Abstract
LED light technology has been used in recent years in plant breeding due to its proven energy efficiency, low cost, and high quality for the enhancement of crops, including some aromatic medicinal plants (AMPs). Nonetheless, although several studies have shown that specific wavelengths can increase the content of bioactive compounds used by pharmaceutical, medical, and perfumery industries, there is limited information on this topic and the possible implications for plant stress in AMPs. The current systematic review focused on the effects of LED light on the physiological response, metabolite synthesis, and flowering induction in three important AMP genera: Lavandula, Salvia, and Thymus, belonging to the Lamiaceae family. A literature search was performed in the Web of Science and Scopus databases. This review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The bibliographic analysis highlights the significant variation in physiological responses to different light spectra between species, even within the same genera, implying a need to optimize light conditions in each species to achieve the best results. Finally, this review provides essential information for laying the groundwork for future research focused on enhancing AMPs using LED light to overcome various types of stress.
1. Introduction
Aromatic medicinal plants (AMPs) are widely recognized as the main source of essential oils, which are extensively used in various industries, from perfumery and agri-food to pharmaceuticals and medicine, leading to an increased global demand in recent years []. Additionally, it has been estimated that there are about 17,000 AMP species worldwide, representing around 10% of all members of the plant kingdom, and they are mostly naturally distributed in warm regions [].
Within the AMP families, the Lamiaceae is one of the largest, consisting of approximately 7100 species grouped into 230 genera [], and it is the most commercially exploited family for a range of applications. Specifically, the genera Lavandula, Salvia, and Thymus have been reported to have useful properties such as antibacterial, antispasmodic, antioxidant, anti-inflammatory, analgesic, and anti-flatulence effects due to the production of key secondary metabolites [,,,,,,].
The synthesis of secondary metabolites (especially volatile compounds) in AMPs is related to seasonal and genetic factors. Nonetheless, it has been recognized that environmental factors also significantly impact the quality and yield of essential oils [,,]. In particular, in addition to some biotic agents, the main environmental conditions that substantially influence the behavior of AMPs are humidity, nutrition, CO2, temperature, and light [,].
Light is essential for the growth and development of plants, which are influenced by factors such as its intensity, spectrum, and photoperiod []. Additionally, light is fundamental for various processes, such as seed germination, leaf formation, and flowering, but it is primarily used in photosynthesis []. Photosynthetic activity depends on photosynthetically active radiation (PAR), generally defined as the light spectrum between 400 and 700 nm, that produces a high quantum yield in CO2 assimilation []. Nonetheless, the quantum yield curve is mostly influenced by two peaks in the light spectra, the red (R) and the blue (B) ranges, which are the main energy sources for CO2 assimilation in photosynthesis [].
Plants can recognize different spectra of light through structures called photoreceptors, which are activated as a function of the wavelength []. The B light spectrum is detected by cryptochromes, phototropins, and Zeitlupes, which can recognize wavelengths from 400 nm to 500 nm, while phytochromes can absorb wavelengths between 650 nm (R light) and 740 nm (far-red, FR) [,]. By the recognition of different wavelengths, photoreceptors can generate a specific physiological response in plants. For instance, cryptochromes are involved in de-etiolation, shade avoidance, stomatal opening, and photoperiodic flowering [], while phytochromes participate in the movement of leaves, stomatal development, flowering transition, control germination, and senescence []. According to Park et al. [], when phytochromes recognize the FR spectrum, they control certain signaling pathways that contribute to leaf expansion, and, in particular, influence flowering. On the other hand, this spectrum is closely related to R light since, depending on the R:FR ratio, plants can either activate or deactivate certain physiological processes [].
Additionally, flowering is a response to both environmental and internal signals. To ensure successful reproduction, flowering must occur during the appropriate season and in response to one or more of these signals. The photoperiod is the main signal that controls when plants flower []. During the development and growth of AMPs, flowers play a crucial role in their life cycle []. They are involved in the production of volatile compounds that have additional functions (beyond those mentioned above), such as promoting organ growth, facilitating pollinator activity, defending against pathogens, and coping with abiotic stresses [,].
Currently, to replicate the environmental light conditions found in the field, LED technology is increasingly used in greenhouses, vertical farming, and growth cabinets, with or without natural daylight, maintaining a specific light spectrum, namely, PAR (400–700 nm), over a chosen photoperiod []. In addition, LEDs have shown several advantages, such as low heat emission, wavelength specificity, adjustable light intensity, long lifetime, low energy costs, and spectral composition control [,,].
Systematic reviews and meta-analyses are considered among the most effective, complete, and repeatable, as well as the least biased types of literature reviews, allowing evidence-based conclusions []. We therefore sought to conduct a systematic review to explore the potential of LED light as an innovative technology to improve the performance of AMPs. To this end, we gathered evidence on the effect of different LED light treatments on plant morphology, physiology, production of bioactive compounds, and flowering induction in AMPs, specifically species belonging to Lavandula, Salvia, and Thymus genera.
2. Results
Despite the Lavandula, Thymus, and Salvia genera being among the most commercially important due to the numerous species that have been studied for medical applications, the systematic review revealed a lack of information regarding the effects of LED light treatment for improving these species. That is, overall, this review identifies a need for further research to determine the potential benefits of using this technology in these plants.
2.1. Study Selection
Figure 1 shows the flow of publications through the different phases of the systematic review and corresponding screening steps.
