar] [CrossRef]
- Łapczuk-Romańska, J.; Droździk, M.; Oswald, S.; Droździk, M. Kidney Drug Transporters in Pharmacotherapy. Int. J. Mol. Sci. 2023, 24, 2856. [Google Scholar] [CrossRef]
- Deng, J.; Li, L.; Feng, Y.; Yang, J. Comprehensive Management of Blood Pressure in Patients with Septic AKI. J. Clin. Med. 2023, 12, 1018. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H. Primary Role of the Kidney in Pathogenesis of Hypertension. Life 2024, 14, 119. [Google Scholar] [CrossRef]
- Päivärinta, J.; Anastasiou, I.A.; Koivuviita, N.; Sharma, K.; Nuutila, P.; Ferrannini, E.; Solini, A.; Rebelos, E. Renal Perfusion, Oxygenation and Metabolism: The Role of Imaging. J. Clin. Med. 2023, 12, 5141. [Google Scholar] [CrossRef]
- Fularski, P.; Czarnik, W.; Frankenstein, H.; Gąsior, M.; Młynarska, E.; Rysz, J.; Franczyk, B. Unveiling Selected Influences on Chronic Kidney Disease Development and Progression. Cells 2024, 13, 751. [Google Scholar] [CrossRef]
- Hansen, K.L.; Nielsen, M.B.; Ewertsen, C. Ultrasonography of the Kidney: A Pictorial Review. Diagnostics 2016, 6, 2. [Google Scholar] [CrossRef]
- Ham, Y.R.; Lee, E.J.; Kim, H.R.; Jeon, J.W.; Na, K.R.; Lee, K.W.; Choi, D.E. Ultrasound Renal Score to Predict the Renal Disease Prognosis in Patients with Diabetic Kidney Disease: An Investigative Study. Diagnostics 2023, 13, 515. [Google Scholar] [CrossRef]
- Maralescu, F.-M.; Bende, F.; Sporea, I.; Popescu, A.; Sirli, R.; Schiller, A.; Petrica, L.; Miutescu, B.; Borlea, A.; Popa, A.; et al. Non-Invasive Evaluation of Kidney Elasticity and Viscosity in a Healthy Cohort. Biomedicines 2022, 10, 2859. [Google Scholar] [CrossRef]
- Russo, E.; Tagliafico, A.S.; Derchi, L.; Bignotti, B.; Tosto, S.; Martinoli, C.; Signori, A.; Brigati, F.; Viazzi, F. Role of Renal Parenchyma Attenuation and Perirenal Fat Stranding in Chest CT of Hospitalized Patients with COVID-19. J. Clin. Med. 2023, 12, 929. [Google Scholar] [CrossRef]
- Kochupillai, N. The Physiology of Vitamin D: Current Concepts. Indian J. Med. Res. 2008, 127, 256–262. [Google Scholar]
- Koushanpour, E.; Kriz, W. An Overview of the Structural and Functional Organization of the Kidney. In Renal Physiology: Principles, Structure, and Function; Koushanpour, E., Kriz, W., Eds.; Springer: New York, NY, USA, 1986; pp. 41–52. ISBN 978-1-4757-1912-3. [Google Scholar]
- Fleet, J.C. The Role of Vitamin D in the Endocrinology Controlling Calcium Homeostasis. Mol. Cell. Endocrinol. 2017, 453, 36–45. [Google Scholar] [CrossRef]
- Khammissa, R.a.G.; Fourie, J.; Motswaledi, M.H.; Ballyram, R.; Lemmer, J.; Feller, L. The Biological Activities of Vitamin D and Its Receptor in Relation to Calcium and Bone Homeostasis, Cancer, Immune and Cardiovascular Systems, Skin Biology, and Oral Health. BioMed Res. Int. 2018, 2018, 9276380. [Google Scholar] [CrossRef]
- Dusso, A.S.; Brown, A.J.; Slatopolsky, E. Vitamin D. Am. J. Physiol. Ren. Physiol. 2005, 289, F8–F28. [Google Scholar] [CrossRef]
- Lips, P. Vitamin D Physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Slominski, A.T.; Janjetovic, Z.; Fuller, B.E.; Zmijewski, M.A.; Tuckey, R.C.; Nguyen, M.N.; Sweatman, T.; Li, W.; Zjawiony, J.; Miller, D.; et al. Products of Vitamin D3 or 7-Dehydrocholesterol Metabolism by Cytochrome P450scc Show Anti-Leukemia Effects, Having Low or Absent Calcemic Activity. PLoS ONE 2010, 5, e9907. [Google Scholar] [CrossRef]
- Brandi, M.L. Indications on the Use of Vitamin D and Vitamin D Metabolites in Clinical Phenotypes. Clin. Cases Min. Bone Metab. 2010, 7, 243–250. [Google Scholar]
- Heaney, R.P.; Weaver, C.M. Calcium and Vitamin D. Endocrinol. Metab. Clin. N. Am. 2003, 32, 181–194. [Google Scholar] [CrossRef]
- Bargagli, M.; Ferraro, P.M.; Vittori, M.; Lombardi, G.; Gambaro, G.; Somani, B. Calcium and Vitamin D Supplementation and Their Association with Kidney Stone Disease: A Narrative Review. Nutrients 2021, 13, 4363. [Google Scholar] [CrossRef]
- Heaney, R.P. The Calcium Economy. In Calcium in Human Health; Weaver, C.M., Heaney, R.P., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 145–162. ISBN 978-1-59259-961-5. [Google Scholar]
- Szeto, C.-C.; Li, P.K.-T. The Use of Vitamin D Analogues in Chronic Kidney Diseases: Possible Mechanisms beyond Bone and Mineral Metabolism. NDT Plus 2009, 2, 205–212. [Google Scholar] [CrossRef][Green Version]
- Selamet, U.; Katz, R.; Ginsberg, C.; Rifkin, D.E.; Fried, L.F.; Kritchevsky, S.B.; Hoofnagle, A.N.; Bibbins-Domingo, K.; Drew, D.; Harris, T.; et al. Serum Calcitriol Concentrations and Kidney Function Decline, Heart Failure, and Mortality in Elderly Community-Living Adults: The Health, Aging, and Body Composition Study. Am. J. Kidney Dis. 2018, 72, 419–428. [Google Scholar] [CrossRef]
- Hamzawy, M.; Gouda, S.A.A.; Rashed, L.; Morcos, M.A.; Shoukry, H.; Sharawy, N. 22-Oxacalcitriol Prevents Acute Kidney Injury via Inhibition of Apoptosis and Enhancement of Autophagy. Clin. Exp. Nephrol. 2019, 23, 43–55. [Google Scholar] [CrossRef]
- Grieff, M.; Dusso, A.; Mori, T.; Nishii, Y.; Slatopolsky, E.; Brown, A.J. 22-Oxacalcitriol Suppresses 25-Hydroxycholecalciferol-1 Alpha-Hydroxylase in Rat Kidney. Biochem. Biophys. Res. Commun. 1992, 185, 191–196. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Morrissey, J.; Finch, J.L.; Martin, D.R.; Liapis, H.; Akizawa, T.; Slatopolsky, E. Combination Therapy with an Angiotensin-Converting Enzyme Inhibitor and a Vitamin D Analog Suppresses the Progression of Renal Insufficiency in Uremic Rats. J. Am. Soc. Nephrol. 2007, 18, 1796–1806. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.-Y.; Jia, Y.; Wu, M.-Y.; Sun, Y.-Y.; Ma, F.-Z. Efficacy and Safety of Paricalcitol in Patients Undergoing Hemodialysis: A Meta-Analysis. Drug Des. Devel. Ther. 2019, 13, 999–1009. [Google Scholar] [CrossRef]
- Gembillo, G.; Siligato, R.; Amatruda, M.; Conti, G.; Santoro, D. Vitamin D and Glomerulonephritis. Medicina 2021, 57, 186. [Google Scholar] [CrossRef]
- Ganimusa, I.; Chew, E.; Lu, E.M.-C. Vitamin D Deficiency, Chronic Kidney Disease and Periodontitis. Medicina 2024, 60, 420. [Google Scholar] [CrossRef]
- Hsu, S.; Vervloet, M.G.; Boer, I.H. de Vitamin D in CKD: An Unfinished Story. Am. J. Kidney Dis. 2023, 82, 512–514. [Google Scholar] [CrossRef]
- Perwad, F.; Zhang, M.Y.H.; Tenenhouse, H.S.; Portale, A.A. Fibroblast Growth Factor 23 Impairs Phosphorus and Vitamin D Metabolism in Vivo and Suppresses 25-Hydroxyvitamin D-1alpha-Hydroxylase Expression in Vitro. Am. J. Physiol. Ren. Physiol. 2007, 293, F1577–F1583. [Google Scholar] [CrossRef]
- Evenepoel, P.; Jørgensen, H.S.; Bover, J.; Davenport, A.; Bacchetta, J.; Haarhaus, M.; Hansen, D.; Gracia-Iguacel, C.; Ketteler, M.; McAlister, L.; et al. Recommended Calcium Intake in Adults and Children with Chronic Kidney Disease-a European Consensus Statement. Nephrol. Dial. Transpl. 2024, 39, 341–366. [Google Scholar] [CrossRef]
- Moorthi, R.N.; Moe, S.M. CKD–Mineral and Bone Disorder: Core Curriculum 2011. Am. J. Kidney Dis. 2011, 58, 1022–1036. [Google Scholar] [CrossRef]
- Schwarz, U.; Amann, K.; Orth, S.R.; Simonaviciene, A.; Wessels, S.; Ritz, E. Effect of 1,25(OH)2 Vitamin D3 on Glomerulosclerosis in Subtotally Nephrectomized Rats. Kidney Int. 1998, 53, 1696–1705. [Google Scholar] [CrossRef]
- Caravaca, F.; Caravaca-Fontán, F.; Azevedo, L.; Luna, E. Changes in Renal Function after Discontinuation of Vitamin D Analogues in Advanced Chronic Kidney Disease. Nefrología 2018, 38, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Moe, S.M.; Chen, N.X. Mechanisms of Vascular Calcification in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2008, 19, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Zaslow, S.J.; Oliveira-Paula, G.H.; Chen, W. Magnesium and Vascular Calcification in Chronic Kidney Disease: Current Insights. Int. J. Mol. Sci. 2024, 25, 1155. [Google Scholar] [CrossRef]
- Chebotareva, N.V.; Vinogradov, A.; Brzhozovskiy, A.G.; Kashirina, D.N.; Indeykina, M.I.; Bugrova, A.E.; Lebedeva, M.; Moiseev, S.; Nikolaev, E.N.; Kononikhin, A.S. Potential Urine Proteomic Biomarkers for Focal Segmental Glomerulosclerosis and Minimal Change Disease. Int. J. Mol. Sci. 2022, 23, 12607. [Google Scholar] [CrossRef]
- Armaly, Z.; Abu-Rahme, M.; Kinaneh, S.; Hijazi, B.; Habbasshi, N.; Artul, S. An Innovative Ultrasound Technique for Early Detection of Kidney Dysfunction: Superb Microvascular Imaging as a Reference Standard. J. Clin. Med. 2022, 11, 925. [Google Scholar] [CrossRef] [PubMed]
- Chhuon, C.; Herrera-Marcos, L.V.; Zhang, S.-Y.; Charrière-Bertrand, C.; Jung, V.; Lipecka, J.; Savas, B.; Nasser, N.; Pawlak, A.; Boulmerka, H.; et al. Proteomics of Plasma and Plasma-Treated Podocytes: Application to Focal and Segmental Glomerulosclerosis. Int. J. Mol. Sci. 2023, 24, 12124. [Google Scholar] [CrossRef]
- Marcos González, S.; Rodrigo Calabia, E.; Varela, I.; Červienka, M.; Freire Salinas, J.; Gómez Román, J.J. High Rate of Mutations of Adhesion Molecules and Extracellular Matrix Glycoproteins in Patients with Adult-Onset Focal and Segmental Glomerulosclerosis. Biomedicines 2023, 11, 1764. [Google Scholar] [CrossRef]
- den Braanker, D.J.W.; Maas, R.J.H.; van Mierlo, G.; Parr, N.M.J.; Bakker-van Bebber, M.; Deegens, J.K.J.; Jansen, P.W.T.C.; Gloerich, J.; Willemsen, B.; Dijkman, H.B.; et al. Primary Focal Segmental Glomerulosclerosis Plasmas Increase Lipid Droplet Formation and Perilipin-2 Expression in Human Podocytes. Int. J. Mol. Sci. 2023, 24, 194. [Google Scholar] [CrossRef] [PubMed]
- Veissi, S.T.; Smeets, B.; van Wijk, J.A.E.; Classens, R.; van der Velden, T.J.A.M.; Jeronimus-Klaasen, A.; Veltkamp, F.; Mak-Nienhuis, E.M.; Morello, W.; Montini, G.; et al. Circulating Permeability Factors in Focal Segmental Glomerulosclerosis: In Vitro Detection. Kidney Int. Rep. 2022, 7, 2691–2703. [Google Scholar] [CrossRef] [PubMed]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef]
- Kadatane, S.P.; Satariano, M.; Massey, M.; Mongan, K.; Raina, R. The Role of Inflammation in CKD. Cells 2023, 12, 1581. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Lloberas, N.; Torras, J.; Herrero-Fresneda, I.; Cruzado, J.M.; Riera, M.; Hurtado, I.; Grinyó, J.M. Postischemic Renal Oxidative Stress Induces Inflammatory Response through PAF and Oxidized Phospholipids. Prevention by Antioxidant Treatment. FASEB J. 2002, 16, 908–910. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Srivastava, A.; Cruz-Gregorio, A.; Pedraza-Chaverri, J.; Mulay, S.R.; Scholze, A. Involvement of Inflammasome Components in Kidney Disease. Antioxidants 2022, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Anders, H.-J. Of Inflammasomes and Alarmins: IL-1β and IL-1α in Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 2564–2575. [Google Scholar] [CrossRef]
- Xiang, H.; Zhu, F.; Xu, Z.; Xiong, J. Role of Inflammasomes in Kidney Diseases via Both Canonical and Non-Canonical Pathways. Front. Cell Dev. Biol. 2020, 8, 106. [Google Scholar] [CrossRef]
- Komada, T.; Muruve, D.A. The Role of Inflammasomes in Kidney Disease. Nat. Rev. Nephrol. 2019, 15, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Islamuddin, M.; Qin, X. Renal Macrophages and NLRP3 Inflammasomes in Kidney Diseases and Therapeutics. Cell Death Discov. 2024, 10, 229. [Google Scholar] [CrossRef]
- Xiong, W.; Meng, X.-F.; Zhang, C. NLRP3 Inflammasome in Metabolic-Associated Kidney Diseases: An Update. Front. Immunol. 2021, 12, 714340. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Chiang, C.-K. Uremic Toxins and Protein-Bound Therapeutics in AKI and CKD: Up-to-Date Evidence. Toxins 2021, 14, 8. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef]