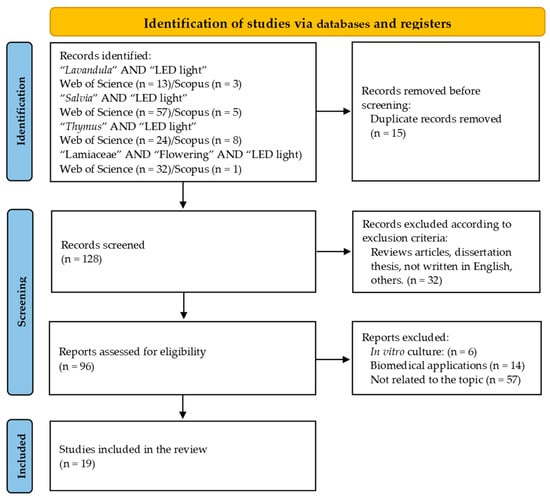
Figure 1.
Preferred Reporting Items in Systematic Reviews and Meta-Analyses (PRISMA) flowchart showing the process of the literature search and publication selection.
The initial search found a total of 143 relevant publications from the last 10 years. Among these, 126 were from WOS and 17 from Scopus. The elimination of duplicates left 128 publications. Out of these, 32 were excluded, for being review articles, dissertation theses, or not written in English, among other reasons. Finally, after removing 87 publications that did not meet other selection criteria, we were left with a sample of 19 publications for inclusion in the review.
Out of the 19 publications that were reviewed systematically, three related to the genus Lavandula, 10 to the genus Salvia, and two to the genus Thymus. All these articles are summarized in Table 1, including the species studied, photosynthetic photon flux density (PPFD), and light spectrum combination used for the LED light treatment, and the main experimental results, as well as the reference.

Table 1.
Main results obtained under different LED light treatments in three genera of aromatic plants: Lavandula, Salvia, and Thymus.
The search also found four articles that discussed the effect of LED light on the flowering of plants belonging to the Lamiaceae family. Data from these articles are compiled in Table 2 including species, experimental conditions, and the results.

Table 2.
Main effects of LED light treatments on flowering in plants belonging to the Lamiaceae family.
2.2. Effects of LED Light on Plant Morphology and Physiology
The use of white (W) LED light with a small amount of the R and FR spectrum was reported to greatly improve the rooting rate in L. dentata []. In addition, W light with a moderate level of R, B, and FR light improved the number of leaves, leaf area, shoot height, and the fresh weight of overground parts in S. fruticosa []. Further, this spectrum (W light) combined with a small amount of FR light was shown to produce better light-use efficiency (mg⋅mol−1) while reducing (NO3)-leaching.
In the case of R light, Wollaeger and Runkle [,] described improvements in the biomass, height, leaf area, and root system of S. plebeia and S. splendens. Likewise, R light was shown to increase the yield of essential oils and some of the main volatile compounds in T. migricus, T. kotschyanus, and T. carmanicus [].
On the other hand, Cáceres-Cevallos et al. [] observed that the exposure of L. latifolia to 100% B light was associated with an increase in leaf area. Similarly, compared to other light regimes (such as R and W light), B light had a positive effect on L. angustifolia, strengthening its root system [].
Further, our review revealed that research found that light with a B:R ratio of 30:70 had positive effects on the photosynthetic rate and stomatal conductance of S. plebeia []. Additionally, this light composition was shown to enhance the quality index and relative chlorophyll content in S. splendens [].
According to Heo et al. [], exposure of S. splendens to a balanced mix of B and R light (50:50 ratio) may increase the photochemical efficiency of PSII. Moreover, the study also revealed that when B light is combined with FR light (50B:50FR), it increases the relative growth rate, as well as the number of flowering buds. Furthermore, a similar effect can also be achieved by using 50R:50FR, resulting not only in more flowering buds but also greater stem elongation.
The literature search also identified two combinations that enabled the improvement of physiological and anatomical parameters in two Salvia species. The first study by Zhang et al. [] on S. miltiorrhiza showed an increase in the growth index and a decrease in the stomatal conductance and transpiration rate using 10B:90R light. On the other hand, the second study conducted by Dorais et al. [] on S. officinalis showed that W light with supplementary 85B:15R lighting led to an improvement in the total biomass.
2.3. Effects of LED Light on the Production of Bioactive Compounds
Bioactive compounds are known to be produced by secondary metabolism, giving plants certain characteristics such as their aroma, color, and taste, and even enhancing their resistance against biotic stress []. Moreover, as a strategy to overcome oxidative damage by reactive oxygen species (ROS), AMPs seem to produce elevated levels of phenolic compounds [,], especially flavonoids and polyphenols []. On the other hand, abiotic stress may decrease the content and richness of the phenolic compounds [], diminishing the essential oil quality [].
R light has been found to increase the levels of phytonutrients and antioxidant activity in Thymus species. For example, studies conducted on T. vulgaris by Seyedi et al. (2024) [] and Tohidi et al. [] observed an enhancement in the levels of important volatile compounds such as α-terpinene and limonene compared to those in plants grown under usual greenhouse conditions. Similarly, Tohidi et al. [] observed that the R light spectrum had a positive effect on the essential oil yield of three Thymus species. Nonetheless, responses to the R light spectrum varied between species, leading to different outcomes in the production of volatile compounds. For instance, the content of p-cymene increased in T. migricus, and that of limonene in T. carmanicus, whereas T. kotschyanus exhibited higher levels of α-terpinene, limonene, and γ-terpinene. On the other hand, Lee [] observed that total phenolics and flavonoid content fell in S. plebeia exposed to R light, leading to a drop in antioxidant activity.