- Gu, Y.-Y.; Liu, X.-S.; Huang, X.-R.; Yu, X.-Q.; Lan, H.-Y. Diverse Role of TGF-β in Kidney Disease. Front. Cell Dev. Biol. 2020, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Ramseyer, V.D.; Garvin, J.L. Tumor Necrosis Factor-α: Regulation of Renal Function and Blood Pressure. Am. J. Physiol. Ren. Physiol. 2013, 304, F1231–F1242. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef]
- Renke, G.; Starling-Soares, B.; Baesso, T.; Petronio, R.; Aguiar, D.; Paes, R. Effects of Vitamin D on Cardiovascular Risk and Oxidative Stress. Nutrients 2023, 15, 769. [Google Scholar] [CrossRef]
- Fernandez-Robredo, P.; González-Zamora, J.; Recalde, S.; Bilbao-Malavé, V.; Bezunartea, J.; Hernandez, M.; Garcia-Layana, A. Vitamin D Protects against Oxidative Stress and Inflammation in Human Retinal Cells. Antioxidants 2020, 9, 838. [Google Scholar] [CrossRef]
- Bergandi, L.; Palladino, G.; Meduri, A.; De Luca, L.; Silvagno, F. Vitamin D and Sulforaphane Decrease Inflammatory Oxidative Stress and Restore the Markers of Epithelial Integrity in an In Vitro Model of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2024, 25, 6404. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Holick, M.F. The One-Hundred-Year Anniversary of the Discovery of the Sunshine Vitamin D3: Historical, Personal Experience and Evidence-Based Perspectives. Nutrients 2023, 15, 593. [Google Scholar] [CrossRef]
- DeLuca, H.F. History of the Discovery of Vitamin D and Its Active Metabolites. Bonekey Rep. 2014, 3, 479. [Google Scholar] [CrossRef]
- Jones, G. 100 Years of Vitamin D: Historical Aspects of Vitamin D. Endocr. Connect. 2022, 11, e210594. [Google Scholar] [CrossRef] [PubMed]
- Steenbock, H.; Black, A. Fat-soluble vitamins: XVII. the induction of growth-promoting and calcifying properties in a ration by exposure to ultra-violet light. J. Biol. Chem. 1924, 61, 405–422. [Google Scholar] [CrossRef]
- Askew, F.A.; Bourdillon, R.B.; Bruce, H.M.; Jenkins RG, C.; Webster, T.A. The Distillation of Vitamin D. Proc. R. Soc. Lond. B 1930, 107, 76–90. [Google Scholar] [CrossRef]
- Windaus, A.; Schenck, F.; Werder, F.T. Über Das Antirachitisch Wirksame Bestrahlungsprodukt Ans 7-Dehydro-Cholesterin. Biol. Chem. 1936, 241, 100–103. [Google Scholar] [CrossRef]
- Balachandar, R.; Pullakhandam, R.; Kulkarni, B.; Sachdev, H.S. Relative Efficacy of Vitamin D2 and Vitamin D3 in Improving Vitamin D Status: Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3328. [Google Scholar] [CrossRef] [PubMed]
- Bosworth, C.; de Boer, I.H. Impaired Vitamin D Metabolism in CKD. Semin. Nephrol. 2013, 33, 158–168. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, X.; Song, F.; Xue, D.; Wang, Y. Vitamin D3 Promotes Autophagy in THP-1 Cells Infected with Mycobacterium Tuberculosis. Exp. Ther. Med. 2022, 23, 240. [Google Scholar] [CrossRef]
- Chen, D.-P.; Ma, Y.-P.; Zhuo, L.; Zhang, Z.; Zou, G.-M.; Yang, Y.; Gao, H.-M.; Li, W.-G. 1,25-Dihydroxyvitamin D3 Inhibits the Proliferation of Rat Mesangial Cells Induced by High Glucose via DDIT4. Oncotarget 2018, 9, 418. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-C.; Liao, M.-T.; Hsiao, P.-J.; Lu, C.-L.; Hsu, Y.-J.; Lu, K.-C.; Chu, P. Antiproteinuria Effect of Calcitriol in Patients With Chronic Kidney Disease and Vitamin D Deficiency: A Randomized Controlled Study. J. Ren. Nutr. 2020, 30, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Oristrell, J.; Oliva, J.C.; Subirana, I.; Casado, E.; Domínguez, D.; Toloba, A.; Aguilera, P.; Esplugues, J.; Fafián, P.; Grau, M. Association of Calcitriol Supplementation with Reduced COVID-19 Mortality in Patients with Chronic Kidney Disease: A Population-Based Study. Biomedicines 2021, 9, 509. [Google Scholar] [CrossRef]
- Negrea, L. Active Vitamin D in Chronic Kidney Disease: Getting Right Back Where We Started From? Kidney Dis. 2018, 5, 59–68. [Google Scholar] [CrossRef]
- Sari, D.C.R.; Putri, M.W.; Leksono, T.P.; Chairunnisa, N.; Reynaldi, G.N.; Simanjuntak, B.C.; Debora, J.; Yunus, J.; Arfian, N. Calcitriol Ameliorates Kidney Injury Through Reducing Podocytopathy, Tubular Injury, Inflammation and Fibrosis in 5/6 Subtotal Nephrectomy Model in Rats. Kobe J. Med. Sci. 2020, 65, E153–E163. [Google Scholar] [PubMed]
- Matsui, I.; Hamano, T.; Tomida, K.; Inoue, K.; Takabatake, Y.; Nagasawa, Y.; Kawada, N.; Ito, T.; Kawachi, H.; Rakugi, H.; et al. Active Vitamin D and Its Analogue, 22-Oxacalcitriol, Ameliorate Puromycin Aminonucleoside-Induced Nephrosis in Rats. Nephrol. Dial. Transpl. 2009, 24, 2354–2361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Wen, Y.; Zhang, M.; Liu, S.; Xiao, H. Vitamin D Ameliorates Podocyte Injury by Enhancing Autophagy Activity in Diabetic Kidney Disease. Kidney Blood Press Res. 2023, 48, 314–325. [Google Scholar] [CrossRef]
- Verouti, S.N.; Tsoupras, A.B.; Alevizopoulou, F.; Demopoulos, C.A.; Iatrou, C. Paricalcitol Effects on Activities and Metabolism of Platelet Activating Factor and on Inflammatory Cytokines in Hemodialysis Patients. Int. J. Artif. Organs 2013, 36, 87–96. [Google Scholar] [CrossRef]
- Verma, V.; Lamture, Y.; Ankar, R. Management of Uremic Xerosis and Chronic Kidney Disease (CKD)-Associated Pruritus (CKD-Ap) With Topical Preparations: A Systematic Review and Implications in the Indian Context. Cureus 2023, 15, e42587. [Google Scholar] [CrossRef]
- Jung, K.E.; Woo, Y.R.; Lee, J.S.; Shin, J.H.; Jeong, J.U.; Koo, D.W.; Bang, K.T. Effect of Topical Vitamin D on Chronic Kidney Disease-Associated Pruritus: An Open-Label Pilot Study. J. Dermatol. 2015, 42, 800–803. [Google Scholar] [CrossRef]
- Vervloet, M. Clinical Uses of 1-Alpha-Hydroxycholecalciferol. Curr Vasc Pharmacol 2014, 12, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D. A Randomised Clinical Study of Alfacalcidol and Paricalcitol. Dan. Med. J. 2012, 59, B4400. [Google Scholar]