In the case of B light, exposure to this part of the spectrum was associated with an enhancement in the production of rosmarinic acid as well as an increase in antioxidant activity in L. latifolia, as indicated by Medik. et al []. Notably, Chen et al. [] observed similar results in S. miltiorrhiza, although this treatment was also associated with a decrease in the content of diterpenoid tanshinone IIA, which is known for its high biological activities, including as an inhibitor of atheromatous plaque formation, as well as anti-inflammatory and anti-tumor activity. On the other hand, regarding the effect of B light on thyme essential oil and chemotype, Tohidi et al. [] observed that T. migricus and T. carmanicus had higher levels of thymol, while T. kotschyanus showed a rise in the synthesis of p-cymene and carvacrol.
The combination of different spectra has been associated with several effects in species of the genera Salvia and Thymus. For instance, treatment with B30:R70 light increased the production of phenolic compounds in S. plebeia []. Similarly, according to Tohidi et al. [], this light composition led to increased production of p-cymene, γ-terpinene, and carvacrol in T. vulgaris, T. migricus, and T. carmanicus, respectively. Moreover, the B:R ratio of 70:30 seems to be effective in enhancing the production of several acids such as danshensu, caffeic, 4-coumaric, and rosmarinic in S. miltiorrhiza Bunge []. A similar effect has been observed in T. vulgaris [], in which this combination of light spectra led to an increase in anthocyanins and the volatile compounds myrcene and γ-terpinene.
While using limited light spectra alone can generate a range of benefits in various species, some research has also explored using LED light to supplement W light. In particular, supplementing W light with a 15B:85R ratio led to a rise in phenolic content in S. officinalis [].
2.4. Effects of LED Light on the Induction of the Flowering Stage
Since photoperiod is a crucial factor for inducing the flowering stage in AMPs, LED light has been used to speed up the time to flowering. For example, Costine [] observed a shortening in the number of days to flowering in two species of Scutellaria when exposed to different light spectra. According to the study conducted by these authors, the application of B light spectra, along with a decrease in PPD from the first month (300 µmol⋅m−2⋅s−1) to the second month (200 µmol⋅m−2⋅s−1), resulted in the flowering of S. baicalensis in the fifth week and S. lateriflora in the seventh week, which was faster than that observed under the full-spectrum W light treatment.
In separate studies conducted by Sabzalian et al. [] and D’Aquino et al. [], it was found that basil flowers bloomed earlier, with time to flowering reduced by half, when grown using an approximate R:B ratio of 70:30, compared to those grown in a greenhouse or under W LED light, respectively. Similarly, SharathKumar et al. [] discovered that Perilla frutescens produced flowers under a short day (11 h of light) when exposed to the R and B light at a ratio of 60:40.
3. Discussion
Our systematic review found that LED light does affect the morphology and physiology of plants belonging to the Lamiaceae family, in particular, those in the Salvia, Thymus, and Lavandula genera.
Specifically, the use of W LED light improved certain morphological and physiological traits in various species from the Lamiaceae family, consistent with W light treatment being beneficial for photosynthesis as it may penetrate through the canopy to lower leaves []. Furthermore, supplementing W light with other spectra has been shown to enhance yield and production in S. officinalis [], as observed in other aromatic crops such as Ocimum basilicum [,]. Further, supplementing W light with R LED light has reported benefits in some crops, such as increases in yield in Solanum lycopersicum and the production of polyphenolics in Lactuca sativa and β-carotene in Pisum sativum []. In fact, the levels and yield of bioactive compounds are related to the spectrum and quality of light to which plants are exposed, as shown by Dou et al. []. Given this, the use of different light spectra beyond W light to obtain a high content of desirable phytonutrients has been studied in AMPs []. In particular, Nguyen and Saleh [] indicated that the use of a single R or B light source may improve the efficiency of some important processes such as photosynthesis, enhancing plant production, and this has been observed in L. latifolia, L. angustifolia, S. plebeia, S. splendens, T. migricus, T. kotschyanus, and T. carmanicus (Table 1).
In the case of the use of single R light, Dou et al. [] pointed out that it may improve physiological parameters in certain aromatic plants including essential oil yield and accumulation of phenolic content. This behavior may be attributed to the fact that R light can activate photoprotective mechanisms, leading to the production of antioxidant compounds [].
Likewise, using only B light can have certain benefits in crops since this part of the spectrum is involved in regulating plant development and physiological processes through photoreceptors []. Indeed, B light has been found to have several advantages in plants belonging to the Lamiaceae family, such as improving their economic value by stimulating plant metabolism []. Furthermore, previous research has shown that exposure to these wavelengths (R and B light) could increase the production of secondary metabolites when applied only as supplementary light []. Specifically, Bantis et al. [] observed that applying B light led to an increase in the production of rosmarinic acid, which is a potent antioxidant.
Although B, W, or R light spectra alone have been successfully used to enhance physiological and anatomical characteristics in plants, especially in plants belonging to the Lamiaceae family [,,,], some research suggests that their combination is even more effective for acquiring desirable parameters []. For instance, Ahmadi et al. [] showed that mixed B and R light (B30:R70) in Melissa officinalis increased the activity of phenylalanine ammonia-lyase (PAL), which is involved in the biosynthesis of secondary metabolites (flavonoids, anthocyanins, coumarins, and phenolic acids). Likewise, increases in biosynthesis have been observed in other species belonging to the Lamiaceae family [,,,].
In line with this, our systematic review found evidence of the positive effects of combining R and B light at different ratios on various plants, including S. splendens, Salvia plebeia, S. miltiorrhiza, T. vulgaris, T. migricus, and T. carmanicus. Specifically, a B:R ratio of 30:70, which is the most commonly used combination, has previously been shown to provide certain benefits for several species apart from AMP crops [,,,]. This is likely because a combination of different spectra can excite photoreceptors more efficiently, leading to an increase in photosynthesis and plant growth [].