- Dheerendra, P.C.; Sakhuja, V.; Kohli, H.S.; Jha, V. Efficacy and Safety of Oral Doxercalciferol in the Management of Secondary Hyperparathyroidism in Chronic Kidney Disease Stage 4. Indian J. Nephrol. 2013, 23, 271–275. [Google Scholar] [CrossRef]
- Coburn, J.W.; Maung, H.M.; Elangovan, L.; Germain, M.J.; Lindberg, J.S.; Sprague, S.M.; Williams, M.E.; Bishop, C.W. Doxercalciferol Safely Suppresses PTH Levels in Patients with Secondary Hyperparathyroidism Associated with Chronic Kidney Disease Stages 3 and 4. Am. J. Kidney Dis. 2004, 43, 877–890. [Google Scholar] [CrossRef][Green Version]
- Zand, L.; Kumar, R. The Use of Vitamin D Metabolites and Analogs in the Treatment of Chronic Kidney Disease. Endocrinol. Metab. Clin. N. Am. 2017, 46, 983–1007. [Google Scholar] [CrossRef] [PubMed]
- Sparaco, M.; Bonavita, S. Vitamin D Supplementation: Effect on Cytokine Profile in Multiple Sclerosis. J. Clin. Med. 2024, 13, 835. [Google Scholar] [CrossRef]
- Roffe-Vazquez, D.N.; Huerta-Delgado, A.S.; Castillo, E.C.; Villarreal-Calderón, J.R.; Gonzalez-Gil, A.M.; Enriquez, C.; Garcia-Rivas, G.; Elizondo-Montemayor, L. Correlation of Vitamin D with Inflammatory Cytokines, Atherosclerotic Parameters, and Lifestyle Factors in the Setting of Heart Failure: A 12-Month Follow-Up Study. Int. J. Mol. Sci. 2019, 20, 5811. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Ge, X.; Du, J.; Deb, D.K.; Li, Y.C. Vitamin D Receptor Inhibits Nuclear Factor κB Activation by Interacting with IκB Kinase β Protein. J. Biol. Chem. 2013, 288, 19450–19458. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; Moore, S.M.; Kishi, N.; Macklis, J.D.; MacDonald, J.L. Vitamin D Supplementation Rescues Aberrant NF-κB Pathway Activation and Partially Ameliorates Rett Syndrome Phenotypes in Mecp2 Mutant Mice. eNeuro 2020, 7, ENEURO.0167-20.2020. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C. Chapter 45—Vitamin D and the Renin-Angiotensin System. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 825–847. ISBN 978-0-12-809965-0. [Google Scholar]
- McMullan, C.J.; Borgi, L.; Curhan, G.C.; Fisher, N.; Forman, J.P. The Effect of Vitamin D on Renin-Angiotensin-System Activation and Blood Pressure—A Randomized Control Trial. J. Hypertens. 2017, 35, 822–829. [Google Scholar] [CrossRef]
- Ajabshir, S.; Asif, A.; Nayer, A. The Effects of Vitamin D on the Renin-Angiotensin System. J. Nephropathol. 2014, 3, 41–43. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Peng, L. Vitamin D and Pulmonary Fibrosis: A Review of Molecular Mechanisms. Int. J. Clin. Exp. Pathol. 2019, 12, 3171–3178. [Google Scholar] [PubMed]
- Chen, T.; Zuo, X.; Wang, S.; Yu, P.; Yuan, J.; Wei, S.; Chen, J.; Sun, Y.; Gao, Y.; Li, X. The Effect of Vitamin D Supplementation on the Progression of Fibrosis in Patients with Chronic Liver Disease. Medicine 2020, 99, e20296. [Google Scholar] [CrossRef]
- Konijeti, G.G.; Arora, P.; Boylan, M.R.; Song, Y.; Huang, S.; Harrell, F.; Newton-Cheh, C.; O’Neill, D.; Korzenik, J.; Wang, T.J.; et al. Vitamin D Supplementation Modulates T Cell–Mediated Immunity in Humans: Results from a Randomized Control Trial. J. Clin. Endocrinol. Metab. 2016, 101, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Ghaseminejad-Raeini, A.; Ghaderi, A.; Sharafi, A.; Nematollahi-Sani, B.; Moossavi, M.; Derakhshani, A.; Sarab, G.A. Immunomodulatory Actions of Vitamin D in Various Immune-Related Disorders: A Comprehensive Review. Front. Immunol. 2023, 14, 950465. [Google Scholar] [CrossRef]
- Dalan, R.; Liew, H.; Tan, W.K.A.; Chew, D.E.K.; Leow, M.K.-S. Vitamin D and the Endothelium: Basic, Translational and Clinical Research Updates. IJC Metab. Endocr. 2014, 4, 4–17. [Google Scholar] [CrossRef]
- Kim, D.-H.; Meza, C.A.; Clarke, H.; Kim, J.-S.; Hickner, R.C. Vitamin D and Endothelial Function. Nutrients 2020, 12, 575. [Google Scholar] [CrossRef]
- Bhutia, S.K. Vitamin D in Autophagy Signaling for Health and Diseases: Insights on Potential Mechanisms and Future Perspectives. J. Nutr. Biochem. 2022, 99, 108841. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, Y.; Liang, Y.; Fu, S.; Yang, C.; Liu, K.; Chen, Y. Vitamin D Protects Glomerular Mesangial Cells from High Glucose-Induced Injury by Repressing JAK/STAT Signaling. Int. Urol. Nephrol. 2021, 53, 1247–1254. [Google Scholar] [CrossRef]
- Souza, C.S.; Deluque, A.L.; Oliveira, B.M.; Maciel, A.L.D.; Giovanini, C.; Boer, P.A.; de Paula, F.J.A.; Costa, R.S.; Franscecato, H.D.C.; de Almeida, L.F.; et al. Vitamin D Deficiency Contributes to the Diabetic Kidney Disease Progression via Increase ZEB1/ZEB2 Expressions. Nutr. Diabetes 2023, 13, 9. [Google Scholar] [CrossRef]
- Wu, W.; Li, X.; Di, J.; Zhou, H.; Niu, H.; Yang, M. Dietary Inflammatory Index Is Associated with Vitamin D in CKD Patients. Int. Urol. Nephrol. 2024, 56, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Krajewska, M.; Witkowska-Sędek, E.; Rumińska, M.; Stelmaszczyk-Emmel, A.; Sobol, M.; Majcher, A.; Pyrżak, B. Vitamin D Effects on Selected Anti-Inflammatory and Pro-Inflammatory Markers of Obesity-Related Chronic Inflammation. Front. Endocrinol. 2022, 13, 920340. [Google Scholar] [CrossRef] [PubMed]
- Brito, R.B.D.O.; Rebello, J.F.; Grabulosa, C.C.; Pinto, W.; Morales, A.; Elias, R.M.; Moyses, R.M.A.; Dalboni, M.A. 25-Vitamin D Reduces Inflammation in Uremic Environment. Sci. Rep. 2020, 10, 128. [Google Scholar] [CrossRef] [PubMed]
- da Silva, E.C.; Taminato, M.; da Fonseca, C.D.; de Moraes, G.M.; Longo, M.C.B.; Grothe, C.E.; Belasco, A.G.S.; Barbosa, D.A. Use of Vitamin D and Infection in Patients with Chronic Kidney Disease. Rev. Bras. Enferm. 2018, 71, 2792–2799. [Google Scholar] [CrossRef]
- Li, Y.C. Vitamin D: Roles in renal and cardiovascular protection. Curr. Opin. Nephrol. Hypertens. 2012, 21, 72–79. [Google Scholar] [CrossRef]