Nonetheless, some studies have reported contrasting results when combining R and B light. For instance, Nguyen and Oh [] reported that red perilla produced less shoot and root fresh and dry weight when treated with certain proportions of R and B, than R or B light alone. On the other hand, Chen et al. [] described an inhibition of chlorogenic acid synthesis in Peucedanum japonicum when applying mixed R and B light with a high proportion of R light.
Additionally, one of the key benefits of using LED lights is the early stimulation of flowering. However, this is a stage that was previously difficult to induce under controlled conditions due to complex environmental factors that control it in the real world [].
Notably, Park and Runkle [] observed that plants in a juvenile phase are not competent to initiate the bloom stage, and even the requirements for flower formation are related to environmental and endogenous cues, with the most important being the photoperiod [].
In this regard, there is evidence that exposure to R light may inhibit flowering, which is necessary for the action of FR light to reverse this effect []. Such observations may be explained by phytochrome B (which recognizes R light) being able to inhibit flowering induction, while phytochrome A (which recognizes FR light) promotes flowering [].
Nonetheless, our systematic review indicates that a mixture of R70:B30 may cause basil flowers to bloom earlier [,], and in certain cases, monochromatic B light may promote earlier flowering in S. baicalensis and S. lateriflora []. On the other hand, SharathKumar et al. [] found that Perilla frutescens flowered well under short-day mixed R and B light, but not under extended B or long-day light treatments. These mixed results underline the need for more research in this field.
The positive influence of treatments containing B light on the flowering could be explained by the fact that cryptochromes (that sense B light) promote the accumulation of CO protein [], which is a zinc finger transcriptional regulator and plays an important role in flowering pathways []. It is regulated by light and the circadian clock []. Apart from this, flowering and fruit sets can be affected by abiotic stress, such as drought, heat, and high salinity, which influence plant maturation and functioning []. In this context, the use of LED light may be an important tool to improve blossom in AMPs, thereby helping to achieve the best point for harvesting and mitigate the adverse effects of extreme conditions.
4. Materials and Methods
4.1. Data Acquisition
A literature search was carried out electronically, seeking to identify studies that had been conducted during the last 10 years and met the following criteria:
- (1)
- they referred to one of three genera, namely, Lavandula, Salvia, or Thymus;
- (2)
- they included LED light treatments;
- (3)
- they assessed flowering in relation to LED light exposure in the Lamiaceae family.
4.2. Search Strategy
This systematic review was conducted by electronically searching two databases: Scopus and Web of Science. For the application of LED light in the genera or species of interest, we used the search string “Genus or species [Topic]” AND “LED light [Topic]”, while for the induction of flowering, we employed the search string “Lamiaceae [Topic]” AND “Flowering [Topic]” AND “LED light [Topic]”. Given that the word “thymus” is also used in human anatomy, the use of this term retrieves numerous publications irrelevant to our study. To avoid this, we opted to use “thyme” rather than “Thymus” for the literature search.
4.3. Selection Criteria
In this systematic review, only studies concerning the application of LED light treatment to species in our genera of interest were considered eligible. Nonetheless, we did include research that used other light sources, such as fluorescent or high-pressure sodium, as a control treatment.
After removing duplicates, we excluded reviews, publications that were not primary studies (e.g., editorials, comments, or letters), posters, conference abstracts, studies not written in English, and studies that did not explore induction of flowering with LED light. Subsequently, the remaining publications were screened in more detail and we excluded studies carried out in vitro, studies focused on biomedical applications, and any unrelated to the topic of interest.
4.4. Study Selection and Data Extraction
The study selection and data extraction are reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations. Two of the authors (G.J.C.-C. and M.J.J.) independently screened the studies retrieved applying the aforementioned selection criteria. In the event of discrepancies, the disagreements were resolved between the authors.
5. Conclusions
This systematic review summarizes information from data produced and reported between 2014 and 2024 on the effects of LED light on three important genera (Salvia, Lavandula, and Thymus) belonging to the Lamiaceae family. LED light technology has proven to be a highly valuable tool in agronomy, with a range of applications in crop improvement and offering several advantages over alternative approaches. Nonetheless, the data available concerning AMP crops are scarce despite their substantial economic potential.
In AMPs, controlled LED light has already demonstrated considerable benefits, including improved growth and increased biomass production, leading to higher essential oil yield, especially in plants belonging to the Thymus genus. This innovative technology can also increase the levels of important phenolic compounds, including monoterpenes and anthocyanins, enhancing their bioactivity and increasing the overall value of crop production. Additionally, LED light has proven to be effective in shortening the time to the flowering of some plants in the Lamiaceae family, enabling the plants to produce seeds for future plant breeding purposes.
Nevertheless, it is important to highlight that each species has shown a specific response that varies with the light treatment applied, indicating the need to examine and standardize light conditions (including spectra, PPFDs, and photoperiods) on a species-by-species basis to attain optimal results for the application of interest.
Author Contributions
Conceptualization, G.J.C.-C. and M.J.J.; methodology, G.J.C.-C.; validation, M.J.J.; formal analysis, G.J.C.-C. and M.J.J.; investigation, G.J.C.-C. and M.J.J.; resources, G.J.C.-C.; writing—original draft preparation, G.J.C.-C.; writing—review and editing, G.J.C.-C. and M.J.J.; supervision, M.J.J.; funding acquisition, M.J.J. All authors have read and agreed to the published version of the manuscript.