- Bai, Y.-J.; Li, Y.-M.; Hu, S.-M.; Zou, Y.-G.; An, Y.-F.; Wang, L.-L.; Shi, Y.-Y. Vitamin D Supplementation Reduced Blood Inflammatory Cytokines Expression and Improved Graft Function in Kidney Transplant Recipients. Front. Immunol. 2023, 14, 1152295. [Google Scholar] [CrossRef]
- Xu, S.; Chen, Y.-H.; Tan, Z.-X.; Xie, D.-D.; Zhang, C.; Zhang, Z.-H.; Wang, H.; Zhao, H.; Yu, D.-X.; Xu, D.-X. Vitamin D3 Pretreatment Regulates Renal Inflammatory Responses during Lipopolysaccharide-Induced Acute Kidney Injury. Sci. Rep. 2015, 5, 18687. [Google Scholar] [CrossRef]
- Mazanova, A.; Shymanskyi, I.; Lisakovska, O.; Labudzynskyi, D.; Khomenko, A.; Veliky, M. The Link between Vitamin D Status and NF-κB-Associated Renal Dysfunction in Experimental Diabetes Mellitus. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2022, 1866, 130136. [Google Scholar] [CrossRef]
- Zomorodian, S.A.; Shafiee, M.; Karimi, Z.; Masjedi, F.; Roshanshad, A. Assessment of the Relationship between 25-Hydroxyvitamin D and Albuminuria in Type 2 Diabetes Mellitus. BMC Endocr. Disord. 2022, 22, 171. [Google Scholar] [CrossRef]
- Wu, C.-C.; Lu, K.-C.; Wu, C.-C.; Lu, K.-C. Pleiotropic Effects of Vitamin D in Kidney Disease. In A Critical Evaluation of Vitamin D—Clinical Overview; IntechOpen: London, UK, 2017; ISBN 978-953-51-3086-4. [Google Scholar]
- Li, L.; Zhang, Y.-L.; Liu, X.-Y.; Meng, X.; Zhao, R.-Q.; Ou, L.-L.; Li, B.-Z.; Xing, T. Periodontitis Exacerbates and Promotes the Progression of Chronic Kidney Disease Through Oral Flora, Cytokines, and Oxidative Stress. Front. Microbiol. 2021, 12, 656372. [Google Scholar] [CrossRef] [PubMed]
- Petchey, W.G.; Hickman, I.J.; Duncan, E.; Prins, J.B.; Hawley, C.M.; Johnson, D.W.; Barraclough, K.; Isbel, N.M. The Role of 25-Hydroxyvitamin D Deficiency in Promoting Insulin Resistance and Inflammation in Patients with Chronic Kidney Disease: A Randomised Controlled Trial. BMC Nephrol. 2009, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Gallo, D.; Baci, D.; Kustrimovic, N.; Lanzo, N.; Patera, B.; Tanda, M.L.; Piantanida, E.; Mortara, L. How Does Vitamin D Affect Immune Cells Crosstalk in Autoimmune Diseases? Int. J. Mol. Sci. 2023, 24, 4689. [Google Scholar] [CrossRef]
- Khashim Alswailmi, F.; Shah, S.I.A.; Nawaz, H.; Al-Mazaideh, G.M. Molecular Mechanisms of Vitamin D-Mediated Immunomodulation. Galen. Med. J. 2021, 10, e2097. [Google Scholar] [CrossRef]
- Bscheider, M.; Butcher, E.C. Vitamin D Immunoregulation through Dendritic Cells. Immunology 2016, 148, 227–236. [Google Scholar] [CrossRef]
- Drozdenko, G.; Scheel, T.; Heine, G.; Baumgrass, R.; Worm, M. Impaired T Cell Activation and Cytokine Production by Calcitriol-Primed Human B Cells. Clin. Exp. Immunol. 2014, 178, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Peruzzu, D.; Dupuis, M.L.; Pierdominici, M.; Fecchi, K.; Gagliardi, M.C.; Ortona, E.; Pagano, M.T. Anti-Inflammatory Effects of 1,25(OH)2D/Calcitriol in T Cell Immunity: Does Sex Make a Difference? Int. J. Mol. Sci. 2022, 23, 9164. [Google Scholar] [CrossRef]
- Gonzalez-Curiel, I.; Marin-Luevano, P.; Trujillo, V.; Enciso-Moreno, J.A.; Gonzalez-Castillo, C.; Rivas-Santiago, B. Calcitriol Prevents Inflammatory Gene Expression in Macrovascular Endothelial Cells. Br. J. Biomed. Sci. 2016, 73, 74–78. [Google Scholar] [CrossRef]
- Rafique, A.; Etzerodt, A.; Graversen, J.H.; Moestrup, S.K.; Dagnæs-Hansen, F.; Møller, H.J. Targeted Lipid Nanoparticle Delivery of Calcitriol to Human Monocyte-Derived Macrophages in Vitro and in Vivo: Investigation of the Anti-Inflammatory Effects of Calcitriol. Int. J. Nanomed. 2019, 14, 2829–2846. [Google Scholar] [CrossRef]
- Lopez, D.V.; Al-Jaberi, F.A.H.; Woetmann, A.; Ødum, N.; Bonefeld, C.M.; Kongsbak-Wismann, M.; Geisler, C. Macrophages Control the Bioavailability of Vitamin D and Vitamin D-Regulated T Cell Responses. Front. Immunol. 2021, 12, 722806. [Google Scholar] [CrossRef]
- Ong, L.T.C.; Schibeci, S.D.; Fewings, N.L.; Booth, D.R.; Parnell, G.P. Age-Dependent VDR Peak DNA Methylation as a Mechanism for Latitude-Dependent Multiple Sclerosis Risk. Epigenetics Chromatin 2021, 14, 9. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes. Front. Immunol. 2019, 10, 2211. [Google Scholar] [CrossRef]
- França, L.F.C.; Vasconcelos, A.C.C.G.; da Silva, F.R.P.; Alves, E.H.P.; Carvalho, J.S.; Lenardo, D.D.; de Souza, L.K.M.; Barbosa, A.L.R.; Medeiros, J.-V.R.; de Oliveira, J.S.; et al. Periodontitis Changes Renal Structures by Oxidative Stress and Lipid Peroxidation. J. Clin. Periodontol. 2017, 44, 568–576. [Google Scholar] [CrossRef]
- Ding, R.; Jiang, Y.; Yang, Y.; Shi, Y.; Ji, Y.; Zhen, T.; Fu, Z.; Bao, X.; Tan, J.; Zhang, S.; et al. Calcitriol Ameliorates Renal Injury with High-Salt Diet-Induced Hypertension by Upregulating GLIS2 Expression and AMPK/mTOR-Regulated Autophagy. Gene 2022, 820, 146239. [Google Scholar] [CrossRef]
- Oliveira, B.M.; de Almeida, L.F.; Deluque, A.L.; Souza, C.S.; Maciel, A.L.D.; Francescato, H.D.C.; Costa, R.S.; Giovanini, C.; de Paula, F.J.A.; Coimbra, T.M. Calcitriol Reduces the Inflammation, Endothelial Damage and Oxidative Stress in AKI Caused by Cisplatin. Int. J. Mol. Sci. 2022, 23, 15877. [Google Scholar] [CrossRef]
- Annamalai, C.; Seth, R.; Viswanathan, P. Ferrotoxicity and Its Amelioration by Calcitriol in Cultured Renal Cells. Anal. Cell. Pathol. 2021, 2021, 6634429. [Google Scholar] [CrossRef]
- Olsen, B.; Bodea, J.; Garcia, A.; Beebe, K.; Campbell, C.; Schwalbach, C.; Salzberg, D.; Miller, H.; Adams, R.; Mirea, L.; et al. Vitamin D Supplementation: Association with Serum Cytokines in Pediatric Hematopoietic Stem Cell Transplantation. Front. Pediatr. 2022, 10, 913586. [Google Scholar] [CrossRef]
- Trachtman, H. Emerging Drugs for Treatment of Focal Segmental Glomerulosclerosis. Expert. Opin. Emerg. Drugs 2020, 25, 367–375. [Google Scholar] [CrossRef]
- Beaudreuil, S.; Lorenzo, H.K.; Elias, M.; Nnang Obada, E.; Charpentier, B.; Durrbach, A. Optimal Management of Primary Focal Segmental Glomerulosclerosis in Adults. Int. J. Nephrol. Renov. Dis. 2017, 10, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.E.; Maes, M.; Godeny, P.; Matsumoto, A.K.; Barbosa, D.S.; da Silva, T.A.F.; Souza, F.H.M.O.; Delfino, V.D.A. Addition of Vitamin D Reverses the Decline in GFR Following Treatment with ACE Inhibitors/Angiotensin Receptor Blockers in Patients with Chronic Kidney Disease. Life Sci. 2017, 191, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Legarth, C.; Grimm, D.; Wehland, M.; Bauer, J.; Krüger, M. The Impact of Vitamin D in the Treatment of Essential Hypertension. Int. J. Mol. Sci. 2018, 19, 455. [Google Scholar] [CrossRef] [PubMed]
- Malek Mahdavi, A. A Brief Review of Interplay between Vitamin D and Angiotensin-converting Enzyme 2: Implications for a Potential Treatment for COVID-19. Rev. Med. Virol. 2020, 30, e2119. [Google Scholar] [CrossRef] [PubMed]
- Tylicki, P.; Polewska, K.; Och, A.; Susmarska, A.; Puchalska-Reglińska, E.; Parczewska, A.; Biedunkiewicz, B.; Szabat, K.; Renke, M.; Tylicki, L.; et al. Angiotensin Converting Enzyme Inhibitors May Increase While Active Vitamin D May Decrease the Risk of Severe Pneumonia in SARS-CoV-2 Infected Patients with Chronic Kidney Disease on Maintenance Hemodialysis. Viruses 2022, 14, 451. [Google Scholar] [CrossRef] [PubMed]
- Verdoia, M.; Nardin, M.; Rolla, R.; Negro, F.; Gioscia, R.; Saghir Afifeh, A.M.; Viglione, F.; Suryapranata, H.; Marcolongo, M.; De Luca, G. Vitamin D Levels Condition the Outcome Benefits of Renin-Angiotensin System Inhibitors (RASI) among Patients Undergoing Percutaneous Coronary Intervention. Pharmacol. Res. 2020, 160, 105158. [Google Scholar] [CrossRef]
- Charoenngam, N.; Jaroenlapnopparat, A.; Mettler, S.K.; Grover, A. Genetic Variations of the Vitamin D Metabolic Pathway and COVID-19 Susceptibility and Severity: Current Understanding and Existing Evidence. Biomedicines 2023, 11, 400. [Google Scholar] [CrossRef]
- Voltan, G.; Cannito, M.; Ferrarese, M.; Ceccato, F.; Camozzi, V. Vitamin D: An Overview of Gene Regulation, Ranging from Metabolism to Genomic Effects. Genes 2023, 14, 1691. [Google Scholar] [CrossRef]
- Mazahery, H.; Von Hurst, P.R. Factors Affecting 25-Hydroxyvitamin D Concentration in Response to Vitamin D Supplementation. Nutrients 2015, 7, 5111–5142. [Google Scholar] [CrossRef]
- Ramasamy, I. Vitamin D Metabolism and Guidelines for Vitamin D Supplementation. Clin. Biochem. Rev. 2020, 41, 103–126. [Google Scholar] [CrossRef]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.-F. Vitamin D and Obesity/Adiposity—A Brief Overview of Recent Studies. Nutrients 2022, 14, 2049. [Google Scholar] [CrossRef]
- Yang, L.; Sato, M.; Saito-Abe, M.; Miyaji, Y.; Sato, C.; Nishizato, M.; Kumasaka, N.; Mezawa, H.; Yamamoto-Hanada, K.; Ohya, Y. Smoking Exposure Is Associated with Serum Vitamin D Deficiency in Children: Evidence from the Japan Environment and Children’s Study. Nutrients 2022, 14, 3121. [Google Scholar] [CrossRef]
- Hansen, A.L.; Ambroziak, G.; Thornton, D.M.; Mundt, J.C.; Kahn, R.E.; Dahl, L.; Waage, L.; Kattenbraker, D.; Grung, B. Vitamin D Status and Physical Activity during Wintertime in Forensic Inpatients—A Randomized Clinical Trial. Nutrients 2021, 13, 3510. [Google Scholar] [CrossRef]
- Kannan, T.; Foster, Y.; Ho, D.J.; Gelzinnis, S.J.; Merakis, M.; Wynne, K.; Balogh, Z.J.; Bendinelli, C. Post-Operative Permanent Hypoparathyroidism and Preoperative Vitamin D Prophylaxis. J. Clin. Med. 2021, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Hermanns, C.; Lauer, A.A.; Reichrath, J.; Erhardt, T.; Hartmann, T.; Grimm, M.O.W.; Grimm, H.S. Vitamin D and Its Analogues: From Differences in Molecular Mechanisms to Potential Benefits of Adapted Use in the Treatment of Alzheimer’s Disease. Nutrients 2023, 15, 1684. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Cooper, S.C.; Ghosh, S.; Hewison, M. The Role of Vitamin D in Inflammatory Bowel Disease: Mechanism to Management. Nutrients 2019, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Vernia, F.; Valvano, M.; Longo, S.; Cesaro, N.; Viscido, A.; Latella, G. Vitamin D in Inflammatory Bowel Diseases. Mechanisms of Action and Therapeutic Implications. Nutrients 2022, 14, 269. [Google Scholar] [CrossRef]
- Bendix, M.; Dige, A.; Jørgensen, S.P.; Dahlerup, J.F.; Bibby, B.M.; Deleuran, B.; Agnholt, J. Seven Weeks of High-Dose Vitamin D Treatment Reduces the Need for Infliximab Dose-Escalation and Decreases Inflammatory Markers in Crohn’s Disease during One-Year Follow-Up. Nutrients 2021, 13, 1083. [Google Scholar] [CrossRef]
- Escobedo-Monge, M.F.; Marcos-Temprano, M.; Parodi-Román, J.; Escobedo-Monge, M.A.; Alonso-Vicente, C.; Torres-Hinojal, M.C.; Marugán-Miguelsanz, J.M. Calcium, Phosphorus, and Vitamin D Levels in a Series of Cystic Fibrosis Patients: A Cross-Sectional Study. Int. J. Mol. Sci. 2024, 25, 1900. [Google Scholar] [CrossRef]
- Gao, S.; Sun, C.; Kong, J. Vitamin D Attenuates Ulcerative Colitis by Inhibiting ACSL4-Mediated Ferroptosis. Nutrients 2023, 15, 4845. [Google Scholar] [CrossRef]
- Infantino, C.; Francavilla, R.; Vella, A.; Cenni, S.; Principi, N.; Strisciuglio, C.; Esposito, S. Role of Vitamin D in Celiac Disease and Inflammatory Bowel Diseases. Nutrients 2022, 14, 5154. [Google Scholar] [CrossRef]
- Muñoz, A.; Grant, W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients 2022, 14, 1448. [Google Scholar] [CrossRef]