Funding
We thank the European Comission ERDF/European Regional Development Fund Operational Programme for Murcia 2021–2027, which financed 60% of the project No. 50467, under which this study was conducted.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors are grateful to the Murcia Institute of Agri-Food and Environmental Research (IMIDA), Murcia, Spain for proofreading and other technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the Consequence of Environmental Stress for Accumulation of Secondary Metabolites in Medicinal and Aromatic Plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Fitsiou, E.; Pappa, A. Anticancer Activity of Essential Oils and Other Extracts from Aromatic Plants Grown in Greece. Antioxidants 2019, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Rahimmalek, M.; Afshari, M.; Sarfaraz, D.; Miroliaei, M. Using HPLC and Multivariate Analyses to Investigate Variations in the Polyphenolic Compounds as Well as Antioxidant and Antiglycative Activities of Some Lamiaceae Species Native to Iran. Ind. Crops Prod. 2020, 154, 112640. [Google Scholar] [CrossRef]
- Kulak, M. Recurrent Drought Stress Effects on Essential Oil Profile of Lamiaceae Plants: An Approach Regarding Stress Memory. Ind. Crops Prod. 2020, 154, 112695. [Google Scholar] [CrossRef]
- Mocan, A.; Babotă, M.; Pop, A.; Fizeșan, I.; Diuzheva, A.; Locatelli, M.; Carradori, S.; Campestre, C.; Menghini, L.; Sisea, C.R.; et al. Chemical Constituents and Biologic Activities of Sage Species: A Comparison between Salvia officinalis L., S. glutinosa L. and S. transsylvanica (Schur Ex Griseb. & Schenk) Schur. Antioxidants 2020, 9, 480. [Google Scholar] [CrossRef]
- Caser, M.; D’Angiolillo, F.; Chitarra, W.; Lovisolo, C.; Ruffoni, B.; Pistelli, L.; Pistelli, L.; Scariot, V. Ecophysiological and Phytochemical Responses of Salvia sinaloensis Fern. to Drought Stress. Plant Growth Regul. 2018, 84, 383–394. [Google Scholar] [CrossRef]
- Khajuria, A.K.; Bisht, N.; Bhagat, N. In Vitro Organogenesis and Plant Regeneration of Thymus serpyllum L.: An Important Aromatic Medicinal Plant. Vitr. Cell. Dev. Biol.-Plant 2020, 56, 652–661. [Google Scholar] [CrossRef]
- Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Regulation of Essential Oil in Aromatic Plants under Changing Environment. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100441. [Google Scholar] [CrossRef]
- Tabbert, J.M.; Schulz, H.; Krähmer, A. Increased Plant Quality, Greenhouse Productivity and Energy Efficiency with Broad-Spectrum LED Systems: A Case Study for Thyme (Thymus vulgaris L.). Plants 2021, 10, 960. [Google Scholar] [CrossRef]
- Radi, F.Z.; Bouhrim, M.; Mechchate, H.; Al-zahranu, M.; Qurtam, A.A.; Aleissa, A.M.; Drioiche, A.; Handaq, N.; Zair, T. Phytochemical Analysis, Antimicrobial and Antioxidant Properties of Thymus zygis L. and Thymus willdenowii Boiss. Essential Oils. Plants 2022, 11, 15. [Google Scholar] [CrossRef]
- Fernández-Sestelo, M.; Carrillo, J. Environmental Effects on Yield and Composition of Essential Oil in Wild Populations of Spike Lavender (Lavandula latifolia Medik.). Agriculture 2020, 10, 626. [Google Scholar] [CrossRef]
- Karalija, E.; Dahija, S.; Tarkowski, P.; Ćavar Zeljković, S. Influence of Climate-Related Environmental Stresses on Economically Important Essential Oils of Mediterranean Salvia Sp. Front. Plant Sci. 2022, 13, 864807. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, A. Secondary Metabolites and Their Antioxidant Capacity of Caucasian Endemic Thyme (Thymus transcaucasicus Ronn.) as Affected by Environmental Stress. J. Appl. Res. Med. Aromat. Plants 2019, 13, 100209. [Google Scholar] [CrossRef]
- Seyedi, F.S.; Nafchi, M.G.; Reezi, S. Effects of Light Spectra on Morphological Characteristics, Primary and Specialized Metabolites of Thymus vulgaris L. Heliyon 2024, 10, e23032. [Google Scholar] [CrossRef]
- Pérez-Romero, L.F.; Stirling, P.J.; Hancock, R.D. Light-Emitting Diodes Improve Yield, Quality and Inhibitory Effects on Digestive Enzymes of Strawberry. Sci. Hortic. 2024, 332, 113192. [Google Scholar] [CrossRef]
- Solbach, J.A.; Fricke, A.; Stützel, H. Seasonal Efficiency of Supplemental LED Lighting on Growth and Photomorphogenesis of Sweet Basil. Front. Plant Sci. 2021, 12, 609975. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.C. Long-Term Effects of Red-and Blue-Light Emitting Diodes on Leaf Anatomy and Photosynthetic Efficiency of Three Ornamental Pot Plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; van Iersel, M.W. Photosynthetic Physiology of Blue, Green, and Red Light: Light Intensity Effects and Underlying Mechanisms. Front. Plant Sci. 2021, 12, 619987. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Van Brenk, J.B.; Courbier, S.; Kleijweg, C.L.; Verdonk, J.C.; Marcelis, L.F.M. Paradise by the Far-Red Light: Far-Red and Red:Blue Ratios Independently Affect Yield, Pigments, and Carbohydrate Production in Lettuce, Lactuca sativa. Front. Plant Sci. 2024, 15, 1383100. [Google Scholar] [CrossRef]