- Gnagnarella, P.; Muzio, V.; Caini, S.; Raimondi, S.; Martinoli, C.; Chiocca, S.; Miccolo, C.; Bossi, P.; Cortinovis, D.; Chiaradonna, F.; et al. Vitamin D Supplementation and Cancer Mortality: Narrative Review of Observational Studies and Clinical Trials. Nutrients 2021, 13, 3285. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Cameselle, C.; Otero, P.; Simal-Gandara, J. The Impact of Vitamin D and Its Dietary Supplementation in Breast Cancer Prevention: An Integrative Review. Nutrients 2024, 16, 573. [Google Scholar] [CrossRef]
- Ahmed, M.; Mital, D.; Abubaker, N.E.; Panourgia, M.; Owles, H.; Papadaki, I.; Ahmed, M.H. Bone Health in People Living with HIV/AIDS: An Update of Where We Are and Potential Future Strategies. Microorganisms 2023, 11, 789. [Google Scholar] [CrossRef] [PubMed]
- Mavar, M.; Sorić, T.; Bagarić, E.; Sarić, A.; Matek Sarić, M. The Power of Vitamin D: Is the Future in Precision Nutrition through Personalized Supplementation Plans? Nutrients 2024, 16, 1176. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.; Morelli, M.E.; Caruso, P. Vitamin D in Neurological Diseases: A Rationale for a Pathogenic Impact. Int. J. Mol. Sci. 2018, 19, 2245. [Google Scholar] [CrossRef] [PubMed]
- Plantone, D.; Primiano, G.; Manco, C.; Locci, S.; Servidei, S.; De Stefano, N. Vitamin D in Neurological Diseases. Int. J. Mol. Sci. 2023, 24, 87. [Google Scholar] [CrossRef]
- Sailike, B.; Onzhanova, Z.; Akbay, B.; Tokay, T.; Molnár, F. Vitamin D in Central Nervous System: Implications for Neurological Disorders. Int. J. Mol. Sci. 2024, 25, 7809. [Google Scholar] [CrossRef]
- Cui, X.; Eyles, D.W. Vitamin D and the Central Nervous System: Causative and Preventative Mechanisms in Brain Disorders. Nutrients 2022, 14, 4353. [Google Scholar] [CrossRef]
- Pludowski, P. Supplementing Vitamin D in Different Patient Groups to Reduce Deficiency. Nutrients 2023, 15, 3725. [Google Scholar] [CrossRef]
- Pludowski, P.; Marcinowska-Suchowierska, E.; Togizbayev, G.; Belaya, Z.; Grant, W.B.; Pilz, S.; Holick, M.F. Daily and Weekly “High Doses” of Cholecalciferol for the Prevention and Treatment of Vitamin D Deficiency for Obese or Multi-Morbidity and Multi-Treatment Patients Requiring Multi-Drugs—A Narrative Review. Nutrients 2024, 16, 2541. [Google Scholar] [CrossRef]
- Płudowski, P.; Kos-Kudła, B.; Walczak, M.; Fal, A.; Zozulińska-Ziółkiewicz, D.; Sieroszewski, P.; Peregud-Pogorzelski, J.; Lauterbach, R.; Targowski, T.; Lewiński, A.; et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients 2023, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Borchert, M.L.; DeLuca, H.F. Identification of the Vitamin D Receptor in Various Cells of the Mouse Kidney. Kidney Int. 2012, 81, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Mihajlovic, M.; Fedecostante, M.; Oost, M.J.; Steenhuis, S.K.P.; Lentjes, E.G.W.M.; Maitimu-Smeele, I.; Janssen, M.J.; Hilbrands, L.B.; Masereeuw, R. Role of Vitamin D in Maintaining Renal Epithelial Barrier Function in Uremic Conditions. Int. J. Mol. Sci. 2017, 18, 2531. [Google Scholar] [CrossRef] [PubMed]
- Finch, J.L.; Suarez, E.B.; Husain, K.; Ferder, L.; Cardema, M.C.; Glenn, D.J.; Gardner, D.G.; Liapis, H.; Slatopolsky, E. Effect of Combining an ACE Inhibitor and a VDR Activator on Glomerulosclerosis, Proteinuria, and Renal Oxidative Stress in Uremic Rats. Am. J. Physiol. Ren. Physiol. 2012, 302, F141–F149. [Google Scholar] [CrossRef]
- Gonçalves, J.G.; Canale, D.; de Bragança, A.C.; Seguro, A.C.; Shimizu, M.H.M.; Volpini, R.A. The Blockade of TACE-Dependent EGF Receptor Activation by Losartan-Erlotinib Combination Attenuates Renal Fibrosis Formation in 5/6-Nephrectomized Rats Under Vitamin D Deficiency. Front. Med. 2020, 7, 609158. [Google Scholar] [CrossRef]
- Sonneveld, R.; Ferrè, S.; Hoenderop, J.G.J.; Dijkman, H.B.; Berden, J.H.M.; Bindels, R.J.M.; Wetzels, J.F.M.; van der Vlag, J.; Nijenhuis, T. Vitamin D Down-Regulates TRPC6 Expression in Podocyte Injury and Proteinuric Glomerular Disease. Am. J. Pathol. 2013, 182, 1196–1204. [Google Scholar] [CrossRef]
- Garsen, M.; Sonneveld, R.; Rops, A.L.W.M.M.; Huntink, S.; van Kuppevelt, T.H.; Rabelink, T.J.; Hoenderop, J.G.J.; Berden, J.H.M.; Nijenhuis, T.; van der Vlag, J. Vitamin D Attenuates Proteinuria by Inhibition of Heparanase Expression in the Podocyte. J. Pathol. 2015, 237, 472–481. [Google Scholar] [CrossRef]
- Yu, Q.; Qiao, Y.; Liu, D.; Liu, F.; Gao, C.; Duan, J.; Liang, L.; Di, X.; Yuan, Y.; Gao, Y.; et al. Vitamin D Protects Podocytes from Autoantibodies Induced Injury in Lupus Nephritis by Reducing Aberrant Autophagy. Arthritis Res. Ther. 2019, 21, 19. [Google Scholar] [CrossRef]
- Fukuyo, Y.; Nakamura, T.; Bubenshchikova, E.; Powell, R.; Tsuji, T.; Janknecht, R.; Obara, T. Nephrin and Podocin Functions Are Highly Conserved between the Zebrafish Pronephros and Mammalian Metanephros. Mol. Med. Rep. 2014, 9, 457–465. [Google Scholar] [CrossRef]
- Martin, C.E.; Jones, N. Nephrin Signaling in the Podocyte: An Updated View of Signal Regulation at the Slit Diaphragm and Beyond. Front. Endocrinol. 2018, 9, 302. [Google Scholar] [CrossRef]
- Sari, F.T.; Sari, F.T.; Sari, F.T.; Arfian, N.; Sari, D.C.R. Effect of Kidney Ischemia/Reperfusion Injury on Proliferation, Apoptosis, and Cellular Senescence in Acute Kidney Injury in Mice. Med. J. Malays. 2020, 75, 20–23. [Google Scholar]
- Nascimento, F.A.M.; Ceciliano, T.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Maternal Vitamin D Deficiency Delays Glomerular Maturity in F1 and F2 Offspring. PLoS ONE 2012, 7, e41740. [Google Scholar] [CrossRef] [PubMed]
- Denburg, M.R.; Kalkwarf, H.J.; de Boer, I.H.; Hewison, M.; Shults, J.; Zemel, B.S.; Stokes, D.; Foerster, D.; Laskin, B.; Ramirez, A.; et al. Vitamin D Bioavailability and Catabolism in Pediatric Chronic Kidney Disease. Pediatr. Nephrol. 2013, 28, 1843–1853. [Google Scholar] [CrossRef]