- Andrés, F.; Coupland, G. The Genetic Basis of Flowering Responses to Seasonal Cues. Nat. Publ. Gr. 2012, 13, 627–639. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Blue Radiation Attenuates the Effects of the Red to Far-Red Ratio on Extension Growth but Not on Flowering. Environ. Exp. Bot. 2019, 168, 103871. [Google Scholar] [CrossRef]
- Silva, T.D.; Batista, D.S.; Fortini, E.A.; de Castro, K.M.; Felipe, S.H.S.; Fernandes, A.M.; de Jesus Sousa, R.M.; Chagas, K.; da Silva, J.V.S.; de Freitas Correia, L.N.; et al. Blue and Red Light Affects Morphogenesis and 20-Hydroxyecdisone Content of In Vitro Pfaffia glomerata Accessions. J. Photochem. Photobiol. B Biol. 2020, 203, 111761. [Google Scholar] [CrossRef]
- Park, W.T.; Yeo, S.K.; Sathasivam, R.; Park, J.S.; Kim, J.K.; Park, S.U. Influence of Light-Emitting Diodes on Phenylpropanoid Biosynthetic Gene Expression and Phenylpropanoid Accumulation in Agastache rugosa. Appl. Biol. Chem. 2020, 63, 25. [Google Scholar] [CrossRef]
- SharathKumar, M.; Luo, J.; Xi, Y.; van Ieperen, W.; Marcelis, L.F.M.; Heuvelink, E. Several Short-Day Species Can Flower under Blue-Extended Long Days, but This Response Is Not Universal. Sci. Hortic. 2024, 325, 112657. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.; Xie, Q.; Yu, F. Understanding and Engineering of Aroma Compounds in Crops. Seed Biol. 2024, 3, e001. [Google Scholar] [CrossRef]
- Caven-Quantrill, D.J.; Buglass, A.J. Determination of Volatile Organic Compounds in English Vineyard Grape Juices by Immersion Stir Bar Sorptive Extraction Gas Chromatography-Mass Spectrometry. Flavour Fragr. J. 2007, 22, 206–213. [Google Scholar] [CrossRef]
- Kalaitzoglou, P.; van Ieperen, W.; Harbinson, J.; van der Meer, M.; Martinakos, S.; Weerheim, K.; Nicole, C.C.S.; Marcelis, L.F.M. Effects of Continuous or End-of-Day Far-Red Light on Tomato Plant Growth, Morphology, Light Absorption, and Fruit Production. Front. Plant Sci. 2019, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Lin, C.-C. Red Light-Emitting Diode Light Irradiation Improves Root and Leaf Formation in Difficult-to-Propagate Protea cynaroides L. Plantlets In Vitro. HortScience 2012, 47, 1490–1494. [Google Scholar] [CrossRef]
- Kusaka, M.; Samborska, I.A. Measuring Light Spectrum as a Main Indicator of Artificial Sources Quality. J. Coast. Life Med. 2015, 3, 400–406. [Google Scholar] [CrossRef]
- Koutsos, T.M.; Menexes, G.C.; Dordas, C.A. An Efficient Framework for Conducting Systematic Literature Reviews in Agricultural Sciences. Sci. Total Environ. 2019, 682, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, A.; Van Labeke, M.C.; Gobin, B.; Van Huylenbroeck, J. Rooting of Ornamental Cuttings Affected by Spectral Light Quality. Acta Hortic. 2015, 1104, 219–224. [Google Scholar] [CrossRef]
- Cáceres-Cevallos, G.; Martínez-Conesa, C.; García-Aledo, I.; Quílez-Simón, M.; Romero-Espinar, P.; Jordán, M.J. Lavandula latifolia Medik. Grown with Led Emitting Diode Light Technology, an Initial Approach. Acta Hortic. 2023, 1358, 303–309. [Google Scholar] [CrossRef]
- Peçanha, D.A.; Peña, J.Á.M.; Freitas, M.S.M.; Chourak, Y.; Urrestarazu, M. Effect of Light Spectra on Stem Cutting Rooting and Lavender Growth. Acta Sci.-Agron. 2023, 45, e58864. [Google Scholar] [CrossRef]
- Heo, W.J.; Lee, W.C.; Paek, Y.K. Influence of Mixed LED Radiation on the Growth of Annual Plants. J. Plant Biol. 2006, 49, 286–290. [Google Scholar] [CrossRef]
- Randall, W.C.; Lopez, R.G. Comparison of Supplemental Lighting from High-Pressure Sodium Lamps and Light-Emitting Diodes during Bedding Plant Seedling Production. HortScience 2014, 49, 589–595. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth and Acclimation of Impatiens, Salvia, Petunia, and Tomato Seedlings to Blue and Red Light. HortScience 2015, 50, 522–529. [Google Scholar] [CrossRef]
- Wollaeger, H.M.; Runkle, E.S. Growth of Impatiens, Petunia, Salvia, and Tomato Seedlings under Blue, Green, and Red Light-Emitting Diodes. HortScience 2014, 49, 734–740. [Google Scholar] [CrossRef]
- Chen, I.G.J.; Lee, M.S.; Lin, M.K.; Ko, C.Y.; Chang, W. Te Blue Light Decreases Tanshinone IIA Content in Salvia miltiorrhiza Hairy Roots via Genes Regulation. J. Photochem. Photobiol. B Biol. 2018, 183, 164–171. [Google Scholar] [CrossRef]
- Bantis, F.; Radoglou, K. Testing the Potential of LEDs to Enhance Growth and Quality Characteristics of Salvia fruticosa. Hortic. Sci. 2019, 46, 98–106. [Google Scholar] [CrossRef]
- Lee, H.R.; Kim, H.M.; Jeong, H.W.; Hwang, S.J. Growth and Bioactive Compounds of Salvia Plebeia r. Br. Grown under Various Ratios of Red and Blue Light. Horticulturae 2020, 6, 35. [Google Scholar] [CrossRef]
- Zhang, S.; Ma, J.; Zou, H.; Zhang, L.; Li, S.; Wang, Y. The Combination of Blue and Red LED Light Improves Growth and Phenolic Acid Contents in Salvia miltiorrhiza Bunge. Ind. Crops Prod. 2020, 158, 112959. [Google Scholar] [CrossRef]
- Dorais, M.; Brégard, A.; Ménard, C.; Dansereau, B.; Zyromski, N.; Pepin, S. Indoor Living Green Walls of Aromatic Plants Lit with LEDs. Acta Hortic. 2020, 1287, 117–125. [Google Scholar] [CrossRef]
- Dorais, M.; Brégard, A.; Ménard, C.; Dansereau, B.; Zyromski, N.; Pepin, S. Production of Organic Potted Herbs with LED Supplementary Lighting: Effect on Plant Biomass and Phenols. Acta Hortic. 2020, 1286, 47–54. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A.; Sabzalian, M.R.; Antolín, M.C.; Fiasconaro, M.L.; Sánchez-Díaz, M.; Živčák, M.; Olšovská, K.; Slamka, P.; et al. Thymol, Carvacrol, and Antioxidant Accumulation in Thymus Species in Response to Different Light Spectra Emitted by Light-Emitting Diodes. Food Chem. 2020, 307, 125521. [Google Scholar] [CrossRef]
- Sabzalian, M.R.; Heydarizadeh, P.; Zahedi, M.; Boroomand, A.; Agharokh, M.; Sahba, M.R.; Schoefs, B. High Performance of Vegetables, Flowers, and Medicinal Plants in a Red-Blue LED Incubator for Indoor Plant Production. Agron. Sustain. Dev. 2014, 34, 879–886. [Google Scholar] [CrossRef]
- D’Aquino, L.; Lanza, B.; Gambale, E.; Sighicelli, M.; Menegoni, P.; Modarelli, G.C.; Rimauro, J.; Chianese, E.; Nenna, G.; Fasolino, T.; et al. Growth and Metabolism of Basil Grown in a New-Concept Microcosm under Different Lighting Conditions. Sci. Hortic. 2022, 299, 111035. [Google Scholar] [CrossRef]
- Costine, B.; Zhang, M.; Pearson, B.; Nadakuduti, S.S. Impact of Blue Light on Plant Growth, Flowering and Accumulation of Medicinal Flavones in Scutellaria baicalensis and S. Lateriflora. Horticulturae 2022, 8, 1141. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An Overview of LEDs’ Effects on the Production of Bioactive Compounds and Crop Quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef] [PubMed]
- Szabó, K.; Radácsi, P.; Rajhárt, P.; Ladányi, M.; Németh, É. Stress-Induced Changes of Growth, Yield and Bioactive Compounds in Lemon Balm Cultivars. Plant Physiol. Biochem. 2017, 119, 170–177. [Google Scholar] [CrossRef]
- Askary, M.; Behdani, M.A.; Parsa, S.; Mahmoodi, S.; Jamialahmadi, M. Water Stress and Manure Application Affect the Quantity and Quality of Essential Oil of Thymus Daenensis and Thymus Vulgaris. Ind. Crops Prod. 2018, 111, 336–344. [Google Scholar] [CrossRef]
- Caser, M.; Chitarra, W.; D’Angiolillo, F.; Perrone, I.; Demasi, S.; Lovisolo, C.; Pistelli, L.; Pistelli, L.; Scariot, V. Drought Stress Adaptation Modulates Plant Secondary Metabolite Production in Salvia dolomitica Codd. Ind. Crops Prod. 2019, 129, 85–96. [Google Scholar] [CrossRef]
- Albergaria, E.T.; Oliveira, A.F.M.; Albuquerque, U.P. The Effect of Water Deficit Stress on the Composition of Phenolic Compounds in Medicinal Plants. S. Afr. J. Bot. 2020, 131, 12–17. [Google Scholar] [CrossRef]
- Shelepova, O.V.; Baranova, E.N.; Tkacheva, E.V.; Evdokimenkova, Y.B.; Ivanovskii, A.A.; Konovalova, L.N.; Gulevich, A.A. Aromatic Plants Metabolic Engineering: A Review. Agronomy 2022, 12, 3131. [Google Scholar] [CrossRef]
- Li, R.; Long, J.; Yan, Y.; Luo, J.; Xu, Z.; Liu, X. Addition of White Light to Monochromatic Red and Blue Lights Alters the Formation, Growth, and Dormancy of In Vitro-Grown Solanum tuberosum L. Microtubers. HortScience 2020, 55, 71–77. [Google Scholar] [CrossRef]
- Bantis, F.; Ouzounis, T.; Radoglou, K. Artificial LED Lighting Enhances Growth Characteristics and Total Phenolic Content of Ocimum basilicum, but Variably Affects Transplant Success. Sci. Hortic. 2016, 198, 277–283. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current Review of the Modulatory Effects of LED Lights on Photosynthesis of Secondary Metabolites and Future Perspectives of Microgreen Vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Ginzburg, D.N.; Klein, J.D. LED Pre-Exposure Shines a New Light on Drought Tolerance Complexity in Lettuce (Lactuca sativa) and Rocket (Eruca sativa). Environ. Exp. Bot. 2020, 180, 104240. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsinia, F.; Gianquinto, G. Beyond Vegetables: Effects of Indoor LED Light on Specialized Metabolite Biosynthesis in Medicinal and Aromatic Plants, Edible Flowers, and Microgreens. J. Sci. Food Agric. 2021, 102, 472–487. [Google Scholar] [CrossRef]