- Zaniew, M.; Jarmoliński, T. Vitamin D Status and Bone Density in Steroid-Treated Children with Glomerulopathies: Effect of Cholecalciferol and Calcium Supplementation. Adv. Med. Sci. 2012, 57, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, J.; Minto, A.W.; Hack, B.K.; Alexander, J.J.; Haas, M.; Li, Y.C.; Heilig, C.W.; Quigg, R.J. Altered Vitamin D Metabolism in Type II Diabetic Mouse Glomeruli May Provide Protection from Diabetic Nephropathy. Kidney Int. 2006, 70, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Xiao, C.; Jia, X.; Luo, C.; Shi, L.; Xia, R.; Zhu, J.; Zhang, S. Vitamin D/VDR Protects Against Diabetic Kidney Disease by Restoring Podocytes Autophagy. Diabetes Metab. Syndr. Obes. 2021, 14, 1681–1693. [Google Scholar] [CrossRef]
- Ristov, M.-C.; Lange, T.; Artelt, N.; Nath, N.; Kuss, A.W.; Gehrig, J.; Lindenmeyer, M.; Cohen, C.D.; Gul, S.; Endlich, K.; et al. The ShGlomAssay Combines High-Throughput Drug Screening With Downstream Analyses and Reveals the Protective Role of Vitamin D3 and Calcipotriol on Podocytes. Front. Cell Dev. Biol. 2022, 10, 838086. [Google Scholar] [CrossRef]
- Hamzawy, M.; Gouda, S.A.A.; Rashid, L.; Attia Morcos, M.; Shoukry, H.; Sharawy, N. The Cellular Selection between Apoptosis and Autophagy: Roles of Vitamin D, Glucose and Immune Response in Diabetic Nephropathy. Endocrine 2017, 58, 66–80. [Google Scholar] [CrossRef]
- de Boer, I.H.; Zelnick, L.R.; Ruzinski, J.; Friedenberg, G.; Duszlak, J.; Bubes, V.Y.; Hoofnagle, A.N.; Thadhani, R.; Glynn, R.J.; Buring, J.E.; et al. Effect of Vitamin D and Omega-3 Fatty Acid Supplementation on Kidney Function in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2019, 322, 1899–1909. [Google Scholar] [CrossRef]
- Obaid, A.A.; Almasmoum, H.; Almaimani, R.A.; El-Boshy, M.; Aslam, A.; Idris, S.; Ghaith, M.M.; El-Readi, M.Z.; Ahmad, J.; Farrash, W.F.; et al. Vitamin D and Calcium Co-Therapy Mitigates Pre-Established Cadmium Nephropathy by Regulating Renal Calcium Homeostatic Molecules and Improving Anti-Oxidative and Anti-Inflammatory Activities in Rat. J. Trace Elem. Med. Biol. 2023, 79, 127221. [Google Scholar] [CrossRef]
- BaSalamah, M.A.; Abdelghany, A.H.; El-Boshy, M.; Ahmad, J.; Idris, S.; Refaat, B. Vitamin D Alleviates Lead Induced Renal and Testicular Injuries by Immunomodulatory and Antioxidant Mechanisms in Rats. Sci. Rep. 2018, 8, 4853. [Google Scholar] [CrossRef]
- Fevrier-Paul, A.; Soyibo, A.K.; Mitchell, S.; Voutchkov, M. Role of Toxic Elements in Chronic Kidney Disease. J. Health Pollut. 2018, 8, 181202. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, R.P.; Rayego-Mateos, S.; Alique, M.; Márquez-Expósito, L.; Tejedor-Santamaria, L.; Ortiz, A.; González-Parra, E.; Ruiz-Ortega, M. Vitamin D, Cellular Senescence and Chronic Kidney Diseases: What Is Missing in the Equation? Nutrients 2023, 15, 1349. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, W.; Al Sayeh, A.; del Pino, F.J. Vitamin D in Modulating Liver And Kidney Functions Following Cadmium Exposure In Vivo. J. Angiother. 2024, 8, 1–7. [Google Scholar] [CrossRef]
- Yang, S.P.; Ong, L.; Loh, T.P.; Chua, H.R.; Tham, C.; Meng, K.C.; Pin, L. Calcium, Vitamin D, and Bone Derangement in Nephrotic Syndrome. J. ASEAN Fed. Endocr. Soc. 2021, 36, 50–55. [Google Scholar] [CrossRef]
- Selewski, D.T.; Chen, A.; Shatat, I.F.; Pais, P.; Greenbaum, L.A.; Geier, P.; Nelson, R.D.; Kiessling, S.G.; Brophy, P.D.; Quiroga, A.; et al. Vitamin D in Incident Nephrotic Syndrome: A Midwest Pediatric Nephrology Consortium Study. Pediatr. Nephrol. 2016, 31, 465–472. [Google Scholar] [CrossRef][Green Version]
- Maji, M.; Kumar, M.; Chacham, S.; Mirza, A.A.; Bhat, N.K.; Mandal, S. Severity of Vitamin D Deficiency in Children with Nephrotic Syndrome: A Study from Tertiary Care Center in Northern India. Saudi J. Kidney Dis. Transpl. 2022, 33, 608–616. [Google Scholar] [CrossRef]
- Banerjee, S.; Basu, S.; Akhtar, S.; Sinha, R.; Sen, A.; Sengupta, J. Free Vitamin D Levels in Steroid-Sensitive Nephrotic Syndrome and Healthy Controls. Pediatr. Nephrol. 2020, 35, 447–454. [Google Scholar] [CrossRef]
- Lucisano, S.; Buemi, M.; Passantino, A.; Aloisi, C.; Cernaro, V.; Santoro, D. New Insights on the Role of Vitamin D in the Progression of Renal Damage. Kidney Blood Press. Res. 2013, 37, 667–678. [Google Scholar] [CrossRef]
- Dehghani, M.; Nasri, H. Glomerulonephritis and Impact of Vitamin D; a Short Look to the Recent Evidence. J. Parathyr. Dis. 2023, 11, e11196. [Google Scholar] [CrossRef]
- Muchtar, E.; Drake, M.T.; Leung, N.; Dispenzieri, A.; Lacy, M.Q.; Buadi, F.K.; Dingli, D.; Hayman, S.R.; Kapoor, P.; Hwa, Y.L.; et al. Hypovitaminosis D Is Prevalent in Patients With Renal AL Amyloidosis and Associated With Renal Outcome. Front. Endocrinol. 2022, 13, 891712. [Google Scholar] [CrossRef] [PubMed]
- Rangan, G.K.; Schwensen, K.G.; Foster, S.L.; Korgaonkar, M.S.; Peduto, A.; Harris, D.C. Chronic Effects of Dietary Vitamin D Deficiency without Increased Calcium Supplementation on the Progression of Experimental Polycystic Kidney Disease. Am. J. Physiol. Ren. Physiol. 2013, 305, F574–F582. [Google Scholar] [CrossRef] [PubMed]
- Vendramini, L.C.; Dalboni, M.A.; de Carvalho, J.T.G., Jr.; Batista, M.C.; Nishiura, J.L.; Heilberg, I.P. Association of Vitamin D Levels With Kidney Volume in Autosomal Dominant Polycystic Kidney Disease (ADPKD). Front. Med. 2019, 6, 112. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, X.; Yin, Z.; Zhu, S.; Wu, D.; Chen, X. Screening for Potential Serum Biomarkers in Rat Mesangial Proliferative Nephritis. Proteomics 2016, 16, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hu, R.; Zhang, J.; Pei, T.; He, Z.; Ju, L.; Han, Z.; Wang, M.; Xiao, W. High-Dose Vitamin D3 Supplementation Ameliorates Renal Fibrosis by Vitamin D Receptor Activation and Inhibiting TGF-Β1/Smad3 Signaling Pathway in 5/6 Nephrectomized Rats. Eur. J. Pharmacol. 2021, 907, 174271. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).