- Nguyen, T.L.; Saleh, M.A. Effect of Exposure to Light Emitted Diode (LED) Lights on Essential Oil Composition of Sweet Mint Plants. J. Environ. Sci. Heal.-Part A 2019, 54, 435–440. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Hsu, M.H. Morphological and Physiological Response in Green and Purple Basil Plants (Ocimum basilicum) under Different Proportions of Red, Green, and Blue LED Lightings. Sci. Hortic. 2021, 275, 109677. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, Y.X.; Jung, E.S.; Lee, S.; Park, J.; Lee, C.H.; Sung, J. Characterization of Metabolic Changes under Low Mineral Supply (N, K, or Mg) and Supplemental LED Lighting (Red, Blue, or Red-Blue Combination) in Perilla frutescens Using a Metabolomics Approach. Molecules 2020, 25, 4714. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.B.; Clausen, M.R.; Kjaer, K.H. Spectral Quality of Supplemental LED Grow Light Permanently Alters Stomatal Functioning and Chilling Tolerance in Basil (Ocimum basilicum L.). Sci. Hortic. 2018, 227, 38–47. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Terence Darwell, C.; Mosaleeyanon, K. The Influence of Different Light Spectra on Physiological Responses, Antioxidant Capacity and Chemical Compositions in Two Holy Basil Cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef]
- Araújo, D.X.; Rocha, T.T.; de Carvalho, A.A.; Bertolucci, S.K.V.; Medeiros, A.P.R.; Ribeiro, F.N.S.; Barbosa, S.M.; Pinto, J.E.B.P. Photon Flux Density and Wavelength Influence on Growth, Photosynthetic Pigments and Volatile Organic Compound Accumulation in Aeollanthus suaveolens (Catinga-de-Mulata) under In Vitro Conditions. Ind. Crops Prod. 2021, 168, 113597. [Google Scholar] [CrossRef]
- Naznin, M.T.; Lefsrud, M.; Gravel, V.; Azad, M.O.K. Blue Light Added with Red LEDs Enhance Growth Characteristics, Pigments Content, and Antioxidant Capacity in Lettuce, Spinach, Kale, Basil, and Sweet Pepper in a Controlled Environment. Plants 2019, 8, 93. [Google Scholar] [CrossRef]
- Ahmadi, T.; Shabani, L.; Sabzalian, M.R. LED Light Mediates Phenolic Accumulation and Enhances Antioxidant Activity in Melissa officinalis L. under Drought Stress Condition. Protoplasma 2020, 257, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Barbi, S.; Barbieri, F.; Bertacchini, A.; Montorsi, M. Statistical Optimization of a Hyper Red, Deep Blue, and White Leds Light Combination for Controlled Basil Horticulture. Appl. Sci. 2021, 11, 9279. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of Phenolic Acid and Flavonoid Synthesis to Blue and Blue-Violet Light Depends on Plant Species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Długosz-grochowska, O.; Kołton, A.; Zupnik, M. Effects of LED Supplemental Lighting on Yield and Some Quality Parameters of Lamb’s Lettuce Grown in Two Winter Cycles. Sci. Hortic. 2015, 187, 80–86. [Google Scholar] [CrossRef]
- Mitchell, C.A.; Both, A.; Bourget, C.M.; Burr, J.F.; Kubota, C.; Lopez, R.G.; Morrow, R.C.; Runkle, E.S. LEDs: The Future of Greenhouse Lighting! Chron. Horticult. 2012, 52, 6–12. [Google Scholar]
- Huang, L.; Xiao, Y.; Ran, J.; Wei, L.; Li, Z.; Li, Y.; Zhang, X.; Liao, L.; Wang, D.; Zhao, X.; et al. Drought Tolerance of Faba Bean (Vicia faba L.) Can Be Improved by Specific LED Light Wavelengths. Photosynthetica 2020, 58, 1040–1052. [Google Scholar] [CrossRef]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M.R. Impact of Light-Emitting Diode Irradiation on Photosynthesis, Phytochemical Composition and Mineral Element Content of Lettuce Cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Nguyen, T.K.L.; Oh, M.M. Physiological and Biochemical Responses of Green and Red Perilla to LED-Based Light. J. Sci. Food Agric. 2021, 101, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Agrawal, D.C.; Lee, M.R.; Lee, R.J.; Kuo, C.L.; Wu, C.R.; Tsay, H.S.; Chang, H.C. Influence of LED Light Spectra on In Vitro Somatic Embryogenesis and LC-MS Analysis of Chlorogenic Acid and Rutin in Peucedanum japonicum Thunb.: A Medicinal Herb. Bot. Stud. 2016, 57, 9. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.G.; Meng, Q.; Runkle, E.S. Blue Radiation Signals and Saturates Photoperiodic Flowering of Several Long-Day Plants at Crop-Specific Photon Flux Densities. Sci. Hortic. 2020, 271, 2–6. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, M.; Zhang, J.; Song, Z. Involvement of CONSTANS-like Proteins in Plant Flowering and Abiotic Stress Response. Int. J. Mol. Sci. 2023, 24, 16585. [Google Scholar] [CrossRef]
- Zahid, N.; Alowaiesh, B.F.; Masood, N.; Shafique, K.; Khalid, S.; Khalid, M.S.; Maqbool, M.; Awan, S.I.; Imtiaz, Z. Multi-Locational Study on Plant Growth Regulators to Minimize Pre-Mature Fruit Drop and Maximize Postharvest Quality of Apples. Cogent Food Agric. 2024, 10, 2300178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).